Abstract
Vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and possible induction of pregnancy complications, including miscarriage, fetal malformations, fetal growth restriction and/or stillbirth, are serious concerns for pregnant individuals with COVID-19. According to clinical information, the incidence of vertical transmission of SARS-CoV-2 is limited to date. However, even if a neonate tests negative for SARS-CoV-2, frequent abnormal findings, including fetal and maternal vascular malperfusion, have been reported in cases of COVID-19-positive mothers. Primary receptor of SARS-CoV-2 is estimated as angiotensin-converting enzyme 2 (ACE2). It is highly expressed in maternal-fetal interface cells, such as syncytiotrophoblasts, cytotrophoblasts, endothelial cells, and the vascular smooth muscle cells of primary and secondary villi. However other route of transplacental infection cannot be ruled out. Pathological examinations have demonstrated that syncytiotrophoblasts are often infected with SARS-CoV-2, but fetuses are not always infected. These findings suggest the presence of a placental barrier, even if it is not completely effective. As the frequency and molecular mechanisms of intrauterine vertical transmission of SARS-CoV-2 have not been determined to date, intensive clinical examinations by repeated ultrasound and fetal heart rate monitoring are strongly recommended for pregnant women infected with COVID-19. In addition, careful investigation of placental samples after delivery by both morphological and molecular methods is also strongly recommended.
Keywords: COVID-19, SARS-CoV-2, Pregnancy, Placental barrier
Highlights
-
•
Vertical transmission of COVID-19 is rare but of serious concern.
-
•
COVID-19 placentas show various histopathological findings.
-
•
The precise mechanism of vertical transmission is so far unknown.
-
•
Though placentas are susceptible to SARS-CoV-2, partially they protect fetuses.
1. Introduction
The outbreak of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has emerged as the most critical global public health problem in 2020. For pregnant individuals infected with SARS-CoV-2, vertical transmission and subsequent pregnancy complications, such as miscarriage, fetal malformations, fetal growth restriction and/or stillbirth, are serious concerns.
It is well known that some viral species can cause congenital viral infections and affect the health status of the fetus [1]. For instance, rubella virus infection during pregnancy causes congenital rubella syndrome, which includes cataracts, cardiac abnormalities and sensorineural deafness, and genital herpes simplex virus infection during vaginal delivery presents a risk of neonatal herpes simplex virus (HSV) infections. The transmission route of a virus to the feto-maternal unit depends on the viral species. Diverse viruses can infect several components of the feto-maternal unit, including syncytiotrophoblasts, cytotrophoblasts, endothelial cells, hematopoietic cells, and the fetal membrane.
From the limited information, the incidence of vertical transmission of SARS-CoV-2 has been considered rare; however, some cases have been reported [[2], [3], [4]]. At this time, we do not have specific medicines or effective vaccines for COVID-19. Therefore, to reduce the incidence of vertical transmission of COVID-19, it is important to understand the mechanisms of SARS-CoV-2 intrauterine infection. In this review, we discuss the possibility of vertical transmission of COVID-19, particularly regarding the placental barrier.
2. Placental pathology in COVID-19-positive mothers
The establishment of vertical transmission of COVID-19 is limited to date. According to a systematic review by Lamouroux, 4 neonates among 68 deliveries and 71 neonates were positive for COVID-19 [5]. A systematic review by Yang showed that among 83 neonates, 9 had evidence of SARS-CoV-2 infection [6]. Zaigham et al. reviewed 18 articles reporting data from 108 pregnancies and found one neonatal death and one intrauterine fetal demise (IUFD), in which vertical transmission could not be ruled out [7].
However, frequent abnormal findings in placental pathology have been reported among COVID-19-positive mothers (Table 1 ). The most common finding is vascular malperfusion. Mulvey et al. investigated five placentas from COVID-19 patients who delivered at term [8]. All five placentas showed fetal vascular malperfusion (FVM) with multiple thromboses [8]. Similarly, Baergen et al. reported that among 20 cases, FVM was observed in 9 cases [9]. Shanes et al. examined 16 placentas from patients with COVID-19, including a second-trimester placenta after IUFD at 16 weeks of gestation [10]. Among 15 third-trimester placentas, FVM and maternal vascular malperfusion were present in 12 cases [10]. The placenta from the patient with IUFD showed villous edema and a retroplacental hematoma [10]. Findings such as thrombosis, intramural fibrin deposition, villous stromal-vascular karyorrhexis, and villous infarction were commonly observed [[8], [9], [10]]. Interestingly, acute and chronic inflammatory reactions were rarely observed [[8], [9], [10]]. This finding could be explained by the presence of regulatory cytokines produced by placental and decidual immune cells. Furthermore, the active replication and release of the SARS-CoV-2 cause pyroptosis [11], which is a highly inflammatory pathway in the infected cells. In human trophoblast, pyroptosis also reported as a critical inflammatory form to induce adverse pregnant outcomes [12]. One possible hypothesis is SARS-CoV-2 can infect and replicate in the trophoblast cells but cannot be released. Therefore, inflammatory findings might be hardly observed in the placenta with SARS-CoV-2. However, the placentas of SARS-CoV-2-positive neonates showed chronic intervillositis with the presence of macrophages [3].
Table 1.
Placental pathology in COVID-19 positive mothers.
| Study | N | GA | COVID-19 | Histological findings |
||
|---|---|---|---|---|---|---|
| FVM | Other findings | |||||
| 1 | Mulvey et al. [7] | 5 | 38w–40w | 0 | Thrombosis, Intrauterine fibrin deposition, Villous stromal-vascular karyorrhexis |
Focal increase in perivillous fibrin chorangiosis, Meconium |
| 2 | Bargen et al. [8] | 20 | 33w–40w | 0 | Thrombosis, Intrauterine fibrin deposition, Villous stromal-vascular karyorrhexis, |
Focal increase in fibrin, Intervillous thrombus, Focal chorangiosis Meconium, Maternal vascular malperfusion |
| 3 | Shanes et al. [9] | 16 | 33w–40w | 0 | Fetal vessel-mural fibrin, Clustered avascular villi, Delayed villous maturation, Chorangiosis |
Maternal vascular malperfusion |
| 16w(IUFD) | Villous edema, Retroplacental hematoma | |||||
N, total number of placentas; GA, weeks of gestation; COVID-19, number of COVID-19-positive neonates; FVM, fetal vascular malperfusion.
3. Detection of SARS-CoV-2 antigens in the placental tissue of COVID-19 positive mothers
The placenta functions as a physical barrier between a mother and a fetus. Specifically, syncytiotrophoblasts are the front line of defense against vertical transmission because maternal and fetal tissues are separated by syncytiotrophoblast layers. Currently, there are several reports suggesting placental infection with SARS-CoV-2 (Table 2 ). Patane et al. reported two cases of COVID-19 vertical transmission with placental pathological findings [3]. Through in situ hybridization, SARS-CoV-2 spike protein mRNA was observed in the syncytiotrophoblasts of placentas obtained from mothers with COVID-19-positive neonates, while no SARS-CoV-2 spike protein mRNA was observed in the positive mother's placenta with a COVID-19-negative neonate [3]. Algarroba et al. presented electron microscopy findings of the placenta of a COVID-19-positive patient. They suggested the localization of coronaviral virions in the cytoplasm of syncytiotrophoblasts despite the neonate testing negative for COVID-19 [13]. Penfield et al. reported that three of 11 COVID-19-positive placental swabs were positive according to SARS-CoV-2 PCR testing; however, all of the neonates were negative according to SARS-CoV-2 RNA testing, and none demonstrated clinical symptoms of COVID-19 infection [14]. Mulvey et al. reported that viral spike protein and viral RNA staining within COVID-19-positive placental tissue was rare [8]. These reports suggest that even though SARS-CoV-2 was present in the placenta, the incidence of vertical transmission may be limited. We need to investigate the maternal-fetal interface in SARS-CoV-2 infection cases in more detail.
Table 2.
Detection of SARS-CoV-2 antigens in the placental tissue of COVID-19 positive mothers.
| Study | N | COVID-19 | GA | Histological findings Histological findings | SARS-CoV-2 detection method and findings | |
|---|---|---|---|---|---|---|
| 1 | Patane et al. [3] | 22 | 2 | 37w6da 35w1da |
Chronic intervillosits with macrophages |
Single-molecule RNA in situ hybridization SARS-CoV-2 spike antigen in villous syncytiotrophoblasts |
| 2 | Algarroba et al. [10] | 1 | 0 | 28w4d | Focal villous edema Decidual vasculopathy |
Transmission electron microscopy Virions were visible in syncytiotrophoblasts and villi |
| 3 | Penfield et al. [11] | 11 | 0 | 26w–41w | NA |
PCR Three of 11 COVID-19 positive placental swabs were positive |
N, total number of placentas; GA, weeks of gestation; COVID-19, number of COVID-19-positive neonates.
GA indicates the gestational weeks for COVID-19-positive neonates only.
4. The expression of angiotensin-converting enzyme 2 (ACE2) in the feto-placental unit
ACE2 is a zinc metalloprotease attached to the cell membrane. The main function of ACE2 is the regulation of blood pressure by catalyzing the hydrolysis of angiotensin I to angiotensin II. In addition, ACE2 works as a SARS-CoV-2 receptor for cell entry [15,16]. The expression of ACE2 is observed in various cells, including alveolar epithelial cells, endothelial cells, nephrocytes, and intestinal epithelial cells. Moreover, ACE2 is widely expressed in cells in the female genital tract and feto-placental unit, such as syncytiotrophoblasts, cytotrophoblasts, endothelial cells, and the vascular smooth muscles of primary and secondary villi [17]. A single-cell transcriptome study revealed ACE2 expression at the maternal-fetal interface and in fetal organs [18]. According to RefEx, which is the reference expression dataset, the expression of ACE2 in the reproductive tract is greater than that detected in the lung. Despite the finding that the mRNA expression level of ACE2 was higher in the early gestation placenta than the term placenta [19], clinical data suggest no evidence of an increase in spontaneous abortion cases in patients infected with SARS-CoV-2 in early pregnancy. The aberrant expression of ACE2 is associated with pregnancy complications. For instance, the expression of ACE2 in the arterial endothelium of the umbilical cord was increased in preeclamptic placentas compared with normal placentas [17]. This finding suggests that preeclampsia patients might be a high-risk group of COVID-19 vertical transmission. The expression levels of ACE2 are variable between trophoblast types and differentiation [19], further it can be influenced by pathophysiological conditions such as gestational diabetes mellitus (GDM) and preeclampsia. These changes must be examined carefully to evaluate possible risks of vertical transmission.
ACE2 is also expressed in the vaginal mucosa; however, SARS-CoV-2 was not detectable in the vaginal fluid of women with severe COVID-19 infections in several studies [[20], [21], [22]].
5. Mechanisms of vertical transmission
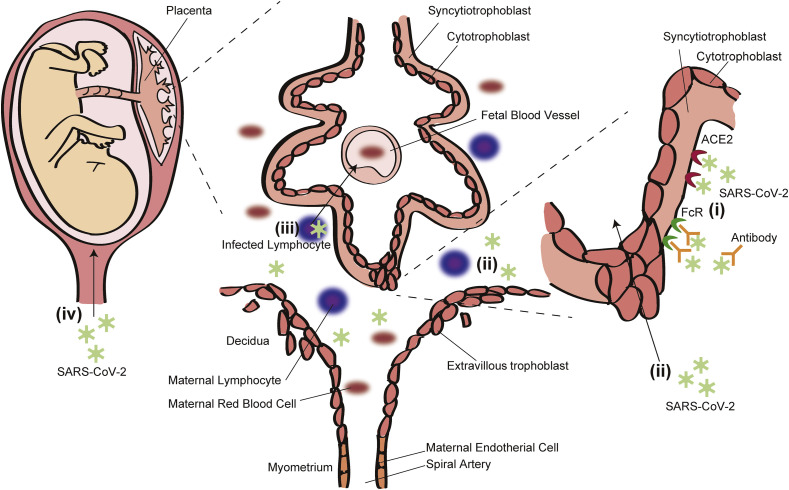
Although the precise mechanisms employed by SARS-CoV-2 to cross the placental barrier are unknown so far, the following possibilities have been considered: (i) direct infection of syncytiotrophoblasts and breach through the syncytial layers via ACE2 and Fc receptor (FcR), (ii) passage through the maternal circulation to extravillous trophoblasts or other placental cells, (iii) passage through maternal immune cells, and (iv) ascending infection via the maternal vaginal tract (Fig. 1 ). According to recent reports, SARS-CoV-2 mRNA or virions were detected in syncytiotrophoblasts [3,13], and transplacental infection of SARS-CoV-2 is strongly suggested. However, direct infection of syncytiotrophoblasts by SARS-CoV-2 must be rare because the incidence of viremia in symptomatic SARS-CoV-2-positive adults was approximately 1% [23]. In addition, the susceptibility and kinetics of SARS-CoV-2 in trophoblasts are not well known. We should continue careful observation of pregnant women with COVID-19 and promote in vitro studies with a trophoblast cell line or primary trophoblast.
Fig. 1.
Possible mechanisms of SARS-CoV-2 vertical transmission. (i) Direct infection of syncytiotrophoblasts and breach through the syncytial layers, (ii) passage through the maternal circulation to extravillous trophoblasts or other placental cells, (iii) passage through maternal immune cells, and (iv) ascending infection via the maternal vaginal tract.
Transmission during vaginal delivery is another possible route of SARS-CoV-2 infection of the neonate. Recent systematic reviews showed that 9.6% [6] to 21.9% [24] of COVID-19 pregnant patients delivered vaginally. A report from Italy concluded that vaginal delivery may be associated with a low risk of intrapartum SARS-CoV-2 transmission to the neonate [25]. In Italy, some researchers recommend routine SARS-CoV-2 PCR assay testing with nasopharyngeal, vaginal and rectal swabs in each pregnant patient before delivery to determine the indication of safe vaginal delivery for patients with negative PCR test results for vaginal and rectal samples [26]. However, the low sensitivity of PCR examination, as well as the financial cost, and limited medical resources make this approach impractical.
Another possible mechanism of vertical transmission is via maternal immune cells. SARS-CoV-2 was reported to infect T lymphocytes through spike protein-mediated membrane fusion [27], and maternal lymphocytes occasionally migrate into the fetal circulation, which is observed as microchimerism.
6. Diagnosis of vertical transmission
There have been several reports of vertical COVID-19 transmission based on positive IgM antibodies in cord blood samples collected at the time of delivery [22]. Due to its large molecular size, IgM cannot pass the placental barrier. Thus, the presence of specific anti-SARS-CoV-2 IgM antibodies suggests intrauterine infection [22]. However, due to the high false positive rates of antibody testing, we must be careful to correctly diagnose intrauterine infection for neonates without any symptoms or positive PCR test results. The detection of viral genomes or viral antigens in neonatal samples is stronger evidence of vertical transmission. However, the site of sampling is an important issue because neonates might be infected with SARS-CoV-2 via the maternal bloodstream. They are not expected to secrete viruses in the sputum, saliva or stools in high copy numbers without prominent inflammation.
7. Possible harms of COVID-19 infection in the early pregnancy
Fortunately, there have been little reports of COVID-19-induced early pregnancy losses, which was reported with SARS [28]. However, we have no information about the effects of SARS-CoV-2 on early embryogenesis and organogenesis. In the Zika virus (ZKV) endemic in 2015, an increase in neural malformations, including microcephaly, was reported several months after the local endemic in Brazil. In this sense, clinicians are requested to perform intensive ultrasound monitoring of the fetuses of mothers infected with COVID-19 in early and mid-pregnancy. Placental investigation after delivery by both morphological and molecular methods is also strongly recommended.
8. Viral and host immune factors that might affect vertical transmission
From its emergence in December 2019, SARS-CoV-2 has constantly mutated [29,30]. There have been several subtypes with different virulence and tropism [31]. These mutations possibly explain different clinical outcomes and rates of mortality between Asian and Caucasian people. In addition, several HLA haplotypes and polymorphisms of immunoregulatory genes, including Toll-like receptor (TLR) promoters and interferon response genes, might affect the susceptibility of the patients, severity of the infection, and rates of vertical transmission. We reported an association between the vertical transmission rates of HIV and several mutations in the viral genome [32]. The role of decidual and intervillous lymphocytes to control local viral transmission is unknown so far. Natural killer cells and cytotoxic T cells are expected to destroy virus-infected cells, but several studies have suggested harmful roles of the cellular immune response and subsequent tissue damage [33,34]. Antibody production might be another double edged sword because some antibodies neutralize viral particles, while others do not inactivate them and are easily captured by Fc receptor-rich target cells, such as endothelial cells and syncytiotrophoblasts, as pointed out in ZKV infection [35].
9. Conclusion
To date, the clinical importance of vertical transmission of SARS-CoV-2 through the placenta remains controversial. The low incidence of COVID-19 vertical transmission may suggest that the placenta functions as a barrier even if it does not completely separate the maternal and fetal circulation. In addition, we have no information about viral factors that might affect the rates of in utero transmission. To confirm the mechanisms of vertical transmission and the placental barrier, extensive investigations are required.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank American Journal Experts for English editing.
References
- 1.Leon-Juarez M., Martinez-Castillo M., Gonzalez-Garcia L.D., Helguera-Repetto A.C., Zaga-Clavellina V., Garcia-Cordero J., Flores-Pliego A., Herrera-Salazar A., Vazquez-Martinez E.R., Reyes-Munoz E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017;75(7) doi: 10.1093/femspd/ftx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 2020;37(8):861. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patane L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM. 2020:100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayakawa S., Komine-Aizawa S., Mor G. COVID-19 pandemic and pregnancy. J. Obstet. Gynaecol. Res. 2020 doi: 10.1111/jog.14384. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamouroux A., Attie-Bitach T., Martinovic J., Leruez-Ville M., Ville Y. Evidence for and against vertical transmission for SARS-CoV-2 (COVID-19) Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z., Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am. J. Perinatol. 2020 doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020 doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulvey J.J., Magro C.M., Ma L.X., Nuovo G.J., Baergen R.N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 2020;46:151530. doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23(3):177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020 doi: 10.1101/2020.05.08.20093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S.B., Nakashima A., Huber W.J., Davis S., Banerjee S., Huang Z., Saito S., Sadovsky Y., Sharma S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019;10(12):927. doi: 10.1038/s41419-019-2162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Algarroba G.N., Rekawek P., Vahanian S.A., Khullar P., Palaia T., Peltier M.R., Chavez M.R., Vintzileos A.M. Visualization of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J., Roman A.S. Detection of SARS-COV-2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020:100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdes G., Neves L.A., Anton L., Corthorn J., Chacon C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27(2–3):200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PloS One. 2020;15(4):e0230295. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pringle K.G., Tadros M.A., Callister R.J., Lumbers E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32(12):956–962. doi: 10.1016/j.placenta.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Qiu L., Liu X., Xiao M., Xie J., Cao W., Liu Z., Morse A., Xie Y., Li T., Zhu L. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., Bao Y., Sun Y., Huang J., Guo Y., Yu Y., Wang S. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effects of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet. Gynecol. 2020 doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S., Bianchi S., Ciriello E., Facchinetti F., Gervasi M.T., Iurlaro E., Kustermann A., Mangili G., Mosca F., Patane L., Spazzini D., Spinillo A., Trojano G., Vignali M., Villa A., Zuccotti G.V., Parazzini F., Cetin I. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020 doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delfino M., Guida M., Patri A., Spirito L., Gallo L., Fabbrocini G. SARS-CoV-2 possible contamination of genital area: implications for sexual and vertical transmission routes. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z., Xie Y., Zhang R., Jiang S., Lu L. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C., Ng P.C., Lam P.W., Ho L.C., To W.W., Lai S.T., Yan W.W., Tan P.Y. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.G. Editors of Infection, Evolution, SARS-CoV-2 and COVID-19: a genetic, epidemiological, and evolutionary perspective. Sironi M., Hasnain S.E., Rosenthal B., Phan T., Luciani F., Shaw M.A., Sallum M.A., Mirhashemi M.E., Morand S., Gonzalez-Candelas F., editors. Infect. Genet. Evol. 2020;84:104384. doi: 10.1016/j.meegid.2020.104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect. Genet. Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becerra-Flores M., Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int. J. Clin. Pract. 2020:e13525. doi: 10.1111/ijcp.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinh Q.D., Pham N.T., Le Nguyen N.T., Lam B.Q., Le Phan K.T., Truong K.H., Izumi Y., Komine-Aizawa S., Mizuguchi M., Ushijima H., Hayakawa S. Short communication: drug resistance mutations in the HIV type 1 protease and reverse transcriptase genes in antiretroviral-naive Vietnamese children. AIDS Res. Hum. Retrovir. 2012;28(10):1305–1307. doi: 10.1089/AID.2011.0280. [DOI] [PubMed] [Google Scholar]
- 33.Yougbare I., Tai W.S., Zdravic D., Oswald B.E., Lang S., Zhu G., Leong-Poi H., Qu D., Yu L., Dunk C., Zhang J., Sled J.G., Lye S.J., Brkic J., Peng C., Hoglund P., Croy B.A., Adamson S.L., Wen X.Y., Stewart D.J., Freedman J., Ni H. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat. Commun. 2017;8(1):224. doi: 10.1038/s41467-017-00269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Zwan A., van der Meer-Prins E.M.W., van Miert P., van den Heuvel H., Anholts J.D.H., Roelen D.L., Claas F.H.J., Heidt S. Cross-reactivity of virus-specific CD8+ T cells against allogeneic HLA-C: possible implications for pregnancy outcome. Front. Immunol. 2018;9:2880. doi: 10.3389/fimmu.2018.02880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuwa K., Hayakawa S. Mechanisms and possible controls of the in utero Zika virus infection: where is the Holy Grail? Am. J. Reprod. Immunol. 2017;77(2) doi: 10.1111/aji.12605. [DOI] [PubMed] [Google Scholar]