Significance
This study identifies a mechanism by which 17β estradiol (estrogen) contributes to peripheral tolerance via modulation of regulatory T cell (Treg) differentiation and function. Our results show that estrogen signaling through the nuclear receptor ERβ is required for TGF-β–dependent differentiation of Tregs, a critical pathway for peripheral Treg differentiation in the gut. Female patients with Crohn’s disease express significantly lower levels of ERβ in peripheral T cells and the intestinal mucosae. Tregs deficient in ERβ express aberrant levels of Tsc22d3 (GILZ), a protein not normally expressed in Tregs and that interferes with functional Treg suppression. Our findings support a pathway by which ERβ-specific signaling normally functions to limit GILZ expression in Tregs, thus maintaining peripheral tolerance in the gut.
Keywords: estrogen, regulatory T cell, inflammatory bowel disease, Crohn’s disease, inflammation
Abstract
Signaling of 17β-estradiol (estrogen) through its two nuclear receptors, α and β (ERα, ERβ), is an important mechanism of transcriptional regulation. Although ERs are broadly expressed by cells of the immune system, the mechanisms by which they modulate immune responses remain poorly understood. ERβ-specific signaling is reduced in patients with chronic inflammatory diseases, including systemic lupus erythematosus and inflammatory bowel disease, and our previous work suggests that dysregulation of ERβ-specific signaling contributes to enhanced intestinal inflammation in female SAMP/YitFC mice, a spontaneous model of Crohn’s disease-like ileitis. The present study builds on these prior observations to identify a nonredundant, immunoprotective role for ERβ-specific signaling in TGF-β–dependent regulatory T cell (Treg) differentiation. Using a strain of congenic SAMP mice engineered to lack global expression of ERβ, we observed dramatic, female-specific exacerbation of intestinal inflammation accompanied by significant reductions in intestinal Treg frequency and function. Impaired Treg suppression in the absence of ERβ was associated with aberrant overexpression of Tsc22d3 (GILZ), a glucocorticoid-responsive transcription factor not normally expressed in mature Tregs, and ex vivo data reveal that forced overexpression of GILZ in mature Tregs inhibits their suppressive function. Collectively, our findings identify a pathway of estrogen-mediated immune regulation in the intestine, whereby homeostatic expression of ERβ normally functions to limit Treg-specific expression of GILZ, thereby maintaining effective immune suppression. Our data suggest that transcriptional cross-talk between glucocorticoid and steroid sex hormone signaling represents an important and understudied regulatory node in chronic inflammatory disease.
Sex-based differences are evident in many physiological and pathological processes, including immune responses to foreign and self-antigens. Women generally mount more robust immune responses than men (1), resulting in heightened immunity to pathogens but also increased prevalence and severity of many chronic inflammatory diseases (2). Accumulating evidence suggests that steroid sex hormone signaling, such as that of 17β estradiol (estrogen, E2), is an important biological variable contributing to these differences. In the case of Crohn’s disease (CD), a chronic form of inflammatory bowel disease (IBD), population studies have linked the use of oral contraceptive pills (OCPs) that raise serum E2 levels to significantly increased risk of disease (3) as well as worsened clinical symptoms (4, 5). Additionally, CD demonstrates an interesting shift in prevalence at the time of puberty; whereas males are at increased risk of pediatric disease, females are at increased risk beginning in the teenage years (6). Collectively, these observations suggest a role for E2 signaling in the development of CD in women.
Aside from its primary roles in female sexual development and function, E2 exerts important gene regulatory effects on diverse cell types throughout the body. E2 signals through two ligand-bound nuclear receptors, α and β (ERα, ERβ), and the membrane-bound G protein-coupled receptor 30 [GPR30 (7), renamed G protein-coupled estrogen receptor 1 (GPER-1)]. Whereas signaling via GPER-1 leads to rapid induction of second messengers (including cAMP, PI3, and Ca3+) and signaling cascades (including MAPK/ERK) (8), signaling via nuclear receptors ERα and ERβ results in significant transcriptional changes in target cells. More than 600 estrogen-responsive genes are directly regulated (positively or negatively) via binding of ERα− or ERβ− homo- or hetero-dimers to DNA of target gene promoters. Transcription of many other genes is indirectly regulated by E2, as ERα and ERβ participate in transcriptional complexes with numerous coregulatory factors (9, 10). When coexpressed in the same cell, ERβ acts as a dominant-negative regulator of ERα signaling (11–14), and therefore, the relative expression (ratio) of ERα and ERβ in a given cell is critical for determining E2’s ultimate cellular effects.
ERα and ERβ are broadly expressed by cells of the immune system, including CD4+ and CD8+ lymphocytes, suggesting that T cells are highly susceptible to regulation by estrogen (15, 16). Indeed, ERα-specific signaling has been shown to be proinflammatory in T cells (17), leading to increased expression of the Th1-associated transcription factor Tbx21 (Tbet) (18) and enhanced production of IFN-γ (19–21). T cell-specific ERα was recently shown to contribute to a murine colitis model by promoting T cell activation and proliferation (22), suggesting a potential pathogenic role for enhanced ERα-specific signaling in T cells. Our recent findings showed that deletion of ERα protects female mice from experimental, dextran sulfate sodium-induced colitis (23), suggesting that enhanced signaling downstream of ERα may be particularly deleterious for females. In contrast, relatively little is known regarding ERβ-specific signaling in cells of the immune system. Mice lacking global expression of ERβ [ERβ-KO (24)] exhibit spontaneous myeloproliferative disease with lymphoid blast crisis (25), suggesting that ERβ has nonredundant roles in immune homeostasis; however, these mechanisms remain poorly understood.
ERβ expression is reduced in the ileal mucosae and peripheral blood T cells of patients with active CD, and is restored to physiologic levels in patients responding to anti-TNF therapies (26, 27), suggesting that TNF-directed therapies may exert their effects, in part, by augmenting ERβ-specific signaling. Chronic inflammation persists in IBD and other chronic inflammatory diseases in response to many factors, including insufficient regulatory mechanisms (28). Although regulatory T cells (Tregs) are present in the IBD intestine at normal or potentially elevated frequencies (29–32), they are functionally insufficient to control inflammation (33, 34). We and others have identified an immunoprotective role for ERβ signaling in CD4+ T cells, via expansion of Tregs (35–37). Our previous work in SAMP1/YitFc (“SAMP”) mice, a spontaneous murine model of CD-like ileitis (38), showed that ileitis in male SAMP mice (but not females) can be ameliorated by implantable E2 pellets and suggested that this effect was potentially due to ERβ-dependent expansion of the intestinal Treg pool in male SAMP mice (36). However, the precise sufficiency and requirement of ERβ signaling in peripheral Treg expansion, as well as the mechanisms underlying the failure of female SAMP mice to respond to E2, remained unclear.
Here, we identify significant female-specific reductions in mucosal and peripheral T cell ERβ expression among patients with active CD, leading to a dramatic perturbation of normal E2 signaling in these individuals (6). To address the mechanisms by which reduced ERβ influences intestinal inflammation in vivo, we developed congenic SAMP mice lacking global expression of ERβ (SAMPΔERβ). Unexpectedly, deletion of ERβ in SAMP led to female-specific worsening of experimental ileitis and intestinal Treg function, suggesting that high levels of E2 (i.e., in females) are required for optimal ERβ-mediated immunoprotection. Tregs isolated from SAMPΔERβ female mice and female CD patients expressed unique gene signatures, characterized by elevated expression of Tsc22d3 (encoding glucocorticoid-induced leucine zipper, GILZ). GILZ expression was negligible in mature, functional Tregs isolated from control subjects, but was found to be strongly induced by a combination of high IL-6 and IFN-γ present in the ileal mucosae of young SAMPΔERβ female mice. Notably, sustained GILZ expression was associated with impaired suppressive function in Tregs naturally expressing GILZ (isolated from SAMPΔERβ female mice), as well as those induced to express GILZ ex vivo in response to dexamethasone (DEX) signaling. Collectively, our findings identify a pathway of E2/ERβ-mediated immunoregulation via repression of Treg GILZ expression. The loss of this regulatory pathway in CD patients who express diminished ERβ levels likely contributes to chronic intestinal inflammation via reduction of Treg functional suppression.
Results
ERβ-Specific Signaling Is Required for E2-Mediated Treg Differentiation.
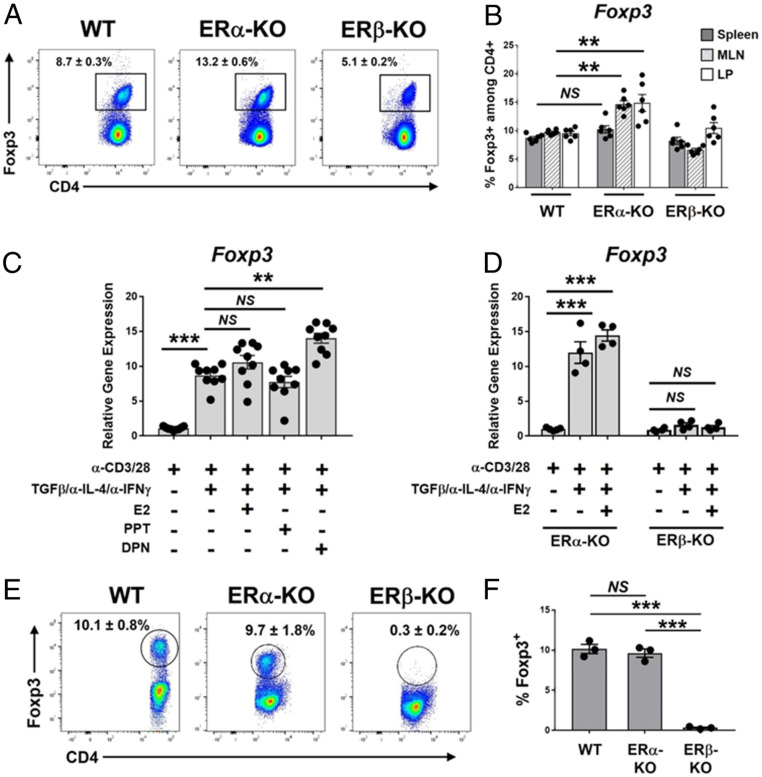
E2 signaling in combination with T cell receptor stimulation has been shown to promote the expression of Foxp3 in naïve CD4+ T cells, contributing to the peripheral differentiation of Tregs (pTregs) (39, 40), and our previous work showed that treatment of male SAMP mice with diarylproprionitrile (DPN), an ERβ-specific agonist, expanded intestinal Tregs in vivo. However, the requirement and sufficiency for ERβ in pTreg differentiation remains unclear. As a first step, we assessed the frequency of Foxp3+ cells within CD4-gated lymphocytes isolated from splenocytes, mesenteric lymph nodes (mLNs), or small intestinal lamina propria (LP) tissue of mice expressing, versus lacking, global expression of ERα or ERβ. We found that mice lacking ERα (ERα-KO) are enriched for CD4+Foxp3+ Tregs in the mLN and LP (Fig. 1 A and B), but not the spleen (Fig. 1B), compared to WT C57BL/6 mice. In contrast, mice lacking global expression of ERβ (ERβ-KO) did not show any significant differences in splenic or intestinal Treg populations (Fig. 1B).
Fig. 1.
Estrogen-mediated differentiation of peripheral Tregs requires ERβ-specific signaling. (A and B) Single-cell suspensions were prepared from ileal LP, spleens, and mLNs of 8- to 10-wk-old C57BL/6 (WT) mice or C57BL/6 mice lacking ERα or ERβ (ERα-KO, ERβ-KO; mixed male and female). Forward scatter/side scatter-gated, singlet cells were analyzed by flow cytometry for expression of CD4 and Foxp3. (A) Representative FACS histograms showing Foxp3 expression among CD4-gated lymphocytes isolated from mLNs of indicated mice. (B) Quantitation of Foxp3 expression among CD4-gated lymphocytes isolated from spleens or mLNs of indicated mice. (C–F) CD4+CD44+ naïve T cells were isolated from spleens of 8- to 10-wk-old (C) C57BL/6 mice or (D–F) ERα-KO or ERβ-KO mice (mixed male and female). Cells were treated ex vivo for 72 h as indicated, followed by analysis of Foxp3 expression. (C and D) Foxp3 gene expression is expressed relative to that of naïve T cells not treated with TGF-β/α-IL-4/α-IFN-γ. (E) TGF-β/α-IL-4/α-IFN-γ–treated cells were permeabilized and stained with mAbs specific to CD4 and Foxp3; representative flow cytometry histograms are shown. (F) Quantitation of results shown in E, representing the percentage of CD4+ cells expressing Foxp3. Data represent the mean ± SEM (NS, not significant; **P ≤ 0.01; ***P ≤ 0.001, n = 3 to 9 per group).
We wished to determine the sufficiency and requirements of ERα- vs. ERβ-specific signaling on E2-mediated pTreg differentiation to determine if these pathways are altered in CD. To test this, we utilized standard TGF-β–dependent ex vivo Treg differentiation assays using naïve T cells (CD4+CD44+) purified from splenocytes of male and female C57BL/6 mice (41). In addition to standard Treg-promoting factors (α-CD3/α-CD28, rmTGF-β, α-IL-4, and α-IFN-γ), cells were cocultured with either E2 or the ER-specific agonists propylpyrazoletriol (PPT, specific for ERα) or DPN (specific for ERβ), all at 5 nM. The addition of DPN, but not PPT or E2, significantly enhanced Foxp3 gene expression above that of TGF-β/α-IL-4/α-IFN-γ alone (Fig. 1C), indicating that ERβ-specific signaling is sufficient to enhance TGF-β–dependent pTreg differentiation.
To determine the requirement of ERα- vs. ERβ-specific signaling in pTreg differentiation, we performed parallel pTreg differentiation assays using naïve T cells isolated from mixed male and female ERα-KO and ERβ-KO mice. Consistent with agonist experiments, T cells from ERα-KO mice (expressing only ERβ) demonstrated an E2-dependent increase in Foxp3 expression, whereas T cells from ERβ-KO mice (expressing only ERα) showed a complete resistance to Foxp3 induction in both the presence and absence of E2 (Fig. 1D). We confirmed our findings at the protein level by intracellular FACS staining of Foxp3 among ex vivo-generated Tregs; similar to gene-expression data, E2 signaling led to induction of Foxp3 protein expression in naïve T cells isolated from WT and ERα-KO mice, but not in cells isolated from ERβ-KO mice (Fig. 1 E and F). Collectively, these data suggest that ERβ-specific signaling is required for TGF-β–dependent Treg differentiation, as well as E2-mediated enhancement of pTreg differentiation.
ERβ Expression Is Selectively Depleted in the Ileal Mucosa and Peripheral Blood T Cells of Female CD Patients.
ERβ is highly expressed by noninflamed/healthy intestinal tissue, where it coordinates intestinal epithelial cell differentiation and proliferation and maintains intestinal barrier function, protecting against exogenous microbes (25, 26, 37, 42). It is therefore unsurprising that IBD patients express reduced levels of intestinal-specific ERβ (26, 27). We asked if there may be a sex difference associated with this effect, and examined gene expression of ERα and ERβ in inflamed ileal biopsy samples from adult males and females with active CD who were free of biologic therapies. Interestingly, female CD patients showed significant reductions in ileal ERβ expression compared to male CD patients and healthy, non-IBD control subjects (68 ± 2% and 72 ± 4% reductions, respectively) (Fig. 2A). We also asked whether the expression of ERβ is altered in T cells from male and female CD patients. We assessed the ratio of ERα to ERβ (ERα:ERβ) among peripheral conventional CD4+ T cells (CD4+CD25−, Tconv) and CD4+CD25highCD127lo Tregs, and observed increased ERα:ERβ ratios (indicating relative enhancement of ERα signaling) in both Tconv and Tregs isolated from female CD patients (Fig. 2B).
Fig. 2.
Female CD patients exhibit reduced ERβ-specific signaling. Gene expression of ERα and ERβ was analyzed in (A) ileal tissue biopsy samples or (B) peripheral blood T cells from indicated donors by qPCR. (A) Relative gene expression of ERα or ERβ is expressed compared to Ctrl males. (B) Ratio of ERα:ERβ expression is expressed for Tconv or Treg cells. Each circle represents an individual donor. Data represent the mean ± SEM (NS, not significant; **P ≤ 0.01; ***P ≤ 0.001, n = 5 to 11 per group).
Global Deletion of ERβ Enhances Ileal Inflammation Selectively in Females.
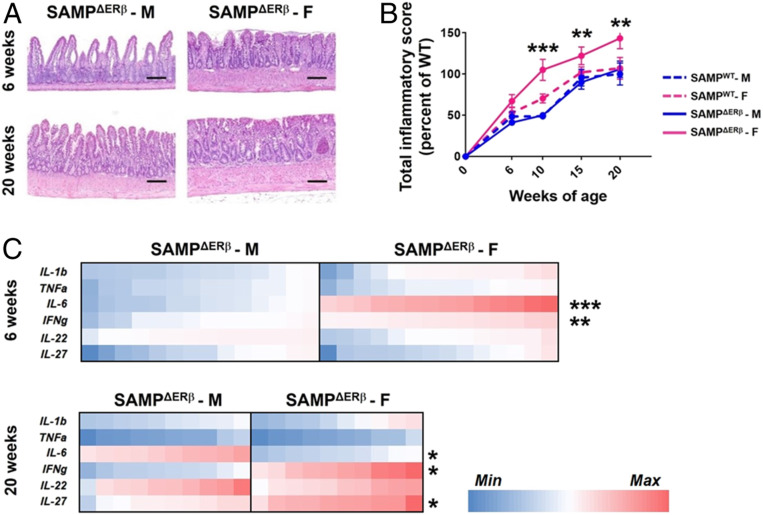
Native SAMP mice exhibit a female sex bias, yet express comparable protein levels of ERα and ERβ to those of AKR/J mice (parental control strain) (36). Thus, to investigate the mechanisms by which altered ERβ signaling contributes to experimental ileitis in vivo, we back-crossed SAMP mice to ERβ-KO mice (24), generating SAMP mice lacking global expression of ERβ (SAMPΔERβ). Histopathological evaluation of ileal tissues from SAMPΔERβ mice revealed significant villus blunting and inflammatory infiltrates in young (6 wk) female SAMPΔERβ mice, which was enhanced at 20 wk of age (Fig. 3A) compared to male SAMPΔERβ mice. Ileal inflammation was assessed by total inflammatory scoring of H&E-stained ileal tissues and was comprised of subscores representing villus distortion, active inflammation, monoinflammation, and chronic inflammation (SI Appendix, Fig. S1). In agreement with our previous observations, native SAMP mice (SAMPWT) displayed a modest female sex bias, which was significant at 10 wk of age (Fig. 3B). However, deletion of ERβ in SAMP mice resulted in dramatically enhanced experimental ileitis in SAMPΔERβ female versus male mice at 10, 15, and 20 wk of age (Fig. 3B). We utilized qPCR to assess the expression of proinflammatory genes in the ileal mucosae of SAMP-ERβ-KO male versus female mice and found a significant increase in IL-6 and IFN-γ in the ileal tissue of young (6 wk) female SAMPΔERβ mice (Fig. 3C). This “high IL-6/IFN-γ” signature was present prior to the exacerbation of the female sex bias (Fig. 3B), suggesting that it may contribute to the worsening ileitis observed in older SAMPΔERβ female mice. IFN-γ remained significantly up-regulated in 20-wk-old female SAMPΔERβ mice, but IL-6 expression was significantly reduced (Fig. 3C). Notably, gene expression of ERα was unchanged between male and female SAMPΔERβ mice (SI Appendix, Fig. S2), suggesting that observed differences are not due to differential compensation of ERα.
Fig. 3.
Global deletion of ERβ exacerbates experimental ileitis in female SAMP mice. SAMP mice lacking global expression of ERβ (SAMPΔERβ mice) were generated by back-crossing SAMP mice with ERβ-KO mice. (A) Representative H&E-stained proximal ileal tissue from 6- and 20-wk-old SAMPΔERβ mice. (Scale bars, 10 μM.) (B) Total inflammatory scores are shown for SAMPΔERβ mice at 6, 10, 15, and 20 wk of age. Data represent the mean ± SEM (**P ≤ 0.01; ***P ≤ 0.001, n = 8 to 14 per group). (C) Relative gene expression of selected proinflammatory genes in full-thickness proximal ileal tissues isolated from 6- or 20-wk old SAMPΔERβ mice is expressed as a heat map. F, female; M, male. *P ≤ 0.05.
Intestinal Tregs from Female SAMPΔERβ Mice Are Functionally Deficient.
Given the requirement for ERβ-specific signaling for E2-mediated Treg differentiation (Fig. 1), together with our previous observations of ERβ-mediated expansion of intestinal Tregs in vivo (36), we hypothesized that SAMPΔERβ mice may exhibit reductions in the overall frequency of intestinal Tregs. FACS analysis of mLN-isolated T cells revealed a significant reduction in the frequency of CD4+-gated Foxp3-expressing cells isolated from SAMPΔERβ female mice compared to SAMPΔERβ male mice (Fig. 4A), in agreement with exacerbated ileal inflammation observed in SAMPΔERβ females (Fig. 3). Notably, whereas the loss of ERβ did not impact the frequency of Foxp3-expressing cells in male SAMP mice, it resulted in a significant reduction in Foxp3-expressing cells in female SAMP (compared to SAMPWT mice) (Fig. 4B). Gene-expression analyses revealed significant sex-dependent skewing of both thymus-derived (t)Tregs and pTregs in response to ERβ deletion in SAMP. Intestinal Tregs from female SAMPΔERβ mice exhibited significantly lower expression of tTreg-associated Ikzf1 (Ikaros) and Ikzf2 (Helios) compared to female SAMPWT mice, as well as lower expression of pTreg-associated Rorc (RORγt). Interestingly, Treg expression of Gata3, also associated with tTregs, was elevated in female SAMPΔERβ mice compared to WT controls (Fig. 4C).
Fig. 4.
Female SAMPΔERβ mice show impaired Treg differentiation and function. (A) Representative FACS histogram showing percentages of CD4+-gated, Foxp3-expressing T cells isolated from mLNs of indicated mice. (B) Quantitation of results shown in A. (C) Relative expression of Treg-associated genes in full-thickness proximal ileal tissues isolated from indicated mice (all 12 to 15 wk of age). (D) Representative FACS plots showing e450 dye dilution of proliferating CD4+CD25− Tconv cells cultured alone (Upper) or cocultured (Lower) with CD4+CD25highCD127lo-expressing Tregs (4:1 Tconv:Treg) isolated from mLNs of male or female SAMP-ERβ-KO mice. (E) Quantitation of results shown in D. (F) Percentage of CD39+/CD73+ coexpressing Tregs from indicated mice, assessed by FACS. Data represents the mean ± SEM (NS, not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001, n = 3 to 8 per group).
To assess the suppressive capacity of intestinal Tregs from SAMPΔERβ mice, eFlour 450-labeled Tconv (CD4+CD25−) were cocultured with mLN-isolated Tregs, and the proliferation of Tconvs in response to anti-CD3/CD28 was assessed by eFluor450 dye dilution. Tregs isolated from SAMPΔERβ female mice were significantly less capable of suppressing Tconv proliferation than those isolated from SAMPΔERβ male mice (Fig. 4D) at all Treg:Tconv ratios tested (Fig. 4E). Hydrolysis of adenosine triphosphate (ATP) to immunosuppressive adenosine (A2) downstream of the purinergic ectoenzymes CD39 and CD73 is an important mechanism by which Tregs mediate immunosuppression (43), and we therefore analyzed the coexpression of CD39/CD73 on mLN-isolated Tregs from SAMPΔERβ vs. SAMPWT mice. Whereas deletion of ERβ did not affect the frequency of CD39/CD73 coexpressing Tregs in male SAMP mice, female SAMPΔERβ mice showed a significant reduction in the frequency of CD39/CD73-expressing intestinal Tregs compared to female SAMPWT mice (Fig. 4F).
Diminished Expression of ERβ Results in Aberrant Induction of Tsc22d3 (GILZ) in Tregs.
To gain insights into the functional differences between male and female SAMPΔERβ Tregs (Fig. 4), we performed global transcriptomic analysis using next-generation RNA sequencing (RNA-seq) (Illumina). CD4+CD25highCD127lo Tregs were FACS-sorted from mLNs of n = 3 mice per group (male and female, SAMPWT and SAMPΔERβ, all 10 to 12 wk of age). An interaction contrast analysis was performed on RNA-seq datasets to identify genes showing significant differences between male and female samples, as well as between ERβ-expressing versus -deficient cells. A heat map based on hierarchical clustering of the top 50 most differentially expressed genes identified by this interaction contrast analysis revealed distinct clusters of genes showing both ERβ- and sex-specific differences (Fig. 5A). We performed gene set enrichment analysis of our RNA-seq dataset, using the Molecular Signatures Database (MSigDB; Broad Institute). MSigDB “Hallmark” and “Immunologic Signatures” gene sets revealed enrichment of several pathways in Tregs isolated from SAMPΔERβ female mice compared to males; these included IFN-α and IFN-γ response pathways [Hallmark gene set (SI Appendix, Fig. S3) and Immunologic Signatures gene set (SI Appendix, Fig. S4)].
Fig. 5.
Impaired expression of ERβ allows aberrant overexpression of Tsc22d3 (GILZ) in Tregs. (A and B) CD4+CD25highCD127lo Tregs were isolated from mLNs of SAMPΔERβ mice and SAMPWT littermate controls by FACS sorting. (A) Tregs were analyzed by next-generation RNA-seq for changes to the global transcriptome, and a dendrogram shows the top 50-most differentially expressed genes by interaction-contrast analysis. (B) Gene expression of Tsc22d3 was analyzed among mLN-isolated Tregs from indicated mice by qPCR. (C and D) CD4+CD44+ naïve T cells were isolated from spleens of SAMPΔERβ male (C) or female (D) mice, then cultured ex vivo with α-CD3/CD28, TGF-β, and neutralizing antibodies α-IL-4 and α-IFN-γ for 5 d. Data represents relative gene expression of Tsc22d3, compared to day 0, in cells from indicated mice. (E) Gene expression of TSC22D3 was analyzed among peripheral blood-derived CD4+CD25highCD127lo Tregs isolated from Ctrl or CD patients. Data represents the mean ± SEM (NS, not significant; **P ≤ 0.01; ***P ≤ 0.001, n = 3 to 8 per group). (F and G) Amnis Imagestream cytometry was used to visualize colocalize ERβ and GILZ in peripheral blood-derived CD4+CD25highCD127lo Tregs isolated from Ctrl or CD patients. (F) Representative Imagestream data from indicated patients is shown; similarity score indicates correlation between MFI (ERβ) and MFI (GILZ). (G) Correlation analysis showing ΔMFI (GILZ) versus ΔMFI (ERβ) for Tregs isolated from male versus female CD patients.
We validated mRNA expression of the 10 most significantly different genes from cluster 1 (genes specifically up-regulated in Tregs from SAMPΔERβ female mice) using qPCR, and identified Tsc22d3 (encoding GILZ) as the most significantly up-regulated gene in SAMPΔERβ female Tregs. Traditional qPCR analysis of a larger cohort of SAMPΔERβ mice confirmed a significant up-regulation of GILZ among mLN-isolated Tregs from SAMPΔERβ female mice (Fig. 5B).
Although GILZ is known to regulate various aspects of T lymphocyte development and function (44–46), including the peripheral differentiation of Tregs, it has not been previously described in mature, fully differentiated Tregs. In fact, GILZ-KO mice exhibit an expanded Treg compartment (47), suggesting that GILZ may normally function as a negative regulator of Tregs. To better understand the normal expression of GILZ throughout the time-course of pTreg differentiation, we isolated naïve T cells (CD4+CD44+) from SAMPWT and SAMPΔERβ mice and performed ex vivo pTreg differentiation, as described for Fig. 1. Cells were sampled at each day (0–5) for gene expression of Tsc22d3, and normalized to expression on day 0 (prior to Treg differentiation). Cells isolated from male and female SAMPWT mice (SI Appendix, Fig. S5) as from male SAMPΔERβ mice showed a temporal reduction in GILZ gene expression throughout the 5-d pTreg differentiation, such that GILZ expression among fully differentiated pTregs (day 5) was ∼30 to 50% of baseline expression values in naïve T cells (day 0) (Fig. 5C). In contrast, cells isolated from female SAMPΔERβ mice maintained a consistent expression of GILZ throughout the 5-d pTreg differentiation (Fig. 5D), suggesting that the normal, temporal regulation of GILZ throughout pTreg differentiation is altered in SAMPΔERβ female mice.
We also analyzed GILZ expression among peripheral blood Tregs (CD4+CD25highCD127lo) isolated from CD patients and healthy controls; we observed that, similar to female SAMPΔERβ mice, female CD patients express significantly higher Treg GILZ (Fig. 5E). Amnis ImageStream cytometry identified a population of CD4+Foxp3+ Tregs expressing high levels of GILZ and low levels of ERβ in peripheral blood lymphocytes isolated from female CD patients (Fig. 5F), in contrast to ERβbright, GILZlow Tregs observed in male CD patients and age-matched, healthy controls (Fig. 5F). Correlation analysis using ΔMFI (mean fluorescence intensity [MFI] of stained sample minus MFI of isotype control) revealed a negative correlation between ERβ and GILZ expression in CD4+Foxp3+-gated Tregs isolated from female, but not male, CD patients (Fig. 5G).
GILZ Expression in Tregs Results in Impaired Suppressive Function.
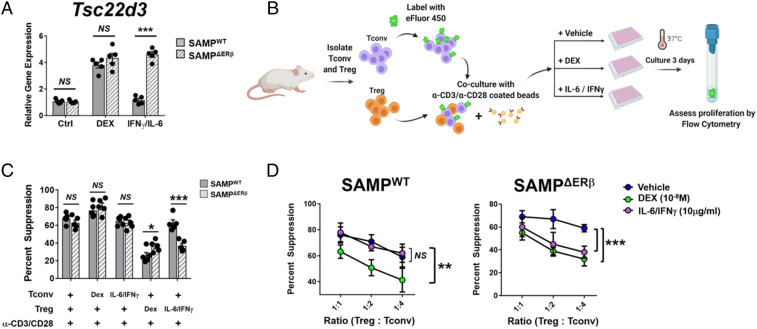
In order to test the functional relevance of aberrant GILZ expression in Tregs, we manipulated GILZ expression in intestinal Tregs isolated from male and female SAMPΔERβ and SAMPWT mice. DEX is known to activate GILZ expression downstream of the glucocorticoid receptor (GR) in T cells (48). First, we assessed the ability of naïve T cells from male and female SAMPWT and SAMPΔERβ mice to up-regulate GILZ in response to DEX, and found that T cells from all groups responded to DEX treatment by inducing GILZ expression (SI Appendix, Fig. S6). We also tested the effects of IFN-γ and IL-6, overexpressed in the ileal mucosa of young SAMPΔERβ female mice (Fig. 3), on T cell GILZ expression. Interestingly, IFN-γ/IL-6 treatment of naïve T cells from SAMPΔERβ mice, but not SAMPWT mice, resulted in an induction of GILZ comparable to that achieved with DEX (Fig. 6A). This suggests that the high levels of IFN-γ and IL-6 present in the inflamed intestine of female SAMPΔERβ mice (Fig. 3) may promote the sustained expression of GILZ in intestinal Tregs.
Fig. 6.
Treg-specific expression of GILZ impairs suppressive function. (A) CD4+CD25highCD127lo Tregs were isolated from mLNs of SAMPΔERβ or SAMPWT mice (mixed male/female) and treated ex vivo as indicated (48 h). Gene expression of Tsc22d3 was determined by qPCR and is expressed relative to Ctrl (untreated) SAMPWT expression values. (B–D) CD4+CD25− Tconv were isolated from spleens of SAMPWT mice (mixed male/female) and cocultured ex vivo with CD4+CD25highCD127lo Tregs isolated from mLNs of SAMPWT or SAMPΔERβ mice as indicated. Either DEX or IL-6/IFN-γ was added to cocultures on day 0. (B) Schematic showing experimental design for Treg suppression assays ± Dex or IL-6/IFN-γ. Figure was created using BioRender. (C) Percent Treg suppression of Tconv proliferation is expressed for the indicated conditions (1:1 Treg:Tconv ratio). (D) Percent Treg suppression of Tconv proliferation is expressed for indicated ratios of Treg:Tconv. Data represent the mean ± SEM (NS, not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001, n = 3 to 5 per group).
Having observed that newly differentiated pTregs from SAMPΔERβ female mice retain high GILZ expression, unlike pTregs from SAMPΔERβ male mice or SAMPWT mice, we asked if sustained expression of GILZ in mature Tregs alters the functional suppressive capacity of these cells. We performed ex vivo Treg suppression assays in which Tconv cells from SAMPWT or SAMPΔERβ mice were cocultured with intestinal Tregs isolated from autologous mice (mixed male and female). Tconv or Treg cells were pretreated with either DEX, IL-6/IFN-γ, or PBS (vehicle) control for 24 h. Cells were then washed and cocultured in suppression assays as described in Fig. 2. Percent Treg suppression was calculated following 3 d of Tconv proliferation in response to anti-CD3/CD28-coated beads, as shown in the schematic in Fig. 6B. Pretreatment of Treg cells with DEX, but not IL-6/IFN-γ, resulted in a reduction of SAMPWT Treg-suppressive function (Fig. 6C). Interestingly, the suppressive function of SAMPΔERβ Tregs was abrogated upon pretreatment with either DEX or IL-6/IFN-γ (Fig. 6C), suggesting that Treg-specific GILZ expression (Fig. 6A) may impair suppressive function.
In parallel assays, either DEX or IFN-γ/IL-6 was added to Tconv/Treg cocultures. Inclusion of either DEX or IFN-γ/IL-6 in the Tconv/Treg coculture led to a marked reduction of SAMPΔERβ Treg suppressive function (reductions of ∼25% at 1:4 Treg:Tconv ratios) (Fig. 6 D, Right). In contrast, cocultures including Tregs isolated from SAMPWT mice were unaffected by the addition of IFN-γ/IL-6 (Fig. 6 D, Left), potentially because SAMPWT T cells do not up-regulate GILZ in response to IFN-γ/IL-6 (Fig. 6A). Collectively, these results suggest that although T cells from both SAMPWT and SAMPΔERβ mice are capable of up-regulating GILZ in response to DEX, the combined effects of IL-6 and IFN-γ can also lead to GILZ up-regulation in the absence of ERβ. Thus, high IL-6/IFN-γ in the inflamed intestine of young female SAMPΔERβ mice likely acts as a “natural inducer” of GILZ in situ, leading to sustained GILZ expression in intestinal Tregs, reduced Treg-suppressive capacity, and perpetuation of chronic inflammation (summarized graphically in Fig. 7).
Fig. 7.
Graphical summary of proposed ERα-ERβ-GILZ signaling axis in Tregs. (Left) In the healthy, noninflamed intestinal mucosae, ERα and ERβ are expressed at physiological levels by immune cells including Tregs. Soluble E2 diffuses across the plasma membrane and binds ERα and ERβ in the cytoplasm, after which ERα/ERβ heterodimers form. These dimers then translocate to the nucleus and bind estrogen response elements (EREs) in target gene promoters. ERβ-containing dimers inhibit the transcriptional activation of Tsc22d3 (GILZ) in Tregs under noninflamed conditions. (Right) In the chronically inflamed intestine, expression of ERβ is significantly reduced in Tregs. Additional signals from cytokines such as IL-6 and IFN-γ contribute to a chronic inflammatory environment and may interact with ER signaling to influence target gene transcription. In the absence of ERβ, ERα/ERα homodimers permit Tsc22d3 transcription in Tregs, leading to loss of functional Treg suppression. Figure was created using BioRender.
Discussion
Intestinal homeostasis and mucosal immune responses are exquisitely regulated in order to limit potentially harmful responses to commensal microbes and self-antigens, including dietary antigens (49). IBD represents one particularly serious consequence of disruptions to the intestinal mucosal immune system; however, pathogenesis is complex and involves host genetics, the gut microbiome, and environmental factors such as diet. Sex differences have been reported in IBD, with females first exhibiting an increased risk of developing CD during adolescence, concomitant with rising estrogen levels (50, 51). In fact, several studies have linked the use of OCPs, that raise serum estrogen levels, to the development and exacerbation of CD (3–5). A study of two large prospective IBD cohorts in the United States found that females’ risk of developing CD was significantly increased (two- to threefold) upon the commencement of OCPs, compared to never-users (5). Interestingly, although menopause does not seem to affect IBD symptoms for a majority of women (52, 53), hormone replacement therapy may provide some protective effects for postmenopausal women; a retrospective study found a significant beneficial effect on disease activity for postmenopausal, hormone replacement therapy-exposed patients, although not for OCP-exposed patients (52). Collectively, these observations suggest that estrogen represents an important environmental risk factor for IBD.
Here, we demonstrate that female CD patients exhibit significant reductions in expression of normally protective ERβ in the inflamed ileum and in peripheral CD4+ T cells, predisposing these cells to heightened ERα-specific signaling in response to estrogen. Similar observations have been made for systemic lupus erythematosus (SLE) (54), suggesting that female-specific reduction of ERβ or increase in ERα may be a common mechanism in chronic inflammatory disorders. Using the SAMP1/YitFc mouse model of spontaneous, CD-like ileitis, we demonstrate that reductions in ERβ signaling, leading to an ERα-dominated milieu, result in aberrant differentiation and function of intestinal Tregs characterized by sustained expression of Tsc22d3 (GILZ), a transcriptional regulator not normally expressed in Tregs. Although the precise biological roles of Treg-specific GILZ remain unclear, our findings suggest that this transcriptional regulator may represent an important link between dysregulated estrogen signaling and loss of intestinal tolerance in females with CD.
The genomic and nongenomic effects of ERα- vs. ERβ-specific signaling are distinct and often antagonistic (11–14); therefore, the ratio of ERα:ERβ expression in a given cell is important for the ultimate cellular effects of estrogen. IBD patients have been reported to express lower levels of ERβ in inflamed mucosal tissues (26, 27), suggesting that ERα-specific signaling is enhanced in these individuals. The ratio of circulating ERα and ERβ has also been found to correlate with endoscopic activity in CD, but not ulcerative colitis, suggesting that it may have benefit as a biomarker of disease activity (55) that can distinguish CD from ulcerative colitis. Consistent with the notion of enhanced ERα/diminished ERβ augmenting intestinal inflammation, we have shown a significant, sex-specific elevation of T cell-specific ERα:ERβ ratios in female CD patients, who often have more severe clinical symptoms compared to males. Similar observations have been reported for SLE, a strongly female-biased inflammatory disease (54). Whether ERβ’s salutary effects in T cells are direct consequences of ERβ-specific genomic and nongenomic signaling, versus antagonism of ERα-specific effects, remains unclear and will be an important area for future study, especially in the context of chronic inflammatory disease.
ERβ is normally expressed at high levels by colonic epithelial cells, where it has essential protective roles in maintaining epithelial cell homeostasis (42) and intestinal permeability (26). ERβ-KO mice exhibit profound abnormalities in colonic epithelial cell homeostasis, including de-differentiation, hyperproliferation, and resistance to apoptosis (42). Accompanying these epithelial changes, thought to represent a premalignant phenotype, is significant accumulation of lymphocytes in the intestinal mucosae (42). Although ERβ-KO mice fail to develop overt signs of IBD, they develop spontaneous myeloproliferative disease with lymphoid blast crisis (25), suggesting that ERβ has critical roles in hematopoietic cell homeostasis.
Despite these previous studies, relatively little is known regarding the mechanisms underlying ERβ-mediated regulation of immune cells. Both ERα and ERβ are expressed in T cells (56), although ERα expression exceeds that of ERβ. T cell-specific deletion of ERα impairs Th1 and Th17 differentiation and enhances pTreg differentiation, contributing to protection against experimental colitis (22); however, whether these effects are the result of enhanced ERβ-specific signaling, versus the loss of ERα, remain unclear. In a Helicobacter hepaticus model of intestinal inflammation, ERβ signaling was found to be protective, although the mechanism was T cell-independent (57). Further complicating matters is the potential contribution of signaling through GPER-1, which is broadly expressed on immune cells (58) and was recently shown to mediate remission in CD (59). The relative activity of GPER-1 in conditions of altered ERα:ERβ ratios is unknown and represents an important area for future study.
Estrogen is known to induce the peripheral differentiation of Tregs from naïve T cells (39, 40), although the requirement for ERα- vs. ERβ-specific signaling in this process has not been well defined. Our studies show that signaling through ERβ, but not ERα, promotes the expansion of Foxp3+ Tregs in WT mice (Fig. 1). Outside of the reproductive tract, E2 mediates many of its molecular and cellular effects at extremely low, picomole concentrations (60) that are present in both sexes (61). Here, we show that both male and female mice lacking ERα (ERα-KO) exhibit increased intestinal Treg frequencies compared to WT and ERβ-KO mice (Fig. 1 A and B). These observations suggest that local concentrations of E2 in the small intestine are sufficient to mediate differentiation of Foxp3+ Tregs and that E2/ERβ-specific signaling likely represents an important mechanism of intestinal Treg homeostasis in both males and females.
Interestingly, despite the presence of Foxp3+ Tregs in ERβ-KO mice, naïve T cells isolated from ERβ-KO mice were completely resistant to ex vivo, TGF-β–dependent Treg differentiation (Fig. 1 D–F). This resistance was observed in both the presence and absence of exogenous E2, suggesting that ligand-independent mechanisms may underlie ERβ’s contribution to TGF-β–dependent induction of Foxp3. Previous reports have shown that ERα-specific signaling causes degradation of Smad proteins (62) and ligand-independent inhibition of PAI-1 (63), thus inhibiting TGF-β signaling. Thus, we propose that reductions in ERβ expression, as observed in female CD patients (Fig. 2), contribute to enhanced ERα signaling and resistance to TGF-β–dependent Treg differentiation.
Because of the central importance of TGF-β to Treg differentiation in the intestine and other peripheral tissues (64), alterations in ER signaling that reduce the efficiency of TGF-β signaling are likely to have significant consequences on intestinal Treg homeostasis. Our previous work described ERβ-mediated expansion of the intestinal Treg pool in vivo (36), and the present study builds upon this observation to demonstrate not only sufficiency, but a requirement for ERβ-specific signaling in estrogen-mediated enhancement of pTreg differentiation. It is unsurprising, therefore, that in an environment of reduced ERβ signaling, such as the inflamed female intestine, estrogen-mediated Treg differentiation is significantly diminished, potentially altering the overall make-up of the peripheral Treg pool. This raises the important question of how estrogen-induced Tregs may functionally differ from other Treg subsets that are not induced by estrogen. Indeed, pTregs are highly heterogeneous, particularly in the dynamic environment of the intestinal mucosa (28, 65). Estrogen is one of several paracrine factors known to induce pTregs, but abundant evidence that Tregs are not “one size fits all” leads us to hypothesize that estrogen-induced Tregs likely have specific physiological roles that have yet to be elucidated. Interestingly, estrogen-induced pTregs are protective against experimental autoimmune encephalitis in mice, suggesting that endogenous E2 signaling to T cells may be protective against chronic inflammation (39). Inhibition experiments in previous studies used the antiestrogen ICI182,780 (Fulvestrant) (40), which exhibits antagonism toward both ERα and ERβ (66) and also activates GPER-1, a structurally distinct, transmembrane ER (67).
Our global transcriptomic analysis of ERβ-deficient intestinal Tregs from SAMP mice revealed a unique, female-specific induction of Tsc22d3, encoding the leucine zipper protein GILZ, in intestinal Tregs. GILZ is a glucocorticoid-induced, estrogen-regulated gene with broad tissue distribution (68) that, to our knowledge and relevant to the present study, has not previously been described in mature Tregs. The identification of up-regulated GILZ among intestinal Tregs from female SAMPΔERβ mice was therefore unexpected, especially since the most well-characterized physiologic roles of GILZ are antiinflammatory, including the induction of T cell anergy via inhibition of T cell activation (45, 69). However, GILZ’s role in inflammation may be more complex: Tsc22d3 was identified as a component of the Th17 transcriptional network (46), and mice overexpressing GILZ are more susceptible to Imiquimod-induced skin inflammation (70). These findings suggest that GILZ’s functional role in an inflamed tissue environment may be distinct from its role in homeostatic conditions. Our present observations suggest that the female-specific induction of Treg GILZ in SAMPΔERβ mice may be a result of high IL-6/IFN-γ, since these cytokines were found to induce ex vivo GILZ expression as efficiently as did DEX. The inflamed intestinal mucosa of female SAMPΔERβ mice is significantly enriched for both IL-6 and IFN-γ relative to that of male SAMPΔERβ mice, especially at early ages; the combined actions of these cytokines may synergize with dysregulated estrogen signaling to induce the expression of GILZ expression where it would not otherwise occur. Interestingly, IL-6 has been shown to negatively regulate ERβ expression in the intestinal epithelium as well as T cells (27), suggesting that efforts to modulate intestinal IL-6 may have efficacy in restoring ERβ expression and ameliorating intestinal inflammation. Given the complexity of steroid receptor signaling and established cross-talk between ERs and the GR (71, 72), further studies are needed to investigate the mechanisms by which ERs coordinate with GILZ to regulate target gene expression in the presence, versus absence, of inflammatory cytokine signals.
Sex-specific differences that alter disease pathogenesis, such as what we report in the present study, have important clinical implications, particularly for the management of female versus male patients and the design of future treatment modalities that may have a sex-biased impact. Corticosteroids and immunosuppressive agents are the mainstays for IBD treatment. In particular, biologic agents targeting TNF remain the most popular choice for induction of IBD remission (73). However, up to 30% of IBD patients choose to discontinue anti-TNF therapy due to lack of responsiveness or adverse side effects (74). Female IBD patients are significantly more likely than males to discontinue adalimumab (P = 0.03), although the reasons for this remain unclear (74). The rise of gender medicine, the study of male- vs. female-specific aspects of disease prevention, progression, and psycho-social impact (75), speaks to the medical community’s growing realization that successful therapeutic strategies are not “one drug fits all.” Observations such as ours, describing an ERβ-GILZ axis in Tregs that contributes to tissue-specific inflammation, will provide candidates for the design of future therapeutics and provide much-needed options to patients suffering from chronic inflammatory conditions such as IBD.
Materials and Methods
Mice.
Equal numbers of age- and sex-matched ERβ-KO, ERα-KO, SAMPΔERβ, and SAMPWT mice were used in all experiments. Female ERα-KO and ERβ-KO mice have impaired fertility (76, 77), and therefore mice heterozygous for ERα or ERβ were used for breeding. Heterozygous ERβ+/− mice were back-crossed onto SAMP1/YitFc mice for a minimum of eight generations to generate SAMP mice heterozygous for ERβ; these were then bred to generate experimental SAMPΔERβ and SAMPWT mice. All mice were genotyped by PCR-based assays of genomic tail DNA, and deletion of ERβ in SAMPΔERβ mice was confirmed by Western blot. Mice were bred and housed under 12-h light/dark cycles in special pathogen-free conditions in the Animal Resource Center of Case Western Reserve University. Mice had ad libitum access to water and were fed with a standard laboratory rodent diet (Harlan Teklad). All procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University and conformed to guidelines established by the American Association for Accreditation of Laboratory Animal Care.
Histologic Evaluation of Experimental Murine Ileitis.
For histologic evaluation of experimental murine ileitis, 10 cm of tissue was harvested from the distal ilea of SAMPΔERβ or SAMPWT mice and flushed with PBS to remove fecal contents. Ilea were opened longitudinally and fixed overnight in Bouin’s solution. Tissues were then embedded in paraffin and 3-μm sections were cut and stained with H&E. Experimental ileitis was evaluated by a board-certified pathologist (Wei Xin, Department of Pathology, University Hospitals Cleveland Medical Center, Cleveland, OH) using an established, semiquantitative histologic scoring system (78). Briefly, scores ranged from 0 (normal histology) to 3 (maximum severity) for each of three criteria: Active inflammation (neutrophilic infiltration), chronic inflammation (presence of lymphocytes, plasma cells, and macrophages in the mucosa and submucosa), and villus distortion. Total inflammatory scores represent the sum of the three subscores. The pathologist was blinded to mouse genotype and sex at all times. Images were obtained on an Olympus VS120 slide scanner using a 10× objective and 2/3-inch high sensitivity/high resolution CCD camera (Olympus Life Science).
Human Samples.
Informed consent was obtained from all human subjects prior to participation in the study. The study was approved by the Institutional Review Boards of University Hospitals Cleveland Medical Center and Case Western Reserve University. Ileal mucosal biopsies were obtained from healthy adult volunteers (ages 18 to 50 y, premenopausal) or patients diagnosed with ileal CD during endoscopy following informed consent. Healthy volunteers consisted of individuals without a diagnosis of IBD and who showed no endoscopic or pathological abnormalities of the ileal mucosa. CD patient biopsies were obtained from regions of active inflammation. All patients and control volunteers were free of biologic therapies and corticosteroids for a minimum of 3 wk prior to biopsy. Biopsy specimens were immediately placed in RNAlater Solution (ThermoFisher) and stored at −20 °C until RNA was isolated as described below. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood by density centrifugation. PBMCs were stained with mAbs specific for CD4 and CD25 (Biolegend), and Tconv (CD4+CD25−) and Treg (CD4+CD25highCD127lo) subsets were isolated by flow cytometric sorting using a BD FACSAria (BD Biosciences). This study was conducted in compliance with good clinical practice and according to the principles of the Declaration of Helsinki.
RNA Extraction, Reverse Transcription, and Real-Time qPCR.
Total RNA was isolated from ileal tissue samples using TRIzol (ThermoFisher) or from primary T cells using the High Pure RNA Isolation Kit (Roche Life Science, for samples >105 cells) or NucleoSpin XS RNA kit (Macherey-Nagel, for samples <105 cells). Reverse-transcription was performed using M-MuLV reverse transcriptase (New England Biolabs) with random hexamer and oligo(dT) primers. Real-time qPCR was performed using Taqman gene-expression assays (ThermoFisher) on an Applied Biosystems Step One Plus real-time PCR system. Gene-expression values were normalized to those of β2-microglobulin or GAPDH (housekeeping genes) and fold-change values were calculated using the ΔΔCT method (79). PCR array data were obtained using the RT2 Profiler mouse inflammatory response array (Qiagen) on a Step One Plus instrument, according to the manufacturer’s instructions.
In Vitro Treg Differentiation Assays.
Spleens or mLNs were harvested from 8- to 12-wk-old mice and depleted of red blood cells (RBCs) using RBC Lysis Buffer (Sigma-Aldrich). Untouched CD4+CD44− T cells were isolated by negative magnetic selection using the Naïve CD4+ T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. Cells were cultured for up to 5 d in Gibco RPMI medium 1640 (ThermoFisher) supplemented with 10% FBS, 100 U/mL penicillin, 10 µg/mL streptomycin, and 2 mM glutamine, together with α-CD3/CD28, rIL-2, rTGF-β, and neutralizing antibodies against IL-4 and IFN-γ (all Biolegend), as previously described (41). Where indicated, 17β-estradiol (E2) or estrogen receptor-specific agonists PPT (specific for ERα) or DPN (specific for ERβ) were added to cultures. Resulting Tregs were harvested and assessed for Foxp3 gene and protein expression as described below.
Flow Cytometry.
Primary T cells were stained with mAbs specific for CD4 (clone GK1.5), CD25 (clone PC61), and CD127 (clone A7R34) (all Biolegend). Cells were fixed and permeabilized using the eBioscience Foxp3/Transcription Factor Staining Buffer Set (ThermoFisher) and stained with α-mouse/rat Foxp3 (clone FJK-16s, eBioscience/ThermoFisher). Data were acquired on a BD LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (FlowJo).
In Vitro T Cell Proliferation and Suppression Assays.
CD4+CD25− Tconv and CD4+CD25highCD127lo Tregs were FACS-sorted from RBC-depleted splenocytes or mLN cells, as described above. Tconv were labeled with the cell proliferation dye eFluor 670 (0.5 μM; eBioscience/ThermoFisher), according to the manufacturers’ instructions, and cultured with or without Tregs at indicated ratios. Next, 1 μg/mL α-CD3/CD28-coated beads (Miltenyi Biotec) were added to cultures at a ratio of 1:4 bead:Tconv. Tconv proliferation was assessed after 3 d of culture by flow cytometric dye dilution. To calculate percent Tconv proliferation and percent Treg suppression, two gates are drawn for each sample: 1) Total Tconv, excluding eFluor-negative (unlabeled) Tregs; and 2) actively proliferating Tconv, defined as Tconv falling between the Treg (negative) gate and the nondividing, eFluor 450-bright gate (established by negative control samples of labeled Tconv in the absence of αCD3/28 beads). Proliferation is calculated as the percentage of proliferating Tconv divided by total Tconv. Suppression is calculated as the percentage of proliferation in Tconv/Treg cocultures divided by proliferation of Tconv alone.
Transcriptome Analysis.
Total RNA was isolated from FACS-sorted CD4+CD25highCD127lo Tregs as described above, using the Nucleospin XS kit. cDNA libraries were prepared using the Illumina TruSeq Stranded Total RNA Sample Preparation kit according to the manufacturer’s instructions (Illumina). Next-generation RNA-seq was performed using an Illumina HiSEq. 2500 system with TruSeq technology (Illumina). Transcripts were analyzed via paired-end, 100-bp RNA-seq runs (10 samples per lane), ensuring a minimum of 15 × 106 mapped reads per sample. Transcripts passing quality control were aligned to GRCm38 using HISAT2 (80), and then analyzed for differential expression using edgeR (81).
Imaging Flow Cytometry.
Data were collected using an Amnis ImageStream cytometer and analyzed using IDEAS software v6.0. Human CD4+ lymphocytes were stained for intracellular expression of Foxp3, ERβ, and GILZ. Analyzed events were selected on 1) focused events, 2) doublet exclusion, and 3) Foxp3bright-expressing cells. Representative events are shown for each cohort (male and female, control [Ctrl] and CD). Similarity scores were calculated based on MFI of GILZ and ERβ staining in representative cells.
Data Analysis and Statistics.
Data were analyzed using GraphPad Prism 7 (GraphPad Software). Unless otherwise noted, data are presented as mean ± SEM. Significance was assessed using two-tailed Student’s t-tests with Bonferroni’s corrections for multiple comparisons. P values <0.05 were considered significant.
Data Availability.
Next-generation RNA-seq datasets are freely available through the Gene Expression Omnibus (GEO) database at https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE151863).
Supplementary Material
Acknowledgments
We thank Dr. Jeffry Katz, Medical Director for Inflammatory Bowel Disease at University Hospitals Cleveland Medical Center (UHCMC), for recruitment of patients and access to clinical samples; Dr. Fabio Cominelli, Division Chief of Gastroenterology at UHCMC, for use of the Olympus VS120 slide scanner and helpful discussions on this project; the technical assistance of R. Michael Sramkoski and Allison Kipling, Case Comprehensive Cancer Center Cytometry & Microscopy Core; and Wei Xin, Danian Che, and Carlo DeSalvo, Cleveland Digestive Disease Research Core Center histology core. This work was primarily supported by grants to W.A.G. and T.T.P. from the NIH: NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant K01 DK105138 (to W.A.G.), NIH/NIDDK Grant P01 DK091222 (to T.T.P.), and NIH/NIDDK Grant P30 DK097948 (to T.T.P.). W.A.G. was also supported by funding from the Crohn’s & Colitis Foundation (Career Development Award 329284 and Senior Research Award 635911). S.M.B. was supported by the NIH/NIDDK-funded Case Medical Student Summer Research Program (T35 DK111373) and a Crohn’s & Colitis Foundation Student Research Fellowship (585026).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (accession no. GSE151863).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002266117/-/DCSupplemental.
References
- 1.Fish E. N., The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 8, 737–744 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein S. L., Flanagan K. L., Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Ortizo R. et al., Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: A meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 29, 1064–1070 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Khalili H. et al., Association between long-term oral contraceptive use and risk of Crohn’s disease complications in a nationwide study. Gastroenterology 150, 1561–1567.e1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalili H. et al., Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 62, 1153–1159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S. C. et al., Sex-based differences in incidence of inflammatory bowel diseases-pooled analysis of population-based studies from Western countries. Gastroenterology 155, 1079–1089.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Carmeci C., Thompson D. A., Ring H. Z., Francke U., Weigel R. J., Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45, 607–617 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman M. A., Budish R. A., Kashyap S., Lindsey S. H., GPER-novel membrane oestrogen receptor. Clin. Sci. (Lond.) 130, 1005–1016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björnström L., Sjöberg M., Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 19, 833–842 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Marino M., Galluzzo P., Ascenzi P., Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics 7, 497–508 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindberg M. K. et al., Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol. Endocrinol. 17, 203–208 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Liu M. M. et al., Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J. Biol. Chem. 277, 24353–24360 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Paech K. et al., Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277, 1508–1510 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Weihua Z. et al., Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc. Natl. Acad. Sci. U.S.A. 97, 5936–5941 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan D., Ansar Ahmed S., The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 6, 635 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovats S., Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 294, 63–69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maret A. et al., Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 33, 512–521 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Karpuzoglu E., Phillips R. A., Gogal R. M. Jr., Ansar Ahmed S., IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: Role of IL-27 but not IL-12. Mol. Immunol. 44, 1808–1814 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox H. S., Bond B. L., Parslow T. G., Estrogen regulates the IFN-gamma promoter. J. Immunol. 146, 4362–4367 (1991). [PubMed] [Google Scholar]
- 20.Grasso G., Muscettola M., The influence of beta-estradiol and progesterone on interferon gamma production in vitro. Int. J. Neurosci. 51, 315–317 (1990). [DOI] [PubMed] [Google Scholar]
- 21.Karpuzoglu-Sahin E., Hissong B. D., Ansar Ahmed S., Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J. Reprod. Immunol. 52, 113–127 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Mohammad I. et al., Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci. Signal. 11, eaap9415 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Goodman W. A. et al., Estrogen receptor alpha loss-of-function protects female mice from DSS-induced experimental colitis. Cell Mol. Gastroenterol. Hepatol. 5, 630–633.e1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krege J. H. et al., Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. U.S.A. 95, 15677–15682 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim G. J. et al., Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc. Natl. Acad. Sci. U.S.A. 100, 6694–6699 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Looijer-van Langen M. et al., Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G621–G626 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Pierdominici M. et al., Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget 6, 40443–40451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman W. A., Pizarro T. T., Regulatory cell populations in the intestinal mucosa. Curr. Opin. Gastroenterol. 29, 614–620 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Lord J. D., Shows D. M., Chen J., Thirlby R. C., Human blood and mucosal regulatory T cells express activation markers and inhibitory receptors in inflammatory bowel disease. PLoS One 10, e0136485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord J. D., Valliant-Saunders K., Hahn H., Thirlby R. C., Ziegler S. F., Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2. Dig. Dis. Sci. 57, 2846–2855 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maul J. et al., Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Sun X. et al., Analysis of murine and human Treg subsets in inflammatory bowel disease. Mol. Med. Rep. 16, 2893–2898 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Eastaff-Leung N., Mabarrack N., Barbour A., Cummins A., Barry S., Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 30, 80–89 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa D. et al., Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. 6, 267–275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggelakopoulou M., Kourepini E., Paschalidis N., Panoutsakopoulou V., ERβ in CD4+ T cells is crucial for ligand-mediated suppression of central nervous system autoimmunity. J. Immunol. 196, 4947–4956 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Goodman W. A. et al., Loss of estrogen-mediated immunoprotection underlies female gender bias in experimental Crohn’s-like ileitis. Mucosal Immunol. 7, 1255–1265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W. F. et al., Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 110, 3543–3548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizarro T. T. et al., SAMP1/YitFc mouse strain: A spontaneous model of Crohn’s disease-like ileitis. Inflamm. Bowel Dis. 17, 2566–2584 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polanczyk M. J. et al., Cutting edge: Estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 173, 2227–2230 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Tai P. et al., Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 214, 456–464 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Fantini M. C., Dominitzki S., Rizzo A., Neurath M. F., Becker C., In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat. Protoc. 2, 1789–1794 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Wada-Hiraike O. et al., Role of estrogen receptor beta in colonic epithelium. Proc. Natl. Acad. Sci. U.S.A. 103, 2959–2964 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borsellino G. et al., Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 110, 1225–1232 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Asselin-Labat M. L. et al., GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood 104, 215–223 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Ayroldi E. et al., Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood 98, 743–753 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Yosef N. et al., Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones S. A. et al., Glucocorticoid-induced leucine zipper (GILZ) inhibits B cell activation in systemic lupus erythematosus. Ann. Rheum. Dis. 75, 739–747 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Bruscoli S. et al., Genomic and non-genomic effects of different glucocorticoids on mouse thymocyte apoptosis. Eur. J. Pharmacol. 529, 63–70 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Turner J. R., Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Chouraki V. et al., The changing pattern of Crohn’s disease incidence in northern France: A continuing increase in the 10- to 19-year-old age bracket (1988-2007). Aliment. Pharmacol. Ther. 33, 1133–1142 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Molinié F. et al., Opposite evolution in incidence of Crohn’s disease and ulcerative colitis in Northern France (1988-1999). Gut 53, 843–848 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kane S. V., Reddy D., Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am. J. Gastroenterol. 103, 1193–1196 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Rolston V. S. et al., The influence of hormonal fluctuation on inflammatory bowel disease symptom severity-A cross-sectional cohort study. Inflamm. Bowel Dis. 24, 387–393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maselli A. et al., Low expression of estrogen receptor β in T lymphocytes and high serum levels of anti-estrogen receptor α antibodies impact disease activity in female patients with systemic lupus erythematosus. Biol. Sex Differ. 7, 3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linares P. M. et al., Ratio of circulating estrogen receptors beta and alpha (ERβ/ERα) indicates endoscopic activity in patients with Crohn’s disease. Dig. Dis. Sci. 62, 2744–2754 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Mor G. et al., The role of the Fas/Fas ligand system in estrogen-induced thymic alteration. Am. J. Reprod. Immunol. 46, 298–307 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Cook L. C. et al., The role of estrogen signaling in a mouse model of inflammatory bowel disease: A Helicobacter hepaticus model. PLoS One 9, e94209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prossnitz E. R., Barton M., The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 7, 715–726 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacenik D. et al., G protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn’s disease. Sci. Rep. 9, 6749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson C. S., Jeng Y. J., Kochukov M. Y., Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 22, 3328–3336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooke P. S., Nanjappa M. K., Ko C., Prins G. S., Hess R. A., Estrogens in male physiology. Physiol. Rev. 97, 995–1043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito I. et al., Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J. Biol. Chem. 285, 14747–14755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stope M. B., Popp S. L., Knabbe C., Buck M. B., Estrogen receptor alpha attenuates transforming growth factor-beta signaling in breast cancer cells independent from agonistic and antagonistic ligands. Breast Cancer Res. Treat. 120, 357–367 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Coombes J. L. et al., A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanoue T., Atarashi K., Honda K., Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 16, 295–309 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Pastore M. B., Talwar S., Conley M. R., Magness R. R., Identification of differential ER-alpha versus ER-beta mediated activation of eNOS in ovine uterine artery endothelial cells. Biol. Reprod. 94, 139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ariazi E. A. et al., The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 70, 1184–1194 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bereshchenko O., Migliorati G., Bruscoli S., Riccardi C., Glucocorticoid-induced leucine zipper: A novel anti-inflammatory molecule. Front. Pharmacol. 10, 308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Adamio F. et al., A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity 7, 803–812 (1997). [DOI] [PubMed] [Google Scholar]
- 70.Carceller E. et al., Overexpression of glucocorticoid-induced leucine zipper (GILZ) increases susceptibility to Imiquimod-induced psoriasis and involves cutaneous activation of TGF-β1. Sci. Rep. 6, 38825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karmakar S., Jin Y., Nagaich A. K., Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) α and activator protein 1 (AP1) in dexamethasone-mediated interference of ERα activity. J. Biol. Chem. 288, 24020–24034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vahrenkamp J. M. et al., Clinical and genomic crosstalk between glucocorticoid receptor and estrogen receptor α in endometrial cancer. Cell Rep. 22, 2995–3005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ben-Horin S., Chowers Y., Tailoring anti-TNF therapy in IBD: Drug levels and disease activity. Nat. Rev. Gastroenterol. Hepatol. 11, 243–255 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Laganà B. et al., Sex differences in response to TNF-inhibiting drugs in patients with spondyloarthropathies or inflammatory bowel diseases. Front. Pharmacol. 10, 47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baggio G., Corsini A., Floreani A., Giannini S., Zagonel V., Gender medicine: A task for the third millennium. Clin. Chem. Lab. Med. 51, 713–727 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Binder A. K. et al., The absence of ER-β results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology 154, 2174–2187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lubahn D. B. et al., Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. U.S.A. 90, 11162–11166 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burns R. C. et al., Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology 121, 1428–1436 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Livak K. J., Schmittgen T. D., Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Kim D., Langmead B., Salzberg S. L., HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robinson M. D., McCarthy D. J., Smyth G. K., edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Next-generation RNA-seq datasets are freely available through the Gene Expression Omnibus (GEO) database at https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE151863).