Abstract
Water and nitrogen availability limit crop productivity globally more than most other environmental factors. Plant availability of macronutrients such as nitrate is, to a large extent, regulated by the amount of water available in the soil, and, during drought episodes, crops can become simultaneously water and nitrogen limited. In this review, we explore the intricate relationship between water and nitrogen transport in plants, from transpiration-driven mass flow in the soil to uptake by roots via membrane transporters and channels and transport to aerial organs. We discuss the roles of root architecture and of suberized hydrophobic root barriers governing apoplastic water and nitrogen movement into the vascular system. We also highlight the need to identify the signalling cascades regulating water and nitrogen transport, as well as the need for targeted physiological analyses of plant traits influencing water and nitrogen uptake. We further advocate for incorporation of new phenotyping technologies, breeding strategies, and agronomic practices to improve crop yield in water- and nitrogen-limited production systems.
Keywords: Ammonium, aquaporins, DRO1, nitrate, nitrogen transport, phenotyping, root architecture, root barriers, suberin, water transport
Given the critical importance and interconnectedness of water and nitrogen in determining crop yield, there is great impetus to understand and optimize their uptake. We review this intersection and provide proposals for improving these critical crop traits.
Introduction
The two resources with the greatest influence on crop productivity are water and nitrogen (N). Food security is becoming more difficult to realize each year as the global population continues to increase, a problem compounded by the loss of arable land to urban sprawl, land degradation, and environmental limitations to agricultural production. Climate change has already had measurable impacts, increasing global temperatures and creating changing weather patterns. The impacts of these changes are only beginning to be understood, but it is clear that many agricultural regions will be impacted by increasingly hotter and drier conditions and more frequent weather extremes (IPCC, 2015). More than two-thirds of freshwater withdrawals and some 90% of total water consumption through human use globally are attributable to irrigation of crops, and water tables have been dropping at alarming rates in many parts of the world due to such water withdrawals (Britto and Kronzucker, 2018). Increasing global scarcity of water will also impact the way in which N fertilizer is accessed by plants and profoundly compromise crop productivity (Swarbreck et al., 2019).
Water is critical to all stages of crop development. Upon sowing, seeds will not germinate without the presence of water. In dryland agriculture, sowing may occur into completely dry soil, but this is a strategy developed by farmers to ensure the seed is ready for germination upon the first rains. Quick emergence of seedlings from the soil is essential to maximize the capture of light, and roots must develop to secure water for this to occur. Plants in the vegetative growth stage are typically characterized by exponential growth, and this requires a large amount of water to sustain. Cell turgor, ion transport, enzyme function, and many other critical physiological roles within the plant are simply not possible without the presence of water. As water is a limiting resource in many regions of the world, the improvement of water-use efficiency (WUE) by crops is critical to maintaining food security. However, WUE is an extremely complex trait and has been relatively recalcitrant to manipulation efforts (Hatfield and Dold, 2019). It is clear that improvement of WUE in crops must be accompanied by agronomic strategies to reduce water requirements in agriculture.
Vast quantities of N fertilizer are applied by farmers every year to crops to maximize growth and yield. Among all nutrients, nitrogen is present in the largest quantities in plant tissue and is crucial to the development of plant structure, nucleotides, and enzymes, among many other central roles. However, crop plants are inherently poor at accessing applied N, taking up only 40–50% of the amount applied, or less (Raun and Johnson, 1999; Kronzucker et al., 2000; Coskun et al., 2017a, b). A large portion of the remaining amount can be lost to the environment in the form of volatilization of gaseous N compounds, such as ammonia and nitrous oxides, or washed out and leached to waterways, in particular in the form of nitrate, producing a substantial pollution load (Wang et al., 2019). The production of N fertilizer through the Haber–Bosch process is also extremely energy demanding, as the process consumes an estimated 2% of the world’s energy supply (Erisman et al., 2008; Pfromm, 2017). As a result, N fertilizer is expensive to produce and is the second greatest expense for farmers, next to fuel, resulting in large losses of potential income. Thus, it is clear that N-use efficiency (NUE) or crops must be improved for sustainable agricultural production; however, as for WUE, efforts to improve NUE have been stymied by the complexity of the trait in plants. Agronomic improvements in both traits are clearly necessary.
Several factors render improvements in WUE and NUE in crop plants challenging, and these include the multigenic nature of the traits, the need for phenotyping of traits throughout the crop’s life cycle, above- and below-ground, and under changing environments, and the fact that both traits are heavily influenced by interactions with environmental conditions and agronomic management practices (the so-called G×E×M interactions). As such, efforts to discover the genetic architecture of WUE and NUE in the field require the use of multiple field sites across a range of agro-climatic zones, over several seasons, to gain any statistical confidence in experimental outcomes. Compounding these practical difficulties is the lack of accurate phenotyping methods to measure complex traits in a meaningful manner. Both WUE and NUE can be broken down into a series of subtraits which can be measured with some accuracy. However, it is uncertain whether selecting varieties based on these subtraits will be useful in identifying improved WUE and NUE (Zubaidi et al., 1999; Sadras, 2004, 2005; Sadras and Rodriguez, 2010; Garnett and Rebetzke, 2013; Asplund et al., 2014; Sadras and Richards, 2014). Compounding this difficulty is that WUE and NUE are highly interdependent traits, which is not surprising, given that N compounds move through the soil as solutes. We will not review N or water transport individually, given the large number of reviews available on these topics (Britto and Kronzucker, 2002; Chaumont and Tyerman, 2014; Maurel et al., 2015; Plett et al., 2018).
In this review, we explore the role of water and N in determining crop yield, particularly where these roles intersect. Given their critical importance to plant growth and productivity, it can be difficult to disentangle the role of each individually, but this makes it all the more necessary to understand the influence of the interaction. We then offer suggestions for methods to improve water and N uptake in crops, as it is clear that both goals much be achieved on the road to improved crop yield.
The intricate interaction between nitrogen uptake and water transport
Mobility of nitrogen in soil by transpiration-driven mass flow and simple diffusion
While most N is taken up by higher plants from the soil as nitrate (NO3−) or ammonium (NH4+), most plants prefer NO3− as long as the pH in the rooting zone remains favourable (Kronzucker et al., 1997; Britto et al., 2001; Britto and Kronzucker, 2002). The mobility of NO3− ions in the soil is mainly governed by electrostatic interactions between negatively charged NO3− ions and either soil minerals or soil organic matter (Allred et al., 2007). The adsorption of anions to soil particles occurs when NO3− ions become attached to positively charged exchange sites of soil. A significant percentage of exchange sites on soil mineral and organic matter are pH dependent. Under low-pH conditions, positively charged hydrogen ions (H+) become attached to certain exchange site functional groups, thereby causing these exchange sites to become positively charged (Foth, 1978; Bohn et al., 2001). However, the mobility of abundant free NO3– ions in soil depends on the water status or soil moisture content, which is a critical factor for the movement of not only NO3– but also other mobile ions in the soil by both transpiration-driven mass flow and simple diffusion.
The extracellular matrix of the walls around living cells or the ‘apoplast’ is porous, the pores being filled with water in all but very exceptional circumstances (Münch, 1930; Steudle, 2000; Kim et al., 2018). It is a physical continuum through which water and ions can freely move either by bulk/mass flow in the presence of a transpirational force, where ions, including NO3– and NH4+, can be dragged by water (‘solvent drag’), or by simple diffusion in the absence of transpirational force. The tension created by the shoot during transpiration should propagate to the soil through roots due to a soil–plant–air continuum (SPAC). However, there is no evidence that mass flow plays a direct role in NO3– uptake across the plasma membrane; the increased NO3 concentration in the rhizosphere due to solvent drag may enhance membrane NO3– transport (Cernusak et al., 2011; Matimati et al., 2014). Thus, transpirational water fluxes appear to play a fundamental role in the acquisition of NO3– and NH4+ as well as other mobile nutrients, explaining the functional up-regulation of membrane-embedded transporter proteins in plants grown in nutrient-deprived soils (Wilkinson et al., 2007; Kupper et al., 2012). Apparently, high transpirational water fluxes are primarily important for the acquisition of mobile nutrients or in zones where roots are sparsely distributed in the soil profile (Scholz et al., 2007; Cramer et al., 2008; Kupper et al., 2012). Even though mathematical models have been used to predict and estimate the spatial extent of nutrient depletion around the rhizosphere (Rengel, 1993; Syring and Claassen, 1995), the magnitude of the distance over which mass/bulk flow is effective remains unknown. Knowledge of the spatial scale over which mass flow operates in soil is highly relevant to our understanding of plant nutrient acquisition from the soil, and there is a clear need for further research in the future.
Ammonium in the paddy rice system
Nitrification and denitrification are the two major processes in determining the availability of soil N for crop plants during their growth and development (Cai, 2002; Kirk and Kronzucker, 2005). High-yielding lowland rice cultivars are often grown in waterlogged paddy fields. The rate of oxygen (O2) diffusion in waterlogged soil is 10 000 times lower than in air due to significantly lower diffusion coefficients (Nobel, 2009; Tuchscherr et al., 2010). When O2 is depleted in the waterlogged soil, the remaining NO3− is used by many microorganisms as a terminal electron acceptor, which results in the reduction of NO3− to NH4+ (Tiedje et al., 1982; Silver et al., 2001).
Generally, the efficiency of N fertilizer uptake in lowland rice is poor, which is approximately as low as 20–40%, whereas upland crops often use 40–60% of the N applied to the soil (Vlek and Byrnes, 1986). Applied N fertilizer can also be lost by denitrification which is known to be one of the main pathways to lose N in flooded lowland rice fields (Reddy and Patrick, 1986; Chen et al., 2013). Usually, ammonium-based fertilizers are the most common N fertilizers applied to lowland waterlogged rice fields (Chen et al., 2013) and it is the commonly available N fertilizer for rice. Ammonium-based fertilizers such as urea can be biologically oxidized into NO3– in the hypoxic top layers of the waterlogged soil due to nitrification; however, they undergo denitrification when NO3– diffuses into anaerobic bulk soils (Tiedje et al., 1982). This coupled nitrification and denitrification in flooded soils (i.e. rice paddies) has been demonstrated by many research groups (Reddy and Patrick, 1986; van Luijn et al., 1996). Using mathematical calculations, it has been found that the diffusion of NH4+ and nitrification would minimize N loss from most flooded lowland soils, where NO3− diffusion and denitrification usually occur at a rapid rate and are not likely to limit the overall process (Reddy and Patrick, 1986). The nitrification process in lowland rice is triggered by draining of floodwater, and accumulated NO3– will be lost by alternate re-flooding and drainage (Reddy and Patrick, 1975; Cai, 2002). During rice fertilization, deep placement of NH4+-based N fertilizers into the anaerobic zones is recommended because it will reduce NH4+ nitrification thereby reducing N loss from the paddy soil (Bouldin, 1986), although spatial heterogeneity of N-enriched zones in rice paddy soils post-fertilization can be pronounced and must be considered in evaluating the effectiveness of fertilizer placements (Y. Li et al., 2016). Although nitrification occurs in hypoxic zones of top layers and denitrification occurs in anaerobic zones of deep layers, NH4+ and NO3− are usually co-present in lowland rice fields (Kronzucker et al., 2000; Kirk and Kronzucker, 2005), and NO3− can reach millimolar concentrations following fertilization (Arth et al., 1998; Cai, 2002). However, the abundant and major form of inorganic N in lowland paddy fields is NH4+.
In addition to its abundance in paddy fields, NH4+ is the preferred N source over NO3− for rice and many other plant species, which has often been attributed to the lower energy requirement for assimilation by roots (Bloom et al., 1992; Balkos et al., 2010; Ranathunge et al., 2014). Rice also shows a superior tolerance to high NH4+ compared with other crop plants (Britto et al., 2001; Britto and Kronzucker, 2002). However, NH4+ acquisition and translocation to the shoot can be enhanced by NO3−, and co-provision of the two N sources in the root zone can produce significant synergistic growth and yield effects (Kronzucker et al., 1999).
Effects of nitrate and ammonium supply on water transport/uptake of roots
Even 50 years after the pioneering research that discovered that water fluxes were important for nutrient uptake by mass/bulk flow and diffusion (Barber, 1962), the role of nutrients in regulating water fluxes in plants remains poorly understood (Raven, 2008). Several studies have suggested a possible role for xylem N concentration as a signal for the regulation of water fluxes in plants (Wilkinson et al., 2007; Cramer et al., 2009; Matimati et al., 2014), but this idea lacks substantial experimental data support. Cramer et al. (2009) proposed a model of N regulation in which NO3– modulates root hydraulic conductance through its control of plasma membrane-bound aquaporins, and foliar nitric oxide (NO) application modulates stomatal conductance (gs). These proposed models have emphasized the role of NO3– in regulating water fluxes in plants (Wilkinson et al., 2007; Kupper et al., 2012) but neglected the potential regulatory effects of NH4+. However, given the importance of NH4+ fertilizers used in agriculture fields around the world, understanding of the regulatory effects of water fluxes by NH4+ fertilizers is critical and important.
Recently, significant progress has been made in understanding the regulation of hydrauli c conductivity of roots (Lpr) and plasma membrane-bound aquaporins under different NO3− treatments in the model system Arabidopsis (G. Li et al., 2016; Tyerman et al., 2017). Comprehensive studies and investigations of the Lpr of Arabidopsis NO3– transporter mutants including the transporters NRT1.1 (dual affinity) and NRT2.1 (HATS) showed that only the loss of NRT2.1 reduced the Lpr. Even though Lpr was reduced in the NRT2.1 knockout mutant, it still responded to low NO3– supply. In the NRT1.1 knockout mutant, there was a correlation between Lpr and shoot NO3– concentration, but there was no apparent correlation between Lpr and root NO3– concentration (Tyerman et al., 2017). In the NRT2.1 mutant, the transcript levels of PIP1;1, PIP1;2, PIP2;1, and PIP2;3 showed a clear positive correlation between changes in Lpr and different NO3– treatments (Tyerman et al., 2017). Not only the transcript levels but also both PIP1 and PIP2 protein abundance were correlated with Lpr. Tyerman et al. (2017) also showed that root aquaporins which drive water flow through the transmembrane path were regulated by (i) shoot to root signal communication; (ii) shoot NO3– status; and (iii) the function of the NRT2.1 gene.
There is clear evidence that aquaporins play a role in the response to N, as indicated above. However, the change in transcriptional responses of root aquaporins to different external N status/concentrations is not instantaneous, but typically occurs over a period of several days (Tyerman et al., 2017). Further, more caution is needed as transcription and protein amounts are not always correlated (Hachez et al., 2012). For example, in tomato, altered Lpr to N changes in the medium were apparent within a very short time (Gorska et al., 2008a, 2010), but aquaporin gene expression was not seen until 48 h after the treatment (Wang et al., 2001). It is likely that Lpr changes in the early stages, due to modulation of water flow in the other pathways, namely symplastic through plasmodesmata and extracellular/apoplastic. In rice, switching from 10 ppm NH4+ to 0.5 ppm NO3− resulted in a slight repression of OsPIP1;1, OsPIP2;3, OsTIP1;1, and OsTIP2;2 expression. However, there was a major reduction in expression observed for OsPIP2;4 and OsPIP2;5, whereas there was an induction for OsTIP2;1 and OsPIP2;6 (Tyerman et al., 2017). In maize, addition of NO3− was not reported to change PIP gene expression in roots within a 4 h time frame, while tungstate treatment, known as a potent inhibitor of nitrate reductase (de la Haba et al., 1990; Britto and Kronzucker, 2005), greatly inhibited the expression of most PIP genes (Gorska et al., 2008b). In Arabidopsis, a switch in exposure to NO3− from NH4+ resulted in repression of AtNIP2;1 gene expression, but the expression levels of all other aquaporins remained unchanged (Wang et al., 2003). In another experiment, resupply of NO3− to N-starved plants strongly induced a TIP member, and several others were induced weakly in Arabidopsis roots (Scheible et al., 2004). These findings confirm that plant root water uptake can be altered by N but that this depends on N form, applied amount or concentration, and plant species. It remains unknown how these changes in membrane water permeability regulated by PIP aquaporins affect apoplastic and/or symplastic water flows. NRT1.2 seems to be an important candidate in the signalling of NO3− to aquaporins, in a probably post-translational regulation mode affecting the activity of aquaporins modulating water flow through the plasma membrane. Physiological data suggest that potassium (K+) can directly reduce aquaporin-mediated N flow, while simultaneously improving plant WUE (Szczerba et al., 2008; Balkos et al., 2010; ten Hoopen et al., 2010; Coskun et al., 2013b; Britto et al., 2014), offering another potential precedent of direct aquaporin regulation by a principal macronutrient ion. However, further comprehensive research is necessary to address whether (i) NO3− and NH4+ affect the activity of PIP and TIP aquaporins in roots directly (e.g. by allosteric means); (ii) water flow through the apoplast and plasmodesmata responds to changing NO3− and NH4+ levels; and (iii) signal transduction cascades are involved in aquaporin activity regulation in relation to shoot and root N levels.
Formation of root apoplastic barriers made of suberin and lignin
Transport properties of roots are strongly related to their anatomy, and interpretation of water and ion transport measurement data requires detailed knowledge of root structure in order to understand function properly (Steudle and Peterson, 1998; Steudle, 2000; Ranathunge et al., 2011b). In the past, and largely due to the difficulty of visualizing root structures in situ and accessing them without inflicting damage, scientists often left root structure/anatomy as ‘black boxes’. However, in the recent past, considerable progress has been made linking root transport properties with root structure. The ‘composite anatomical structure’ of roots results in ‘composite transport’ of both water and nutrient ions, including N (Steudle and Peterson, 1998; Ranathunge et al., 2017). The parallel arrangement of the apoplastic (cell wall and extracellular) and symplastic (transmembrane and cell to cell) paths, and switches between these paths, are important features of this model (Steudle, 2000; Kim et al., 2018). By switching between apoplastic and symplastic paths, depending on the prevailing resistances to water and nutrient flow, the composite transport model allows for an adjustment and for regulation of water and nutrient uptake driven by shoot demand. The apoplastic component of water and solute flow may be restricted by the existence of barriers such as Casparian bands. Along the cell to cell path (transmembrane and symplastic flow via plasmodesmata), aquaporins, ion transporters, plasmodesmata, and suberin lamellae all engage in the regulation of the intensity of water and solute flow (Tyerman et al., 1999, 2017; Roberts and Oparka, 2003; Ranathunge et al., 2004).
Enhanced cell wall suberization and lignification are the most common and efficient strategies for sealing of roots under adverse conditions of both a biotic and abiotic nature. Suberization and lignification of roots are known to increase with age or developmental stage, and also during exposure to abiotic stresses (salinity, osmotic stress, drought, anoxia, heavy metals, nutrient stress, etc.) (Lux et al., 2004; Kotula et al., 2009; Krishnamurthy et al., 2009, 2011; Ranathunge et al., 2011a; Kreszies et al., 2019).
Our understanding of suberized and lignified apoplastic barrier deposition in roots, and the functions of these barriers, has made great progress in the past decade, but many gaps remain in our understanding of the metabolic and cellular processes of suberin and lignin formation. The relationship between suberin deposition and water transport is not always negatively correlated and this deserves further investigation to understand more completely (Kreszies et al., 2018). Importantly, the impact of N supply on the formation of root barriers is not well understood (Schreiber et al., 2005; Ranathunge et al., 2016). In addition, no major studies have hitherto explored how combinations of stresses, such as N and water stress, will impact on the formation of root barriers. We have attempted to synthesize information about the putative combined effects of N and water on barrier formation in Fig. 1. Recently developed analytical methods, and the increasing availability of molecular tools for model systems in suberin and lignin research, will help fill these gaps in the future. Some variability in the characterization of barrier properties of suberized and lignified tissues indicates that the root barriers established in cell walls are complex and that there is no simple generalization to be made. A multifaceted approach is required, combining molecular genetics, analytical chemistry, and structural analysis with quantitative physiological transport studies to better understand the physiological importance of suberized and lignified cell walls in plants.
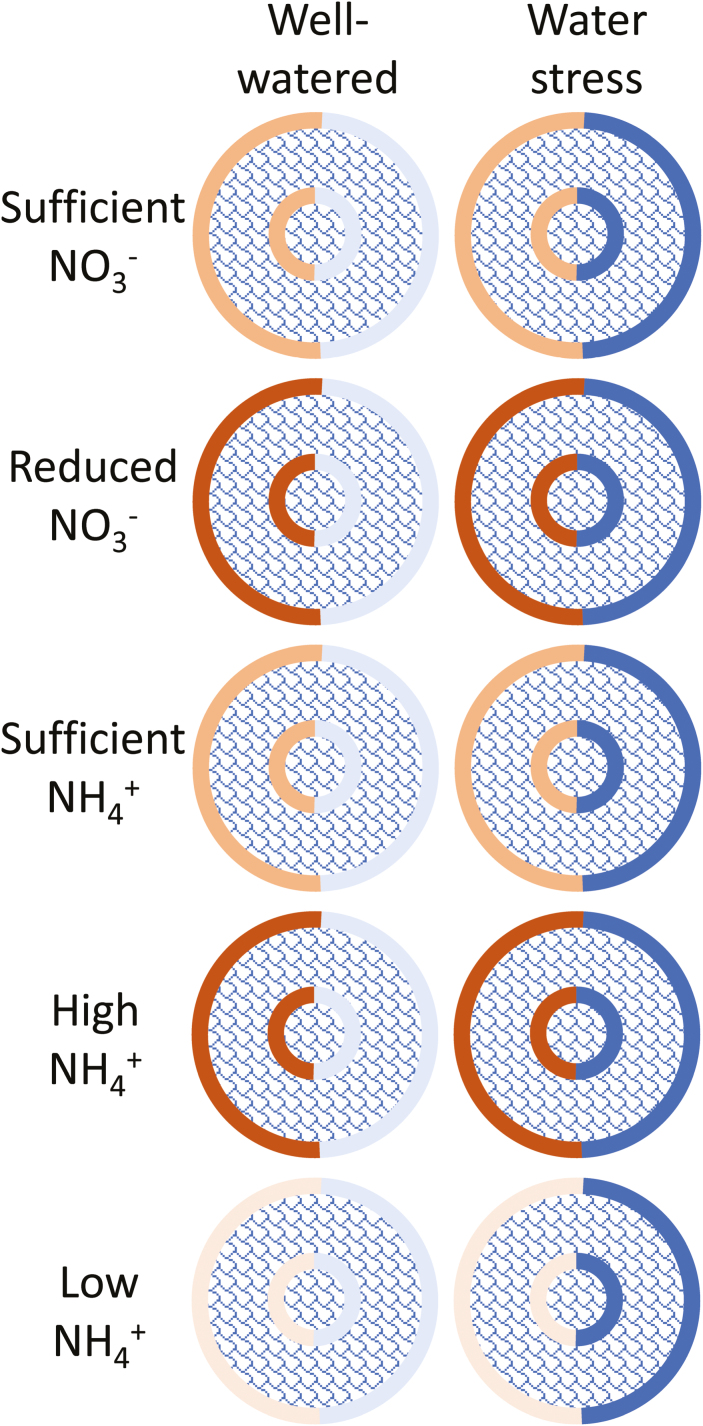
Fig. 1.
The putative impact of water and N supply on the development of plant root barriers. The outer and inner rings represent the exodermal and endodermal cell layers, respectively. The matrix represents the combinations of different water availability (columns) and different N sources and supplies (rows). Darker rings indicate induced root barriers which have increased suberin and lignin depositions in the cell walls, decreasing apoplastic water and N transport, while the reverse is depicted by lighter rings. The effect of N (left) and water (right) supply is represented on opposite sides of each ring. Future work must address whether an increase in barrier development influences N and water transport similarly, in particular where available water and N are having opposite influences on barrier development (e.g. low NH4+ combined with water stress, as depicted in the bottom right of the figure).
C3 versus C4 plants—nitrogen and photosynthesis
Plants possessing the C4 photosynthetic machinery are able to concentrate CO2 around the Rubisco enzyme. This means that C4 species are able to reduce photorespiratory losses via this mechanism compared with C3 plants. The CO2-concentrating mechanism also allows for stomatal aperture to be reduced, resulting in less water loss via the transpiration stream, thereby increasing photosynthetic WUEs by 1.5–4 times over C3 species in similar conditions (Vogan and Sage, 2011). Related to this, photosynthetic N-use efficiency (PNUE) is generally 50–100% higher in C4 versus C3 plants (Sage et al., 1987; Sage and Pearcy, 1987a, b; Ripley et al., 2008; Vogan and Sage, 2011). However, in rice, a C3 species, there is evidence that N source can impact photosynthetic efficiency under water stress conditions. The provision of NH4+, as opposed to NO3–, under drought stress [simulated by polyethylene glycol (PEG)] allowed rice to maintain photosynthetic rate and Rubisco content (Guo et al., 2007). However, in durum wheat, the capacity of N supply to increase photosynthetic parameters is heavily influenced by water supply, indicating the extent to which these two important determinants of crop productivity are linked (Shangguan et al., 2000).
Drought stress suppresses symbiotic nitrogen fixation
Legumes acquire N from symbiotic interactions with N2-fixing bacteria (rhizobia). The establishment of this symbiosis requires dual recognition and chemical communication that leads to the stimulation of nodule organogenesis (Oldroyd et al., 2009). Once residing within the nodule, the infecting rhizobia can convert atmospheric N2 to ammonia. Grain legume species differ in their tolerance to drought stress, and the reader is directed to a number of excellent reviews (Subbarao et al., 1995; Turner et al., 2001; Turner, 2003). However, universally, the establishment of infection and symbiotic N2 fixation is very sensitive to drought stress (Sprent, 1971; Gil-Quintana et al., 2013). A number of metabolic changes in the nodule of legumes exposed to water stress have been reported, including a decline in starch content and an increase in sucrose, decreased total free amino acid and ureide content (González et al., 1995), proteolysis, and a decline in the content of the oxygen carrier leghaemoglobin (Guérin et al., 1991), the latter causing poor oxygen diffusion in the nodule. It was suggested that ureides, which are N-rich compounds exported from the nodules of N2-fixing-tropical legumes (i.e. soybean and common bean), are involved in feedback regulation of the nitrogenase enzyme which is responsible for reduction of N2 to ammonia (Sinclair and Serraj, 1995; Serraj et al., 1999). A more recent proteomic and metabolic approach suggests that this is unlikely (Gil-Quintana et al., 2013). A more likely indirect regulation of nitrogenase via interaction between the ureide allantoin and abscisic acid (ABA) has been suggested. Allantoin was demonstrated to activate ABA production in Arabidopsis through increased transcription of NCED3, encoding an enzyme required for ABA biosynthesis and through post-translational activation of ABA (Watanabe et al., 2014). The interaction between allantoin and ABA is also supported in legumes where exogenous supply of ABA to peas was shown to inhibit nodulation (Philips, 1971) and regulate nodule formation via suppression of Nod factor (a bacterial chemical signal released in the rhizosphere of a host plant), signal transduction, and cytokinin induction of the nodule primordia (Ding et al., 2008). ABA likewise suppresses bacterial infection of the nodules (Ding et al., 2008) and reduces N fixation rates by up to 80% (González et al., 2001). The specific drought-induced suppression of nitrogenase activity via ureide-mediated induction of ABA is an area that requires further exploration.
Nitrogen assimilation and remobilization under drought
Ammonium generated from metabolic processes such as RNA turnover (Zrenner et al., 2006), protein turnover (Tabuchi et al., 2007), and photorespiration (Mattsson et al., 1997), as well as from uptake of external NH4+, is assimilated into organic N. Cytosolic NH4+ concentrations in the low to medium millimolar range are toxic to plants, although species differ in their sensitivity. Given that long-distance translocation of unassimilated NH4+ from the roots to the shoots rarely occurs, localized assimilation of NH4+ into organic N requires access to carbon skeletons, thereby inducing a localized carbon deprivation that contributes to the toxicity symptoms (Britto et al., 2001; Britto and Kronzucker, 2002). Additionally, some plant species, such as barley, experience a high energetic burden associated with the futile cycling of NH4+, with NH4+ efflux constituting as much as 80% of primary influx (Britto et al., 2001); such futile cycling, under special conditions, can also involve the NH3 species and passage through aquaporins (Coskun et al., 2013a), pointing at another potential link to water fluxes.
Drought stress induces recycling of NH4+ via premature leaf senescence and enhanced photorespiration (Wingler et al., 1999). The first step in recycling reduced N from NH4+ into organic molecules is catalysed by glutamine synthetase (GS). There are two isoforms of GS, cytosolic GS1 and plastidic GS2, both catalysing ATP-dependent condensation of NH4+ to the δ-carboxyl group of glutamate to form glutamine. GS has an important role in senescence-induced nutrient remobilization in cereal leaves (see reviews by Habash et al., 2001 and Hirel et al., 2007). Drought-afflicted rice plants have been reported to possess reduced total GS activity as the result of reduced transcript and protein levels of OsGS2, with the drought-tolerant rice cultivar Khitish better able to maintain total leaf GS activity than the drought-sensitive IR-64, over 12 d of water stress (Singh and Ghosh, 2013). It is understood that plants exposed to abiotic stresses such as drought undergo chloroplast dismantling into catabolic products such as amino acids and lipids, and further into nutrients that can be recycled and mobilized to sink organs (Otegui, 2018). It would be necessary to see if changes in GS2 protein levels, such as those reported by Singh and Ghosh (2013), are the result of plastidic protein turnover. Delaying chlorophyll degradation has been suggested to be a viable means of enhancing stress tolerance. Abiotic stresses induce expression of the chloroplast vesiculation pathway leading to chloroplast destabilization and the formation of vesicles. Silencing of the chloroplast vesiculation gene, CV, which interacts with the PSII subunit PsbO1, was shown to enhance drought tolerance of Arabidopsis (Wang and Blumwald, 2014) and rice (Sade et al., 2018).
‘Haying-off’
Rainfall in dryland agricultural regions is infrequent, and water shortages are common. Crops are generally produced in winter months when the majority of the rainfall events occur; however, this often results in crops maturing during months that are warmer and drier than those in which vegetative growth occurs. As a result, heat and drought stress are major factors during reproductive growth and often produce yield-limiting conditions. Of particular concern for farmers in these agro-climatic regions are production years with average or above-average rainfall during the vegetative growth stages which finish with drought conditions (Fig. 2). Crop plants produce vigorous vegetative growth and, as a result, use available soil water more quickly. Coupled with a dry finish to the season, this means crops are prone to ‘haying-off’, which refers to plants with a large biomass without accompanying large grain yield. The problem is compounded as the plants also produce grain with low quality. In the case of wheat crops, grain protein is critical to grain price; that is, low yields are exacerbated by reduced value per volume. Producers in such areas have adapted agronomic methods to deal with this type of growth environment. N fertilizers are applied in split applications over the course of the growing season, with limited amounts applied at sowing. This limits the vegetative growth in the early part of the season, and decisions to apply further applications are made based on rainfall events. If sufficient water will be available at the end of the season, producers will apply extra fertilizer to ensure the crops have nutrients available to maximize yield and grain quality. In drought years, these extra applications will not be applied to ensure some yield is achieved and the grain is of high quality. It should be noted that the converse situation can also occur in seasons with a wet finish, meaning the crops put on good yield with the extra water, but, if the producer has not applied sufficient N, the grain quality will have low protein content and the price per volume will be decreased as a result. (van Herwaarden et al., 1998a, b; Garnett and Rebetzke, 2013)
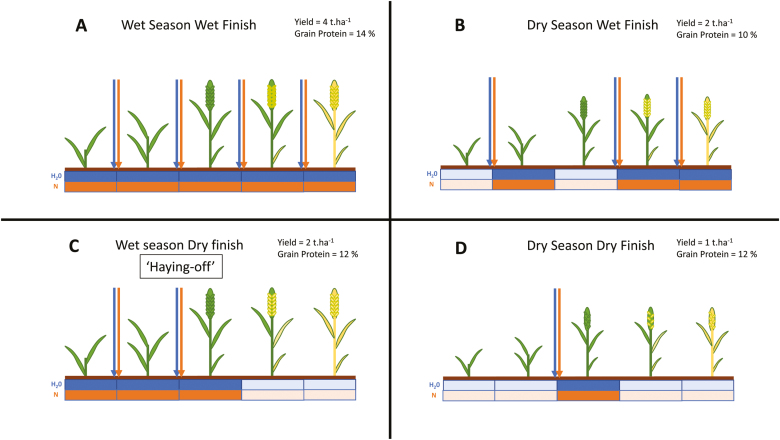
Fig. 2.
The importance of timing N application with water availability in dryland agriculture. Five stages of cereal crop development are represented in each panel. Arrows represent rainfall events (blue) and N applications (orange). Soil water (blue) and N (orange) for each development stage are represented by boxes beneath each developmental stage, indicating high water or N (dark), or reduced water or N (light). Size of plants and number of grains on each spike represent actual biomass and grain production of plants in each situation. Relative grain yields and protein content are provided for each of the four growth seasons. (A) A season with regular rainfall events; (B) a season with few rainfall events during vegetative growth, but regular rainfall during reproductive growth; (C) a season with regular rainfall events during vegetative growth, but few rainfall events during reproductive growth; (D) a ‘drought’ season with few rainfall events.
Molecular links
Water availability regulates nitrate transporters
The way in which N supply affects the N uptake system has been well characterized; in general the system is up-regulated by limiting N availability. It is also clear that, in the soil, N (particularly NO3–) supply is limited by water availability for movement of N towards the roots for uptake. The question of whether water limitation or the impact of drought on plants has an effect on the NO3– transport systems has not been explored in depth. However, recent work suggests that mimicking the osmotic potential stress associated with drought by PEG treatment impacts the components of the NO3– uptake system directly in rice. Expression levels of several NRT2 genes were decreased, while the expression of the NAR2 genes (NRT3 genes) was increased by PEG treatment. Overexpression of OsNAR2.1 had a positive impact on vegetative growth following treatment with PEG, and the transgenic lines had greater grain yield after drought treatment in a pot trial. Expression of genes associated with osmotic regulation in plants was altered by the overexpression of OsNAR2.1, suggesting that there are molecular links between the two regulatory systems (Chen et al., 2019). Increased NH4+ supply improved the rate of water uptake and root hydraulic conductance, and increased transcript levels of several aquaporin genes in rice (Ishikawa-Sakurai et al., 2014; Ren et al., 2015). Further work suggests the signalling for these responses is ABA related (Ding et al., 2016), as is the case for NO3– (see below ‘Nitrate–ABA crosstalk’). It should be noted that urea and NH4+ (or ammonia) can be transported by certain aquaporins, and this represents a direct link between water and N transport (Liu et al., 2003; Coskun et al., 2013a; Kirscht et al., 2016), and, while no plant example of NO3–-permeable aquaporins have been found, they do exist in mammals (Yasui et al., 1999). Finally, NRT1.1 is expressed in root tips, but it is also expressed in guard cells, indicating a putative link between N supply and water transport in Arabidopsis (Guo et al., 2003).
Recycling nitrogen under drought
The catabolism of nucleic acids and purine nucleotides in particular serves a housekeeping function, to recycle and remobilize nutrients during senescence (Taylor et al., 1993; Hillwig et al., 2011), thereby supporting plant growth and development (Zrenner et al., 2006). Oxidation of the purine catabolite xanthine to glyoxylate liberates three molecules of CO2 and four molecules of NH4+. Purine catabolism via turnover of RNA is also induced under nutrient depletion stress (Taylor et al., 1993; Melino et al., 2018; Casartelli et al., 2019). Nitrogen-starved wheat plants exogenously supplied with the purine catabolites xanthine and allantoin grew and photosynthesized as well as plants re-supplied with NO3–, suggesting that they can support plant growth (Melino et al., 2018).
A correlation between the accumulation of ureide compounds, allantoin and allantoate, in response to drought stress has been demonstrated in a number of legumes, including common bean (Coleto et al., 2014), soybean (Silvente et al., 2012), and French bean (Alamillo et al. 2010), although this appears to be a response of only drought-sensitive genotypes (Coleto et al., 2014). In comparison, allantoin accumulates in drought-tolerant cultivars of rice (Wang et al., 2012; Degenkolbe et al., 2013; Casartelli et al., 2018) and wheat (Bowne et al., 2012; Casartelli et al., 2019). In fact, allantoin accumulation is understood to provide protection to non-leguminous plant species via induction of ABA as previously described (Watanabe et al., 2014). This apparent contrast between the response of the ureide metabolic pathway in leguminous and non-leguminous plant species in response to water stress is unrelated to the de novo synthesis of ureides in nodules evidenced by the fact that ureides accumulate to levels in non-nodulated, NO3–-fed plants similar to those grown in symbiotic N-fixing conditions (Alamillo et al., 2010). The accumulation of allantoin under drought in non-leguminous plant species, when carbon skeletons are limited for assimilation into organic N, has been suggested to prevent loss of that N as ammonia gas (Casartelli et al., 2019) (Fig. 3).
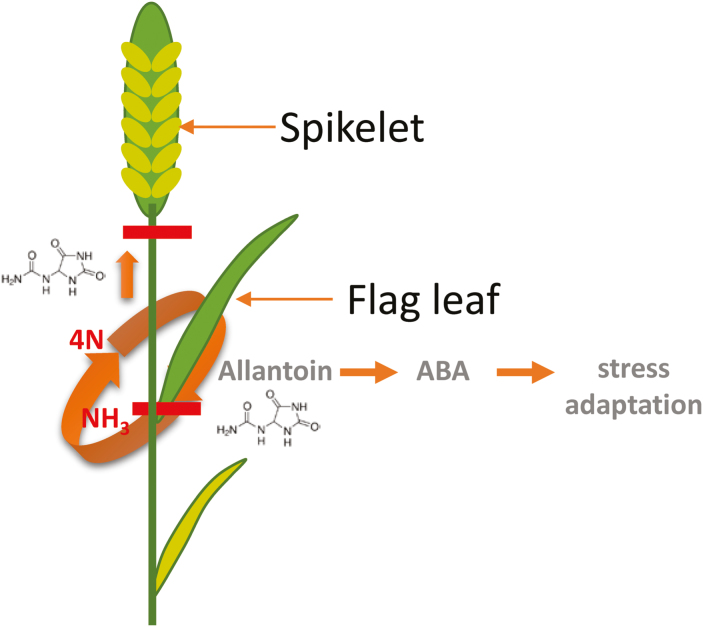
Fig. 3.
A plant metabolic link between adaption to drought and reduced N stress. Allantoin catabolism is restricted in drought-stressed plants. Allantoin accumulation both induces de novo synthesis of ABA and activates ABA from the inactive glycosylated form. Regulation of these processes may prevent loss of N as NH3 gas when carbon skeletons are in short supply. In contrast, under low N conditions, allantoin is catabolized (Melino et al., 2018) and recycled to NH3 which can be reduced by N-assimilatory enzymes or instead serve as a cheap N storage form for translocation to the grain where it represents a significant portion of the soluble N pool (Casartelli et al., 2019).
Nitrate–ABA crosstalk
Soil moisture content can be heterogenous in distribution, and particularly so when partial root drying techniques are used to restrict crop water use. Movement of ABA from the roots, sensing water availability, to the leaves is an important coordinator of plant response to the environment (Dodd et al., 2008). Likewise, soil N is often distributed heterogeneously in the soil, so that, when roots are grown under low-NO3– conditions (e.g. 0.01 mM NO3–), lateral root branching is stimulated, particularly from the roots in direct contact with the NO3– supply (1 mM NO3–) (Hackett, 1972; Drew et al., 1973; Forde, 2014), although the response is genotype dependent (Liao et al., 2006; Melino et al., 2015). High concentrations of external NO3– (>10 mM) inhibit lateral root development across the whole root, and this involves ABA signalling. Genetic dissection of the role of ABA in mediating the inhibitory effects of high NO3– on root branching in Arabidopsis demonstrated the requirement for an ABA signal transduction pathway involving pathway genes ABI4 and ABI5 (Signora et al., 2001). Signals such as dehydration stress (Xu et al., 2012) or NO3– (Ondzighi-Assoume et al., 2016) also stimulate the release of bioactive ABA via β-glucosidase (BG1 or BG2). Conjugated forms of ABA (ABA-glucose ester, ABA-GE) are stored in the vacuole and transported in the xylem, and the active form of ABA must be released from the inactive conjugated state. Exposing Arabidopsis roots to an increased concentration of NO3– (from 20 mM to 30 mM) led to a 3-fold increased ABA signal, with most of the accumulation localized to the endodermis and the stele of the growing tip as determined visually using a novel immunocytochemistry technique (Ondzighi-Assoume et al., 2016). Genetic dissection of the NO3–-stimulated ABA accumulation demonstrated that it occurred even in the absence of de novo ABA biosynthesis and was dependent on an active BG1 which stimulated the release of ABA from ABA-GE. Nitrate was shown to regulate BG1 at the transcriptional level (Ondzighi-Assoume et al., 2016).
It has also been suggested that stress transduction pathways can regulate NO3– sensing and signalling of the NO3– transporters. Two members of the NO3– transport peptide (NPF) family, Medicago truncatula NPF6.8 (Pellizzaro et al., 2014) and Arabidopsis NPF4.6 (Kanno et al., 2012), transport both NO3– and ABA. Additionally, another member of this family, NPF6.3 (formerly NRT1.1 or CHL1), functions as an NO3– sensor and transporter; the kinase CIPK23 and the calcium sensor CBL9 form a complex to phosphorylate NPF6.8 and activate NO3– uptake at low external concentrations whilst dephosphorylation of NPF6.8 leads to a switch to low-affinity NO3– uptake mode (Ho et al., 2009). A protein phosphatase 2C family member, AtABI2, has been reported to be a positive regulator of AtNPF6.3 via interaction with and dephosphorylation of CIPK23 and CBL1. The ABA-insensitive Arabidopsis mutant, abi2-2, was shown to be defective in NO3– perception (Léran et al., 2015). It is interesting to consider that ABA, which is produced under drought stress, could restrict NO3– sensing via ABI2 and NPF6.3 and thereby result in reduced NO3– uptake (Léran et al., 2015).
Pathways to improving nitrogen and water uptake
Better physiology
Uptake of water and nitrogen from the soil
It is clear that there is much left to understand about N movement in the soil and how this is influenced by the availability of water. Conversely, equally i mportant is the consideration of how soil water movement is regulated by plant nutritional status and the soil nutrient profile. Collaboration between root biologists and soil scientists will be required to truly unpack this interaction. The problem is complex given the connectivity and variation in soil types and characteristics, plant species, root ideotypes, crop physiology, environmental conditions, and agronomic management practices. It is clear that multi-level modelling will be useful in this regard, something which has only begun to be attempted in the context of the way plant water flux is regulated by nutritional factors (Cramer et al., 2009).
Improved physiological techniques
A better understanding of the so-called subtraits making up WUE and NUE is required. A biomarker trait would be extremely useful in efforts to improve WUE and NUE in crops. To what extent the large phenotypes can be dissected into smaller and more easily measurable traits is unclear. In the case of NUE, we know that N uptake efficiency (NUpE) and N utilization efficiency (NUtE) (and remobilization efficiency in the case of grain protein crops) are important traits to improve, but to what extent improving GS activity, for example, will impact on NUE, is less understood. Complicating matters is that feedback inhibition of improved subtraits may make the improvements impossible to measure accurately in the manipulated plants. For example, measurement of integrative traits such as stable isotope discrimination for carbon and oxygen have shown some promise in their capacity to identify germplasms with superior WUE in field experiments (Cabrera-Bosquet et al., 2011; Yousfi et al., 2012), although the value of this relationship has shown variable results across studies (Condon et al., 2004). Equally important is the identification of biomarkers that are actually useful in field-based experiments, as hydroponics and the use of PEG to mimic drought conditions can only be regarded as poor substitutes for the real-life interactions of water and N in soil.
Understanding the interaction of nitrogen and water uptake at the cellular level
Much remains to be explored regarding the interaction of N and water transport at the cellular level. For example, we need to understand the root zone and cell membrane localization of water and N transport more completely to determine if there is a common link with aquaporins, and new techniques such as single-cell transcriptomics, and measurement techniques to resolve co-location of transporters and physiological fluxes of water and N are required to answer these important questions. These types of integrated studies will allow identification of molecular identities of the basic machinery co-regulating water and N transport.
Improved WUE of crops through fine-tuning nitrogen supply
The understanding of the physiological, biochemical, and molecular mechanisms that control water use and WUE under N fertilization is critical for the development of new efficiencies of water use in agriculture. Even though grain yield and WUE in crops are primarily limited by the soil water deficit, higher yields are often achieved by using a higher dose of N fertilizer, especially in developing countries (Zhu and Chen, 2002; Wang et al., 2018). However, this practice may result in negative environmental consequences. The understanding of the mechanisms that control water use and WUE under N fertilization is therefore not only critical for water-scarce areas, such as semi-arid and arid regions, but much more broadly.
Several studies have shown that N supply enhances plant productivity by improving WUE through: (i) reducing water loss by regulating stomatal conductance without impacting the assimilation rate (Toft et al., 1989; Guehl et al., 1995); (ii) increasing the assimilation rate as a result of increased N investment in the photosynthetic apparatus (Ranjith et al., 1995) with no counterbalancing effect on stomatal conductance (Liu and Dickmann, 1996; Harvey and Van Den Driessche, 1999; Welander and Ottosson, 2000); (iii) causing a moderate increase in assimilation rate with a slight decrease in stomatal conductance (Wang et al., 1998); or (iv) increasing root growth and root length density (RLD) in deeper soil layers (Zhang et al., 2012). Further, several investigations showed that there is a positive correlation between NUE (van der Werf et al., 1993) and drought tolerance in cereal crops. In winter wheat, lines with greater NUE showed higher drought tolerance ability under soil water deficit (Fan and Li, 2001). In maize, cultivars with either high NUpE and NUtE that linked to greater drought-tolerant ability produced consistently higher yields (Kamara et al., 2014). In sweet sorghum, improved WUE and NUE under water stress contributed to the high degree of physiological acclimation to drought (Wang et al., 2014). This finding highlights that higher NUE could help plants have a higher ability to tolerate drought stress.
In rice, high rates of N application to high-yielding rice increased WUE in conventionally flooded rice (Zhang et al., 2012). Similarly, in wheat , application of N fertilizer significantly enhances root growth and RLD in deeper soil layers in wheat mainly because of (i) an increase of mineral N in deeper soil layers and (ii) a decrease in root mass per unit root length and average root diameter (Scott Russell, 1977; Liao et al., 2004). N supply increased root growth and RLD in deeper layers of the soil profile, namely 80–140 cm, and improved water uptake, above-ground biomass, and WUE during the vegetative growth stage of wheat. Enhanced RLD in deeper layers of the soil profile proved to be a beneficial trait in environments that are prone to end-of-season drought or terminal drought because roots at deeper layers of the soil profile are able to extract available water from deep layers (Kirkegaard et al., 2007; Palta et al., 2011; Wang et al., 2018). In wheat, the activity of these roots was evident as N applications enhanced water absorption of roots from deep soil layers more than in non-N supply treatments. In contrast, application of N fertilizer reduced the proportion of total biomass allocated to the root system, presumably because of the decreased average root diameter (RD) and root mass per unit of root length (RML) (Ercoli et al., 2008; Kamiji et al., 2014). The resulting low RD and RML by higher N fertilizer application enhanced RLD without increasing root biomass; on the other hand, it significantly increased water uptake and above-ground biomass (Wang et al., 2018). The proportion of the total biomass allocated to the root system was reduced by N fertilizer application compared with no N treatment. This lower partitioning of assimilates to roots has been positively correlated with higher grain yield and WUE (Fang et al., 2010; Hu et al., 2014).
Root traits that could improve water and nitrogen uptake
The genetic improvement of root traits could be valuable to enhance acquisition of water and nutrients because these resources are heterogeneously distributed in the soil (Hodge, 2004; Kitomi et al., 2018; Y. Li et al., 2016). In dryland agriculture, the water in the soil moves to the deep soil layers following gravity; that is, NO3– dissolved in soil water is also leached by precipitation into deep soil layers. Therefore, deeper rooting represents an advantageous trait to capture water and N from subsoil (Trachsel et al., 2013; Lynch and Wojciechowski, 2015). Under drought conditions, deep roots are especially advantageous in obtaining water efficiently from the subsoil (Rich and Watt, 2013). For example, a rice near-isogenic line (Dro1-NIL) which expresses deeper roots caused by a functional allele of DRO1, which is a quantitative trait locus (QTL) controlling root growth angle, had higher grain yield than the parent variety with shallow roots under drought condition (Uga et al., 2013) (Fig. 4). In maize, the steep, cheap, and deep root ideotype consisting of specific architectural (reduced crown root number; longer, but fewer lateral roots) and anatomical (increased root cortical aerenchyma, altered root barriers) traits could also be useful to capture N efficiently from subsoil (Lynch, 2013; Lynch and Wojciechowski, 2015). Higher root length density also enhances N acquisition in some crops by increasing the root surface area (Garnett et al., 2009). Whether the increased surface area contributes to N absorption depends largely on the soil environment; however, even in paddy fields where water and NH4+ are relatively equally distributed, increased deep roots in the lower soil layer enhanced grain yield in rice (Kawata et al., 1978; Morita et al., 1986, 1988). The N uptake from the lower soil layer is important for grain filling in the maturity stage when N is often depleted in the upper soil layers (Toriyama, 2001). In fact, experiments using Dro1-NIL showed that deep rooting by DRO1 improved N uptake after heading, resulting in better grain filling in a paddy field (Arai-Sanoh et al., 2014). Root system architecture (RSA), which determines the extent of the root zone, has the greatest influence on a plant’s water and N acquisition area from the soil. However, efforts to improve crop uptake efficiency for water and N must consider the physiological function of roots as well as lateral roots and root hairs, and root anatomical traits. Since the interaction between roots, soil, and microorganisms is complex, there is no simple RSA ideotype for improving acquisition efficiency of water and N. To construct an RSA model adapted to each environment, it is necessary not only to characterize RSA of each crop and variety but also to understand soil conditions in the target environment. Wild accessions may be useful in this regard, but has been a relatively underexplored resource for improving root traits.
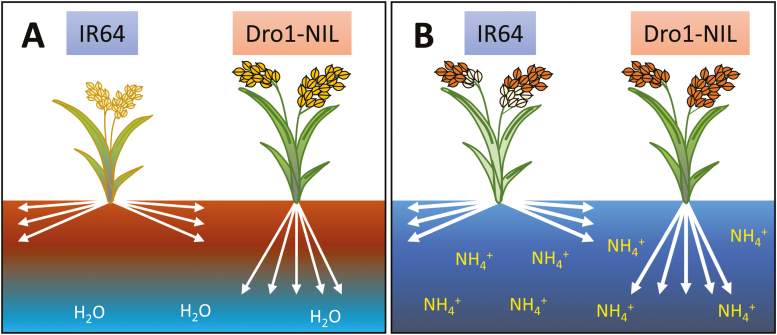
Fig. 4.
The beneficial impacts of deeper roots on water and N uptake in rice. Dro1-NIL has deeper rooting compared with the IR64 parental lowland cultivar. (A) The DRO1 locus allows rice roots to explore deeper subsoil for water in a drought that IR64 cannot access, allowing Dro1-NIL to continue to grow and produce grain in drought seasons. (B) The deeper roots of Dro1-NIL allow the plants to access NH4+ in deeper subsoils, meaning the plants can access N later in the growing season to improve grain yield and quality as compared with IR64.
Understanding crosstalk between the two uptake systems
Our understanding of whole-plant molecular physiological links between water and N transport is rudimentary. Signalling cascades linking the potentiation of stress detection with action responses may occur across organs. For example, shoot-based signals indicating water or N deficiency must be transported to the roots to enhance uptake, therefore requiring an understanding and synthesis of the molecular events occurring across plant tissues and organs. Recent work identified EPIDERMAL PATTERNING FACTOR1 (OsEPF1) in rice, which regulates the stomatal patterning in leaves, but also controls the development of aerenchyma cells in roots (Mohammed et al., 2019). This work represents an important link between regulation of gas exchange and potential drought adaptation with root transport physiology given the importance of root cortical development in the transport of nutrients, including N (Postma and Lynch, 2011; Hu et al., 2014; Saengwilai et al., 2014; Schneider et al., 2017).
Better phenotyping and breeding
Better phenotyping technology—both in the field and in controlled environments
High-throughput phenotyping technologies that can accurately measure physiological and morphological traits would be beneficial to efforts to improve the uptake efficiency of water and N. The choice between phenotyping in the field and controlled environments depends on the purpose and/or target traits. Since field conditions are heterogeneous, the data must be interpreted by taking into account the effects of the natural environment. Compounding this issue, phenotyping of large field trials requires a significant amount of resources in terms of labour, cost, and time. Nevertheless, field trials are indispensable for phenotypic selection in crop breeding. Recently, several types of field-based high-throughput phenotyping platforms have been established, from ground- to aerial-based platforms (Araus and Cairns, 2014; Shakoor et al., 2017; Araus et al., 2018). These platforms can acquire large amounts of phenotypic data non-destructively at one time with decreased labour and time costs compared with conventional methods. Ground-based platforms called ‘phenomobiles’ are vehicles with a range of on-board technologies such as navigation devices and sensors (Li et al., 2019; Qiu et al., 2019). The ground-based platforms are limited to measurement of a single or a few plots at a time. However, unmanned aerial platforms with multiple sensors, which can scan an entire trial in a short amount of time, have been developed (Zaman-Allah et al., 2015; Santesteban et al., 2017). This remote sensing technology based on visible/near-infrared spectroradiometry, infrared thermometry, and RGB colour cameras can acquire data on the physiological state of the plant body non-destructively, including water and N status, by different vegetation indices, such as the normalized difference vegetation index (NDVI) (Araus and Cairns, 2014; Kusnierek and Korsaeth, 2015).
Phenotyping of RSA in the field is more challenging because roots must be accessed from soil. Several sampling methods have been developed to date: the trench method for observation of vertical root distribution in the soil (Nemoto et al., 1998; Uga et al., 2013); methods of square or round monolith (Abe and Morita, 1994; Kano et al., 2011); and soil-core methods for quantification of root parameters such as root volume or length for each soil depth. Other unique methods for quantifying root traits other than root volume and length include the basket method for measuring root growth angle (Uga et al., 2011) and ‘shovelomics’ for scoring of several traits of basal roots after root sampling by shovels (Trachsel et al., 2011). No method enables quantification of the entire RSA in the field at one time (Topp et al., 2016). To do so, it is necessary to estimate the RSA by combining several methods (e.g. Nagel et al., 2012; Guo and York, 2019). Development of high-throughput technology that can measure RSA non-destructively in the field will greatly facilitate efforts to improve traits such as nutrient capture.
In controlled environments, several types of high-throughput phenotyping platforms for above-ground traits have been established (Junker et al., 2014; Halperin et al., 2017; Czedik-Eysenberg et al., 2018). Similar to the field, imaging data can be automatically acquired using several sensors fixed in a system installed in the greenhouse or growth chamber. These platforms enable non-destructive acquisition of data on plant traits including abiotic stresses such as water and N deficiency (Neilson et al., 2015; Ge et al., 2016). Various phenotyping methods or evaluating root traits related to RSA have also been developed for controlled environments using pot, box-pinboards, rhizotrons, and polyvinyl chloride (PVC) tubes, and hydroponic culture (Shashidhar et al., 2012; Downie et al., 2015). These culture conditions generally provide a limited root zone, which can result in different root phenotypes compared with field-grown plants. Rice, which often grows in hypoxic conditions, is amenable to several high-throughput phenotyping systems for RSA that have been established based on 3-D imaging of roots developed in gel media or hydroponic growth systems (Iyer-Pascuzzi et al., 2010; Clark et al., 2011; Fang et al., 2013; Uga et al., 2018). Just as for root phenotyping in the field, however, these methods do not allow measurement of the whole picture of 3-D RSA in the soil. To address this problem, 3-D root image analysis using X-ray computed tomography (CT) and MRI have been developed (Metzner et al., 2015; van Dusschoten et al., 2016; Atkinson et al., 2019). Phenotyping systems using X-ray CT and MRI imaging are still low throughput because their scanning and 3-D reconstruction require significant time for data acquisition and analysis. To use these methods practically, the speed and efficiency of their scanning and 3-D reconstruction require improvement.
Incorporation of multiple site–year trials into breeding efforts to improve WUE and NUE is required
Given the significant G×E×M interaction for both traits, it is unclear whether QTLs showing effects on improving the traits will be expressed or beneficial across different agro-climatic regions. As a result, models incorporating the G×E×M information may be useful in designing the genetic architecture of new varieties targeted to different environments. It is also clear, especially in dryland agricultural settings, that selecting for germplasms with superior NUE is not possible, and potentially pointless, unless the accompanying improvements in WUE are also selected. Efforts have been made to utilize existing networks for trials with proper characterization of climate and management variables and to model metadata (e.g. National Variety Trials in Australia; or the Wheat Genetic Improvement Network in the UK); however, there is room for improvement in this regard. Incorporation of new breeding targets may be required, for example breeding for growth habits such as altering maturity to take advantage of rainfall events. The complexity of the WUE–NUE interaction is emerging from efforts to understand the role of selection in the development of new varieties (Bänzinger et al., 2000; Ribaut et al., 2007; Sadras and Lawson, 2013; Elazab et al., 2016; Prey et al., 2018).
Better agronomy
Farmers in dryland agricultural environments already manage drought by applying N in split applications only when there is sufficient soil water present to support vegetative or reproductive growth. These decisions are based on a coarse understanding of the effect of timing of N application on yield and require further study to evaluate the implications of various nutrient management decisions (Abid et al., 2016). There is potential to prime the crop plants for various degrees of water availability based on the nutrient profile of fertilizer applied to induce useful modifications of root architecture. Similarly, in irrigated cropping environments, the capacity of crops to access fertilizer can be managed by managing irrigation decisions to maximize beneficial root architecture development (van Herwaarden et al., 1998a, b; Garnett and Rebetzke, 2013). A similar optimization of both N and water acquisition may be achieved in flooded systems, such as irrigated rice fields, by imposing alternate wetting and drying protocols, which have been shown to shift soil microbial communities and optimize ratios of NH4+ and NO3– in soil water by favouring nitrification during drying periods and inhibiting it during flooding periods while reducing water consumption (Kronzucker et al., 1999; Kirk and Kronzucker, 2005). Novel precision agriculture approaches in these areas carry much promise.
Foliar application of fertilizer is much less dependent on the availability of water than soil application. Work has been carried out to evaluate the efficiency of foliar uptake compared with root uptake, the role of the type of N (NO3–, NH4+, urea) preferred by plants, and the composition of the application solution (e.g. use of adjuvants) versus soil application (Woolfolk et al., 2002).
The potential impact of novel fertilizer technologies and soil amendments (e.g. silicon, ammonium chloride, biological nitrification inhibitors, novel slow-release fertilizer coatings or carriers, such as graphene) on NUpE is only beginning to be explored, but they have shown promise (Snyder, 2017). Technologies which time fertilizer release with water availability would have obvious benefit for crop growth and would reduce losses associated with N that has not been taken up by the crop.
Conclusions and future work
Given the fundamental importance of water and N supply to the success of sustainable crop production and our ability to feed the world, it is daunting to realize how much is left to discover regarding the uptake of these resources by plants. The genetic regulation of water and N uptake individually is complex, but it is clear that efforts to improve N uptake must also take into consideration the intricate ties with water availability and uptake. It also must be acknowledged that climate change will alter agricultural systems in complex ways, and these changes must be understood as part of programmes to improve N and water transport in crops. While our understanding of the machinery of transport systems has grown significantly, the signalling pathways regulating the uptake of water and N are not yet sufficiently understood. Similarly, the responses of root barrier formation to water and N supply and how changes in both resources together affect this formation require further investigation. Despite the enormity of the task of improving WUE and NUE in crops, with well-designed physiological studies, improved phenotyping and breeding capacity, and development of cutting-edge agronomic solutions, we are confident substantial gains will be realized in the coming years.
References
- Abe J, Morita S. 1994. Growth direction of nodal roots in rice: its variation and contribution to root system formation. Plant and Soil 165, 333–337. [Google Scholar]
- Abid M, Tian Z, Ata-Ul-Karim ST, Cui Y, Liu Y, Zahoor R, Jiang D, Dai T. 2016. Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Frontiers in Plant Science 7, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamillo JM, Díaz-Leal JL, Sánchez-Moran MV, Pineda M. 2010. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant, Cell & Environment 33, 1828–1837. [DOI] [PubMed] [Google Scholar]
- Allred JB, Brown OG, Bigham MJ. 2007. Nitrate mobility under unsaturated flow conditions in four initially dry soils. Soil Science 172, 27–41. [Google Scholar]
- Arai-Sanoh Y, Takai T, Yoshinaga S, Nakano H, Kojima M, Sakakibara H, Kondo M, Uga Y. 2014. Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Scientific Reports 4, 5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Cairns JE. 2014. Field high-throughput phenotyping: the new crop breeding frontier. Trends in Plant Science 19, 52–61. [DOI] [PubMed] [Google Scholar]
- Araus JL, Kefauver SC, Zaman-Allah M, Olsen MS, Cairns JE. 2018. Translating high-throughput phenotyping into genetic gain. Trends in Plant Science 23, 451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arth I, Frenzel P, Conrad R. 1998. Denitrification coupled to nitrification in the rhizosphere of rice. Soil Biology and Biochemistry 30, 509–515. [Google Scholar]
- Asplund L, Bergkvist G, Weih M. 2014. Proof of concept: nitrogen use efficiency of contrasting spring wheat varieties grown in greenhouse and field. Plant and Soil 374, 829–842. [Google Scholar]
- Atkinson JA, Pound MP, Bennett MJ, Wells DM. 2019. Uncovering the hidden half of plants using new advances in root phenotyping. Current Opinion in Biotechnology 55, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkos KD, Britto DT, Kronzucker HJ. 2010. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant, Cell & Environment 33, 23–34. [DOI] [PubMed] [Google Scholar]
- Bänzinger M, Edmeades GO, Beck D, Bellon M. 2000. Breeding for drought and nitrogen stress tolerance in maize. From theory to practice. Mexico: CIMMYT. [Google Scholar]
- Barber AS. 1962. A diffusion and mass-flow concept in soil nutrient availability. Soil Science 93, 39–49. [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL. 1992. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiology 99, 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn HL, McNeal BL, O’Connor GA. 2001. Soil chemistry. New York: John Wiley & Sons. [Google Scholar]
- Bouldin DR. 1986. The chemistry and biology of flooded soils in relation to the nitrogen economy in rice fields. Fertilizer research 9, 1–14. [Google Scholar]
- Bowne JB, Erwin TA, Juttner J, Schnurbusch T, Langridge P, Bacic A, Roessner U. 2012. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Molecular Plant 5, 418–429. [DOI] [PubMed] [Google Scholar]
- Britto DT, Balkos KD, Becker A, Coskun D, Huynh WQ, Kronzucker HJ. 2014. Potassium and nitrogen poising: physiological changes and biomass gains in rice and barley. Canadian Journal of Plant Science 94, 1085–1089. [Google Scholar]
- Britto DT, Kronzucker HJ. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159, 567–584. [Google Scholar]
- Britto DT, Kronzucker HJ. 2005. Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new views on old paradigms. Plant, Cell & Environment 28, 1396–1409. [Google Scholar]
- Britto DT, Kronzucker HJ. 2018. From aquaporin to ecosystem: plants in the water cycle. Journal of Plant Physiology 227, 1–2. [DOI] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass AD, Kronzucker HJ. 2001. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proceedings of the National Acadademy of Sciences, USA 98, 4255–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Albrizio R, Nogués S, Araus JL. 2011. Dual Ä 13C/ä 18O response to water and nitrogen availability and its relationship with yield in field-grown durum wheat. Plant, Cell & Environment 34, 418–433. [DOI] [PubMed] [Google Scholar]
- Cai ZC. 2002. Ammonium transformation in paddy soils affected by the presence of nitrate. Nutrient Cycling In Agroecosystems 63, 267–274. [Google Scholar]
- Casartelli A, Melino VJ, Baumann U, et al. 2019. Opposite fates of the purine metabolite allantoin under water and nitrogen limitations in bread wheat. Plant Molecular Biology 99, 477–497. [DOI] [PubMed] [Google Scholar]
- Casartelli A, Riewe D, Hubberten HM, Altmann T, Hoefgen R, Heuer S. 2018. Exploring traditional aus-type rice for metabolites conferring drought tolerance. Rice 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Turner BL. 2011. Transpiration modulates phosphorus acquisition in tropical tree seedlings. Tree Physiology 31, 878–885. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD. 2014. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology 164, 1600–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Guo S, Kronzucker HJ, Shi W. 2013. Nitrogen use efficiency (NUE) in rice links to NH4+ toxicity and futile NH4+ cycling in roots. Plant and Soil 369, 351. [Google Scholar]
- Chen J, Qi T, Hu Z, Fan X, Zhu L, Iqbal MF, Yin X, Xu G, Fan X. 2019. OsNAR2.1 positively regulates drought tolerance and grain yield under drought stress conditions in rice. Frontiers in Plant Science 10, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RT, MacCurdy RB, Jung JK, Shaff JE, McCouch SR, Aneshansley DJ, Kochian LV. 2011. Three-dimensional root phenotyping with a novel imaging and software platform. Plant Physiology 156, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleto I, Pineda M, Rodiño AP, De Ron AM, Alamillo JM. 2014. Comparison of inhibition of N2 fixation and ureide accumulation under water deficit in four common bean genotypes of contrasting drought tolerance. Annals of Botany 113, 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Li M, Becker A, Kronzucker HJ. 2013a Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiology 163, 1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Li M, Oh S, Kronzucker HJ. 2013b Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiology 162, 496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Shi W, Kronzucker HJ. 2017a How plant root exudates shape the nitrogen cycle. Trends in Plant Science 22, 661–673. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Shi W, Kronzucker HJ. 2017b Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nature Plants 3, 17074. [DOI] [PubMed] [Google Scholar]
- Cramer MD, Hawkins HJ, Verboom GA. 2009. The importance of nutritional regulation of plant water flux. Oecologia 161, 15–24. [DOI] [PubMed] [Google Scholar]
- Cramer MD, Hoffmann V, Verboom GA. 2008. Nutrient availability moderates transpiration in Ehrharta calycina. New Phytologist 179, 1048–1057. [DOI] [PubMed] [Google Scholar]
- Czedik-Eysenberg A, Seitner S, Güldener U, Koemeda S, Jez J, Colombini M, Djamei A. 2018. The ‘PhenoBox’, a flexible, automated, open-source plant phenotyping solution. New Phytologist 219, 808–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe T, Do PT, Kopka J, Zuther E, Hincha DK, Köhl KI. 2013. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS One 8, e63637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Haba P, Agüera E, Maldonado J. 1990. Differential effects of ammonium and tungsten on nitrate and nitrite uptake and reduction by sunflower plants. Plant Science 70, 21–26. [DOI] [PubMed] [Google Scholar]
- Ding L, Li Y, Wang Y, Gao L, Wang M, Chaumont F, Shen Q, Guo S. 2016. Root ABA accumulation enhances rice seedling drought tolerance under ammonium supply: interaction with aquaporins. Frontiers in Plant Science 7, 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, Harris JM, Oldroyd GE. 2008. Abscisic acid coordinates Nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. The Plant Cell 20, 2681–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. 2008. Abscisic acid signalling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits abscisic acid export to the shoots. Plant, Cell & Environment 31, 1263–1274. [DOI] [PubMed] [Google Scholar]
- Downie HF, Adu MO, Schmidt S, Otten W, Dupuy LX, White PJ, Valentine TA. 2015. Challenges and opportunities for quantifying roots and rhizosphere interactions through imaging and image analysis. Plant, Cell & Environment 38, 1213–1232. [DOI] [PubMed] [Google Scholar]
- Drew MC, Saker LR, Ashley TW. 1973. Nutrient supply and growth of seminal root system in barley. 1. Effect of nitrate concentration on growth of axes and laterals. Journal of Experimental Botany 24, 1189–1202. [Google Scholar]
- Elazab A, Serret MD, Araus JL. 2016. Interactive effect of water and nitrogen regimes on plant growth, root traits and water status of old and modern durum wheat genotypes. Planta 244, 125–144. [DOI] [PubMed] [Google Scholar]
- Ercoli L, Lulli L, Mariotti M, Masoni A, Arduini I. 2008. Post-anthesis dry matter and nitrogen dynamics in durum wheat as affected by nitrogen supply and soil water availability. European Journal of Agronomy 28, 138–147. [Google Scholar]
- Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W. 2008. How a centrury of ammonia synthesis changed the world. Nature Geoscience 1, 636. [Google Scholar]
- Fan XL, Li YK. 2001. Effect of drought stress and drought tolerance heredity on nitrogen efficiency of winter wheat. In: Horst WJ, Schenk MK, Bürkert A, et al. , eds. Plant nutrition: food security and sustainability of agro-ecosystems through basic and applied research. Dordrecht: Springer Netherlands, 62–63. [Google Scholar]
- Fang S, Clark RT, Zheng Y, Iyer-Pascuzzi AS, Weitz JS, Kochian LV, Edelsbrunner H, Liao H, Benfey PN. 2013. Genotypic recognition and spatial responses by rice roots. Proceedings of the National Academy of Sciences, USA 110, 2670–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Xu B, Turner NC, Li F. 2010. Does root pruning increase yield and water-use efficiency of winter wheat? Crop and Pasture Science 61, 899–910. [Google Scholar]
- Forde BG. 2014. Nitrogen signalling pathways shaping root system architecture: an update. Current Opinion in Plant Biology 21, 30–36. [DOI] [PubMed] [Google Scholar]
- Foth HD. 1978. Fundamentals of soil science. Soil Science 125, 272. [Google Scholar]
- Garnett T, Conn V, Kaiser BN. 2009. Root based approaches to improving nitrogen use efficiency in plants. Plant, Cell & Environment 32, 1272–1283. [DOI] [PubMed] [Google Scholar]
- Garnett TP, Rebetzke GJ. 2013. Improving crop nitrogen use in dryland farming. In: Rengel Z, ed. Improving water and nutrient-use efficiency in food production systems. Chichester, UK: John Wiley & Sons, Inc., 123–144. [Google Scholar]
- Ge Y, Bai G, Stoerger V, Schnable JC. 2016. Temporal dynamics of maize plant growth, water use, and leaf water content using automated high throughput RGB and hyperspectral imaging. Computers and Electronics in Agriculture 127, 625–632. [Google Scholar]
- Gil-Quintana E, Larrainzar E, Seminario A, Díaz-Leal JL, Alamillo JM, Pineda M, Arrese-Igor C, Wienkoop S, González EM. 2013. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. Journal of Experimental Botany 64, 2171–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González EM, Gálvez L, Arrese-Igor C. 2001. Abscisic acid induces a decline in nitrogen fixation that involves leghaemoglobin, but is independent of sucrose synthase activity. Journal of Experimental Botany 52, 285–293. [DOI] [PubMed] [Google Scholar]
- González EM, Gordon AJ, James CL, Arrese-lgor C. 1995. The role of sucrose synthase in the response of soybean nodules to drought. Journal of Experimental Botany 46, 1515–1523. [Google Scholar]
- Górska A, Lazor J, Zwieniecka A, Benway C, Zwieniecki M. 2010. The capacity for nitrate regulation of root hydraulic properties correlates with species’ nitrate uptake rates. Plant and Soil 337, 447–455. [Google Scholar]
- Gorska A, Ye Q, Holbrook NM, Zwieniecki MA. 2008a Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiology 148, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska A, Zwieniecka A, Holbrook NM, Zwieniecki MA. 2008b Nitrate induction of root hydraulic conductivity in maize is not correlated with aquaporin expression. Planta 228, 989–998. [DOI] [PubMed] [Google Scholar]
- Guehl JM, Fort C, Ferhi A. 1995. Differential response of leaf conductance, carbon isotope discrimination and water-use efficiency to nitrogen deficiency in maritime pine and pedunculate oak plants. New Phytologist 131, 149–157. [Google Scholar]
- Guérin V, Pladys D, Trinchant J-C, Rigaud J. 1991. Proteolysis and nitrogen fixation in faba-bean (Vicia faba) nodules under water stress. Physiologia Plantarum 82, 360–366. [Google Scholar]
- Guo FQ, Young J, Crawford NM. 2003. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. The Plant Cell 15, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, York LM. 2019. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. Journal of Experimental Botany 70, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Chen G, Zhou Y, Shen Q. 2007. Ammonium nutrition increases photosynthesis rate under water stress at early development stage of rice (Oryza sativa L.). Plant and Soil 296, 115–124. [Google Scholar]
- Habash DZ, Massiah AJ, Rong HL, Wallsgrove RM, Leigh RA. 2001. The role of cytosolic glutamine synthetase in wheat. Annals of Applied Biology 138, 83–89. [Google Scholar]
- Hachez C, Veselov D, Ye Q, Reinhardt H, Knipfer T, Fricke W, Chaumont F. 2012. Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant, Cell & Environment 35, 185–198. [DOI] [PubMed] [Google Scholar]
- Hackett C. 1972. A method of applying nutrients locally to roots under controlled conditions, and some morphological effects of locally applied nitrate on the branching of wheat roots. Australian Journal of Biological Sciences 25, 1169–1180. [Google Scholar]
- Halperin O, Gebremedhin A, Wallach R, Moshelion M. 2017. High-throughput physiological phenotyping and screening system for the characterization of plant–environment interactions. The Plant Journal 89, 839–850. [DOI] [PubMed] [Google Scholar]
- Harvey HP, Van Den Driessche R. 1999. Nitrogen and potassium effects on xylem cavitation and water-use efficiency in poplars. Tree Physiology 19, 943–950. [DOI] [PubMed] [Google Scholar]
- Hatfield JL, Dold C. 2019. Water-use efficiency: advances and challenges in a changing climate. Frontiers in Plant Science 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillwig MS, Contento AL, Meyer A, Ebany D, Bassham DC, Macintosh GC. 2011. RNS2, a conserved member of the RNase T2 family, is necessary for ribosomal RNA decay in plants. Proceedings of the National Academy of Sciences, USA 108, 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Le Gouis J, Ney B, Gallais A. 2007. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. Journal of Experimental Botany 58, 2369–2387. [DOI] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. 2009. CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162, 9–24. [Google Scholar]
- Hu B, Henry A, Brown KM, Lynch JP. 2014. Root cortical aerenchyma inhibits radial nutrient transport in maize (Zea mays). Annals of Botany 113, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2015. IPCC expert meeting on climate change, food, and agriculture. Mastrandrea MD, Mach KJ, Barros VR, et al. , eds. http://www.ipcc.ch/.
- Ishikawa-Sakurai J, Hayashi H, Murai-Hatano M. 2014. Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant and Soil 379, 289–300. [Google Scholar]
- Iyer-Pascuzzi AS, Symonova O, Mileyko Y, Hao Y, Belcher H, Harer J, Weitz JS, Benfey PN. 2010. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiology 152, 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker A, Muraya MM, Weigelt-Fischer K, Arana-Ceballos F, Klukas C, Melchinger AE, Meyer RC, Riewe D, Altmann T. 2014. Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems. Frontiers in Plant Science 5, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamara MM, El-Degwy IS, Koyama H. 2014. Estimation combining ability of some maize inbred lines using line×tester mating design under two nitrogen levels. Australian Journal of Crop Science 8, 1336–1342. [Google Scholar]
- Kamiji Y, Pang J, Milroy SP, Palta JA. 2014. Shoot biomass in wheat is the driver for nitrogen uptake under low nitrogen supply, but not under high nitrogen supply. Field Crops Research 165, 92–98. [Google Scholar]
- Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M. 2012. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proceedings of the National Academy of Sciences, USA 109, 9653–9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Inukai Y, Kitano H, Yamauchi A. 2011. Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant and Soil 342, 117–128. [Google Scholar]
- Kawata S-i, Soejima M, Yamazaki K. 1978. The superficial root formation and yield of hulled rice. Japanese Journal of Crop Science 47, 617–628. [Google Scholar]
- Kim YX, Ranathunge K, Lee S, Lee Y, Lee D, Sung J. 2018. Composite transport model and water and solute transport across plant roots: an update. Frontiers in Plant Science 9, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk GJ, Kronzucker HJ. 2005. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Annals of Botany 96, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard J, Lilley J, Howe G, Graham J. 2007. Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315. [Google Scholar]
- Kirscht A, Kaptan SS, Bienert GP, Chaumont F, Nissen P, de Groot BL, Kjellbom P, Gourdon P, Johanson U. 2016. Crystal structure of an ammonia-permeable aquaporin. PLOS Biology 14, e1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitomi Y, Itoh J-I, Uga Y. 2018. Genetic mechanisms involved in the formation of root system architecture. In: Sasaki T, Ashikari M, eds. Rice genomics, genetics and breeding. Singapore: Springer Singapore, 241–274. [Google Scholar]
- Kotula L, Ranathunge K, Schreiber L, Steudle E. 2009. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany 60, 2155–2167. [DOI] [PubMed] [Google Scholar]
- Kreszies T, Schreiber L, Ranathunge K. 2018. Suberized transport barriers in Arabidopsis, barley and rice roots: from the model plant to crop species. Journal of Plant Physiology 227, 75–83. [DOI] [PubMed] [Google Scholar]
- Kreszies T, Shellakkutti N, Osthoff A, Yu P, Baldauf JA, Zeisler-Diehl VV, Ranathunge K, Hochholdinger F, Schreiber L. 2019. Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: analysis of chemical, transcriptomic and physiological responses. New Phytologist 221, 180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK. 2009. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230, 119–134. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK. 2011. Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). Journal of Experimental Botany 62, 4215–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY, Kirk GJD. 2000. Comparative kinetic analysis of ammonium and nitrate acquisition by tropical lowland rice: implications for rice cultivation and yield potential. New Phytologist 145, 471–476. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. 1997. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385, 59–61. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass AD, Kirk GJ. 1999. Nitrate–ammonium synergism in rice. A subcellular flux analysis. Plant Physiology 119, 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper P, Rohula G, Saksing L, Sellin A, Lõhmus K, Ostonen I, Helmisaari H-S, Sõber A. 2012. Does soil nutrient availability influence night-time water flux of aspen saplings? Environmental and Experimental Botany 82, 37–42. [Google Scholar]
- Kusnierek K, Korsaeth A. 2015. Simultaneous identification of spring wheat nitrogen and water status using visible and near infrared spectra and Powered Partial Least Squares Regression. Computers and Electronics in Agriculture 117, 200–213. [Google Scholar]
- Léran S, Edel KH, Pervent M, Hashimoto K, Corratgé-Faillie C, Offenborn JN, Tillard P, Gojon A, Kudla J, Lacombe B. 2015. Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Science Signaling 8, ra43. [DOI] [PubMed] [Google Scholar]
- Li G, Tillard P, Gojon A, Maurel C. 2016. Dual regulation of root hydraulic conductivity and plasma membrane aquaporins by plant nitrate accumulation and high-affinity nitrate transporter NRT2.1. Plant & Cell Physiology 57, 733–742. [DOI] [PubMed] [Google Scholar]
- Li X, Ingvordsen CH, Weiss M, Rebetzke GJ, Condon AG, James RA, Richards RA. 2019. Deeper roots associated with cooler canopies, higher normalized difference vegetation index, and greater yield in three wheat populations grown on stored soil water. Journal of Experimental Botany 70, 4963–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kronzucker HJ, Shi W. 2016. Microprofiling of nitrogen patches in paddy soil: analysis of spatiotemporal nutrient heterogeneity at the microscale. Scientific Reports 6, 27064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Fillery IRP, Palta JA. 2004. Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Functional Plant Biology 31, 121–129. [DOI] [PubMed] [Google Scholar]
- Liao MT, Palta JA, Fillery IRP. 2006. Root characteristics of vigorous wheat improve early nitrogen uptake. Australian Journal of Agricultural Research 57, 1097–1107. [Google Scholar]
- Liu LH, Ludewig U, Gassert B, Frommer WB, von Wirén N. 2003. Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiology 133, 1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dickmann DI. 1996. Effects of water and nitrogen interaction on net photosynthesis, stomatal conductance, and water-use efficiency in two hybrid poplar clones. Physiologia Plantarum 97, 507–512. [Google Scholar]
- Lux A, Sottníková A, Opatrná J, Greger M. 2004. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiologia Plantarum 120, 537–545. [DOI] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Wojciechowski T. 2015. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66, 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matimati I, Verboom GA, Cramer MD. 2014. Nitrogen regulation of transpiration controls mass-flow acquisition of nutrients. Journal of Experimental Botany 65, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson M, Hausler RE, Leegood RC, Lea PJ, Schjoerring JK. 1997. Leaf–atmosphere NH3 exchange in barley mutants with reduced activities of glutamine synthetase. Plant Physiology 114, 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L. 2015. Aquaporins in plants. Physiological Reviews 95, 1321–1358. [DOI] [PubMed] [Google Scholar]
- Melino VJ, Casartelli A, George J, Rupasinghe T, Roessner U, Okamoto M, Heuer S. 2018. RNA catabolites contribute to the nitrogen pool and support growth recovery of wheat. Frontiers in Plant Science 9, 1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino VJ, Fiene G, Enju A, Cai J, Buchner P, Heuer S. 2015. Genetic diversity for root plasticity and nitrogen uptake in wheat seedlings. Functional Plant Biology 42, 942–956. [DOI] [PubMed] [Google Scholar]
- Metzner R, Eggert A, van Dusschoten D, Pflugfelder D, Gerth S, Schurr U, Uhlmann N, Jahnke S. 2015. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: potential and challenges for root trait quantification. Plant Methods 11, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed U, Caine RS, Atkinson JA, Harrison EL, Wells D, Chater CC, Gray JE, Swarup R, Murchie EH. 2019. Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Scientific Reports 9, 5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Iwabuchi A, Yamazaki K. 1986. Relationships between the growth direction of primary roots and yield in rice plants. Japanese Journal of Crop Science 55, 520–525. [Google Scholar]
- Morita S, Suga T, Yamazaki K. 1988. The relationship between root length density and yield in rice plants. Japanese Journal of Crop Science 57, 438–443. [Google Scholar]
- Münch E. 1930. Die stoffbewegungen in der pflanze. Jena: G. Fischer. [Google Scholar]
- Nagel KA, Putz A, Gilmer F, et al. 2012. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39, 891–904. [DOI] [PubMed] [Google Scholar]
- Neilson EH, Edwards AM, Blomstedt CK, Berger B, Møller BL, Gleadow RM. 2015. Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a C4 cereal crop plant to nitrogen and water deficiency over time. Journal of Experimental Botany 66, 1817–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto H, Suga R, Ishihara M, Okutsu Y. 1998. Deep rooted rice varieties detected through the observation of root characteristics using the trench method. Japanese Journal of Breeding 48, 321–324. [Google Scholar]
- Nobel PS. 2009. Physicochemical and environmental plant physiology. Burlington, MA: Elsevier Academic Press. [Google Scholar]
- Oldroyd GE, Harrison MJ, Paszkowski U. 2009. Reprogramming plant cells for endosymbiosis. Science 324, 753–754. [DOI] [PubMed] [Google Scholar]
- Ondzighi-Assoume CA, Chakraborty S, Harris JM. 2016. Environmental nitrate stimulates abscisic acid accumulation in Arabidopsis root tips by releasing it from inactive stores. The Plant Cell 28, 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS. 2018. Vacuolar degradation of chloroplast components: autophagy and beyond. Journal of Experimental Botany 69, 741–750. [DOI] [PubMed] [Google Scholar]
- Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M. 2011. Large root systems: are they useful in adapting wheat to dry environments? Functional Plant Biology 38, 347–354. [DOI] [PubMed] [Google Scholar]
- Pellizzaro A, Clochard T, Cukier C, et al. 2014. The nitrate transporter MtNPF6.8 (MtNRT1.3) transports abscisic acid and mediates nitrate regulation of primary root growth in Medicago truncatula. Plant Physiology 166, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfromm PH. 2017. Towards sustainable agriculture: fossil-free ammonia. Journal of Renewable and Sustainable Energy 9, 034702. [Google Scholar]
- Philips DA. 1971. Abscisic acid inhibition of root nodule initiation in Pisum sativum. Planta 100, 181–190. [DOI] [PubMed] [Google Scholar]
- Plett DC, Holtham LR, Okamoto M, Garnett TP. 2018. Nitrate uptake and its regulation in relation to improving nitrogen use efficiency in cereals. Seminars in Cell & Developmental Biology 74, 97–104. [DOI] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. 2011. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiology 156, 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prey L, Kipp S, Hu Y, Schmidhalter U. 2018. Nitrogen use efficiency and carbon traits of high-yielding European hybrid vs. line winter wheat cultivars: potentials and limitations. Frontiers in Plant Science 9, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q, Sun N, Bai H, Wang N, Fan Z, Wang Y, Meng Z, Li B, Cong Y. 2019. Field-based high-throughput phenotyping for maize plant using 3D lidar point cloud generated with a ‘Phenomobile’. Frontiers in Plant Science 10, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, El-Kereamy A, Gidda S, Bi YM, Rothstein SJ. 2014. AMT1;1 transgenic rice plants with enhanced NH4+ permeability show superior growth and higher yield under optimal and suboptimal NH4+ conditions. Journal of Experimental Botany 65, 965–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Kim YX, Wassmann F, Kreszies T, Zeisler V, Schreiber L. 2017. The composite water and solute transport of barley (Hordeum vulgare) roots: effect of suberized barriers. Annals of Botany 119, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Kotula L, Steudle E, Lafitte R. 2004. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. Journal of Experimental Botany 55, 433–447. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Lin J, Steudle E, Schreiber L. 2011a Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant, Cell & Environment 34, 1223–1240. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L, Bi YM, Rothstein SJ. 2016. Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 243, 231–249. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L, Franke R. 2011b Suberin research in the genomics era—new interest for an old polymer. Plant Science 180, 399–413. [DOI] [PubMed] [Google Scholar]
- Ranjith SA, Meinzer FC, Perry MH, Thom M. 1995. Partitioning of carboxylase activity in nitrogen-stressed sugarcane and its relationship to bundle sheath leakiness to CO2, photosynthesis and carbon isotope discrimination. Functional Plant Biology 22, 903–911. [Google Scholar]
- Raun WR, Johnson GV. 1999. Improving nitrogen use efficiency for cereal production. Agronomy Journal 91, 357–363. [Google Scholar]
- Raven JA. 2008. Transpiration: how many functions? New Phytologist 179, 905–907. [DOI] [PubMed] [Google Scholar]
- Reddy KR, Patrick WH. 1975. Effect of alternate aerobic and anaerobic conditions on redox potential, organic matter decomposition and nitrogen loss in a flooded soil. Soil Biology and Biochemistry 7, 87–94. [Google Scholar]
- Reddy KR, Patrick WH. 1986. Denitrification losses in flooded rice fields. Fertilizer Research 9, 99–116. [Google Scholar]
- Ren B, Wang M, Chen Y, Sun G, Li Y, Shen Q, Guo S. 2015. Water absorption is affected by the nitrogen supply to rice plants. Plant and Soil 396, 397–410. [Google Scholar]
- Rengel Z. 1993. Mechanistic simulation models of nutrient uptake: a review. Plant and Soil 152, 161–173. [Google Scholar]
- Ribaut J-M, Fracheboud Y, Monneveux P, Banziger M, Vargas M, Jiang C. 2007. Quantitative trait loci for yield and correlated traits under high and low soil nitrogen conditions in tropical maize. Molecular Breeding 20, 15–29. [Google Scholar]
- Rich SM, Watt M. 2013. Soil conditions and cereal root system architecture: review and considerations for linking Darwin and Weaver. Journal of Experimental Botany 64, 1193–1208. [DOI] [PubMed] [Google Scholar]
- Ripley BS, Abraham TI, Osborne CP. 2008. Consequences of C4 photosynthesis for the partitioning of growth: a test using C3 and C4 subspecies of Alloteropsis semialata under nitrogen-limitation. Journal of Experimental Botany 59, 1705–1714. [DOI] [PubMed] [Google Scholar]
- Roberts A, Oparka K. 2003. Plasmodesmata and the control of symplastic transport. Plant, Cell and Environment 26, 103. [Google Scholar]
- Sade N, Umnajkitikorn K, Rubio Wilhelmi MDM, Wright M, Wang S, Blumwald E. 2018. Delaying chloroplast turnover increases water-deficit stress tolerance through the enhancement of nitrogen assimilation in rice. Journal of Experimental Botany 69, 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadras VO. 2004. Yield and water-use efficiency of water- and nitrogen-stressed wheat crops increase with degree of co-limitation. European Journal of Agronomy 21, 455–464. [Google Scholar]
- Sadras VO. 2005. A quantitative top-down view of interactions between stresses: theory and analysis of nitrogen–water co-limitation in Mediterranean agro-ecosystems. Australian Journal of Agricultural Research 56, 1151–1157. [Google Scholar]
- Sadras VO, Lawson C. 2013. Nitrogen and water-use efficiency of Australian wheat varieties released between 1958 and 2007. European Journal of Agronomy 46, 34–41. [Google Scholar]
- Sadras VO, Richards RA. 2014. Improvement of crop yield in dry environments: benchmarks, levels of organisation and the role of nitrogen. Journal of Experimental Botany 65, 1981–1995. [DOI] [PubMed] [Google Scholar]
- Sadras VO, Rodriguez D. 2010. Modelling the nitrogen-driven trade-off between nitrogen utilisation efficiency and water use efficiency of wheat in Eastern Australia. Field Crops Research 118, 297–305. [Google Scholar]
- Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP. 2014. Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW. 1987a The nitrogen use efficiency of C3 and C4 plants: I. Leaf nitrogen, growth, and biomass partitioning in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 84, 954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW. 1987b. The nitrogen use efficiency of C3 and C4 plants. II. Leaf nitrogen effects on the gas exchange characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 84, 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR. 1987. The nitrogen use efficiency of C3 and C4 plants: III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 85, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesteban LG, Di Gennaro SF, Herrero-Langreo A, Miranda C, Royo JB, Matese A. 2017. High-resolution UAV-based thermal imaging to estimate the instantaneous and seasonal variability of plant water status within a vineyard. Agricultural Water Management 183, 49–59. [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M. 2004. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiology 136, 2483–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider HM, Postma JA, Wojciechowski T, Kuppe C, Lynch JP. 2017. Root cortical senescence improves growth under suboptimal availability of N, P, and K. Plant Physiology 174, 2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F. 2007. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiology 27, 551–559. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Franke R, Hartmann K. 2005. Effects of NO3 deficiency and NaCl stress on suberin deposition in rhizo- and hypodermal (RHCW) and endodermal cell walls (ECW) of castor bean (Ricinus communis L.) roots. Plant and Soil 269, 333–339. [Google Scholar]
- Scott Russell R. 1977. Plant root systems: their function and interaction with the soil. London: McGraw-Hill. [Google Scholar]
- Serraj R, Vadez VV, Denison RF, Sinclair TR. 1999. Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiology 119, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor N, Lee S, Mockler TC. 2017. High throughput phenotyping to accelerate crop breeding and monitoring of diseases in the field. Current Opinion in Plant Biology 38, 184–192. [DOI] [PubMed] [Google Scholar]
- Shangguan ZP, Shao MA, Dyckmans J. 2000. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environmental and Experimental Botany 44, 141–149. [DOI] [PubMed] [Google Scholar]
- Shashidhar HE, Henry A, Hardy B, eds. 2012. Methodologies for root drought studies in rice. Manila: International Rice Research Institute. [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang H. 2001. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. The Plant Journal 28, 655–662. [DOI] [PubMed] [Google Scholar]
- Silver WL, Herman DJ, Firestone MK. 2001. Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology 82, 2410–2416. [Google Scholar]
- Silvente S, Sobolev AP, Lara M. 2012. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS One 7, e38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Serraj R. 1995. Legume nitrogen fixation and drought. Nature 378, 344. [Google Scholar]
- Singh KK, Ghosh S. 2013. Regulation of glutamine synthetase isoforms in two differentially drought-tolerant rice (Oryza sativa L.) cultivars under water deficit conditions. Plant Cell Reports 32, 183–193. [DOI] [PubMed] [Google Scholar]
- Snyder CS. 2017. Enhanced nitrogen fertiliser technologies support the ‘4R’ concept to optimise crop production and minimise environmental losses. Soil Research 55, 463–472. [Google Scholar]
- Sprent JI. 1971. The effects of water stress on nitrogen-fixing root nodules. New Phytologist 70, 9–17. [Google Scholar]
- Steudle E. 2000. Water uptake by roots: effects of water deficit. Journal of Experimental Botany 51, 1531–1542. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49, 775–788. [Google Scholar]
- Subbarao GV, Johansen C, Slinkard AE, Nageswara Rao RC, Saxena NP, Chauhan YS, Lawn RJ. 1995. Strategies for improving drought resistance in grain legumes. Critical Reviews in Plant Sciences 14, 469–523. [Google Scholar]
- Swarbreck SM, Wang M, Wang Y, Kindred D, Sylvester-Bradley R, Shi W, Varinderpal-Singh, Bentley AR, Griffiths H. 2019. A roadmap for lowering crop nitrogen requirement. Trends in Plant Science 24, 892–904. [DOI] [PubMed] [Google Scholar]
- Syring K, Claassen N. 1995. Estimation of the influx and the radius of the depletion zone developing around a root during nutrient uptake. Plant and Soil 175, 115–123. [Google Scholar]
- Szczerba MW, Britto DT, Ali SA, Balkos KD, Kronzucker HJ. 2008. NH4+-stimulated and -inhibited components of K+ transport in rice (Oryza sativa L.). Journal of Experimental Botany 59, 3415–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi M, Abiko T, Yamaya T. 2007. Assimilation of ammonium ions and reutilization of nitrogen in rice (Oryza sativa L.). Journal of Experimental Botany 58, 2319–2327. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Bariola PA, delCardayré SB, Raines RT, Green PJ. 1993. RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proceedings of the National Academy of Sciences, USA 90, 5118–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP. 2010. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. Journal of Experimental Botany 61, 2303–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje JM, Sexstone AJ, Myrold DD, Robinson JA. 1982. Denitrification: ecological niches, competition and survival. Antonie van Leeuwenhoek 48, 569–583. [DOI] [PubMed] [Google Scholar]
- Toft NL, Anderson JE, Nowak RS. 1989. Water use efficiency and carbon isotope composition of plants in a cold desert environment. Oecologia 80, 11–18. [DOI] [PubMed] [Google Scholar]
- Topp CN, Bray AL, Ellis NA, Liu Z. 2016. How can we harness quantitative genetic variation in crop root systems for agricultural improvement? Journal of Integrative Plant Biology 58, 213–225. [DOI] [PubMed] [Google Scholar]
- Toriyama K. 2001. Soil and plant nutrition science now developed from field study: aquisition and analysis of data from the new view point: growth of rice plant in the large size paddy field and the variability in soil nitrogen fertility. Japanese Journal of Soil Science and Plant Nutrition 72, 453–458. [Google Scholar]
- Trachsel S, Kaeppler S, Brown K, Lynch J. 2011. Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant and Soil 341, 75–87. [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP. 2013. Maize root growth angles become steeper under low N conditions. Field Crops Research 140, 18–31. [Google Scholar]
- Tuchscherr L, Löffler B, Buzzola FR, Sordelli DO. 2010. Staphylococcus aureus adaptation to the host and persistence: role of loss of capsular polysaccharide expression. Future Microbiology 5, 1823–1832. [DOI] [PubMed] [Google Scholar]
- Turner NC. 2003. Drought resistance: a comparison of two research frameworks. In: Saxena NP, ed. Management of agricultural drought: agronomic and genetic options. Enfield, NH: Science Publishers, 89–102. [Google Scholar]
- Turner NC, Wright GC, Siddique KHM. 2001. Adaptation of grain legumes (pulses) to water-limited environments. Advances in Agronomy 71, 193–231. [Google Scholar]
- Tyerman SD, Bohnert H, Maurel C, Steudle E, Smith J. 1999. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. Journal of Experimental Botany 50, 1055–1071. [Google Scholar]
- Tyerman SD, Wignes JA, Kaiser BN. 2017. Plant aquaporins from transport to signaling. Cham: Springer International Publishing. [Google Scholar]
- Uga Y, Assaranurak I, Kitomi Y, Larson BG, Craft EJ, Shaff JE, McCouch SR, Kochian LV. 2018. Genomic regions responsible for seminal and crown root lengths identified by 2D & 3D root system image analysis. BMC Genomics 19, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Okuno K, Yano M. 2011. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. Journal of Experimental Botany 62, 2485–2494. [DOI] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, et al. 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- van der Werf A, van Nuenen M, Visser AJ, Lambers H. 1993. Contribution of physiological and morphological plant traits to a species’ competitive ability at high and low nitrogen supply: a hypothesis for inherently fast- and slow-growing monocotyledonous species. Oecologia 94, 434–440. [DOI] [PubMed] [Google Scholar]
- van Dusschoten D, Metzner R, Kochs J, Postma JA, Pflugfelder D, Bühler J, Schurr U, Jahnke S. 2016. Quantitative 3D analysis of plant roots growing in soil using magnetic resonance imaging. Plant Physiology 170, 1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herwaarden AF, Angus JF, Richards RA, Farquhar GD. 1998a ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser II. Carbohydrate and protein dynamics. Australian Journal of Agricultural Research 49, 1083–1094. [Google Scholar]
- van Herwaarden AF, Farquhar GD, Angus JF, Richards RA, Howe GN. 1998b ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser. I. Biomass, grain yield, and water use. Australian Journal of Agricultural Research 49, 1067–1082. [Google Scholar]
- van Luijn F, Boers PCM, Lijklema L. 1996. Comparison of denitrification rates in lake sediments obtained by the N2 flux method, the 15N isotope pairing technique and the mass balance approach. Water Research 30, 893–900. [Google Scholar]
- Vlek P, Byrnes B. 1986. 7. The efficacy and loss of fertilizer N in lowland rice. Fertilizer research 9, 131–147. [Google Scholar]
- Vogan PJ, Sage RF. 2011. Water-use efficiency and nitrogen-use efficiency of C3–C4 intermediate species of Flaveria Juss. (Asteraceae). Plant, Cell & Environment 34, 1415–1430. [DOI] [PubMed] [Google Scholar]
- Wang JR, Hawkins CDB, Letchford T. 1998. Photosynthesis, water and nitrogen use efficiencies of four paper birch (Betula papyrifera) populations grown under different soil moisture and nutrient regimes. Forest Ecology and Management 112, 233–244. [Google Scholar]
- Wang L, Palta JA, Chen W, Chen Y, Deng X. 2018. Nitrogen fertilization improved water-use efficiency of winter wheat through increasing water use during vegetative rather than grain filling. Agricultural Water Management 197, 41–53. [Google Scholar]
- Wang M, Chen Y, Xia X, Li J, Liu J. 2014. Energy efficiency and environmental performance of bioethanol production from sweet sorghum stem based on life cycle analysis. Bioresource Technology 163, 74–81. [DOI] [PubMed] [Google Scholar]
- Wang P, Kong CH, Sun B, Xu XH. 2012. Distribution and function of allantoin (5-ureidohydantoin) in rice grains. Journal of Agricultural and Food Chemistry 60, 2793–2798. [DOI] [PubMed] [Google Scholar]
- Wang R, Min J, Kronzucker HJ, Li Y, Shi W. 2019. N and P runoff losses in China’s vegetable production systems: loss characteristics, impact, and management practices. The Science of the Total Environment 663, 971–979. [DOI] [PubMed] [Google Scholar]
- Wang R, Okamoto M, Xing X, Crawford NM. 2003. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiology 132, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Blumwald E. 2014. Stress-induced chloroplast degradation in Arabidopsis is regulated via a process independent of autophagy and senescence-associated vacuoles. The Plant Cell 26, 4875–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Garvin DF, Kochian LV. 2001. Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiology 127, 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Matsumoto M, Hakomori Y, Takagi H, Shimada H, Sakamoto A. 2014. The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant, Cell & Environment 37, 1022–1036. [DOI] [PubMed] [Google Scholar]
- Welander NT, Ottosson B. 2000. The influence of low light, drought and fertilization on transpiration and growth in young seedlings of Quercus robur L. Forest Ecology and Management 127, 139–151. [Google Scholar]
- Wilkinson S, Bacon MA, Davies WJ. 2007. Nitrate signalling to stomata and growing leaves: interactions with soil drying, ABA, and xylem sap pH in maize. Journal of Experimental Botany 58, 1705–1716. [DOI] [PubMed] [Google Scholar]
- Wingler A, Quick WP, Bungard RA, Bailey KJ, Lea PJ, Leegood RC. 1999. The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant, Cell & Environment 22, 361–373. [Google Scholar]
- Woolfolk CW, Raun WR, Johnson GV, Thomason WE, Mullen RW, Wynn KJ, Freeman KW. 2002. Influence of late-season foliar nitrogen applications on yield and grain nitrogen in winter wheat. Agronomy Journal 94, 429–434. [Google Scholar]
- Xu ZY, Lee KH, Dong T, et al. 2012. A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. The Plant Cell 24, 2184–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. 1999. Rapid gating and anion permeability of an intracellular aquaporin. Nature 402, 184–187. [DOI] [PubMed] [Google Scholar]
- Yousfi S, Serret MD, Márquez AJ, Voltas J, Araus JL. 2012. Combined use of δ¹³C, δ18O and δ15N tracks nitrogen metabolism and genotypic adaptation of durum wheat to salinity and water deficit. New Phytologist 194, 230–244. [DOI] [PubMed] [Google Scholar]
- Zaman-Allah M, Vergara O, Araus JL, et al. 2015. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tang Q, Peng S, Xing D, Qin J, Laza RC, Punzalan BR. 2012. Water use efficiency and physiological response of rice cultivars under alternate wetting and drying conditions. TheScientificWorldJournal 2012, 287907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZL, Chen DL. 2002. Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutrient Cycling in Agroecosystems 63, 117–127. [Google Scholar]
- Zrenner R, Stitt M, Sonnewald U, Boldt R. 2006. Pyrimidine and purine biosynthesis and degradation in plants. Annual Review of Plant Biology 57, 805–836. [DOI] [PubMed] [Google Scholar]
- Zubaidi A, McDonald GK, Hollamby GJ. 1999. Nutrient uptake and distribution by bread and durum wheat under drought conditions in South Australia. Australian Journal of Experimental Agriculture 39, 721–732. [Google Scholar]