Abstract
Studies on earthworms using molecular markers are rare in Africa except a handful from South Africa. Reports on Libyodrilus violaceous, an earthworm found in West Africa are available including their metal tolerance and bioaccumulation capacity but their molecular characterization and ecotoxicology studies are scarce. In this study, triplicate L. violaceous specimens were collected from four locations within a petroleum polluted site and one in a control site, ≃1Km away from point of spill. DNA was extracted and 18S rRNA and 16S rRNA genes were amplified and sequenced. DNA methylation of their 18S rRNA gene was determined using Methylation specific PCR (MSP) method. Phylogenetic trees generated for 18S rRNA and 16S rRNA genes grouped L. violaceous within the Eudrilidae family concurrent with its conventional grouping and MSP results indicate no methylation in L. violaceous population from this site.
Key Words: Oil spill, Pollution, Natural attenuation, DNA Barcode, Phylogeny
INTRODUCTION
Earthworms are ecologically important soil fauna hence their role as terrestrial bioindicators. The estimated earthworm species worldwide was approximated as 3000 in 2007 [1] and 3700 species in 2008 [2]. With molecular methods, there are estimated; 67 species in Greece [3], 90 species in Balkan Peninsula [4], 28 species in Guadeloupe [5] and 212 species in Vietnam [6]. Figures on indigenous African earthworm species are lacking and their sequences are few in repositories.
The conventional earthworms’ taxonomy is labour-intensive, takes time and requires expertise. Some features of earthworms used for earthworm taxonomy at family level are now unreliable [1]. Molecular approach for characterization and earthworm ecological studies outweighs that of conventional methods [7-9]. Others argue that there are earthworm identification mismatch in both methods. Depending on one DNA region for molecular identification could be inaccurate as regions like mitochondria are known for their paraphyly and polyphyly [10-12]. More studies to investigate and corroborate previous classifications are therefore necessary.
DNA barcoding [13] using 18S rRNA and 16S rRNA genes and DNA methylation profiling (MSP) are the molecular methods used in classification and ecotoxicology in this study. 18S rRNA gene is recommended for taxonomic groupings of eukaryotes [14] while 16SrRNA gene are commonly used in classifying prokaryotes [15] and such sequences are found in the GenBank [16, 17]. Methylation occurs in the DNA as organisms respond to environmental stress like heavy metal pollution [18, 19] resulting in gene expressions or repressions [20]. Kille, showed DNA methylation among earthworm species in arsenic polluted site [21]. Gross also showed the variability of the 18S rRNA locus of three fish species seen in their chromosomal rearrangement [22] and Li established cysteine methylation of 18SrDNA gene in Arabidopsis from Nickel polluted soil [23].
The most common African earthworm family is Eudrilidae [24], endemic to tropical and sub-tropical soils, a taxa with 45 genera and 350 species [25] but only 34 nucleotide sequences and 11 protein sequences of the Eudrilidae family are found in NCBI /DDBJ/EMBL/GenBank. Beddard [26] first described Libyodrilus violaceous as a West African earthworm species isolated from Lagos, conventionally classified under the family Eudrilidae [24], subfamily Polytoreutinae and genus Libyodrilus. They have ‘acorn-shaped’ enlarged central chamber [27], ventrally located tubular structured prostrates and prostratic pores with male pore at the 17/18 intersegmental furrow. Their spermatheca also combines with ovarian duct as an organ at segment 14. Unlike other members of the family, they lack dorsal pores and nephridiopore [26]. Other genera in the family Eudrillidae include Hyperiodrilus and Polytoreutus.
L. violaceous are found in muddy soils abundantly during the wet seasons [28]. They appear purplish and luminescent occurring mostly twined together forming rope-like bundles [26, 29-32]. The species is found in Cameroon and distributed from the middle belt spanning down the southern part of Nigeria [32]. The importance of this earthworm species are highlighted in investigations including physiology [33], ecology [28], nutrition [34], ecotoxicology [35-39] especially its ability to tolerate polluted soils [28, 38] and metal bioaccumulation potential [40]. Except from a few molecular reports on L. violaceous [38, 40], there is dearth of information in its molecular characterization and ecotoxicology. This study involved molecular characteriza-tion of L. violaceous using 16SrRNA and 18SrRNA genes and determination of DNA methyla-tion in L. violaceous specimens from a site eight years post impact of oil spills in a pipeline vandalized area in Lagos State, Nigeria [28].
MATERIALS AND METHODS
Molecular characterization Earthworm Collection: Fifteen L. violaceous samples were collected from the oil polluted site at Agaye, Ije-Ododo, Alimosho LGA of Lagos State Nigeria (Lat. 06o 29'N and Long. 03o 15' E). Triplicate samples were collected from five different points ≃100m apart: LV1 (N 06o 29. 40', E003o 15. 23'), LV2 (N 06o29.41', E003o 15.20'), LV3 (N 06o 29.42', E003o15.24') and LV4 (N 06o29.44', E003o 15.19') within the site and LV5 (N 06o 30.00', E003o15.25') ≃1km away from point of spill as control. Earthworms were collected by hand from 0 – 15cm depth of soil surface and allowed to depurate for 24hrs in the laboratory. 3-5mm of the anterior end of each earthworm was cut and remaining gut content removed; this portion was preserved in RNA shield (ZYMO RESEARCH) for further molecular procedures. Earthworm specimens were identified conventionally by Prof.Owa, an earthworm taxonomist from the Zoology Department, Osun State University, Nigeria [28].
DNA Extraction, PCR amplification and Sequencing of 18S rRNA and 16S rRNA genes: ≤25mg anterior portion of each sample was cut and grinded in Liquid Nitrogen. DNA extraction was done using Quick-DNATM Universal kit (ZYMO RESEARCH) following the manufacturer’s procedure. Isolated DNA were amplified with final volume of 20 µl using 2 µl (10mM) of primers: 16SrRNA; F: CGA CTG TTT AAC AAA AAC AT (ewA) and R: CGC GGT CTG AAC TCA GCT CAT G (ewF) (≃ 520 bp) [41] and 18SrRNA; F: CAG CAG CCG CGG TAA TTC C (F-566), R: CCC GTG TTG AGT CAA ATT AAG C (R-1200) (≃650 bp) [42]. Also 2µl DNA template, 10µl PCR Master mix (Phusion High-Fidelity) and 4 µl nuclease H2O. The PCR conditions were as follows; one cycle of 10 mins at 95oC 34 cycles of 30s at 95oC 40s at 48oC and 50oC for 16S rRNA and 18S rRNA respectively, 1 min of 72oC; then 5 mins of 72oC. Gel electrophoresis using 1% (w/v) Agarose gel stained with ethidiumbromide (10mg/ml) was done to visualize the PCR amplicons. Representative PCR amplicons were sequenced using Sanger sequencer (Central analytical facility, Stellenbosch University).
Phylogenetic analyses : The 18S rRNA and 16S rRNA nucleotide sequence reads were compared with reference sequences obtained from NCBI GenBank using the Basic Local Alignment Search Tool (BLAST). Sequence alignments were done with MAFFT program using the L-INS-i algorithm (maximum likelihood) (ultrafast bootstrap with 1000 replicates). The Phylogenetic trees were generated with the command-line version of the IQ-tree software version 1.5.5 [43- 46].
Methylation specific PCR (MSP): DNA extracts from the specimens (LV1 - LV5) were subjected to bisulphite conversion using EZ DNA Methylation lightening kit (Zymo research, Irvine, CA USA) following the manufacturer’s instruction. CpG islands with high CpG density were identified in 18SrRNA nucleotide of L. violaceous and two sets of primers each (Forward and reverse primers) specific for methylated and unmethylated cytosine targeting the CpG islands were designed using Meth Primer 2 design software. Each PCR amplification were done using 50 µl mixture with 25 µl of GoTaqHot Start Green Master mix (Promega, coop), 0.75µl forward and 0.75µl reverse primers both specific for methylated and unmethylated, 2µl of bisulphite converted DNA template and 21.5 µl of nuclease free water. The PCR condition was one cycle of 10mins at 95oC; 35 cycles of 30s at 95oC,40s at 44oC and 42oC for methylated and unmethylated respectively, 1min of 72oC;then 7 mins of 72oC. PCR products were resolved by electrophoresis on 2% (w/v) ethidium bromide stained agarose gel.
RESULTS
16S rRNA and 18S rRNA genes were successfully amplified and viewed at the expected sizes (≃520bp and ≃650bp respectively) [41-42]. The partial nucleotide sequences of the two genes of the representative L.violaceous specimens are deposited in DDBJ/EMBL/GenBank with accession numbers KY114795.1 (18SrRNA) and KY114796.1 (16S rRNA). The 18S rRNA nucleotide sequence of L.violaceous shows this earthworm species had close genetic relationship with Eudriloides sp. Polytorentus finni and Hyperiodrilus sp. (Table 1). Similarly, the 16SrRNA nucleotide sequence BLAST indicates close identities with Eudriloides sp.; Hyperiodrilus sp; Metaphire sieboldi; Dichogaster sp and P. finni (Table 2).
Table 1.
L. violaceous 18S rRNA nucleotide sequence BLAST percentage coverage and identity compared with genetically closely related earthworm species
| Organisms | Accession number | Percentage Identity | Percentage coverage |
|---|---|---|---|
| L. violaceous | KY114795.1 | 100% | 100% |
| Eudriloides sp. Ke | HQ728924.1 | 99% | 100% |
| Polytorentus finni | HQ728926.1 | 98% | 100% |
| Hyperiodrilus sp. Gh | HQ728925.1 | 98% | 100% |
Table 2.
L. violaceous 16S rRNA nucleotide sequence BLAST percentage coverage and identity compared with genetically closely related earthworm species
| Organisms | Accession number | Percentage Identity | Percentage coverage |
|---|---|---|---|
| L. violaceous | KY114796.1 | 100% | 100% |
| Eudriloides sp. Ke | JF267879.1 | 82% | 97% |
| Hyperiodrilus sp. Gh | JF267877.1 | 82% | 97% |
| Dichogaster sp | JF267876.1 | 81% | 96% |
| Metaphire sieboldi | AB534992.1 | 82% | 92% |
| P. finni | JF267878.1 | 80% | 72% |
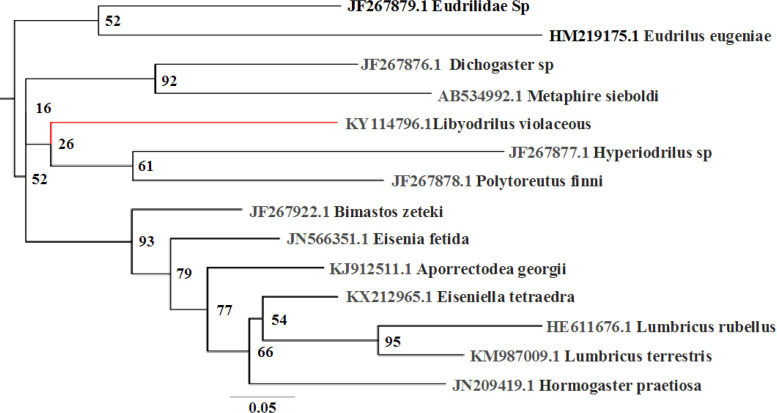
The molecular classification of L. violaceous with 18S rRNA gene is depicted in the phylogenetic tree below (Fig. 1). The tree has two major clusters, the first comprising of the Eudrilidae group with the species; L. violaceous, P. finni, Hyperiodrilus sp. and Eudriloides sp. and the second with species of the families Microchaetidae, Tritogeniidae, Hormogastridae, Glossoscolecidae, Megascolecidae, and Acanthodrilidae of the superfamily Megascolecoidae. The molecular classification of L.violaceous using16S rRNA gene is depicted in the phylogenetic tree below (Fig. 2). The tree shows two major sister clusters, the first, a clade consisting of Eudrilidae sp. and Eudrilus eugeniae with a common root. The second sister cluster has three sub-clusters with the first having Dichogaster sp. and Metaphire sieboldi nested in one clade and the second clade having L. violaceous, Hyperiodrilus sp. and P. finni. The third subcluster shows the remaining reference earthworm spp. There was no variation in the specimens of L. violaceous within the polluted site compared to the control.
Figure 1.
Phylogenetic tree of 18S rRNA nucleotide sequence of L. violaceous (indicated in red) compared with other earthworms’18SrRNA nucleotide sequences
Figure 2.
Phylogenetic tree of 16S RNA nucleotide sequence of L. violaceous (indicated in red) compared with some earthworms’16S rRNA nucleotide sequences
DISCUSSION
The 16S rRNA and 18S rRNA genes were successfully amplified. The percentage coverage and identity of the sequence reads of both genes and phylogenetic trees corroborate the conventional classification of L. violaceous as a Eudrillidae.
The closest relative of L. violaceous is Eudriloides sp. It originates from Kenya, central Africa. James and Davidson [47] had reported Eudriloides sp. as more evolutionary related with Hyperiodrilous sp. in the 18S+28S partitioned analysis, Bayesian phylogram. They also reported this species to be equally related to P. finni and Hyperiodrilus africanus in a 28S gene tree. P. finni and Hyperiodrilus sp. are of the genus Polytoreutus and Hyperiodrilus, respectively, of the family Eudrilidae [24]; all four species are seen clustered in one clade in our study (Fig. 1) and is consistent with the conventional grouping of the species [26, 29].
The second cluster of the phylogram (Fig. 1). has two subclusters with the first showing a clade of two species of the family Microchaetidae; Microchaetus papillatus and Proandricus thornvillensis. Also within this subcluster is Trigenia lunata of the family Tritogeniidae formerly of Microchaetidae [47-51] recently allotted its own family Tritogeniidae by Plisko in 2013 [52]. The species Hormogaster samnitica is also within this subcluster. Plisko [53] had suggested Hormogastridae having genetic relationship with Tritogeniidae.
The second subcluster shows a clade of Glossoscolecidae; Fimoscolex sp., and Glossodrilus sp. [53, 54]; The closeness of Glossoscolecidae to Eudrilidae had earlier been highlighted as they have similar ejaculatory structure [47, 55] and they possess euprostate and paired dorsal calcium carbonate gland in either segment 12 or 13 [47]. The second subcluster also shows a clade of the family Megascolecidaeis; Amynthus sp, Metaphire exilis and Terrisswakerius sp. and another of the Acanthodrilidae family; Dichogaster saliens and Dichogaster sp. All species in the second subcluster are from the super family Megascolecidae. The phylogenetic tree generated clusters that indicate a common origin of all species being oligochaetes; of the polyphyletic order Crassiclitellata [56-57].
The phylogenetic tree for the 16S rRNA gene (fig. 2) shows weak clustering of Eudrilidae sp. and Eudrilus eugeniae species separate from the other clade of L. violaceous, Hyperiodrilus sp. and P. finni although they are all of the Eudrilidae family. This could be due to the polyphyletic nature of the Eudrilidae family [47, 51], it could also be because 16S rRNA gene is best for prokaryotic classification [15]. Dichogaster sp. and Metaphire sieboldi in the second cluster are conventionally of the super family Megascolecidae. The third sub-clade clusters Aporrectodea georgii, Eisenia fetida, Bimastos zeteki, Eiseniella tetraedra, Lumbricus rubellus and Lumbricus terristris (of the family Lumbricidae) with Hormogaster praetiosa (of the same super family Lumbricoidea).
The phylogenetic grouping in this study (Fig. 1, 2) supports the conventional classification of L. violaceous with the18S rRNA gene as more reliable than the 16S rRNA gene for its molecular taxonomic grouping. 18S rRNA gene is acclaimed as more appropriate for eukaryotic classification than 16S rRNA gene [14]. Among the reference earthworm species, the closest evolutionary relation of L. violaceous for 18S rRNA partial nucleotide sequence was Eudrilidae sp, although the taxonomy to the specie level of Eudrilidae sp. still remains imprecise. Chang [58] had alleged many incorrect earthworm species sequences in the GenBank which results in misleading conclusions. This however, cannot be adjudged so in our conclusion since the phylograms for L. violaceous 18S rRNA and 16S rRNA indicate evolutionary differences between L. violaceous and Eudrilidae sp.
Although pollutants like metals are known to modify DNA [59] or cause epigenetic responses like methylation and locus variability [21-23], there were no methylations in the specimens sampled in this study. The sampling site is an oil polluted site post incident of oil spill and inferno earlier reported to have heavy metal concentrations [60], it possibly had undergone natural attenuation over the 8yrs [61, 62]. From the earlier report, levels of Zn, Cd, Mn, Ni, V, Pb, Cu and Cr in both polluted and control sites were all below the FEPA standard threshold value [62] hence could be why the metals did not cause epigenetic impact on the 18S rRNA gene of this earthworm species. It could also be due to the fact that the metals monitored do not cause epigenetic impact on earthworm species since none of these metals have been reported to cause methylation of 18SrRNA gene in any available literature. Arsenic is one of the few metals reported to cause epigenetic responses resulting in variation among species of organisms [21].
Acknowledgements:
We thank the Schlumberger faculty of the future foundation for the financial support of this work. We also thank Prof.Owa an earthworm taxonomist for his role in the earlier identification of L. violaceous.
Conflict of Interest:
The authors declare no conflicts of interest
References
- 1.Huang J, Xu Q, Sun ZJ, Tang GL, Su ZY. Identifying earthworms through DNA barcodes. Pedobi. 2007;(51):301–309. [Google Scholar]
- 2.Hendrix PF, Callaham Jr MA, Drake JM, Huang CY, James SW, Snyder BA, Zhang W. Pandora's box contained bait: the global problem of introduced earthworms. Annu Rev Ecol Syst. 2008;(39):593–613. [Google Scholar]
- 3.Szederjesi T, Vavoulidou E, Chalkia C, Dányi L, Csuzdi C. An annotated checklist of earthworms of Greece (Clitellata: Megadrili) Zootaxa . 2017;(4272):57–82. doi: 10.11646/zootaxa.4272.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Trakić T, Valchovski H, Stojanović M. Endemic earthworms (Oligochaeta: Lumbricidae) of the Balkan Peninsula: a review. Zootaxa . 2016;4189:251–274. doi: 10.11646/zootaxa.4189.2.3. [DOI] [PubMed] [Google Scholar]
- 5.James SW, Gamiette F. New species of Dichogaster Beddard, 1888 (Clitellata: Benhamiidae) with additional records of earthworms from Guadeloupe (French West Indies) Zootaxa. 2016;4178:391–408. doi: 10.11646/zootaxa.4178.3.5. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TT, Nguyen AD, Tran BT, Blakemore RJ. A comprehensive checklist of earthworm species and subspecies from Vietnam (Annelida: Clitellata: Oligochaeta: Almidae, Eudrilidae, Glossoscolecidae, Lumbricidae, Megascolecidae, Moniligastridae, Ocnerodrilidae, Octochaetidae) Zootaxa. 2016;4140:1–92. doi: 10.11646/zootaxa.4140.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Palumbi AR, Cipriano F. Species identification using genetic tools: the value of nuclear and mitochondrial gene sequences in whale conservation. J Hered . 1998;89:459–464. doi: 10.1093/jhered/89.5.459. [DOI] [PubMed] [Google Scholar]
- 8.Symondson WO. Molecular identification of prey in predator diets. Mol Ecol. 2002;11:627–641. doi: 10.1046/j.1365-294x.2002.01471.x. [DOI] [PubMed] [Google Scholar]
- 9.Yadav S, Mullah M. A review on molecular markers as tools to study earthworm diversity. Int J Pure Appl Zool. 2017;5:62–69. [Google Scholar]
- 10.Sperling F. DNA barcoding: deuset machine. Newsl Biol Surv Canada (Terr. Arthropods). 2003;22:50–53. [Google Scholar]
- 11.Will KW, Rubinoff D. Myth of the molecule: DNA barcodes for species cannot replace morphology for identification and classification. Cladistics. 2004;20:47–55. doi: 10.1111/j.1096-0031.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 12.Szederjesi T, Pop VV, PavlÍČek T, MÁrton O, KrÍzsik V, Csuzdi C. Integrated taxonomy reveals multiple species in the Dendrobae nabyblica (Rosa, 1893) complex (Oligochaeta: Lumbricidae) Zool J Linn Soc. 2017;182:500–516. [Google Scholar]
- 13.Hebert PD, Cywinska A, Ball SL, De Waard JR. Biological identifications through DNA barcodes. Proc Biol Sci. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Xiong J, Yu Y. Taxonomic resolutions based on 18S rRNA genes: a case study of subclass copepoda. PLoS One. 2015;10:e0131498. doi: 10.1371/journal.pone.0131498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stackebrandt E, Goebel BM. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 16.Pop AA, Cech G, Wink M, Csuzdi C, Pop VV. Application of 16S, 18S rDNA and COI sequences in the molecular systematics of the earthworm family Lumbricidae (Annelida, Oligochaeta) Eur J Soil Biol. 2007;43:S43–S52. [Google Scholar]
- 17.Klarica J, Kloss-Brandstätter A, Traugott M, Juen A. Comparing four mitochondrial genes in earthworms-implications for identification, phylogenetics, and discovery of cryptic species. Soil Biol Biochem. 2012;45:23–30. [Google Scholar]
- 18.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 19.Wrobel K, Wrobel K, Caruso JA. Epigenetics: an important challenge for ICP-MS in metallomics studies. Anal Bioanal Chem. 2009;393:481–486. doi: 10.1007/s00216-008-2472-3. [DOI] [PubMed] [Google Scholar]
- 20.Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. 2012;20:339–349. doi: 10.1016/j.joca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Kille P, Andre J, Anderson C, Angx HN, Bruford MW, Bundy JG, Donnelly R, Hodson ME, Juma G, Lahive E, Morgan AJ, Sturzenbaum SR, Spurgeon DJ. DNA sequence variation and methylation in an arsenic tolerant earthworm population. Soil Biology and Biochemistry. 2013;57:524–532. [Google Scholar]
- 22.Gross MC, Schneider CH, Valente GT, Martins C, Feldberg E. Variability of 18S rDNA locus among Symphysodon fishes: chromosomal rearrangements. J Fish Biol. 2010;76:1117–1127. doi: 10.1111/j.1095-8649.2010.02550.x. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Chen X, Li S, Wang Z. Effect of nickel chloride on Arabidopsis genomic DNA and methylation of 18S rDNA. Electronic Journal of Biotechnology. 2015;18:51–57. [Google Scholar]
- 24.Claus C. 1880. Main features of zoology. Fourth completely reworked and improved edition. Marburg: N.G. Elwert'scheVerlagsbuchhandlung. [Google Scholar]
- 25.Plisko JD, Nxele TC. An annotated key separating foreign earthworm species from the indigenous South African taxa (Oligochaeta: Acanthodrilidae, Eudrilidae, Glossoscolecidae, Lumbricidae, Megascolecidae, Microchaetidae, Ocnerodrilidae and Tritogeniidae) African Invertebrates. 2015;56:663–708. [Google Scholar]
- 26.Beddard FE. Memoirs: On the structure of an earthworm allied to Nemertodrilus Mich with observations on the post-embryonic development of certain organs. J Cell Sci. 1891;2:539–585. [Google Scholar]
- 27.Sims RW, Gerard BM. Earthworms: keys and notes for the identification and study of the species. Brill Archive. 1985;Vol 31 [Google Scholar]
- 28.Ogunlaja A, Morenikeji OA. Species diversity and population size of earthworms after oil spillage in a pipeline vandalized area in Lagos State, Nigeria. Afr J Agri Res. 2013;8:10–19. [Google Scholar]
- 29.Owa SO. A new earthworm genus (Eudrilidae: Oligochaeta) from Nigeria. J Nat Hist. 1995;29:571–579. [Google Scholar]
- 30.Bamgbose O, Odukoya OO, Arowolo TOA. Earthworms bioindicators of metal pollutions in dumpsites of Abekuta city, Nigeria. Rev Biol Trop. 2000;48:1–13. [Google Scholar]
- 31.Ogunlaja A. Earthworm ecology, heavy metals and total petroleum hydrocarbon assessment of an oil spill site in Agaye, Lagos State, Nigeria. Doctoral dissertation. Nigeria : University of Ibadan; 2014. [Google Scholar]
- 32.Dada EO, Njoku KL, Osuntoki AA, Akinola MO. Evaluation of the responses of a wetland, tropical earthworm to heavy metal contaminated soil. Analysis. 2013;1:47–52. [Google Scholar]
- 33.Idowu AB, Edema MO, Adeyi AO. Distribution of bacteria and fungi in the earthworm Libyodrillus violaceous (Annelida: Oligochaeta), a native earthworm from Nigeria. Rev Biol Trop. 2006;54:49–58. doi: 10.15517/rbt.v54i1.13991. [DOI] [PubMed] [Google Scholar]
- 34.Dedeke GA, Owa SO, Olurin KB, Akinfe AO, Awotedu OO. Partial replacement of fish meal by earthworm meal (Libyodrilus violaceus) in diets for African catfish, Clariasgariepinus. Int J Fish Aquac. 2013;5:229–233. [Google Scholar]
- 35.Ogunlaja A, Ogunlaja OO. Stress indicators and behavioural end-points of Libyiodrillus violaceous exposed to petroleum products contaminated soil. J Environ Anal Toxicol. 2012;2:1–4. [Google Scholar]
- 36.Bamidele JA, Idowu AB, Ademolu KO, Atayese AO. Microbial diversity and digestive enzyme activities in the gut of earthworms found in sawmill industries in Abeokuta, Nigeria. Rev Biol Trop. 2014;62:1241–1249. [PubMed] [Google Scholar]
- 37.Ossai EK, Iwegbue CM, Nwajei GE. Trace Elements in Water, Soil, Earthworm and Fishes from Otokutu End of Warri River, Delta State, Nigeria. Pak J Biol Sci. 2014;17:1136–1140. doi: 10.3923/pjbs.2014.1136.1140. [DOI] [PubMed] [Google Scholar]
- 38.Dedeke GA, Owagboriaye FO, Adebambo AO, Ademolu KO. Earthworm metallothionein production as biomarker of heavy metal pollution in abattoir soil. Appl Soil Ecol. 2016;104:42–47. [Google Scholar]
- 39.Ojo OF, Adewumi DG, Oluwatoyin AK. Glutathione-S-transferase production in earthworm (Annelida: Eudrilidae) as a tool for heavy metal pollution assessment in abattoir soil. Rev Biol Trop. 2016;64:779–789. [PubMed] [Google Scholar]
- 40.Dedeke GA, Iwuchukwu PO, Aladesida AA, Afolabi TA, Ayanda IO. Impact of heavy metal bioaccumulation on antioxidant activities and DNA profile in two earthworm species and freshwater prawn from Ogun River. Sci Total Environ. 2018;624:576–585. doi: 10.1016/j.scitotenv.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 41.Bienert F, De Danieli S, Miquel C, Coissac E, Poillot C, Brun JJ, Taberlet P. Tracking earthworm communities from soil DNA. Mol Ecol . 2012;21:2017–2030. doi: 10.1111/j.1365-294X.2011.05407.x. [DOI] [PubMed] [Google Scholar]
- 42.Hadziavdic K, Lekang K, Lanzen A, Jonassen I, Thompson EM, Troedsson C. Characterization of the 18S rRNA gene for designing universal eukaryote specific primers. PLoS One . 2014;9:e87624. doi: 10.1371/journal.pone.0087624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katoh K, Misawa K, Kuma KI, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic acids Res . 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. Model Finder: fast model selection for accurate phylogenetic estimates. Nat Methods . 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minh BQ, Nguyen MAT, Haeseler AV. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol . 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James SW, Davidson SK. Molecular phylogeny of earthworms (Annelida: Crassiclitellata) based on 28S, 18S and 16S gene sequences. Invertebrate Systematics . 2012;26:213–229. [Google Scholar]
- 48.Blakemore RJ. The major megadrile families of the world reviewed again on their taxonomic types (Annelida: Oligochaeta: Megadrilacea) Opusc Zool Budapest. 2013;44:107–127. [Google Scholar]
- 49.Kinberg JGH, Annulata nova, Ofersigt af Kungl. Ventenskaps-Akademiens Forhandlingar 1866. Stockholm. 1867;23:97–103. [Google Scholar]
- 50.Michaelsen W. Oligochaeta. Das Tierreich. 1900;10:1–575. [Google Scholar]
- 51.Plisko JD. An annotated checklist of the South African Acanthodrilidae (Oligochaeta: Acanthodrilidae: Acanthodrilinae, Benhamiinae) Zootaxa. 2012;3458:4–58. [Google Scholar]
- 52.Plisko JD. A new family Tritogeniidae for the genera Tritogenia and Michalakus, earlier accredited to the composite Microchaetidae (Annelida: Oligochaeta) Afr Invertebr. 2013;54:69–93. [Google Scholar]
- 53.Plisko JD. Michalakus, a remarkable new genus of microchaetid earthworm from South Africa (Oligochaeta: Microchaetidae) Ann Natal Mus. 37:287–293. [Google Scholar]
- 54.Cognetti de Martiis L. The oligochaetes of the neotropicale region. Pt.1. Mem Reale Ace Sci Torino. 1905;55:1–72. [Google Scholar]
- 55.Benham WB. A new English genus of aquatic Oligochaeta (Sparganophilus) belonging to the family Rhinodrilidae. Quart J Micros Sci. 1892;34:155–179. [Google Scholar]
- 56.Jamieson BGM, Tillier S, Tillier A, Justine JL, Ling E, James SW, MacDonald KR, Hugall AF. Phylogeny of the megascolecidae and crassiclitellata (Annelida, Oligochaeta): combined versus partitioned analysis using nuclear (28S) and mitochondrial (12S, 16S) rDNA. Zoosystema . 2002;24:707–734. [Google Scholar]
- 57.Omodeo P. Evolution and biogeography of megadriles(Annelida, Clitellata) Ital J Zool. 2000;67:179–201. [Google Scholar]
- 58.Chang CH, Shen HP, Chen JH. Earthworm fauna of Taiwan. National Taiwan University Press; 2009. [Google Scholar]
- 59.Noel S, Rath SK. Randomly amplified polymorphic DNA as a tool for genotoxicity: an assessment. Toxicol Ind Health . 2006;22:267–275. doi: 10.1191/0748233706th267oa. [DOI] [PubMed] [Google Scholar]
- 60.Ogunlaja A, Ogunlaja OO, Okewole DM, Morenikeji OA. Risk assessment and source indentification of heavy metal contamination by multivariate and hazard index analysis of a pipeline vandalized area in Lagos State, Nigeria. Sci Tot Env. 2019;651:2943–2952. doi: 10.1016/j.scitotenv.2018.09.386. [DOI] [PubMed] [Google Scholar]
- 61.Fukushi K, Sasaki M, Sato T, Yanase N, Amano H, Ikeda H. A natural attenuation of arsenic in drainage from an abandoned arsenic mine dump. Appl Geochem. 2003;18:1267–1278. [Google Scholar]
- 62.Mulligan CN, Yong RN. Natural attenuation of contaminated soils. Environ Int. 2004;30:587–601. doi: 10.1016/j.envint.2003.11.001. [DOI] [PubMed] [Google Scholar]