Entrapment neuropathies are a heterogenous condition reflecting distinct underlying pathomechanisms. A contemporary assessment aimed at identifying potential mechanisms may help target management for these patients.
Keywords: Entrapment neuropathies, Compression neuropathies, Carpal tunnel syndrome, Sciatica, Radiculopathy, Radicular pain, Pathomechanisms, Assessment, Diagnosis, Management, Treatment
Abstract
Entrapment neuropathies such as carpal tunnel syndrome, radiculopathies, or radicular pain are the most common peripheral neuropathies and also the most common cause for neuropathic pain. Despite their high prevalence, they often remain challenging to diagnose and manage in a clinical setting. Summarising the evidence from both preclinical and clinical studies, this review provides an update on the aetiology and pathophysiology of entrapment neuropathies. Potential mechanisms are put in perspective with clinical findings. The contemporary assessment is discussed and diagnostic pitfalls highlighted. The evidence for the noninvasive and surgical management of common entrapment neuropathies is summarised and future areas of research are identified.
Key Points
The wide variety of pathomechanisms at play in entrapment neuropathy may account for patient heterogeneity.
Contemporary clinical assessment involves a detailed history, clinical examination, and additional tests if required.
Noninvasive management is the first-line treatment for patients with entrapment neuropathies.
Future research is required to determine whether patient stratification can improve outcomes in patients with entrapment neuropathies.
1. Introduction
Entrapment neuropathies are caused by compression and/or irritation of peripheral nerves as they travel through narrow anatomical spaces. The most common entrapment neuropathy is carpal tunnel syndrome (CTS) with a lifetime risk of 10%, which increases to a staggering 84% in patients with diabetes.135 The second most common entrapment neuropathy is cubital tunnel syndrome.100 Another common condition is “sciatica,” with reported prevalence values ranging from 1.6% to 43%.76 The striking variation in prevalence has been attributed to the varying definitions of “sciatica.”76 Indeed, “sciatica” is an arcane term which, depending on its interpretation, may include somatic referred pain, radicular pain (pain evoked by ectopic discharges from a dorsal root or its ganglion),19 and radiculopathy (conduction block along a spinal nerve or its roots manifesting clinically with dermatomal sensory loss, myotomal weakness, or reflex changes).19,129 Here, we use “sciatica” as an umbrella term for all 3 conditions, but use the more accurate descriptions (eg, radiculopathy, radicular pain) when studies specifically defined their patient population.
The aetiology of entrapment neuropathies remains largely unknown. They share several risk factors across conditions, such as increased body mass index,115,134 occupational or physical factors,78,115 and predisposing systemic diseases such as diabetes or hypothyroidism.115,118 Recently, genetic predisposition is emerging as one of the strongest risk factors for entrapment neuropathies.2,82,86,165 In addition to rare variants,158 genome-wide association studies have identified several genetic susceptibility loci for entrapment neuropathies.2,82,86,165 Intriguingly, many of the genes are related to connective tissue and extracellular matrix architecture. It currently remains unclear whether these genes increase vulnerability by altering the nerve itself (as a substantial proportion consists of connective tissue) or the environment through which the nerve travels (eg, the tunnels).165
Over the past decade, significant advances have been made in our understanding of the pathophysiology of entrapment neuropathies and their assessment and management.
This article summarises the current knowledge and highlights the challenges faced when diagnosing and managing patients with entrapment neuropathies.
2. Pathophysiology
Our understanding of neuropathic pain is largely based on preclinical models involving acute and severe nerve injuries. However, human entrapment neuropathies are quite distinct from these preclinical models because their onset is mostly slow, and the neural injury is often of mild but chronic nature. We130 and others36,62 have therefore refined animal models that closely mimic human entrapment neuropathies. Below, we summarise the main pathomechanisms that are associated with nerve compression in preclinical and clinical studies (Fig. 1) and provide potential links to the clinical presentation.
Figure 1.
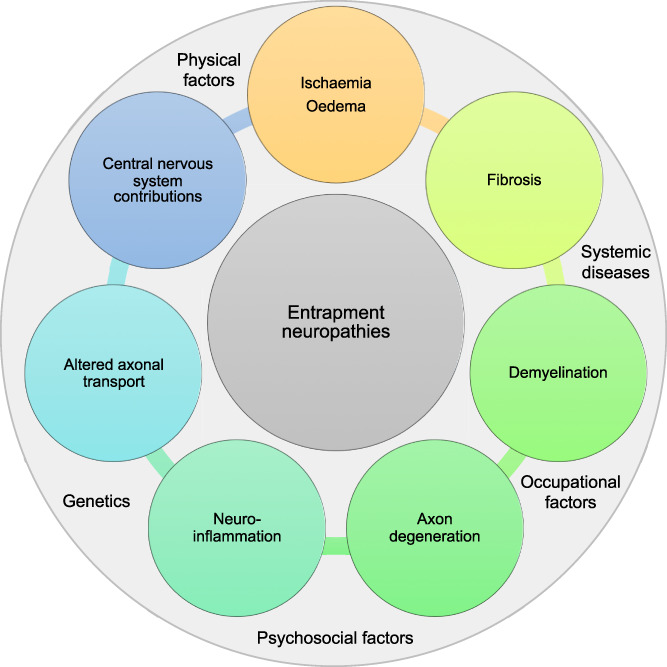
Potential pathomechanisms and risk factors contributing to entrapment neuropathies.
2.1. Ischaemia, oedema, and intraneural fibrosis
Intraneural ischaemia is typical of mild entrapment neuropathies. Animal models demonstrate that extraneural pressures as low as 20 to 30 mm Hg disrupt intraneural venous circulation.125 These pressures are often reached in patients with entrapment neuropathies.31,60,132,139 The ensuing reversal of the pressure gradient necessary to assure adequate blood supply could explain the sometimes intermittent paraesthesia, which occur at night, in static positions or with end of range positions (eg, Phalen test for CTS) and may reduce or disappear with movement.
Prolonged ischaemia is likely to induce a compromise of the blood nerve interface90,170 with subsequent oedema formation. Clinically, the presence of oedema is apparent by an enlargement of the compressed nerves103,169 and increases in signal intensity on specialised magnetic resonance sequences.32,83,136 Persistent oedema may eventually lead to intraneural and extraneural fibrotic changes,92 which is a feature of both radiculopathies68 and entrapment neuropathies of distal nerve trunks.1,28 Extraneural fibrotic changes are thought to explain the reduced gliding of human compressed nerves that is apparent during limb movements.46
2.2. Demyelination and axon degeneration
Prolonged ischaemia and mechanical compromise may induce downstream effects such as demyelination and eventually axon degeneration. Focal demyelination is a hallmark of entrapment neuropathies,58,63,91,92,107,109,130 which are often characterised by nerve conduction slowing or block.95 Ischaemia in the absence of demyelination may, however, also contribute to nerve conduction changes.25,73
Together with demyelination, the architecture of nodes of Ranvier changes after nerve compression,131 including both downregulation and upregulation of existing ion channels as well as expression of novel channels.24,42,49,71,85 Such changes have been implicated with spontaneous ectopic generation of action potentials,6,26 and may contribute to spontaneous electric shock-like pain or symptoms provoked upon Tinel testing.
If nerve compression/irritation perseveres, axons may eventually degenerate. It is commonly believed that entrapment neuropathies predominantly affect the large myelinated fibres (eg, demyelination and axon damage)36,92 and that small axons are relatively resistant to compression.36 Indeed, large myelinated Aβ-fibre-mediated numbness to light touch is common as is motor neuron dysfunction apparent by myotomal muscle weakness, atrophy, and reflex changes. Guidelines on the diagnosis of entrapment neuropathies such as CTS or cervical radiculopathy thus focus heavily on large fibre tests.5,20 Recent evidence, however, suggests that small nerve fibre function and structure are affected in entrapment neuropathies8,29,50,74,80,126,142,146,155,167 and may even precede large fibre changes.9,130,131,141 These findings suggest that (early) diagnosis of entrapment neuropathies should include tests for small fibre function.
2.3. Neuroinflammation
There is a wealth of preclinical data confirming an important role of neuroinflammation in the generation and maintenance of neuropathic pain [for review, see Refs. 99, 151, 163, 166]. Neuroinflammation is characterised by activation of immune cells (eg, macrophages and T-lymphocytes) at the site of damaged axons. Immune cells release inflammatory mediators (eg, cytokines, chemokines, and lipid mediators), which induce a breakdown of the blood–nerve barrier resulting in further immune cell influx and swelling. Importantly, neuroinflammation sensitises injured and uninjured axons and nociceptors in target tissue, contributing to neuropathic pain initiation and maintenance. Whereas most evidence for a link between neuroinflammation and neuropathic pain stems from acute and severe nerve injury models, there is a growing body of evidence suggesting that neuroinflammation is also a feature of mild chronic nerve compression.61,112,130,133 Intriguingly though, the neuroinflammation does not remain restricted to the lesion site, but can be found in associated dorsal root ganglia after peripheral nerve compression130 or nerve root compromise.112 Animal models of radiculopathy also demonstrate an activation of glial cells in the dorsal horn of the spinal cord.122,123,140 A similar glial cell reaction has also been reported for severe distal nerve injuries involving substantial axonal damage,67 whereas compression injuries with only minimal axonal damage seem insufficient to induce overt spinal cord neuroinflammation.130 The presence of local and remote immune inflammation has now been confirmed in patients with lumbar radicular pain using combined positron emission tomography and magnetic resonance imaging (MRI)3 The presence of remote neuroinflammation could explain the often observed spread of symptoms beyond affected dermatomes or innervation territories in patients with entrapment neuropathies23,102,172 (see https://www.youtube.com/watch?v=BZYtAR4zUpg).
Of note, mild experimental nerve compression can also induce an inflammatory reaction within the epineurium.130 Such epineural inflammation may sensitise the nervi nervorum21 and induce spontaneous or evoked activity in nociceptive axons resulting in mechanical hypersensitivity despite the absence of frank axonal damage.40 Arguably, such extraneural mechanisms may explain why a subgroup of patients with entrapment neuropathies has pain with a predominant nociceptive rather than neuropathic character.
2.4. Changes to axonal transport
Peripheral nerve compression7,33–35,76 and inflammation10 impair retrograde and anterograde axonal transport. Its blockage can result in increased nerve mechanosensitivity,39 presumably by the accumulation and insertion of ion channels at the lesion site (see changes to nodal architecture above), and may contribute to symptoms in patients with entrapment neuropathies.
2.5. Central nervous system contributions
Because the peripheral and central nervous system form a functional entity, injuries of peripheral nerves inevitably initiate central changes. Central changes after severe experimental nerve injury include, but are not limited to, central immune-inflammatory mechanisms,81,101,122,123 central sensitisation,80 and changes to cortical representations.43,104,105,150 Clinical hallmarks of central mechanisms including bilateral sensory deficits in unilateral painful entrapment neuropathies,29,37,59,142 widespread hypersensitivity,37,53,142,171,172 and impaired conditioned pain modulation138 have been described in patients with entrapment neuropathies. Furthermore, cortical changes have been reported including functional and structural changes of the somatosensory cortex in patients with CTS43,93,104,150 and changes in cortical morphometry in patients with lumbar radicular pain.87 Clinically, such cortical changes may manifest in an impairment in left/right judgement tasks such as found in patients with CTS.128
It is subject of ongoing debate, whether central changes are dependent on peripheral drivers or can drive symptoms independently.12 Patients with entrapment neuropathies have continued abnormal input from the peripheral nervous system (too much or too little), which may perpetuate central adaptations. The importance of peripheral drivers in entrapment neuropathies becomes clear from clinical observations of often immediate relief of focal and widespread symptoms after decompression surgery15,145,155 or local steroid injections.97
2.6. Psychosocial factors
Psychosocial factors often play a role as risk factors for the development or persistence of pain. To date, the psychosocial contributions to entrapment neuropathies have not been evaluated in detail. A recent systematic review suggests that there is only limited and inconsistent evidence for a positive association of psychosocial risk factors and CTS.96 In patients with “sciatica,” the role of psychosocial features as prognostic factors for conservative management has either not been studied11 or remains controversial.57,77 Undoubtingly though, entrapment neuropathies can have strong psychosocial consequences as apparent in lumbar radicular pain affecting many aspects of life, including psychological status.124 Future work is required to conclusively determine the role of psychosocial factors in the generation, maintenance, and resolution of symptoms in patients with entrapment neuropathies.
3. Clinical presentation
The diagnosis and management of these patients can be challenging due to the heterogeneity of pathomechanisms and varied clinical presentation. Interestingly, nerve compression may be asymptomatic, as documented by false positive nerve root compression seen on MRI.48 In some patients, nerve entrapment may cause pain in the innervation territory of the affected nerve55; however, extradermatomal pain distribution is common (CTS up to 70%23,172; radicular pain 64%−70%).102 Therefore, clinicians should not rule out entrapment neuropathies in the absence of clearly defined dermatomal/peripheral symptom referral patterns. Nerve entrapment may or may not cause nerve fibre damage; it may affect large and/or small fibres, and the extent of sensory fibre damage may account for heterogeneity of symptoms, ie, sensory loss and/or enhanced pain sensitivity.131 The heterogeneity of pathomechanisms likely explains the identification of subgroups of patients with radicular pain13,94,142 with differing somatosensory profiles, based on quantitative sensory testing13,142 and neuropathic pain screening tools.94
The core sign of nerve damage in entrapment neuropathies is loss of function due to reduced action potential conduction caused by the nerve lesion.146 Various positive sensory symptoms and signs indicating gain of function have been reported, including paraesthesia, pain attacks, and evoked pain (hyperalgesia and allodynia).94 Self-reported pain profiles differ between patients with distal (CTS) vs proximal (radiculopathy) entrapment neuropathies.146 Patients with radiculopathy show an increased pain attack severity and pressure, potentially reflecting distinct underlying pain mechanisms. Another feature of gain of function seen in patients with nerve-related pain is heightened nerve mechanosensitivity, which is characterised clinically by pain to mechanical provocation of neural structures (eg, elongation of the affected nerve during straight leg raise testing or nerve compression with manoeuvres such as Spurling test.4,29,48,108,157 Historically, neural provocation tests were thought to be diagnostic for entrapment neuropathies; however, heightened mechanosensitivity can occur in the absence of a nerve lesion,4,143,144,157 and vice versa, patients with confirmed entrapment neuropathies may present without signs of heightened mechanosensitivity.14 Nevertheless, neural provocation tests form an integral part of the clinical examination (see below) as the presence of heightened mechanosensitivity may guide physiotherapeutic management.127
Although entrapment neuropathies are considered the most common cause of neuropathic pain, not all patients have obvious signs of nerve damage. For instance, radicular pain from nerve root compression can exist as a discrete disorder without a nerve root lesion and associated loss of function, ie, radiculopathy.19 Furthermore, only 30% of patients with cervical radiculopathy are classified as having neuropathic pain based on the painDETECT questionnaire.144 However, it has to be acknowledged that neuropathic screening tools were developed before the new definition of neuropathic pain and their validity may be in question.143,147,148
4. Assessment
4.1. Neuropathic pain grading system
The Neuropathic Pain Special Interest Group of the International Association of the Study of Pain introduced a grading system to assist clinicians and researchers determining the certainty of neuropathic pain (Fig. 2).55 The level of “probable” neuropathic pain is suggested to be sufficient to initiate pharmacological treatment for neuropathic pain.55
Figure 2.
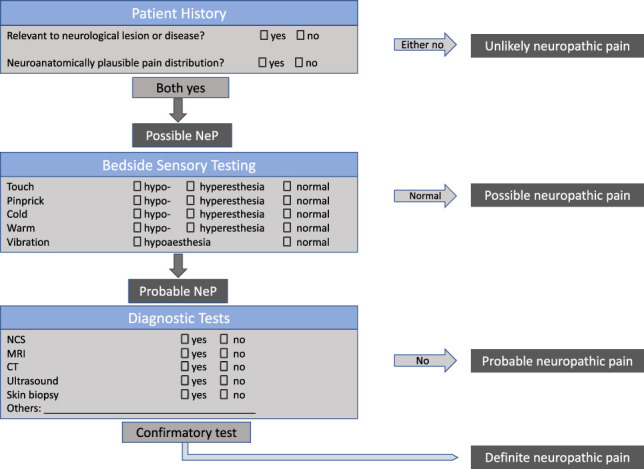
Neuropathic pain grading system adapted from 55.
The application of the grading system may, however, be problematic in patients with entrapment neuropathies because (1) symptoms often spread beyond the affected dermatome or innervation territories of the affected nerve, (2) patients may have very subtle sensory loss that may not be identified with bedside sensory testing and/or patients may present with mainly gain of function, and (3) diagnostic tests may not be indicated (eg, skin biopsies) or may be negative (see imaging and nerve conduction studies below). It therefore often remains unclear whether patients with entrapment neuropathies should receive management according to the neuropathic pain guidelines.44
4.2. Clinical assessment
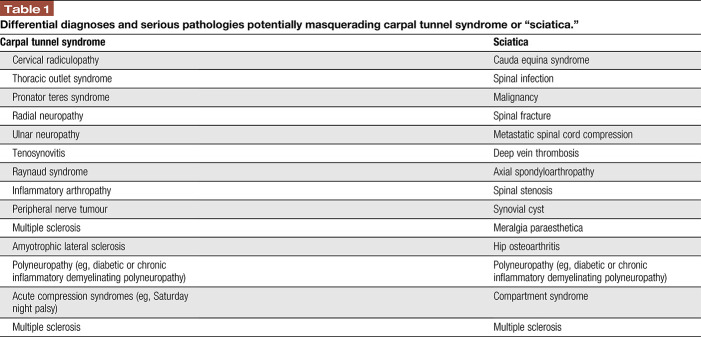
The examination of patients with suspected entrapment neuropathies comprises a comprehensive subjective assessment, including the localisation and distribution of symptoms on a body chart as well as their quality, intensity, and behaviour over 24 hours. The medical history provides clues about potential mechanisms of injury or causes leading to pain or neurological lesions and disorder progression. The history may also provide important information on psychological or behavioural factors (orange, yellow flags), social and economic factors (blue flags) as well as occupational factors (black flags) that may contribute to the presentation.72 Importantly, clinicians are encouraged to evaluate the presence of differential diagnoses and serious pathologies (red flags) masquerading as entrapment neuropathies (Table 1).56
Table 1.
Differential diagnoses and serious pathologies potentially masquerading carpal tunnel syndrome or “sciatica.”
The physical examination includes the assessment of both musculoskeletal and related neural tissue, as well as a detailed neurological bedside examination of sensory and motor function to ascertain the presence of a nerve lesion. Results of medical investigations may assist in the diagnostic work-up. For a detailed discussion on clinical assessment of entrapment neuropathies with a focus on spinally referred pain, please consult the study by Schmid and Tampin.129
4.2.1. Neurological examination
Motor function assessment comprises the examination of reflex responses and muscle strength testing. Sensory examination must include the assessment of large and small sensory fibres because both can be affected in patients with nerve entrapment.142,146 Figure 3 outlines various tools for bedside sensory testing that assess loss and gain of function in distinct sensory fibre populations. The validity of low-cost bedside sensory testing compared to quantitative sensory testing or skin biopsy has been documented in recent studies.120,173
Figure 3.
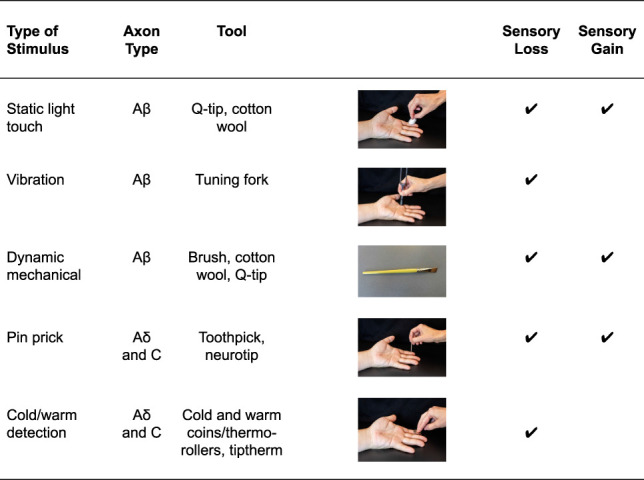
Bedside sensory testing tools.
4.2.2. Neural mechanosensitivity
Several provocation tests are designed to detect nerve mechanosensitivity such as the Tinel sign or Phalen test for CTS or the Spurling test for cervical radiculopathy. However, the measurement properties of these positional tests vary greatly between studies, indicating uncertainty in their diagnostic utility.88,153
The straight leg raise test has been widely used to assess heightened nerve mechanosensitivity in the lower limb.38,59 Although the upper-limb analogue (upper-limb neurodynamic test)47 is less known in medicine, it is extensively applied in physiotherapy practice.108 These tests comprise passive joint movements that cause movement/elongation or tension on neural structures. An abnormal response to the tests is defined as at least partial reproduction of the patient's symptoms with positive structural differentiation.108 Structural differentiation uses movements at a site remote to the painful area to further load or unload neural structures, thus differentiating symptoms of neural origin from local musculoskeletal origin.64 For detailed description of nerve mechanosensitivity testing, we refer the readers to the studies by Butler,22 Schmid and Tampin,129 and Tampin.149
4.2.3. Imaging and nerve conduction studies
Additional examinations such as MRI, ultrasound, or nerve conduction studies may help in the diagnostic workup. Although nerve conduction tests are particularly useful in the diagnosis of distal entrapment neuropathies (CTS and cubital tunnel syndrome), their value for more proximal neuropathies has not been fully established.30 It should be noted that in the early stages of CTS, nerve conduction studies may be unable to detect nerve injury.131 MRI is commonly used for proximal entrapment neuropathies (eg, “sciatica”), but often results in false negatives or positives and the affected level identified on imaging and clinical examination often does not match.159 Ultrasonography is often preferred over MRI for distal entrapment neuropathies due to its cost-effectiveness, ease of access, and possibility of quick contralateral comparison. The screening for an enlarged nerve diameter might be useful in the diagnosis of CTS121 or cubital tunnel syndrome.27 In addition to being confirmatory tests, imaging and nerve conduction tests can be important for differential diagnosis and the exclusion of serious pathologies.
5. Natural history, prognosis, and management
Due to the scarcity of evidence in less common entrapment neuropathies, we report findings from CTS and “sciatica” only. For ethical reasons, studying the natural history of entrapment neuropathies is challenging. In CTS patients who have not received treatment over an average of 2 years, ∼23% of patients deteriorated, ∼29% remained stable, and ∼48% showed symptom recovery.111 A similar pattern presents in patients with “sciatica,” where the clinical course is generally considered favourable; however, at least one-third will develop persistent pain and disability lasting a year or more.65,69,77,156,161,164 Unfortunately, prognostic factors for pain persistence remain elusive11; the most comprehensive cohort study to date in “sciatica” showed only modest associations with poor outcome for shorter pain duration, belief that symptoms will not last long and overall impact of “sciatica.”77 Unexpectedly, the strongest predictive factor (odds ratio 5.62, 95% confidence interval 1.76–17.92) was myotomal weakness, which was, however, associated with a good prognosis. Given the failure or only weak predictive ability of traditional prognostic factors (psychosocial features, MRI, pain severity etc), novel approaches must be considered.
5.1. Management
Independent of the type of entrapment neuropathy, clinical guidelines suggest a course of conservative treatment before more invasive options are considered.20,120,174
5.1.1. Physiotherapy/occupational therapy for carpal tunnel syndrome
For mild to moderate CTS, therapy usually includes advice, splinting, electrophysiological agents as well as manual therapy and/or exercises.51 There is currently limited evidence for a short-term benefit of splinting compared to no treatment.113 Splinting is recommended at night only.51 A Cochrane review reported that there is limited evidence for a beneficial effect of exercise and mobilisation in the management of patients with CTS.114 This was supported by another review suggesting that neural mobilisation did not improve clinical outcomes compared to other treatments.16 However, more recent randomised controlled trials suggest a benefit of more comprehensive hand therapy packages.52,54,84 Given the limited literature available for the effectiveness of therapeutic management of CTS, the decision to provide physiotherapy/occupational therapy management should be based on the clinicians' expertise and patients' preferences114 while following current treatment recommendations.51
5.1.2. Physiotherapy for “sciatica”
Physiotherapy is recommended as the first-line treatment for patients with “sciatica.”106 Based on studies with mostly high risk of bias and heterogeneity of physiotherapy interventions, we found inadequate evidence to make clinical recommendations on physiotherapy for patients with “sciatica.”41 Importantly, “sciatica” definitions in available studies varied significantly and the studied populations were heterogeneous making firm conclusions difficult. There is some indication that distinct subgroups of patients may react better to certain physiotherapy treatments. For instance, patients with heightened neural mechanosensitivity seem to benefit most from neural mobilisations compared to patients with radiculopathy or radicular pain.127 Large, well-designed trials are required to determine the most effective physiotherapy approach and whether treatment stratification may improve outcome.
5.1.3. Pharmacological interventions
Oral medications do not seem to be beneficial in patients with CTS, with nonsteroidal anti-inflammatories, diuretics, or vitamin B6 not being superior to placebo.110 Local steroid injections seem to have a short-term benefit only, which exceeds the effect of orally taken steroids.97 Similar results are reported by several meta-analyses for patients with “sciatica,” suggesting that nonsteroidal anti-inflammatories,119 oral corticosteroids,116 Tumour necrosis factor blockers,162 opioids,116 and specific neuropathic pain medications (eg, anticonvulsants and antidepressants) do not provide better symptom relief than placebo.116 Again, corticosteroid injections seem to provide a small benefit in the short but not long term.117
This disappointing pharmacological efficacy in patients with entrapment neuropathies may in part be due to our limited understanding of the exact pathomechanisms. Importantly, the heterogeneity of patients within one diagnosis may mask potential beneficial effects of specific subgroups. Future work is required to determine whether stratification methods could be used to identify distinct populations that may benefit from specific pharmacological interventions.
5.1.4. Surgery for carpal tunnel syndrome
Carpal tunnel decompression is the most common upper-limb surgery45 and surgery rates are predicted to double over the coming decade,17 posing a significant challenge to public health systems. The 2 surgical approaches used are endoscopic and open carpal tunnel release, with no strong evidence indicating one technique to be more superior.160 Although CTS surgery is usually successful, ∼25% of patients do not benefit.18 Potential complications include scar tenderness, persistent symptoms, neurovascular injury, wound complication, and reduced grip strength.98 Indications for surgical consultation include moderate-to-severe or deteriorating symptoms, daily symptoms, frequent night waking, persistent symptoms causing functional impairment, not responding to nonsurgical treatments (120), and patients' preference.152
5.1.5. Surgery for “sciatica”
Lumbar microdiscectomy is the most common type of surgery performed to relieve nerve root irritation or compression due to a herniated disk. Pooled data of >13,000 patients undergoing surgery for “sciatica” demonstrated that although surgery is followed by a rapid decrease in pain and disability by 3 months, patients still experienced mild-to-moderate pain and disability 5 years after surgery.89 Of note, ∼4% of patients are worse after surgery137 and reoperation rates of ∼12% within 4 years have been reported after discectomy.66 Factors associated with negative postoperative outcomes include intact annulus fibrosus, longer duration of sick-leave, worker's compensation, and greater severity of baseline symptoms.168 Two systematic reviews suggest that surgery may yield faster pain relief and perceived recovery than physiotherapy or physical activity for patients with “sciatica;” however, long-term outcomes are comparable.70 Given the nonsuperiority of surgery in the long-term and the significant risks and side effects, conservative management should remain the first-line treatment. However, surgery is indicated in the presence of severe or progressive neurological deficits or persistent symptoms that are unresponsive to conservative treatment.106 Future research is needed to better understand appropriate patient selection and timelines for surgery to improve outcomes and patient satisfaction.
6. Future challenges and conclusion
The somewhat disappointing evidence of pharmacological, physiotherapeutic and surgical management of entrapment neuropathies clearly highlights that a one-size-fits-all approach is not successful. Considering the distinct pathomechanisms and contributing factors, contemporary management should be personalised, considering the multidimensional profile of an individual patient (eg, different pain mechanisms at play, contextual factors, cognitive emotional drivers, comorbidities, Fig. 4).154 As such, individualised management will vary in patients with entrapment neuropathy and should target the predominant dimensions. For instance, tissue-specific interventions (eg, specific exercises, postural modification, mobilisations, targeted pharmacology, injection, and surgery) may be indicated in patients with clear nociceptive, heightened neural mechanosensitivity or neuropathic components. By contrast, multidisciplinary systemic treatments (eg, cognitive behavioural and functional approaches, general exercise) may be more suitable for patients with strong cognitive–emotional, contextual, and nociplastic drivers. Future research is required to further understand specific drivers at play in individual patients, their utility for predicting outcome and treatment stratification, and the effect of personalised treatment on outcomes before a truly evidence-based management of patients with entrapment neuropathies is possible.
Figure 4.
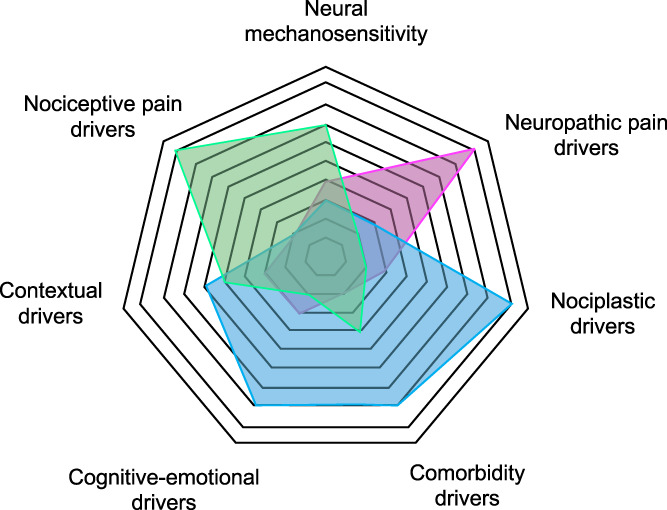
Potential drivers contributing to specific multidimensional profiles in patients with entrapment neuropathies. The spiderweb highlights how distinct drivers may be more prominent in 3 distinct patient presentations (green, blue, and pink). The weighting of these drivers in individual patients may contribute to the design of personalized management for patients with entrapment neuropathies.
Disclosures
The authors have no conflict of interest to declare.
A.B. Schmid is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). Brigitte Tampin was supported by the Government of Western Australia, Department of Health, and Raine Medical Research Foundation. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Abzug JM, Jacoby SM, Osterman AL. Surgical options for recalcitrant carpal tunnel syndrome with perineural fibrosis. Hand (N Y) 2012;7:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med 2002;34:42–7. [DOI] [PubMed] [Google Scholar]
- [3].Albrecht DS, Ahmed SU, Kettner NW, Borra RJH, Cohen-Adad J, Deng H, Houle TT, Opalacz A, Roth SA, Melo MFV, Chen L, Mao J, Hooker JM, Loggia ML, Zhang Y. Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. PAIN 2018;159:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allison GT, Nagy BM, Hall T. A randomized clinical trial of manual therapy for cervico-brachial pain syndrome—a pilot study. Man Ther 2002;7:95–102. [DOI] [PubMed] [Google Scholar]
- [5].American Academy of Orthopaedic Surgeons. Clinical practice guideline on the diagnosis of carpal tunnel syndrome. Rosemont, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci 1999;19:8589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ando Y. Experimental study on chronic entrapment neuropathy [in Japanese]. Nippon Seikeigeka Gakkai Zasshi 1990;64:633–47. [PubMed] [Google Scholar]
- [8].Andrasinova T, Kalikova E, Kopacik R, Srotova I, Vlckova E, Dusek L, Bednarik J, Adamova B. Evaluation of the neuropathic component of chronic low back pain. Clin J Pain 2019;35:7–17. [DOI] [PubMed] [Google Scholar]
- [9].Arendt-Nielsen L, Gregersen H, Toft E, Bjerring P. Involvement of thin afferents in carpal tunnel syndrome: evaluated quantitatively by argon laser stimulation. Muscle Nerve 1991;14:508–14. [DOI] [PubMed] [Google Scholar]
- [10].Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Lee M, Chhith S, Gomariz RP, Waschek JA. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience 2004;129:93–9. [DOI] [PubMed] [Google Scholar]
- [11].Ashworth J, Konstantinou K, Dunn KM. Prognostic factors in non-surgically treated sciatica: a systematic review. BMC Musculoskelet Disord 2011;12:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol 2013;74:630–6. [DOI] [PubMed] [Google Scholar]
- [13].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baselgia LT, Bennett DL, Silbiger RM, Schmid AB. Negative neurodynamic tests do not exclude neural dysfunction in patients with entrapment neuropathies. Arch Phys Med Rehabil 2017;98:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baskozos G, Sandy-Hindmarch O, Clark A, Windsor K, Karlsson P, Weir G, McDermott L, Burchall J, Wiberg A, Furniss D, Bennett D, Schmid A. Molecular and cellular correlates of human nerve regeneration: ADCYAP1 encoding PACAP enhances sensory neuron outgrowth. Brain 2020;143:2009–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Basson A, Olivier B, Ellis R, Coppieters M, Stewart A, Mudzi W. The effectiveness of neural mobilization for neuromusculoskeletal conditions: a systematic review and meta-analysis. J Orthop Sports Phys Ther 2017;47:593–615. [DOI] [PubMed] [Google Scholar]
- [17].Bebbington E, Furniss D. Linear regression analysis of Hospital Episode Statistics predicts a large increase in demand for elective hand surgery in England. J Plast Reconstr Aesthet Surg 2015;68:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve 2007;36:167–71. [DOI] [PubMed] [Google Scholar]
- [19].Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. PAIN 2009;147:17–19. [DOI] [PubMed] [Google Scholar]
- [20].Bono CM, Ghiselli G, Gilbert TJ, Kreiner DS, Reitman C, Summers JT, Baisden JL, Easa J, Fernand R, Lamer T, Matz PG, Mazanec DJ, Resnick DK, Shaffer WO, Sharma AK, Timmons RB, Toton JF, North American Spine S. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J 2011;11:64–72. [DOI] [PubMed] [Google Scholar]
- [21].Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol 2003;90:1949–55. [DOI] [PubMed] [Google Scholar]
- [22].Butler DS. The sensitive nervous system. Adelaide: NOIgroup publications, 2000. [Google Scholar]
- [23].Caliandro P, La Torre G, Aprile I, Pazzaglia C, Commodari I, Tonali P, Padua L. Distribution of paresthesias in Carpal Tunnel Syndrome reflects the degree of nerve damage at wrist. Clin Neurophysiol 2006;117:228–31. [DOI] [PubMed] [Google Scholar]
- [24].Calvo M, Richards N, Schmid AB, Barroso A, Zhu L, Ivulic D, Zhu N, Anwandter P, Bhat MA, Court FA, McMahon SB, Bennett DL. Altered potassium channel distribution and composition in myelinated axons suppresses hyperexcitability following injury. Elife 2016;5:e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cappelen-Smith C, Lin CS, Burke D. Activity-dependent hyperpolarization and impulse conduction in motor axons in patients with carpal tunnel syndrome. Brain 2003;126:1001–8. [DOI] [PubMed] [Google Scholar]
- [26].Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. Eur J Pain 1998;2:165–78. [DOI] [PubMed] [Google Scholar]
- [27].Chen IJ, Chang KV, Wu WT, Ozcakar L. Ultrasound parameters other than the direct measurement of ulnar nerve size for diagnosing cubital tunnel syndrome: a systemic review and meta-analysis. Arch Phys Med Rehabil 2019;100:1114–30. [DOI] [PubMed] [Google Scholar]
- [28].Chhabra A, Wadhwa V, Thakkar RS, Carrino JA, Dellon AL. Recurrent ulnar nerve entrapment at the elbow: correlation of surgical findings and 3-Tesla magnetic resonance neurography. Can J Plast Surg 2013;21:186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chien A, Eliav E, Sterling M. Whiplash (grade II) and cervical radiculopathy share a similar sensory presentation: an investigation using quantitative sensory testing. Clin J Pain 2008;24:595–603. [DOI] [PubMed] [Google Scholar]
- [30].Cho SC, Ferrante MA, Levin KH, Harmon RL, So YT. Utility of electrodiagnostic testing in evaluating patients with lumbosacral radiculopathy: an evidence-based review. Muscle Nerve 2010;42:276–82. [DOI] [PubMed] [Google Scholar]
- [31].Coppieters MW, Schmid AB, Kubler PA, Hodges PW. Description, reliability and validity of a novel method to measure carpal tunnel pressure in patients with carpal tunnel syndrome. Man Ther 2012;17:589–92. [DOI] [PubMed] [Google Scholar]
- [32].Cudlip SA, Howe FA, Clifton A, Schwartz MS, Bell BA. Magnetic resonance neurography studies of the median nerve before and after carpal tunnel decompression. J Neurosurg 2002;96:1046–51. [DOI] [PubMed] [Google Scholar]
- [33].Dahlin LB, McLean WG. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurol Sci 1986;72:19–30. [DOI] [PubMed] [Google Scholar]
- [34].Dahlin LB, Rydevik B, McLean WG, Sjostrand J. Changes in fast axonal transport during experimental nerve compression at low pressures. Exp Neurol 1984;84:29–36. [DOI] [PubMed] [Google Scholar]
- [35].Dahlin LB, Sjostrand J, McLean WG. Graded inhibition of retrograde axonal transport by compression of rabbit vagus nerve. J Neurol Sci 1986;76:221–30. [DOI] [PubMed] [Google Scholar]
- [36].Dahlin LB, Shyu BC, Danielsen N, Andersson SA. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol Scand 1989;136:97–105. [DOI] [PubMed] [Google Scholar]
- [37].de la Llave-Rincon AI, Fernandez-de-Las-Penas C, Fernandez-Carnero J, Padua L, Arendt-Nielsen L, Pareja JA. Bilateral hand/wrist heat and cold hyperalgesia, but not hypoesthesia, in unilateral carpal tunnel syndrome. Exp Brain Res 2009;198:455–63. [DOI] [PubMed] [Google Scholar]
- [38].Deville WL, van der Windt DA, Dzaferagic A, Bezemer PD, Bouter LM. The test of Lasegue: systematic review of the accuracy in diagnosing herniated discs. Spine (Phila Pa 1976) 2000;25:1140–7. [DOI] [PubMed] [Google Scholar]
- [39].Dilley A, Bove GM. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol 2008;586:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dilley A, Lynn B, Pang SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. PAIN 2005;117:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dove L, Jones G, Kelsey L, Cairns M, Schmid AB. How effective is physiotherapy for sciatica? A systematic review and meta-analysis. 7th International Congress on Neuropathic Pain, London, 2019.
- [42].Drummond PD, Drummond ES, Dawson LF, Mitchell V, Finch PM, Vaughan CW, Phillips JK. Upregulation of alpha1-adrenoceptors on cutaneous nerve fibres after partial sciatic nerve ligation and in complex regional pain syndrome type II. PAIN 2014;155:606–16. [DOI] [PubMed] [Google Scholar]
- [43].Druschky K, Kaltenhauser M, Hummel C, Druschky A, Huk WJ, Stefan H, Neundorfer B. Alteration of the somatosensory cortical map in peripheral mononeuropathy due to carpal tunnel syndrome. Neuroreport 2000;11:3925–30. [DOI] [PubMed] [Google Scholar]
- [44].Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, Levy RM, Backonja M, Baron R, Harke H, Loeser JD, Treede RD, Turk DC, Wells CD. International association for the study of pain neuropathic pain special interest G. Interventional management of neuropathic pain: NeuPSIG recommendations. PAIN 2013;154:2249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Elective JM. Surgery waiting times 2016–2017: Australian hospital statistics. Australian Institute of Health and Wellness; Available at: https://www.aihw.gov.au/reports/hospitals/elective-surgery-waiting-times-ahs-2016-17/data. Accessed March 7, 2018. [Google Scholar]
- [46].Ellis R, Blyth R, Arnold N, Miner-Williams W. Is there a relationship between impaired median nerve excursion and carpal tunnel syndrome? A systematic review. J Hand Ther 2017;30:3–12. [DOI] [PubMed] [Google Scholar]
- [47].Elvey RL. Physical evaluation of the peripheral nervous system in disorders of pain and dysfunction. J Hand Ther 1997;10:122–9. [DOI] [PubMed] [Google Scholar]
- [48].Endean A, Palmer KT, Coggon D. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: a systematic review. Spine (Phila Pa 1976) 2011;36:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].England JD, Gamboni F, Ferguson MA, Levinson SR. Sodium channels accumulate at the tips of injured axons. Muscle Nerve 1994;17:593–8. [DOI] [PubMed] [Google Scholar]
- [50].Erdem Tilki H, Coskun M, Unal Akdemir N, Incesu L. Axon count and sympathetic skin responses in lumbosacral radiculopathy. J Clin Neurol 2014;10:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Erickson M, Lawrence M, Jansen CWS, Coker D, Amadio P, Cleary C. Hand pain and sensory deficits: carpal tunnel syndrome. J Orthop Sports Phys Ther 2019;49:CPG1–CPG85. [DOI] [PubMed] [Google Scholar]
- [52].Fernandez-de-Las Penas C, Ortega-Santiago R, de la Llave-Rincon AI, Martinez-Perez A, Fahandezh-Saddi Diaz H, Martinez-Martin J, Pareja JA, Cuadrado-Perez ML. Manual physical therapy versus surgery for carpal tunnel syndrome: a randomized parallel-group trial. J Pain 2015;16:1087–94. [DOI] [PubMed] [Google Scholar]
- [53].Fernandez-de-las-Penas C, de la Llave-Rincon AI, Fernandez-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain 2009;132:1472–9. [DOI] [PubMed] [Google Scholar]
- [54].Fernandez-de-Las-Penas C, Cleland J, Palacios-Cena M, Fuensalida-Novo S, Pareja JA, Alonso-Blanco C. The effectiveness of manual therapy versus surgery on self-reported function, cervical range of motion, and pinch grip force in carpal tunnel syndrome: a randomized clinical trial. J Orthop Sports Phys Ther 2017;47:151–61. [DOI] [PubMed] [Google Scholar]
- [55].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Finucane L, Downie A, Mercer C, Greenhalgh S, Boissonnault WG, Pool-Goudzwaard A, Beneciuk JM, Leech R, Selfe J. International Framework for red flags for potential serious spinal pathologies. J Orthop Sports Phys Ther 2020;50:350–72. [DOI] [PubMed] [Google Scholar]
- [57].Fjeld O, Grotle M, Siewers V, Pedersen LM, Nilsen KB, Zwart JA. Prognostic factors for persistent leg-pain in patients hospitalized with acute sciatica. Spine (Phila Pa 1976) 2017;42:E272–9. [DOI] [PubMed] [Google Scholar]
- [58].Foix C, Marie P. Atrophie isolée de l'éminence thénar d'origin névritique. Rôle du ligament annulaire antérieur du carpe dans la pathogénie de la lésion. Rev Neurol 1913;26:647–8. [Google Scholar]
- [59].Freynhagen R, Rolke R, Baron R, Tolle TR, Rutjes AK, Schu S, Treede RD. Pseudoradicular and radicular low-back pain—a disease continuum rather than different entities? Answers from quantitative sensory testing. PAIN 2008;135:65–74. [DOI] [PubMed] [Google Scholar]
- [60].Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am 1981;63:380–3. [PubMed] [Google Scholar]
- [61].Gupta R, Channual JC. Spatiotemporal pattern of macrophage recruitment after chronic nerve compression injury. J Neurotrauma 2006;23:216–26. [DOI] [PubMed] [Google Scholar]
- [62].Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol 2003;461:174–86. [DOI] [PubMed] [Google Scholar]
- [63].Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol 2004;187:500–8. [DOI] [PubMed] [Google Scholar]
- [64].Hall TM, Elvey RL. Nerve trunk pain: physical diagnosis and treatment. Man Ther 1999;4:63–73. [DOI] [PubMed] [Google Scholar]
- [65].Haugen AJ, Brox JI, Grovle L, Keller A, Natvig B, Soldal D, Grotle M. Prognostic factors for non-success in patients with sciatica and disc herniation. BMC Musculoskelet Disord 2012;13:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Heindel P, Tuchman A, Hsieh PC, Pham MH, D'Oro A, Patel NN, Jakoi AM, Hah R, Liu JC, Buser Z, Wang JC. Reoperation rates after single-level lumbar discectomy. Spine (Phila Pa 1976) 2017;42:E496–501. [DOI] [PubMed] [Google Scholar]
- [67].Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 2007;21:599–616. [DOI] [PubMed] [Google Scholar]
- [68].Ido K, Urushidani H. Fibrous adhesive entrapment of lumbosacral nerve roots as a cause of sciatica. Spinal Cord 2001;39:269–73. [DOI] [PubMed] [Google Scholar]
- [69].Iversen T, Solberg TK, Wilsgaard T, Waterloo K, Brox JI, Ingebrigtsen T. Outcome prediction in chronic unilateral lumbar radiculopathy: prospective cohort study. BMC Musculoskelet Disord 2015;16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jacobs WC, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, Koes B, Peul WC. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J 2011;20:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. PAIN 2008;137:495–506. [DOI] [PubMed] [Google Scholar]
- [72].Kendall N, Linton S, Main C. Guide to assessing psychosocial yellow flags in acute low back pain: risk factors for long-term disability and work loss. Wellington: Accident Rehabilitation and Compensation Insurance Corporation of New Zealand and the National Health Committee, 1997. [Google Scholar]
- [73].Kiernan MC, Mogyoros I, Burke D. Conduction block in carpal tunnel syndrome. Brain 1999;122(pt 5):933–41. [DOI] [PubMed] [Google Scholar]
- [74].Kiylioglu N. Sympathetic skin response and axon counting in carpal tunnel syndrome. J Clin Neurophysiol 2007;24:424; author reply 424. [DOI] [PubMed] [Google Scholar]
- [75].Kobayashi S, Kokubo Y, Uchida K, Yayama T, Takeno K, Negoro K, Nakajima H, Baba H, Yoshizawa H. Effect of lumbar nerve root compression on primary sensory neurons and their central branches: changes in the nociceptive neuropeptides substance P and somatostatin. Spine (Phila Pa 1976) 2005;30:276–82. [DOI] [PubMed] [Google Scholar]
- [76].Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976) 2008;33:2464–72. [DOI] [PubMed] [Google Scholar]
- [77].Konstantinou K, Dunn KM, Ogollah R, Lewis M, van der Windt D, Hay EM, Team AS. Prognosis of sciatica and back-related leg pain in primary care: the ATLAS cohort. Spine J 2018;18:1030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kozak A, Schedlbauer G, Wirth T, Euler U, Westermann C, Nienhaus A. Association between work-related biomechanical risk factors and the occurrence of carpal tunnel syndrome: an overview of systematic reviews and a meta-analysis of current research. BMC Musculoskelet Disord 2015;16:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kuwabara S, Tamura N, Yamanaka Y, Misawa S, Isose S, Bae JS, Hattori T, Asahina M. Sympathetic sweat responses and skin vasomotor reflexes in carpal tunnel syndrome. Clin Neurol Neurosurg 2008;110:691–5. [DOI] [PubMed] [Google Scholar]
- [80].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].LeBlanc BW, Zerah ML, Kadasi LM, Chai N, Saab CY. Minocycline injection in the ventral posterolateral thalamus reverses microglial reactivity and thermal hyperalgesia secondary to sciatic neuropathy. Neurosci Lett 2011;498:138–42. [DOI] [PubMed] [Google Scholar]
- [82].Lemmela S, Solovieva S, Shiri R, Benner C, Heliovaara M, Kettunen J, Anttila V, Ripatti S, Perola M, Seppala I, Juonala M, Kahonen M, Salomaa V, Viikari J, Raitakari OT, Lehtimaki T, Palotie A, Viikari-Juntura E, Husgafvel-Pursiainen K. Genome-wide meta-analysis of sciatica in Finnish population. PLoS One 2016;11:e0163877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lewis AM, Layzer R, Engstrom JW, Barbaro NM, Chin CT. Magnetic resonance neurography in extraspinal sciatica. Arch Neurol 2006;63:1469–72. [DOI] [PubMed] [Google Scholar]
- [84].Lewis KJ, Coppieters MW, Ross L, Hughes I, Vicenzino B, Schmid AB. Group education, night splinting and home exercises reduce conversion to surgery for carpal tunnel syndrome: a multicentre randomised trial. J Physiother 2020;66:97–104. [DOI] [PubMed] [Google Scholar]
- [85].Liu X, Zhou JL, Chung K, Chung JM. Ion channels associated with the ectopic discharges generated after segmental spinal nerve injury in the rat. Brain Res 2001;900:119–27. [DOI] [PubMed] [Google Scholar]
- [86].Lozano-Calderon S, Anthony S, Ring D. The quality and strength of evidence for etiology: example of carpal tunnel syndrome. J Hand Surg Am 2008;33:525–38. [DOI] [PubMed] [Google Scholar]
- [87].Luchtmann M, Steinecke Y, Baecke S, Lutzkendorf R, Bernarding J, Kohl J, Jollenbeck B, Tempelmann C, Ragert P, Firsching R. Structural brain alterations in patients with lumbar disc herniation: a preliminary study. PLoS One 2014;9:e90816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].MacDermid J. Accuracy of clinical tests used in the detection of carpal tunnel syndrome: a literature review. J Hand Ther 1991;4:169–76. [Google Scholar]
- [89].Machado GC, Witzleb AJ, Fritsch C, Maher CG, Ferreira PH, Ferreira ML. Patients with sciatica still experience pain and disability 5 years after surgery: a systematic review with meta-analysis of cohort studies. Eur J Pain 2016;20:1700–9. [DOI] [PubMed] [Google Scholar]
- [90].Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Chronic nerve compression—an experimental model in the rat. Ann Plast Surg 1984;13:112–20. [DOI] [PubMed] [Google Scholar]
- [91].Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Chronic human nerve compression-a histological assessment. Neuropathol Appl Neurobiol 1986;12:547–65. [DOI] [PubMed] [Google Scholar]
- [92].Mackinnon SE. Pathophysiology of nerve compression. Hand Clin 2002;18:231–41. [DOI] [PubMed] [Google Scholar]
- [93].Maeda Y, Kettner N, Sheehan J, Kim J, Cina S, Malatesta C, Gerber J, McManus C, Mezzacappa P, Morse LR, Audette J, Napadow V. Altered brain morphometry in carpal tunnel syndrome is associated with median nerve pathology. NeuroImage Clin 2013;2:313–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mahn F, Hullemann P, Gockel U, Brosz M, Freynhagen R, Tolle TR, Baron R. Sensory symptom profiles and co-morbidities in painful radiculopathy. PLoS One 2011;6:e18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mallik A, Weir AI. Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry 2005;76(suppl 2):ii23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mansfield M, Thacker M, Sandford F. Psychosocial risk factors and the association with carpal tunnel syndrome: a systematic review. Hand (N Y) 2018;13:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2007:CD001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Middleton SD, Anakwe RE. Carpal tunnel syndrome. BMJ 2014;349:g6437. [DOI] [PubMed] [Google Scholar]
- [99].Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006;51:240–64. [DOI] [PubMed] [Google Scholar]
- [100].Mondelli M, Giannini F, Ballerini M, Ginanneschi F, Martorelli E. Incidence of ulnar neuropathy at the elbow in the province of Siena (Italy). J Neurol Sci 2005;234:5–10. [DOI] [PubMed] [Google Scholar]
- [101].Mor D, Bembrick AL, Austin PJ, Keay KA. Evidence for cellular injury in the midbrain of rats following chronic constriction injury of the sciatic nerve. J Chem Neuroanat 2011;41:158–69. [DOI] [PubMed] [Google Scholar]
- [102].Murphy DR, Hurwitz EL, Gerrard JK, Clary R. Pain patterns and descriptions in patients with radicular pain: does the pain necessarily follow a specific dermatome? Chiropr Osteopat 2009;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nakamichi KI, Tachibana S. Enlarged median nerve in idiopathic carpal tunnel syndrome. Muscle Nerve 2000;23:1713–18. [DOI] [PubMed] [Google Scholar]
- [104].Napadow V, Kettner N, Ryan A, Kwong KK, Audette J, Hui KK. Somatosensory cortical plasticity in carpal tunnel syndrome-a cross-sectional fMRI evaluation. Neuroimage 2006;31:520–30. [DOI] [PubMed] [Google Scholar]
- [105].Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, Makris N, Audette J, Hui KK. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. PAIN 2007;130:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].National Guideline Centre. NICE guideline NG59: Low back pain and sciatica in over 16s: assessment and management. London, United Kingdomn: National Institute for Health and Care Excellence UK, 2016. [Google Scholar]
- [107].Neary D. The pathology of ulnar nerve compression in men. Neuropathol Appl Neurobiol 1975;1:69–88. [Google Scholar]
- [108].Nee RJ, Jull GA, Vicenzino B, Coppieters MW. The validity of upper-limb neurodynamic tests for detecting peripheral neuropathic pain. J Orthop Sports Phys Ther 2012;42:413–24. [DOI] [PubMed] [Google Scholar]
- [109].O'Brien JP, Mackinnon SE, MacLean AR, Hudson AR, Dellon AL, Hunter DA. A model of chronic nerve compression in the rat. Ann Plast Surg 1987;19:430–5. [DOI] [PubMed] [Google Scholar]
- [110].O'Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev 2003;2003:CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ortiz-Corredor F, Enriquez F, Diaz-Ruiz J, Calambas N. Natural evolution of carpal tunnel syndrome in untreated patients. Clin Neurophysiol 2008;119:1373–8. [DOI] [PubMed] [Google Scholar]
- [112].Otoshi K, Kikuchi S, Konno S, Sekiguchi M. The reactions of glial cells and endoneurial macrophages in the dorsal root ganglion and their contribution to pain-related behavior after application of nucleus pulposus onto the nerve root in rats. Spine (Phila Pa 1976) 2010;35:264–71. [DOI] [PubMed] [Google Scholar]
- [113].Page MJ, Massy-Westropp N, O'Connor D, Pitt V. Splinting for carpal tunnel syndrome. Cochrane Database Syst Rev 2012;7:CD010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst Rev 2012;6:CD009899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Parreira P, Maher CG, Steffens D, Hancock MJ, Ferreira ML. Risk factors for low back pain and sciatica: an umbrella review. Spine J 2018;18:1715–21. [DOI] [PubMed] [Google Scholar]
- [116].Pinto RZ, Maher CG, Ferreira ML, Ferreira PH, Hancock M, Oliveira VC, McLachlan AJ, Koes B. Drugs for relief of pain in patients with sciatica: systematic review and meta-analysis. BMJ 2012;344:e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, Koes B, Ferreira PH. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med 2012;157:865–77. [DOI] [PubMed] [Google Scholar]
- [118].Pourmemari MH, Shiri R. Diabetes as a risk factor for carpal tunnel syndrome: a systematic review and meta-analysis. Diabet Med 2016;33:10–16. [DOI] [PubMed] [Google Scholar]
- [119].Rasmussen-Barr E, Held U, Grooten WJ, Roelofs PD, Koes BW, van Tulder MW, Wertli MM. Nonsteroidal anti-inflammatory drugs for sciatica: an updated Cochrane review. Spine (Phila Pa 1976) 2017;42:586–94. [DOI] [PubMed] [Google Scholar]
- [120].Ridehalgh C, Sandy-Hindmarch OP, Schmid AB. Validity of clinical small-fiber sensory testing to detect small-nerve fiber degeneration. J Orthop Sports Phys Ther 2018;48:767–74. [DOI] [PubMed] [Google Scholar]
- [121].Roll SC, Case-Smith J, Evans KD. Diagnostic accuracy of ultrasonography vs. electromyography in carpal tunnel syndrome: a systematic review of literature. Ultrasound Med Biol 2011;37:1539–53. [DOI] [PubMed] [Google Scholar]
- [122].Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res 2007;1181:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rothman SM, Nicholson KJ, Winkelstein BA. Time-dependent mechanics and measures of glial activation and behavioral sensitivity in a rodent model of radiculopathy. J Neurotrauma 2010;27:803–14. [DOI] [PubMed] [Google Scholar]
- [124].Ryan C, Roberts L. Life on hold: the lived experience of radicular symptoms. A qualitative, interpretative inquiry. Musculoskelet Sci Pract 2019;39:51–7. [DOI] [PubMed] [Google Scholar]
- [125].Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood blow. An in vivo study on rabbit tibial nerve. J Hand Surg Am 1981;6:3–12. [DOI] [PubMed] [Google Scholar]
- [126].Samuelsson L, Lundin A. Thermal quantitative sensory testing in lumbar disc herniation. Eur Spine J 2002;11:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Schafer A, Hall T, Muller G, Briffa K. Outcomes differ between subgroups of patients with low back and leg pain following neural manual therapy: a prospective cohort study. Eur Spine J 2011;20:482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Schmid AB, Coppieters MW. Left/right judgment of body parts is selectively impaired in patients with unilateral carpal tunnel syndrome. Clin J Pain 2012;28:615–22. [DOI] [PubMed] [Google Scholar]
- [129].Schmid AB, Tampin B. Spinally referred back and leg pain. In: Lumbar spine online textbook. ISftSotL Spine, 2018. Available at: http://www.wheelessonline.com/ISSLS/section-10-chapter-10-spinally-referred-back-and-leg-pain/. Accessed 1 September 2019. [Google Scholar]
- [130].Schmid AB, Coppieters MW, Ruitenberg MJ, McLachlan EM. Local and remote immune-mediated inflammation after mild peripheral nerve compression in rats. J Neuropathol Exp Neurol 2013;72:662–80. [DOI] [PubMed] [Google Scholar]
- [131].Schmid AB, Bland JD, Bhat MA, Bennett DL. The relationship of nerve fibre pathology to sensory function in entrapment neuropathy. Brain 2014;137:3186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Seradge H, Jia YC, Owens W. In vivo measurement of carpal tunnel pressure in the functioning hand. J Hand Surg Am 1995;20:855–9. [DOI] [PubMed] [Google Scholar]
- [133].Shamji MF, Allen KD, So S, Jing L, Adams SB, Jr, Schuh R, Huebner J, Kraus VB, Friedman AH, Setton LA, Richardson WJ. Gait abnormalities and inflammatory cytokines in an autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa 1976) 2009;34:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Shiri R, Pourmemari MH, Falah-Hassani K, Viikari-Juntura E. The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev 2015;16:1094–104. [DOI] [PubMed] [Google Scholar]
- [135].Singh R, Gamble G, Cundy T. Lifetime risk of symptomatic carpal tunnel syndrome in Type 1 diabetes. Diabet Med 2005;22:625–30. [DOI] [PubMed] [Google Scholar]
- [136].Sirvanci M, Kara B, Duran C, Ozturk E, Karatoprak O, Onat L, Ulusoy OL, Mutlu A. Value of perineural edema/inflammation detected by fat saturation sequences in lumbar magnetic resonance imaging of patients with unilateral sciatica. Acta Radiol 2009;50:205–11. [DOI] [PubMed] [Google Scholar]
- [137].Solberg TK, Nygaard OP, Sjaavik K, Hofoss D, Ingebrigtsen T. The risk of getting worse after lumbar microdiscectomy. Eur Spine J 2005;14:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Soon B, Vicenzino B, Schmid AB, Coppieters MW. Facilitatory and inhibitory pain mechanisms are altered in patients with carpal tunnel syndrome. PLoS One 2017;12:e0183252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Takahashi K, Shima I, Porter RW. Nerve root pressure in lumbar disc herniation. Spine (Phila Pa 1976) 1999;24:2003–6. [DOI] [PubMed] [Google Scholar]
- [140].Takahata S, Takebayashi T, Terasima Y, Tanimoto K, Wada T, Sohma H, Kokai Y, Yamashita T. Activation of glial cells in the spinal cord of a model of lumbar radiculopathy. J Orthopaedic Sci 2011;16:313–20. [DOI] [PubMed] [Google Scholar]
- [141].Tamburin S, Cacciatori C, Praitano ML, Cazzarolli C, Foscato C, Fiaschi A, Zanette G. Median nerve small- and large-fiber damage in carpal tunnel syndrome: a quantitative sensory testing study. J Pain 2010;12:205–12. [DOI] [PubMed] [Google Scholar]
- [142].Tampin B, Slater H, Hall T, Lee G, Briffa NK. Quantitative sensory testing somatosensory profiles in patients with cervical radiculopathy are distinct from those in patients with nonspecific neck-arm pain. PAIN 2012;153:2403–14. [DOI] [PubMed] [Google Scholar]
- [143].Tampin B, Briffa NK, Slater H. Self-reported sensory descriptors are associated with quantitative sensory testing parameters in patients with cervical radiculopathy, but not in patients with fibromyalgia. Eur J Pain 2013;17:621–33. [DOI] [PubMed] [Google Scholar]
- [144].Tampin B, Slater H, Briffa NK. Neuropathic pain components are common in patients with painful cervical radiculopathy, but not in patients with nonspecific neck-arm pain. Clin J Pain 2013;29:846–56. [DOI] [PubMed] [Google Scholar]
- [145].Tampin B, Slater H, Jacques A, Lind C. Association of quantitative sensory testing parameters with clinical outcome in patients with lumbar radiculopathy undergoing microdiscectomy. European Journal of Pain 2020. doi: 10.1002/ejp.1586 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Tampin B, Vollert J, Schmid AB. Sensory profiles are comparable in patients with distal and proximal entrapment neuropathies, while the pain experience differs. Curr Med Res Opin 2018;34:1899–1906. [DOI] [PubMed] [Google Scholar]
- [147].Tampin B, Broe RE, Seow LL, George SG, Tan J, Menon R, Jacques A, Slater H. Field testing of the revised neuropathic pain grading system in a cohort of patients with neck and upper limb pain. Scand J Pain 2019;19:523–32. [DOI] [PubMed] [Google Scholar]
- [148].Tampin B, Royle J, Bharat C, Trevenen M, Olsen L, Goucke R. Psychological factors can cause false pain classification on painDETECT. Scand J Pain 2019;19:501–12. [DOI] [PubMed] [Google Scholar]
- [149].Tampin B. Integration of peripheral nerves in the examination of the locomotor system. In: Hueter-Becker A, Doelken M, editors. Physical therapy examination and assessment. Stuttgart: Thieme, 2015. p. 76–104. [Google Scholar]
- [150].Tecchio F, Padua L, Aprile I, Rossini PM. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp 2002;17:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg 2007;105:838–47. [DOI] [PubMed] [Google Scholar]
- [152].The Royal College of Surgeons of England and British Orthopaedic Association. Commissioning guide: Treatment of carpal tunnel syndrome; London, United Kingdom: 2017. [Google Scholar]
- [153].Thoomes EJ, van Geest S, van der Windt DA, Falla D, Verhagen AP, Koes BW, Thoomes-de Graaf M, Kuijper B, Scholten-Peeters WGM, Vleggeert-Lankamp CL. Value of physical tests in diagnosing cervical radiculopathy: a systematic review. Spine J 2018;18:179–89. [DOI] [PubMed] [Google Scholar]
- [154].Tousignant-Laflamme Y, Martel MO, Joshi AB, Cook CE. Rehabilitation management of low back pain—it's time to pull it all together! J Pain Res 2017;10:2373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Tschugg A, Lener S, Hartmann S, Neururer S, Wildauer M, Thome C, Loscher WN. Improvement of sensory function after sequestrectomy for lumbar disc herniation: a prospective clinical study using quantitative sensory testing. Eur Spine J 2016;25:3543–9. [DOI] [PubMed] [Google Scholar]
- [156].Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol 2004;57:174–9. [DOI] [PubMed] [Google Scholar]
- [157].van der Heide B, Bourgoin C, Eils G, Garnevall B, Blackmore M. Test-retest reliability and face validity of a modified neural tissue provocation test in patients with cervicobrachial pain syndrome. J Man Manipulative Ther 2006;14:30–6. [Google Scholar]
- [158].van Paassen BW, van der Kooi AJ, van Spaendonck-Zwarts KY, Verhamme C, Baas F, de Visser M. PMP22 related neuropathies: charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies. Orphanet J Rare Dis 2014;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].van Rijn JC, Klemetso N, Reitsma JB, Majoie CB, Hulsmans FJ, Peul WC, Bossuyt PM, Heeten GJ, Stam J. Symptomatic and asymptomatic abnormalities in patients with lumbosacral radicular syndrome: clinical examination compared with MRI. Clin Neurol Neurosurg 2006;108:553–7. [DOI] [PubMed] [Google Scholar]
- [160].Vasiliadis HS, Georgoulas P, Shrier I, Salanti G, Scholten RJ. Endoscopic release for carpal tunnel syndrome. Cochrane Database Syst Rev 2014:CD008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Vroomen PC, de Krom MC, Slofstra PD, Knottnerus JA. Conservative treatment of sciatica: a systematic review. J Spinal Disord 2000;13:463–9. [DOI] [PubMed] [Google Scholar]
- [162].Wang YF, Chen PY, Chang W, Zhu FQ, Xu LL, Wang SL, Chang LY, Luo J, Liu GJ. Clinical significance of tumor necrosis factor-alpha inhibitors in the treatment of sciatica: a systematic review and meta-analysis. PLoS One 2014;9:e103147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 2002;82:981–1011. [DOI] [PubMed] [Google Scholar]
- [164].Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine (Phila Pa 1976) 1993;18:1433–8. [PubMed] [Google Scholar]
- [165].Wiberg A, Ng M, Schmid AB, Smillie RW, Baskozos G, Holmes MV, Kunnapuu K, Magi R, Bennett DL, Furniss D. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat Commun 2019;10:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates pain: physiological and pathological consequences. Brain Behav Immun 2005;19:104–11. [DOI] [PubMed] [Google Scholar]
- [167].Wilder-Smith EP, Fook-Chong S, Chew SE, Chow A, Guo Y. Vasomotor dysfunction in carpal tunnel syndrome. Muscle Nerve 2003;28:582–6. [DOI] [PubMed] [Google Scholar]
- [168].Wilson CA, Roffey DM, Chow D, Alkherayf F, Wai EK. A systematic review of preoperative predictors for postoperative clinical outcomes following lumbar discectomy. Spine J 2016;16:1413–22. [DOI] [PubMed] [Google Scholar]
- [169].Yoon JS, Walker FO, Cartwright MS. Ulnar neuropathy with normal electrodiagnosis and abnormal nerve ultrasound. Arch Phys Med Rehabil 2010;91:318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Yoshii Y, Nishiura Y, Terui N, Hara Y, Saijilafu ON. The effects of repetitive compression on nerve conduction and blood flow in the rabbit sciatic nerve. J Hand Surg Eur 2010;35:269–78. [DOI] [PubMed] [Google Scholar]
- [171].Zanette G, Marani S, Tamburin S. Extra-median spread of sensory symptoms in carpal tunnel syndrome suggests the presence of pain-related mechanisms. PAIN 2006;122:264–70. [DOI] [PubMed] [Google Scholar]
- [172].Zanette G, Cacciatori C, Tamburin S. Central sensitization in carpal tunnel syndrome with extraterritorial spread of sensory symptoms. PAIN 2010;148:227–36. [DOI] [PubMed] [Google Scholar]
- [173].Zhu GC, Bottger K, Slater H, Cook C, Farrell SF, Hailey L, Tampin B, Schmid AB. Concurrent validity of a low-cost and time-efficient clinical sensory test battery to evaluate somatosensory dysfunction. Eur J Pain 2019;23:1826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zyluk A, Kosowiec L. Regional sympathetic disturbances in carpal tunnel syndrome—a review [in Polish]. Chir Narzadow Ruchu Ortop Pol 2008;73:30–6. [PubMed] [Google Scholar]