Abstract
Objectives
Antimicrobial resistance (AMR) in Neisseria gonorrhoeae, compromising gonorrhoea treatment, is a threat to reproductive health globally. South-East and East Asia have been major sources of emergence and subsequent international spread of AMR gonococcal strains during recent decades. We investigated gonococcal isolates from 2011 and 2015–16 in Vietnam using AMR testing, WGS and detection of AMR determinants.
Methods
Two hundred and twenty-nine gonococcal isolates cultured in 2015–16 (n = 121) and 2011 (n = 108) in Vietnam were examined. AMR testing was performed using Etest and WGS with Illumina MiSeq.
Results
Resistance among the 2015–16 isolates was as follows: ciprofloxacin, 100%; tetracycline, 79%; benzylpenicillin, 50%; cefixime, 15%; ceftriaxone, 1%; spectinomycin, 0%; and 5% were non-WT to azithromycin. Eighteen (15%) isolates were MDR. The MIC range for gentamicin was 2–8 mg/L. Among the 2015–16 isolates, 27% (n = 33) contained a mosaic penA allele, while no isolates had a mosaic penA allele in 2011. Phylogenomic analysis revealed introduction after 2011 of two mosaic penA-containing clones (penA-10.001 and penA-34.001), which were related to cefixime-resistant strains spreading in Japan and Europe, and a minor clade (eight isolates) relatively similar to the XDR strain WHO Q.
Conclusions
From 2011 to 2015–16, resistance in gonococci from Vietnam increased to all currently and previously used antimicrobials except ceftriaxone, spectinomycin and tetracycline. Two mosaic penA-containing clones were introduced after 2011, explaining the increased cefixime resistance. Significantly increased AMR surveillance, antimicrobial stewardship and use of WGS for molecular epidemiology and AMR prediction for gonococcal isolates in Vietnam and other Asian countries are crucial.
Introduction
Neisseria gonorrhoeae has developed antimicrobial resistance (AMR) to all drugs used in the treatment of gonorrhoea using mainly all known AMR mechanisms.1–3 The extended-spectrum cephalosporin (ESC) ceftriaxone is the last option for first-line empirical monotherapy; however, gonococcal resistance to ceftriaxone and occasional treatment failures with ceftriaxone have been verified.3 Ceftriaxone (250–500 mg) plus azithromycin (1–2 g orally) is the empirical first-line treatment in most high-income countries, but some countries recommend high-dose (1 g) ceftriaxone monotherapy, i.e. when chlamydia has been excluded.4–9 Worryingly, international spread of a ceftriaxone-resistant strain (FC428), initially identified in Japan,10 has been verified since 2015.11–18 The first gonorrhoea treatment failure with ceftriaxone plus azithromycin was reported in 2016,19 and in 2018 the first gonococcal strain with ceftriaxone resistance combined with high-level azithromycin resistance was identified in the UK and Australia (WHO Q).20–22 This development emphasizes the urgent need for new antimicrobials for gonorrhoea treatment23 and increased AMR surveillance globally.2,3
During the past several decades, South-East and East Asia have had high gonorrhoea prevalences and been major sources of emergence and subsequent international spread of gonococcal AMR.3,24–26 Most of the ceftriaxone-resistant cases during recent years have been associated with travel to South-East or East Asia.3,10–22 In Vietnam, quality-assured surveillance of gonococcal AMR has been very limited, and non-prescribed antimicrobials are widely available over the counter.27 The recommended treatment for uncomplicated gonorrhoea in Vietnam is 400 mg of cefixime, 250 mg of ceftriaxone, 2 g of spectinomycin or 1 g of cefotaxime, and all these are given together with 200 mg of doxycycline daily for 7 days.28 Worryingly, one recent study reported exceedingly high rates of gonococcal AMR, including 5% ceftriaxone resistance, in 2011 in Hanoi, Vietnam.27
WGS, due to its high resolution, is ideal for enhanced understanding of the gonococcal population, for molecular epidemiology of AMR gonococcal strains and to detect AMR determinants and predict AMR in gonococci nationally and internationally.29–35 However, WGS has been conducted on few gonococcal isolates from Asia, and no WGS of isolates from Vietnam has been published.
Our aims were to investigate: (i) resistance to previously and currently used gonorrhoea therapeutic antimicrobials in gonococcal isolates cultured in Vietnam in 2015–16 compared with 2011;27 and (ii) phylogenomics and AMR determinants among all these isolates using WGS.
Materials and methods
N. gonorrhoeae isolates
Two-hundred and twenty-nine clinical gonococcal isolates from Hanoi, Vietnam were examined. Of these, 121 isolates were cultured in 2015–16 from urethral swabs from 117 males and cervical swabs from four females. The mean (median) age was 30 (29) years for males (range: 16–61 years) and 28 (26) years for females (range: 23–34 years). All patients were diagnosed and treated at the National Hospital of Dermatology and Venereology, Hanoi, Vietnam. Additionally, 108 isolates cultured in the same setting in 2011 and previously published with regard to AMR27 were included in the WGS analysis. All isolates from 2011 and 2015–16 were cultured and preserved as part of the routine diagnostics in Vietnam. No patient identification data, except age and gender, were available; accordingly, ethical approval was not required. Prior to AMR testing and WGS, all isolates were cultured on Difco GC Medium Base agar (BD, Diagnostics, Sparks, MD, USA) supplemented with 1% haemoglobin (BD), 10% horse serum and 1% IsoVitalex (BD).
The 2016 WHO F gonococcal reference strain36 was used as reference for mapping and was excluded prior to building the phylogenomic trees. For comparison, the internationally spreading ceftriaxone-resistant strain FC42810 and WHO Q with ceftriaxone resistance and high-level azithromycin resistance, identified in 2018 and associated with travel to South-East Asia,20–22 were included in the WGS analysis. All Vietnamese isolates were compared with previously published cefixime-resistant isolates from Japan37 (n = 69; 2015) and 11 European countries32 (n = 42; 2013). The 2016 WHO gonococcal reference strains36 were used for phenotypic and genetic quality controls.
AMR testing
AMR testing for ceftriaxone, cefixime, azithromycin, spectinomycin, ciprofloxacin, benzylpenicillin, tetracycline and gentamicin was performed using the Etest (bioMérieux, Marcy-l’Étoile, France). MICs (mg/L) were interpreted according to the EUCAST clinical breakpoints,38 where available. For azithromycin, the EUCAST azithromycin epidemiological cut-off (ECOFF) of 1 mg/L (www.eucast.org/clinical_breakpoints) was used to identify non-WT isolates for azithromycin. Additionally, an ESC MIC of 0.125 mg/L was considered as decreased ESC susceptibility because isolates with this MIC have been associated with ESC treatment failures.3,25
WGS
DNA was extracted from bacterial suspensions using a customized QIAsymphony (QIAGEN GmbH, Hilden, Germany) DNA extraction protocol. Quality controls of the extracted DNA and the Nextera XT libraries (Illumina, Inc., San Diego, CA, USA) were performed using Qubit (Thermo Fisher Scientific, Waltham, MA, USA) and Tapestation (Agilent Technologies, Santa Clara, CA, USA).
Sequencing libraries were prepared using the Nextera XT DNA library preparation kit (Illumina, Inc.) and WGS was performed using Illumina MiSeq (Illumina, Inc.), as previously described.31 Illumina reads were quality controlled, Illumina adaptors removed, and reads were trimmed according to Phred quality score Q30, mapped to different references for additional contamination control and assembled; and AMR determinants and genes included in the MLST and N. gonorrhoeae multiantigen sequence typing (NG-MAST) schemes were extracted using a customized CLC Genomics Workbench (v9.5.3) (QIAGEN GmbH) workflow (Figure S1, available as Supplementary data at JAC Online). The frequency of 23S rRNA allele mutations was identified using mapping and quality-based variant detection (Neighborhood Quality Standard algorithm).36 Reads from all isolates were mapped to the genome sequence of the WHO F gonococcal reference strain36 using Burrows–Wheeler Aligner (BWA) v0.7.17,39 SNPs were called and recombinant regions were removed using Gubbins v1.4.10.40 Subsequently, a maximum-likelihood phylogenetic tree based on inherited SNPs was obtained using RAxML v8.2.8.41 All Vietnamese isolates were compared with cefixime-resistant isolates from Japan37 and Europe32 in a phylogenetic tree created as described above without removing the recombinant regions and visualized in Microreact.42
All WGS sequence reads of the Vietnamese isolates are available from the ENA (PRJEB34425).
Results
AMR
In vitro resistance among the 2015–16 isolates (n = 121) was as follows: ciprofloxacin, 100% (n = 121); tetracycline, 79% (n = 95); benzylpenicillin, 50% (n = 60); cefixime, 15% (n = 18); ceftriaxone, 1% (n = 1); spectinomycin, 0% (n = 0); and 5% (n = 6) were non-WT to azithromycin (Table 1). Fifteen percent (n = 18) of the isolates were MDR.43 For gentamicin, the MIC range (2–8 mg/L), MIC50 (4 mg/L) and MIC90 (8 mg/L) showed high in vitro susceptibility. The AMR results for the isolates from 2011 have been previously published.27 Briefly, from 2011 to 2015–16, resistance to all examined antimicrobials except ceftriaxone, spectinomycin and tetracycline increased (Table 1). One (0.8%) isolate in 2015–16 was resistant to ceftriaxone compared with five (4.6%) isolates in 2011.27 Furthermore, 15 (12.4%) of the 2015–16 isolates had a decreased susceptibility to ceftriaxone compared with 25 (23.1%) isolates in 2011. In contrast, 18 (14.9%) isolates from 2015–16 were resistant to cefixime compared with only 1 isolate (0.9%) from 2011,27 which was the only XDR43 isolate in this study. Additionally, 12.4% of the 2015–16 isolates had a decreased susceptibility to cefixime compared with only 7.4% of the isolates from 2011,27 and the MIC90 of cefixime had increased from 0.064 mg/L in 2011 to 0.25 mg/L in 2015–16.
Table 1.
Antimicrobial susceptibility of N. gonorrhoeae isolates cultured in Vietnam in 2011 (n = 108)27 and 2015–16 (n = 121)
| Antimicrobial (breakpoints, mg/L) | MIC range 2011/2015–16 | MIC50 2011/2015–16 | MIC90 2011/2015–16 | S/I (D)/R % 2011 | S/I (D)/R % 2015–16 |
|---|---|---|---|---|---|
| Cefixime (S <0.125, R >0.125) | <0.016 to 0.25/<0.016 to 0.5 | 0.032/0.064 | 0.125/0.25 | 92/7/1 | 73/12/15 |
| Ceftriaxone (S <0.125, R >0.125) | <0.002 to 0.25/0.004 to 0.25 | 0.064/0.064 | 0.125/0.064 | 72/23/5 | 87/12/1 |
| Azithromycin (non-WT >1) | 0.032 to 4/0.032 to 2 | 0.25/0.5 | 1/1 | 97/NA/3 | 95/NA/5 |
| Spectinomycin (S ≤64, R >64) | 4 to 16/8 to 32 | 16/16 | 16/16 | 100/NA/0 | 100/NA/0 |
| Ciprofloxacin (S ≤0.032, R >0.064) | 0.008 to >32/0.5 to >32 | >32/24 | >32/>32 | 2/0/98 | 0/0/100 |
| Tetracycline (S ≤0.5, R >1) | 0.25 to >256/0.75 to >256 | 32/4 | 128/256 | 6/12/82 | 0/21/79 |
| Benzylpenicillin (S ≤0.064, R >1) | 0.064 to >32/0.25 to >32 | 1/1 | >32/>32 | 2/51/47 | 0/50/50 |
| Gentamicin (NA) | 0.032 to 8/2 to 8 | 4/4 | 4/8 | NA | NA |
S, susceptible; I (D), intermediate (decreased); R, resistant; NA, not applicable.
Molecular epidemiology, AMR determinants and phylogenomics
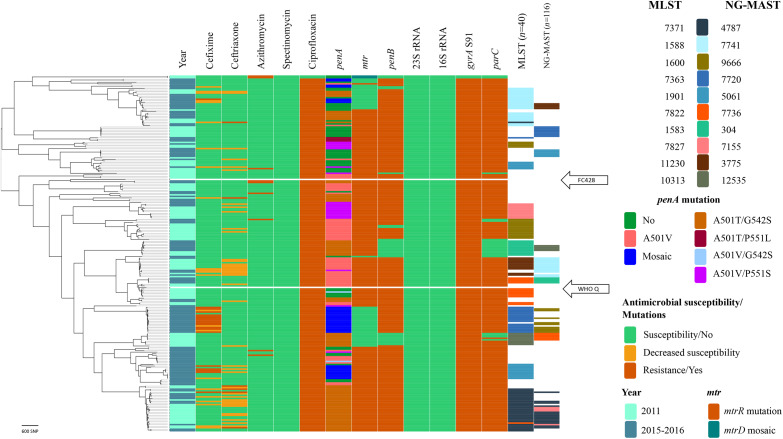
The genomic heterogeneity among all isolates (n = 229) was large (Figure 1). One hundred and nineteen NG-MAST STs were identified; 52 new STs and 64 represented by single isolates. The most common NG-MAST STs were ST4787 (n = 14), ST7741 (n = 12), ST9666 (n = 9), ST7720 (n = 7) and ST5061 (n = 5). Forty MLST STs, including two new STs and 12 STs represented by single isolates, were found. The most common MLST STs were ST7371 (n = 28), ST1588 (n = 22), ST1600 (17), ST7363 (n = 17) and ST1901 (n = 15).
Figure 1.
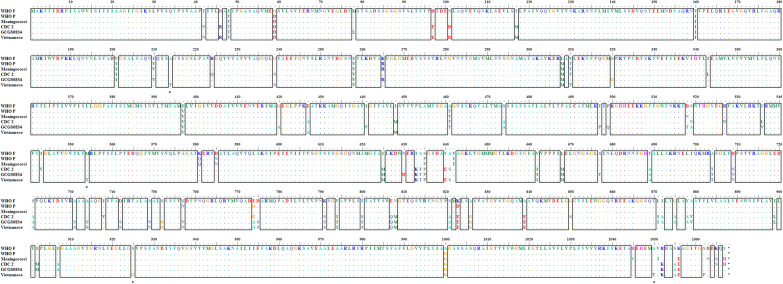
Amino acid sequences of MtrD in WHO F (azithromycin susceptible)36 and WHO P (azithromycin non-WT)36 and a meningococcal MtrD sequence (included as reference). GCGS083445 and CDC 246 were included as the two previously described mosaic MtrD sequences most similar to the Vietnamese MtrD sequence, which had only four unique amino acid alterations (asterisks). Conserved regions are boxed. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A mosaic penA allele or PBP2 A501 amino acid substitutions, which decrease the susceptibility to ESCs,1,32–35 were found in 14.4% (n = 33) and 65.1% (n = 149) of all isolates, respectively. All isolates with a mosaic penA allele [penA-10.001 (n = 20), penA-34.001 (n = 11) and penA-92.001 (n = 2)]44 were isolated in 2015–16. The majority (75.8%) of these isolates clustered in the phylogenomic tree and belonged to MLST ST7363 (n = 16) and ST1901 (n = 9). The amino acid substitution in PBP2 A501 (n = 149) was A501T (n = 73) or A501V (n = 76). A501T was always in combination with PBP2 G542S (n = 70) or PBP2 P551L (n = 3), while isolates with A501V were less frequent in combination with G542S (n = 2) or P551S (n = 21). AMR mutations in mtrR (increasing antimicrobial efflux through the MtrCDE efflux pump), penB (decreasing antimicrobial intake through PorB1b) and ponA [encoding PBP1 (L421P), associated with penicillin resistance]1,29,31–35 were found in 79.0% (n = 181; 87.0% in 2011 and 71.9% in 2015–16), 88.2% (n = 202; 84.3% in 2011 and 91.7% in 2015–16) and 94.8% (n = 217; 95.4% in 2011 and 94.2% in 2015–16), respectively, of all isolates. Furthermore, two isolates from 2011 had an identical mosaic mtrD sequence causing increased antimicrobial MtrCDE efflux and non-WT to azithromycin (MIC = 2 mg/L and MIC = 4 mg/L), and the MtrD sequence differed by only four amino acid alterations compared with some previously described mosaic MtrD sequences45,46 (Figure 1). No isolates contained any target determinants for azithromycin resistance in 23S rRNA or for spectinomycin resistance in 16S rRNA or the rpsE gene. Fluoroquinolone resistance mutations in gyrA (GyrA S91) were found in 99.1% (n = 227; 98.1% in 2011 and 100.0% in 2015–16) and in parC in 90.8% (n = 208; 89.8% in 2011 and 91.7% in 2015–16) of isolates. The rpsJ V57M mutation, involved in tetracycline resistance, was found in 98.7% (n = 226) of isolates. Finally, tetM-carrying plasmids and blaTEM-carrying β-lactamase plasmids were found in 42.4% (n = 97; 50.0% in 2011 and 35.5% in 2015–16) and 28.4% (n = 65; 25.9% in 2011 and 30.6% in 2015–16) isolates, respectively.
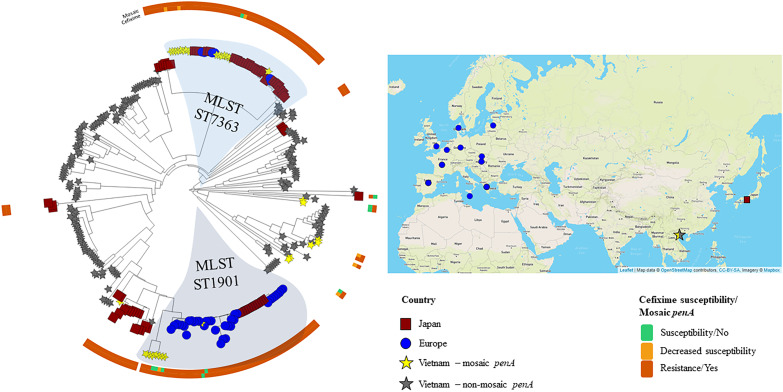
In Figure 2, the phylogenomic structure and phenotypic AMR for all isolates (n = 229; 108 in 2011 and 121 in 2015–16), including their AMR determinants, for the therapeutically most relevant antimicrobials are illustrated. Two new clades in 2015–16 [42 (18.3%) of the 229 isolates] included the majority (94.4%) of the cefixime-resistant isolates and most (78.8%) of the isolates with a mosaic penA allele. No isolate genomically similar to the FC428 strain10 was found. However, a minor clade of isolates (n = 8) from 2011 had relatively close relationships to WHO Q (Figure 1),20–22 differing by 137–195 SNPs. The Vietnamese isolates carrying mosaic penA, mostly MLST ST7363 and ST1901, were related to cefixime-resistant isolates spreading in Japan in 2015 (mostly with mosaic penA-10)37 and in 11 European countries in 2013 (mostly NG-MAST genogroup 1407 with mosaic penA-34)32 (Figure 3).
Figure 2.
Phylogenomic tree with susceptibility to the most therapeutically relevant antimicrobials, AMR determinants, the most common MLST STs and N. gonorrhoeae multiantigen sequence typing (NG-MAST) STs for N. gonorrhoeae isolates from Vietnam in 2011 (n = 108) and 2015–16 (n = 121). The WHO Q20–22 reference strain and FC42810 are included for comparison and are marked with white bars. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Circular phylogenomic tree visualized using Microreact42 of all isolates from Vietnam (n = 229) compared with cefixime-resistant isolates (MIC >0.125 mg/L) from Japan (n = 69)37 and 11 European countries (n = 42).32 Coloured bars represent cefixime susceptibility and the presence of the mosaic penA allele. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Concordance of resistance phenotype and genotype for the therapeutically most relevant antimicrobials
All ciprofloxacin-resistant isolates (n = 227; 99.1%) had the main fluoroquinolone resistance determinant, i.e. the gyrA S91F mutation, and all ciprofloxacin-susceptible isolates (n = 2; 0.9%) were lacking mutations in GyrA amino acid positions S91 and D95. Eighteen (94.7%) of the cefixime-resistant isolates (n = 19; 8.3%) had a mosaic penA allele [penA-10.001 (n = 14) and penA-34.001 (n = 4)] and the remaining cefixime-resistant isolate had a non-mosaic penA-18.001 encoding a PBP2 with A501T plus G542S mutations. Nevertheless, eight (3.5%) additional isolates had a mosaic penA allele [penA-34.001 (n = 5) and penA-10.001 (n = 3)], but were susceptible to cefixime (MICs = 0.032–0.064 mg/L). All six ceftriaxone-resistant isolates contained penA-18.001, encoding PBP2 with A501T and G542S amino acid alterations, mtrR and penB AMR determinants, although belonging to different clades in the tree. However, 27 other isolates with PBP2 A501T plus G542S amino acid alterations, mtrR and penB AMR determinants remained ceftriaxone susceptible. Finally, none of the nine (3.9%) azithromycin-non-WT isolates had any macrolide resistance-associated mutation in the 23S rRNA gene (A2059G or C2611T). However, two closely related isolates from 2011 had identical mosaic mtrD genes (Figure 1) and the remaining seven azithromycin-non-WT isolates had the characteristic A deletion in the mtrR promoter sequence (Figure 2), both causing an overexpression of the MtrCDE efflux pump.
Discussion
Our findings show that cefixime, azithromycin, benzylpenicillin, tetracycline and ciprofloxacin should not be used for empirical first-line monotherapy in Vietnam as in most other countries globally.1–3 Nevertheless, in Vietnam and many additional countries, particularly (but not exclusively) in South-East and East Asia, enhanced surveillance of gonococcal AMR, antimicrobial stewardship and molecular epidemiology are crucial.
The decrease in ceftriaxone-resistant gonococcal isolates from 2011 (5%) to 2015–16 (1%) in Vietnam is promising. However, the level of cefixime-resistant isolates increased from 1% to 15%, which was mainly due to the introduction and clonal expansion of two cefixime-resistant gonococcal mosaic penA-carrying clones that were related to cefixime-resistant strains spreading in Japan37 and Europe32 for many years. Cefixime has been frequently used for gonorrhoea treatment in Vietnam,27 which has probably facilitated the dissemination of these cefixime-resistant strains. Worryingly, these mosaic penA alleles only need one SNP to also develop high-level resistance to ceftriaxone. Of the 2015–16 isolates (n = 121), 18 (14.9%) were resistant to cefixime and all contained a mosaic penA allele, which affects the MIC of cefixime more than the MIC of ceftriaxone. In contrast, all six ceftriaxone-resistant isolates (4.6% in 2011 and 0.8% in 2015–16) had a non-mosaic penA allele encoding a PBP2 with A501T and G542S amino acid alterations, which together with overexpression of the MtrCDE efflux pump and decreased ESC intake through PorB significantly increases the MIC of ceftriaxone in particular. Furthermore, despite low resistance (1%) to ceftriaxone in 2015–16, 12.4% (n = 15) of the 2015–16 isolates had a decreased susceptibility to ceftriaxone and all these isolates contained PBP2 with an A501 (80.0%) alteration or a mosaic penA allele (20%). All isolates were susceptible to spectinomycin, and it would be valuable to have spectinomycin widely available internationally again.
WGS is very valuable for molecular epidemiology (emergence, transmission and evolution of strains and their AMR), nationally and internationally, and for detection of AMR determinants to predict AMR.29–37 For N. gonorrhoeae, prediction of ciprofloxacin resistance can be effectively conducted by detection of mutations in GyrA codon S91. Furthermore, it can be important to survey for specific penA alleles, ideally in combination with ceftriaxone and cefixime susceptibility data. However, despite the fact that most gonococcal isolates with cefixime resistance harbour a mosaic penA allele, many gonococcal isolates with mosaic penA alleles do not show in vitro and/or clinical resistance to cefixime.1,32–35 In the present study, no mutations in the azithromycin resistance determinant 23S rRNA were found, but 3% and 5% of the isolates from 2011 and 2015–16, respectively, were non-WT to azithromycin. This resistance might be due to overexpression of the MtrCDE efflux pump in particular, as shown, but also to additional unknown macrolide resistance mechanisms. Using a more comprehensive geographical, temporal and genomically diverse collection of gonococcal isolates could improve surveillance strategies and AMR prediction based on WGS. It was recently reported that real-time WGS using the small hand-held MinION can rapidly and relatively accurately sequence genomes and predict resistance/non-WT to ciprofloxacin and azithromycin, and decreased susceptibility to ESCs in gonococcal isolates.47 Accordingly, rapid and reliable prediction of gonococcal AMR using WGS might be available in routine diagnostic laboratories in the near future.
In Vietnam, the recommended treatment for uncomplicated gonorrhoea is 400 mg of cefixime, 250 mg of ceftriaxone, 2 g of spectinomycin or 1 g of cefotaxime plus, and all these are given together with 200 mg of doxycycline daily for 7 days.28 Nevertheless, the compliance with these treatment recommendations in the public and, in particular, the private sector is unknown and other antimicrobials and doses are also used for monotherapy of some patients. First-line empirical dual therapy with at a minimum 500 mg of ceftriaxone combined with azithromycin should be considered in Vietnam and many additional countries in South-East and East Asia.
The main limitations of this study included the relatively low number of gonococcal isolates which were only from the capital city Hanoi, the lack of epidemiological data concerning the patients (e.g. identification of risk groups such as MSM and place of infection) and the low number of isolates from females.
In conclusion, from 2011 to 2015–16 an increased resistance to all currently and previously used antimicrobials except ceftriaxone, spectinomycin and tetracycline was identified among the heterogeneous N. gonorrhoeae isolates obtained in Vietnam. After 2011, cefixime resistance has rapidly increased in Vietnam, which was shown to be due to the introduction and subsequent clonal expansion of two mosaic penA-containing strains, which were genomically similar to cefixime-resistant gonococcal strains spreading in Europe32 and Japan37 for many years. Furthermore, a minor clade (eight isolates) relatively similar to the XDR strain WHO Q was identified; however, these isolates were not resistant to ceftriaxone due to the lack of mosaic penA-60. Isolates similar to WHO Q have also been identified in, for example, China and Japan,21 and, for improved understanding of emergence and spread of ceftriaxone resistance, it is important to identify the origin of such XDR strains. Continued and significantly expanded surveillance of gonococcal AMR and molecular epidemiology, using WGS, and improved antimicrobial stewardship in Vietnam and other Asian countries are crucial.
Supplementary Material
Funding
The present work was supported by the Örebro County Council Research Committee, Örebro, Sweden, and the Foundation for Medical Research at Örebro University Hospital, Örebro, Sweden.
Transparency declarations
None to declare.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online.
References
- 1. Unemo M, Shafer WM.. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27: 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wi T, Lahra MM, Ndowa F. et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14: e1002344.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unemo M, Lahra MM, Cole M. et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; doi:10.1071/SH19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. WHO Guidelines for the Treatment of Neisseria gonorrhoeae 2016. http://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. [PubMed]
- 5. Bignell C, Unemo M; European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2013; 24: 85–92. [DOI] [PubMed] [Google Scholar]
- 6. Workowski KA, Bolan GA.. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64: 1–137. [PMC free article] [PubMed] [Google Scholar]
- 7. Romanowski B, Robinson J, Wong T, Gonococcal infections chapter In: Canadian Guidelines on Sexually Transmitted Infections. Public Health Agency of Canada, 2013. www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf. [Google Scholar]
- 8.Australasian Sexual Health Alliance (ASHA). Gonorrhoea. In: Australian STI Management Guidelines for Use in Primary Care 2018. www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management.
- 9. Fifer H, Saunders J, Soni S. et al. British Association for Sexual Health and HIV National Guideline for the Management of Infection with Neisseria gonorrhoeae (2019). https://www.bashhguidelines.org/media/1208/gc-2019.pdf.
- 10. Nakayama S-I, Shimuta K, Furubayashi K-I. et al. New ceftriaxone and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 2016; 60: 4339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terkelsen D, Tolstrup J, Johnsen CH. et al. Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill 2017; 22: pii=17-00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lahra MM, Martin I, Demczuk W. et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 2018; 24: doi:10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poncin T, Fouere S, Braille A. et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill 2018; 23: pii=1800264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golparian D, Rose L, Lynam A. et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill 2018; 23: pii=1800617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee K, Nakayama SI, Osawa K. et al. Clonal expansion and spread of the ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, identified in Japan in 2015, and closely related isolates. J Antimicrob Chemother 2019; 74: 1812–19. [DOI] [PubMed] [Google Scholar]
- 16. Lefebvre B, Martin I, Demczuk W. et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis 2018; 24: 381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen SC, Han Y, Yuan LF. et al. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis 2019; 25: 1427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko KKK, Chio MTW, Goh SS. et al. First case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore. Antimicrob Agents Chemother 2019; 63: e02624-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fifer H, Natarajan U, Jones L. et al. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016; 374: 2504–6. [DOI] [PubMed] [Google Scholar]
- 20. Jennison AV, Whiley D, Lahra MM. et al. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill 2019; 24: pii=1900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eyre DW, Sanderson ND, Lord E. et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23: pii=1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whiley DM, Jennison A, Pearson J. et al. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18: 717–18. [DOI] [PubMed] [Google Scholar]
- 23. Alirol E, Wi TE, Bala M. et al. Multidrug-resistant gonorrhea: a research and development roadmap to discover new medicines. PLoS Med 2017; 14: e1002366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tapsall JW, Ndowa F, Lewis DA. et al. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther 2009; 7: 821–34. [DOI] [PubMed] [Google Scholar]
- 25. Unemo M, Nicholas RA.. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012; 7: 1401–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rowley J, Vander Hoorn S, Korenromp E. et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97: 548–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen B, Pham TL, Golparian D. et al. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis 2013; 13: 40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health, Vietnam. National Clinical Guidelines on Reproductive Health Care Services. 2016. http://soytecaobang.gov.vn/SiteFolders/syt/uploads/tai-lieu-chuyen-mon/2017_05/qd_4128_2016_byt_huong_dan_quoc_gia_ve_dich_vu_skss_.pdf.
- 29. Demczuk W, Martin I, Peterson S. et al. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 2016; 54: 1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Silva D, Peters J, Cole K. et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis 2016; 16: 1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobsson S, Golparian D, Cole M. et al. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother 2016; 71: 3109–16. [DOI] [PubMed] [Google Scholar]
- 32. Harris SR, Cole MJ, Spiteri G. et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis 2018; 18: 758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grad YH, Kirkcaldy RD, Trees D. et al. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 2014; 14: 220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grad YH, Harris SR, Kirkcaldy RD. et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 2016; 214: 1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eyre DW, De Silva D, Cole K. et al. WGS to predict antibiotic MICs for Neisseria gonorrhoeae. J Antimicrob Chemother 2017; 72: 1937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Unemo M, Golparian D, Sanchez-Buso L. et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 2016; 71: 3096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yahara K, Nakayama SI, Shimuta K. et al. Genomic surveillance of Neisseria gonorrhoeae to investigate the distribution and evolution of antimicrobial-resistance determinants and lineages. Microb Genom 2018; 4: doi:10.1099/mgen.0.000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.1 2019; http://www.eucast.org.
- 39. Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013; 1303.3997v1. https://arxiv.org/abs/1303.3997. [Google Scholar]
- 40. Croucher NJ, Page AJ, Connor TR. et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 2015; 43: e15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30: 1312–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Argimón S, Abudahab K, Goater RJE. et al. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb Genom 2016; 2: e000093.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gianecini RA, Golparian D, Zittermann S. et al. Genome-based epidemiology and antimicrobial resistance determinants of Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina in 2011–16. J Antimicrob Chemother 2019; 74: 1551–9. [DOI] [PubMed] [Google Scholar]
- 44. Demczuk W, Sidhu S, Unemo M. et al. Neisseria gonorrhoeae Sequence Typing for Antimicrobial Resistance (NG-STAR), a novel antimicrobial resistance multilocus typing scheme for tracking the global dissemination of N. gonorrhoeae strains. J Clin Microbiol 2017; 55: 1454–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wadsworth CB, Arnold BJ, Sater MRA. et al. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 2018; 9: e01419-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rouquette-Loughlin CE, Reimche JL, Balthazar JT. et al. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 2018; 9: e02281-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Golparian D, Donà V, Sánchez-Busó L. et al. Antimicrobial resistance prediction and phylogenetic analysis of Neisseria gonorrhoeae isolates using the Oxford Nanopore MinION sequencer. Sci Rep 2018; 8: 17596.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.