Abstract
Objective
Idiopathic inflammatory myositis-associated interstitial lung disease (IIM-ILD) significantly increases morbidity and mortality. Lung ultrasound B-lines and Krebs von den Lungen-6 (KL-6) are identified as new sonographic and serum markers of ILD, respectively. The aim of our work was to assess the role of B-lines and KL-6 as markers of the severity of IIM-ILD. For this purpose, the correlation among B-lines score, serum KL-6 levels, high-resolution CT (HRCT) score, and pulmonary function tests were investigated in IIM-ILD patients.
Methods
Thirty-eight patients with IIM-ILD underwent chest HRCT scans, lung ultrasound and pulmonary function tests (independently performed within 1 week) examination. To assess severity and extent of ILD at HRCT, the Warrick score was used. The B-lines score denoting the extension of ILD was calculated by summing the number of B-lines on a total of 50 scanning sites. Serum KL-6 levels (U/ml) was measured by chemiluminescent enzyme immunoassay.
Results
A significant correlation was found between the B-lines score and serum KL-6 levels (r = 0.43, P < 0.01), and between the Warrick score and serum KL-6 levels (r = 0.45, P < 0.01). A positive correlation between B-lines score and the Warrick score (r = 0.87, P < 0.0001) was also confirmed. Both B-lines score and KL-6 levels inversely correlated to diffusion capacity for carbon monoxide (r = −0.77, P < 0.0001 and r = −0.42, P < 0.05, respectively) and total lung capacity (r = −0.73, P < 0.0001 and r = −0.36, P < 0.05, respectively). Moreover, B-lines correlated inversely with forced vital capacity (r = −0.73, P < 0.0001), forced expiratory volume in 1 s (r = −0.69, P < 0.0001).
Conclusion
B-lines score and serum KL-6 levels correlate with HRCT findings and pulmonary function tests, supporting their use as measures of IIM-ILD severity.
Keywords: B-lines, high-resolution CT, idiopathic inflammatory myositis-associated interstitial lung disease, KL-6, lung ultrasound, pulmonary function tests
Rheumatology key messages
B-lines score, serum KL-6 levels are correlated with HRCT and PFTs in IIM-ILD patients.
B-lines combined with KL-6 could be helpful in the assessment of IIM-ILD severity.
B-lines and KL-6 are promising tools to closely monitor the rapidly progressive IIM-ILD.
Introduction
Idiopathic inflammatory myositis (IIM) is a systematic, heterogeneous autoimmune disease including different subsets [1]. Usually, IIM is characterized by skeletal muscle inflammation and involvement of other organs such as skin, joints and lungs. Interstitial lung disease (ILD) is the most frequent pulmonary complication, particularly in the anti-synthetase syndrome and anti-melanoma differentiation-associated gene 5 (MDA-5) antibody positive clinically amyopathic dermatomyositis (CADM), significantly increased substantial morbidity and mortality [2, 3]. Although the precise pathophysiology of IIM-associated ILD (IIM-ILD) is not fully understood, early screening, prompt therapeutic interventions, and close follow-up are of paramount importance [4]. At present, chest high-resolution CT (HRCT) and pulmonary function tests (PFTs) are fundamental for ILD diagnosis and follow-up. However, the radiation load of HRCT may limit its clinical application to closely monitor ILD [5].
In the past decade, several data have identified that lung ultrasound (LUS) B-lines and Krebs von den Lungen-6 (KL-6) may be used as sonographic and serum markers, respectively, for connective tissue diseases associated ILD (CTD-ILD) [6–10]. These two radiation-free and non-invasive markers have shown a good sensitivity to detect early pulmonary changes in CTD-ILD [11, 12]. However, little is known regarding the use of these two markers to assess IIM-ILD.
The aim of this study was to evaluate the significance of B-lines and KL-6 for the evaluation of the severity of ILD in IIM patients.
Methods
Patients
Thirty-eight consecutive IIM-ILD patients (including PM, DM and CADM) were enrolled in the study from 2016 to 2019. PM and DM were classified according to Bohan and Peter’s criteria [13], while CADM diagnosis was based on Sontheimer’s criteria [14]. All patients underwent chest HRCT scans, LUS and PFTs (independently performed within 1 week) examination. The presence and pattern of ILD were defined by HRCT findings assessed by a radiologist [15]. The study was approved by the Shantou Central Hospital Ethics Committee. All investigations were conducted in compliance with the Declaration of Helsinki. All patients provided written informed consent. Patients with a history of asthma, chronic obstructive pulmonary disease, lung neoplasm, occupational lung disease, radiation lung disease, heart failure, renal failure, other rheumatic diseases and pregnancy were excluded from the study.
Measurement of KL-6
Serum KL-6 concentration (U/ml) was measured with chemiluminescent enzyme immunoassay method (LUMIPULSE G2100, Japan) in the study population.
Lung ultrasound
Commercially available ultrasound equipment with a 2.5–3.5 MHz cardiac sector transducer was used in this study (Siemens Medical Solutions, Erlangen, Germany). Two senior ultrasound physicians were blinded to any information about chest HRCT results and clinical data of the patients. Ultrasound images were obtained by recording the number of B-lines in a total of 50 scanning sites [16]. The sum of B-lines yielded a score reflecting ILD extent [17]. A B-line was defined as a discrete laser-like vertical hyperechoic reverberation artefact arising from the pleural line, extending to the bottom of the screen without fading, and moving synchronously with respiration [18].
Chest HRCT was performed on a dual-source 128-section multi-detector CT system (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany). The scan range was from the level of the pulmonary apex to the costophrenic angle, and all patient scans were acquired by holding their breath at the end of deep inspiration during the examination. Examination parameters were as follows: sequential mode, 1 mm collimation and 10 mm interval, 120 kV tube voltage and 110 reference mAS. Edge-enhancing B 70 f kernel was obtained by using filtered back projection for clinical reading with lung window. No intravenous contrast agent was employed. The duration of the CT acquisition was 35–45 s. The matrix was 512 × 512 and the effective dose was in the range of 3–5 mSv. The Warrick score was used to assess radiographic ILD severity and extent [19].
Pulmonary function tests
Standard spirometric measurements of total lung capacity, flow indices and diffusion capacity of carbon monoxide (DLco) were performed in the Lung Function Laboratory of the Pulmonary Department. Forced vital capacity and forced expiratory volume in 1 s were measured with a computerized lung analyser (Masterscreen; Viasys Jaeger, Höechberg, Germany). The DLco was determined as the single-breath diffusing lung capacity and corrected for haemoglobin and carbon monoxide levels. The results were expressed as percentages of predicted values.
Statistical analysis
Significant differences of continuous variables were calculated by independent sample t test, while categorical variables were calculated by χ2 using SPSS version 16 (SPSS, Chicago, IL, USA). Correlation among total B-lines number, serum KL-6 level, Warrick score and PFT variables was assessed with Pearson correlation using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). A P-value of <0.05 was regarded as significant.
Results
A total of 38 IIM-ILD patients (29 female and 9 male, mean age 54.6 (9.9) years) were enrolled in the study. The patients’ demographic features are presented in Table 1. HRCT scans showed three ILD patterns, encompassing non-specific interstitial pneumonia, organizing pneumonia and usual interstitial pneumonia. Twenty-eight out of 38 cases (73.7%) showed a radiological non-specific interstitial pneumonia pattern. Four cases (10.5%) showed non-specific interstitial pneumonia overlapped with organizing pneumonia. Three cases (7.9%) revealed predominant organizing pneumonia. Usual interstitial pneumonia was diagnosed in three patients (7.9%). The X-ray findings are summarized in Table 1. Feasibility of LUS was 100%. Due to the exacerbation of ILD, four patients did not accomplish the PFTs examination.
Table 1.
Patients’ clinical features
| IIM-ILD patients (n = 38) | |
|---|---|
| Age, mean (s.d.), years | 54.6 (9.9) |
| Sex (female/male), n | 29/9 |
| Duration of disease, mean (s.d.), years | 2.2 (2.0) |
| Diagnosis, n (%) | |
| PM | 18 (47.4) |
| DM | 18 (47.4) |
| CADM | 2 (5.2) |
| HRCT pattern, n (%) | |
| NSIP | 28 (73.7) |
| NSIP + OP | 4 (10.5) |
| OP | 3 (7.9) |
| UIP | 3 (7.9) |
| KL-6 serum level, U/ml | 650 (323–1304)a |
| B-lines total number, mean (s.d.) | 200.5 (126.9) |
| Warrick score, mean (s.d.) | 15.7 (7.5) |
| FEV1% predicted, mean (s.d.) | 72.9 (22.6) |
| FVC% predicted, mean (s.d.) | 71.2 (21.0) |
| DLco% predicted, mean (s.d.) | 50.5 (23.6) |
| TLC% predicted, mean (s.d.) | 73.6 (16.5) |
| Treatment, n (%) | |
| Glucocorticoid | 38 (100.0) |
| Immunosuppressant | 35 (92.1) |
| Anti-fibrotic agents | 12 (31.6) |
The median value (interquartile range).
CADM: clinically amyopathic dermatomyositis; DLco: diffusion capacity of carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; HRCT: high-resolution CT; IIM-ILD: idiopathic inflammatory myositis-associated interstitial lung disease; KL-6: Krebs von den Lungen-6; NSIP: non-specific interstitial pneumonia; OP: organizing pneumonia; TLC: total lung capacity; UIP: usual interstitial pneumonia.
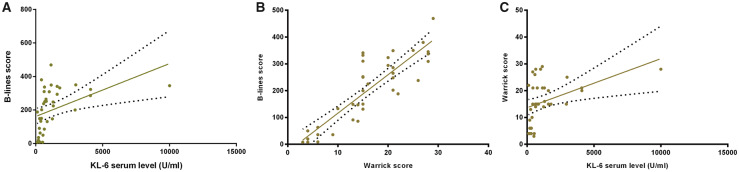
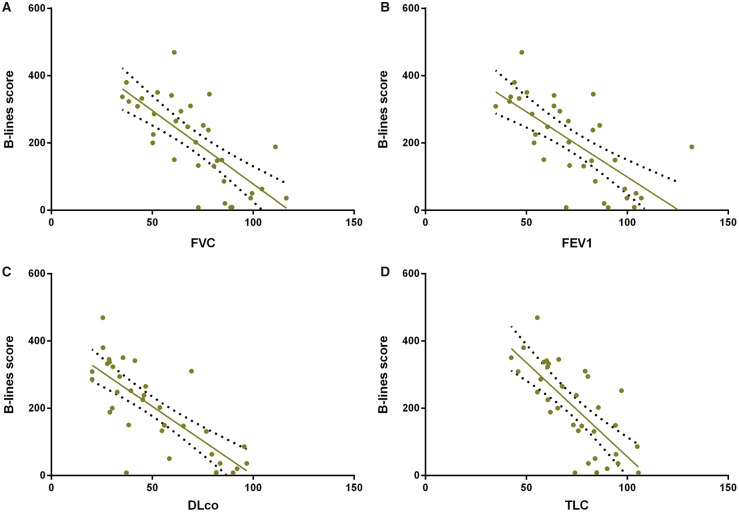
A significant correlation was found between the B-lines score and serum KL-6 levels (r = 0.43, P < 0.01), and between the Warrick score and serum KL-6 levels (r = 0.45, P < 0.01). A positive correlation between B-lines score and the Warrick score (r = 0.87, P < 0.0001) was also confirmed. Both B-lines score and KL-6 levels inversely correlated to diffusion capacity for carbon monoxide (r = −0.77, P < 0.0001 and r = −0.42, P < 0.05, respectively) and total lung capacity (r = −0.73, P < 0.0001 and r = −0.36, P < 0.05, respectively). Moreover, B-lines correlated inversely with forced vital capacity (r = −0.73, P < 0.0001), and forced expiratory volume in 1 s (r = −0.69, P < 0.0001, Figs 1 and 2 and Supplementary Fig. S1, available at Rheumatology online).
Fig. 1.
Correlation among the B-lines score, the serum level of KL-6 and Warrick score
(A) Correlation of B-lines score and the serum level of KL-6; (B) correlation of B-lines score and Warrick score; (C) correlation of the serum level of KL-6 and Warrick score. KL-6: Krebs von den Lungen-6.
Fig. 2.
Significant inverse correlation between B-lines score and pulmonary function tests
(A) Forced vital capacity (FVC); (B) forced expiratory volume in 1 s (FEV1); (C) diffusing capacity for carbon monoxide (DLco); (D) total lung capacity (TLC).
Discussion
Our findings show that LUS B-lines are a useful sonographic marker for detecting and scoring IIM-ILD. Moreover, B-lines were strongly associated with the HRCT severity score and PFTs parameters, and moderately correlated with the serum level of KL-6. Our results are in agreement with previous findings showing that in SSc B-lines significantly and directly correlate with Warrick scores (r = 0.72; P < 0.001), and inversely correlate with DLco (r = −0.6; P < 0.05) [17]. These data have also been confirmed by three other independent studies including a total of 137 SSc patients [20–22]. In RA-associated ILD, it has been demonstrated that the B-lines score is directly correlated with the Warrick score (r = 0.81) [23]. In the past decade, robust data have suggested B-lines as a new, sensitive and promising sonographic marker of CTD-ILD [6]. However, the majority of these previous studies were focused on SSc and RA. To the best of our knowledge, this is the first study evaluating LUS in IIM-ILD, though our data concern a small sample size. In comparison with other CTD-ILD, IIM-ILD has a more aggressive progression with a substantially poorer prognosis, but despite this evidence, no guidelines for the monitoring and treatment of IIM-ILD have been provided [24]. In fact, today the treatment is largely based on the individual physician’s experience and published case reports and series [3]. In rapidly progressing IIM-ILD cases, the combination therapy with high-dose corticosteroids and immunosuppressive agents should be considered as an early intervention. Therefore, a careful evaluation of CTD-ILD could be critical to improve the clinical outcome of these patients [8]. Today, HRCT is still a ‘gold standard’ to diagnose and follow the ILD morphological changes. However, the radiological burden, and the large, non-portable equipment limit HRCT clinical application to closely monitor the lung [25]. Furthermore, respiratory failure due to acute exacerbation of ILD may sometimes inhibit patients from properly performing PFTs. In this clinical context, LUS are a promising tool for an accurate assessment of the severity of rapidly progressive ILD. In fact, LUS are sensitive to parenchymal modifications, are obtained in real time, and are non-invasive, low-cost and radiation-free.
KL-6 is a mucin-like, high-molecular weight glycoprotein expressed on the surface membrane of alveolar epithelial cells II and bronchiolar epithelial cells, and increases following cellular injury and/or regeneration [26]. Its role in patients with IIM-ILD has been extensively studied and KL-6 is considered a useful biomarker of lung fibrosis and disease severity [27, 28]. Previous studies mainly investigated the correlation between serum KL-6 levels with HRCT findings and PFT variables. In IIM patients, KL-6 concentrations were found to be significantly higher in patients with ILD compared with those without ILD, and the KL-6 levels were correlated with the ILD course [29–31]. Furthermore, KL-6 was shown to be a predictive value for the development of ILD [32, 33]. To date, few studies have addressed the relationship between serum KL-6 levels and B-lines in IIM-ILD. Our results clearly show that serum KL-6 values directly correlate with B-lines score, in agreement with the data we have provided in a previous study encompassing 60 patients with CTD-ILD (r = 0.54, P < 0.0001) [34]. Moreover, we also confirmed the significant correlation between KL-6, the Warrick score and the DLco value. In our patients, eight cases had extremely high KL-6 levels (>1500 U/l, ranging from 1599 to 10 000 U/l). Among them, two cases were MDA-5 antibody positive, six were anti-aminoacyl-tRNA synthetase antibody positive. This sub-group also had significantly higher total B-lines number (308.9 (46.6) vs 171.6 (125.9), P < 0.05) and HRCT Warrick score (18.9 (3.4) vs 14.8 (8.0), P < 0.05). Additionally, three out of eight patients (one MDA-5 positive, two Jo-1 positive) initially presented with rapidly progressive ILD. Aggressive therapy including high-dose glucocorticoids, intravenous cyclophosphamide and ciclosporin A were given and were successful. While we do not have data to describe what constitutes high enough for extra vigilance, this can be the impetus for further examination of such outliers.
In a recently published IIM-ILD case, B-lines and KL-6 were used to detect and follow anti-MDA-5 positive CADM-associated rapid progressive ILD. In this case, the increase of B-lines numbers and the serum levels of KL-6 were consistent with HRCT changes and clinical features. The treatment option was decided according to the close follow-up performed by monitoring B-lines numbers and serum KL-6 level. The patient was successfully treated and chest ultrasound was employed for detecting and following the rapidly progressing ILD, in particular combining LUS and serum biomarkers [25].
Although the data is encouraging, we would not convey the message that the two biomarkers could replace HRCT at baseline. To date, HRCT is still the gold standard to diagnose ILD, as it could identify the definite radiographic pattern of ILD, comprehensively assess the extent and severity of ILD. B-lines and KL-6 have the advantage of being very sensitive, non-invasive and radiation-free. They are more appropriate to screen and follow up early ILD change, and closely monitor the rapidly progressing ILD dynamic change during the aggressive treatment.
The major limitation of this retrospective study is that the data are obtained from a single centre with a relatively small sample size. Therefore, a prospective, large sample and multicentre studies are warranted to confirm our results.
In conclusion, our preliminary data indicate that LUS B-lines and serum KL-6 could be useful markers for detecting and evaluating the severity of IIM-ILD. This finding suggests that a combined use of non-invasive, radiation-free imaging markers and serum biomarkers might be helpful in following IIM-ILD. This hypothesis will need to be tested in the future.
Supplementary Material
Acknowledgements
Y.-K.W., S.-Q.C., L.G., M.M.-C. and D.E.F. designed the study. S.-Q.C., X.-Z.X. and S.-J.H. performed lung ultrasound. G.-Z.D. performed chest HRCT. J.-Q.L. performed serum KL-6 level analyses. X.-F.H. performed pulmonary function test. Q.-S.L. and K.-D.Z. collected patient samples and clinical information. G.-H.Z. analysed and interpreted data. Y.-K.W., S.-Q.C. and L.G. wrote the manuscript. All authors have read and contributed to the final text and also approved the submitted version.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: the authors have declared no conflicts of interest.
References
- 1. Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;372:1734–47. [DOI] [PubMed] [Google Scholar]
- 2. Barba T, Fort R, Cottin V. et al. Treatment of idiopathic inflammatory myositis associated interstitial lung disease: a systematic review and meta-analysis. Autoimmun Rev 2019;18:113–22. [DOI] [PubMed] [Google Scholar]
- 3. Morisset J, Johnson C, Rich E, Collard HR, Lee JS.. Management of myositis-related interstitial lung disease. Chest 2016;150:1118–28. [DOI] [PubMed] [Google Scholar]
- 4. Doyle TJ, Dellaripa PF.. Lung manifestations in the rheumatic diseases. Chest 2017;152:1283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Picano E, Matucci-Cerinic M.. Unnecessary radiation exposure from medical imaging in the rheumatology patient. Rheumatology 2011;50:1537–9. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Gargani L, Barskova T, Furst DE, Cerinic MM.. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther 2017;19:206.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferro F, Delle Sedie A.. The use of ultrasound for assessing interstitial lung involvement in connective tissue diseases. Clin Exp Rheumatol 2018;36(Suppl 114):165–70. [PubMed] [Google Scholar]
- 8. Lee JS, Lee EY, Ha YJ. et al. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 2019;21:58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumanovics G, Minier T, Radics J. et al. Comprehensive investigation of novel serum markers of pulmonary fibrosis associated with systemic sclerosis and dermato/polymyositis. Clin Exp Rheumatol 2008;26:414–20. [PubMed] [Google Scholar]
- 10. Gargani L, Volpicelli G.. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014;12:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song G, Bae SC, Lee YH.. Diagnostic accuracy of lung ultrasound for interstitial lung disease in patients with connective tissue diseases: a meta-analysis. Clin Exp Rheumatol 2016;34:11–6. [PubMed] [Google Scholar]
- 12. Hant FN, Ludwicka-Bradley A, Wang HJ. et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol 2009;36:773–80. [DOI] [PubMed] [Google Scholar]
- 13. Bohan A, Peter JB.. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- 14. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol 2002;46:626–36. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS) and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–64. [DOI] [PubMed] [Google Scholar]
- 16. Gutierrez M, Salaffi F, Carotti M. et al. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders—preliminary results. Arthritis Res Ther 2011;13:R134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gargani L, Doveri M, D'Errico L. et al. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009;48:1382–7. [DOI] [PubMed] [Google Scholar]
- 18. Volpicelli G, Elbarbary M, Blaivas M. et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577–91. [DOI] [PubMed] [Google Scholar]
- 19. Warrick JH, Bhalla M, Schabel SI, Silver RM.. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520–8. [PubMed] [Google Scholar]
- 20. Barskova T, Gargani L, Guiducci S. et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann Rheum Dis 2013;72:390–5. [DOI] [PubMed] [Google Scholar]
- 21. Gigante A, Rossi Fanelli F, Lucci S. et al. Lung ultrasound in systemic sclerosis: correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern Emerg Med 2016;11:213–7. [DOI] [PubMed] [Google Scholar]
- 22. Tardella M, Di Carlo M, Carotti M. et al. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: cut-off point definition for the presence of significant pulmonary fibrosis. Medicine 2018;97:e0566.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cogliati C, Antivalle M, Torzillo D. et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology 2014;53:1497–503. [DOI] [PubMed] [Google Scholar]
- 24. Wallace B, Vummidi D, Khanna D.. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol 2016;28:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Chen S, Lin Z. et al. Utilize lung ultrasound B-lines and KL-6 to monitor anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis-associated interstitial lung disease: a case report and literature review. Clin Rheumatol 2019;38:1433–6. [DOI] [PubMed] [Google Scholar]
- 26. Ishikawa N, Hattori N, Yokoyama A, Kohno N.. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3–13. [DOI] [PubMed] [Google Scholar]
- 27. Bandoh S, Fujita J, Ohtsuki Y. et al. Sequential changes of KL-6 in sera of patients with interstitial pneumonia associated with polymyositis/dermatomyositis. Ann Rheum Dis 2000;59:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takanashi S, Nishina N, Nakazawa M, Kaneko Y, Takeuchi T.. Usefulness of serum Krebs von den Lungen-6 for the management of myositis-associated interstitial lung disease. Rheumatology 2019;58:1034–9. [DOI] [PubMed] [Google Scholar]
- 29. Fathi M, Barbasso Helmers S, Lundberg IE.. KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 2012;271:589–97. [DOI] [PubMed] [Google Scholar]
- 30. Kubo M, Ihn H, Yamane K. et al. Serum KL-6 in adult patients with polymyositis and dermatomyositis. Rheumatology 2000;39:632–6. [DOI] [PubMed] [Google Scholar]
- 31. Arai S, Kurasawa K, Maezawa R. et al. Marked increase in serum KL-6 and surfactant protein D levels during the first 4 weeks after treatment predicts poor prognosis in patients with active interstitial pneumonia associated with polymyositis/dermatomyositis. Mod Rheumatol 2013;23:872–83. [DOI] [PubMed] [Google Scholar]
- 32. Chen F, Lu X, Shu X. et al. Predictive value of serum markers for the development of interstitial lung disease in patients with polymyositis and dermatomyositis: a comparative and prospective study. Intern Med J 2015;45:641–7. [DOI] [PubMed] [Google Scholar]
- 33. Isoda K, Kotani T, Takeuchi T. et al. Potential of Krebs von den Lungen-6 as a predictor of relapse in interstitial pneumonia with anti-aminoacyl tRNA synthetase antibodies-positive dermatomyositis. Clin Respir J 2018;12:2235–41. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Chen S, Lin Z. et al. Imaging and serum biomarkers in connective tissue disease-associated interstitial lung diseases: correlation between lung ultrasound B-lines and KL-6 levels. Ann Rheum Dis 2019;78:573–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.