Abstract
Objectives
This article estimates the frequency of polyautoimmunity and associated factors in a large retrospective cohort of patients with SLE.
Methods
RELESSER (Spanish Society of Rheumatology Lupus Registry) is a nationwide multicentre, hospital-based registry of SLE patients. This is a cross-sectional study. The main variable was polyautoimmunity, which was defined as the co-occurrence of SLE and another autoimmune disease, such as autoimmune thyroiditis, RA, scleroderma, inflammatory myopathy and MCTD. We also recorded the presence of multiple autoimmune syndrome, secondary SS, secondary APS and a family history of autoimmune disease. Multiple logistic regression analysis was performed to investigate possible risk factors for polyautoimmunity.
Results
Of the 3679 patients who fulfilled the criteria for SLE, 502 (13.6%) had polyautoimmunity. The most frequent types were autoimmune thyroiditis (7.9%), other systemic autoimmune diseases (6.2%), secondary SS (14.1%) and secondary APS (13.7%). Multiple autoimmune syndrome accounted for 10.2% of all cases of polyautoimmunity. A family history was recorded in 11.8%. According to the multivariate analysis, the factors associated with polyautoimmunity were female sex [odds ratio (95% CI), 1.72 (1.07, 2.72)], RP [1.63 (1.29, 2.05)], interstitial lung disease [3.35 (1.84, 6.01)], Jaccoud arthropathy [1.92 (1.40, 2.63)], anti-Ro/SSA and/or anti-La/SSB autoantibodies [2.03 (1.55, 2.67)], anti-RNP antibodies [1.48 (1.16, 1.90)], MTX [1.67 (1.26, 2.18)] and antimalarial drugs [0.50 (0.38, 0.67)].
Conclusion
Patients with SLE frequently present polyautoimmunity. We observed clinical and analytical characteristics associated with polyautoimmunity. Our finding that antimalarial drugs protected against polyautoimmunity should be verified in future studies.
Keywords: polyautoimmunity, systemic lupus erythematosus, multiple autoimmune syndrome
Rheumatology key messages
Antimalarial drugs could delay the development of polyautoimmunity in SLE patients. Anti-Ro/SSA, anti-La/SSB and anti-RNP antibodies were independently associated with polyautoimmunity in SLE patients.
Polyautoimmunity in SLE patients was associated with Jaccoud arthropathy, RP and interstitial lung disease.
Introduction
SLE is a systemic autoimmune rheumatic disease (SARD) that can affect any tissue and lead to a wide range of clinical manifestations that are shared by other inflammatory diseases [1]. Polyautoimmunity is the co-occurrence of more than one well-defined autoimmune disease in the same patient. The co-occurrence of three or more autoimmune diseases is known as multiple autoimmune syndrome (MAS), which is an extreme phenotype of polyautoimmunity. Patients with SLE often present with autoimmune thyroiditis, SS, RA, autoimmune hepatitis or APS [2]. Overlap syndrome is defined as the co-occurrence of manifestations of several recognized and diagnostically confirmed autoimmune diseases in the same patient [3], although this area remains open to debate [4]. One example would be MCTD, which shares clinical characteristics with SLE, SSc, PM/DM and RA. Patients with this condition also have high ANA titres (anti-RNP), and their main symptoms are polyarthritis, hand oedema, RP, sclerodactyly, myositis and oesophageal hypomobility [5]. The subject of whether this disease is a separate entity or a transitional syndrome remains controversial [6]. The main objections are as follows: (i) patients can progress to another well-defined SARD; (ii) anti-RNP antibodies are not exclusive to this condition, and the most typical clinical manifestations of MCTD co-occur with other antibodies; and (iii) there are no widely accepted classification criteria [7, 8].
Both polyautoimmunity and overlap syndromes, together with familial aggregation of autoimmune diseases, represent phenotypic expressions of an identical or similar genotype and of ‘immune tautology’. Study of some of the characteristics of patients with SLE, polyautoimmunity and overlap syndromes (epidemiology, familial coaggregation, shared pathophysiology, genetic factors and similar treatments) has revealed no clear distinction between phenotypic subgroups [4, 9, 10]; therefore, it would be interesting to know how polyautoimmunity affects patients with SLE. Increased knowledge would provide us with information on the frequency and clinical characteristics of the condition and pave the way for new lines of research into the clinical significance of and mechanisms involved in autoimmunity.
The aims of the present study were as follows: (i) to investigate the prevalence of polyautoimmunity and MAS in a large cohort of patients with SLE in Spain; (ii) to describe the characteristics of subphenotypes; and (iii) to identify factors associated with polyautoimmunity in patients with SLE.
Methods
RELESSER Registry
We performed a retrospective evaluation of all adult patients in the RELESSER Registry (Spanish Society of Rheumatology Lupus Registry) to determine the prevalence of polyautoimmunity and MAS in patients with SLE.
The RELESSER Registry is a nationwide multicentre, hospital-based registry involving a cross-sectional stage designed by the Systemic Autoimmune Diseases Working Group of the Spanish Society of Rheumatology [11]. The methodologic and general characteristics of the RELESSER Registry have been published elsewhere [12, 13]. The registry includes cumulative clinical data for patients with SLE in clinical settings. The Research Unit of the Spanish Society of Rheumatology was the coordinating centre and was responsible for providing expert methodological support at all stages of the project, monitoring the study, and identifying and resolving inconsistencies.
Written commitment was obtained from all investigators prior to participation. The study was approved by the Ethics Committee at Dr Negrín University Hospital, Gran Canaria, Spain, and then by the institutional research review boards at all participating centres. Informed consent was obtained from the patients. The study was conducted in accordance with the Declaration of Helsinki.
Study population
We selected adult patients with SLE defined according to the 1997 criteria of the ACR [14]. All patients had been attended and followed at any one of 45 Spanish rheumatology teaching centres with experience in the treatment of SLE. Patients were widely and homogeneously distributed throughout Spain in order to prevent selection bias.
Outcomes and definitions
Main outcome
Patients were considered to have polyautoimmunity when they simultaneously fulfilled the classification criteria for SLE classified according to the ACR 1997 criteria [14] and another autoimmune disease, such as autoimmune thyroiditis (abnormal thyroid function with antithyroid antibodies), MCTD according to the criteria of Alarcón-Segovia and Cardiel [15], other autoimmune diseases such as RA according to the criteria of the ACR [16], SSc [17] and inflammatory myopathy [18]. MAS was defined as the co-occurrence of SLE and at least two other autoimmune diseases (see above).
Other outcomes
We also recorded whether patients had secondary SS according to the criteria of the 2002 American-European Consensus Group [19], APS according to the Sydney criteria [20] or a family history (first- or second-degree) of SARD. However, these forms were not counted as polyautoimmunity but as secondary syndromes.
We also recorded demographic, clinical, analytical and treatment data. Demographic and clinical variables included age (in years), sex, comorbidities included in the Charlson index [21], date of onset of the disease (date the patient first fulfilled four or more ACR or SLE criteria), duration of the disease (calculated from diagnosis to the most recent visit or death, if applicable), and all of the parameters covered in the ACR and SLE classification criteria. Analytical data were assessed in the local laboratory. We recorded all present or past drugs and the reasons for interrupting them, nonpharmacological treatments and mortality. At the last visit, we retrospectively recorded the activity index Safety of Oestrogens in Lupus Erythematosus: National Assessment-SLEDAI [22], the damage index SLICC/ACR Damage Index [23] and the Katz severity index [24].
Statistical analysis
We performed a descriptive analysis of the clinical, epidemiological, autoimmune and therapeutic characteristics of all patients with SLE with or without polyautoimmunity. Qualitative variables were expressed as absolute numbers and percentages; quantitative variables were expressed as mean (s.d.) or median [interquartile range (IQR)], as applicable.
The χ2 and an ANOVA or Kruskal–Wallis test (depending on normality) were performed to compare the main characteristics between four groups of patients: (i) patients with SLE and no other autoimmune disease; (ii) patients with a family history of SLE; (iii) patients with autoimmune thyroiditis; and (iv) patients with another SARD. Survival was analysed based on Kaplan–Meier curves in order to compare time on antimalarial drugs in patients with SLE with and without polyautoimmunity or positive antibody titres. Finally, a binary logistic regression analysis was performed (dependent variable: polyautoimmunity) to explore variables associated with polyautoimmunity identified in the bivariate analysis and/or of clinical interest. Multicollinearity of independent variables was verified using the Pearson correlation coefficient (r > 0.4). The analysis was performed using Rcomander.
Results
Of the 4037 patients with SLE recruited in RELESSER-TRANS, 3679 (91.1%) fulfilled four or more ACR criteria for SLE. Most patients were women (90.3%) and white (93.1%), and the median (IQR) age was 44.0 (35.0–56.0) years.
Polyautoimmunity
A total of 502 of the 3679 (13.6%) patients with SLE had polyautoimmunity, and 51 (1.4%) had MAS. Most patients with polyautoimmunity were white women (94.4%) with long-term disease. The most common clinical manifestations in patients with polyautoimmunity were arthritis, cutaneous abnormalities and RP. Most patients (99%) had positive ANA titres, with a predominance of anti-DNA, anti-Ro and anti-RNP antibodies. As for treatment, the vast majority had received CS and antimalarial drugs at some time during the course of their disease. Table 1 shows a comparison of the results for the whole SLE group (n = 3679) and the polyautoimmunity and SLE paients (n = 502).
Table 1.
Characteristics of whole SLE group (n = 3679) with the polyautoimmunity and SLE patients (n = 502)
| Variable | Whole SLE group (n = 3679) | Polyautoimmunity (n = 502) | P-value |
|---|---|---|---|
| Epidemiological characteristics | |||
| Sex (female), n (%) | 3315 (90.3) | 473 (94.4) | 0.001 |
| Age at SLE diagnosis (years), mean (s.d.) | 34.6 (14.6) | 36.7 (14.2) | 0.220 |
| Age at the time of inclusion (years), mean (s.d.) | 46.2 (14.8) | 48.8 (14.6) | 0.189 |
| Disease duration (months), median (IQR) | 165.4 (82.0–234.0) | 162.0 (83.0–243.0) | 0.159 |
| Family historya, n (%) | 433 (16.0) | 60 (15.7) | 0.902 |
| Clinical manifestations | |||
| SS, n (%) | 517 (14.4) | 127 (25.7) | <0.001 |
| APS, n (%) | 505 (13.9) | 74 (14.9) | 0.486 |
| Malar rash, n (%) | 2004 (55.2) | 253 (50.8) | 0.100 |
| Discoid lupus, n (%) | 753 (21.0) | 94 (19.1) | 0.265 |
| Photosensitivity, n (%) | 2172 (60.8) | 293 (59.7) | 0.569 |
| Ulcer, n (%) | 1645 (46.1) | 218 (44.4) | 0.414 |
| Arthritis, n (%) | 2827 (77.9) | 393 (79.4) | 0.415 |
| Jaccoud arthropathy, n (%) | 363 (10.0) | 90 (18.1) | 0.005 |
| Pleuritis, n (%) | 826 (23.0) | 105 (21.3) | 0.357 |
| Pericarditis, n (%) | 579 (16.1) | 86 (17.3) | 0.404 |
| Neurologicb, n (%) | 331 (9.1) | 45 (9.1) | 0.989 |
| Hematologicc, n (%) | 2371 (66.0) | 320 (64.9) | 0.568 |
| RP, n (%) | 1200 (33.9) | 226 (45.8) | <0.001 |
| Nephritis, n (%) | 1101 (30.6) | 131 (26.5) | 0.035 |
| Proteinuria, n (%) | 1170 (32.2) | 132 (26.6) | 0.013 |
| Interstitial lung disease, n (%) | 73 (2.0) | 25 (5.0) | 0.010 |
| Pulmonary hypertension, n (%) | 8 (2.4) | 17 (3.4) | 0.157 |
| Antibody profile | |||
| ANA, n (%) | 3637 (99.1) | 497 (99.0) | 0.892 |
| Anti-dsDNA antibody positivity, n (%) | 2629 (73.3) | 350 (71.0) | 0.208 |
| Anti-Sm antibody positivity, n (%) | 737 (21.2) | 110 (22.8) | 0.337 |
| Anti-RNP antibody positivity, n (%) | 891 (25.2) | 164 (34.1) | <0.001 |
| Anti-Ro antibody positivity, n (%) | 1350 (36.0) | 193 (39.9) | 0.099 |
| Anti-La antibody positivity, n (%) | 690 (18.8) | 104 (21.4) | 0.117 |
| LA, n (%) | 638 (23.9) | 70 (20.3) | 0.114 |
| aCL positivity, n (%) | 759 (20.6) | 96 (19.1) | 0.678 |
| Anti-beta 2 glycoprotein 1 positivity, n (%) | 442 (12.0) | 59 (11.8) | 0.802 |
| Severity indexes | |||
| SLICC-ACR, median (IQR) | 1.1 (0.0–2.0) | 1.0 (0.0–2.0) | 0.108 |
| Katz index, median (IQR) | 2.5 (1.0–3.0) | 2.0 (1.0–3.0) | 0.915 |
| Mortality, n (%) | 211 (6.6) | 43 (8.4) | 0.124 |
| Treatment | |||
| Glucocorticoids, n (%) | 3112 (88.9) | 439 (91.1) | 0.224 |
| MTX, n (%) | 579 (16.6) | 120 (24.7) | <0.001 |
| Antimalarials, n (%) | 2899 (83.3) | 369 (76.7) | <0.001 |
| Time on antimalarials (months), median (IQR) | 123 (62.0–204.0) | 113.0 (50.0–192.0) | |
| AZA, n (%) | 1143 (33.0) | 173 (36.0) | 0.129 |
| CYC, n (%) | 780 (22.5) | 95 (19.7) | 0.126 |
| Mycophenolate, n (%) | 525 (15.2) | 60 (12.4) | 0.075 |
| Rituximab, n (%) | 227 (6.5) | 44 (9.1) | 0.170 |
| Immunoglobulin, n (%) | 154 (4.5) | 23 (4.8) | 0.721 |
Family history: family history of systemic autoimmune rheumatic disease. bNeurologic: seizure and psychosis. cHematologic: haemolytic anaemia, leukopoenia and thrombocytopenia. IQR: interquartile range.
The autoimmune diseases most commonly associated with SLE were autoimmune thyroiditis [289/3679 (7.9%)] and other autoimmune diseases [227/3679 (6.2%)]. In the latter group, 97/3679 (2.6%) had MCTD and 130/3679 (3.5%) had RA, SSc or inflammatory myopathy. A total of 517/3679 (14.1%) patients had secondary SS and 505/3679 (13.7%) had secondary APS. A family history of SARD was recorded in 433 patients (11.8%) with SLE.
Characteristics of the subtypes of polyautoimmunity
Table 2 shows the characteristics of the various subgroups associated with polyautoimmunity compared with patients with SLE who did not have polyautoimmunity. As shown, almost all differences were concentrated in patients with polyautoimmunity associated with another SARD, whereas patients with autoimmune thyroiditis or a family history of SARD had similar characteristics to patients with SLE but not polyautoimmunity.
Table 2 Characteristics of the different phenotypes of patients with SLE
| Variable | Phenotype | ||||
|---|---|---|---|---|---|
| SLE ≥4 criteriaa (n = 3177) | SLE + family history of systemic autoimmune disease (n = 433) | SLE + autoimmune thyroiditis (n = 289) | SLE + other SARD (n = 227) | P-value | |
| Epidemiological characteristics | |||||
| Sex (female), n (%) | 2842 (89.6) | 402 (92.8) | 275 (95.5) | 212 (94.0) | 0.006 |
| Age at SLE diagnosis (years), mean (s.d.) | 34.6 (14.6) | 31.3 (13.5) | 36.1 (13.8) | 37.7 (14.6) | 0.051 |
| Age at the time of inclusion (years), mean (s.d.) | 42.2 (13.8) | 47.0 (14.1) | 51.3 (14.8) | 46.2 (14.8) | 0.010 |
| Disease duration (months), median (IQR) | 148.0 (82.0–234.0) | 144.0 (81.0–231.0) | 143.0 (69.0–233.0) | 180.5 (106.5–259.2) | 0.001 |
| Clinical manifestations | |||||
| Malar rash, n (%) | 1751 (55.9) | 254(59.1) | 156 (54.5) | 102 (44.9) | 0.013 |
| Discoid lupus, n (%) | 659 (21.3) | 94 (22.1) | 61 (21.6) | 35 (15.6) | 0.161 |
| Photosensitivity, n (%) | 1879 (61.0) | 264 (62.9) | 181 (64.9) | 121 (53.3) | 0.023 |
| Oral ulcers, n (%) | 1427 (46.4) | 224 (52.8) | 123 (43.5) | 101 (45.5) | 0.348 |
| Arthritis, n (%) | 2434 (77.7) | 338 (79.5) | 218 (77.0) | 187 (82.7) | 0.179 |
| Jaccoud arthropathy, n (%) | 315 (9.9) | 46 (10.7) | 22 (7.7) | 72 (31.9) | <0.001 |
| Pleuritis, n (%) | 721 (23.2) | 88 (20.9) | 57 (20.2) | 49 (21.7) | 0.532 |
| Pericarditis, n (%) | 493 (15.9) | 70 (16.6) | 47 (16.5) | 40 (17.7) | 0.476 |
| Proteinuria, n (%) | 1001 (32.2) | 126 (29.6) | 87 (30.4) | 45 (20.1) | 0.011 |
| Neurologicb, n (%) | 286 (9.1) | 44 (10.3) | 20 (7.5) | 26 (11.0) | 0.500 |
| Hematologicc, n (%) | 2031 (65.5) | 277 (64.9) | 182 (64.3) | 134 (60.3) | 0.985 |
| SS, n (%) | 390 (12.6) | 54 (12.7) | 62 (21.9) | 72 (31.9) | <0.001 |
| APS, n (%) | 431 (13.7) | 65 (15.2) | 43 (15.0) | 34 (15.2) | 0.843 |
| RP, n (%) | 974 (31.9) | 159 (37.0) | 88 (31.1) | 148 (66.4) | <0.001 |
| Nephritis, n (%) | 970 (31.3) | 124 (28.9) | 79 (27.7) | 51 (23.0) | 0.133 |
| Interstitial lung disease, n (%) | 48 (1.5) | 10 (2.3) | 2 (0.7) | 23 (10.2) | <0.001 |
| Pulmonary hypertension, n (%) | 71 (2.3) | 13 (3.1) | 5 (1.7) | 11 (4.9) | <0.001 |
| Antibody profile | |||||
| ANA, n (%) | 3140 (99.1) | 428 (99.1) | 288 (99.7) | 223 (98.2) | 0.356 |
| Anti-dsDNA antibody positivity, n (%) | 2279 (73.7) | 320 (76.4) | 206 (73.0) | 154 (68.4) | 0.050 |
| Anti-Sm antibody positivity, n (%) | 627 (21.0) | 108 (26.4) | 58 (21.2) | 56 (25.6) | 0.435 |
| Anti-RNP antibody positivity, n (%) | 727 (23.8) | 114 (27.3) | 55 (20.2) | 117 (52.5) | <0.001 |
| Anti-Ro antibody positivity, n (%) | 1210 (38.1) | 185 (44.7) | 111 (40.4) | 90 (41.5) | 0.179 |
| Anti-La antibody positivity, n (%) | 586 (18.4) | 96 (23.1) | 63 (22.9) | 43 (19.3) | 0.057 |
| aCL positivity, n (%) | 728 (25.1) | 117 (26.3) | 70 (24.6) | 48 (22.0) | 0.518 |
| Anti-beta 2 glycoprotein 1 positivity, n (%) | 270 (14.2) | 56 (12.5) | 30 (15.0) | 13 (11.0) | 0.166 |
| LA, n (%) | 568 (24.4) | 94 (27.4) | 42 (20.1) | 27 (22.5) | 0.255 |
| Treatment | |||||
| Antimalarials, n (%) | 2530 (84.3) | 356 (86.0) | 231 (83.4) | 151 (69.0) | <0.001 |
| Time on antimalarials (months), median (IQR) | 60.0 (25.0–120.0) | 58.0 (27.5–109.5) | 48.0 (22.5–79.5) | 36.0 (13.2–108.0) | 0.008 |
| MTX, n (%) | 459 (15.3) | 81 (19.8) | 48 (17.1) | 76 (34.9) | <0.001 |
| AZA, n (%) | 970 (32.5) | 135 (32.8) | 82 (29.7) | 95 (44.0) | 0.001 |
| CYC, n (%) | 685 (22.9) | 85 (20.9) | 52 (18.7) | 44 (20.2) | 0.339 |
| Mycophenolate, n (%) | 465 (15.7) | 80 (19.7) | 40 (14.3) | 20 (9.2) | 0.145 |
| Rituximab, n (%) | 183 (6.1) | 31 (7.6) | 22 (7.9) | 23 (10.6) | 0.038 |
| Immunoglobulins, n (%) | 131 (4.4) | 22 (5.4) | 18 (6.5) | 5 (2.3) | 0.210 |
The P-value is generated based on the χ2 test, ANOVA or Kruskal–Wallis test (depending on normality). aSLE ≥4 criteria without polyautoimmunity. bNeurologic: seizure and psychosis. cHematologic: haemolytic anaemia, leukopoenia and thrombocytopenia. SARD: systemic autoimmune rheumatic disease; IQR: interquartile range.
Female sex was generally more frequent in all polyautoimmunity subphenotypes, whereas age and duration of SLE were higher in patients with polyautoimmunity and associated SARDs. However, other epidemiological characteristics such as race and smoking did not differ significantly between groups. As for clinical manifestations, patients with polyautoimmunity and another SARD less frequently had photosensitivity, malar rash, proteinuria and kidney disease. However, Jaccoud arthropathy, RP, interstitial lung disease, pulmonary hypertension and secondary SS were much more common.
The abovementioned associations were observed in patients with MAS as an extreme subphenotype of polyautoimmunity, although the association was stronger: they were all women (100%), had a lower frequency of kidney disease (16.0 vs 31.3%; P = 0.024), and had double the number of cases with secondary SS [n (%) =51.0 vs 12.6; P < 0.00], RP (87.8 vs 31.9; P < 0.001), interstitial lung disease (6.0 vs 1.5; P < 0.001) and Jaccoud arthropathy (28.0 vs 8.7; P < 0.001), with respect to patients with SLE but no polyautoimmunity.
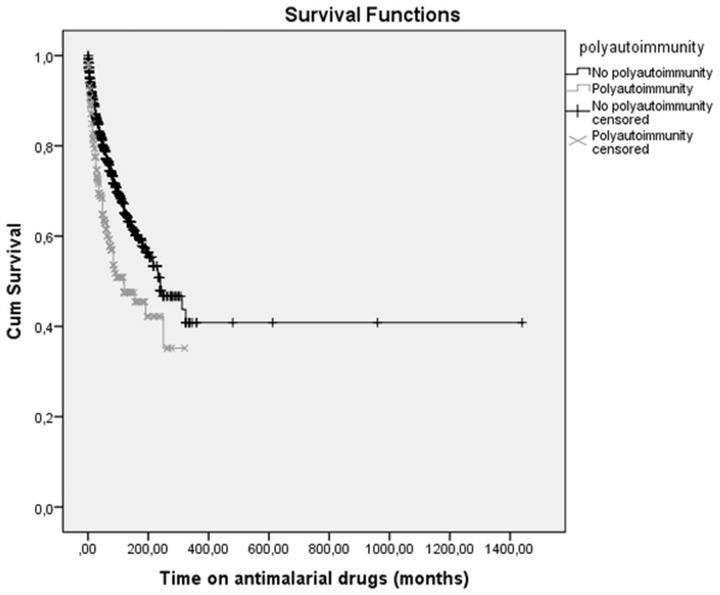
Polyautoimmunity was associated with anti-Ro/SSA antibodies (P = 0.012), and MAS was associated with anti-RNP antibodies (P < 0.001). Other antibodies such as aPL did not differ significantly between groups. As for treatment, patients with polyautoimmunity, including those with MAS, more commonly received MTX (P < 0.001), AZA (P = 0.019) and rituximab (P = 0.043). However, they took antimalarial agents less frequently (P < 0.001) or for less time [median (IQR): 56.6 (31.2–179.3) vs 185.9 (27.5–294.0) months; P < 0.001] (Fig. 1). Similarly, less exposure to antimalarial drugs was associated with a greater frequency of positive anti-Ro and anti-La titres [median (IQR): 157.0 (101.2–212.7) vs 240.0 (187.3–292.6) months; P = 0.003].
Fig. 1.
Kaplan–Meier survival estimates
Multivariate analysis
The results of the multivariate analysis in Table 3 show that polyautoimmunity was associated with female sex, Jaccoud arthropathy, RP, interstitial lung disease, and positive titres for anti-Ro/SSA, anti-La/SSB and anti-RNP antibodies. Similarly, patients with polyautoimmunity were 67% more likely to be treated with MTX and 50% less likely to receive antimalarial drugs. However, variables of severity such as mortality or damage were not associated with polyautoimmunity.
Table 3.
Binary logistic regression analysis of the characteristics associated with polyautoimmunity in patients with SLE
| Predictor | OR (95% CI) | P-value |
|---|---|---|
| Female sex | 1.721 (1.074, 2.729) | 0.024 |
| RP | 1.630 (1.292, 2.056) | <0.001 |
| Interstitial lung disease | 3.350 (1.840, 6.009) | <0.001 |
| Anti-RNP positivity | 1.486 (1.163, 1.900) | 0.002 |
| Anti-Ro and/or anti-La antibody positivity | 2.039 (1.555, 2.673) | <0.001 |
| Jaccoud arthropathy | 1.921 (1.402, 2.634) | <0.001 |
| Antimalarials | 0.509 (0.384, 0.675) | <0.001 |
| MTX | 1.666 (1.268, 2.189) | <0.001 |
Nagelkerke R2 =0.122. Variables included: sex, age at the time of RELESSER-TRANS inclusion, duration of disease, proteinuria, anti-Ro and anti-La antibodies, RP, interstitial lung disease, anti-RNP, use of MTX, use of antimalarial treatment, Jaccoud arthropathy, mortality, SLICC-ACR. Dependent variable: polyautoimmunity. OR: odds ratio; RELESSER-TRANS: Spanish Society of Rheumatology Lupus Registry, cross-sectional phase.
Discussion
Classic systemic autoimmune diseases are characterized by very heterogeneous clinical phenotypes and by the fact that they share clinical manifestations and pathogenic mechanisms. This clinical and aetiological–pathogenic overlap has been referred to by some authors as ‘autoimmune tautology’ [25]. The best clinical evidence of this tautology arises from the co-occurrence of several autoimmune diseases in the same patient (i.e. polyautoimmunity), the progression from one phenotype to another during the clinical course (e.g. progression from MCTD to lupus) or the presence of overlap syndromes (e.g. antisynthetase syndrome) [26, 27]. In accordance with this perspective, we found the prevalence of polyautoimmunity to be 14% in patients with SLE and <2% in MAS. These percentages are lower than those reported by Rojas-Villarraga et al. [25, 28], who found a greater prevalence of polyautoimmunity in patients with SLE (around 40%) and of MAS (5.1%). The difference arises from the fact that the authors considered SS and APS to be independent entities and not secondary disorders. While this approach has recently been supported by other authors [29, 30], in the present study we adopted a traditional approach and treated them as being secondary to SLE. However, aside from this consideration, the percentages reported by these authors for SS and APSndrome were similar to ours. In addition, the results of our multivariate model remained unchanged irrespective of which approach was used.
As for diseases associated with SLE, autoimmune thyroiditis was as frequent in the present study as the other SARDs together, although its importance in the clinical and epidemiological characteristics of the patients was negligible. Autoimmune thyroiditis is the most prevalent autoimmune disease in the general population [25, 31]. Although their aetiology is still poorly understood, genetic, immunological, hormonal and environmental factors are major predisposing and triggering factors [32].
The finding that polyautoimmunity has higher female representation than SLE is probably driven by the high prevalence of thyroid autoimmunity, which has a much higher female predominance than lupus, on the order of 30–40:1 vs 9:1 [33]. Female sex is also associated with a greater frequency of MAS, which was recorded in 100% of cases in the present study. While this finding could be biased owing to the composition of the RELESSER Registry itself (90% are women), it has been reported in other studies and attributed to hormones, genetics [34] and gender-related lifestyle [35].
Rojas-Villarraga et al. [28] also highlighted that a family history of autoimmune disease was a key risk factor for polyautoimmunity. While this observation has been reported in other autoimmune diseases [36–39], we were unable to confirm it in our study, not even when SS and APS were considered as not being secondary to SLE.
Anti-Ro/SSA and anti-La/SSB antibodies are associated with SS, SLE and neonatal lupus. In the present study, these autoantibodies were also independently associated with polyautoimmunity. Despite the fact that this finding has been reported in studies that consider SS to be a separate entity [28, 29], we found that the association persisted even with autoimmune diseases other than this syndrome, such as autoimmune thyroiditis, RA, scleroderma, MCTD and inflammatory myositis. Other authors found that polyautoimmunity was more frequent in patients with SSc and positive anti-Ro titres [40]. The association between these antibodies and polyautoimmunity could be explained by the process known as ‘epitope spreading’, in which an immune response is modified over time and the changes are associated with changes in the clinical picture [41, 42].
Another interesting aspect of the determinants of poly-autoimmunity is their influence on prognosis. While patients with polyautoimmunity in the present study less frequently had nephritis, our multivariate analysis showed that they were three times more likely to have interstitial lung disease and twice as likely to have Jaccoud arthropathy and RP. Interstitial lung disease and RP have been reported to be more frequent in patients with SLE and overlapping SS, SSc and MCTD [43, 44]. Our data confirm this observation. Jaccoud arthropathy is sometimes observed in patients with long-term SLE and is associated with disability. SLE has previously been reported to overlap with antisynthetase syndrome, RP and scleroderma [45], in addiction to MCTD [46]. In all cases, as in our study, this clinical problem was associated with long-term disease and inflammation of the joints and tendons, which was sometimes subclinical [47]. Rojas-Villarraga et al. [28] found that joint involvement was a risk factor associated with polyautoimmunity and reported that patients with SLE and joint involvement were twice as likely to develop polyautoimmunity as patients with SLE without arthritis.
Lastly, a key aspect of systemic autoimmune diseases as a specific group is that they share similar therapies. Therefore, a detailed knowledge of the aetiology and pathogenesis of polyautoimmunity could enable the development of new therapeutic targets in these diseases. In this sense, we found that patients with polyautoimmunity were almost 50% less likely to receive antimalarial drugs and that patients who took these drugs did so for shorter periods. While the design of our study does not allow us to establish causality, this finding leads us to take two aspects into consideration. First, antimalarial drugs have a wide range of beneficial effects in patients with SLE and other autoimmune diseases (e.g. SS, DM). A particularly interesting effect for us is that patients with incomplete lupus (i.e. fewer than four criteria) treated with HCQ take longer to develop SLE than those who do not receive the drug [48]. Second, antimalarial agents are less frequently indicated in patients with polyautoimmunity, owing to the poorer response or the presence of manifestations such as Jaccoud arthropathy that lead physicians to prefer other drugs, such as MTX. Nevertheless, MTX seems less likely, because antimalarial drugs are the agents of choice for treatment of joint manifestations and because they tend to be used in combination with other drugs, even in severe complications [49]. Unfortunately, the relationship between polyautoimmunity and treatments received in SLE, as well as in other systemic autoimmune diseases, has not been as well studied as other aetiological, epidemiological and clinical-analytical factors.
Although polyautoimmunity has been addressed from various angles by a small number of authors, our study analyses a large number of patients, serves to validate current knowledge and provides data on a predominantly white Spanish population. However, our study is subject to the methodological limitations that are inherent to all cross-sectional analyses of national registries. Over 40 centres throughout Spain participated in the registry. Since there was no centralized laboratory, the accuracy of the serological data may have been affected. It is also limited by the fact that most of the comorbid autoimmune diseases were not collected exhaustively or independently. Nevertheless, the diseases we did record account for 90% of those diseases that are commonly associated with patients who have SLE.
In conclusion, polyautoimmunity in the present cohort of patients with SLE was not uncommon and was associated with female sex, manifestations of poor prognosis (interstitial lung disease, Jaccoud arthropathy and RP), and specific antibody profiles (anti-Ro/SSA, anti-La/SSB and anti-RNP). Patients received more immunosuppressants and fewer antimalarial drugs. While this finding has to be confirmed, the lower frequency of antimalarial drugs in patients with polyautoimmunity suggests that these agents could have a protective effect against polyautoimmunity.
Funding: The RELESSER Registry was partially funded by GlaxoSmithKline (GSK), Roche, Union Chimique Belge (UCB), Lilly and Novartis. The sponsors had no role in the study design, data collection, analysis or interpretation, in writing the report, or in the decision to submit the article for publication. J.M.P.-R. is supported by grant 316265 (BIOCAPS) from the European Union 7th Framework Program (FP7/REGPOT-2012-2013.1). The study was supported by FIS Grant PI11/02857 (Instituto Carlos III, Fondos FEDER). Grant for medical researchers of the ‘Fundación Española de Reumatología’.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Aringer M, Schneider M. [ Systemic lupus erythematosus]. Dtsch Med Wochenschr 2016;141:537–43. [DOI] [PubMed] [Google Scholar]
- 2. Cojocaru M, Cojocaru IM, Silosi I.. Multiple autoimmune syndrome. Maedica (Buchar) 2010;5:132–4. [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly A, Panush RS.. Diagnostic uncertainty and epistemologic humility. Clin Rheumatol 2017;36:1211–4. Germany [DOI] [PubMed] [Google Scholar]
- 4. Anaya JM. The autoimmune tautology. A summary of evidence. Joint Bone Spine 2017;84:251–3. [DOI] [PubMed] [Google Scholar]
- 5. Ortega-Hernandez OD, Shoenfeld Y.. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol 2012;26:61–72. [DOI] [PubMed] [Google Scholar]
- 6. Missoum H, Alami M, Bachir F. et al. Prevalence of autoimmune diseases and clinical significance of autoantibody profile: data from National Institute of Hygiene in Rabat, Morocco. Hum Immunol 2019;80:523–32. [DOI] [PubMed] [Google Scholar]
- 7. Smolen JS, Steiner G.. Mixed connective tissue disease: to be or not to be? Arthritis Rheum 1998;41:768–77. [DOI] [PubMed] [Google Scholar]
- 8. Swanton J, Isenberg D.. Mixed connective tissue disease: still crazy after all these years. Rheum Dis Clin North Am 2005;31:421–36, v. [DOI] [PubMed] [Google Scholar]
- 9. Wallace B, Vummidi D, Khanna D.. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol 2016;28:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace DJ. Diagnosis and differential diagnosis of systemic lupus erythematosus in adults. UpToDate. Waltham, MA: UpToDate 2017 [uptodate.com]. https://scholar.google.es/scholar? q=Wallace+DJ.+Diagnosis+and+ differential+diagnosis+of+systemic+lupus+ erythematosus+ in+adults+2017&hl=en&as_sdt=0&as_vis=1&oi=scholart.
- 11. Rúa-Figueroa I, López-Longo FJ, Calvo-Alén J. et al. National registry of patients with systemic lupus erythematosus of the Spanish Society of Rheumatology: objectives and methodology. Reumatol Clin 2014;10:17–24. [DOI] [PubMed] [Google Scholar]
- 12. Fernández-Nebro A, Rúa-Figueroa I, López-Longo FJ. et al. Cardiovascular events in systemic lupus erythematosus: a nationwide study in Spain from the RELESSER registry. Medicine (Baltimore) 2015;94:e1183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Narváez J, Borrell H, Sánchez-Alonso F. et al. Primary respiratory disease in patients with systemic lupus erythematosus: data from the Spanish rheumatology society lupus registry (RELESSER) cohort. Arthritis Res Ther 2018;20:280.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725.. [DOI] [PubMed] [Google Scholar]
- 15. Alarcón-Segovia D, Cardiel MH.. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol 1989;16:328–34. [PubMed] [Google Scholar]
- 16. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 17. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohan A, Peter JB.. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. [DOI] [PubMed] [Google Scholar]
- 19. Vitali C, Bombardieri S, Jonsson R. et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyakis S, Lockshin MD, Atsumi T. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 22. Petri M, Kim MY, Kalunian KC. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 23. Gladman D, Ginzler E, Goldsmith C. et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 24. Katz JD, Senecal J-L, Rivest C, Goulet J-R, Rothfield N.. A simple severity of disease index for systemic lupus erythematosus. Lupus 1993;2:119–23. [DOI] [PubMed] [Google Scholar]
- 25. Rojas-Villarraga A, Amaya-Amaya J, Rodriguez-Rodriguez A, Mantilla RD, Anaya JM.. Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Dis 2012;2012:254319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anaya J-M, Castiblanco J, Rojas-Villarraga A. et al. The multiple autoimmune syndromes. A clue for the autoimmune tautology. Clin Rev Allergy Immunol 2012;43:256–64. [DOI] [PubMed] [Google Scholar]
- 27. Anaya JM, Corena R, Castiblanco J, Rojas-Villarraga A, Shoenfeld Y.. The kaleidoscope of autoimmunity: multiple autoimmune syndromes and familial autoimmunity. Expert Rev Clin Immunol 2007;3:623–35. [DOI] [PubMed] [Google Scholar]
- 28. Rojas-Villarraga A, Toro CE, Espinosa G. et al. Factors influencing polyautoimmunity in systemic lupus erythematosus. Autoimmun Rev 2010;9:229–32. [DOI] [PubMed] [Google Scholar]
- 29. Anaya JM, Rojas-Villarraga A, Mantilla RD, Arcos-Burgos M, Sarmiento-Monroy JC.. Polyautoimmunity in Sjögren syndrome. Rheum Dis Clin North Am 2016;42:457–72. [DOI] [PubMed] [Google Scholar]
- 30. Shiboski SC, Shiboski CH, Criswell L. et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: A data‐driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012; 64; 475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lazúrová I, Benhatchi K, Rovenský J. et al. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Ann N Y Acad Sci 2009;1173:211–6. [DOI] [PubMed] [Google Scholar]
- 32. Szyper-Kravitz M, Marai I, Shoenfeld Y.. Coexistence of thyroid autoimmunity with other autoimmune diseases: friend or foe? Additional aspects on the mosaic of autoimmunity. Autoimmunity 2005;38:247–55. [DOI] [PubMed] [Google Scholar]
- 33. Franco J-S, Amaya-Amaya J, Anaya JM. Thyroid disease and autoimmune diseasesIn: Anaya JM, Shoenfeld Y, Rojas-Villarraga A et al., eds. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013. Jul 18. Chapter 30. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459466/. [PubMed]
- 34. Ginn LR, Lin JP, Plotz PH, Bale SJ. et al. Familial autoimmunity in pedigrees of idiopathic inflammatory myopathy patients suggests common genetic risk factors for many autoimmune diseases. Arthritis Rheum 1998;41:400–5.9506566 [Google Scholar]
- 35. Oliver JE, Silman AJ.. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther 2009;11:252.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rojas-Villarraga A, Diaz FJ, Calvo-Páramo E, Salazar JC. et al. Familial disease, the HLA-DRB1 shared epitope and anti-CCP antibodies influence time at appearance of substantial joint damage in rheumatoid arthritis. J Autoimmun 2009;32:64–9. [DOI] [PubMed] [Google Scholar]
- 37. Hudson M, Rojas-Villarraga A, Coral-Alvarado P. et al. Polyautoimmunity and familial autoimmunity in systemic sclerosis. J Autoimmun 2008;31:156–9. [DOI] [PubMed] [Google Scholar]
- 38. Anaya JM, Tobon GJ, Vega P, Castiblanco J.. Autoimmune disease aggregation in families with primary Sjogren’s syndrome. J Rheumatol 2006;33:2227–34. [PubMed] [Google Scholar]
- 39. Anaya JM, Castiblanco J, Tobón GJ. et al. Familial clustering of autoimmune diseases in patients with type 1 diabetes mellitus. J Autoimmun 2006;26:208–14. [DOI] [PubMed] [Google Scholar]
- 40. Sharma S, Kumar U.. Scleroderma overlap syndromes. Int J Rheum Dis 2016;19:831–3. [DOI] [PubMed] [Google Scholar]
- 41. Routsias JG, Tzioufas AG.. B-cell epitopes of the intracellular autoantigens Ro/SSA and La/SSB: tools to study the regulation of the autoimmune response. J Autoimmun 2010;35:256–64. [DOI] [PubMed] [Google Scholar]
- 42. Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y.. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol 2012;2012:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scherlinger M, Guillotin V, Truchetet ME. et al. Systemic lupus erythematosus and systemic sclerosis: all roads lead to platelets. Autoimmun Rev 2018;17:625–35. [DOI] [PubMed] [Google Scholar]
- 44. Medlin JL, Hansen KE, McCoy SS, Bartels CM.. Pulmonary manifestations in late versus early systemic lupus erythematosus: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oddis CV, Medsger TA Jr, Cooperstein LA.. A subluxing arthropathy associated with the anti-Jo-1 antibody in polymyositis/dermatomyositis. Arthritis Rheum 1990;33:1640–5. [DOI] [PubMed] [Google Scholar]
- 46. Gunashekar S, Prakash M, Minz RW. et al. Comparison of articular manifestations of mixed connective tissue disease and systemic lupus erythematosus on clinical examination and musculoskeletal ultrasound. Lupus 2018;27:2086–92. [DOI] [PubMed] [Google Scholar]
- 47. Piga M, Gabba A, Congia M. et al. Predictors of musculoskeletal flares and Jaccoud’s arthropathy in patients with systemic lupus erythematosus: a 5-year prospective study. Semin Arthritis Rheum 2016;46:217–24. [DOI] [PubMed] [Google Scholar]
- 48. James JA, Kim-Howard XR, Bruner BF. et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 2007;16:401–9. [DOI] [PubMed] [Google Scholar]
- 49. Kasitanon N, Fine DM, Haas M, Magder LS, Petri M.. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus 2006;15:366–70. [DOI] [PubMed] [Google Scholar]