Abstract
Cardiovascular diseases (CVD) are more prevalent among HIV-infected individuals. We examined the associations between carotid artery intima-media thickness (CIMT), conventional CVD risk factors, and HIV-related risk factors among Hispanics with HIV infection. This cross-sectional study involved 96 consecutive HIV patients on stable antiretroviral therapy and without history of CVD in a university-based outpatient clinic who underwent carotid ultrasound evaluation. Increased CIMT was defined as common carotid artery (CCA)-CIMT values ≥75th percentile for the patient’s age, sex, and race/ethnicity based on CIMT nomograms from large population studies. The sample was comprised of 96 Hispanic Americans aged 39.7±11.9, 89% of whom were men, 64% were on a protease inhibitor, and 11% had increased CIMT (95% CI, 5.9–19.6%). In univariable analysis, increased CIMT was significantly associated (p<0.05) with older age, metabolic syndrome, intermediate/high Framingham risk score, HIV infection duration ≥5 years, integrase inhibitors, and protease inhibitors. In multivariable analysis, only Framingham risk score (p=0.009) was independently associated with increased CIMT. The median CCA-CIMT value was significantly greater in patients with intermediate/high compared with those with low Framingham risk score (0.60 versus 0.49 mm; p<0.001). In conclusion, given the significant association between increased CIMT and Framingham risk score, adherence to prevention guidelines to reduce CVD risk factor burden in this population is strongly recommended.
Keywords: Atherosclerosis, Human immunodeficiency virus, Carotid intima-media thickness, Cardiovascular disease
INTRODUCTION
The growing prevalence of HIV worldwide is partly from the increased longevity due to combined antiretroviral therapy.1 Among the approximately 36.7 million HIV-infected individuals,2 over 1.2 million reside in the US.3 These patients are at an increased risk for age-related health conditions, especially cardiovascular disease (CVD),4 as a result of chronic inflammatory disease and immune activation.5–6 Identifying CVD-free HIV patients who might benefit the most from risk-reduction interventions is challenging using conventional tools,3,7 thus supporting vascular imaging techniques,8 such as those measuring carotid intima-media thickness (CIMT), as a predictor of future CVD.9,10 Furthermore, CVD burden has not been well assessed in Hispanic patients with HIV. The associations between CIMT, conventional CVD risk factors, and HIV-related risk factors in Hispanic patients with HIV were assessed in this study.
METHODS
This was a cross-sectional study of consecutive outpatients recruited from the HIV clinic at the Texas Tech University Health Sciences Center, El Paso, Texas, between 03/01/2013 and 02/28/2014. To be enrolled in this study, patients must have been ≥18 years old with HIV infection, on combined antiretroviral therapy for at least 3 months, and had given consent to undergo carotid ultrasound evaluation for cardiovascular risk assessment. Patients were considered to have HIV infection if the screening test (ELISA test kit) and confirmatory test (Western blot) were both positive. Patients with recent change in combination antiretroviral therapy (<3 months), recent diagnosis of AIDS (<3 months), or a known history of atherosclerotic vascular disease (coronary artery disease, cerebrovascular disease, peripheral artery disease, or the presence of carotid plaque on carotid ultrasound examination) were excluded. The institutional review board of Texas Tech University Health Science Center, El Paso approved this study, and each patient signed a written informed consent. Once eligible, patients were given an appointment for carotid ultrasound imaging
Detailed medical history was collected using the clinic’s electronic medical records (EMR) and in person at the time of enrollment. Medication use, fasting blood glucose, lipid profiles, and HIV-related measures (CD4 cell count and HIV-RNA copies) were obtained from the office visit closest to the time of enrollment (≤4 month before or after the date of enrollment). Other cardiovascular risk factors recorded were hypertension, diabetes mellitus, body mass index (BMI), current/former cigarette smoking, and metabolic syndrome. Diabetes mellitus was defined as having ≥2 fasting blood glucose measures >126 mg/dl or treatment with glucose-lowering medication. BMI was defined as weight in kilograms divided by height in meters squared. Based on the National Cholesterol Education Program-Adult Treatment Panel III criteria, metabolic syndrome was defined as the presence of ≥3 of the following components: 1) waist circumference ≥88 cm in women or ≥102 cm in men; 2) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or use of antihypertensive medications; 3) fasting triglyceride ≥150 mg/dl or use of lipid lowering medications; 4) fasting blood glucose ≥100 mg/dl or use of glucose-lowering medications; 5) HDL-C <50 mg/dl in women or <40 mg/dl in men.11 Framingham Risk Score was calculated using the established calculator.12,13 Scores of 10–20% were considered to be intermediate while scores >20% were classified as high-risk for predicting the 10-year risk of cardiovascular events (myocardial infarction, death due to coronary heart disease, or ischemic stroke).
After enrollment, subjects underwent high resolution scanning of the bilateral common carotid arteries (CCA) using a 7 MHz (Philips IU22, Bothell, WA, USA) linear array transducer probe following the recommendations of the consensus statement of the American Society of Echocardiography Carotid Intima-Media Thickness (CIMT) Task Force.9 The posterior (far) arterial wall was imaged 1.5 cm from the bifurcation of the CCA. The far wall intimal media thickness (IMT) was measured offline by a single reader blinded to subject characteristics using a semi-automated and validated algorithm (QLab 4.2.1 with IMT plug-in, version 1.1; Philips Ultrasound, Bothell, WA, USA). The software calculates the mean and standard deviation (SD) for the IMT thickness in a region-of interest box. Cine clips were obtained of the bilateral carotid arteries and images were gated to diastole. The presence of carotid plaque was noted and defined as CIMT greater than 1.5 mm or CIMT more than 50% greater than the adjacent vessel wall. Subjects with carotid plaque were excluded from analyses.
Our main outcome of interest was increased CIMT, defined as an average mean far wall CCA-CIMT value ≥75th percentile for the patient’s age, sex, and race/ethnicity based on CIMT nomograms from large population studies.9 The mean CCA-CIMT was used as the outcome because of its reproducibility compared with other CIMT measures. Furthermore, CIMT values ≥75th percentile of normative data sets from observational studies were considered to be high and to indicate increased cardiovascular risk.9
Because our study is mainly in Hispanic Americans, we used the Multi-Ethnic Study of Atherosclerosis Study (MESA) data to determine the CCA-CIMT cut-off values in patients ≥45 years old.9,14 For patients <45 years old, we used data from the Bogalusa Heart Study, the Carotid Atherosclerosis Progression Study (CAPS), and the AXA Study.15–17
Quantitative variables were summarized using mean ±SD while categorical variables were summarized using frequency and percentages. The mean CCA-CIMT, maximal CCA-CIMT, and CCA-CIMT ≥75th percentile were estimated and reported along with the 95% confidence intervals (CI) using normal distribution or binomial distribution. Quantitative variables were compared between patients with CCA-CIMT ≥75th percentile and patients with CCA-CIMT <75th percentile using the two-sample t-test, while Fisher’s exact test was used to compare the categorical variables between those 2 groups. Given the sample size and number of patients with CCA-CIMT ≥75th percentile, the Firth’s logistic regression analyses using penalized maximum likelihood approach as opposed to conventional logistic regression models were conducted to identify the factors associated with having CCA-CIMT ≥75th percentile. The Firth’s logistic regression allows to account for small number of cases of binary outcomes to avoid small-sample bias. Initially, we conducted a full multivariable model with removing any non-significant variables (full model). Thereafter, backward variable selection with α = 0.15 for inclusion and to stay was used to develop the multivariable model (final multivariable model 2).18 CCA-CIMT (mm) did not follow a normal distribution in our study population, and hence, significance of difference in the CCA-CIMT (mm) between the groups of patients with low and intermediate/high Framingham risk score was evaluated using Mann-Whitney test, with p-value <0.05 considered significant. Statistical analyses were carried out using Stata version 15 software (StataCorp, College Station, Texas).
RESULTS
Of the 110 consented patients, 14 (13%) patients did not complete carotid ultrasound imaging and were excluded. Thus, we analyzed the data of 96 (87%) patients with complete data (Figure 1). Overall, 89% were men, the entire population was Hispanic American, and 62% were overweight/obese. 31% of the patients had metabolic syndrome, 60% had a low Framingham risk score, and 64% were receiving a protease inhibitor. Of the patients, 12% had diabetes mellitus, 53% were current/former cigarette smokers, and 45% reported the use of alcohol.
Figure 1.
Study participant flow chart. CIMT, carotid intima-media thickness; US, ultrasonography.
In the unadjusted analysis, the mean age was significantly higher for the patients with increased CCA-CIMT. Higher prevalence of metabolic syndrome was seen in the subjects with increased CCA-CIMT. Among the individual components of metabolic syndrome, the proportion of patients with triglycerides ≥150 trended to be higher in the patients who had CCA-CIMT ≥75th percentile (p=0.059). LDL cholesterol also trended to be higher in the patients with increased CCA-CIMT (0.084). Patients with CCA-CIMT ≥75th percentile had significantly higher intermediate or high Framingham risk scores compared with patients with CCA-CIMT <75th percentile (p<0.001); 73% of the patients with increased CCA-CIMT had HIV for more than 5 years, while only 33% of the patients with CCA-CIMT <75th percentile had HIV for more than 5 years (p=0.018). All the patients with CCA-CIMT ≥75th percentile were not treated with integrase inhibitors, while all of them were treated with protease inhibitors (PI) (Table 1).
Table 1.
Characteristics of patients and risk factors for increased carotid intima-media thickness in patients with HIV infection.
| Variable | Total Population | CIMT ≥75th percentile | CIMT <75th percentile | p-value |
|---|---|---|---|---|
| (N = 96) | (N= 11) | (N= 85) | ||
| Age (years) | 39.7±11.9 | 49.3±8.9 | 38.5±11.7 | 0.004 |
| Men | 85 (89%) | 11 (100%) | 74 (87%) | 0.35 |
| Hispanic | 96 (100%) | 11 (100%) | 85 (100%) | |
| Metabolic syndrome | 30 (31%) | 7 (64%) | 23 (27%) | 0.032 |
| BMI ≥25 (kg/ m2) | 59 (62%) | 9 (82%) | 50 (59%) | 0.20 |
| Waist circumference ≥88 (female) or ≥102 (Male) (cm) | 27 (28%) | 5 (46%) | 22 (26%) | 0.28 |
| SBP ≥130 or DBP ≥85(mmHg) | 34 (35%) | 5 (46%) | 29 (34%) | 0.51 |
| Triglycerides ≥150 (mg/dl) | 43 (45%) | 8 (73%) | 35 (41%) | 0.059 |
| HDL cholesterol <50 (female) or <40 (men) (mg/dl) | 41 (43%) | 4 (36%) | 37 (44%) | 0.75 |
| Fasting blood glucose ≥100 (mg/dl) | 25 (26%) | 5 (46%) | 20 (24%) | 0.15 |
| LDL cholesterol (mg/dl) | 89.6±30.7 | 108.9±44.2 | 87.1±27.9 | 0.084 |
| Diabetes Mellitus | 11 (12%) | 2 (18%) | 9 (11%) | 0.61 |
| Current/former smoking | 51 (53%) | 6 (55%) | 45 (53%) | 1.00 |
| Current/former alcohol use | 43 (45%) | 4 (36%) | 39 (46%) | 0.75 |
| Framingham risk score 10-year risk) | <.001 | |||
| High | 19 (20%) | 7 (64%) | 12 (14%) | |
| Intermediate | 19 (20%) | 3 (27%) | 16 (19%) | |
| Low | 58 (60%) | 1 (9%) | 57 (67%) | |
| CD4 cell count ≥200 (cells/μl) | 82 (85%) | 10 (91%) | 72 (85%) | 1.00 |
| HIV infection duration ≥5 (years) | 36 (38%) | 8 (73%) | 28 (33%) | 0.018 |
| HIV-RNA ≥200 (copies/ml) | 26 (27%) | 3 (27%) | 23 (27%) | 1.00 |
| Treatment | ||||
| Integrase inhibitors | 28 (29%) | 0 (0%) | 28 (33%) | 0.030 |
| NNRTI | 13 (14%) | 0 (0%) | 13 (15%) | 0.35 |
| NRTI | 89 (93%) | 10 (91%) | 79 (93%) | 0.59 |
| Protease inhibitors (PI) | 61 (64%) | 11 (100) | 50 (59%) | 0.006 |
Continuous variables are presented as mean ± SD, all others an n (%). BMI=body mass index; CIMT=carotid artery intima-media thickness; CVD=cardiovascular disease; DBP=diastolic blood pressure; HDL=high-density lipoprotein; LDL=low-density lipoprotein; NNRTI=Non-nucleoside reverse-transcriptase inhibitors; NRTI=Nucleoside reverse-transcriptase inhibitors; RNA= ribonucleic acid; SBP= systolic blood pressure; SD=standard deviation.
The average mean and average maximal CCA-CIMTs were 0.55 (95% CI, 0.53–0.57) and 0.59 (95% CI, 0.56–0.62) mm, respectively. The proportion of patients with average mean CCA-CIMT ≥75th percentile was 11/96 or 11% (95% CI, 5.9%−19.6%). Of the 96 patients, 60% had average mean and average maximal CCA-CIMT values less than 0.6 mm. (Table 2a and Table 2b).
Table 2a.
Distribution of carotid intima-media thickness in the study population.
| Range (mm) | Average maximal CIMT | Average mean CIMT | ||
|---|---|---|---|---|
| Count by group | Percent by group | Count by group | Percent by group | |
| 0.4–0.445 | 9 | (9%) | 19 | (20%) |
| 0.45–0.495 | 13 | (14%) | 19 | (20%) |
| 0.5–0.545 | 17 | (18%) | 19 | (20%) |
| 0.55–0.595 | 21 | (22%) | 15 | (16%) |
| 0.6–0.645 | 11 | (12%) | 10 | (10%) |
| 0.65–0.69 | 11 | (12%) | 3 | (3%) |
| 0.7–0.745 | 1 | (1%) | 2 | (2%) |
| 0.75–0.79 | 4 | (4%) | 3 | (3%) |
| 0.8–0.845 | 3 | (3%) | 4 | (4%) |
| 0.85–0.9 | 4 | (4%) | 1 | (1%) |
| >0.9 | 2 | (2%) | 1 | (1%) |
CIMT = carotid artery intima-media thickness.
Table 2b:
Carotid artery intima-media thickness values in the study population.
| Variable | Mean CIMT | 95% Confidence interval | Median CIMT | Range (maximum-minimum) CIMT | ≥75% percentile CIMT value for age and gender |
|---|---|---|---|---|---|
| (N=96) | (N=96) | (N=96) | (N=96) | (N=96) | |
| Right maximal value (mm) | 0.58 ±0.13 | [0.55–0.61] | 0.55 | 0.65 (1.05–0.40) | 6 |
| Right mean value (mm) | 0.54 ±0.12 | [0.52–0.56] | 0.51 | 0.60 (1.00–0.40) | 48 |
| Left maximal value (mm) | 0.60 ±0.15 | [0.57–0.63] | 0.58 | 0.67 (1.07–0.40) | 8 |
| Left mean value (mm) | 0.56 ±0.13 | [0.53–0.59] | 0.53 | 0.62 (1.02–0.40) | 36 |
| Average mean value (mm) | 0.55 ±0.12 | [0.53–0.57] | 0.53 | 0.57 (0.97–0.40) | 11 |
| Average maximal value (mm) | 0.59 ±0.13 | [0.56–0.62] | 0.57 | 0.61 (1.02–0.41) | N/A* |
CIMT=carotid intima-media thickness.
None of the large population studies have reported maximum far wall common carotid artery carotid intima-media thickness values for patients <60 years old.
Table 3 shows the multivariable penalized maximum likelihood logistic regression models for the association between risk factors and increased CCA-CIMT. Age, sex, metabolic syndrome, current/former smoking, current/former alcohol use, LDL cholesterol, diabetes, Framingham risk score, CD4 cell count, HIV infection duration ≥5 years, and HIV-RNA copies (viral load) were included in the full multivariable model. After backward variable selection, Framingham risk score and HIV infection duration ≥5 years remained in the final model. Among all considered variables, Framingham risk score was found to be the only statistically significant variable. Patients with high or intermediate Framingham risk score were more likely to have increased CCA-CIMT (adjusted odds ratio [OR], 10.95; 95% CI, 1.83–65.37; p=0.009) after adjustment. Figure 2 shows that the median CCA-CIMT value was significantly greater in patients with intermediate/high Framingham risk score compared to patients with low Framingham risk score (0.60 mm versus 0.49 mm; p<0.001).
Table 3.
Multivariable penalized maximum likelihood logistic regression models for the association between risk factors and increased carotid intima-media thickness (α = 0.15).
| Cofactor | Full model | Final model | ||
|---|---|---|---|---|
| OR [95% CI] | p-value | OR [95% CI] | p-value | |
| Age (years) | 1.05 [0.97–1.14] | 0.25 | ||
| Men | 2.25 [0.09–55.58] | 0.62 | ||
| Metabolic syndrome | 3.37 [0.68–16.81] | 0.14 | ||
| Current/ former smoking | 1.19 [0.27–5.32] | 0.82 | ||
| Current/ former alcohol use | 0.9 [0.22–3.63] | 0.88 | ||
| LDL cholesterol (mg/dl) | 1.02 [1.00–1.04] | 0.12 | ||
| Diabetes | 1.63 [0.25–10.57] | 0.61 | ||
| Framingham risk score: High or intermediate | 3.13 [0.33–29.72] | 0.32 | 10.95 [1.83–65.37] | 0.009 |
| CD4 cell count ≥ 200 (cells/μl) | 0.28 [0.02–3.40] | 0.32 | ||
| HIV infection duration ≥ 5 (years) | 2.01 [0.47–8.66] | 0.35 | 3.21 [0.79–13.05] | 0.10 |
| HIV-RNA (copies/ml) | 1.57 [0.27–9.32] | 0.62 | ||
CI=Confidence interval; HIV=Human immunodeficiency virus; LDL=low-density lipoprotein; OR, Odds ratio; RNA, ribonucleic acid.
Figure 2.
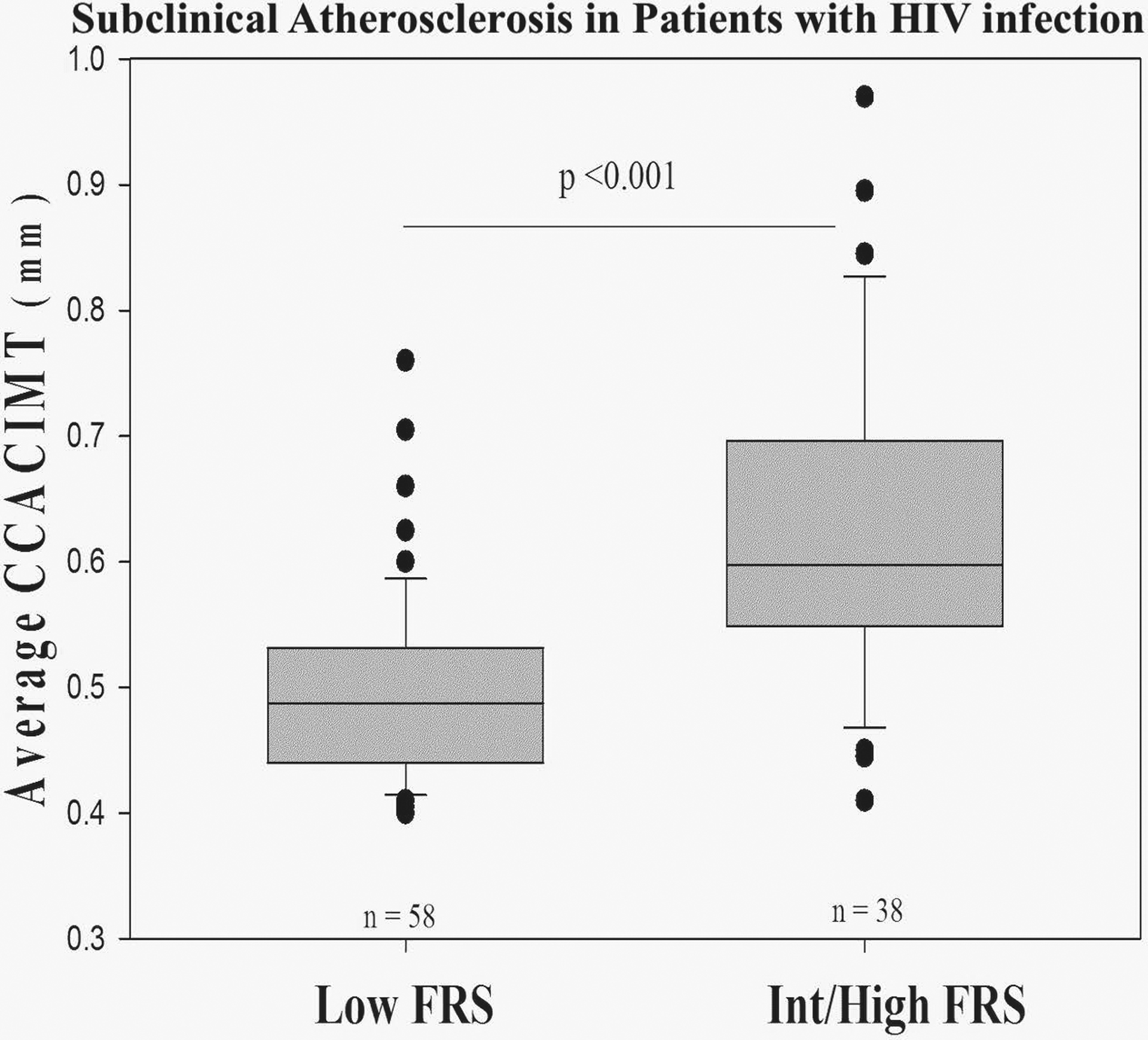
Subclinical Atherosclerosis in Patients with HIV infection: The box plot shows median and minimum to maximum range of CCA-CIMT (mm) in groups of HIV patients with low (n=58) and intermediate/high (n=38) Framingham risk score. Two-tailed p-value was calculated using Mann-Whitney rank-sum test. CCA, common carotid artery; CIMT, carotid intima-media thickness; FRS, Framingham risk score; •, outlier values of the average CCA-CIMT (mm).
DISCUSSION
Our results show that 11% of the study population had increased CCA-CIMT, a noninvasive measure of early systemic atherosclerotic vascular disease that independently predicts incident CVD events. HIV patients at high or intermediate CVD risk using the Framingham risk score were more likely to have increased CCA-CIMT.
The etiology of CVD in patients with HIV is multifactorial and includes HIV infection itself, chronic inflammation, immune dysfunction, and metabolic abnormalities due to certain antiretroviral therapies. These factors are further confounded by higher rates of smoking, dyslipidemia, metabolic syndrome, and diabetes among patients with HIV.19 Although significant progress has been made in the management of patients with HIV, treatment with combined antiretroviral therapy alone does not effectively prevent CVD in patients with HIV. In fact, previous studies have suggested that certain antiretroviral therapies are associated with increased cardiovascular adverse effects such as dyslipidemia, metabolic syndrome, and diabetes.20,21 Thus, the presence of HIV infection continues to be an important CVD risk factor despite the use of effective antiretroviral therapy. A recent meta-analysis has reported that HIV infection is associated with an increased incidence of CVD that is of a magnitude comparable to that of diabetes mellitus.4 Indeed, implementation of prevention strategies to simultaneously manage multiple risks factors would offer a valuable opportunity to reduce CVD burden in this population.
The use of rosuvastatin in patients with HIV on antiretroviral therapy was previously reported to slow the progression of CCA-CIMT in a randomized clinical trial.22 In another randomized clinical trial to assess the cardiovascular benefits of statin therapy in this population, the authors demonstrated that noncalcified coronary plaque volume and high-risk coronary plaque features were reduced with the use of atorvastatin during a one-year period.23 Furthermore, the benefit of statin therapy in patients with HIV infection for primary prevention is supported by the American College of Cardiology/American Heart Association (AHA/ACC) in the recently released 2018 guidelines for the management of blood cholesterol.24
In our study we also found an independent association between high or intermediate CVD risk using the Framingham risk score and increased CCA-CIMT which are consistent with other studies. The PARC study evaluated the association between CCA-CIMT and the Framingham risk score in a large HIV-negative population with and without risk factors for atherosclerosis. They found a significant association between CCA-CIMT and the Framingham risk score.25 A cross-sectional study of CVD risk among 331 HIV patients showed a significant association between high or intermediate CVD risk using the Framingham risk score and increased CCA-CIMT.26 Our findings are consistent with those of Stein et al. and suggest that conventional CVD risk factors as reflected by the Framingham risk score are strongly associated with increased CCA-CIMT in this population.
We found older age was not a strong independent predictor of increased CCA-CIMT in our adjusted analyses, whereas Mercie et al.27 found the opposite. Mercie et al. in a multi-center study of 423 HIV patients with a mean age of 41 years, found a significant correlation between increased CIMT and older age. The patients recruited in our analyses were slightly younger (mean 39.7 years) and hence, one might expect the increased CCA-CIMT in our cohort was mainly driven by factors other than older age. Additionally, owing to the small sample size of our study population (wide CI) metabolic syndrome and higher LDL cholesterol remained non-significant in the final multivariable analyses. Metabolic syndrome among HIV patients is associated with a number of factors, such as chronic inflammation, antiretroviral therapy, older age, and Framingham risk score ≥10%.28 Both metabolic syndrome and higher LDL cholesterol have also been associated with increased CCA-CIMT.26
Our study has some limitations. This study was designed to assess the association between conventional CVD risk factors, HIV-related risk factors, and increased CCA-CIMT in an understudied and underserved population of patients with HIV. Given the nature of this study, the potential for detection bias could not be ruled out. In an attempt to lessen the impact of this bias, we implemented a consecutive sampling strategy to recruit a representative sample of the overall eligible patients. Since our study included only Hispanic Americans, our results may be less generalizable to other racial groups. Additionally, due to the small sample size, it was difficult to comment on the association between CCA-CIMT, antiretroviral therapy, and novel biomarkers.6 Lastly, this is a cross-sectional study; well designed, longitudinal, multiracial studies on CVD risk factors in patients with HIV would be useful.
In conclusion, in this analysis of HIV patients, the prevalence of increased CIMT is high and is associated with Framingham risk score. Physicians who care for HIV patients should adhere to the current guideline recommendations to reduce the CVD risk factor burden in this population.
Disclosures
Kyari Sumayin Ngamdu reports receiving postdoctoral training grant in cardiovascular research from the National Institute of Health (grant T32 HL007604).
Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, PLx Pharma, Takeda.
Acknowledgments
The authors thank Jorge Sarmiento, MD of Professional Radiology PLLC, 10400 Vista del Sol Dr Ste 104, El Paso, TX 79925, USA, for interpreting the carotid ultrasounds. This work was supported by a seed grant from the Department of Internal Medicine, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, El Paso, Texas.
Role of the Sponsor
Texas Tech University Health Sciences Center had no role in the design and conduct of study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Submission of Declaration
This manuscript has not been published in part or in whole and is not under consideration for publication elsewhere. All authors have read and agreed to its submission/publication.
References
- 1.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, Wang H, Duber HC, Naghavi M, Dicker D, Dandona L, Salomon JA, Heuton KR, Foreman K, Phillips DE, Fleming TD, Flaxman AD, Phillips BK, Johnson EK, Coggeshall MS, Abd-Allah F, Abera SF, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Adeyemo AO, Adou AK, Adsuar JC, Agardh EE, Akena D, Al Kahbouri MJ, Alasfoor D, Albittar MI, Alcala-Cerra G, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Amini H, Ammar W, Anderson BO, Antonio CA, Anwari P, Arnlov J, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Banerjee A, Basu S, Beardsley J, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Abdulhak AB, Binagwaho A, Blore JD, Basara BB, Bose D, Brainin M, Breitborde N, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang JC, Chiang PP, Chuang TW, Colomar M, Cooper LT, Cooper C, Courville KJ, Cowie BC, Criqui MH, Dandona R, Dayama A, De Leo D, Degenhardt L, Del Pozo-Cruz B, Deribe K, Des Jarlais DC, Dessalegn M, Dharmaratne SD, Dilmen U, Ding EL, Driscoll TR, Durrani AM, Ellenbogen RG, Ermakov SP, Esteghamati A, Faraon EJ, Farzadfar F, Fereshtehnejad SM, Fijabi DO, Forouzanfar MH, Fra Paleo U, Gaffikin L, Gamkrelidze A, Gankpe FG, Geleijnse JM, Gessner BD, Gibney KB, Ginawi IA, Glaser EL, Gona P, Goto A, Gouda HN, Gugnani HC, Gupta R, Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Haro JM, Havmoeller R, Hay SI, Hedayati MT, Pi IB, Hoek HW, Hornberger JC, Hosgood HD, Hotez PJ, Hoy DG, Huang JJ, Iburg KM, Idrisov BT, Innos K, Jacobsen KH, Jeemon P, Jensen PN, Jha V, Jiang G, Jonas JB, Juel K, Kan H, Kankindi I, Karam NE, Karch A, Karema CK, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Keren A, Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Khonelidze I, Kinfu Y, Kinge JM, Knibbs L, Kokubo Y, Kosen S, Defo BK, Kulkarni VS, Kulkarni C, Kumar K, Kumar RB, Kumar GA, Kwan GF, Lai T, Balaji AL, Lam H, Lan Q, Lansingh VC, Larson HJ, Larsson A, Lee JT, Leigh J, Leinsalu M, Leung R, Li Y, De Lima GM, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, Lotufo PA, Machado VM, Maclachlan JH, Magis-Rodriguez C, Majdan M, Mapoma CC, Marcenes W, Marzan MB, Masci JR, Mashal MT, Mason-Jones AJ, Mayosi BM, Mazorodze TT, McKay AC, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Melaku YA, Memish ZA, Mendoza W, Miller TR, Mills EJ, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Moore AR, Mori R, Moturi WN, Mukaigawara M, Murthy KS, Naheed A, Naidoo KS, Naldi L, Nangia V, Narayan KM, Nash D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nisar MI, Nolte S, Norheim OF, Nowaseb V, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira DM, Pervaiz A, Pesudovs K, Petzold M, Pourmalek F, Qato D, Quezada AD, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Ur Rahman S, Raju M, Rana SM, Razavi H, Reilly RQ, Remuzzi G, Richardus JH, Ronfani L, Roy N, Sabin N, Saeedi MY, Sahraian MA, Samonte GM, Sawhney M, Schneider IJ, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Sheikhbahaei S, Shibuya K, Shin HH, Shiue I, Shivakoti R, Sigfusdottir ID, Silberberg DH, Silva AP, Simard EP, Singh JA, Skirbekk V, Sliwa K, Soneji S, Soshnikov SS, Sreeramareddy CT, Stathopoulou VK, Stroumpoulis K, Swaminathan S, Sykes BL, Tabb KM, Talongwa RT, Tenkorang EY, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Towbin JA, Traebert J, Tran BX, Dimbuene ZT, Tsilimbaris M, Uchendu US, Ukwaja KN, Uzun SB, Vallely AJ, Vasankari TJ, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Waller S, Wallin MT, Wang L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman R, White RA, Wilkinson JD, Williams TN, Woldeyohannes SM, Wong JQ, Xu G, Yang YC, Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis M, Yu C, Jin KY, El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD, Vos T. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:1005–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Global AIDS update 2016. Joint United Nations Programme on HIV/AIDS. 2016. [Google Scholar]
- 3.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, Budoff MJ, Mathews WC, Kitahata MM, Saag MS, Eron JJ, Moore RD, Achenbach CJ, Lloyd-Jones DM, Crane HM. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol 2017;2:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, Rajagopalan S, Kottilil S, Nair H, Newby DE, McAllister DA, Mills NL. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–489c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W, Wang X, Deik AA, Hanna DB, Wang T, Haberlen SA, Shah SJ, Lazar JM, Hodis HN, Landay AL, Yu B, Gustafson D, Anastos K, Post WS, Clish CB, Kaplan RC, Qi Q. Elevated Plasma Ceramides Are Associated With Antiretroviral Therapy Use and Progression of Carotid Artery Atherosclerosis in HIV Infection. Circulation 2019;139:2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson-Paul AM, Lichtenstein KA, Armon C, Palella FJ Jr., Skarbinski J, Chmiel JS, Hart R, Wei SC, Loustalot F, Brooks JT, Buchacz K Cardiovascular Disease Risk Prediction in the HIV Outpatient Study. Clin Infect Dis 2016;63:1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor AJ, Merz CN, Udelson JE. 34th Bethesda Conference: Executive summary--can atherosclerosis imaging techniques improve the detection of patients at risk for ischemic heart disease? J Am Coll Cardiol 2003;41:1860–1862. [DOI] [PubMed] [Google Scholar]
- 9.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 11.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 13.Pursnani A, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Guideline-Based Statin Eligibility, Coronary Artery Calcification, and Cardiovascular Events. Jama 2015;314:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113:30–37. [DOI] [PubMed] [Google Scholar]
- 15.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, Chen W, Berenson GS, Stein JH. Distribution and predictors of carotid intima-media thickness in young adults. Prev Cardiol 2007;10:181–189. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87–92. [DOI] [PubMed] [Google Scholar]
- 17.Denarie N, Gariepy J, Chironi G, Massonneau M, Laskri F, Salomon J, Levenson J, Simon A. Distribution of ultrasonographically-assessed dimensions of common carotid arteries in healthy adults of both sexes. Atherosclerosis 2000;148:297–302. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–936. [DOI] [PubMed] [Google Scholar]
- 19.Triant VA. Epidemiology of coronary heart disease in patients with human immunodeficiency virus. Rev Cardiovasc Med 2014;15 Suppl 1:S1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012;13:453–468. [DOI] [PubMed] [Google Scholar]
- 21.Bavinger C, Bendavid E, Niehaus K, Olshen RA, Olkin I, Sundaram V, Wein N, Holodniy M, Hou N, Owens DK, Desai M. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One 2013;8:e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. Aids 2016;30:2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L Jr, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation 2019. June 18;139(25):e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touboul PJ, Vicaut E, Labreuche J, Belliard JP, Cohen S, Kownator S, Portal JJ, Pithois-Merli I, Amarenco P. Correlation between the Framingham risk score and intima media thickness: the Paroi Arterielle et Risque Cardio-vasculaire (PARC) study. Atherosclerosis 2007;192:363–369. [DOI] [PubMed] [Google Scholar]
- 26.Stein JH, Brown TT, Ribaudo HJ, Chen Y, Yan M, Lauer-Brodell E, McComsey GA, Dube MP, Murphy RL, Hodis HN, Currier JS. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. Aids 2013;27:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercie P, Thiebaut R, Lavignolle V, Pellegrin JL, Yvorra-Vives MC, Morlat P, Ragnaud JM, Dupon M, Malvy D, Bellet H, Lawson-Ayayi S, Roudaut R, Dabis F. Evaluation of cardiovascular risk factors in HIV-1 infected patients using carotid intima-media thickness measurement. Ann Med 2002;34:55–63. [DOI] [PubMed] [Google Scholar]
- 28.Bonfanti P, De Socio GV, Ricci E, Antinori A, Martinelli C, Vichi F, Penco G, Madeddu G, Orofino G, Valsecchi L, Rusconi S, Menzaghi B, Pocaterra D, Quirino T. The feature of Metabolic Syndrome in HIV naive patients is not the same of those treated: results from a prospective study. Biomed Pharmacother 2012;66:348–353. [DOI] [PubMed] [Google Scholar]


