Abstract
Objective
To evaluate subsequent rate of thrombosis among obstetric antiphospholipid syndrome (Ob-APS) women in a multicenter database of antiphospholipid antibody (aPL)-positive patients; and clinical utility of adjusted Global Antiphospholipid Syndrome Score (aGAPSS), a validated tool to assess the likelihood of developing new thrombosis, in this group of patients.
Design
Retrospective study.
Setting
APS Alliance For Clinical Trials & International Networking (APS ACTION) Clinical Database And Repository.
Population
Women with Ob-APS.
Methods
Comparison of clinical and laboratory characteristics; measurement of aGAPSS of Ob-APS women with or without thrombosis after initial pregnancy morbidity (PM).
Main Outcome Measures
Risk factors for thrombosis, aGAPSS.
Results
Of 550 patients, 126 had Ob-APS; 74/126 (59%) presented thrombosis, and 47 (63%) of them developed thrombosis after initial PM, in a mean time of 7.6 ± 8.2 years (4.9/100 patient years). Younger age of Ob-APS, additional cardiovascular risk factors, superficial vein thrombosis, heart valve disease, and multiple aPL positivity increased the risk of first thrombosis after PM. Women with thrombosis after PM had higher aGAPSS compared to those with Ob-APS alone ([median 11.5 [4-16] vs 9 [4-13], P = 0.0089]).
Conclusion
Based on retrospective analysis of our multicenter aPL database, 63% of Ob-APS women developed thrombosis after initial obstetric morbidity; additional thrombosis risk factors, selected clinical manifestations, and high-risk aPL profile increased risk. Women with subsequent thrombosis after Ob-APS had higher aGAPSS score at registry entry. We believe that aGAPSS is a valid tool to improve risk stratification in aPL-positive women. There was no funding for this study.
Keywords: Antiphospholipid antibodies, antiphospholipid syndrome, thrombosis, preeclampsia, abortion, fetal death
Tweetable abstract
More than 60% of obstetric antiphospholipid syndrome women had thrombosis after initial pregnancy morbidity
Introduction:
Antiphospholipid syndrome (APS) is a multisystem disease that can present with thrombosis and/or obstetric complications in patients with persistently positive antiphospholipid antibodies (aPL).1 Based on the Updated Sapporo APS classification criteria, obstetric APS (Ob-APS) is defined as: one or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation (fetal loss); one or more premature births of a morphologically normal neonate before the 34th week of gestation due to eclampsia or severe preeclampsia; or three or more unexplained consecutive spontaneous abortions before the 10th week of gestation.1
While recent studies suggest that women with pure Ob-APS are at increased risk for future thrombosis compared to women without APS2-5, identifying the subgroup of these patients who are at higher risk for future thrombosis is an unmet clinical need. Concomitant systemic lupus erythematosus diagnosis, cardiovascular disease risk factors, or high-risk aPL profile may increase the risk of thrombosis after an aPL-related pregnancy morbidity.3,6-8 In this context, the use of a thrombosis scoring system, such as the Global Antiphospholipid Syndrome Score (GAPSS), may help risk stratify Ob-APS women for future thrombosis risk by subgroups based on traditional cardiovascular risk factors and aPL profile.
The objectives of this retrospective study were to evaluate the subsequent rate of thrombosis among Ob-APS women in a multicenter database of aPL-positive patients, and to evaluate the clinical utility of GAPSS as a tool to identify women at higher future thrombosis risk after presenting with Ob-APS. Our hypotheses are that women presenting with an aPL-related pregnancy morbidity are at increased risk for future thrombosis, and GAPSS is a useful tool to identify the subgroup of these high-risk patients.
Methods:
APS ACTION Clinical Database and Repository (“Registry”):
The APS ACTION Registry was created to study the natural disease course over at least 10 years in persistently aPL-positive patients with/without other systemic autoimmune diseases.9 Each center had ethics committee approval and all patients signed informed consent before enrolling the registry. A web-based data capture system is used to store patient demographics, aPL-related history, and medications. The inclusion criteria are positive aPL based on the Updated Sapporo APS Classification Criteria1 at least twice, greater than 12 weeks apart, within one year prior to enrollment. For the purpose of this retrospective baseline registry analysis, we included Ob-APS women with or without thrombosis after the initial diagnosis of pregnancy morbidity. The retrospective study follow-up period is from the first Ob-APS manifestation to thrombosis or registry entry.
Data retrieved were age and type of first pregnancy morbidity (embryonic loss before 10 weeks of gestation, fetal loss after ten weeks of gestation, premature birth, and preeclampsia), age and type of thrombosis (arterial or venous), other autoimmune diseases, cardiovascular risk factors (hypertension on medication, diabetes on medication, hyperlipidemia on medication, obesity [BMI > 30], and smoking) at the time of the registry entry, non-criteria manifestations of APS (thrombocytopenia, hemolytic anemia, livedo reticularis, aPL, nephropathy, and valve disease); aPL data, and medications. There was no funding and patients were not involved in this study.
Global Antiphospholipid Syndrome Score (GAPSS):
Global Antiphospholipid Syndrome Score is a validated tool to assess the likelihood of developing new thrombosis, which was originally developed based on lupus patients10 and then validated in primary APS patients.11 Global Antiphospholipid Syndrome Score includes the following points based on a linear transformation derived from the B regression: positive anticardiolipin antibody IgG/M is scored five points; antβ2 glycoprotein-I IgG/M four points; lupus anticoagulant test four points; anti-phosphatidylserine/prothrombin antibodies (aPS/PT) IgG/IgM three points; hyperlipidemia three points; and arterial hypertension one point. For the purpose of our analysis, we used the adjusted version of GAPSS (aGAPSS), which excludes aPS-PT, as this test was not available for most of the registry patients.
The primary study outcome was documented thrombosis (venous and/or arterial), confirmed by imaging studies.
Statistical analysis:
Although patients included in APS ACTION registry are followed prospectively, in this retrospective study we analyzed the baseline clinical and laboratory characteristics of aPL-positive women presenting with pregnancy morbidity with a comparison between those with and without subsequent thromboses. We also calculated the mean cumulative adjusted GAPSS (aGAPSS) for each group.10
The univariate analysis was performed using the Pearson, χ2 and Fisher exact tests to assess the association between thrombosis and risk factors. The demographic, clinical and serologic parameters considered in the univariate analysis are listed in Table 1. Multivariate logistic regression analysis was performed to identify significant independent factors adjusted for the potential confounding risk factors able to predict thrombosis. The final multivariate logistic regression model included the following variables: age, diagnosis of concomitant autoimmune disease, cardiovascular risk factors, aPL profile, type of pregnancy morbidity, and treatment. The forward conditional techniques were used to finalize the model.
Table 1.
Clinical and Laboratory Characteristics of Women with Obstetric Antiphospholipid Syndrome (Obs-APS) in APS ACTION registry with/without Subsequent Non-gravid Thrombosis.
| Variables, n (%) | Obstetric APS only (n=52) |
Obstetric APS followed by Thrombosis (n=47) |
p value |
|---|---|---|---|
| Demographics | |||
| Age of first pregnancy morbidity | 28.9 ± 6.77 | 26.25 ± 5.52 | 0.03 |
| Associated Autoimmune Disease | |||
| No other autoimmune disease | 28 (53.8%) | 29 (61.7%) | 0.21 |
| SLE | 12 (23.0%) | 8 (17.0%) | 0.22 |
| Lupus-like disease (3 American College of Rheumatology criteria for lupus) | 6 (11.5%) | 2 (4.2%) | 0.09 |
| Other | 6 (11.5%) | 8 (17.0%) | 0.43 |
| Vascular Events | |||
| Venous Thrombosis | NA | 25 (53.1%) | NA |
| Arterial Thrombosis | NA | 17 (36.1%) | NA |
| Venous and Arterial Thrombosis | NA | 5 (10.6%) | NA |
| Cardiovascular Risk Factors at registry entry | |||
| Hypertension on medication | 11 (21.1%) | 20 (42.5) | 0.01 |
| Diabetes on medication | 1 (1.9%) | 3 (6.3%) | 0.13 |
| Hyperlipidemia on medication | 3 (5.7%) | 8 (17.0%) | 0.03 |
| Obesity (BMI > 30) | 6 (11.5%) | 11 (23.4%) | 0.06 |
| Smoking (ever) | 9 (17.3%) | 18 (38.2%) | 0.009 |
| First Pregnancy Morbidity | |||
| Fetal Loss | 34 (65.3%) | 30 (63.8%) | 0.43 |
| Premature Birth < 34 week | 14 (26.9%) | 12 (25.5%) | 0.43 |
| ≥ Three (pre)-embryonic loss | 4 (7.6%) | 5 (10.6%) | 0.30 |
| Non-Criteria Manifestations | |||
| Superficial Vein Thrombosis | 1 (1.9%) | 6 (12.7%) | 0.01 |
| Transient Ischemic Attack | 4 (7.6%) | 7 (14.8%) | 0.12 |
| Livedo | 6 (11.5%) | 11 (23.4%) | 0.06 |
| Thrombocytopenia | 12 (23.0%) | 10 (21.2%) | 0.41 |
| Hemolytic Anemia | 3 (5.7%) | 4 (8.5%) | 0.29 |
| Heart Valve Disease | 1 (1.9%) | 6 (12.7%) | 0.01 |
| Skin Ulcer | 0 | 4 (8.5%) | NA |
| aPL-Nephropathy | 2 (3.8%) | 0 | NA |
| Laboratory parameters | |||
| Lupus Anticoagulant (alone or with other autoantibodies) | 35 (67.3%) | 42 (89.3%) | 0.004 |
| Triple Positivity | 17 (32.6%) | 13 (27.6%) | 0.29 |
Results:
Of 550 patients included in the APS ACTION registry as of May 2015, 419 (76%) were female. We excluded 131 (31%) women with no pregnancy history, and 162 (39%) with history of pregnancy but who did not fulfill the Updated APS Classification Criteria for Ob-APS (with/without any morbidity).1 Of the remaining 126 (30%) women with Ob-APS, 74 (59%) had a history of thrombosis at time of cohort entry (venous: 43; arterial: 22; and both: 9): 47 (64%) after pregnancy morbidity and 27 (36%) before Ob-APS. For the purpose of this study, only women with vascular thrombosis after the initial pregnancy morbidity (n = 47) and those with Ob-APS without thrombosis (n = 52) were included.
The clinical and laboratory characteristics of Ob-APS women with or without thrombosis after pregnancy morbidity are described in Table 1. Fetal loss was the most common pregnancy morbidity in both groups (65% and 64%, respectively). The clinical and laboratory characteristics of women were not different except women with thrombosis after Ob-APS, compared to those with pure Ob-APS: a) had the first pregnancy morbidity at a younger age (26.2 ± 5.5 vs 28.9 ± 6.7 years, p=0.03); b) more frequently had superficial vein thrombosis (6 vs 1, p=0.01) and heart valve disease (6 vs 1, p=0.01); c) more frequently had hypertension (21 vs 11, p=0.01), hyperlipidemia (8 vs 3, p=0.03), and smoking history (18 vs 9, p=0.009) at study entry; and d) more frequently were positive for lupus anticoagulant (alone or with other aPL) (42 vs 35, p=0.004). The mean age of inclusion in the registry of women with Ob-APS without thrombosis was 40.8 years (± 9.8).
Among Ob-APS women with subsequent thrombosis, the mean time between pregnancy morbidity and thrombosis was 7.6 ± 8.2 years (4.9 per 100 patient years) (figure S1). Based on the registry entry data, at least one cardiovascular risk factor and multiple aPL positivity (defined as positivity for more than one aPL criteria test1) were identified using stepwise multivariate logistic regression analysis as independent risk factors for thrombosis (Table S1).
Obstetric-APS women with subsequent thrombosis after pregnancy morbidity had higher aGAPSS than those with Ob-APS alone ([median 11.5 [4-16] vs 9 [4-13], p = 0.0089], data shown as box-and-whisker plot in Figure 1). Higher aGAPSS were also shown after a subgroup analysis of the type of thrombosis (12 [4-16] for arterial thrombosis, 11 [4-13] for venous thrombosis, and 9 [4-13] for Ob-APS alone, p = 0.038 and p = 0.044, respectively).
Figure 1. Global Antiphospholipid Syndrome (APS) Score Based on Obstetric APS (OAPS) versus Obstetric and Thrombotic APS (O+T APS).
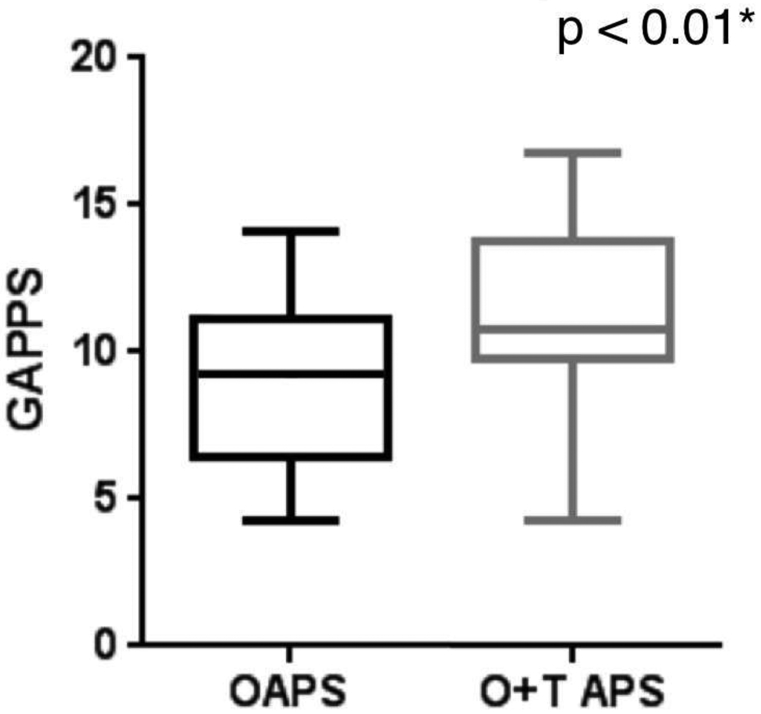
Data are shown as box plots, where each box represents the 25th–75th percentiles: lines inside the box represent the median. The whiskers represent the 95% CI.
* Assessed by t-test
Discussion:
Main Findings
This is the first multicenter international large scale analysis of Ob-APS women for their risk of first thrombosis after the initial pregnancy morbidity. In addition, our study is the first attempt to quantify the thrombosis risk of these women. In our cohort, we observed that 63% of APS women presenting with pregnancy morbidity eventually developed thrombosis after a mean time of 7.6 years (4.9 per 100 patient years), which was independently associated with multiple aPL positivity. We also found that pregnancy morbidity at a younger age, concomitant cardiovascular risk factors, and non-criteria manifestations (namely superficial vein thrombosis and heart valve disease) were predictors of new thrombosis.
Strengths and Limitations
Our study has several strengths and limitations. Although our study is one of the largest international analyses of the association between Ob-APS and subsequent thrombosis, the study is limited by retrospective, case control study design. Similarly the retrospective assessment of cardiovascular disease risk factors at the time of the registry entry, but not at the time of thrombosis, limits the accuracy of aGAPSS.
Interpretation
The increased risk of thrombosis following pregnancy morbidity in aPL-positive women, compared to general population, has been previously described both retrospectively2 and prospectively3; although not all studies agree4. A 10-year prospective study of 1,592 women with three consecutive spontaneous abortions before the 10th week of gestation or one fetal death at or beyond the 10th week of gestation compared the frequencies of thrombosis among women with pregnancy morbidity with positive aPL (n: 517), women carrying the coagulation factor polymorphisms F5 6025 or F2 rs1799963 (n: 279), and women with negative thrombophilia screening results (n: 796).3 Annual rates of deep vein thrombosis (1.46%; range: 1.15%-1.82%), pulmonary embolism (0.43%; range: 26%-0.66%), superficial vein thrombosis (0.44%; range: 0.28%-0.68%), and cerebrovascular events (0.32%; range: 0.18%-0.53%) were significantly higher in women with aPL than in the other groups, despite low-dose aspirin. On the other hand, one study described a thrombosis rates after fetal loss in women with APS to be of 1.3 and 7.4 per 100 patient-years in aspirin-treated and untreated women, respectively.5 A retrospective cohort of 32 women with Obs-APS treated with aspirin reported an overall thrombosis rate of 3.3 per 100 patient-year; however, thrombosis rate with double or triple aPL positivity was 4.6 per patient-year (n:7 and n:14, respectively), and 10 per 100 patient-years with SLE-associated Ob-APS.12
The clinical utility of the adjusted GAPSS in assessing the thrombotic risk in different clinical scenarios has been previously described and validated, as recently summarized in a systematic review.13 In the first description of patients with SLE; it was observed that GAPSS values ≥ 10 had the best diagnostic accuracy for APS. In patients with primary APS, GAPSS values ≥11 were strongly associated with a higher risk of recurrence [OR 18.27 (95% CI 3.74, 114.5)], showing the best accuracy in terms of sensitivity and specificity.14 More recently, in a cohort of patients with autoimmune disease, Fernandez Mosteirin et al. showed that aGAPSS values≥ 5 had the best diagnostic accuracy (AUC = 0.661; p< 0.001) for any thrombotic event.15 Cut-off values may differ in different of cohorts,14,16 which suggests that baseline characteristics in divergent groups of patients can account for differences in cut-off values of GAPSS.
Several studies also demonstrated that aGAPSS seems to be a valid tool to assess the likelihood of developing new thrombotic events in patients with APS and may guide pharmacological treatment for high-risk patients. This score has been independently validated in different APS populations11,14,17 and also in specific groups, such as young APS patients with acute myocardial infarction.16
In a recent study, aGAPSS baseline values were statistically higher in patients with APS and history of thrombosis compared with those without.15 A Chinese cohort reported a higher aGAPSS in patients with thrombosis than those with pregnancy morbidity only, but patients with both thrombosis and pregnancy morbidity had no statistical difference in aGAPSS when compared to those with Ob-APS only.18 We showed that Ob-APS women who experience thrombosis after initial pregnancy morbidity have higher aGAPSS values, when compared to those without thrombosis.
Conclusion
Our retrospective analysis of a large scale aPL registry suggests that: a) among women with both thrombotic and Ob-APS, more than half developed thrombosis after an initial aPL-related pregnancy morbidity; and b) younger age at the time of onset for Ob-APS related event, additional cardiovascular risk factors, superficial vein thrombosis, heart valve disease and multiple aPL positivity increased the risk of the first thrombosis after pregnancy morbidity. In addition, the aGAPSS may be a valid tool for a substantial improvement in risk stratification for thrombosis in women with Ob-APS and to identify women who might benefit from tailored a management approach.
Supplementary Material
Acknowledgement:
The authors thank all members of APS Action for the valuable help with data acquisition. For a full list of members please see apsaction.org.
Funding
There was no funding for this study.
Footnotes
Disclosure of interests
Roger Abramino Levy is a licensed professor of Rheumatology at Universidade do Estado do Rio de Janeiro, currently working as global medical expert for GlaxoSmithKlinein Upper Providence, PA, USA. The other authors declare that there is no conflict of interest. Completed disclosure of interest forms are available to view online as supporting information.
Ethics approval
This study was approved by Hospital Universitário Pedro Ernesto’s Ethics Committee in October 18th of 2012, approval number 02190912.6.1001.5259.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/1471-0528.15469
Contributor Information
Guilherme Ramires de Jesús, Department of Obstetrics, Universidade do Estado do Rio de Janeiro.Rio de Janeiro, Brazil..
Savino Sciascia, Center of Research of Immunopathology and Rare Diseases, Department of Clinical and Biological Sciences, University of Turin. Turin, Italy..
Danieli Andrade, Departament of Rheumatology, Universidade de São Paulo. São Paulo, Brazil..
Iana Souza Nascimento, Departament of Rheumatology, Universidade de São Paulo. São Paulo, Brazil..
Renata Rosa, Departament of Rheumatology, Universidade de São Paulo. São Paulo, Brazil..
Medha Barbhaiya, Department of Medicine, Division of Rheumatology, Hospital for Special Surgery. New York, NY, United States.
Doruk Erkan, Department of Medicine, Division of Rheumatology, Hospital for Special Surgery. New York, NY, United States.
Maria Tektonidou, Rheumatology Unit, First Department of Propaedeutic Internal Medicine, University of Athens. Athens, Greece..
Alessandra Banzato, Department of Cardiac Thoracic and Vascular Sciences, University of Padova. Padova, Italy..
Vittorio Pengo, Department of Cardiac Thoracic and Vascular Sciences, University of Padova. Padova, Italy..
Lanlan Ji, Rheumatology and Immunology Department, Peking University First Hospital. Beijing, China..
Pier Luigi Meroni, Department of Rheumatology, University of Milan. Milan, Italy..
Amaia Ugarte, Autoimmune Diseases Research Unit, Department of Internal Medicine, Hospital Universitario Cruces. Barakaldo, Spain..
Hannah Cohen, Department of Haematology, University College London. London, England..
D. Ware Branch, Department of Obstetrics and Gynecology, University of Utah Health Sciences and Intermountain Healthcare. Salt Lake City, Utah, United States..
Laura Andreoli, Rheumatology and Clinical Immunology, Department of Clinical and Experimental Sciences, University of Brescia. Brescia, Italy.
H. Michael Belmont, Division of Rheumatology, NYU School of Medicine. New York, NY, United States..
Paul R. Fortin, Division of Rheumatology, Centre Hospitalier de l'Université Laval. Québec, QC, Canada.
Michelle Petri, Division of Rheumatology, John Hopkins University. Baltimore, MD, United States..
Esther Rodriguez, Rheumatology Department, Hospital 12 de Octubre. Madrid, Spain..
Ricard Cervera, Department of Autoimmune Diseases, Hospital Clínic. Barcelona, Spain..
Jason S. Knight, Division of Rheumatology, University of Michigan. Ann Arbor, MI, United States.
Tatsuya Atsumi, Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University. Sapporo, Japan..
Rohan Willis, Antiphospholipid Standardization Laboratory, Internal Medicine, University of Texas Medical Branch. Galveston, TX, United States..
Roger A. Levy, Department of Rheumatology, Universidade do Estado do Rio de Janeiro. Rio de Janeiro, Brazil. GlaxoSmithKline Immunology and Inflammation, Upper Providence, PA, USA.
References:
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. [DOI] [PubMed] [Google Scholar]
- 2.Drozidnsky G, Hadar E, Shmueli A, Gabbay-Benziv R, Shiber S. Obstetric antiphospholipid syndrome and long term arterial thrombosis risk. J Thromb Thrombolysis. 2017;44(3):371–375 [DOI] [PubMed] [Google Scholar]
- 3.Gris J-C, Bouvier S, Molinari N, Galanaud J-P, Cochery-Nouvellon E, Mercier E, et al. Comparative incidence of a first thrombotic event in purely obstetric antiphospholipid syndrome with pregnancy loss: the NOH-APS observational study. Blood. 2012;119(11):2624–32. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Zamora MA, Peralta S, Creus M, Tassies D, Reverter JC, Espinosa G, et al. Risk of thromboembolic events after recurrent spontaneous abortion in antiphospholipid syndrome: a case-control study. Ann Rheum Dis. 2012;71(1):61–6. [DOI] [PubMed] [Google Scholar]
- 5.Erkan D, Harrison MJ, Levy R, Peterson M, Petri M, Sammaritano L, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: A randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody–positive individuals. Arthritis Rheum. 2007;56(7):2382–91. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber K, Radin M, Sciascia S. Current insights in obstetric antiphospholipid syndrome. Curr Opin Obstet Gynecol. 2017;29(6):397–403 [DOI] [PubMed] [Google Scholar]
- 7.Lefèvre G, Lambert M, Bacri J-L, Dubucquoi S, Quemeneur T, Caron C, et al. Thrombotic events during long-term follow-up of obstetric antiphospholipid syndrome patients. Lupus. 2011;20(8):861–5. [DOI] [PubMed] [Google Scholar]
- 8.Ruffatti A, Tonello M, Del Ross T, Cavazzana A, Grava C, Noventa F, et al. Antibody profile and clinical course in primary antiphospholipid syndrome with pregnancy morbidity. Thromb Haemost. 2006;96(3):337–41. [DOI] [PubMed] [Google Scholar]
- 9.Erkan D, Lockshin M, APS ACTION members. APS ACTION - AntiPhospholipid Syndrome Alliance For Clinical Trials and International Networking. Lupus. 2012;21(7):695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: The global anti-phospholipid syndrome score. Rheumatol (Oxford). 2013;52(8):1397–403. [DOI] [PubMed] [Google Scholar]
- 11.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. The global anti-phospholipid syndrome score in primary APS. Rheumatology (Oxford). 2015;54(1):134–8. [DOI] [PubMed] [Google Scholar]
- 12.Lefèvre G, Lambert M, Bacri J-L, Dubucquoi S, Quemeneur T, Caron C, et al. Thrombotic events during long-term follow-up of obstetric antiphospholipid syndrome patients. Lupus. 2011;20(8):861–5. [DOI] [PubMed] [Google Scholar]
- 13.Sciascia S, Radin M, Sanna G, Cecchi I, Roccatello D, Bertolaccini ML. Clinical utility of the global anti-phospholipid syndrome score for risk stratification: a pooled analysis. Rheumatology. 2018. April 1;57(4):661–5. [DOI] [PubMed] [Google Scholar]
- 14.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. The global anti-phospholipid syndrome score in primary APS. Rheumatology. 2014;54(1):134–8. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez Mosteirin N, Saez Comet L, Salvador Osuna C, Calvo Villas JM, Velilla Marco J. Independent validation of the adjusted GAPSS. Role of thrombotic risk assessment in the real-life setting. Lupus. 2017;26(12):1328–1332. [DOI] [PubMed] [Google Scholar]
- 16.Radin M, Schreiber K, Costanzo P, Cecchi I, Roccatello D, Baldovino S, et al. The adjusted Global AntiphosPholipid Syndrome Score (aGAPSS) for risk stratification in young APS patients with acute myocardial infarction. International Journal of Cardiology. 2017;240:72–7. [DOI] [PubMed] [Google Scholar]
- 17.Sciascia S, Cuadrado MJ, Sanna G, Murru V, Roccatello D, Khamashta MA, et al. Thrombotic risk assessment in systemic lupus erythematosus: validation of the global antiphospholipid syndrome score in a prospective cohort. Arthritis Care Res (Hoboken). 2014;66(12):1915–20. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Y, Li C, Karp DR LZ. Clinical and Epidemiological Correlates of the Adjusted Global Anti-Phospholipid Syndrome Score in a Large Cohort of Chinese APS Patients. Arthritis Rheumatol. 2015;67(suppl 10). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


