Abstract
Objective
This study aimed to describe the effectiveness and optimum use of tocilizumab (TCZ) treatment by the support of clinical, laboratory and radiologic observations.
Methods
All patients were followed up in the hospital with daily interleukin-6 (IL-6), C-reactive protein (CRP), ferritin, d-dimer, full blood count, and procalcitonin. Thoracic computed tomography (CT) was performed on admission, when oxygen support was necessary, and seven days after TCZ started. Disease course of the patients was grouped as severe or critical, according to their clinical, laboratory and radiologic evaluations.
Results
Forty-three patients were included: 70% were male; the median age was 64 years (minimum–maximum: 27–94); and six (14%) patients died. The median duration of oxygen support before the onset of TCZ was shorter among the severe patient group than the critical patient group (1 vs. 4 days, p < 0.001). Three cases of 21 (14%) who received TCZ in the ward were transferred to ICU, and none of them died. The levels of IL-6, CRP, ferritin, d-dimer, and procalcitonin were significantly lower in the severe cases group than the critical cases group (p = 0.025, p = 0.002, p = 0.008, p = 0.002, and p = 0.001, respectively). Radiological improvement was observed in severe cases on the seventh day of TCZ. Secondary bacterial infection was detected in 41% of critical cases, but none of the severe ones.
Conclusion
Earlier use of TCZ in COVID-19 infection was beneficial for survival, length of hospitalization and duration of oxygen support. The recommendation for administration of TCZ was based on an increase in requirement of oxygen support, progression in thoracic CT, and elevation of inflammation markers, including IL-6, CRP, ferritin, and d-dimer, and decrease in % lymphocytes.
Keywords: Tocilizumab, COVID-19, Cytokines, Interleukin-6, Therapy
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus, which is the cause of pneumonia that was first seen in Wuhan, China, in December 2019 (Wang et al., 2020a). SARS-CoV-2 pneumonia has been reported in many countries around the world, and the World Health Organization (WHO) declared the outbreak as a pandemic on 11 March 2020. Patients diagnosed with COVID-19 may have a wide range of symptoms from mild to severe. Fever, cough and shortness of breath are the most common symptoms that have been reported (Ciotti et al., 2020). Advanced age, underlying comorbidities such as hypertension, diabetes, cardiovascular disease, and cerebrovascular disease are the risk factors that have been reported for developing into severe to critical cases (Wang et al., 2020b, Huang et al., 2020). Acute respiratory distress syndrome (ARDS) may develop in some patients, who are more likely to have poor outcomes (Ruan et al., 2020). Cytokine storm causes ARDS and multiple organ failure, which occurs as a result of the macrophage activating syndrome (MAS) both in sepsis and COVID-19 (Chousterman et al., 2017, Ye et al., 2020). It has been reported that the serum levels of the cytokines, including interleukin-6 (IL-6), are higher in critical patients with COVID-19 (Chen et al., 2020, Henderson et al., 2020). Hence, suppressing the cytokine storm seems to be an effective way to decrease fatality (Liu et al., 2020).
Tocilizumab (TCZ) is an anti-IL-6 receptor monoclonal antibody that inhibits signal transduction and is used for the treatment of rheumatoid arthritis (Nishimoto et al., 2000, Tanaka et al., 2014). TCZ also has a significant effect on the treatment of infection-induced cytokine storm, and it might be effective in the treatment of critical patients with COVID-19 (Luo et al., 2020, Di Giambenedetto et al., 2020). However, correct use and timing of TCZ in the treatment course is necessary.
This study aimed to detail the benefit and appropriate timing for TCZ treatment in severe to critical cases with COVID-19 pneumonia. It describes the secondary bacterial infections that could be associated with TCZ.
Methods
Patients and study design
In this retrospective study, the patients who were infected with SARS-CoV-2 and received TCZ from 10 March to 14 April 2020 at the American Hospital and Koc University Hospital in Istanbul, Turkey, were included. All patients were followed up for at least 14 days at the hospital after TCZ treatment. A COVID-19 team – including infectious disease, internal medicine, chest medicine, and intensive care unit physicians – managed all the cases with weekly meetings and daily on-call and/or bedside consultations.
Definitions
The WHO case definition was used in diagnosing COVID-19, including probable and confirmed cases (WHO, 2020). Confirmation was defined as a positive laboratory result of COVID-19 infection, whereas a probable case was defined as inconclusive or a suspect case for whom testing could not be confirmed for any reason.
Blood oxygen levels <93% were accepted as decreased oxygen saturation. If necessary, oxygen support was given by nasal cannula, a venturi mask, and non-invasive respiratory support or invasive mechanical ventilation.
Patients were categorized as severe or critical based on the severity of infection (Anon, 2020). The features of the severe cases were respiratory distress (≥30 breaths/minute) or oxygen saturation ≤93% or arterial partial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg. The features of the critical cases were respiratory failure requiring mechanical ventilation or shock or organ failure requiring ICU.
Primary outcomes were duration of oxygen requirement, being transferred to ICU and fatality. Secondary outcomes were the duration of oxygen support after using TCZ and length of hospitalization.
Treatment algorithm and follow-up
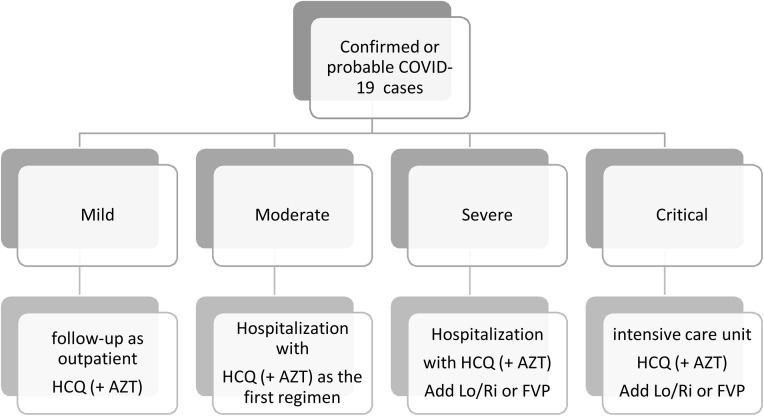
All suspected or confirmed adult cases with COVID-19 received hydroxychloroquine (or plus azithromycin) on admission, according to the national protocol of Ministry of Health (Figure 1 ). By 01 April 2020, since it became available, favipiravir started to be used as an alternative in cases of progression in radiological imaging or requiring oxygen support. TCZ was started when cytokine storm was suspected, by evaluating: radiological progression, requirement of oxygen support, C-reactive protein (CRP), IL-6, procalcitonin, lactate dehydrogenase (LDH), ferritin, d-dimer level, leucocyte, lymphocyte and platelet counts. The initial dose of TCZ was 8 mg/kg, based on Chinese guidelines (Anon, 2020). If the first dose was ineffective, one more dose was given at least 12 h later. Informed consent was obtained from the cases if unavailable from their relatives. The cases were followed up in terms of adverse events with biosafety monitoring document.
Figure 1.
Flow diagram for the management among patient groups.
Laboratory studies
Nasopharyngeal and oropharyngeal samples were tested for SARS-CoV-2 by real-time polymerase chain reaction in the laboratory of Koç University Hospital in Istanbul. Mediated amplification and detection of B-βCoV (target E gene) specific RNA and SARS CoV-2 (target S gene), QIAamp® Viral RNA Mini Kit (Qiagen, Hilden, Germany) for nucleic acid extraction and then the RealStar® SARS-CoV-2 RT-PCR Kit 1.0 (Altona Diagnostics, Hamburg, Germany) on Rotor-Gene® Q5/6 plex Platform (Qiagen, Hilden, Germany) were used. IL-6, CRP, procalcitonin, aspartate transaminase (AST), alanine transaminase (ALT), ferritin, d-dimer, LDH, leucocytes, neutrophil, lymphocyte, and platelet counts were used daily in follow-up. Thoracic computed tomography (CT) was used before the initiation of TCZ.
Statistical analysis
Categorical variables were compared by using Chi-square test. For continuous variables, the non-parametric Kruskal-Wallis test was used because of the small sample size. STATA 15v was used for statistical analysis and statistical significance was set as p-value < 0.05. The Institutional Review Board of Koç University approved the study with the reference number 2020.143.IRB1.033.
Results
Forty-three patients were included: 70% were male; the median age was 64 years (minimum–maximum: 27–94); 63% were >60 years (Table 1 ). Fever (91%) and cough (60%) were the most common symptoms. All fatal cases were male and aged >60 years; the median age of fatal cases was older than those who survived (75 vs. 61 years, p = 0.016, Table 1). Hypertension and diabetes mellitus were the most commonly detected comorbid diseases, and the comorbidities were similar between two groups (Table 1).
Table 1.
The Univariate Analysis for the fatality of COVID-19 patients.
| Total N = 43 (%) | Fatal N = 6 (%) | SurvivedN = 37 (%) | p | |
|---|---|---|---|---|
| Median age | 64 (27–94) | 75 (64–82) | 61 (27–94) | 0.016 |
| > 60 years | 27 (62.8) | 6 (100) | 18 (56.8) | 0.04 |
| Male gender | 31 (72) | 6 (100) | 25 (67.6) | 0.1 |
| Comorbidities | ||||
| Hypertension | 19 (44) | 3 (50) | 16 (43) | 0.75 |
| Diabetes mellitus | 13 (30) | 2 (33) | 11 (29.7) | 0.85 |
| Obesity | 5 (11.6) | – | 5 (13.5) | 0.33 |
| Chronic obstructive pulmonary disease | 3 (7) | – | 3 (8) | 0.47 |
| Coronary artery disease | 8 (19) | 2 (33) | 6 (16) | 0.31 |
| Laboratory (on the day of TCZ) | ||||
| CRP (mg/L) | 188 (1–380) | 214 (109–318) | 182 (1–380) | 0.34 |
| Ferritin (ng/mL) | 1197 (250–52,765) | 1884 (713–10,143) | 844 (250–52,765) | 0.1 |
| D-Dimer (μg/L) | 993 (360–100,000) | 2645 (790–56,200) | 953 (360–100,000) | 0.13 |
| IL-6 (pg/mL) | 133 (18–50,000) | 337 (93–50,000) | 126 (18–3011) | 0.04 |
| ALT (U/L) | 61 (14–1578) | 64 (38–84) | 78 (14–1578) | 0.97 |
| AST (U/L) | 60 (24–2045) | 101 (62–180) | 66 24–2045) | 0.18 |
| LDH (U/L)) | 373 (197–5770) | 381 (366–783) | 367 (197–5770) | 0.35 |
| Procalcitonin (ng/mL) | 0.23 (0.04–100) | 0.43 (0.07–72.8) | 0.22 (0.04–100) | 0.34 |
| Leucocyte (K/μL) | 7.0 (1.7–19.1) | 6.75 (4.2–11.4) | 7.0 (1.7–19.1) | 0.96 |
| Lymphocyte (%) | 12.5 (3–47) | 9 (3–26) | 13 (3–47) | 0.25 |
| Platelet (K/μL) | 242 (62–582) | 183 (114–227) | 254 (62–582) | 0.026 |
| Clinical | ||||
| Oxygen support duration before tocilizumab (days) | 2 (0–13) | 3.5 (2–6) | 2 (0–13) | 0.12 |
| Duration from onset of symptoms to tocilizumab (days) | 10 (5–26) | 9 (7–13) | 10 (5–26) | 0.41 |
| Severe case | 21 (48.8) | 0 | 21 (56) | 0.01 |
| Intensive care unit admission | 25 (58) | 6 (100) | 19 (51.4) | 0.02 |
| Secondary bacterial infection | 9 (21) | 4 (67) | 5 (13.5) | 0.03 |
| Pneumonia | 6 (14) | 2 (33) | 4 (11) | 0.14 |
| Bacteremia | 5 (12) | 2 (33) | 3 (8) | 0.074 |
Abbreviations: CRPC-reactive protein; IL-6interleukin-6; ASTaspartate transaminase; ALTalanine transaminase; LDHlactate dehydrogenase.
The median duration of oxygen support before the onset of TCZ was 3.5 days in fatal cases and 2 days in those who survived (p = 0.12). The duration from the onset of symptoms to the start of TCZ was similar among fatal and survived cases. None of the severe cases and three critical cases died (p = 0.01, Table 1).
The laboratory results of the patients on the day of TCZ administration among fatal and non-fatal cases are summarized in Table 1. IL-6 level was 337 pg/mL in fatal cases, while it was 126 pg/mL in those who survived (p = 0.04). Ferritin, CRP and d-dimer were found to be higher among the fatal cases than those who survived, with no statistical significance (Table 1).
The severe and critical cases were compared (Table 2 ). The groups were similar in terms of age and comorbid diseases, while male gender was higher among critical cases (91% to 52%, p = 0.005). Favipiravir use among the severe and critical cases was similar (p = 0.23, Table 2). The median duration of oxygen support before the onset of TCZ was shorter among the severe cases than critical cases (1 vs. 4 days, p < 0.001). The median length of stay, duration of oxygen support and the total duration of hospitalization after administration of TCZ was shorter among the severe cases than critical cases (p < 0.001, p = 0.028 and p = 0.001, respectively, Table 2). Three severe cases of 21 (14%) were transferred to the ICU, and none of them died. In laboratory analysis, IL-6 was 115 in severe cases and 168 in critical cases (p = 0.025). CRP, ferritin, d-dimer, and procalcitonin were significantly lower in severe cases (p = 0.0029, p = 0.008, p = 0.002, and p = 0.001, respectively). The lymphocyte percentage was 13.5% in severe cases and 11% in critical ones (p = 0.007). Radiological imaging with CT was also examined before the onset of TCZ in most of the cases, and radiological improvement was also observed on the seventh day of TCZ in severe cases (Figure 3).
Table 2.
Comparison of the patients who were given tocilizumab based on the severity of cases.
| Tocilizumab in severe cases N = 21 (%) | Tocilizumab in critical cases N = 22 (%) | p | |
|---|---|---|---|
| Mean age | 61 (sd: 15) | 66 (sd: 11) | 0.198 |
| Male gender | 11 (52) | 20 (91) | 0.005 |
| Confirmed COVID-19 | 17 (81) | 18 (82) | 0.9 |
| Hypertension | 9 (43) | 10 (45) | 0.864 |
| Diabetes mellitus | 7 (33) | 6 (27) | 0.665 |
| Obesity | 3 (14) | 2 (9) | 0.595 |
| Chronic obstructive pulmonary disease | 2 (10) | 1 (5) | 0.522 |
| Coronary artery disease | 6 (29) | 2 (9) | 0.101 |
| Laboratory (on the day of TCZ) (median, min–max) | |||
| CRP (mg/L) | 88 (1–299) | 233 (25–380) | 0.0029 |
| Ferritin (ng/mL) | 575 (273–1991) | 1884 (250–52,765) | 0.008 |
| D-Dimer (μg/L) | 870 (360–2300) | 1710 (620–100,000) | 0.002 |
| IL-6 (pg/mL) | 115 (18–942) | 168 (27–50,000) | 0.025 |
| ALT (U/L) | 78 (14–428) | 58 (17–1578) | 0.796 |
| AST (U/L) | 58 (27–129) | 64 (24–2045) | 0.354 |
| LDH (U/L)) | 358 (205–544) | 381 (197–5770) | 0.183 |
| Procalcitonin (ng/mL) | 0.15 (0.04–0.44) | 0.38 (0.07–100) | 0.001 |
| Leucocyte (K/μL) | 6.38 (2.25–9.6) | 7.98 (1.74–19.1) | 0.96 |
| Lymphocyte (%) | 13.5 (7–47) | 11 (3–26) | 0.007 |
| Platelet (K/μL) | 242 (62–582) | 227 (97–473) | 0.52 |
| Clinical support | |||
| Oxygen support duration before tocilizumab (days) | 1 (0–7) | 4 (1–13) | <0.001 |
| Duration from onset of symptoms to tocilizumab (days) | 9.5 (5–18) | 10 (5–26) | 0.76 |
| Favipiravir | 15 (71) | 19 (86) | 0.23 |
| Hydroxychloroquine | 18 (86) | 20 (91) | 0.6 |
| Azithromycin | 14 (67) | 11 (50) | 0.27 |
| Lopinavir/Ritonavir | 0 | 7 (32) | 0.005 |
| Outcomes | |||
| Duration of oxygen support after tocilizumab | 5 (0–16) | 12 (6–30) | <0.001 |
| Length of stay after tocilizumab (days) | 7.5 (2–21) | 12 (4–30) | 0.028 |
| Length of stay | 12.5 (6–32) | 20 (8–43) | 0.009 |
| Secondary bacterial infection | – | 9 (41) | 0.001 |
| Pneumonia | – | 6 (27.3) | 0.01 |
| Corynebacterium spp. | – | 4 | |
| Bacteremia | – | 5 (22.7) | 0.02 |
| Fatal | 0 (0) | 6 (27) | 0.01 |
Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6; AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase.
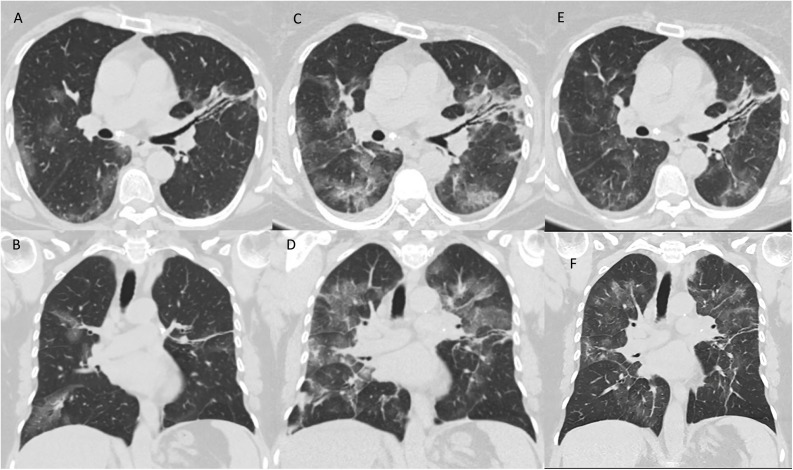
Figure 3.
A 59-year-old woman with Covid-19. Same level of mid-axial (A, C and E) and mid-coronal (B, D and F) chest CT scans on admission (A–B), on the day of tocilizumab given because of requirement of oxygen support and progressive lung imaging (C–D) and 7 days after onset of tocilizumab (E–F), retrospectively.
A–B: Focal peripheral ground-glass opacities. The left upper lobe lesions were accompanied by consolidation with bronchiectasis.
C–D: Chest CT showed that the lesions had become larger with some peripheral newer small consolidation.
E–F: The lesions were suppressed and improved.
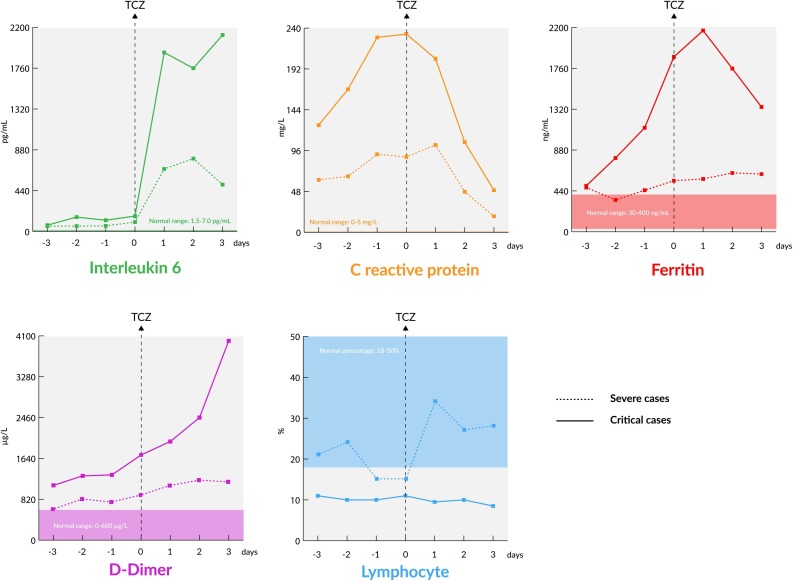
After the use of TCZ, the earliest change in laboratory tests was observed in % lymphocytes, which increased within the first day. The CRP sharply declined one day after TCZ. Two days after TCZ, IL-6 declined sharply, but ferritin and d-Dimer slightly declined (Table 3, Figure 2 ).
Figure 2.
Laboratory parameters 3 days before and after tocilizumab among severe and critical cases.
During ICU stay, secondary bacterial infections were detected in nine patients (41%). In five cases (22%), bloodstream infections of Gram-negative, Gram-positive bacteria and Candida spp. were detected. Among six (27.3%) cases with pneumonia, Corynebacterium striatum and Corynebacterium jeikeum were isolated in four cases (67%).
Discussion
Clinical presentation of COVID-19 varies from mild symptoms to ARDS and death. This study analyzed TCZ treatment in 43 severe and critical patients with COVID-19 pneumonia. Earlier use of TCZ had significantly better outcomes with low ICU admissions, lower duration of oxygen requirement and no fatality. The adaptive immune response plays a crucial role in this wide range of outcomes (Fung et al., 2020). A large amount of cytokine release explains the pathogenesis of dyspnea and ARDS in COVID-19 after the virus binds to alveolar epithelial cells. As a result of over-inflammation, vascular permeability increases and this leads to the alveoli filling with a massive amount of fluid (Zhang et al., 2020). IL-6 plays a significant role in cytokine storm (Hunter and Jones, 2015). TCZ is an anti-inflammatory drug that has the potential to decrease MAS-induced cytokine storm, and was shown to be beneficial in COVID-19 in some case series (Luo et al., 2020, Xu et al., 2020, Sciascia et al., 2020) and a recent systematic review (Alzghari and Acuna, 2020). The current study observed that IL-6 was higher in fatal than survived cases. In the early days of the COVID-19 pandemic, because of the off-label use and insufficient data on efficacy and safety of TCZ, it could not be used in the early stages of the disease; however, it was commonly used in ICU, where the cases could be intubated. Later on, access to the drug became easier, then it was started earlier on the ward. After this no fatal cases and very low levels of ICU admissions were observed (Table 2).
The critical decision for TCZ use was the onset of cytokine storm. After cytokine storm had started, oxygen saturation decreased within hours to days by the increase in lung involvement. Inflammation parameters such as % lymphocytes, IL-6, CRP, ferritin, and d-dimer were compared before and after administration of TCZ. Among severe cases, the % lymphocytes increased in one day, CRP declined after one day, IL-6 sharply declined after two days, and ferritin and d-Dimer slightly declined after two days (Fig. 2). However, among the critical cases, IL-6 levels did not decline and % lymphocytes did not increase (Fig. 2).
Among nine of 22 (41%) patients who were admitted to the ICU, secondary bacterial infections were detected. Secondary bacterial infections could not be solely related to TCZ use. However, secondary bacterial and fungal infections, including tuberculosis, should be kept in mind. Patients whose QuantiFERON test is positive should be evaluated for isoniazid prophylaxis (Cantini et al., 2017).
One limitation of this study is that it was an observational pre-post study. During the study period, TCZ started being used earlier in the later phase compared with the early phase. However, patient distribution in severe and critical groups (Table 1) enabled comparison of starting TCZ in severe or critical patient groups. In clinical practice the patients were grouped according to clinical, radiological and laboratory criteria, and the categorization was in parallel with Chinese guidelines, although this was not yet validated. There is no doubt about the need of randomized clinical studies for definite conclusions. Adverse events related to TCZ, including secondary infections, should be detailed in further studies.
In conclusion, earlier use of TCZ in COVID-19 infection was beneficial for survival, length of hospitalization and duration of oxygen support. This recommendation for the administration of TCZ was based on the increase in requirement of oxygen support, progression of thoracic CT, and elevation of inflammation markers including IL-6, CRP, ferritin, d-dimer, and decrease in % lymphocytes. Secondary bacterial infections should be borne in mind after TCZ use.
Author contributions
Conceptualization: ŞK, BS, CÇ, ÖE
Data curation: ŞK, VOB, CÇ, ÖE
Formal analysis: ŞK, VOB, ÖE
Investigation: ŞK, ÖE
Methodology: ŞK, MK, ST, CÇ, Pİ, ÖE
Project administration: ÖE
Supervision: ÖE
Visualization: Ali Pekşen.
Writing - review & editing: ŞK, ÖE
Funding source
No funding was received.
Ethical approval
IRB of Koç University School of Medicine approved the study.
Conflict of interest
No conflict of interest was reported by the authors.
Acknowledgement
We thank the administrators of hospital management, Erhan Bulutçu, Ömür Erçelen, İsmail Bozkurt, Erdal Aksoy, and laboratory manager Erhan Palaoğlu. We are thankful to Ali Pekşen for his drawing.
References
- Alzghari S.K., Acuna V.S. Supportive treatment with Tocilizumab for COVID-19: a systematic review. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon . 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. [Google Scholar]
- Cantini F., Nannini C., Niccoli L., Petrone L., Ippolito G., Goletti D. Risk of tuberculosis reactivation in patients with Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Receiving Non-Anti-TNF-Targeted Biologics. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/8909834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Ciotti M., Angeletti S., Minieri M., Giovannetti M., Benvenuto D., Pascarella S. COVID–19 Outbreak: an overview. Chemotherapy. 2020:1–9. doi: 10.1159/000507423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E. Off-label use of tocilizumab in Patients with SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Kishimoto T., Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis. 2000;59(Suppl 1) doi: 10.1136/ard.59.suppl_1.i21. i21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia S., Apra F., Baffa A., Baldovino S., Boaro D., Boero R. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin Exp Rheumatol. 2020 [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Ogata A., Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26:88–96. doi: 10.1016/j.smim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Global Surveillance for COVID-19 Caused by Human Infection with COVID-19 Virus. [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]