Abstract
People living with HIV (PLWH) in the antiretroviral therapy (ART) era may lose more life-years to tobacco use than to HIV. Yet, smoking rates are more than twice as high among PLWH than the general population, contributing not just to mortality but to other adverse health outcomes, including neurocognitive deficits (neuroHIV). There is growing evidence that synergy with chronic inflammation and immune dysregulation that persists despite ART may be one mechanism by which tobacco smoking contributes to neuroHIV. This review will summarize the differential effects of nicotine vs tobacco smoking on inflammation in addition to the effects of tobacco smoke components on HIV disease progression. We will also discuss biomarkers of inflammation via neuroimaging as well as biomarkers of nicotine dependence (e.g., nicotine metabolite ratio). Tobacco smoking and nicotine may impact ART drug metabolism and conversely, certain ARTs may impact nicotine metabolism. Thus, we will review these bidirectional relationships and how they may contribute to neuroHIV and other adverse outcomes. We will also discuss the effects of tobacco use on the interaction between peripheral organs (lungs, heart, kidney) and subsequent CNS function in the context of HIV. Lastly, given the dramatic rise in the use of electronic nicotine delivery systems, we will discuss the implications of vaping on these processes. Despite the growing recognition of the importance of addressing tobacco use among PLWH, more research is necessary at both the preclinical and clinical level to disentangle the potentially synergistic effects of tobacco use, nicotine, HIV, cognition and immune dysregulation, as well as identify optimal approaches to reduce tobacco use.
Keywords: Nicotine, Tobacco, HIV, neuroHIV, HIV-associated neurocognitive disorder, Inflammation, Immune function
Introduction
The widespread use of anti-retroviral therapy (ART) for people living with HIV (PLWH) has substantially improved life expectancy (Antiretroviral Therapy Cohort 2008), with certain sub-groups of PLWH showing a life expectancy similar to that of the general population (Samji et al. 2013). Nevertheless, the annual number of new HIV infections has remained constant, increasing the number of people living with HIV/AIDS (Hall et al. 2008; Palella et al. 1998; WHO 2019). Between 2001 and 2008, the percentage of adults in the US living with HIV who are aged 50 or older has increased from 17% to 31% and the rates of new infections among 25–29 year old increased between 2012 and 2016 (CDC 2017; Mahy et al. 2014). Thus, there is a critical need to address modifiable health risk behaviors, most notably tobacco use. Unfortunately, a study with a large nationally representative sample reported that 42% of PLWH were current smokers (Mdodo et al. 2015). Although the rate of smoking among PLWH declined by ~5% from 2009 to 2014, it was still more than 2-times greater than the rate in the general population (Frazier et al. 2018). PLWH now lose more life-years to tobacco use than to their HIV infection (Helleberg et al. 2013; Helleberg et al. 2015).
Fortunately, advances in smoking cessation research over the past several decades have led to the development of treatment models that can yield quit rates approaching 40% in smokers without HIV (Pacek and Crum 2015). Further, interest in quitting smoking is high among PLWH (Frazier et al. 2018; Pacek and Cioe 2015; Pacek and Crum 2015), particularly when HIV treatment is initiated (Vidrine et al. 2018) and when tobacco use treatment is integrated with HIV care (Pacek et al. 2017). However, PLWH may also experience unique barriers to smoking cessation. For instance, depression and deficits in cognitive function are risk factors for relapse (Hitsman et al. 2013; Loughead et al. 2015) and are more prevalent among PLWH than smokers without HIV (Heaton et al. 2015; Nanni et al. 2015). Moreover, PLWH report that nicotine dependence, concerns about cravings, weight gain, and the ability to manage stress, as well as having a social network of smokers, are barriers to smoking cessation (Cioe et al. 2018; Weinberger et al. 2018).
Several reviews have concluded that there are insufficient data to indicate that tobacco dependence interventions that are efficacious in the general population are as efficacious for PLWH (Ledgerwood and Yskes 2016; Pacek and Cioe 2015; Pool et al. 2016). Indeed, two of the most effective pharmacotherapies for nicotine dependence – nicotine patches and varenicline – appear to be less effective in PLWH compared to the general population (Ashare et al. 2019b; Humfleet et al. 2013; Mercie et al. 2018). For example, a recent randomized controlled trial of varenicline for smokers with HIV, we found that varenicline increased abstinence rates relative to placebo at end-of-treatment, but, quit rates for those treated with varenicline were lower than we see in the general population and this effect subsided by 6 months (Ashare et al. 2019b). However, treatment utilization remains low with only 1 in 5 clinicians recommending varenicline to their patients with HIV who smoke and fewer than 4% of PLWH reporting varenicline use (Pacek et al. 2017). Moreover, while behavioral smoking cessation interventions yield moderate effects vs. standard medical care (Keith et al. 2016), no specific behavioral intervention has been identified as being uniquely effective for treating tobacco use among PLWH.
Given the high smoking rates and limited smoking cessation treatment utilization and efficacy, it is not surprising that tobacco use among PLWH has significant adverse health outcomes. It increases the risk for cancer (Altekruse et al. 2018; Reddy et al. 2017), cardiovascular disease (CVD) (Friis-Moller et al. 2003), respiratory conditions such as COPD and pneumonia (Pacek and Crum 2015), and cognitive deficits observed in HIV-associated neurocognitive disorder (HAND) (Bryant et al. 2013; Durazzo et al. 2007; Harrison et al. 2017). In the current ART era, a common feature underlying many comorbidities including HAND, is persistent inflammation that does not completely reverse with viral suppression (Butler et al. 2011; Hunt et al. 2016; Lederman et al. 2013). Thus, a shared mechanism by which tobacco smoking may exacerbate these conditions may be through tobacco’s effects on inflammation (Ambrose and Barua 2004; USDHHS 2010). This review provides a brief summary of the evidence from preclinical and clinical studies demonstrating the impact of nicotine and tobacco smoking on neurocognition and inflammation in the context of HIV. The specific effects of nicotine on neuroHIV/neuroAIDS (Han et al. 2018) and the role of nicotinic receptors in HAND and inflammation (Capo-Velez et al. 2018a) have been reviewed elsewhere. The primary goal of this review is to focus on: (1) the putative paradoxical effects of nicotine (which may be neuroprotective) vs tobacco smoking (which has harmful effects) on inflammation; (2) the effects of components of tobacco smoke on HIV disease progression; (3) biomarkers of inflammation (e.g., using neuroimaging) and nicotine dependence (e.g., nicotine metabolism) that may be useful for understanding mechanisms of tobacco dependence and/or neuroHIV; (4) the interactions between ARTs and nicotine and tobacco smoking; (5) the effects of tobacco smoking on peripheral organs (lungs, heart, kidney) and subsequent central nervous system (CNS) function; and (6) the impact of alternative nicotine delivery systems (i.e., e-cigarettes) on these processes. Figure 1 depicts a proposed model for the complex relationships among HIV, nicotine/tobacco use, inflammation/immune function, and cognition. Although some of these relationships are supported by evidence reviewed below, we also include proposed relationships among these variables that require evaluation. In each section, we will review the existing evidence and will discuss gaps in the research that provide directions for future studies.
Figure 1. Proposed model of the relationships among HIV, ART, smoking, inflammation, and neurocognition.
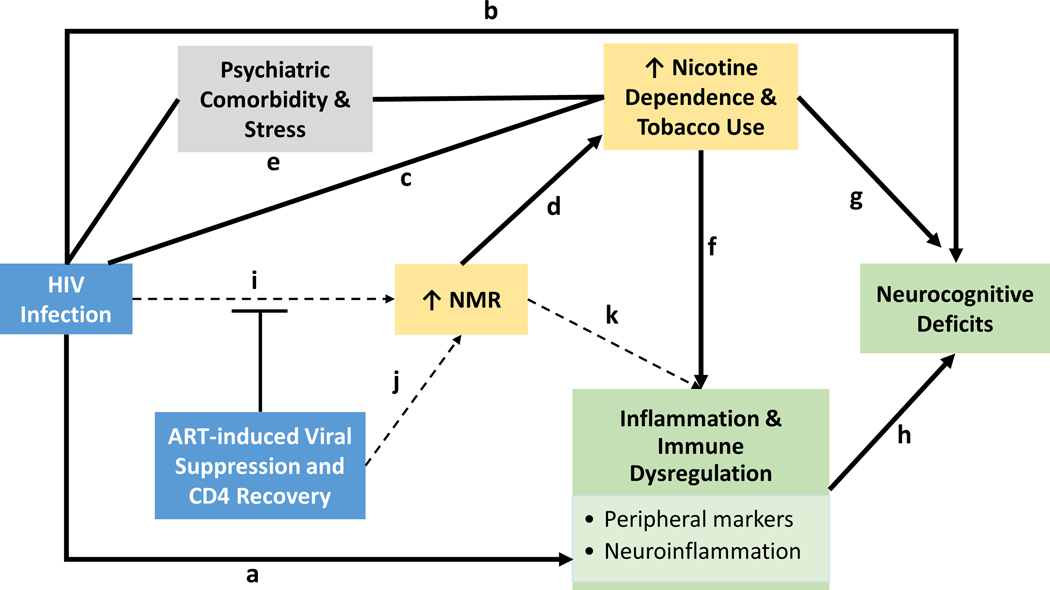
Solid lines represent relationships supported by evidence. Dashed lines represent relationships that have not yet been studied or there is not enough evidence to make a conclusion. (a) HIV infection produces elevated levels of inflammation and immune dysregulation even among virally suppressed individuals. (b) HIV is associated with deficits in cognition function. (c) Smoking rates are significantly higher among PLWH, compared to the general population. (d) The nicotine metabolite ratio (NMR) is strongly associated with smoking behavior (i.e., faster metabolizers tend to smoke more cigarettes, are more nicotine dependent, and have less success at quitting smoking compared to slower metabolizers). (e) HIV and tobacco use are both associated with higher rates of psychiatric comorbidities, such as depression, and elevated levels of chronic stress. These factors may represent other mechanisms linking HIV and tobacco use. (f) The relationship between nicotine, tobacco smoking, and inflammation is complex, but it is well-established that smoking induces inflammation; the evidence for nicotine as anti-inflammatory is supported in some studies, but not others. (g) Similar to the relationship between tobacco use and inflammation, the relationship with neurocognition may differ for the effects of nicotine (acute nicotine use may have beneficial effects) vs. tobacco smoking (chronic use may impair cognition). (h) There is evidence that elevated levels of inflammation are associated with deficits in cognition. (i) There is some evidence that PLWH metabolize nicotine faster than those without HIV; the mechanism is not yet known and the finding needs validation in larger samples. We also hypothesize that if HIV-infection increases nicotine metabolism, then we should observe an attenuation effect once ART is initiated. (j) It is possible that the increase in NMR is due to ART effects on CYP2A6. Therefore, depending on whether a particular ART induces, inhibits or is a substrate of CYP2A6, the direction of the effect on NMR may change. (k) We hypothesize that faster nicotine metabolism may result in higher levels of inflammation since nicotine has anti-inflammatory properties.
Clinical Correlates of Neurocognitive and Neuropsychiatric Comorbidities in Smokers with HIV
As mentioned above, depression and deficits in cognitive function are two known barriers to cessation or risk factors for smoking relapse (Hitsman et al. 2013; Loughead et al. 2015) that are also more common among smokers with HIV than smokers without HIV (Heaton et al. 2015; Nanni et al. 2015). Since the advent of ART, the incidence of HIV-associated dementia (HAD), the most severe form of HAND, has substantially decreased (Heaton et al. 2010; Heaton et al. 2011; Sacktor et al. 2016). Yet, mild neurocognitive disorder (MND) and asymptomatic neurocognitive impairment (ANI) persist despite ART, and 25–47% of PLWH exhibit deficits in multiple cognitive domains including memory, verbal fluency, processing speed, and executive function (Heaton et al. 2015; Sacktor et al. 2016). These cognitive deficits are associated with functional disabilities including unemployment, difficulty driving, and poor ART adherence (Doyle et al. 2013; Schouten et al. 2011; Thames et al. 2013). Importantly, abundant evidence suggests that chronic smoking increases the risk of neurocognitive dysfunction and global cognitive impairment in the general population (Durazzo et al. 2012; Paul et al. 2006; Weiser et al. 2010). Similar findings have been observed among PLWH (Bryant et al. 2013; Chang et al. 2017; Durazzo et al. 2007; Monnig et al. 2016), though not in all studies (Tsima et al. 2018). Data from our group suggests that smokers with HIV perform worse on working memory and attention tasks compared to smokers without HIV (Harrison et al. 2017). In addition, smokers with HIV have demonstrated decreased cognitive flexibility (as measured by the Wisconsin Card Sort Task) and worse decision-making (as measured via the Iowa Gambling task) compared to smokers without HIV and nonsmokers with HIV (Chang et al. 2017). In one study using data from the Multicenter AIDS Cohort Study (MACS), cumulative amount of smoking (i.e., pack-years) was associated with a greater decline in cognitive performance suggesting that even reducing smoking may reduce risk of neuroHIV (Akhtar-Khaleel et al. 2017). Moreover, nicotine withdrawal produces deficits in cognitive function (Ashare et al. 2014) that are similar to those observed in neuroHIV (Antinori et al. 2007; McArthur et al. 2010; Robertson and Yosief 2014; Woods et al. 2004) and are predictive of relapse (Culhane et al. 2008; Krishnan-Sarin et al. 2007; Patterson et al. 2010; Powell et al. 2004). Therefore, it is possible that a bidirectional relationship exists between neuroHIV and tobacco use creating a dual challenge for smokers with HIV during a quit attempt. Specifically, the cognitive deficits observed in PLWH may be exacerbated during early nicotine withdrawal, thereby making it more difficult to maintain abstinence.
Depression is another common comorbidity associated with both smoking (Forman-Hoffman et al. 2016; Hitsman et al. 2013) and HIV (Nanni et al. 2015). The prevalence of tobacco dependence among adults with depression is 3–4 times higher than that among the U.S. population (40–60% vs. 15%) (Jamal et al. 2016). Moreover, up to 43% of individuals with major depressive disorder (MDD) are daily smokers (Pratt and Brody 2010) and they are less likely to quit than those with no history of MDD (Hitsman et al. 2009; Hitsman et al. 2013; Ziedonis et al. 2008). Similarly, rates of depression are approximately 3 times higher among PLWH compared to the general population (26.5% vs. 9.1%) (Do et al. 2014). Although few studies have investigated the combined effects of smoking and HIV on depression, at least one study found that smokers with HIV reported the highest rates of psychopathology symptoms compared to smokers without HIV and nonsmokers with HIV (Chang et al. 2017). In addition to the association with both HIV and smoking, depression is also strongly associated with elevated levels of inflammation (Herron et al. 2018; Rosenblat et al. 2014) as well as deficits in cognition (Rubin and Maki 2019). Thus, the combined effects of tobacco smoking, HIV, and depression may yield synergistic effects on inflammation, which may have further consequences for neurocognition.
Differential Effects of Nicotine (NIC) vs. Cigarette Smoke Extracts (CSE) on Inflammation
Numerous studies have demonstrated that smokers exhibit elevated levels of inflammation across a variety of markers, relative to non-smokers. Tobacco-induced inflammation is believed to be one of the primary pathways by which tobacco smoking contributes to acute and chronic inflammatory diseases, including CVD, COPD, acute respiratory distress syndrome, and lung cancer (Ambrose and Barua 2004). Although this topic has been reviewed in depth elsewhere (Arnson et al. 2010; Goncalves et al. 2011; Stampfli and Anderson 2009), CRP, cytokines (e.g., IL6), markers of oxidative stress (nitric oxide), cell adhesion molecules (e.g., ICAM), and markers associated with vascular inflammation (e.g., MMP-9) have all demonstrated differences between smokers and non-smokers (Kianoush et al. 2017; McEvoy et al. 2015; Shiels et al. 2014; Sopori 2002; Tibuakuu et al. 2017). Indeed, cigarette smoke condensate upregulates pro-inflammatory cytokines including IL1α, IL1β, IL6 and IL8 in vitro and in vivo, and mRNA and protein expression levels, in a dose- and time-dependent manner (Kodidela et al. 2018; Shizu et al. 2008). Wang et al reported higher levels of TNFα and IL1β and lower expression of β2 adrenergic receptors in alveolar macrophages and lung tissue from rats exposed to tobacco smoke (Wang et al. 2015). Ghosh et al demonstrated that specific components of tobacco smoke extract activate microglia in vitro and in vivo and lead to neuronal damage (Ghosh et al. 2009). Tobacco smoke may also downregulate expression of genes involved with regulation of immune function (Spira et al. 2004). In addition, there is evidence that chronic tobacco exposure compromises the integrity of the blood-brain barrier (BBB) and increases the likelihood of monocyte transmigration into the brain and/or direct CNS exposure to tobacco constituents which, in turn, enhances the risk for neurodegeneration (Hossain et al. 2009; Manda et al. 2010b; Naik et al. 2014). This may be a particularly relevant point of synergy with neuroHIV, which is driven by myeloid cell accumulation and activation in the CNS.
There is some evidence that smoking burden (e.g., number of cigarettes smoked per day and/or number of years smoked) is associated with inflammation (Asthana et al. 2010; Kianoush et al. 2017; King et al. 2017), although others have found no relationship (Shiels et al. 2014). Importantly, former smokers exhibit lower levels of inflammation relative to current smokers (Shiels et al. 2014) and inflammation decreases following smoking cessation, particularly markers reflecting oxidative stress (King et al. 2017; Van Keulen et al. 2017). While the precise time course for reduced inflammation following cessation remains an important research question, several studies have shown a relationship between time since cessation and reductions in inflammation, with times ranging from 4 months to 20 years (Peres et al. 2017; Shiels et al. 2014; Van Keulen et al. 2017). Therefore, the adverse effects of tobacco smoking on inflammation are clearly at least in part reversible.
Despite the adverse effects of tobacco use on inflammation, nicotine alone, the main psychoactive ingredient in cigarettes, may have opposite effects (Han et al. 2018). For instance, nicotine attenuates pro-inflammatory cytokines interleukin-1β (IL1β), IL6, and tumor necrosis factor alpha (TNF)-α (Wei et al. 2015). In addition, nicotine may alter expression of genes involved with immune function, upregulating some genes (e.g., TGF-β1, IL4, CCR2, CXCR6, IL1α) and downregulating others (e.g., TNFα, CCL2, IL8, IL10, CXCR4, IRF4) (Rock et al. 2008; Yang et al. 2016). Many of nicotine’s anti-inflammatory effects occur in the CNS (Shi et al. 2009), which may contribute to nicotine’s neuroprotective properties (Baez-Pagan et al. 2015; Kalkman and Feuerbach 2016; Quik et al. 2012). Specifically, nicotine binds to and activates nicotinic acetylcholine receptors (nAChRs) leading to an influx of calcium, particularly through the α7 nAChR. This activates downstream signaling cascades such as the NFκB (Yoshikawa et al. 2006), Akt and CREB signaling pathway (reviewed in Shimohama and Kawamata 2018). Nicotine has been known to modulate innate immune pathways, also through the α7 nAChR (reviewed in Cui and Li 2010). For instance, activation of α7 nAChRs suppresses abnormal activation of microglia and expression of CD14, ICAM-1, CD40, and TNFα (De Simone et al. 2005; Hamano et al. 2006; Suzuki et al. 2006), and the α7 nAChR antagonist mecamylamine reverses these effects (Hamano et al. 2006).
Components of Tobacco Smoke and HIV Disease Progression
Immunopathogenesis
In addition to nicotine, tobacco and tobacco smoke contain over 8000 compounds from different classes produced over different phases of tobacco burning (Rodgman and Perfetti 2013). These compounds include carcinogens such as 1–3 butadiene and polycyclic aromatic hydrocarbons (PAH), respiratory irritants such as acrolein and acetaldehyde, agents that increase cardiovascular risks such as cyanide, arsenic, cresols, and tar to name a few (Rodgman and Perfetti 2013). Cigarette smoke extract is comprised of various combustion products of tobacco, highly reactive free radicals, gases and heavy metals (Feldman and Anderson 2013). Tobacco smoke has also been shown to contain low levels of bacterial endotoxins that can be pro-inflammatory (Hasday et al. 1999; Larsson et al. 2012). Although increasing evidence suggests that tobacco smoking alters HIV disease progression among ART-treated individuals (Gamarel et al. 2018) and those who have not yet initiated ART (Ande et al. 2013), the exact contribution of nicotine vs. the other components of tobacco smoke extract remains elusive.
Tobacco smoking may contribute to HIV-1 replication (see Figure 2 for model). For example, smokers with HIV were found to have a higher viral load when compared to non-smokers with HIV (Ande et al. 2015; Pollack et al. 2017). This would be consistent with the fact that HIV replicates most efficiently in activated CD4+ T cells, and longstanding observations that immune activation from various causes (tuberculosis, vaccination) can enhance viral replication and/or disease progression in vivo (Modjarrad and Vermund 2010; Whalen et al. 1995; Yek et al. 2016). Several studies have shown that smoking enhances HIV-1 replication in alveolar macrophages, microglia and T-cells in vitro. Notably, HIV-1 inoculation of alveolar macrophages isolated from smokers were shown to exhibit elevated p24 antigen levels compared with macrophages isolated from non-smokers (Abbud et al. 1995). Alveolar macrophages from smokers may have increased levels of iron that contribute to increased oxidative stress (Ande et al. 2013; Boelaert et al. 1996). Oxidative stress, in turn, activates and increases HIV-1 replication via the NFκB pathway (Boelaert et al. 1996) as the long terminal repeat (LTR) region of the viral genome consists of two or more NFκB binding sites (Stroud et al. 2009). In another study, benzo(a)pyrene (BaP) from tobacco smoke was shown to enhance HIV-1 replication in primary human macrophages and U1 cells. BaP, a PAH present in tobacco smoke, induces the CYP1A1-mediated oxidative stress pathway followed by NFκB activation (Ranjit et al. 2018). Dioxin, another component of tobacco smoke also increases HIV-1 gene expression and replication that is mediated via oxidative stress (Yao et al. 1995). Indeed, cigarette smoke condensate increases levels of CYP1A1 and CYP1B1, leading to oxidative stress, increased caspase 3 mediated apoptosis and elevated HIV-1 replication in human monocytoid cells (Rao et al. 2016). Another possible mechanism for increased HIV-1 replication associated with smoking is increased CCR5 expression. Tobacco smoke been reported to potentiate HIV-1 infection in bronchial epithelial cells by upregulating CD4 and CCR5 receptor expression (Chinnapaiyan et al. 2018). Interestingly, Zao et al demonstrated that tobacco smoke extract, but not nicotine alone, increased HIV-1 replication in TZM-bl and Jurkat T-cell lines (Zhao et al. 2010). These studies highlight the importance of considering both nicotine and cigarette smoke extract separately and combined in order to understand their differential effects on HIV-1 replication and pathogenesis.
Figure 2. Proposed molecular mechanism underlying effects of tobacco smoking on HIV neuropathogenesis.
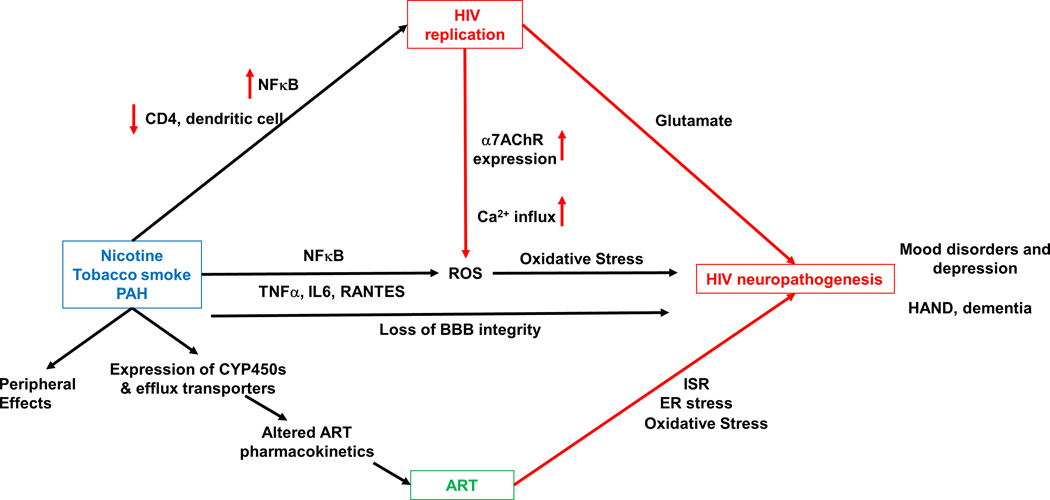
Tobacco smoke and its components increase HIV replication via increased transcription of HIV proteins mediated by NFκB pathways, and decreased function of immune cells including CD4+ T-cells and dendritic cells. In addition, tobacco smoking increases levels of pro-inflammatory cytokines and oxidative stress and leads to loss of BBB integrity, further contributing to HIV neuropathogenesis. Increased HIV replication increases oxidative stress via the α7 nAChR, that has been shown to increase expression during HIV infection. Tobacco smoking also affects the periphery including the lungs, cardiovascular system and the renal system. Importantly, tobacco smoke induces expression of cytochrome-P450s and efflux transporters, that alter pharmacokinetics, and hence the efficacy of antiretrovirals. ARTs themselves can have neurotoxic effects that are mediated by the ER stress, the integrated stress response (ISR), and oxidative stress.
In addition to the general effects on immune activation and function, tobacco use also alters these processes in the context of HIV. For instance, smokers have demonstrated a more rapid loss of CD4+ T cells during HIV-1 infection (Feldman et al. 2006). Wojna et al showed that among HIV-seropositive women, current smokers had a higher plasma viral load than non-smokers, and history of smoking negatively correlated with CD4+ T cell counts (Wojna et al. 2007). Valiathan et al showed that both HIV and smoking increase the expression of activation markers on T cells, smoking also impairs T cell function in PLWH on ART (Valiathan et al. 2014). In addition, elevated levels of microbial products in the bloodstream of smokers were associated with immune activation (Valiathan et al. 2014).
Neuropathogenesis
Besides T cells, smoking also affects dendritic cells. In fact, stimulation of human monocyte-derived dendritic cells by cigarette smoke extract inhibits the T cell priming function of dendritic cells, and skews immunity by suppressing dendritic cell-mediated Th-1 responses (Vassallo et al. 2005). Nicotine also suppresses dendritic cell-mediated T cell priming and proliferation, and reduces IFN-γ production via upregulation of PPRγ pathways (Yanagita et al. 2012). Exposure of dendritic cells to tobacco smoke promotes expression levels of pro-inflammatory chemokines including CXCL8 via oxidative stress (Vassallo et al. 2008).
As shown in Figure 2, another mechanism that might affect HIV progression in smokers includes altered blood brain barrier (BBB) permeability. A multitude of studies have demonstrated that nicotine plays a bimodal role in BBB permeability. Specifically, chronic nicotine exposure to endothelial cells increases oxidative and nitrosative stress resulting in loss of BBB integrity. In contrast, acute or chronic low dose nicotine either have no effect or prevent BBB disruption, indicating a dose-response type effect (reviewed in Feldman and Anderson 2013). Disruption of the BBB by nicotine in tobacco smoke (Hawkins et al. 2004; Manda et al. 2010a) might facilitate recruitment of HIV-1 infected macrophages and HIV progression in the CNS.
As noted above, nicotine and tobacco smoke have distinct effects on inflammation, which are also observed in the context of HIV. Steel et al studied the augmentative effect of tobacco smoking and ART on systemic inflammation in HIV-1 infected patients, and found significantly higher circulating levels of β2-microglobulin, cyclophilin A and RANTES irrespective of ART status (Steel et al. 2018). Furthermore, as shown in Figure 2, the α7 nAChR, which is thought to play a role in nicotine’s effects on immune pathways, has been implicated in HAND pathology as HIV-1 coat protein gp120 binds to α7 nAChR (Bracci et al. 1992). Although nicotine is shown to be neuroprotective, this effect may vary in the context of HIV. For example, pretreating human microglia with nicotine potentiates HIV-1 expression in a concentration dependent manner (Rock et al. 2008). Midde et al showed that transgenic rats expressing HIV-1 viral proteins (HIV-1Tg) had attenuated nicotine-induced behavioral sensitization assayed by horizontal activity following nicotine or saline administration (Midde et al. 2011). In another study, chronic nicotine exposure attenuated the effect of HIV-1 viral proteins on cognitive function as assayed by the y-maze and passive avoidance tests. They further observed differential expression of genes involved in synaptic plasticity in a brain-region specific manner that varied in HIV-1Tg and control F344 rats (Nesil et al. 2015). Yang et al demonstrated that HIV-1Tg rats show greater novelty seeking behavior, and that nicotine administration altered expression levels of different GABA and dopamine receptors in the ventral tegmental area (VTA), nucleus accumbens (Nac) and prefrontal cortex (PFC) of HIV-1Tg compared to control F344 rats (Yang et al. 2017). Changes in the dopaminergic and GABAergic receptor expression levels in the PFC-VTA-Nac circuit suggests changes in reward perception, goal-directed behavior and habit formation in the presence of HIV-1 proteins. Although these paradigms are useful for assessing drug effects, self-administration paradigms would be more informative as a parallel to nicotine consumption in humans.
Biomarkers
Neuroimaging Biomarkers: Nicotinic Receptors
Neuroimaging offers a powerful tool for understanding vulnerability to CNS disorders, facilitating treatment development (Borsook et al. 2006), and may therefore shed light on the mechanisms that underlie the interaction between tobacco smoking, HIV, and cognitive function. There are several techniques for studying the underlying neural effects including functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). In this review, we focus on PET imaging because of its capacity to target specific receptors in the brain (i.e., nAChRs) as well as neuroinflammation. Indeed, significant technological advances in radiotracer development (i.e., the incorporation of a radioisotope into the molecular structure of an epitope that binds to a specific biologic target) and instrumentation have enabled PET brain imaging to deepen our understanding of drug abuse, including nicotine dependence. For example, the [F-18] labeled radiotracer 85380 (also known as 2[F-18]FA) targets the α4β2-nAChR subtype (Gallezot et al. 2005; Horti et al. 1998; Sullivan et al. 1996; Valette et al. 1999). Although its kinetics are suboptimal (i.e., long time to achieve homeostasis throughout the brain), quantification techniques have been developed that permit robust α4β2-nAChR measurements without invasive arterial blood sampling and labor intensive high performance liquid chromatography (HPLC) required for metabolite analysis (Kimes et al. 2008; Lotfipour et al. 2011). More recently, new radiotracers ([F-18]flubatine and [F-18] AZAN) have been developed (Deuther-Conrad et al. 2008; Kuwabara et al. 2012) that target the α4β2-nAChR and demonstrate excellent reproducibility and more desirable kinetics in human brain than 2[F-18]FA (Coughlin et al. 2018; Hillmer et al. 2016; Sabri et al. 2015; Wong et al. 2013).
Despite its limitations, 2[F-18]FA has been a valuable tool for understanding the acute effects of nicotine, the effects of smoking cessation treatments that target nAChRs, and the effects of nicotine withdrawal. Even a small amount of nicotine successfully competes for receptors (Brody et al. 2006) and does so in a dose-dependent manner. For instance, sub-cigarette nicotine doses delivered by low or de-nicotinized cigarettes showed displacement of 2[F-18]FA proportional to nicotine dose (Brody et al. 2009). Pharmacological challenge studies revealed that second hand smoke also occupies nAChRs (Brody et al. 2011). Importantly, the use of 2[F-18]FA has validated that varenicline – one of the most effective smoking cessation medications and an α4β2 nAChR agonist – occupies nAChR α4β2* in vivo (Lotfipour et al. 2012). More recently, a newer tracer, [F-18]flubatine, has been studied with alternative nicotine delivery systems and has shown that α4β2 nAChRs are occupied following electronic cigarette vaping (Baldassarri et al. 2018).
In addition to studying acute drug effects, PET imaging techniques are also useful for studying different states of drug dependence (e.g., nicotine withdrawal) as well as individual differences. One study found that, during nicotine withdrawal, nAChR availability, measured via SPECT (Single Photon Emission Computed Tomography) imaging, peaked one week following initiation of abstinence (Cosgrove et al. 2009). This finding was important because it suggested that nAChR receptors remained occupied by nicotine far longer than expected given nicotine’s short half-life of ~2 hours (Benowitz et al. 2009). There are also individual differences in receptor availability. For example, male (vs female) smokers have greater nAChR availability after 7–9 days of nicotine abstinence (Cosgrove et al. 2012). nAChR α4β2* PET imaging has demonstrated that nAChR availability normalizes upon smoking cessation regardless of the type of treatment used (bupropion vs cognitive-behavioral therapy vs placebo) providing insight into the neural basis of effective smoking cessation treatments (Brody et al. 2013). Importantly, nAChR α4β2* availability has demonstrated utility as a predictive biomarker for successful cessation therapy. Specifically, smokers who exhibit less nAChR upregulation are more likely to maintain abstinence (Brody et al. 2014). However, there are no studies that we know of on nAChR availability in PLWH, and given the potential role for nAChRs in inflammatory processes associated with HAND (Baez-Pagan et al. 2015; Wei et al. 2015; Wittebole et al. 2007), this is a key knowledge gap and an important area for future research.
Neuroimaging Biomarkers: Neuroinflammation
In addition to radioisotopes that bind to nAChRs, ligands have been developed as a means to quantify inflammation in the brain. To date, PET probes for imaging neuroinflammation have targeted upregulation of the translocator protein (TSPO), which is thought to reflect activation of microglia (Doorduin et al. 2009; Kreisl et al. 2013; Venneti et al. 2006). First generation TSPO tracers (e.g., [11C]R-PK11195) have yielded conflicting results regarding differences in binding levels between ART-treated PLWH and healthy controls, but this may be due to poor performance of the radiotracer (Garvey et al. 2014; Hammoud et al. 2005; Wiley et al. 2006). Although second generation TSPO tracers (e.g., [11C]DPA-713, [11C]PBR28, and [18F]FEPPA) have demonstrated increased specificity (Brown et al. 2007; Chauveau et al. 2008; Endres et al. 2012; Rusjan et al. 2011), they are limited in that their ability to cross the blood brain barrier is determined by the rs6971 polymorphism in the TSPO gene, producing substantial inter-individual variation in their access to brain (Guo et al. 2013; Kreisl et al. 2013). Despite these caveats, TSPO PET imaging studies have yielded important information regarding the brain regions that may be affected in HIV and nicotine dependence. For example, using the TSPO tracer [11C]DPA-713, differences in binding potential between PLWH and controls were observed in white matter, cingulum, and supramarginal cortex, indicating elevated levels of neuroinflammation (Coughlin et al. 2014). Moreover, TSPO binding in the prefrontal cortex was associated with HAD, though these findings were TSPO-genotype dependent (Coughlin et al. 2014). In a follow-up study using [11C]PBR28, Vera and colleagues (2016) found increased uptake of the TSPO tracer in PLWH compared to controls, particularly in subcortical brain regions (e.g., basal ganglia). Importantly, this study also found that higher TSPO binding was associated with worse cognitive function and peripheral markers of immune activation (e.g., lower CD4/CD8 ratio) (Vera et al. 2016). It is important to note that, in both of these studies, the findings were adjusted for TSPO genotype to account for differences in binding potential (Coughlin et al. 2014; Vera et al. 2016)
More recently, studies have begun to investigate the impact of tobacco use on neuroinflammation using PET imaging (for a review, see Woodcock et al. 2019). For instance, Brody and colleagues (2017) observed lower binding of the TSPO tracer [11C]DAA1106, among smokers compared to nonsmokers. Moreover, higher self-reported cigarettes smoked per day was also associated with lower TSPO uptake. The authors conclude that these findings indicate that tobacco smoking results in global impairment of microglial activation, a marker of impaired inflammatory processes (Brody et al. 2017). In a follow up study, the same group investigated the effects of overnight abstinence on [11C]DAA1106 binding in the brain. This study replicated the previous finding that overnight abstinent smokers exhibited lower TSPO binding compared to nonsmokers, but found no effect of nicotine withdrawal (Brody et al. 2018). Although future studies are needed to assess whether these differences persist over time, these studies demonstrate global differences in microglial activation, which may reflect impaired immune function as a consequence of chronic tobacco use.
Given some of the limitations of the TSPO ligands, newer tracers targeting different markers of neuroinflammation are being developed. Indeed, TSPO levels do not distinguish between pro- and anti-inflammatory processes (Notter et al. 2018), which, as noted above, may be critical for studying the effects of nicotine vs tobacco smoking. Moreover, TSPO is not directly involved in neuroimmune signaling and activated microglia may reflect pathophysiological processes other than neuroimmune activation (Notter et al. 2018). One promising new tracer is [18F]-6-(1/2)(2-fluoro-propyl)-4-methylpyridin-2-amine ([18F]NOS), which is a radiolabeled version of a reversible inducible nitric oxide synthase (iNOS) inhibitor. Nitric oxide (NO), which plays an important role in immune regulation (Alderton et al. 2001), is produced by three nitric oxide synthase (NOS) enzymes: neuronal NOS (nNOS), endothelial NOS (eNOS), and iNOS. iNOS is associated with acute and chronic inflammatory diseases including asthma and COPD (Kaneki et al. 2007; Koch et al. 2007; Lechner et al. 2005; Smith and Lassmann 2002) and is expressed on glial cells in the brain (Esplugues 2002). [18F]NOS readily crosses the blood brain barrier and has good selectivity over other NOS enzymes, permitting the measurement of in vivo iNOS expression via PET imaging (Herrero et al. 2012). Thus, [18F]NOS may be a useful tool for studying neuroinflammation in future studies of HIV and tobacco use.
Although no PET imaging studies that we know of have explicitly tested the combined effects of HIV and tobacco use on neuroinflammation, a recent study utilized diffusion tensor imaging (DTI) to evaluate differences in brain microstructure (Liang et al. 2018). This study found that HIV and tobacco smoking had additive effects on diffusivity in several brain regions suggesting an increased vulnerability to white matter brain injury (Liang et al. 2018). Moreover, cognitive performance was also associated with diffusivity in some brain regions. However, the specificity of using DTI to quantify neuroinflammation is mixed given that several measures (e.g., fractional anisotropy, mean diffusivity) may also be impacted by demyelination and membrane permeability (Jones et al. 2013).
Nicotine Metabolism
An important biomarker of tobacco use is the nicotine metabolite ratio (NMR) (Allenby et al. 2016; Benowitz 2009), which reflects individual differences in the rate at which people metabolize nicotine. Nearly 70–80% of nicotine is metabolized by the P450 liver enzyme CYP2A6 (Hukkanen et al. 2005). The primary metabolite of nicotine is cotinine, which is further metabolized to 3´-hydroxycotinine (3HC). Due to the long half-life of cotinine (13–19 h), the ratio of 3HC to cotinine – the NMR – is a stable measure of CYP2A6-mediated nicotine metabolism that is independent of recent nicotine use (Benowitz and Jacob 2001; Benowitz et al. 2003). It has been validated in numerous studies as a biomarker of nicotine metabolism and has been associated with multiple smoking phenotypes in non-HIV populations (Allenby et al. 2016). Higher NMR values, which are indicative of faster nicotine clearance, are associated with smoking more cigarettes each day, greater nicotine dependence, and more several nicotine withdrawal symptoms (Allenby et al. 2016). Several studies have also demonstrated that the NMR is a viable tool for personalizing treatment for nicotine dependence to improve treatment response (Kaufmann et al. 2015; Lerman et al. 2006; Schnoll et al. 2009), including a recent large smoking cessation clinical trial using prospective NMR stratification (Lerman et al. 2015).
The NMR is influenced by both genetic and environmental factors. Genetic variation in the CYP2A6 gene contributes to differences in enzyme activity – and therefore nicotine metabolism (Piliguian et al. 2014); https://www.pharmvar.org/). There are more than 30 known variations in the CYP2A6 gene; some variants cause functional changes in CYP2A6 activity. Those with CYP2A6 variants that reduce function (i.e., slow metabolizers of nicotine) are generally less nicotine dependent and more likely to quit smoking (Chenoweth et al. 2013; Gu et al. 2000; Kubota et al. 2006). There are substantial population differences in the frequencies of reduced functional variants. African-Americans are more likely to have reduced function variants compared to Caucasians (Zhu et al. 2013) and NMR values generally parallel these population differences. Moreover, estrogen levels, alcohol use, age, body mass index (BMI), and menthol exposure contribute to variation in nicotine metabolism. Overall, higher NMR is associated with being female, older, lower BMI, non-menthol smokers, and greater alcohol consumption (Allenby et al. 2016; Chenoweth et al. 2014). In addition, medications that are substrates, inhibitors, or inducers of CYP2A6 activity may also impact nicotine metabolism, such as tegafur, letrozole, and the ART medication efavirenz (discussed further below) (Tanner and Tyndale 2017).
There is emerging evidence from our group and others that HIV-infected smokers may have higher rates of nicotine metabolism compared to HIV-uninfected smokers (Ashare et al. 2019a; Earla et al. 2014). Preliminary evidence suggested that the concentration of nicotine was lower among HIV-infected smokers but concentrations of its metabolites (e.g., nornicotine and cotinine) were higher, relative to HIV-uninfected smokers (Earla et al. 2014). However, this was a small sample of only six HIV-infected smokers and 17 HIV-uninfected smokers and the authors did not specifically report the NMR. Using data from a clinical trial of varenicline for smoking cessation, we recently found that, HIV-infected smokers (n=131) had a significantly higher NMR compared to HIV-uninfected smokers (n=199) (Ashare et al. 2019a). Although we were able to match the HIV+ and HIV- groups on several key characteristics (e.g., sex, race, BMI and smoking rate), this study was still unable to determine the underlying mechanism that contributed to the faster nicotine metabolism. Follow up studies are needed to: (1) evaluate whether the HIV infection itself increases nicotine metabolism; (2) identify whether certain ARTs that are either substrates, inducers, or inhibitors of CYP2A6 contribute to changes in NMR as well as other metabolizing pathways via glucuronidation (Taghavi et al. 2017; Wassenaar et al. 2015). For instance, concentrations of the ART, efavirenz, which is a substrate of CYP2A6 (in addition to other enzymes), may be associated with variation in CYP2A6 activity (McDonagh et al. 2015); and (3) investigate the consequences of faster nicotine metabolism. Indeed, evidence that CYP2A6 variation can affect HIV pathogenesis through oxidative stress pathways (Ande et al. 2013; Jin et al. 2012) suggests that the higher NMR observed among HIV-infected smokers may have downstream effects on immune function and/or neuroHIV (for a review, see Ande et al. 2013).
Interactions between ARTs and Tobacco Smoking
Nicotine interacts with ARTs, affecting their metabolism by induction or inhibition of cytochrome P-450 (CYPs) and efflux transporters (reviewed in Kumar et al. 2015; Pal et al. 2011). Notably, CYP3A4 metabolizes most commercially available drugs including ARTs (Anzenbacher and Anzenbacherova 2001) to produce ROS that leads to oxidative stress (Ande et al. 2015; Pal and Mitra 2006; Walubo 2007). Also important in the elimination of drugs is P-glycoprotein (P-gp), an ATP-dependent transporter located in the apical membrane of mucosal cells of the gastrointestinal tract, hepatic biliary canaliculi, and proximal tubule epithelial cells. Tobacco smoking can alter the pharmacokinetics of ARTs by induction of CYP3A4 and aryl-hydrocarbon-hydroxylases (D’Arcy 1984; Rahmioglu et al. 2011). Although nicotine is primarily metabolized by CYP2A6 (reviewed above), tobacco smoking can also upregulate levels of extrahepatic CYPs, namely CYP1A1 and CYP1B1 (Ande et al. 2015; Ande et al. 2013; Kumar et al. 2015; Rao et al. 2016; Zevin and Benowitz 1999), that can result in faster clearance of specific ARTs like dolutegravir, an HIV integrase inhibitor (Zhu et al. 2018). ATP-binding cassette transporters that efflux any xenobiotic molecules including drugs are differentially regulated by tobacco smoke (e.g., ABCG2, ABCB6, ABCA13, ABCC1 and ABCC3) (Aguiar et al. 2019; An et al. 2012). Smoking and ARTs can have an additive effect on expression of CYPs and efflux transporters. A recent study by Mu et al showed that cigarette smoke concentrate and the ART lopinavir polarize macrophages from a pro-inflammatory-like M1 to an anti-inflammatory-like M2 phase (Mu et al. 2018). Further, this shift induced expression of drug P-gp, CYP1B1 and CYP2A6 in vitro. Mathematical modeling by Irunde et al showed that tobacco smoking decreases efficacy of ART by inducing metabolism of ARTs by 30–70% (Irunde et al. 2017).
In addition to being a substrate of CYP2A6 (see Biomarker section above), nicotine also induces several drug-metabolizing enzymes including CYP3A4, CYP1A2, CYP2B6, CYP2E1 and UDP- glucuronosyltransferase (UGT) (Pal et al. 2011). Pal et al demonstrated that nicotine can induce expression of CYP3A4 and MDR1 that codes for P-gp in vitro (Pal et al. 2011). Nicotine can also alter pharmacokinetics by impairing BBB integrity. Indeed, both nicotine and protease inhibitor saquinavir disrupt the BBB via their effect on endothelial cells, altering the exposure of the CNS to ARTs and other potential neurotoxins (Manda et al. 2010a; Manda et al. 2010b).
In line with the bidirectional relationship between ART and tobacco use, ARTs also potentially modulate the cholinergic system and affect nicotine metabolism. Indeed, evidence reviewed above suggests that nicotine metabolism may be increased in smokers with HIV on ART compared to smokers without HIV, suggesting a role of increased CYP expression by ART (Ashare et al. 2019a; Earla et al. 2014). For example, we recently demonstrated that individuals taking efavirenz, an ART that is metabolized in part by CYP2A6, had a higher NMR compared to those not taking efavirenz (Schnoll et al. 2019). Further, ARTs can also have an effect on the cholinergic system in the CNS. A recent study by Ekins et al has shown that the HIV protease inhibitor, indinavir acts as a positive allosteric modulator of α7 nAChRs at low concentrations. However, at a higher concentration, indinavir inhibits α7 nAChRs reducing synaptic transmission in the cholinergic system thus contributing to cognitive dysfunction (Ekins et al. 2017). Interactions between ARTs and the cholinergic system can contribute to pathology, especially since HIV alters expression levels of specific nicotinic receptors. The levels of α7 nAChRs have been shown to be increased by HIV in vitro (Delgado-Velez et al. 2015) and in vivo (Capo-Velez et al. 2018b). The HIV envelope glycoprotein gp120 was shown to bind to and upregulate α7 expression levels (Ballester et al. 2012). Further, α7 nAChRs may compromise the BBB (Zhang et al. 2015) and alter calcium signaling (Ramos et al. 2016), potentially exacerbating HAND neuropathology (Liu et al. 2017).
Interestingly, growing evidence suggests that single nucleotide polymorphisms (SNPs) in genes encoding for drug-metabolizing enzymes and transporters add another layer of complexity in drug-drug interactions, especially with ARTs (Berno et al. 2014; Guttman et al. 2019; Tanner and Tyndale 2017; Zanger and Schwab 2013). For example, Feldman et al demonstrated that a genetic variant of CYP1A1 is associated with reduced response to antiretroviral therapy selectively in HIV infected smokers (Feldman et al. 2009). The variant of CYP1A1-m1 has a higher catalytic activity and has been associated with an increase in the metabolic conversion of environmental toxins to DNA modifiers, which may stimulate HIV replication. Thus, this is further evidence of a bidirectional relationship between ARTs and tobacco use.
ARTs also contribute to neurotoxicity via various mechanisms including oxidative stress, activation of endoplasmic stress and integrated stress responses (ISR), and mitochondrial dysfunction (reviewed in Shah et al. 2016; Yuan and Kaul 2019). In fact, some ARTs have been shown to increase neuropsychiatric symptoms (reviewed in Abers et al. 2014). For example, efavirenz has been shown to have anxiogenic (Cavalcante et al. 2017) and psychoactive effects (Gatch et al. 2013), and may produce mild increases in neuropsychiatric symptoms (Van de Wijer et al. 2019). There is also some evidence that efavirenz may have rewarding and addiction-like properties in rats (Möller et al. 2018) and humans (Grelotti et al. 2014). Thus, as depicted in Figure 3, the continuous use of ARTs might contribute to the neuropathology of HAND in the ART era, specifically for PLWH with other behavioral risk factors such as tobacco use or cigarette smoking. Given that tobacco smoking can alter expression levels of various CYPs and efflux transporters, and that ARTs can affect both nicotine metabolism and can interact with the cholinergic system, the interactions between ARTs and tobacco smoking needs to be further examined. This would not only be important to understand nicotine dependence and adherence to ARTs, but also how tobacco smoking alters HIV neuropathology.
Figure 3. Interaction between tobacco smoking, HIV and antiretroviral drugs.
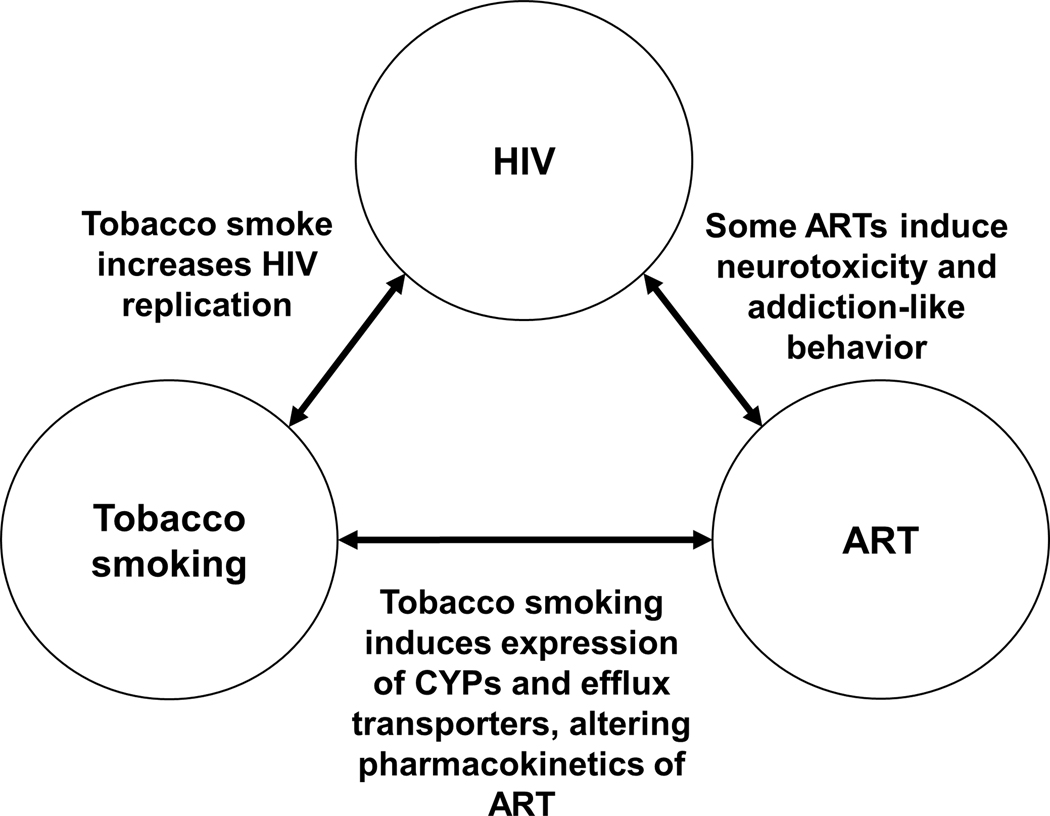
Tobacco smoke has been shown to increase HIV replication and decrease the efficacy of antiretrovirals (ARTs). Even though ARTs are indispensable for management of HIV, some have been shown to have neurotoxic adverse effects.
Effects on Peripheral Organ Systems
Tobacco smoking not only increases the susceptibility to infections in the lungs and other organs (Feldman and Anderson 2013), but also affects the interaction between lungs and other organ systems including the CNS, heart and the kidneys. Several studies have demonstrated that severe pulmonary disorders such as acute lung injury or respiratory distress may be responsible for poor cognitive outcomes (Fries et al. 2005; Hopkins et al. 1999), forming the basis for the lung-brain axis (Stevens and Puybasset 2011). Further, air pollution, specifically ozone, has been linked to increased risk of several CNS disorders, mediated by the priming of microglia through the lung-brain axis (Mumaw et al. 2016). Besides ozone, tobacco smoke components can also activate microglia, potentially contributing to neuropathology of HAND (Ghosh et al. 2009). Interestingly, a study by Gao et al demonstrated that, whereas non-nicotine components of tobacco smoke exacerbate pathology of experimental autoimmune encephalopathy (EAE), an animal model for neuroinflammation, nicotine attenuates symptoms (Gao et al. 2014).
Tobacco smoking can also alter the epigenome in blood and alveolar macrophages, that can be associated with lung function and susceptibility to lung cancer (Armstrong et al. 2019; de Vries et al. 2018; Gao et al. 2016; Imboden et al. 2019). The lungs have been considered an anatomical reservoir for HIV, even in the era of ARTs, and HIV alters pulmonary immunology (reviewed in Almodovar 2014). This is further complicated by tobacco smoking. Chand et al concluded that even in the presence of ART, simian-adapted HIV (SHIV) and tobacco smoke independently and synergistically induced chronic bronchitis and pro-COPD changes in the lungs in macaques (Chand et al. 2018). How the lung-brain axis is altered in HIV remains understudied and is an important are for future investigation.
Additionally, air pollution and tobacco smoking promote cardiovascular diseases via oxidative injury and damage to mitochondria, leading to atherosclerosis (reviewed in Cole and Freeman 2009). Interestingly, tobacco smoke-induced oxidative stress activates resident immune cells in the lungs including macrophages, endothelial cells, and platelets leading to altered expression of adhesion molecules, dysfunctional smooth muscle cells and an increase in pro-inflammatory cytokines (reviewed in Messner and Bernhard 2014). Tobacco smoking also induces the formation of advanced glycation end products (AGE) which increases oxidative stress and NFκB-mediated proinflammatory cytokines, further contributing to cardiovascular diseases (reviewed in Prasad et al. 2015). Thus, future studies should investigate how the effects of tobacco smoking on cardiovascular function interact with HIV and whether there are subsequent effects on CNS function.
Another organ system affected by tobacco use is the kidneys. Smoking is a risk factor associated with chronic kidney disease (CKD) (Hall et al. 2016; Van Laecke and Van Biesen 2017), an effect that is mediated by nicotine (Arany et al. 2016; Hua et al. 2010; Jain and Jaimes 2013). In fact, a study determined smoking as a significant risk factor for CKD within PLWH and observed a dose-response relationship between packs smoked per day and CKD within this population (Miguez-Burbano et al. 2010). Importantly, ARTs including indinavir, tenofovir, atazanavir and lopinavir have also been shown to cause renal tubular dysfunction and increase risk for CKD (reviewed in Yombi et al. 2014). A recent study exploring urine biomarkers for CKD in PLWH determined that tobacco smoking was associated with higher levels of α1-microglobulin within HIV-1 seropositive patients (Muiru et al. 2019).
Recent evidence supports an association between the microbiome and brain function, including neurodegenerative and psychiatric disorders (Kim and Shin 2018; Zhu et al. 2017). Although these relationships remain unexplored in the context of HIV-seropositive smokers, smoking may independently alter the gut biome via changes in mucus and the gut immune system (Allais et al. 2016; Capurso and Lahner 2017). Thus, the gut-brain axis is another potential mechanism linking HIV and tobacco use to neurocognitive function that warrants additional investigation. Importantly, smoking also affects the neuroendocrine or the hypothalamus-pituitary-adrenal (HPA) axis (Mendelson et al. 2005; Rohleder and Kirschbaum 2006; Tweed et al. 2012) which can alter cortisol or stress levels and mood that may be modulated by sex (Badrick et al. 2007; Mendelson et al. 2005; Torres and O’Dell 2016). Indeed, sex modulates the effects of tobacco smoking on inflammation, and can modulate risk for psychiatric disorders (reviewed in Ashare and Wetherill 2018). Thus, in addition to HIV, future studies need to consider sex and external factors, such as stress levels when planning studies of the microbiome.
Alternative Nicotine Delivery Systems
The differential effects of nicotine alone compared to combustible tobacco reviewed above may have important implications for alternative forms of nicotine delivery. This is particularly relevant in light of the dramatic rise in the use of electronic nicotine delivery systems, or ENDS (e.g., e-cigarettes, vape pens), particularly among youth and young adults (Singh et al. 2016). ENDS deliver nicotine via a liquid that is heated to form an aerosol, and thus, deliver nicotine without combusting tobacco. Because ENDS do not burn tobacco, ENDS are often perceived as less harmful than conventional cigarettes and have been proposed as part of a harm reduction approach. Although vaping is often posed as a substitute for combustible tobacco, epidemiological evidence suggests that the majority of those who vape also smoke tobacco. According to the 2016 Behavioral Risk Factor Surveillance System (BRFSS), only 15% of current ENDS users were never-smokers compared to 54.6% who were current smokers and 30.4% who were former smokers (Mirbolouk et al. 2018). There is also emerging evidence that ENDS use is growing among PLWH. A recent survey of men who have sex with men (MSM) found that 47% of HIV+ MSM reported smoking or vaping in the last 12 months, compared to 37% of HIV- MSM. Among HIV+ MSM, 16% reported vaping and the vast majority (96%) of those who vaped, also smoked (Santos et al. 2019). While there are still relatively few randomized controlled trials of e-cigarettes to reduce tobacco smoking, a recent trial found that they increased abstinence rates compared to those who used nicotine replacement therapy (Hajek et al. 2019). However, because there is generally a lack of long-term evidence of the effects of ENDS, many practitioners, including those who provide care to PLWH, are not yet willing to recommend them to their patients who smoke as strategies for reducing cigarette consumption (Bell et al. 2017). Indeed, recent reports of lung disease associated with vaping have raised important questions about their potential adverse health effects (Henry et al. 2019; Layden et al. 2019; Maddock et al. 2019).
In light of the sharp rise in the use of ENDS, there is a rapidly growing body of research investigating the effects of aerosolized nicotine on inflammation and immune function. In fact, emerging data show that ENDS may cause inflammation similar to, yet distinct from, smoking combustible cigarettes (Hom et al. 2016; Ogunwale et al. 2017). Here, we review evidence from studies focused on markers associated with neuroHIV, including oxidative stress and cytokines (e.g., IL6) (Portilla et al. 2019; Robertson et al. 2019; Yuan and Kaul 2019). For instance, in vitro studies have demonstrated that cells exposed to ENDS vapor exhibit higher oxidative stress levels – regardless of whether nicotine was present in the vapor (Scheffler et al. 2015). However, the magnitude of the increase in oxidative stress was smaller than that observed for combusted tobacco. Exposure to ENDS vapor also increased IL6 in mice and in normal human bronchial epithelial (NHBE) cells, though this effect was specific to nicotine-containing vapor (Garcia-Arcos et al. 2016). In clinical studies, adverse effects on markers of cardiovascular disease, including oxidative stress, have been found following habitual and acute e-cigarette use among smokers (Biondi-Zoccai et al. 2019; Moheimani et al. 2017). Similar effects have been observed among non-smokers, though the effects dissipated 6 hours after inhalation (Chatterjee et al. 2019). Of particular relevance to the potential impact on neuroHIV, e-cigarette vapor may compromise the BBB through similar mechanisms as tobacco smoke (Sivandzade and Cucullo 2019). Interestingly, some studies have found that ENDS aerosol exposure increases inflammation, with or without nicotine, suggesting that the chemicals in the aerosol are sufficient to produce pro-inflammatory effects (for a review, see Shields et al. 2017). While this finding is consistent with the evidence reviewed above regarding the possible anti-inflammatory effects of nicotine, other studies have found that the effects of vaping on endothelial function and oxidative stress are driven by nicotine, not e-cigarette aerosol (Chaumont et al. 2018). Importantly, these data suggest that long-term ENDS use may impact CNS function mediated by the detrimental effects on peripheral organs (Eltorai et al. 2019), including the heart and lung.
Other parameters may be important to the inflammatory effects of ENDS including the temperature at which the aerosol is heated, the ingredients used to flavor the aerosol, and the base constituents of e-liquids which are not contained in combustible tobacco (e.g., propylene glycol and vegetable glycerin, vitamin E). When heated, propylene glycol and vegetable glycerin produce carbonyls, including acrolein, formaldehyde, and acetaldehyde, which have adverse effects on lung and cardiovascular function (Qasim et al. 2017; Shields et al. 2017). Moreover, diacetyl, which is used in flavors such as caramel and butterscotch, and benzaldehyde, which is used in fruit flavorings, have been associated with respiratory disease and cytotoxicity (Shields et al. 2017). Although recent evidence suggest potentially harmful effects of vaping on the lungs, there is a paucity of human data on the long-term effects of ENDS use or how vaping may interact with HIV-associated inflammation. Consequently, rigorous research designed to identify the effects of ENDS use is needed.
Conclusions
Figure 1 depicts the complex and often bidirectional relationships among HIV, nicotine/tobacco use, inflammation/immune function, and cognition reviewed above. We have reviewed evidence drawing direct links between HIV and tobacco use (line c), as well as studies pointing to potential mechanisms that may account for the high smoking rates among PLWH (box e, line d). Similarly, our review provides evidence of an association between HIV and neurocognitive deficits (line b) that may be driven by chronic tobacco use (line g) and/or immune dysregulation (line a). The current review also highlighted several important biomarkers of nicotine dependence (e.g., nicotine metabolite ratio) and neuroinflammation (using novel neuroimaging techniques) that may shed light on these relationships. Additional studies are necessary at both the preclinical and clinical level to disentangle the potentially synergistic effects of tobacco use and HIV and cognition and immune dysregulation. Lastly, given the rapid rise in e-cigarettes, we suggest that future studies investigate the short- and long-term health effects of ENDS on immune function, cognition, and HIV-related disease outcomes.
Acknowledgments
Funding
This research was supported by grants from the National Institutes of Health (K24 DA045244; R01 DA042682; R01 DA044906) and through core services and support from the Penn Center for AIDS Research (P30 AI045008) and the Penn Mental Health AIDS Research Center (P30 MH097488).
Footnotes
Conflicts of Interest
Dr. Schnoll receives medication and placebo free of charge from Pfizer for clinical trials and has provided consultation to Pfizer, GlaxoSmithKline, and Curaleaf. Dr. Gross serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to smoking or HIV. Dr. Ashare has an investigator-initiated grant from Novo Nordisk for a drug unrelated to the current paper.
References
- Abbud RA, Finegan CK, Guay LA, Rich EA (1995) Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers J Infect Dis 172:859–863 [DOI] [PubMed] [Google Scholar]
- Abers MS, Shandera WX, Kass JS (2014) Neurological and psychiatric adverse effects of antiretroviral drugs CNS drugs 28:131–145 doi: 10.1007/s40263-013-0132-4 [DOI] [PubMed] [Google Scholar]
- Aguiar JA et al. (2019) The impact of cigarette smoke exposure, COPD, or asthma status on ABC transporter gene expression in human airway epithelial cells Scientific Reports 9:153 doi: 10.1038/s41598-018-36248-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar-Khaleel WZ et al. (2017) Association of midlife smoking status with change in processing speed and mental flexibility among HIV-seropositive and HIV-seronegative older men: the Multicenter AIDS Cohort Study J Neurovirol 23:239–249 doi: 10.1007/s13365-016-0496-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition Biochem J 357:593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allais L et al. (2016) Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut Environ Microbiol 18:1352–1363 doi: 10.1111/1462-2920.12934 [DOI] [PubMed] [Google Scholar]
- Allenby CE, Boylan KA, Lerman C, Falcone M (2016) Precision Medicine for Tobacco Dependence: Development and Validation of the Nicotine Metabolite Ratio J Neuroimmune Pharmacol 11:471–483 doi: 10.1007/s11481-016-9656-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almodovar S (2014) The complexity of HIV persistence and pathogenesis in the lung under antiretroviral therapy: challenges beyond AIDS Viral Immunology 27:186–199 doi: 10.1089/vim.2013.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altekruse SF et al. (2018) Cancer burden attributable to cigarette smoking among HIV-infected people in North America AIDS 32:513–521 doi: 10.1097/QAD.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS (2004) The pathophysiology of cigarette smoking and cardiovascular disease: an update J Am Coll Cardiol 43:1731–1737 doi: 10.1016/j.jacc.2003.12.047 [DOI] [PubMed] [Google Scholar]
- An Y et al. (2012) Cigarette smoke promotes drug resistance and expansion of cancer stem cell-like side population PLOS ONE 7:e47919 doi: 10.1371/journal.pone.0047919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande A et al. (2015) Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals PLoS One 10:e0122402 doi: 10.1371/journal.pone.0122402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande A, McArthur C, Kumar A, Kumar S (2013) Tobacco smoking effect on HIV-1 pathogenesis: role of cytochrome P450 isozymes Expert Opin Drug Metab Toxicol 9:1453–1464 doi: 10.1517/17425255.2013.816285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders Neurology 69:1789–1799 doi: 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort C (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies Lancet (London, England) 372:293–299 doi: 10.1016/S0140-6736(08)61113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzenbacher P, Anzenbacherova E (2001) Cytochromes P450 and metabolism of xenobiotics Cell Mol Life Sci 58:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I, Hall S, Reed DK, Reed CT, Dixit M (2016) Nicotine Enhances High-Fat Diet-Induced Oxidative Stress in the Kidney Nicotine Tob Res 18:1628–1634 doi: 10.1093/ntr/ntw029 [DOI] [PubMed] [Google Scholar]
- Armstrong DA et al. (2019) DNA Methylation Changes in Regional Lung Macrophages Are Associated with Metabolic Differences ImmunoHorizons 3:274–281 doi: 10.4049/immunohorizons.1900042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnson Y, Shoenfeld Y, Amital H (2010) Effects of tobacco smoke on immunity, inflammation and autoimmunity J Autoimmun 34:J258–265 doi: 10.1016/j.jaut.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C (2014) Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment Neuropharmacology 76 Pt B:581–591 doi: 10.1016/j.neuropharm.2013.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL et al. (2019a) Differences in the rate of nicotine metabolism among smokers with and without HIV AIDS 33:1083–1088 doi: 10.1097/qad.0000000000002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL et al. (2019b) Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV Drug Alcohol Depend 200:26–33 doi: 10.1016/j.drugalcdep.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Wetherill RR (2018) The Intersection of Sex Differences, Tobacco Use, and Inflammation: Implications for Psychiatric Disorders Current Psychiatry Reports 20:75 doi: 10.1007/s11920-018-0946-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH (2010) Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers Am Heart J 160:458–463 doi: 10.1016/j.ahj.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M (2007) The relationship between smoking status and cortisol secretion J Clin Endocrinol Metab 92:819–824 doi: 10.1210/jc.2006-2155 [DOI] [PubMed] [Google Scholar]
- Baez-Pagan CA, Delgado-Velez M, Lasalde-Dominicci JA (2015) Activation of the Macrophage alpha7 Nicotinic Acetylcholine Receptor and Control of Inflammation J Neuroimmune Pharmacol 10:468–476 doi: 10.1007/s11481-015-9601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarri SR et al. (2018) Use of Electronic Cigarettes Leads to Significant Beta2-Nicotinic Acetylcholine Receptor Occupancy: Evidence From a PET Imaging Study Nicotine Tob Res 20:425–433 doi: 10.1093/ntr/ntx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester LY et al. (2012) Up-regulation of the neuronal nicotinic receptor alpha7 by HIV glycoprotein 120: potential implications for HIV-associated neurocognitive disorder J Biol Chem 287:3079–3086 doi: 10.1074/jbc.M111.262543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SK, Mena G, Dean J, Boyd M, Gilks C, Gartner C (2017) Vaporised nicotine and tobacco harm reduction for addressing smoking among people living with HIV: A cross-sectional survey of Australian HIV health practitioners’ attitudes Drug Alcohol Depend 177:67–70 doi: 10.1016/j.drugalcdep.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics Annu Rev Pharmacol Toxicol 49:57–71 doi: 10.1146/annurev.pharmtox.48.113006.094742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P 3rd(2009) Nicotine chemistry, metabolism, kinetics and biomarkers Handbook of experimental pharmacology:29–60 doi: 10.1007/978-3-540-69248-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P 3rd (2001) Trans-3’-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking Br J Clin Pharmacol 51:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P, 3rd (2003) Nicotine metabolite ratio as a predictor of cigarette consumption Nicotine Tob Res 5:621–624 [DOI] [PubMed] [Google Scholar]
- Berno G et al. (2014) Analysis of single-nucleotide polymorphisms (SNPs) in human CYP3A4 and CYP3A5 genes: potential implications for the metabolism of HIV drugs BMC Med Genet 15:76 doi: 10.1186/1471-2350-15-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi-Zoccai G et al. (2019) Acute Effects of Heat-Not-Burn, Electronic Vaping, and Traditional Tobacco Combustion Cigarettes: The Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking ( SUR - VAPES ) 2 Randomized Trial J Am Heart Assoc 8:e010455 doi: 10.1161/jaha.118.010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert JR, Piette J, Weinberg GA, Sappey C, Weinberg ED (1996) Iron and oxidative stress as a mechanism for the enhanced production of human immunodeficiency virus by alveolar macrophages from otherwise healthy cigarette smokers J Infect Dis 173:1045–1047 doi: 10.1093/infdis/173.4.1045a [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R (2006) A role for fMRI in optimizing CNS drug development Nat Rev Drug Discov 5:411–424 doi: 10.1038/nrd2027 [DOI] [PubMed] [Google Scholar]
- Bracci L, Lozzi L, Rustici M, Neri P (1992) Binding of HIV-1 gp120 to the nicotinic receptor FEBS Lett 311:115–118 [DOI] [PubMed] [Google Scholar]
- Brody AL et al. (2018) Effect of overnight smoking abstinence on a marker for microglial activation: a [(11)C]DAA1106 positron emission tomography study Psychopharmacology (Berl) 235:3525–3534 doi: 10.1007/s00213-018-5077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL et al. (2017) Effect of Cigarette Smoking on a Marker for Neuroinflammation: A [(11)C]DAA1106 Positron Emission Tomography Study Neuropsychopharmacology 42:1630–1639 doi: 10.1038/npp.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL et al. (2009) Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette Int J Neuropsychopharmacol 12:305–316 doi: 10.1017/S146114570800922X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL et al. (2011) Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain Arch Gen Psychiatry 68:953–960 doi: 10.1001/archgenpsychiatry.2011.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL et al. (2006) Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors Arch Gen Psychiatry 63:907–915 doi: 10.1001/archpsyc.63.8.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL et al. (2014) Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial JAMA Psychiatry 71:797–805 doi: 10.1001/jamapsychiatry.2014.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL et al. (2013) Treatment for tobacco dependence: effect on brain nicotinic acetylcholine receptor density Neuropsychopharmacology 38:1548–1556 doi: 10.1038/npp.2013.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AK et al. (2007) Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation J Nucl Med 48:2072–2079 doi: 10.2967/jnumed.107.044842 [DOI] [PubMed] [Google Scholar]
- Bryant VE, Kahler CW, Devlin KN, Monti PM, Cohen RA (2013) The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS AIDS Care 25:1308–1316 doi: 10.1080/09540121.2013.764965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL et al. (2011) Disease-modifying therapeutic concepts for HIV in the era of highly active antiretroviral therapy J Acquir Immune Defic Syndr 58:297–303 doi: 10.1097/QAI.0b013e31822ccfcc [DOI] [PubMed] [Google Scholar]
- Capo-Velez CM, Delgado-Velez M, Baez-Pagan CA, Lasalde-Dominicci JA (2018a) Nicotinic Acetylcholine Receptors in HIV: Possible Roles During HAND and Inflammation Cell Mol Neurobiol 38:1335–1348 doi: 10.1007/s10571-018-0603-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-Velez CM et al. (2018b) The alpha7-nicotinic receptor contributes to gp120-induced neurotoxicity: implications in HIV-associated neurocognitive disorders Scientific Reports 8:1829 doi: 10.1038/s41598-018-20271-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurso G, Lahner E (2017) The interaction between smoking, alcohol and the gut microbiome Best Practice & Research Clinical Gastroenterology 31:579–588 doi: 10.1016/j.bpg.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Cavalcante GIT et al. (2017) HIV antiretroviral drug Efavirenz induces anxiety-like and depression-like behavior in rats: evaluation of neurotransmitter alterations in the striatum European journal of pharmacology 799:7–15 doi: 10.1016/j.ejphar.2017.02.009 [DOI] [PubMed] [Google Scholar]
- CDC (2017) HIV surveillance reports. Atlanta: Centers for Disease Control and Prevention; 29 [Google Scholar]
- Chand HS et al. (2018) Cigarette smoke and HIV synergistically affect lung pathology in cynomolgus macaques J Clin Invest 128:5428–5433 doi: 10.1172/JCI121935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lim A, Lau E, Alicata D (2017) Chronic Tobacco-Smoking on Psychopathological Symptoms, Impulsivity and Cognitive Deficits in HIV-Infected Individuals J Neuroimmune Pharmacol 12:389–401 doi: 10.1007/s11481-017-9728-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Tao JQ, Johncola A, Guo W, Caporale A, Langham MC, Wehrli FW (2019) Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects American Journal of Physiology - Lung Cellular and Molecular Physiology 317:L155–L166 doi: 10.1152/ajplung.00110.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont M et al. (2018) Differential Effects of E-Cigarette on Microvascular Endothelial Function, Arterial Stiffness and Oxidative Stress: A Randomized Crossover Trial Sci Rep 8:10378 doi: 10.1038/s41598-018-28723-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B (2008) Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers Eur J Nucl Med Mol Imaging 35:2304–2319 doi: 10.1007/s00259-008-0908-9 [DOI] [PubMed] [Google Scholar]
- Chenoweth MJ et al. (2014) Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers Cancer Epidemiol Biomarkers Prev 23:1773–1782 doi: 10.1158/1055-9965.EPI-14-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth MJ, O’Loughlin J, Sylvestre MP, Tyndale RF (2013) CYP2A6 slow nicotine metabolism is associated with increased quitting by adolescent smokers Pharmacogenet Genomics 23:232–235 doi: 10.1097/FPC.0b013e32835f834d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnapaiyan S, Dutta R, Bala J, Parira T, Agudelo M, Nair M, Unwalla HJ (2018) Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function Scientific Reports 8:7984 doi: 10.1038/s41598-018-26095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioe PA, Gordon REF, Guthrie KM, Freiberg MS, Kahler CW (2018) Perceived barriers to smoking cessation and perceptions of electronic cigarettes among persons living with HIV AIDS Care 30:1469–1475 doi: 10.1080/09540121.2018.1489103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MP, Freeman BA (2009) Promotion of cardiovascular disease by exposure to the air pollutant ozone American Journal of Physiology - Lung Cellular and Molecular Physiology 297:L205–208 doi: 10.1152/ajplung.00187.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP et al. (2009) beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking Arch Gen Psychiatry 66:666–676 doi: 10.1001/archgenpsychiatry.2009.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP et al. (2012) Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers Arch Gen Psychiatry 69:418–427 doi: 10.1001/archgenpsychiatry.2011.1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM et al. (2018) (18)F-XTRA PET for Enhanced Imaging of the Extrathalamic alpha4beta2 Nicotinic Acetylcholine Receptor J Nucl Med 59:1603–1608 doi: 10.2967/jnumed.117.205492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM et al. (2014) Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV J Neurovirol 20:219–232 doi: 10.1007/s13365-014-0239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WY, Li MD (2010) Nicotinic modulation of innate immune pathways via alpha7 nicotinic acetylcholine receptor Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology 5:479–488 doi: 10.1007/s11481-010-9210-2 [DOI] [PubMed] [Google Scholar]
- Culhane MA et al. (2008) Predictors of early abstinence in smokers with schizophrenia J Clin Psychiatry 69:1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcy PF (1984) Tobacco smoking and drugs: a clinically important interaction? Drug Intelligence & Clinical Pharmacy 18:302–307 doi: 10.1177/106002808401800405 [DOI] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L (2005) Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures J Neuroinflammation 2:4 doi: 10.1186/1742-2094-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M et al. (2018) From blood to lung tissue: effect of cigarette smoke on DNA methylation and lung function Respiratory Research 19:212 doi: 10.1186/s12931-018-0904-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Velez M et al. (2015) The alpha7-nicotinic receptor is upregulated in immune cells from HIV-seropositive women: consequences to the cholinergic anti-inflammatory response Clinical & Translational Immunology 4:e53 doi: 10.1038/cti.2015.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuther-Conrad W et al. (2008) Norchloro-fluoro-homoepibatidine (NCFHEB) - a promising radioligand for neuroimaging nicotinic acetylcholine receptors with PET Eur Neuropsychopharmacol 18:222–229 doi: 10.1016/j.euroneuro.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Do AN et al. (2014) Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system PLoS One 9:e92842 doi: 10.1371/journal.pone.0092842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study J Nucl Med 50:1801–1807 doi: 10.2967/jnumed.109.066647 [DOI] [PubMed] [Google Scholar]
- Doyle KL et al. (2013) Real-world impact of neurocognitive deficits in acute and early HIV infection J Neurovirol 19:565–573 doi: 10.1007/s13365-013-0218-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ (2012) A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers Drug Alcohol Depend 122:105–111 doi: 10.1016/j.drugalcdep.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ (2007) Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: consequences for neurocognition and brain morphology Alcohol 41:489–501 doi: 10.1016/j.alcohol.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earla R, Ande A, McArthur C, Kumar A, Kumar S (2014) Enhanced nicotine metabolism in HIV-1-positive smokers compared with HIV-negative smokers: simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique Drug Metab Dispos 42:282–293 doi: 10.1124/dmd.113.055186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Mathews P, Saito EK, Diaz N, Naylor D, Chung J, McMurtray AM (2017) alpha7-Nicotinic acetylcholine receptor inhibition by indinavir: implications for cognitive dysfunction in treated HIV disease AIDS 31:1083–1089 doi: 10.1097/QAD.0000000000001488 [DOI] [PubMed] [Google Scholar]
- Eltorai AE, Choi AR, Eltorai AS (2019) Impact of Electronic Cigarettes on Various Organ Systems Respir Care 64:328–336 doi: 10.4187/respcare.06300 [DOI] [PubMed] [Google Scholar]
- Endres CJ, Coughlin JM, Gage KL, Watkins CC, Kassiou M, Pomper MG (2012) Radiation dosimetry and biodistribution of the TSPO ligand 11C-DPA-713 in humans J Nucl Med 53:330–335 doi: 10.2967/jnumed.111.094565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues JV (2002) NO as a signalling molecule in the nervous system Br J Pharmacol 135:1079–1095 doi: 10.1038/sj.bjp.0704569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman C, Anderson R (2013) Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems J Infect 67:169–184 doi: 10.1016/j.jinf.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Feldman DN et al. (2009) CYP1A1 genotype modifies the impact of smoking on effectiveness of HAART among women AIDS education and prevention : official publication of the International Society for AIDS Education 21:81–93 doi: 10.1521/aeap.2009.21.3_supp.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JG et al. (2006) Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study Am J Public Health 96:1060–1065 doi: 10.2105/ajph.2005.062745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman-Hoffman VL, Hedden SL, Glasheen C, Davies C, Colpe LJ (2016) The role of mental illness on cigarette dependence and successful quitting in a nationally representative, household-based sample of U.S. adults Ann Epidemiol 26:447–454 doi: 10.1016/j.annepidem.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J (2018) Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009–2014 Prev Med 111:231–234 doi: 10.1016/j.ypmed.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Fries M et al. (2005) S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury Anesthesiology 102:761–767 doi: 10.1097/00000542-200504000-00011 [DOI] [PubMed] [Google Scholar]
- Friis-Moller N et al. (2003) Combination antiretroviral therapy and the risk of myocardial infarction N Engl J Med 349:1993–2003 doi: 10.1056/NEJMoa030218 [DOI] [PubMed] [Google Scholar]
- Gallezot JD et al. (2005) In vivo imaging of human cerebral nicotinic acetylcholine receptors with 2–18F-fluoro-A-85380 and PET J Nucl Med 46:240–247 [PubMed] [Google Scholar]
- Gamarel KE, Westfall AO, Lally MA, Hosek S, Wilson CM, Adolescent Medicine Trials Network for HIVAI (2018) Tobacco Use and Sustained Viral Suppression in Youth Living with HIV AIDS and behavior 22:2018–2025 doi: 10.1007/s10461-017-1915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Breitling LP, Brenner H (2016) Tobacco smoking and methylation of genes related to lung cancer development Oncotarget 7:59017–59028 doi: 10.18632/oncotarget.10007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Nissen JC, Ji K, Tsirka SE (2014) The experimental autoimmune encephalomyelitis disease course is modulated by nicotine and other cigarette smoke components PLOS ONE 9:e107979 doi: 10.1371/journal.pone.0107979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arcos I et al. (2016) Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner Thorax 71:1119–1129 doi: 10.1136/thoraxjnl-2015-208039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A (2014) Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART AIDS 28:67–72 doi: 10.1097/01.aids.0000432467.54003.f7 [DOI] [PubMed] [Google Scholar]
- Gatch MB et al. (2013) The HIV Antiretroviral Drug Efavirenz has LSD-Like Properties Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38:2373–2384 doi: 10.1038/npp.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Mishra MK, Das S, Kaushik DK, Basu A (2009) Tobacco carcinogen induces microglial activation and subsequent neuronal damage J Neurochem 110:1070–1081 doi: 10.1111/j.1471-4159.2009.06203.x [DOI] [PubMed] [Google Scholar]
- Goncalves RB, Coletta RD, Silverio KG, Benevides L, Casati MZ, da Silva JS, Nociti FH Jr. (2011) Impact of smoking on inflammation: overview of molecular mechanisms Inflamm Res 60:409–424 doi: 10.1007/s00011-011-0308-7 [DOI] [PubMed] [Google Scholar]
- Grelotti DJ et al. (2014) Whoonga: Potential recreational use of HIV antiretroviral medication in South Africa AIDS and behavior 18:511–518 doi: 10.1007/s10461-013-0575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu DF, Hinks LJ, Morton NE, Day IN (2000) The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit Ann Hum Genet 64:383–390 [DOI] [PubMed] [Google Scholar]
- Guo Q et al. (2013) Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: a genetic polymorphism effect on in vivo binding J Nucl Med 54:1915–1923 doi: 10.2967/jnumed.113.121020 [DOI] [PubMed] [Google Scholar]
- Guttman Y, Nudel A, Kerem Z (2019) Polymorphism in Cytochrome P450 3A4 Is Ethnicity Related Front Genet 10:224 doi: 10.3389/fgene.2019.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P et al. (2019) A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy N Engl J Med 380:629–637 doi: 10.1056/NEJMoa1808779 [DOI] [PubMed] [Google Scholar]
- Hall HI et al. (2008) Estimation of HIV incidence in the United States JAMA 300:520–529 doi: 10.1001/jama.300.5.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ME et al. (2016) Cigarette Smoking and Chronic Kidney Disease in African Americans in the Jackson Heart Study Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease 5 doi: 10.1161/JAHA.116.003280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N (2006) Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes Shock 26:358–364 doi: 10.1097/01.shk.0000228168.86845.60 [DOI] [PubMed] [Google Scholar]
- Hammoud DA et al. (2005) Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS J Neurovirol 11:346–355 doi: 10.1080/13550280500187351 [DOI] [PubMed] [Google Scholar]
- Han H, Yang Z, Chang SL, Li MD (2018) Modulatory Effects of Nicotine on neuroHIV/neuroAIDS Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology 13:467–478 doi: 10.1007/s11481-018-9806-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JD et al. (2017) The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV J Neurovirol 23:550–557 doi: 10.1007/s13365-017-0526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W (1999) Bacterial endotoxin is an active component of cigarette smoke Chest 115:829–835 doi: 10.1378/chest.115.3.829 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP (2004) Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution Brain Res 1027:48–58 doi: 10.1016/j.brainres.2004.08.043 [DOI] [PubMed] [Google Scholar]
- Heaton RK et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study Neurology 75:2087–2096 doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors J Neurovirol 17:3–16 doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study Clin Infect Dis 60:473–480 doi: 10.1093/cid/ciu862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M et al. (2013) Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study Clin Infect Dis 56:727–734 doi: 10.1093/cid/cis933 [DOI] [PubMed] [Google Scholar]
- Helleberg M et al. (2015) Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America AIDS 29:221–229 doi: 10.1097/qad.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TS, Kanne JP, Kligerman SJ (2019) Imaging of Vaping-Associated Lung Disease New England Journal of Medicine doi: 10.1056/NEJMc1911995 [DOI] [PubMed] [Google Scholar]
- Herrero P et al. (2012) Feasibility and dosimetry studies for 18F-NOS as a potential PET radiopharmaceutical for inducible nitric oxide synthase in humans J Nucl Med 53:994–1001 doi: 10.2967/jnumed.111.088518 [DOI] [PubMed] [Google Scholar]
- Herron JW, Nerurkar L, Cavanagh J (2018) Neuroimmune Biomarkers in Mental Illness Curr Top Behav Neurosci 40:45–78 doi: 10.1007/7854_2018_45 [DOI] [PubMed] [Google Scholar]
- Hillmer AT et al. (2016) Imaging of cerebral alpha4beta2* nicotinic acetylcholine receptors with (−)-[(18)F]Flubatine PET: Implementation of bolus plus constant infusion and sensitivity to acetylcholine in human brain Neuroimage 141:71–80 doi: 10.1016/j.neuroimage.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Moss TG, Montoya ID, George TP (2009) Treatment of tobacco dependence in mental health and addictive disorders Can J Psychiatry 54:368–378 doi: 10.1177/070674370905400604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B et al. (2013) Past major depression and smoking cessation outcome: a systematic review and meta-analysis update Addiction 108:294–306 doi: 10.1111/add.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA (2016) Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations Platelets 27:694–702 doi: 10.3109/09537104.2016.1158403 [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV (1999) Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome Am J Respir Crit Care Med 160:50–56 doi: 10.1164/ajrccm.160.1.9708059 [DOI] [PubMed] [Google Scholar]
- Horti AG et al. (1998) 2-[18F]Fluoro-A-85380, an in vivo tracer for the nicotinic acetylcholine receptors Nucl Med Biol 25:599–603 doi: 10.1016/s0969-8051(98)00031-6 [DOI] [PubMed] [Google Scholar]
- Hossain M et al. (2009) Tobacco smoke: a critical etiological factor for vascular impairment at the blood-brain barrier Brain Res 1287:192–205 doi: 10.1016/j.brainres.2009.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua P, Feng W, Ji S, Raij L, Jaimes EA (2010) Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers Am J Physiol Renal Physiol 299:F732–739 doi: 10.1152/ajprenal.00293.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P 3rd, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine Pharmacol Rev 57:79–115 doi: 10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Hall SM, Delucchi KL, Dilley JW (2013) A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings Nicotine Tob Res 15:1436–1445 doi: 10.1093/ntr/ntt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Lee SA, Siedner MJ (2016) Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection J Infect Dis 214 Suppl 2:S44–50 doi: 10.1093/infdis/jiw275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden M et al. (2019) Epigenome-wide association study of lung function level and its change The European Respiratory Journal 54 doi: 10.1183/13993003.00457-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irunde JI, Luboobi LS, Nkansah-Gyekye Y (2017) Modeling tobacco smoking effect on HIV antiretroviral therapy Journal of Mathematical and Computational Science 7:1046–1073-1073 [Google Scholar]
- Jain G, Jaimes EA (2013) Nicotine signaling and progression of chronic kidney disease in smokers Biochem Pharmacol 86:1215–1223 doi: 10.1016/j.bcp.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM (2016) Current Cigarette Smoking Among Adults - United States, 2005–2015 MMWR Morb Mortal Wkly Rep 65:1205–1211 doi: 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- Jin M et al. (2012) A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers J Neuroimmune Pharmacol 7:289–299 doi: 10.1007/s11481-011-9283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI Neuroimage 73:239–254 doi: 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Kalkman HO, Feuerbach D (2016) Modulatory effects of alpha7 nAChRs on the immune system and its relevance for CNS disorders Cell Mol Life Sci 73:2511–2530 doi: 10.1007/s00018-016-2175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneki M, Shimizu N, Yamada D, Chang K (2007) Nitrosative stress and pathogenesis of insulin resistance Antioxid Redox Signal 9:319–329 doi: 10.1089/ars.2006.1464 [DOI] [PubMed] [Google Scholar]
- Kaufmann A et al. (2015) Rate of nicotine metabolism and smoking cessation outcomes in a community-based sample of treatment-seeking smokers Addict Behav 51:93–99 doi: 10.1016/j.addbeh.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A, Dong Y, Shuter J, Himelhoch S (2016) Behavioral Interventions for Tobacco Use in HIV-Infected Smokers: A Meta-Analysis J Acquir Immune Defic Syndr 72:527–533 doi: 10.1097/qai.0000000000001007 [DOI] [PubMed] [Google Scholar]
- Kianoush S et al. (2017) Association Between Smoking and Serum GlycA and High-Sensitivity C-Reactive Protein Levels: The Multi-Ethnic Study of Atherosclerosis (MESA) and Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) J Am Heart Assoc 6 doi: 10.1161/jaha.117.006545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Shin C (2018) The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments Curr Neuropharmacol 16:559–573 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Vaupel DB, Stein EA, Mukhin AG (2008) Quantification of nicotinic acetylcholine receptors in the human brain with PET: bolus plus infusion administration of 2-[18F]F-A85380 Neuroimage 39:717–727 doi: 10.1016/j.neuroimage.2007.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CC, Piper ME, Gepner AD, Fiore MC, Baker TB, Stein JH (2017) Longitudinal Impact of Smoking and Smoking Cessation on Inflammatory Markers of Cardiovascular Disease Risk Arterioscler Thromb Vasc Biol 37:374–379 doi: 10.1161/atvbaha.116.308728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A et al. (2007) Inducible NO synthase expression in endomyocardial biopsies after heart transplantation in relation to the postoperative course Eur J Cardiothorac Surg 32:639–643 doi: 10.1016/j.ejcts.2007.06.041 [DOI] [PubMed] [Google Scholar]
- Kodidela S, Ranjit S, Sinha N, McArthur C, Kumar A, Kumar S (2018) Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers PLOS ONE 13:e0201144 doi: 10.1371/journal.pone.0201144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC et al. (2013) A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation J Cereb Blood Flow Metab 33:53–58 doi: 10.1038/jcbfm.2012.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S et al. (2007) Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers Drug Alcohol Depend 88:79–82 doi: 10.1016/j.drugalcdep.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T et al. (2006) CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation Pharmacogenomics J 6:115–119 doi: 10.1038/sj.tpj.6500348 [DOI] [PubMed] [Google Scholar]
- Kumar S, Rao PS, Earla R, Kumar A (2015) Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems Expert Opinion on Drug Metabolism & Toxicology 11:343–355 doi: 10.1517/17425255.2015.996546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara H et al. (2012) PET Imaging of nicotinic acetylcholine receptors in baboons with 18F-AZAN, a radioligand with improved brain kinetics J Nucl Med 53:121–129 doi: 10.2967/jnumed.111.092338 [DOI] [PubMed] [Google Scholar]
- Larsson L, Pehrson C, Dechen T, Crane-Godreau M (2012) Microbiological components in mainstream and sidestream cigarette smoke Tobacco Induced Diseases 10:13 doi: 10.1186/1617-9625-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden JE et al. (2019) Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin — Preliminary Report New England Journal of Medicine doi: 10.1056/NEJMoa1911614 [DOI] [PubMed] [Google Scholar]
- Lechner M, Lirk P, Rieder J (2005) Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin Semin Cancer Biol 15:277–289 doi: 10.1016/j.semcancer.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW (2013) Residual immune dysregulation syndrome in treated HIV infection Adv Immunol 119:51–83 doi: 10.1016/b978-0-12-407707-2.00002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Yskes R (2016) Smoking Cessation for People Living With HIV/AIDS: A Literature Review and Synthesis Nicotine Tob Res 18:2177–2184 doi: 10.1093/ntr/ntw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C et al. (2015) Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial Lancet Respir Med 3:131–138 doi: 10.1016/S2213-2600(14)70294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N (2006) Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation Clin Pharmacol Ther 79:600–608 doi: 10.1016/j.clpt.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Liang H, Chang L, Chen R, Oishi K, Ernst T (2018) Independent and Combined Effects of Chronic HIV-Infection and Tobacco Smoking on Brain Microstructure J Neuroimmune Pharmacol 13:509–522 doi: 10.1007/s11481-018-9810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L et al. (2017) Alpha7 nicotinic acetylcholine receptor is required for amyloid pathology in brain endothelial cells induced by Glycoprotein 120, methamphetamine and nicotine Scientific Reports 7:40467 doi: 10.1038/srep40467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Mandelkern M, Alvarez-Estrada M, Brody AL (2012) A single administration of low-dose varenicline saturates alpha4beta2* nicotinic acetylcholine receptors in the human brain Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37:1738–1748 doi: 10.1038/npp.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Mandelkern M, Brody AL (2011) Quantitative Molecular Imaging of Neuronal Nicotinic Acetylcholine Receptors in the Human Brain with A-85380 Radiotracers Curr Med Imaging Rev 7:107–112 doi: 10.2174/157340511795445676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C (2015) Working memory-related neural activity predicts future smoking relapse Neuropsychopharmacology 40:1311–1320 doi: 10.1038/npp.2014.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, Raman SM, Aberegg SK (2019) Pulmonary Lipid-Laden Macrophages and Vaping New England Journal of Medicine doi: 10.1056/NEJMc1912038 [DOI] [PubMed] [Google Scholar]
- Mahy M, Autenrieth CS, Stanecki K, Wynd S (2014) Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data AIDS 28 Suppl 4:S453–459 doi: 10.1097/QAD.0000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda VK, Mittapalli RK, Bohn KA, Adkins CE, Lockman PR (2010a) Nicotine and cotinine increases the brain penetration of saquinavir in rat J Neurochem 115:1495–1507 doi: 10.1111/j.1471-4159.2010.07054.x [DOI] [PubMed] [Google Scholar]
- Manda VK, Mittapalli RK, Geldenhuys WJ, Lockman PR (2010b) Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity J Neurochem 115:515–525 doi: 10.1111/j.1471-4159.2010.06948.x [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap Ann Neurol 67:699–714 doi: 10.1002/ana.22053 [DOI] [PubMed] [Google Scholar]
- McDonagh EM, Lau JL, Alvarellos ML, Altman RB, Klein TE (2015) PharmGKB summary: Efavirenz pathway, pharmacokinetics Pharmacogenet Genomics 25:363–376 doi: 10.1097/FPC.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JW et al. (2015) Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis Arterioscler Thromb Vasc Biol 35:1002–1010 doi: 10.1161/atvbaha.114.304960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J (2015) Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys Ann Intern Med 162:335–344 doi: 10.7326/M14-0954 [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK (2005) Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 30:1751–1763 doi: 10.1038/sj.npp.1300753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercie P et al. (2018) Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial Lancet HIV 5:e126–e135 doi: 10.1016/s2352-3018(18)30002-x [DOI] [PubMed] [Google Scholar]
- Messner B, Bernhard D (2014) Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis Arterioscler Thromb Vasc Biol 34:509–515 doi: 10.1161/ATVBAHA.113.300156 [DOI] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J (2011) Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats Pharmacol Biochem Behav 98:587–597 doi: 10.1016/j.pbb.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguez-Burbano MJ, Wyatt C, Lewis JE, Rodriguez A, Duncan R (2010) Ignoring the obvious missing piece of chronic kidney disease in HIV: cigarette smoking The Journal of the Association of Nurses in AIDS Care : JANAC 21:16–24 doi: 10.1016/j.jana.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbolouk M et al. (2018) Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016 Ann Intern Med 169:429–438 doi: 10.7326/M17-3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjarrad K, Vermund SH (2010) Effect of treating co-infections on HIV-1 viral load: a systematic review Lancet Infect Dis 10:455–463 doi: 10.1016/S1473-3099(10)70093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR (2017) Increased Cardiac Sympathetic Activity and Oxidative Stress in Habitual Electronic Cigarette Users: Implications for Cardiovascular Risk JAMA Cardiol 2:278–284 doi: 10.1001/jamacardio.2016.5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller M, Fourie J, Harvey BH (2018) Efavirenz exposure, alone and in combination with known drugs of abuse, engenders addictive-like bio-behavioural changes in rats Scientific Reports 8:12837 doi: 10.1038/s41598-018-29978-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Kahler CW, Lee H, Pantalone DW, Mayer KH, Cohen RA, Monti PM (2016) Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men AIDS Care 28:300–305 doi: 10.1080/09540121.2015.1093595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Patters BJ, Midde NM, He H, Kumar S, Cory TJ (2018) Tobacco and Antiretrovirals Modulate Transporter, Metabolic Enzyme, and Antioxidant Enzyme Expression and Function in Polarized Macrophages Curr HIV Res 16:354–363 doi: 10.2174/1570162X17666190130114531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiru AN et al. (2019) Kidney disease risk factors associate with urine biomarkers concentrations in HIV-positive persons; a cross-sectional study BMC Nephrol 20:4 doi: 10.1186/s12882-018-1192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumaw CL et al. (2016) Microglial priming through the lung-brain axis: the role of air pollution-induced circulating factors FASEB J 30:1880–1891 doi: 10.1096/fj.201500047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P et al. (2014) Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci 15:51 doi: 10.1186/1471-2202-15-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L (2015) Depression in HIV infected patients: a review Current Psychiatry Reports 17:530 doi: 10.1007/s11920-014-0530-4 [DOI] [PubMed] [Google Scholar]
- Nesil T, Cao J, Yang Z, Chang SL, Li MD (2015) Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories Molecular Brain 8:43 doi: 10.1186/s13041-015-0134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Sawa A, Meyer U (2018) Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry Mol Psychiatry 23:36–47 doi: 10.1038/mp.2017.232 [DOI] [PubMed] [Google Scholar]
- Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, Fu XA (2017) Aldehyde Detection in Electronic Cigarette Aerosols ACS Omega 2:1207–1214 doi: 10.1021/acsomega.6b00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Cioe PA (2015) Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV Curr HIV/AIDS Rep 12:413–420 doi: 10.1007/s11904-015-0281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Crum RM (2015) A Review of the Literature Concerning HIV and Cigarette Smoking: Morbidity and Mortality, Associations with Individual- and Social-Level Characteristics, and Smoking Cessation Efforts Addict Res Theory 23:10–23 doi: 10.3109/16066359.2014.920013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Rass O, Johnson MW (2017) Positive smoking cessation-related interactions with HIV care providers increase the likelihood of interest in cessation among HIV-positive cigarette smokers AIDS Care 29:1309–1314 doi: 10.1080/09540121.2017.1330532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK (2011) Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals Life Sci 88:959–971 doi: 10.1016/j.lfs.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Mitra AK (2006) MDR- and CYP3A4-mediated drug-drug interactions Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology 1:323–339 doi: 10.1007/s11481-006-9034-2 [DOI] [PubMed] [Google Scholar]
- Palella FJ Jr. et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators N Engl J Med 338:853–860 doi: 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- Patterson F et al. (2010) Working memory deficits predict short-term smoking resumption following brief abstinence Drug Alcohol Depend 106:61–64 doi: 10.1016/j.drugalcdep.2009.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH et al. (2006) Cognitive status of young and older cigarette smokers: data from the international brain database J Clin Neurosci 13:457–465 doi: 10.1016/j.jocn.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Peres FS, Barreto SM, Camelo LV, Ribeiro ALP, Vidigal PG, Duncan BB, Giatti L (2017) Time From Smoking Cessation and Inflammatory Markers: New Evidence From a Cross-Sectional Analysis of ELSA-Brasil Nicotine Tob Res 19:852–858 doi: 10.1093/ntr/ntx032 [DOI] [PubMed] [Google Scholar]
- Piliguian M, Zhu AZ, Zhou Q, Benowitz NL, Ahluwalia JS, Sanderson Cox L, Tyndale RF (2014) Novel CYP2A6 variants identified in African Americans are associated with slow nicotine metabolism in vitro and in vivo Pharmacogenet Genomics 24:118–128 doi: 10.1097/FPC.0000000000000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack TM, Duong HT, Pham TT, Do CD, Colby D (2017) Cigarette smoking is associated with high HIV viral load among adults presenting for antiretroviral therapy in Vietnam PLOS ONE 12:e0173534 doi: 10.1371/journal.pone.0173534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool ER, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K (2016) Interventions for tobacco use cessation in people living with HIV and AIDS Cochrane Database Syst Rev:CD011120 doi: 10.1002/14651858.CD011120.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portilla I et al. (2019) Neurocognitive Impairment in Well-Controlled HIV-Infected Patients: A Cross-Sectional Study AIDS Res Hum Retroviruses 35:634–641 doi: 10.1089/aid.2018.0279 [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF (2004) Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation Addict Behav 29:1407–1426 [DOI] [PubMed] [Google Scholar]
- Prasad K, Dhar I, Caspar-Bell G (2015) Role of Advanced Glycation End Products and Its Receptors in the Pathogenesis of Cigarette Smoke-Induced Cardiovascular Disease Int J Angiol 24:75–80 doi: 10.1055/s-0034-1396413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ (2010) Depression and smoking in the U.S. household population aged 20 and over, 2005–2008 NCHS Data Brief:1–8 [PubMed] [Google Scholar]
- Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ (2017) Impact of Electronic Cigarettes on the Cardiovascular System J Am Heart Assoc 6 doi: 10.1161/jaha.117.006353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Perez XA, Bordia T (2012) Nicotine as a potential neuroprotective agent for Parkinson’s disease Mov Disord 27:947–957 doi: 10.1002/mds.25028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmioglu N et al. (2011) Genetic epidemiology of induced CYP3A4 activity Pharmacogenet Genomics 21:642–651 doi: 10.1097/FPC.0b013e3283498ecf [DOI] [PubMed] [Google Scholar]
- Ramos FM, Delgado-Velez M, Ortiz AL, Baez-Pagan CA, Quesada O, Lasalde-Dominicci JA (2016) Expression of CHRFAM7A and CHRNA7 in neuronal cells and postmortem brain of HIV-infected patients: considerations for HIV-associated neurocognitive disorder J Neurovirol 22:327–335 doi: 10.1007/s13365-015-0401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit S, Sinha N, Kodidela S, Kumar S (2018) Benzo(a)pyrene in Cigarette Smoke Enhances HIV-1 Replication through NF-kappaB Activation via CYP-Mediated Oxidative Stress Pathway Scientific Reports 8:10394 doi: 10.1038/s41598-018-28500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Ande A, Sinha N, Kumar A, Kumar S (2016) Effects of Cigarette Smoke Condensate on Oxidative Stress, Apoptotic Cell Death, and HIV Replication in Human Monocytic Cells PLoS One 11:e0155791 doi: 10.1371/journal.pone.0155791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KP et al. (2017) Lung Cancer Mortality Associated With Smoking and Smoking Cessation Among People Living With HIV in the United States JAMA Intern Med 177:1613–1621 doi: 10.1001/jamainternmed.2017.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Landay A, Miyahara S, Vecchio A, Masters MC, Brown TT, Taiwo BO (2019) Limited correlation between systemic biomarkers and neurocognitive performance before and during HIV treatment J Neurovirol doi: 10.1007/s13365-019-00795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Yosief S (2014) Neurocognitive assessment in the diagnosis of HIV-associated neurocognitive disorders Semin Neurol 34:21–26 doi: 10.1055/s-0034-1372339 [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Aravalli RN, Hu S, Sheng WS, Peterson PK (2008) Potentiation of HIV-1 expression in microglial cells by nicotine: involvement of transforming growth factor-beta 1 J Neuroimmune Pharmacol 3:143–149 doi: 10.1007/s11481-007-9098-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgman A, Perfetti TA (2013) The Chemical Components of Tobacco and Tobacco Smoke. 2nd edn CRC Press, Taylor & Francis Group, [Google Scholar]
- Rohleder N, Kirschbaum C (2006) The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers Int J Psychophysiol 59:236–243 doi: 10.1016/j.ijpsycho.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS (2014) Inflamed moods: a review of the interactions between inflammation and mood disorders Prog Neuropsychopharmacol Biol Psychiatry 53:23–34 doi: 10.1016/j.pnpbp.2014.01.013 [DOI] [PubMed] [Google Scholar]
- Rubin LH, Maki PM (2019) HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy Curr HIV/AIDS Rep 16:82–95 doi: 10.1007/s11904-019-00421-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S, Mizrahi R (2011) Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography J Cereb Blood Flow Metab 31:1807–1816 doi: 10.1038/jcbfm.2011.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O et al. (2015) First-in-human PET quantification study of cerebral alpha4beta2* nicotinic acetylcholine receptors using the novel specific radioligand (−)-[(18)F]Flubatine Neuroimage 118:199–208 doi: 10.1016/j.neuroimage.2015.05.065 [DOI] [PubMed] [Google Scholar]
- Sacktor N et al. (2016) Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study Neurology 86:334–340 doi: 10.1212/wnl.0000000000002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H et al. (2013) Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada PLoS One 8:e81355 doi: 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos G-M, Tan J, Turner C, Raymond HF (2019) Demographic, Behavioral, and Social Characteristics Associated With Smoking and Vaping Among Men Who Have Sex With Men in San Francisco Am J Mens Health 13:1557988319847833 doi: 10.1177/1557988319847833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler S, Dieken H, Krischenowski O, Forster C, Branscheid D, Aufderheide M (2015) Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells Int J Environ Res Public Health 12:3915–3925 doi: 10.3390/ijerph120403915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C (2009) Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study Pharmacol Biochem Behav 92:6–11 doi: 10.1016/j.pbb.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA et al. (2019) Brief Report: Rate of Nicotine Metabolism and Tobacco Use Among Persons With HIV: Implications for Treatment and Research J Acquir Immune Defic Syndr 80:e36–e40 doi: 10.1097/QAI.0000000000001895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P (2011) HIV-1 infection and cognitive impairment in the cART era: a review AIDS 25:561–575 doi: 10.1097/QAD.0b013e3283437f9a [DOI] [PubMed] [Google Scholar]
- Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A (2016) Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics Neurotoxicity research 30:677–697 doi: 10.1007/s12640-016-9646-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ (2009) Nicotinic attenuation of central nervous system inflammation and autoimmunity J Immunol 182:1730–1739 doi: 10.4049/jimmunol.182.3.1730 [DOI] [PubMed] [Google Scholar]
- Shields PG et al. (2017) A Review of Pulmonary Toxicity of Electronic Cigarettes in the Context of Smoking: A Focus on Inflammation Cancer Epidemiol Biomarkers Prev 26:1175–1191 doi: 10.1158/1055-9965.Epi-17-0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS et al. (2014) Cigarette smoking and variations in systemic immune and inflammation markers J Natl Cancer Inst 106 doi: 10.1093/jnci/dju294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S, Kawamata J (2018) Roles of Nicotinic Acetylcholine Receptors in the Pathology and Treatment of Alzheimer’s and Parkinson’s Diseases In: Akaike A, Shimohama S, Misu Y, Akaike A, Shimohama S, Misu Y (eds) Nicotinic Acetylcholine Receptor Signaling in Neuroprotection. Singapore, [Google Scholar]
- Shizu M et al. (2008) Cigarette smoke condensate upregulates the gene and protein expression of proinflammatory cytokines in human fibroblast-like synoviocyte line J Interferon Cytokine Res 28:509–521 doi: 10.1089/jir.2007.0081 [DOI] [PubMed] [Google Scholar]
- Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA (2016) Tobacco use among middle and high school students--United States, 2011–2015. MMWR Morb Mortal Wkly Rep 65:361–367 [DOI] [PubMed] [Google Scholar]
- Sivandzade F, Cucullo L (2019) Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment BMC Neurosci 20:15 doi: 10.1186/s12868-019-0497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Lassmann H (2002) The role of nitric oxide in multiple sclerosis Lancet Neurol 1:232–241 [DOI] [PubMed] [Google Scholar]
- Sopori M (2002) Effects of cigarette smoke on the immune system Nat Rev Immunol 2:372–377 doi: 10.1038/nri803 [DOI] [PubMed] [Google Scholar]
- Spira A et al. (2004) Effects of cigarette smoke on the human airway epithelial cell transcriptome Proc Natl Acad Sci U S A 101:10143–10148 doi: 10.1073/pnas.0401422101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfli MR, Anderson GP (2009) How cigarette smoke skews immune responses to promote infection, lung disease and cancer Nat Rev Immunol 9:377–384 doi: 10.1038/nri2530 [DOI] [PubMed] [Google Scholar]
- Steel HC et al. (2018) Effects of Tobacco Usage and Antiretroviral Therapy on Biomarkers of Systemic Immune Activation in HIV-Infected Participants Mediators Inflamm 2018:8357109 doi: 10.1155/2018/8357109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RD, Puybasset L (2011) The brain-lung-brain axis Intensive Care Medicine 37:1054–1056 doi: 10.1007/s00134-011-2233-1 [DOI] [PubMed] [Google Scholar]
- Stroud JC, Oltman A, Han A, Bates DL, Chen L (2009) Structural basis of HIV-1 activation by NF-kappaB--a higher-order complex of p50:RelA bound to the HIV-1 LTR Journal of molecular biology 393:98–112 doi: 10.1016/j.jmb.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP et al. (1996) A-85380 [3-(2(S)-azetidinylmethoxy) pyridine]: in vitro pharmacological properties of a novel, high affinity alpha 4 beta 2 nicotinic acetylcholine receptor ligand Neuropharmacology 35:725–734 doi: 10.1016/0028-3908(96)84644-2 [DOI] [PubMed] [Google Scholar]
- Suzuki T et al. (2006) Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role J Neurosci Res 83:1461–1470 doi: 10.1002/jnr.20850 [DOI] [PubMed] [Google Scholar]
- Taghavi T, St Helen G, Benowitz NL, Tyndale RF (2017) Effect of UGT2B10, UGT2B17, FMO3, and OCT2 genetic variation on nicotine and cotinine pharmacokinetics and smoking in African Americans Pharmacogenet Genomics 27:143–154 doi: 10.1097/fpc.0000000000000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JA, Tyndale RF (2017) Variation in CYP2A6 Activity and Personalized Medicine J Pers Med 7 doi: 10.3390/jpm7040018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arentoft A, Rivera-Mindt M, Hinkin CH (2013) Functional disability in medication management and driving among individuals with HIV: a 1-year follow-up study J Clin Exp Neuropsychol 35:49–58 doi: 10.1080/13803395.2012.747596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibuakuu M et al. (2017) The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study PLoS One 12:e0184914 doi: 10.1371/journal.pone.0184914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, O’Dell LE (2016) Stress is a principal factor that promotes tobacco use in females Progress in neuro-psychopharmacology & biological psychiatry 65:260–268 doi: 10.1016/j.pnpbp.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsima B, Ratcliffe SJ, Schnoll R, Frank I, Kolson DL, Gross R (2018) Is Tobacco Use Associated with Neurocognitive Dysfunction in Individuals with HIV? J Int Assoc Provid AIDS Care 17:2325958218768018 doi: 10.1177/2325958218768018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweed JO, Hsia SH, Lutfy K, Friedman TC (2012) The endocrine effects of nicotine and cigarette smoke Trends in Endocrinology and Metabolism 23:334–342 doi: 10.1016/j.tem.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS (2010) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta GA: [PubMed] [Google Scholar]
- Valette H et al. (1999) Imaging central nicotinic acetylcholine receptors in baboons with [18F]fluoro-A-85380 J Nucl Med 40:1374–1380 [PubMed] [Google Scholar]
- Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D (2014) Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: a cross-sectional pilot study PLOS ONE 9:e97698 doi: 10.1371/journal.pone.0097698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wijer L et al. (2019) Neuropsychiatric symptoms in Tanzanian HIV-infected children receiving long-term efavirenz treatment: a multicentre, cross-sectional, observational study The lancet HIV 6:e250–e258 doi: 10.1016/S2352-3018(18)30329-1 [DOI] [PubMed] [Google Scholar]
- Van Keulen HV et al. (2017) Serum levels of nitric oxide and cytokines in smokers at the beginning and after 4months of treatment for smoking cessation Int J Cardiol 230:327–331 doi: 10.1016/j.ijcard.2016.12.111 [DOI] [PubMed] [Google Scholar]
- Van Laecke S, Van Biesen W (2017) Smoking and chronic kidney disease: seeing the signs through the smoke? Nephrology Dialysis Transplantation 32:403–405 doi: 10.1093/ndt/gfw448 [DOI] [PubMed] [Google Scholar]
- Vassallo R, Kroening PR, Parambil J, Kita H (2008) Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses Mol Immunol 45:3321–3329 doi: 10.1016/j.molimm.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L (2005) Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming J Immunol 175:2684–2691 doi: 10.4049/jimmunol.175.4.2684 [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA (2006) The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging Prog Neurobiol 80:308–322 doi: 10.1016/j.pneurobio.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera JH et al. (2016) Neuroinflammation in treated HIV-positive individuals: A TSPO PET study Neurology 86:1425–1432 doi: 10.1212/WNL.0000000000002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ et al. (2018) HIV Care Initiation: A Teachable Moment for Smoking Cessation? Nicotine Tob Res 20:1109–1116 doi: 10.1093/ntr/ntx218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walubo A (2007) The role of cytochrome P450 in antiretroviral drug interactions Expert Opinion on Drug Metabolism & Toxicology 3:583–598 doi: 10.1517/17425225.3.4.583 [DOI] [PubMed] [Google Scholar]
- Wang W, Li X, Xu J (2015) Exposure to cigarette smoke downregulates beta2-adrenergic receptor expression and upregulates inflammation in alveolar macrophages Inhalation Toxicology 27:488–494 doi: 10.3109/08958378.2015.1075628 [DOI] [PubMed] [Google Scholar]
- Wassenaar CA et al. (2015) UGT1A and UGT2B genetic variation alters nicotine and nitrosamine glucuronidation in european and african american smokers Cancer Epidemiol Biomarkers Prev 24:94–104 doi: 10.1158/1055-9965.epi-14-0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Liu Q, Li D, Zheng Q, Zhou J, Li J (2015) Acute nicotine treatment attenuates lipopolysaccharide-induced cognitive dysfunction by increasing BDNF expression and inhibiting neuroinflammation in the rat hippocampus Neurosci Lett 604:161–166 doi: 10.1016/j.neulet.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Seng EK, Esan H, Shuter J (2018) Perceived risks and benefits of quitting smoking in a sample of adults living with HIV/AIDS AIDS Care 30:564–568 doi: 10.1080/09540121.2017.1382678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Zarka S, Werbeloff N, Kravitz E, Lubin G (2010) Cognitive test scores in male adolescent cigarette smokers compared to non-smokers: a population-based study Addiction 105:358–363 doi: 10.1111/j.1360-0443.2009.02740.x [DOI] [PubMed] [Google Scholar]
- Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J (1995) Accelerated course of human immunodeficiency virus infection after tuberculosis Am J Respir Crit Care Med 151:129–135 doi: 10.1164/ajrccm.151.1.7812542 [DOI] [PubMed] [Google Scholar]
- WHO (2019) Progress report on HIV, viral hepatitis and sexually transmitted infections. World Health Organization, Geneva [Google Scholar]
- Wiley CA et al. (2006) Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment J Neurovirol 12:262–271 doi: 10.1080/13550280600873868 [DOI] [PubMed] [Google Scholar]
- Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF (2007) Nicotine exposure alters in vivo human responses to endotoxin Clin Exp Immunol 147:28–34 doi: 10.1111/j.1365-2249.2006.03248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojna V et al. (2007) Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women J Neurovirol 13:561–568 doi: 10.1080/13550280701620747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF et al. (2013) PET imaging of high-affinity alpha4beta2 nicotinic acetylcholine receptors in humans with 18F-AZAN, a radioligand with optimal brain kinetics J Nucl Med 54:1308–1314 doi: 10.2967/jnumed.112.108001 [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Hillmer AT, Mason GF, Cosgrove KP (2019) Imaging Biomarkers of the Neuroimmune System among Substance Use Disorders: A Systematic Review Mol Neuropsychiatry 5:125–146 doi: 10.1159/000499621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP et al. (2004) Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV J Clin Exp Neuropsychol 26:759–778 doi: 10.1080/13803390490509565 [DOI] [PubMed] [Google Scholar]
- Yanagita M, Kobayashi R, Kojima Y, Mori K, Murakami S (2012) Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-gamma upregulation Cell Immunol 274:26–33 doi: 10.1016/j.cellimm.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Yang Z, Nesil T, Connaghan KP, Li MD, Chang SL (2016) Modulation Effect of HIV-1 Viral Proteins and Nicotine on Expression of the Immune-Related Genes in Brain of the HIV-1 Transgenic Rats J Neuroimmune Pharmacol 11:562–571 doi: 10.1007/s11481-016-9679-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Nesil T, Wingo T, Chang SL, Li MD (2017) HIV-1 Proteins Influence Novelty-Seeking Behavior and Alter Region-Specific Transcriptional Responses to Chronic Nicotine Treatment in HIV-1Tg Rats Nicotine Tob Res 19:1024–1032 doi: 10.1093/ntr/ntx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Hoffer A, Chang CY, Puga A (1995) Dioxin activates HIV-1 gene expression by an oxidative stress pathway requiring a functional cytochrome P450 CYP1A1 enzyme Environ Health Perspect 103:366–371 doi: 10.1289/ehp.95103366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yek C et al. (2016) Standard vaccines increase HIV-1 transcription during antiretroviral therapy AIDS 30:2289–2298 doi: 10.1097/QAD.0000000000001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yombi JC, Pozniak A, Boffito M, Jones R, Khoo S, Levy J, Post FA (2014) Antiretrovirals and the kidney in current clinical practice: renal pharmacokinetics, alterations of renal function and renal toxicity AIDS 28:621–632 doi: 10.1097/QAD.0000000000000103 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H et al. (2006) Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-?B phosphorylation and nuclear factor-?B transcriptional activity through nicotinic acetylcholine receptor?7 Clinical and Experimental Immunology 146:116–123 doi: 10.1111/j.1365-2249.2006.03169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan NY, Kaul M (2019) Beneficial and Adverse Effects of cART Affect Neurocognitive Function in HIV-1 Infection: Balancing Viral Suppression against Neuronal Stress and Injury J Neuroimmune Pharmacol doi: 10.1007/s11481-019-09868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation Pharmacol Ther 138:103–141 doi: 10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Zevin S, Benowitz NL (1999) Drug interactions with tobacco smoking. An update Clin Pharmacokinet 36:425–438 [DOI] [PubMed] [Google Scholar]
- Zhang B et al. (2015) Alpha7 nicotinic acetylcholine receptor is required for blood-brain barrier injury-related CNS disorders caused by Cryptococcus neoformans and HIV-1 associated comorbidity factors BMC Infect Dis 15:352 doi: 10.1186/s12879-015-1075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Li F, Zhang Y, Elbourkadi N, Wang Z, Yu C, Taylor EW (2010) Mechanisms and genes involved in enhancement of HIV infectivity by tobacco smoke Toxicology 278:242–248 doi: 10.1016/j.tox.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF (2013) Variation in trans-3’-hydroxycotinine glucuronidation does not alter the nicotine metabolite ratio or nicotine intake PLoS One 8:e70938 doi: 10.1371/journal.pone.0070938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J et al. (2018) CYP1A1 and 1B1-mediated metabolic pathways of dolutegravir, an HIV integrase inhibitor Biochem Pharmacol 158:174–184 doi: 10.1016/j.bcp.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Han Y, Du J, Liu R, Jin K, Yi W (2017) Microbiota-gut-brain axis and the central nervous system Oncotarget 8:53829–53838 doi: 10.18632/oncotarget.17754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D et al. (2008) Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report Nicotine Tob Res 10:1691–1715 doi: 10.1080/14622200802443569 [DOI] [PubMed] [Google Scholar]


