Abstract
Objectives
Medical compression therapy is used for non-invasive treatment of venous and lymphatic diseases. Medical compression therapy-associated adverse events and contraindications have been reported, although some contraindications are theoretically based. This consensus statement provides recommendations on medical compression therapy risks and contraindications.
Methods
A systematic literature search of medical compression therapy publications reporting adverse events up until November 2017 was performed. A consensus panel comprising 15 international experts critically reviewed the publications and formulated the recommendations.
Results
Sixty-two publications reporting medical compression therapy adverse events were identified. The consensus panel issued 21 recommendations on medical compression therapy contraindications and adverse event risk mitigation, in addition to reviewing medical compression therapy use in borderline indications. The most frequently reported non-severe medical compression therapy-associated adverse events included skin irritation, discomfort and pain. Very rare but severe adverse events, including soft tissue and nerve injury, were also identified.
Conclusion
This consensus statement summarises published medical compression therapy-associated adverse events and contraindications, and provides guidance on medical compression therapy. Severe medical compression therapy-associated adverse events are very rarely encountered if compression is used correctly and contraindications are considered.
Keywords: Compression, consent, complications, lymphedema, venous disease
Introduction
Medical compression (MC) devices, including MC stockings (MCS), compression bandages (CB), adjustable compression wraps (ACW) and thromboprophylactic stockings (TPS) are basic management options for the non-invasive treatment of venous and lymphatic diseases. A wide range of side effects associated with their use and contraindications have been reported in the literature. These are based mostly on case reports and consensus documents, but some are based solely on theoretical concerns. The aim of this consensus was to review the available literature, to undertake a critical appraisal of reported side effects, and to provide recommendations on contraindications and risks of MC treatment. In this paper, although intermittent pneumatic compression (IPC) is an important component of compression therapy, we report on side effects and contraindications of IPC only when they differ from those of other forms of compression therapy.
Methods
Consensus panel
The consensus panel contributing to the development of this article comprised 15 international experts experienced in compression therapy, representing different medical disciplines (angiology, cardiology, dermatology and vascular surgery). The consensus group is chaired by the primary author, Eberhard Rabe, and comprises a core group (Christopher R Lattimer, Mark H Meissner, Nick Morrison, Eberhard Rabe, and Hugo Partsch) and an extended group, whose members were proposed by the group’s Chair and its co-Chair, Hugo Partsch. The responsibility of the experts, supported by a secretary, Sylvain Gaillard, was to perform and refine the literature searches and to use personal records and knowledge to select and draft the initial recommendations. These were used as a framework for discussion prior to writing the article.
Study design
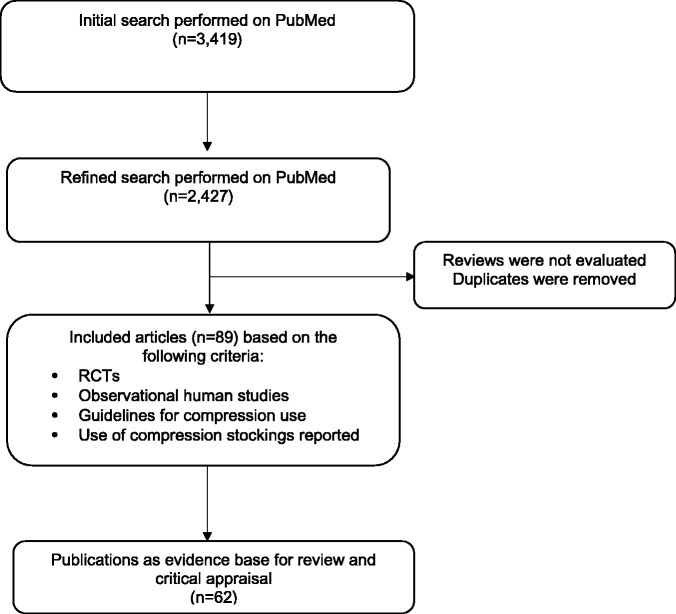
A systematic literature search was performed for articles, guidelines and treatment recommendations published up to November 2017 which reported adverse events related to MC treatment. The primary search terms used were: ‘compression bandages’, ‘medical compression stockings’, ‘graduated elastic compression’, ‘adjustable compression’, ‘thromboprophylactic stockings’ and ‘TPS’. These were cross-matched with: ‘complications’, ‘adverse events’, ‘contraindications’, ‘side effects’ and ‘risks’. The search used the online citation search engine PubMed. The search results are summarised in Figure 1. An additional search performed up to December 2019 did not identify any new publications on the contraindications for compression based on original data.
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram of relevant literature identified.
RCT: randomised controlled trial.
Risks and complications for compression therapy reported in literature
This paper reviews common risks and complications of compression therapy reported in literature (Table 1), including skin irritation and pruritus, rare complications such as superficial venous thrombophlebitis at the upper stocking border, decompensation of heart failure or spread of bacterial and/or fungal infection, and exceptional but potentially devastating complications such as nerve damage, venous thromboembolism, arterial thrombosis and skin or limb necrosis. All of the more severe complications are very rare and occur in predisposed patients or during improper use of compression therapy. Therefore, every patient receiving compression therapy should be screened for conditions that increase the risk of complications, and every compression device should be checked for appropriate fit and application. The use of compression therapy must be considered carefully in the presence of such conditions, and contraindications must be considered. Many of the reported risks are similar in MCS and CB but there are also some considerable differences. For example, in a patient with severe peripheral arterial occlusive disease (PAOD) with an ankle pressure of 60 mmHg for whom a contraindication for MCS exists, well-padded inelastic CB applied with low pressure may be appropriate even with an ankle pressure of 50 mmHg.1
Table 1.
Summary of reported adverse events.
| Reported adverse events | Incidence |
|---|---|
| Non-severe | |
| Skin irritation4,5,20,21,23,25–31,36,37 | Common |
| Allergic skin reaction5,6 | Very rare |
| Discomfort and pain7 | Common |
| Forefoot oedema and lymphoedemaa | Rare |
| Bacterial and fungal infection4,9,10 | Very rare |
| Severe | |
| Soft tissue damage or necrosis20–30,34–37,62 | Very rare |
| Nerve damage31–37 | Very rare |
| Arterial impairment24,77 | Very rare |
| Venous thromboembolism43 | Very rare |
| Cardiac decompensation47 | Very rare |
Frequency grouping: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to < 1/100), rare (≥1/10,000 to <1/1000), very rare (<1/10,000), frequency not known (cannot be estimated from the available data).89
aBased on experts’ experience.
Source: reproduced with permission from European Commission, 2009.89
Recommendation 1
We recommend that every patient receiving compression therapy should be screened for conditions that increase the risk of complications, and every compression device should be checked for appropriate fit and application. Contraindications for compression treatment must be considered to limit the risk of side effects.
Skin irritation
Low-severity skin problems, including itching, feeling cold or warm and dry skin, are frequently associated with compression therapy.2,3 The higher incidence of itching and dryness reported with MCS when compared with TPS may be partially explained by the skin of MCS patients already being compromised due to venous congestion. Skin irritation may be mistaken for allergic reactions, but usually they are self-limiting, can be controlled without interruption of the compression treatment and may be prevented through adequate skin care. A considerable number of potentially treatment-related adverse events, including irritant inflammatory skin reactions below four-layer bandage systems and short-stretch bandaging, has been described in a Canadian trial.4 Delayed bandage changes are contributory, as these prolong contact with irritant topical applications on pre-sensitised skin of venous disease patients. Tension-induced skin stress is a frequent experts’ observation occurring in patients after a well applied bandage or measured stocking has been removed. It is manifest as circumferential red lines or a patch of redness, typically over fulcrum areas, where the fabric of the material rubs against the skin. Whilst not reported as a complication and reversible, it may represent the physical evidence of why compression devices may cause discomfort or irritation.
Recommendation 2
We recommend using adequate skin care to prevent skin irritation in patients with sensitive skin.
Allergic skin reactions
In most cases, inflammatory skin reactions are caused by skin desiccation and skin irritations due to an occlusive barrier effect of the compression material. True allergic skin reactions to compression materials are rare, as allergenic components are usually avoided in actual MC products, but have been noted following the use of rubber-based products.5 Most current compression products no longer contain natural rubber or latex. For the same reason, type IV sensitisation to sulphur-containing catalysers of vulcanisation of natural rubber is of almost no relevance. Sensitisation to paraphenylenediamine, the basic component of black and dark blue dyes, may cause allergic contact dermatitis to compression stockings of dark colours but in many countries, paraphenylenediamine-containing colours are no longer used in clothing materials.6
Recommendation 3
To prevent allergic skin reactions due to compression devices, we suggest avoiding potentially allergenic substances and dyes in compression materials.
Discomfort and pain
The feeling that a compression device, MCS, TPS or CB, is ‘too tight’ is often reported by the patient on first application and may influence compliance. It may take several days before patients get used to wearing compression. Compression discomfort is experienced usually around the ankle or foot. Discomfort or pain below compression may be due to the new experience of ‘pressure on the leg’ but may also be due to incorrect sizing, bandaging technique or indication for the selected pressure level, and allowing patients to select their stocking from an array of choices is an option for improving patient compliance.7
Recommendation 4
In patients with discomfort and/or pain below compression garments, we recommend checking the correct indication, pressure level, material, fitting or bandage techniques as well as the correct donning and doffing.
Forefoot oedema and lymphoedema
Classical compression devices apply low or no pressure to the flat dorsum of the forefoot and toes. According to experts’ experience in susceptible patients, in immobile patients and, in particular, in lymphoedema, this may lead to increased swelling in the forefoot and toe area, with a theoretically increased risk of interdigital fungal infection. Prevention and treatment consist of including the forefoot and the toes in the compression with special additional compression garments. In addition, healthcare professionals (e.g. wound care nurses) should be trained in applying these techniques.
Several cases of genital lymphoedema development or worsening after IPC treatment have been reported in a single retrospective study.8 Possibly related to improvements in IPC systems, more recent studies investigating the safety of IPC treatment have not reported cases of IPC-related genital lymphoedema.
Compression of the lower leg alone may cause increased swelling of the knee region. Based on the experts’ experience, the length of compression garments should be adapted to the region of pathological findings.
Recommendation 5
In patients with, or in those developing, forefoot or toe oedema when wearing compression, we suggest considering forefoot and toe bandaging or forefoot and toe compression pieces in addition to leg compression with a foot piece.
Bacterial and fungal infections
In many conditions treated with compression devices, such as venous ulcers and lymphoedema, concomitant bacterial and fungal colonisation or infection may be present. The occlusive barrier effect of compression devices in the toe area may add to the risk of infection,9 which may be mitigated by toe-free compression. Folliculitis of the skin associated with IPC has also been reported.10
Bacterial colonisation of the skin and chronic venous leg ulcers (VLU) is common and, in the absence of clinical signs of systemic infections, is often treated with topical antiseptics, dedicated dressings and necrotic tissue debridement. Current guidelines suggest no local or systemic antibiotic treatment for bacterial colonisation in cases where no systemic infection is evident.11,12 The evidence strongly supports the use of MC for VLU treatment in patients with local bacterial wound colonisation. There is no evidence that compression may worsen bacterial colonisation or infection. An application of compression together with local wound treatment facilitates and accelerates wound healing in this clinical setting.11,12 Studies have shown that restricting the use of MC in VLU treatment leads to prolonged ulcer healing.13 In certain cases of VLU treatment, local bacterial colonisation or infection may worsen below MC despite the use of appropriate local therapy.
According to experts’ experience, such worsening is usually multifactorial and, in most cases, related to changes in wound condition and bacterial type.
In the Canadian Bandaging Trial, the occurrence of infection was treated as an adverse event.4 However, this interpretation is controversial and comparative data with and without compression are missing. In other studies, the use of compression is associated with shortened ulcer healing times, and local bacterial loads have not been found to affect duration of treatment.11,12,14
Current evidence is lacking for the occurrence of infectious complications of previously uninfected skin after MCS use. In isolated cases, pre-existing bacterial colonisation may exploit changes in the local environment triggered by compression garment materials or by skin injuries caused by folding of ill-fitting MCS.5
Erosive pustular dermatosis (EPD) has been described as a rare, mostly non-infectious condition in association with venous insufficiency and atrophy of the skin of the lower leg during long-term permanent four-layer bandaging. Intermittent compression regimens improved EPD compared with the permanent application of a four-layer bandage system producing sustained compression in patients unresponsive to antibiotics for confirmed bacterial infection of the skin of the lower leg.15
Possible fungal colonisation has also been described in some leg ulcer patients treated with MCS infused with skin care ointment, but it is not clear if this colonisation was due to the ointment or the compression.9
Compression materials may also exert antiseptic effects. Zinc oxide compression dressing was compared with standard wound care for the treatment of sutured excisions on the legs and found to promote superior healing compared with standard wound care.16 Incorporation of antimicrobial substances in MCS products has led to the development of antimicrobial compression garments containing nanoparticles or fibres made of silver.17
There is no evidence that compression principally increases the risk of infection. To the contrary, compression may facilitate healing in conditions such as erysipelas in lymphoedema or in VLU, despite bacterial colonisation. However, in rare cases and under special conditions (e.g. lateral compression of the toes with interdigital maceration, long-lasting occlusive barrier effects beneath four-layer bandages), local compression effects may foster microbiological colonisation.
Recommendation 6
In patients with bacterial or fungal infection beneath the compression device, we recommend considering treatment with topical antiseptics or topical anti-microbiological medication. In patients with systemic symptoms (fever, chills), erysipelas or cellulitis, we recommend that systemic treatment should be given. In other cases of systemic symptoms and severe local wound and tissue infection, the decision on the further treatment, including also MC, should be individualised on the basis of the local and general patient condition evaluation.
Recommendation 7
If the compression application or material is suspected to contribute to the infection (e.g. lateral pressure on toes with interdigital maceration), we suggest a modification of compression.
Mechanical tissue and nerve damage
Compression exerted by CB, TPS, adjustable compression garments and MCS is mediated through tension. As a consequence of the Law of Laplace, areas on the legs with smaller radius and bony or tendinous prominences are subject to higher local pressure than flat areas made up of predominantly softer tissue.18 Higher local pressure may result in pressure necrosis, tissue ischaemia or nerve damage, and is a particular problem in aged, malnourished, or sun-damaged skin with little supporting subcutaneous fat.
A reduced ankle-brachial pressure index (ABPI) is contributory to the risk of tissue damage by compression although it does not measure skin perfusion directly.19 Areas affected include the tibialis anterior tendon, the Achilles tendon, the anterior border of the tibia, the malleoli and the head of the fibula. Prevention involves reducing the inappropriate high pressure by decreasing the locally elevated pressure through appropriately placed soft padding material, using lower pressure levels, taking appropriate circumference measurements so that the compression devices fit properly and using flat-knitted material where round-knitted stockings are not applicable due to high calf/ankle circumference ratios.
Failure to maintain appropriate position on the leg with TPS, MCS or CB, which may lead to strangulation or inappropriate high local pressure values or continuous high pressure values, has been reported in association with tissue damage, and may also lead to arterial compression and necrosis. An additional risk factor for tissue necrosis is severe PAOD or severe microangiopathy, which are common in patients with diabetes.
Recommendation 8
We suggest considering that, according to the Law of Laplace, the local pressure below the compression material may be higher than expected at bony and tendinous prominences such as above ankles, the tibia, the fibular head or above tendons such as the Achilles tendon, and to check those locations for skin lesions due to pressure.
Recommendation 9
To prevent tissue damage or necrosis and nerve damage in regions with a small radius, we suggest protecting these regions (tendons, nerves and bones) from inappropriate high pressure, particularly in patients with sensitive skin, by:
Decreasing the local pressure by inserting soft padding material
Using low overall pressure
Taking appropriate circumference measurements so that the compression devices fit properly
Soft tissue damage or necrosis
Single cases of skin damage and/or skin necrosis due to inappropriate use of TPS in patients with or without pre-existing PAOD have been reported.20,21
Two cases of skin breaks located at the popliteal fossa and the heel, respectively, were related to the use of two inadequately superimposed MCS, which delivered a combined, sustained (day and night) pressure of 40 mmHg at the ankle over a prolonged period of time.22 Pressure ulcers in the popliteal fossa associated with the use of TPS have also been reported.23
A survey among general surgeons in Scotland found that 11% of the respondent surgeons had observed development of tissue necrosis secondary to the use of graduated MCS in their patients.24 In the CLOT I study, which investigated the benefit of TPS in stroke patients, skin breaks, ulcers, blisters, skin necrosis and lower limb amputations were significantly more common in patients treated with TPS.25 Pressure-induced skin lesions, as described above, may be assumed to be more frequently related to the use of high-pressure MC bandages. Distal foot ulcers in patients with normal arterial circulation have been described as a complication of firmly applied MC bandages.26 Tissue damage has also been described by inappropriate use of or malfunctioning IPC devices.27–30
Recommendation 10
We suggest specific precaution (padding, special care of fit, low pressure), and close controls at the initial stages of compression therapy in patients with polyneuropathy and elderly patients with frail, atrophic skin (dermatoporosis).
Nerve damage
Peripheral nerve damage with numbness or palsy following compression treatment has been reported following compression bandaging, IPC and after use of MCS or TPS.31–37 The common peroneal (fibular) nerve at the fibular head was the most frequently affected injury site, and the typical presentation of common peroneal nerve palsy included acute, complete or partial foot drop along with associated numbness in the affected foot or leg.31–33,35–37
In most cases, poorly fitted MCS or TPS and inadequate compression techniques with strangulation resulted in persistent, high focal pressure to a superficial nerve. Insufficient protection of a superficial nerve by padding below the compression in regions at risk was also reported. Symptoms of nerve damage were generally noted within a few hours of increased compression, and significant clinical improvement occurred in approximately three weeks to six months, depending on the extent of nerve damage.
In general, the factors reported as causes of pressure-induced nerve damage are similar to the processes causing soft tissue damage:
Incorrectly sized TPS or MCS,36,37 especially when combined with intraoperative use of IPC devices that provide additional focal compression to a vulnerable area34,37
Slipping and/or rolling of TPS or MCS resulting in increased persistent local compression34
Sensory loss, especially in patients with diabetic neuropathy or following anaesthesia, as a potential cause of non-perception of local pressure damage35
Hereditary disposition to developing pressure palsies37
The risk depends on the duration and pressure of the compression effect as well as patient-specific conditions such as cachexia, anatomic variations and patients with a habit of leg crossing.31,36
Recommendations in the literature for the prevention of nerve palsy include:
Guidelines on correct sizing and application of TPS or MCS should be followed34,36,37
Patients should be routinely questioned about the comfort of their bandaging and symptoms suggestive of neurological disturbance31
The anatomy of the extremities including the nerves should be taught to those applying compression bandaging31
Routine surveillance is required for skin damage and reassessment to ensure correct application of TPS, MCS or bandaging,31,36,37 especially with distant operative sites and in patients with one or more related risk factors34,36,37
Periodic neurological assessment is needed in patients on long-term chronic compression therapy31
Patients should be educated about recognising symptoms suggestive of neurological disturbance and minimisation of leg-crossing habits31
Recommendation 11
We suggest considering that pressure-induced nerve damage may occur at specific points of the leg (e.g. fibular head) mainly in cases with excessive local compression pressure, e.g. due to ill-fitting MCS, TPS or CB. Numbness and nerve palsy may occur. We suggest preventing high or continuous local pressure in regions with a risk of nerve compression as well as correct sizing and application of compression. Patients at higher risk for nerve damage (e.g. patients with diabetes, patients with neuropathy) should be treated with special caution to prevent nerve damage.
PAOD in compression treatment
PAOD with low ankle pressure and low ABPI may interfere with compression treatment and increase the risk of soft tissue damage and necrosis due to insufficient arterial nutrition underneath the compression area. This may happen more often in regions with elevated local pressure beneath compression devices such as ankles, tendons or in the foot (see the section ‘Soft tissue damage or necrosis’). To avoid skin injury by compression in severe PAOD, ankle pressure and ABPI or toe pressure should be measured and calculated before compression treatment is initiated.
Recommendation 12
We recommend checking the arterial circulation status before any kind of compression therapy is initiated. If foot pulse and/or ankle pulse is weak or not palpable, ABPI should be measured and calculated prior to initiating MC therapy.
In severe PAOD, sustained compression is contraindicated if the systolic ankle pressure is <60 mmHg or the toe pressure is <30 mmHg. This is a clear contraindication against compression therapy with MCS. In CB, the applied pressure and the elasticity of the material are important. Inelastic material may provide very low resting pressure and pressure peaks during walking only, which may be well tolerated and even increase arterial flow as oedema subsides.
Recommendation 13
Severe PAOD (systolic ankle pressure <60 mmHg, toe pressure <30 mmHg) is a contraindication against compression therapy with MCS. In CB, the applied pressure and the elasticity of the material are important. This contraindication does not apply to IPC and to patients with non-critical leg ischaemia treated with inelastic material applied with low resting pressure.
Recommendation 14
In every patient with impaired perfusion of the lower limb (ABI <0.9), the clinical effect of the MCS on leg blood supply should be carefully monitored. If the situation is not recognised, there is a possibility of developing non-healing skin breaks even under low pressure MCS.
Compression after arterial bypass surgery or stenting
Most surgical anatomic bypasses for PAOD are located deep below the muscular fascia, and it is unlikely that compression will adversely affect arterial inflow. However, in extra-anatomical bypasses, the anastomosis and the bypass itself may be very superficial, and thus compression of the epifascial bypass conduit should be avoided. Special care should be also taken in patients with in situ venous femoro-popliteal (especially distal popliteal) or tibial bypasses or in patients with very distal lower-leg anastomoses, which are usually located very superficially in the lower leg tissue – in these cases, standard compression should be avoided. No published data of damage by compression treatment in this patient group have been identified.
Recommendation 15
After bypass surgery with improved peripheral arterial pressures, MC treatment may be performed if there is no direct compression effect on the bypass itself. We suggest avoiding the compression of epifascial bypass conduits.
As for all patients with chronic leg ischaemia, the recommendations regarding the use of MC treatment should be followed (see recommendations 12–14).
Venous thromboembolism
Compression is not a contraindication in venous thromboembolic conditions but rather helps to reduce pain and oedema in deep vein thrombosis (DVT) immediately, allowing for comfortable ambulation.38–41 Thromboembolic complications due to MC are rare, most of them are without clinical symptoms.42
MCS and TPS may cause superficial venous thrombosis (SVT) in patients with varicose veins, primarily in regions where they could exert a tourniquet effect. In a study of airline passengers, elastic compression stockings during long-haul air travel were associated with a reduction in symptomless DVT, but 3.8% of patients developed SVT in varicose veins in the knee region, which were compressed by the upper edge of the stocking.43
Recommendation 16
Because of a tourniquet effect, improper compression can cause local SVT, especially in combination with prolonged sitting (long-haul flights). To prevent thromboembolic complications, we recommend avoiding a tourniquet effect and strangulation by inappropriate application of MCS, TPS and bandages.
Cardiac insufficiency
According to national and international guidelines, decompensated cardiac insufficiency is considered to be contraindicative for phlebological and lymphological compression bandaging, MCS and manual lymphatic drainage.44–46
A recent review of 20 international guidelines and consensus papers published between 2009 and 2016 on venous ulcer concluded that only pulmonary oedema should be regarded as a contraindication for compression treatment.47
The results of studies on the redistribution of regional blood volumes by applying compression to the legs of patients in supine position with the help of inflatable rubber boots indicated a reduction in blood volume in the legs and an increased blood volume in the organs of the thorax, abdomen and the liver.48 Similar blood-volume shifts in the legs were identified when classes I and II MCS (18–32 mmHg) were worn on the lower legs.49 Due to the elastic properties of the lower vena cava and the large number of visceral veins, only a fraction of the blood displaced from the legs reaches the right atrium. The volume overload caused by a change in the cardiac output will be compensated by the heart rate. In structural cardiac disease, changes in the behaviour and regulation of the myocardium have been observed.50,51
In patients with long-standing severe congestive heart failure, short-term increases in cardiac return may increase right heart pressures but do not appear to influence left heart haemodynamic indices.51,52 An increased volume in the right atrium produces a local rise in pressure as well as an increased expression of natriuretic peptides (NPs) triggered by a strain on the heart wall as a result of over-distension.53 In a comparative study of patients with chronic venous insufficiency (CVI) with or without cardiac insufficiency (New York Heart Association Functional Classification II (NYHA II))54 who wore class II MCS (25–32 mmHg), a significant rise in NPs due to MCS was reported in the heart disease group, who already had increased baseline values.55 This temporary rise in NPs was not accompanied by haemodynamic changes, and it was concluded that compression therapy using compression class II MCS on the lower leg does not constitute a risk to patients with NYHA II cardiac insufficiency.55
A similar conclusion was reached in a study where right heart catheterisation was used to investigate haemodynamic changes following the application of multilayer bandages on patients with NYHA III and IV-grade cardiac insufficiency in a cardiac intensive care unit. Initial increases in right atrial and ventricular pressure were observed after application of the bandages, followed by a return to baseline values without any long-term clinical impairment.56
An evaluation of the impact of locally applied manual lymphatic drainage on patients with NYHA III and IV-grade cardiac insufficiency concluded that, despite a highly significant reduction in the circumference of the treated extremities, there were no significant changes in haemodynamic parameters other than heart rate, and that using locally applied manual lymphatic drainage in the legs as a decongestive therapy in patients with NYHA III and IV-grade cardiac insufficiency does not constitute a contraindication.57
No pulmonary impairment, observed as pulmonary oedema, was triggered in any of the examined test subjects in a study where CB were applied to patients with decompensated cardiac insufficiency to prevent fainting as a consequence of seating-induced postural hypotension, despite an increase in blood pressure.58
Currently no studies have evaluated the significance of refractory or unstable hypertension. However, studies and consistent observations of volume displacement due to immersion have indicated that compression can contribute to pulmonary oedema in such cases.59,60
Overall, key points to note regarding case reports and experimental studies on cardiac insufficiency and compression therapy include:
Cardiac insufficiency in itself does not constitute a contraindication for compression therapy.
In the disease stages NYHA I and NYHA II, appropriate compression is possible.
In the disease stages NYHA III and IV, careful use of compression therapy is possible to a limited extent if there is a strict indication, clinical and haemodynamic monitoring.
In patients with oedema and cardiac insufficiency, it is recommended to start compression therapy with reduced pressure on one lower leg and slowly progress to stronger pressure applied on both legs.
Recommendation 17
We recommend against applying compression in severe cases of cardiac insufficiency (NYHA IV). We also suggest against routine application of MCS in NYHA III cases. When needed, careful use of compression therapy in this patient group may be considered if there is a strict indication, with clinical and haemodynamic monitoring. In less severe cases, cautious increase of compression pressure only leads to very short phases of increased cardiac load and may lead to a substantial reduction of peripheral oedema.
Borderline indications and previously reported contraindications
Compression may have positive effects in clinical situations, in which compression use was previously considered to be contraindicated, herein referred to as ‘borderline indications’.
A clinical case review noted that layered compression therapy is an effective and safe treatment in patients with DVT and SVT, oedema, venous ulceration or pre-ulceration and diabetes with adequate arterial circulation.61 Reduced compression can be helpful in patients with diabetes, arterial compromise, oedema and venous ulceration or pre-ulceration. Conversely, excessive compression may be disastrous, as shown in a case report of a 76-year-old, overweight patient with diabetes who, following treatment with 40–60 mmHg MCS due to leg ulcers and oedema, developed a deep necrosis over the dorsal tendon of the ankle joint, presumably due to rolling of the stocking over the tissue defect.62
Deep and superficial vein thrombosis
The avoidance of compression in patients with established DVT or SVT appears to be based only on theoretical grounds, as compression might promote the dislodgement of clots and cause pulmonary embolism (PE). There are no data supportive of this theory. Routine lung scans in patients with DVT not treated with compression treatment have demonstrated that PE is found in more than 50% of patients with DVT, thus demonstrating that DVT is a thromboembolic disease. Most of these pulmonary emboli are clinically silent, with the incidence of PE depending on the location of the thrombus.42
In a cohort of patients with acute DVT treated with either low-molecular-weight heparin (LMWH) plus compression and walking or LMWH plus bed rest, new PEs (majority asymptomatic) occurred in <7.4% of patients in both groups.42 Three randomised controlled trials (RCTs) also demonstrated that early mobilisation does not increase the frequency of PE compared with bed rest in patients with DVT treated with anticoagulation.39,63,64 The situation is similar in patients with SVT, as approximately 34% of patients with acute-phase SVT have predominantly asymptomatic PE.65,66
In an RCT of patients with isolated SVT, patients treated with LMWH plus compression stockings demonstrated faster thrombus regression and no increased risk of PE compared with LMWH alone, but did not show any improvement of pain compared with no compression.67
Following a European tradition, compression in acute SVT or DVT belongs to the standard treatment of these diseases, because firm compression leads to an immediate reduction of pain and swelling in patients with acute DVT.39–41,67–69 There are no data suggesting that compression of veins filled with clots may lead to an increased risk of PE or post-thrombotic syndrome (PTS).39,41,63,64,70,71
Recommendation 18
We recommend considering that, in contrast to previous concepts, compression is not contraindicated in acute thrombotic events, but results in favourable clinical outcomes when applied with caution. In the hands of experts, proper compression leads to an immediate improvement of pain and oedema.
Oedema
The most effective treatment to reduce oedema in mobile patients is compression, which is also true for patients with compensated cardiac oedema and in patients with concomitant arterial occlusive disease in which positive effects of compression on oedema may be expected, as long as the external compression pressure does not exceed the perfusion pressure.60 The use of compression to treat oedema must be carefully considered in patients with heart failure, diabetes, mixed pathology CVI or lymphoedema and/or PAOD and after arterial bypass surgery or stenting.
Oedema in heart failure
In patients with compensated heart failure (NYHA I and II) and venous or lymphatic oedema, compression of both legs may lead to a short asymptomatic increase in cardiac preload. In those patients, mild compression should start in the lower legs before it may be extended to the thigh region.51–53,55
Oedema in patients with diabetes
The efficacy and safety of mild compression with ‘diabetic socks’ (18–25 mmHg) in patients with diabetes and mild-to-moderate lower-extremity oedema were demonstrated in a pilot study and in an RCT.72,73
Oedema and/or venous ulcers in mixed pathology CVI or lymphoedema and/or PAOD
Compression does not always reduce arterial inflow, as several experiments have demonstrated that the use of compression can even lead to an increased arterial flow in healthy individuals.74–76 Because arterial occlusive disease is a frequent condition, especially in older age groups, this applies particularly to patients with venous and lymphatic diseases. In a population of 1416 patients with VLUs, 16% had concomitant arterial occlusive disease, 2% had critical ischaemia and 14% had an ABPI between 0.5 and 0.85.77 Critical ischaemia, characterised by ABPI < 0.6 or absolute perfusion pressure values of ≤50 mmHg, is a strict contraindication to compression therapy with MCS. However, less severe cases, with higher perfusion pressure values, which are far more frequent, have been shown to benefit from compression therapy; this was exemplified by a study where inelastic bandages applied with a pressure not exceeding 40 mmHg were able to increase local arterial perfusion and to improve the venous pumping function in patients with mixed, arterial-VLU and an ABPI > 0.5.1
Oedema after leg vein harvesting in bypass surgery
The results of several studies have demonstrated a beneficial postoperative effect of mild compression following harvest of leg veins for bypass graft surgery.78–81 Post-reconstructive oedema is very common after revascularisation and also occurs after bypass surgery. A prospective RCT involving patients with peripheral arterial disease treated with autologous femoro-popliteal bypass reconstruction demonstrated superior oedema reduction with 15–21 mmHg compression stockings compared with IPC treatment.82
Oedema and/or venous ulcers in patients after arterial bypass surgery or stenting
Oedema or venous ulcerations may occur or persist after arterial bypass surgery or arterial stenting.
Inflammatory diseases and infections
Erysipelas and vasculitis
Dermatolymphangioadenitis (DLA) is described as local tenderness and erythema, with possible systemic symptoms, including fever and chills, related to infection and the obstruction of the lymphatic system outflow.83 Erysipelas and cellulitis were considered contraindications to compression therapy due to the risk of MC-facilitated transfer of bacteria into the circulation. However, MC and concomitant antibiotics treatment may deliver synergistic effects by reducing local skin inflammation, improving lymphatic outflow and decreasing local swelling-related symptoms in patients without systemic infection symptoms related to DLA. In some centres, compression is used immediately upon resolution of the fever and systemic infection symptoms.78,84 However, compression needs to be made comfortable if the tissues remain tender.
Despite limited evidence from RCTs, the wide use of compression in patients with lymphoedema decreases oedema development and infections related to swelling, so that application of MC can potentially prevent cellulitis and reduce the risk of recurrent episodes.85 Similar outcomes are expected in other swelling- and lymphoedema-related skin infections, such as erysipelas. Erysipelas, as well as its recurrence with each infection, further impairs the lymphatic system, exposing the affected region to additional infectious episodes. MC in combination with prolonged use of antibiotics has been proposed by some researchers,83,86,87 and may decrease the recurrence and flare-up of infections, and thereby break the cycle. Based on experts’ experience, MC therapy has a potentially positive influence on the successful recovery of patients with pre-existing local skin inflammation and frequent bacterial infections.
Leucocytoclastic vasculitis
The local anti-inflammatory effect of compression may also improve skin changes, in addition to systemic anti-inflammatory treatment.68,88, Compression in this indication is widely used but prospective comparative studies are missing.69
Recommendation 19
We suggest additional compression, in purpura due to leucocytoclastic vasculitis and in leg erysipelas or cellulitis, to reduce inflammation, pain and oedema.
In infectious inflammation, we suggest compression only in combination with antibacterial treatment.
Recommendation 20
Special precautions have to be taken if MC treatment is considered in patients with ‘borderline indications’. Treatment decisions should be taken on a case-by-case basis and under consideration of a careful benefit–risk assessment. In case of a favourable assessment, we suggest the use of low-pressure compression, the use of modified-compression strategies (compression materials) and the use of padding to reduce pressure peaks.
Contraindications for MC treatment
Based on the sections above and the literature review, only a few contraindications for MC remain. These include severe PAOD, compression of epifascial arterial bypasses, severe cardiac insufficiency and true allergy to compression material.
Recommendation 21
We recommend considering the following contraindications for sustained compression with TPS, ACW MCS and elastic CB:
In patients with severe PAOD with any of the following: ABPI <0.6; ankle pressure <60 mmHg; toe pressure <30 mmHg; transcutaneous oxygen pressure < 20 mmHg.
Suspected compression of an existing epifascial arterial bypass
Severe cardiac insufficiency (NYHA IV)
Routine application of MC in NYHA III without strict indication, and clinical and haemodynamic monitoring
Confirmed allergy to compression material
Severe diabetic neuropathy with sensory loss or microangiopathy with the risk of skin necrosis (this may not apply to inelastic compression exerting low levels of sustained compression pressure (modified compression)).
Conclusions
Severe adverse events due to compression treatment, such as skin necrosis, nerve damage or thromboembolic events are rarely encountered if compression is correctly used and contraindications are considered. Discomfort, dry skin and itching are the most frequently reported adverse events related to compression use. To prevent skin irritations in patients with sensitive skin, we propose the use of applicable skin care.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ER reports grants from SIGVARIS during the conduct of studies, and consultancy fees from Eurocom and SIGVARIS outside the submitted work. PHC reports personal fees from SIGVARIS outside the submitted work. FL reports he does not have any conflicts to disclose. GM reports he does not have any conflicts to disclose. HP reports he does not have any conflicts to disclose. JC reports support from Janssen R&D, Pfizer, BMS and Alexion Pharmaceuticals, and personal fees from Sanofi and Recovery Force outside the submitted work. JH reports he does not have any conflicts to disclose. CRL reports he does not have any conflicts to disclose. MJ reports he does not have any conflicts to disclose. MHM reports he does not have any conflicts to disclose. MP reports support received from Bauerfeind outside the submitted work. NM reports grants from Medi and Medtronic, and personal fees from Medtronic and Merz outside the submitted work. SG is an employee of SIGVARIS. SW reports she does not have any conflicts to disclose. TH reports personal fees from SIGVARIS during the conduct of studies, and personal fees from SIGVARIS, Bauerfeind AG, Medi, Lohmann und Rauscher and Juzo outside the submitted work. TU reports he does not have any conflicts to disclose.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SIGVARIS funded the editorial support and medical writing services for this manuscript.
Ethical approval
N/A
Guarantor
ER
Contributorship
ER, HP and SG performed and refined the literature searches, and used their personal records and knowledge to select the evidence for critical appraisal. An initial meeting of a core group of authors (ER, HP, GM, NM, CRL and SG) took place on 3 November 2017, in Austin, TX, USA, to review the selected evidence, to formulate recommendations on contraindications and risks of medical compression treatment, and to assign the remaining authors to write, review and edit the content. All authors reviewed and edited the recommendations, and approved the final version of the manuscript.
Acknowledgements
We thank AXON Communications for editorial support on behalf of the authors and SIGVARIS.
ORCID iDs
Nick Morrison https://orcid.org/0000-0002-1750-6521
Giovanni Mosti https://orcid.org/0000-0001-7639-8463
Christopher R Lattimer https://orcid.org/0000-0002-0282-4826
References
- 1.Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg 2012; 55: 122–128. [DOI] [PubMed] [Google Scholar]
- 2.Dennis M, Cranswick G, Deary A. Thigh-length versus below-knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med 2010; 153: 553–562. [DOI] [PubMed] [Google Scholar]
- 3.Reich-Schupke S, Feldhaus F, Altmeyer P, et al. Efficacy and comfort of medical compression stockings with low and moderate pressure six weeks after vein surgery. Phlebology 2014; 29: 358–366. [DOI] [PubMed] [Google Scholar]
- 4.Harrison MB, Vandenkerkhof EG, Hopman WM, et al. The Canadian Bandaging Trial: evidence-informed leg ulcer care and the effectiveness of two compression technologies. BMC Nurs 2011; 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno J, In-Nami H. Allergic contact dermatitis to synthetic rubber, neoprene in compression stockings [In Japanese]. Masui 2011; 60: 104–106. [PubMed] [Google Scholar]
- 6.Valesky EM, Kaufmann R, Meissner M. Contact allergy to compression stockings: is this possible? Phlebologie 2014; 43: 140–143. [Google Scholar]
- 7.Lattimer CR, Azzam M, Kalodiki E, et al. Compression stockings significantly improve hemodynamic performance in post-thrombotic syndrome irrespective of class or length. J Vasc Surg 2013; 58: 158–165. [DOI] [PubMed] [Google Scholar]
- 8.Boris M, Weindorf S, Lasinski BB. The risk of genital edema after external pump compression for lower limb lymphedema. Lymphology 1998; 31: 15–20. [PubMed] [Google Scholar]
- 9.Hansson C, Faergemann J, Swanbeck G. Fungal infections occurring under bandages in leg ulcer patients. Acta Derm Venereol 1987; 67: 341–345. [PubMed] [Google Scholar]
- 10.Carliota AB, Kaya E, Turgut H, et al. Letter to the editor. Folliculitis associated with intermittant pneumatic compression. Yonsei Med J 2014; 55: 545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Meara S, Al-Kurdi D, Ologun Y, et al. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014: CD003557. [DOI] [PubMed] [Google Scholar]
- 12.Franks PJ, Barker J, Collier M, et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care 2016; 25: S1–S67. [DOI] [PubMed] [Google Scholar]
- 13.O’Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012; 11: CD000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elder DM, Greer KE. Venous disease: how to heal and prevent chronic leg ulcers. Geriatrics 1995; 50: 30–36. [PubMed] [Google Scholar]
- 15.Dawn G, Loney M, Zamiri M, et al. Erosive pustular dermatosis of the leg associated with compression bandaging and fungal infection. Br J Dermatol 2003; 148: 489–492. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CB, Wiemken TL, Brown TS. Effect of postoperative dressing on excisions performed on the leg: a comparison between zinc oxide compression dressings versus standard wound care. Dermatol Surg 2017; 43: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 17.Jaccard Y, Singer E, Degischer S, et al. Effect of silver-threads-containing compression stockings on the cutaneous microcirculation: a double-blind, randomized cross-over study. Clin Hemorheol Microcirc 2007; 36: 65–73. [PubMed] [Google Scholar]
- 18.Basford JR. The Law of Laplace and its relevance to contemporary medicine and rehabilitation. Arch Phys Med Rehabil 2002; 83: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 19.Crawford F, Welch K, Andras A, et al. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev 2016; 9: CD010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath DI, Kent SJ, Johns DL, et al. Arterial thrombosis associated with graduated pressure antiembolic stockings. Br Med J (Clin Res Ed) 1987; 295: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrett ND, Hanel KC. Ischaemic complications of graduated compression stockings in the treatment of deep venous thrombosis. Postgrad Med J 1993; 69: 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creton D. La chirurgie des varices. Complications cutanée dues à la compression par doubles collants postopératoires [In French]. Phlebologie 1998; 51: 363–364. [Google Scholar]
- 23.Ong JC, Chan FC, McCann J. Pressure ulcers of the popliteal fossae caused by thromboembolic deterrent stockings (TEDS). Ir J Med Sci 2011; 180: 601–602. [DOI] [PubMed] [Google Scholar]
- 24.Callam MJ, Ruckley CV, Dale JJ, et al. Hazards of compression treatment of the leg: an estimate from Scottish surgeons. Br Med J (Clin Res Ed) 1987; 295: 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis M, Sandercock PA, Reid J, et al. Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373: 1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan CL, Meyer FJ, Hay RJ, et al. Toe ulceration associated with compression bandaging: observational study. BMJ 2001; 323: 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIlhone S, Ukra H, Karim A, et al. Soft tissue injury to the sole of the foot secondary to a retained AV impulse foot pump. Foot Ankle Surg 2012; 18: 216–217. [DOI] [PubMed] [Google Scholar]
- 28.Anand A. Complications associated with intermittent pneumatic compression devices. Anesthesiology 2000; 93: 1556–1557. [DOI] [PubMed] [Google Scholar]
- 29.Parra RO, Farber R, Feigl A. Pressure necrosis from intermittent-pneumatic-compression stockings. N Engl J Med 1989; 321: 1615. [DOI] [PubMed] [Google Scholar]
- 30.Werbel GB, Shybut GT. Acute compartment syndrome caused by a malfunctioning pneumatic-compression boot. A case report. J Bone Joint Surg Am 1986; 68: 1445–1446. [PubMed] [Google Scholar]
- 31.Usmani N, Baxter KF, Sheehan-Dare R. Partially reversible common peroneal nerve palsy secondary to compression with four-layer bandaging in a chronic case of venous leg ulceration. Br J Dermatol 2004; 150: 1224–1225. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda H. Bilateral peroneal nerve palsy caused by intermittent pneumatic compression. Intern Med 2006; 45: 93–94. [DOI] [PubMed] [Google Scholar]
- 33.McGrory BJ, Burke DW. Peroneal nerve palsy following intermittent sequential pneumatic compression. Orthopedics 2000; 23: 1103–1105. [DOI] [PubMed] [Google Scholar]
- 34.Hirate H, Sobue K, Tsuda T, et al. Peripheral nerve injury caused by misuse of elastic stockings. Anaesth Intensive Care 2007; 35: 306–307. [PubMed] [Google Scholar]
- 35.Kim JH, Kim WI, Kim JY, et al. Peroneal nerve palsy after compression stockings application. Saudi J Anaesth 2016; 10: 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Güzelküçük Ü, Skempes D, Kumnerddee W. Common peroneal nerve palsy caused by compression stockings after surgery. Am J Phys Med Rehabil 2014; 93: 609–611. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien C, Eltigani T. Common peroneal nerve palsy as a possible sequelae of poorly fitting below-knee thromboembolic deterrent stockings (TEDS). Ann Plastic Surg 2006; 57: 356–357. [DOI] [PubMed] [Google Scholar]
- 38.Blättler W, Partsch H. Leg compression and ambulation is better than bed rest for the treatment of acute deep venous thrombosis. Int Angiol 2003; 22: 393–400. [PubMed] [Google Scholar]
- 39.Partsch H, Blättler W. Compression and walking versus bed rest in the treatment of proximal deep venous thrombosis with low molecular weight heparin. J Vasc Surg 2000; 32: 861–869. [DOI] [PubMed] [Google Scholar]
- 40.Partsch H. The role of leg compression in the treatment of deep vein thrombosis. Phlebology 2014; 29: 66–70. [DOI] [PubMed] [Google Scholar]
- 41.Ten Cate-Hoek AJ, Amin EE, Bouman AC, et al. Individualised versus standard duration of elastic compression therapy for prevention of post-thrombotic syndrome (IDEAL DVT): a multicentre, randomised, single-blind, allocation-concealed, non-inferiority trial. Lancet Haematol 2018; 5: e25–e33. [DOI] [PubMed] [Google Scholar]
- 42.Partsch H. Therapy of deep vein thrombosis with low molecular weight heparin, leg compression and immediate ambulation. VASA 2001; 30: 195–204. [DOI] [PubMed] [Google Scholar]
- 43.Scurr JH, Smith PD, Machin S. Deep vein thrombosis in airline passengers – the incidence of deep vein thrombosis and the efficacy of elastic compression stockings. Cardiovasc Surg 2001; 9: 159–161. [DOI] [PubMed] [Google Scholar]
- 44.Jünger M, Partsch H, Kahle B, et al. Phlebologischer Kompressionsverband (PKV) – Leitlinie der Deutschen Gesellschaft für Phlebologie. Phlebologie 2009; 38: 168–171. [Google Scholar]
- 45.AWMF online. S3-Leitlinie Prophylaxe der venösen Thromboembolie (VTE), www.awmf.org/leitlinien/detail/ll/003-001.html (2010, accessed July 2019).
- 46.AWMF online. S2k Leitlinie Diagnostik und Therapie der Lymphödeme AWMF Reg. -Nr. 058-001, www.awmf.org/uploads/tx_szleitlinien/058-001l_S2k_Diagnostik_und_Therapie_der_Lymphoedeme_2017-05.pdf (2017, accessed July 2019).
- 47.Andriessen A, Apelqvist J, Mosti G, et al. Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications – a review of present guidelines. J Eur Acad Dermatol Venereol 2017; 31: 1562–1568. [DOI] [PubMed] [Google Scholar]
- 48.Mostbeck A, Partsch H. Influence of dihydroergotamine and leg compression on the blood volume in different regions of the body [in German]. Med Klin 1978; 73: 801–806. [PubMed] [Google Scholar]
- 49.Lattimer CR, Kalodiki E, Azzam M, et al. Haemodynamic performance of low strength below knee graduated elastic compression stockings in health, venous disease, and lymphoedema. Eur J Vasc Endovasc Surg 2016; 52: 105–112. [DOI] [PubMed] [Google Scholar]
- 50.Azevedo PS, Polegato BF, Minicucci MF, et al. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol 2016; 106: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bain RJ, Tan LB, Murray RG, et al. Central haemodynamic changes during lower body positive pressure in patients with congestive cardiac failure. Cardiovasc Res 1989; 23: 833–837. [DOI] [PubMed] [Google Scholar]
- 52.Dereppe H, Hoylaerts M, Renard M, et al. The effects of pressotherapy on cardiovascular hemodynamics [In French]. Rev Med Brux 1989; 10: 185–186. [PubMed] [Google Scholar]
- 53.Todd J, Austwick T, Berridge D, et al. B-type natriuretic peptide in lymphedema. Lymphology 2011; 44: 29–34. [PubMed] [Google Scholar]
- 54.The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Boston, MA: Little, Brown & Co, 1994, pp.253–256. [Google Scholar]
- 55.Galm O, Jansen-Genzel W, von Helden J, et al. Plasma human atrial natriuretic peptide under compression therapy in patients with chronic venous insufficiency with or without cardiac insufficiency. VASA 1996; 25: 48–53. [PubMed] [Google Scholar]
- 56.Wilputte F, Renard M, Venner JP. Hemodynamic response to multilayered bandages dressed on a lower limb of patients with heart failure. Eur J Lym 2005; XV: 1–4. [Google Scholar]
- 57.Leduc O, Crasset V, Leleu C, et al. Impact of manual lymphatic drainage on hemodynamic parameters in patients with heart failure and lower limb edema. Lymphology 2011; 44: 13–20. [PubMed] [Google Scholar]
- 58.Gorelik O, Almoznino-Sarafian D, Litvinov V, et al. Seating-induced postural hypotension is common in older patients with decompensated heart failure and may be prevented by lower limb compression bandaging. Gerontology 2009; 55: 138–144. [DOI] [PubMed] [Google Scholar]
- 59.Boussuges A, Ayme K, Chaumet G, et al. Observational study of potential risk factors of immersion pulmonary edema in healthy divers: exercise intensity is the main contributor. Sports Med Open 2017; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter HH, Spence AL, Ainslie PN, et al. Differential impact of water immersion on arterial blood flow and shear stress in the carotid and brachial arteries of humans. Physiol Rep 2017; 5: pii: 5/10/e13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowering CK. Use of layered compression bandages in diabetic patients. Experience in patients with lower leg ulceration, peripheral edema, and features of venous and arterial disease. Adv Wound Care 1998; 11: 129–135. [PubMed] [Google Scholar]
- 62.Robertson BF, Thomson CH, Siddiqui H. Side effects of compression stockings: a case report. Br J Gen Pract 2014; 64: 316–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aschwanden M, Labs KH, Engel H, et al. Acute deep vein thrombosis: early mobilization does not increase the frequency of pulmonary embolism. Thromb Haemost 2001; 85: 42–46. [PubMed] [Google Scholar]
- 64.Schellong SM, Schwarz T, Kropp J, et al. Bed rest in deep vein thrombosis and the incidence of scintigraphic pulmonary embolism. Thromb Haemost 1999; 82: 127–129. [PubMed] [Google Scholar]
- 65.Partsch H, Mostbeck A. Early diagnosis of deep venous thrombosis of the lower leg [In German]. Acta Med Austriaca 1979; 4: 159–160. [Google Scholar]
- 66.Verlato F, Zucchetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the thigh. J Vasc Surg 1999; 30: 1113–1115. [DOI] [PubMed] [Google Scholar]
- 67.Boehler K, Kittler H, Stolkovich S, et al. Therapeutic effect of compression stockings versus no compression on isolated superficial vein thrombosis of the legs: a randomized clinical trial. Eur J Vasc Endovasc Surg 2014; 48: 465–471. [DOI] [PubMed] [Google Scholar]
- 68.Abu-Own A, Shami SK, Chittenden SJ, et al. Microangiopathy of the skin and the effect of leg compression in patients with chronic venous insufficiency. J Vasc Surg 1994; 19: 1074–1083. [DOI] [PubMed] [Google Scholar]
- 69.Dini V. Compression in vasculitis. Veins and Lymphatics 2016; 5: 5981. [Google Scholar]
- 70.Roumen-Klappe EM, den Heijer M, van Rossum J, et al. Multilayer compression bandaging in the acute phase of deep-vein thrombosis has no effect on the development of the post-thrombotic syndrome. J Thromb Thrombolysis 2009; 27: 400–405. [DOI] [PubMed] [Google Scholar]
- 71.Amin EE, Joore MA, Ten Cate H, et al. Clinical and economic impact of compression in the acute phase of deep vein thrombosis. J Thromb Haemost 2018; 16: 1555–1563. [DOI] [PubMed] [Google Scholar]
- 72.Wu SC, Crews RT, Najafi B, et al. Safety and efficacy of mild compression (18–25 mm Hg) therapy in patients with diabetes and lower extremity edema. J Diabetes Sci Technol 2012; 6: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu SC, Crews RT, Skratsky M, et al. Control of lower extremity edema in patients with diabetes: double blind randomized controlled trial assessing the efficacy of mild compression diabetic socks. Diabetes Res Clin Pract 2017; 127: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bochmann RP, Seibel W, Haase E, et al. External compression increases forearm perfusion. J Appl Physiol (1985) 2005; 99: 2337–2344. [DOI] [PubMed] [Google Scholar]
- 75.Mayrovitz HN, Macdonald JM. Medical compression: effects on pulsatile leg blood flow. Int Angiol 2010; 29: 436–441. [PubMed] [Google Scholar]
- 76.Fromy B, Legrand MS, Abraham P, et al. Effects of positive pressure on both femoral venous and arterial blood velocities and the cutaneous microcirculation of the forefoot. Cardiovasc Res 1997; 36: 372–376. [DOI] [PubMed] [Google Scholar]
- 77.Humphreys ML, Stewart AH, Gohel MS, et al. Management of mixed arterial and venous leg ulcers. Br J Surg 2007; 94: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 78.Alizadeh-Ghavidel A, Ramezannejad P, Mirmesdagh Y, et al. Prevention of edema after coronary artery bypass graft surgery by compression stockings. Res Cardiovasc Med 2014; 3: e17463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belczak CE, Tyszka AL, Godoy JM, et al. Clinical complications of limb undergone harvesting of great saphenous vein for coronary artery bypass grafting using bridge technique. Rev Bras Cir Cardiovasc 2009; 24: 68–72. [DOI] [PubMed] [Google Scholar]
- 80.Khoshgoftar Z, Ayat Esfahani F, Marzban M, et al. Comparison of compression stocking with elastic bandage in reducing postoperative edema in coronary artery bypass graft patient. J Vasc Nurs 2009; 27: 103–106. [DOI] [PubMed] [Google Scholar]
- 81.Maleti O. Compression after vein harvesting for coronary bypass. Veins and Lymphatics 2016; 5: 5989. [Google Scholar]
- 82.te Slaa A, Dolmans DE, Ho GH, et al. Prospective randomized controlled trial to analyze the effects of intermittent pneumatic compression on edema following autologous femoropopliteal bypass surgery. World J Surg 2011; 35: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olszewski WL. Episodic dermatolymphangioadenitis (DLA) in patients with lymphedema of the lower extremities before and after administration of benzathine penicillin: a preliminary study. Lymphology 1996; 29: 126–131. [PubMed] [Google Scholar]
- 84.Macciò A. Compression in dermato-lymphangio-adenits. Veins and Lymphatics 2016; 5: 5982. [Google Scholar]
- 85.Stalbow J. Preventing cellulitis in older people with persistent lower limb oedema. Br J Nurs 2004; 13: 725–732. [DOI] [PubMed] [Google Scholar]
- 86.Bonnetblanc JM, Bédane C. Erysipelas: recognition and management. Am J Clin Dermatol 2003; 4: 157–163. [DOI] [PubMed] [Google Scholar]
- 87.Villefrance M, Høgh A, Kristensen LH. Compression is important in erysipelas treatment [In Danish]. Ugeskr Laeger 2017; 179: pii: V04170284. [PubMed] [Google Scholar]
- 88.Ligi D, Croce L, Mannello F. Inflammation and compression: state of the art. Veins and Lymphatics 2016; 5: 5980. [Google Scholar]
- 89.European Commission. Consumer Goods – Pharmaceuticals: a guideline on summary of product characteristics (SmPC): section 4.8: undesirable effects, www.ema.europa.eu/en/documents/presentation/presentation-section-48-undesirable-effects_en.pdf (2009, accessed July 2019).