Abstract
Background:
The rapid worldwide spread of COVID-19 has posed a serious threat to patients treated with kidney replacement therapy (KRT). Moreover, the impact of the disease on hemodialysis centers, the patients, and the health care workers is still not completely understood.
Objective:
We present the analysis of a COVID-19 outbreak in a hemodialysis center in Belgium and report the incidence, clinical course, and outcome of the disease.
Design:
A retrospective cross-sectional cohort study.
Setting:
A hemodialysis center during the COVID-19 outbreak.
Patients:
A total of 62 patients on maintenance hemodialysis at a tertiary care center in Belgium attended by 26 health care workers.
Measurements:
Baseline patients’ characteristics were retrieved. The incidence, clinical course, and outcome were reported. The differences between COVID-19 survivors and nonsurvivors were assessed along with the differences between COVID-19-hospitalized and nonhospitalized patients. The incidence of the disease and outcome of health care workers were also reported.
Methods:
Proportions for categorical variables were compared using the Fisher exact test and χ2. The Mann-Whitney rank sum test was used to compare continuous variables. Univariate analysis and a binomial logistic regression were used to explore variables as predictors of death.
Results:
Between March 6 and April 14, 2020, 40 of 62 (65%) patients tested positive for severe acute respiratory syndrome beta coronavirus 2 (SARS-CoV-2) along with 18 of 26 (69%) health care professionals. Twenty-five (63%) of the infected patients were hospitalized with a median time for hospitalization-to-discharge of 8 (interquartile range [IQR] = 4-12) days. Eleven (28%) COVID-19-related deaths were recorded with a median time for onset of symptoms-to-death of 9 (IQR = 5-14) days. Lymphocytopenia was prevalent among the cohort and was found in 9 of 11 (82%) reported deaths (P = .4). There was no influence of the use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers on COVID-19-related deaths (P = .3). Advanced age, cardiovascular disease (CVD), and obstructive sleep apnea syndrome were all found to be significantly related to death. Of the 18 infected health care professionals, 13 (72%) were symptomatic and 2 (11%) were hospitalized. There was no reported death among the health care workers.
Limitations:
Limited follow-up time compared with the course of the disease along with a small sample size.
Conclusions:
Patients treated with KRT show a high mortality rate secondary to COVID-19. CVD and age are shown to impact survival. Proactive measures must be taken to prevent the spread of the virus in such facilities.
Trial Registration:
Not applicable as this is a retrospective study.
Keywords: hemodialysis, COVID-19, SARS-CoV2, mortality, hemodialysis center, end-stage kidney disease
Introduction
Since December 2019, the world has been shaken by the emergence of the Corona Virus Disease 2019 (COVID-19), in Wuhan, Hubei Province, China. COVID-19 is caused by a novel severe acute respiratory syndrome beta coronavirus 2 (SARS-CoV-2).1,2 The pathogen was found to follow an insidious path with a high person-to-person transmission,1,3,4 including asymptomatic infected subjects.5 Due to the rapid spread of the disease across the globe, the World Health Organization declared it a pandemic on March 11, 2020.
Clinical symptoms secondary to COVID-19 range from mild to severe. The most common manifestations are fever and pulmonary symptoms reported in 84% and 31% of cases, respectively.6 About 2% to 10% of patients might also develop gastrointestinal symptoms,7 while others only present with anosmia.8 Large-scale epidemiological data indicate a significantly higher mortality rate due to SARS-CoV-2 infection for elderly patients and/or patients with comorbidities, such as cardiovascular disease (CVD), chronic lung disease, diabetes, and cancer.9,10 Patients treated with kidney replacement therapy (KRT) by maintenance hemodialysis face an increased complication rate due to the advanced age of the population and various comorbid conditions.3,11 Logistics at hemodialysis centers, such as physical proximity and frequent visits, contribute to higher rates of disease transmission.11 Consequently, this situation poses great challenges to patients, health care workers, and hemodialysis centers. The EUDIAL (European Dialysis Working Group) working group recently published recommendations for the prevention of SARS-CoV-2 infection and mitigation among the dialyzed population.12 Other clinical guidance for safeguarding hemodialysis patients were also published from several centers.13,14
To this day, only 1 unpublished series of 230 patients treated with KRT from Wuhan University15 can be found, along with a few scattered published case reports of infected subjects on maintenance hemodialysis.16,17 Very few epidemiological data are available regarding COVID-19 outbreaks in hemodialysis centers, with still unpublished data by Li et al,18 and recently published data by Wang19 along with data from the experience in Italy.20
The first reported case of SARS-CoV-2 infection in Belgium was reported on February 4, 2020. The patient was an asymptomatic worker screened after returning from China, and authorities imposed a lockdown on March 13, 2020, after a rapid spread of the disease.
The objectives of this case series are to (1) describe the clinical characteristics of a cohort of patients treated with KRT treated by chronic hemodialysis and affected, together with their caregivers, by an outbreak of COVID-19; (2) compare the clinical characteristics of the cohort’s COVID-19 survivors versus nonsurvivors; (3) assess the relevant factors that may explain the outbreak and impact the prognosis of these COVID-19 patients along with the temporal pattern of their infection, hospitalization, and death; and (4) present actions taken for the mitigation and containment of this outbreak.
Materials and Methods
The Institutional Review Board (IRB) at the Centre Hospitalier Universitaire et Psychiatrique de Mons-Borinage (CHUPMB) reviewed and approved the study protocol on April 15, 2020. The board waived the requirement for written consent.
Study Design
This is a retrospective, monocentric, cross-sectional case series carried out between March 6 and April 14, 2020, evaluating patients on chronic hemodialysis at a tertiary care center in Belgium. This center follows the Belgian authorities’ recommendations for set-up in terms of layout and health care workers. Three hemodialysis units are available within the hospital site: a main unit, divided into 2 rooms with 10 and 4 machines, respectively; a secondary unit with 6 machines; and a nearby satellite unit with 10 machines. The units are managed by a total of 26 medical professionals.
Primary physicians from the center retrospectively collected and assessed data from clinical medical records of patients treated at the hemodialysis center. Demographic, epidemiological, and clinical information, including laboratory and radiological findings, were obtained. Patients were divided into a COVID-19 survivor group and a COVID-19 nonsurvivor group. Categorical variables were described as frequency rates and percentages, and continuous variables were described using the median (interquartile range [IQR]). Proportions for categorical variables were compared using the Fisher exact test and χ2. The Mann-Whitney rank sum test was used to compare continuous variables. Univariate analysis and a binomial logistic regression were used to explore variables as predictors of death. Variables were chosen for the binomial regression on the basis of the univariate analysis. All variables with a P value of less than .05 in the univariate analysis were included in the binomial logistic regression. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each covariate.
Definition and Laboratory Confirmation
A confirmed COVID-19 case was defined as a symptomatic patient with a positive result by real-time reverse transcription polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 from an upper respiratory specimen (nasopharyngeal swab). Early criteria for a nasopharyngeal swab test were fever and highly suspicious symptoms, including dyspnea, cough, rhinorrhea, sore throat, headache, and diarrhea. A second swab test was performed in case of a negative first test.
Fever was defined as tympanic temperature of at least 38.0°C. Pulmonary symptoms were defined as cough, sputum production, or dyspnea.
The COVID-19 severity index (CSI) used was a pulse O2 saturation (SpO2) of less than 93% on room air/O2 supplement or an arterial oxygen partial pressure to fractional inspired oxygen ratio (PaO2/FiO2) of less than 300.21 Patients who met any of these criteria with a confirmed RT-PCR were considered to exhibit a severe course of the disease.
Results
Demographic and Clinical Characteristics
The studied population included 62 patients treated with maintenance hemodialysis at the center and 26 caregivers. Loss of follow-up was documented for 1 asymptomatic patient who died at home. This patient was known to have a mental disorder and refused dialysis care due to the ongoing pandemic, and did not meet the criteria for a nasopharyngeal swab test. All other patients underwent RT-PCR from nasopharyngeal swabs for the detection of SARS-CoV-2.
Table 1 lists the patients’ overall characteristics. Over a 4-week period after the first positive RT-PCR test, 40 of 62 (65%) patients tested positive. The median age of infected patients was 75 (IQR = 68-83) years, and 23 (58%) of the patients were men. Of the 40 patients, 37 (93%) had systemic hypertension, 26 (65%) had diabetes, and 25 (63%) had concomitant CVD. Fever was reported as a presenting symptom in 23 (58%) patients followed by pulmonary symptoms in 17 (43%). A total of 11 (28%) patients were asymptomatic. Among the 26 health care professionals, 18 (69%) were found to have a positive RT-PCR test, of whom 3 were practitioners, 14 were nurses and 1 was a practical nurse. Of the 18 health care workers, 13 (72%) were symptomatic, including 2 practitioners, 9 nurses, and were instructed to self-isolate during at least 7 days and until respiratory symptoms and fever subsided for 3 consecutive days based on the national recommendations,22 and 2 (11%), including 1 nurse and 1 practical nurse, were hospitalized. There was no reported death among the health care professionals. Following the national recommendations22 and the severe shortage in health care professionals along with a national lack of specific primer probes, the remaining asymptomatic health care workers remained active at the hemodialysis center and were assigned to confirmed infected patients, and a nasopharyngeal swab test was not repeated prior to resuming activity.
Table 1.
Patient Characteristics.
| Parameter | COVID-19 survivor | P value | ||
|---|---|---|---|---|
| Outpatient | Hospitalized | Total | ||
| Part 1: Comparison of COVID-19 Survivors | ||||
| N | 15 | 14 | 29 | |
| Age, y (IQR) | 74 (66-82) | 69 (61-78) | 71 (63-79) | .6 |
| BMI, kg/m2 (IQR) | 29.7 (27.5-31.9) | 28.9 (20.9-37.4) | 29.6 (26.4-32.8) | .5 |
| Sex, No. (%) | ||||
| Male | 6 (40.0) | 8 (57.1) | 14 (48.3) | .4 |
| Female | 9 (60.0) | 6 (42.9) | 15 (51.7) | |
| CVD, No. (%) | 9 (60.0) | 5 (35.7) | 14 (48.3) | .2 |
| Hypertension, No. (%) | 14 (93.3) | 12 (85.7) | 26 (89.3) | .5 |
| Diabetes mellitus, No. (%) | 11 (73.3) | 8 (57.1) | 19 (65.5) | .3 |
| Chronic lung disease, No. (%) | 4 (26.7) | 5 (35.7) | 9 (31.0) | .5 |
| OSAS, No. (%) | 3 (20.0) | 3 (21.4) | 6 (20.7) | .6 |
| Neurological disease, No. (%) | 1 (6.7) | 2 (14.3) | 3 (10.3) | .5 |
| Cognitive impairment, No. (%) | 1 (6.7) | 3 (21.4) | 4 (13.8) | .3 |
| Neoplasm, No. (%) | 1 (6.7) | 1 (7.1) | 2 (6.9) | .7 |
| Hematological disease, No. (%) | 1 (6.7) | 0 (0.0) | 1 (3.4) | .5 |
| Immunodeficiency, No. (%) | 1 (6.7) | 5 (35.7) | 6 (20.1) | .07 |
| Cirrhosis, No. (%) | 2 (13.3) | 1 (7.1) | 3 (10.3) | .5 |
| Smoking, No. (%) | 1 (6.7) | 1 (7.1) | 2 (6.9) | .7 |
| ACE-I/ARB treatment, No. (%) | 5 (33.3) | 5 (35.7) | 10 (34.5) | .6 |
| Inaugural symptoms, No. (%) | ||||
| Fever | 3 (20) | 12 (85.7) | 15 (51.7) | <.001 |
| Pulmonary symptoms | 3 (20) | 7 (50.0) | 10 (34.5) | .1 |
| Asymptomatic | 10 (66.7) | 0 (0.0) | 10 (34.5) | <.001 |
| Lymphocytopenia, No. (%) | 8 (53.3) | 13 (92.9) | 21 (72.4) | .02 |
| Saturation <93% on presentation,a No. (%) | 0 (0.0) | 5 (35.7) | 5 (17.2) | .02 |
| Saturation <93% during the disease’ coursea (CSI), No. (%) | 0 (0.0) | 12 (85.7) | 12 (41.4) | <.001 |
| Positive chest CT-scan,b No. (%) | 0 (0.0) | 8 (57.1) | 8 (27.6) | .001 |
| Hydroxychloroquine treatment, No. (%) | 0 (0.0) | 12 (85.7) | 12 (41.4 | <.001 |
| Antibiotics treatment,c No. (%) | 2 (13.3) | 10 (71.4) | 12 (41.4) | .002 |
| All patients | COVID-19 survivor | COVID-19 nonsurvivor | ||
| Part 2: Comparison of COVID-19 survivors and COVID-19 nonsurvivors | ||||
| N | 40 | 29 | 11 | |
| Age, y (IQR) | 75 (68-83) | 71 (63-79) | 78 (73-82) | .02 |
| BMI, kg/m2 (IQR) | 29.7 (26.1-33.4) | 29.6 (26.4-32.8) | 30.8 (25.2-36.5) | .5 |
| Sex, No. (%) | ||||
| Male | 23 (57.5) | 14 (48.3) | 9 (81.8) | .06 |
| Female | 17 (42.5) | 15 (51.7) | 2 (18.2) | |
| CVD, No. (%) | 25 (62.5) | 14 (48.3) | 11 (100) | .002 |
| Hypertension, No. (%) | 37 (92.5) | 26 (89.3) | 11 (100 | .4 |
| Diabetes mellitus, No. (%) | 26 (65.0) | 19 (65.5) | 7 (63.6) | .6 |
| Chronic lung disease, No. (%) | 16 (40.0) | 9 (31.0) | 7 (63.6) | .07 |
| OSAS, No. (%) | 12 (30.0) | 6 (20.7) | 6 (54.5) | .05 |
| Neurological disease, No. (%) | 4 (10.0) | 3 (10.3 | 1 (9.1) | .7 |
| Cognitive impairment, No. (%) | 5 (12.5) | 4 (13.8) | 1 (9.1 | .6 |
| Neoplasm, No. (%) | 3 (7.5) | 2 (6.9) | 1 (9.1) | .6 |
| Hematological disease, No. (%) | 2 (5.0) | 1 (3.4) | 1 (9.1) | .5 |
| Immunodeficiency, No. (%) | 7 (17.5) | 6 (20.1) | 1 (9.1) | .4 |
| Cirrhosis, No. (%) | 3 (7.5) | 3 (10.3) | 0 (0.0) | .4 |
| Smoking, No. (%) | 3 (7.5) | 2 (6.9) | 1 (9.1) | .6 |
| ACE-I/ARB treatment, No. (%) | 12 (30.0) | 10 (34.5) | 2 (18.2) | .3 |
| Inaugural symptoms, No. (%) | ||||
| Fever | 23 (57.5) | 15 (51.7) | 8 (72.7) | .2 |
| Pulmonary symptoms | 17 (42.5) | 10 (34.5) | 7 (63.6) | .1 |
| Asymptomatic | 11 (27.5) | 10 (34.5) | 1 (9.1) | .1 |
| Lymphocytopenia | 30 (75.0) | 21 (72.4) | 9 (81.8) | .4 |
| Saturation <93% on presentation,a No. (%) | 14 (35.0) | 5 (17.2) | 9 (81.8) | <.001 |
| Saturation <93% during the disease’ coursea (CSI), No. (%) | 22 (55.0) | 12 (41.4) | 10 (90.9) | .005 |
| Positive chest CT-scan,b No. (%) | 11 (27.5) | 8 (27.6) | 3 (27.3) | .7 |
| Hydroxychloroquine treatment, No. (%) | 20 (50.0) | 12 (41.4) | 8 (72.7) | .08 |
| Antibiotics treatment,c No. (%) | 21 (52.5) | 12 (41.4) | 9 (81.8) | .03 |
Note. IQR = interquartile range; BMI = body mass index; CVD = cardiovascular disease; OSAS = obstructive sleep apnea syndrome; ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CSI = COVID-19 severity index; CT-scan = computed tomography scan.
Saturation at room air or on oxygen.
Defined as images suggestive of COVID-19 (peripheral, bilateral ground-glass appearance of infiltrates).23,24
Antibiotics and antiviral treatment.
Treatment and Clinical Outcomes
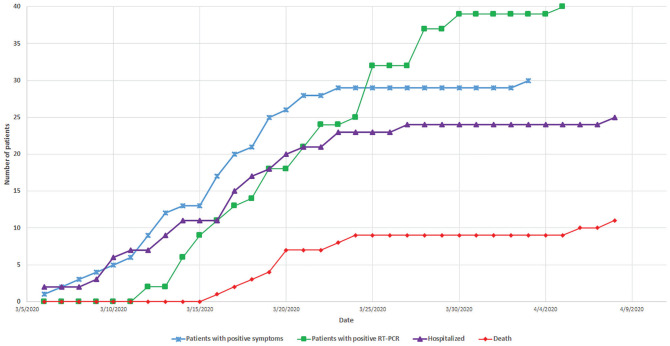
The first symptoms (dyspnea and cough) were reported on March 6 in 1 of 2 already hospitalized patients, coinciding with the development of symptoms in 3 members of the health care workers. The second patient also developed fever and cough later. On March 11, the first swab to detect SARS-CoV-2 infection by RT-PCR was requested following a highly suspicious chest computed tomography scan suggesting COVID-19 pneumonia.24 On March 12, a technical problem with the water treatment system emerged in the main hemodialysis unit leading to the emergent referral of 30 patients to the secondary unit, the nearby satellite unit, and another hospital center. By April 14, 18 of the 26 (69%) members of the health care team had been infected, and 25 (63%) patients had been hospitalized based on the severity of symptoms and clinical deterioration (O2 desaturation <93% on pulse oximetry)21 and/or impaired general status. Among hospitalized patients, 8 (20%) had been discharged with a median hospitalization-to-discharge time of 8 (IQR = 4-12) days and a median onset of symptoms-to-discharge time of 12 (IQR = 8-16) days. A total of 11 (28%) COVID-19-related deaths were recorded. The median time of onset of symptoms-to-death was 9 (IQR = 7-12) days, and the median hospitalization time was 9 (IQR = 3-16) days. One patient was admitted due to elevated C-reactive protein (CRP) levels but was asymptomatic. Three (8%) patients were admitted to the intensive care unit (ICU) (1 reported death) with a median time spent in the ICU of 15 days. Two of these required mechanical ventilation along with vasopressor support. These patients were treated by 4 hours of maintenance hemodialysis thrice a week. There were neither ICU restrictions nor a limited number of ventilators available at our center, but a prior no-escalation of care decision was taken by a multidisciplinary team for 10 of the 11 hospitalized patients on KRT who died based on their baseline ill-prognosis and multiple comorbidities. Figure 1 depicts the progression of the total number of patients by date during the study period.
Figure 1.
Temporal evolution of clinical events.
The use of angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin II receptor blockers (ARBs) did not show any statistically significant difference in COVID-19-related deaths (P = .3). Advanced age was found to be related to a significantly higher rate of death (P = .02), as was CVD (P = .002). A slightly statistically significant higher rate of death in patients with obstructive sleep apnea syndrome (OSAS) was noted (P = .05).
Lymphocytopenia was reported in 30 (75%) patients, and was found in 9 of the 11 (82%) reported deaths (P = .4). Of the 25 hospitalized patients, 20 (80%) received hydroxychloroquine treatment early after hospitalization during the course of the disease, of whom 8 (40%) died (P = .08). Patients who received hydroxychloroquine had a statistically significant higher CSI (P < .001). Three patients among hospitalized and overall patients on KRT who did not receive hydroxychloroquine treatment (n = 5 and 20, respectively) died. These patients had multiple comorbidities along with significant in-hospital clinical deterioration, and a palliative treatment was favored. Two of the remaining hospitalized patients presented mild symptoms and survived. Of the 40 patients, 21 (53%) received antibiotic and/or antiviral therapy, and 9 (42.8%) of these died (P = .025). A binomial logistic regression run from sex, CVD, OSAS, CSI, treatment with hydroxychoroquine, use of antibiotics did not show any significant predictor of death but was borderline for CSI (P = .06).
Discussion
SARS-CoV-2 is currently one of the primary pathogens of respiratory infections worldwide. Although several drugs and vaccines are under investigation, there are no effective therapies or vaccines available yet for COVID-19.25-28 Here, we report the incidence, clinical course, and outcome of a COVID-19 outbreak at our hemodialysis center. These facilities are at high risk of respiratory infection outbreaks,26 as attested to in our study with the high person-to-person transmission rate and a high incidence of infection (63%) found among patients. By April 14, a total of 58 confirmed cases were identified, including both patients and health care workers. The rapid transmission rate in our facility posed a serious threat to this medically vulnerable population and put a strain on the local health care professionals. Due to the technical problem that emerged, several patients were transferred to the satellite unit and another center. This contributed to limiting the spread of the disease. In our patient cohort, the clinical presentation of COVID-19 during the prodromal phase was insidious, leading to a rapid spread of SARS-CoV-2 among the population. The management of patients was challenging due to (1) the fact that the patients had a “silent hypoxemia” phase presenting with mild symptoms, and (2) the multiphasic parity of the disease (including a viremic phase and subsequent adaptive immunity) before deterioration. The recurrent interaction, physical contact/proximity, and the limited qualified health care workers also highly contributed to the higher incidence rate. Hospitalized patients had a more aggressive course of the disease and a higher CSI.
Currently, the approach to COVID-19 is to control the source of infection, reduce the risk of transmission by the use of personal protective equipment (PPE), and test early for diagnosis, isolate, and provide supportive treatments for affected patients. Upon detection of the first laboratory-proven case on March 11, airborne and contact precautions were implemented at our facility as per the guidance of the American Society of Nephrology (ASN),18 the Société Francophone de Néphrologie Dialyse Transplantation (SFNDT),29 Centers for Disease Control and Prevention (CDC),30 the European Centre for Disease Prevention and Control (EDCD),31 and the Canadian Society of Nephrology.32 Also implemented were ongoing patient and health care worker education regarding hand and respiratory hygiene, as well as cough etiquette. Furthermore, health care workers were educated on the selection and use of PPE. The hemodialysis waiting area was adapted to decrease social interaction with a minimal distance of 1.5 m between patients, and when applicable, patients were asked to wait in their cars. A triage plan to identify patients with fever or symptoms of respiratory infection before they entered the treatment area was implemented. Patients were instructed to call ahead to report fever and/or suspicious symptoms, including dyspnea, cough, rhinorrhea, sore throat, headache, and diarrhea. Temperature and pulse oxygen saturation were measured regularly before and after the hemodialysis session. Patients were assigned to separate units according to their infection status: asymptomatic, symptomatic suspected, or confirmed cases. By March 12, due to the lack of appropriate masks, the entire health care professionals team and patients were required to have a surgical mask on during their presence at the hemodialysis unit; in addition, visitors were not allowed in the unit. As of March 17, filtering facepiece masks 2 (FFP2) were used by the circulating nursing staff when caring for suspected and/or confirmed patients. Furthermore, nurses were required to wear protective gowns, gloves, and protective face shields. Protective curtains were placed when the distance between 2 hemodialysis stations was less than 2 m (the distance between 2 stations in the main unit was of 1.4 m). By March 27 and April 2, all patients and health care professionals had been tested, respectively. Patients were considered recovered 28 days after the first positive SARS-CoV-2 swab test and following 2 negative swab tests 48 hours apart.
The mortality rate secondary to SARS-CoV-2 infection has been reported to reach up to 10%,25 with mortality rates of patients admitted to the ICU and in need of mechanical ventilation as high as 78%.33 Our findings demonstrate a high mortality rate (28%) in patients treated with KRT. Older age has been reported in several studies as a risk factor for mortality in COVID-19 patients.33,34 This was confirmed in our study, which showed a statistically significant better survival rate for younger patients. Male sex has also been associated with a higher rate of infection and mortality.34 Hypertension and CVD were linked to higher morbidity and mortality in SARS-CoV-2 infection.33 Our data were also aligned with the literature for CVD, but not for hypertension. No influence was shown regarding the use of ACE-I or ARB, and this has also been suggested by recently published data on their mechanisms during COVID-19.35,36 Obesity was shown to be prevalent among patients admitted to the ICU and has been linked to a more severe course of COVID-19.37 Although our cohort presented a relatively high median body mass index (BMI), no significant difference was found between the 2 groups. We also noted a slightly statistically significant higher rate of death in patients with OSAS. Whether this is due to the pathophysiology of OSAS or the overall chronic lung disease and obesity is still open for investigation.
Of the 62 patients, 40 (65%) were presumed to have been infected at the hemodialysis center, including 2 patients (3%), who were already hospitalized for other reasons, and 18 health care workers (78%). Personnel-to-patient and patient-to-patient transmission are also presumed to have occurred.
Finally, hydroxychloroquine was administered in 20 hospitalized patients with an aggressive course of the disease according to the interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium.21 Hydroxychloroquine failed to show any statistically significant difference between the 2 groups of our cohort. Twenty patients were started on antibiotics for suspected bacterial pneumonia or another bacterial infection, and 1 other patient received oseltamivir for a positive influenza A virus RT-PCR test. These patients were also found to have a higher CSI on diagnosis.
To the best of our knowledge, this is the first report of an outbreak of COVID-19 in a hemodialysis center in Belgium. Our results are in line with the recently published data from Lombardy, Italy, showing a high in-hospital mortality rate (52%).20 Besides unpublished data of 230 hemodialysis patients with 37 patients infected with SARS-CoV-2 (16%) without any reported death along with 4 of the staff members (12%),15 there are limited data on COVID-19 mortality in patients treated by KRT. A larger-scale study should be undertaken for further investigation of this predisposed and frail population.
Limitations
This case series has several limitations. First, it was a retrospective review. Second, the follow-up time was relatively short compared with the course of the disease, and the reported mortality data and length of stay data reported in this study could change. Third, the sample size was limited.
Conclusion
In this single-center case series of 40 SARS-CoV-2-infected patients treated with maintenance hemodialysis, the high mortality rate was linked to advanced age and CVD. In the current context of rapidly emerging outbreaks around the globe, proactive measures should be taken in facilities to prevent the introduction and spread of the virus among more vulnerable populations.
Acknowledgments
We thank Natalie Dickson from the Centre de Langues Vivantes of UMONS (Université de Mons) for her careful review of our manuscript.
Footnotes
Ethics Approval and Consent to Participate: The Institutional Review Board (IRB) at the Centre Hospitalier Universitaire et Psychiatrique de Mons-Borinage (CHUPMB) reviewed and approved the study protocol on April 15, 2020. The board waived the requirement for written consent.
Consent for Publication: Not applicable.
Availability of Data and Materials: The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Philippe Delmotte is partially supported by a grant from UMHAP (Université de Mons Hôpital Ambroise Paré) research council.
ORCID iDs: Lionel Mazzoleni  https://orcid.org/0000-0002-0154-7812
https://orcid.org/0000-0002-0154-7812
Chadi Ghafari  https://orcid.org/0000-0002-5971-5073
https://orcid.org/0000-0002-5971-5073
Stéphane Carlier  https://orcid.org/0000-0001-7787-1937
https://orcid.org/0000-0001-7787-1937
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706-711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciotti M, Minieri M, Angeletti S, et al. COVID-19 outbreak : an overview. Chemotherapy. 2019;64:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019-nCoV patients. MedRxiv. 2020. doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 10. Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikizler TA. COVID-19 and dialysis units: what do we know now and what should we do? Am J Kidney Dis. 2020;76:1-3. doi: 10.1053/j.ajkd.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basile C, Combe C, Pizzarelli F, et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meijers B, Messa P, Ronco C. Safeguarding the maintenance hemodialysis patient population during the coronavirus disease 19 pandemic. Blood Purif. 2020;49:259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikizler TA, Kliger AS. Minimizing the risk of COVID-19 among patients on dialysis. Nat Rev Nephrol. 2020;16:311-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma Y, Diao B, Lv X, et al. 2019. novel coronavirus disease in hemodialysis (HD) patients: report from one HD center in Wuhan, China. MedRxiv. doi: 10.1101/2020.02.24.20027201. [DOI] [Google Scholar]
- 16. Ferrey AJ, Choi G, Hanna RM, et al. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol. 2020;51:337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu D, Yang B, Xu J, Mao Z, Zhou C, Xue C. COVID-19 infection in a patient with end-stage kidney disease. Nephron. 2020;144:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Xu G. Lessons from the experience in Wuhan to reduce risk of COVID-19 infection in patients undergoing long-term hemodialysis. Clin J Am Soc Nephrol. 2020;15:717-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H. Maintenance Hemodialysis and Coronavirus Disease 2019 (COVID-19): Saving Lives With Caution, Care, and Courage. [published online ahead of print March 26, 2020]. Kidney Med. doi: 10.1016/j.xkme.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. La Milia V, Bacchini G, Carla Bigi M, et al. COVID-19 outbreak in a large hemodialysis centre in Lombardy, Italy. Kidney Int Reports. 2020;5:1095-1099. doi: 10.1016/j.ekir.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziekenhuis U. Interim clinical guidance for patients suspected of/confirmed with covid-19 in Belgium. www.notifieruneffetindesirable.be. Published 2020. Accessed April 16 2020.
- 22. Procédure Pour Les Hôpitaux : Prise En Charge D’Un Patient Possible Ou Confirmé De Covid-19. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_procedure_hospitals_FR.pdf. Published 2020. Accessed June 5 2020.
- 23. Hani C, Trieu NH, Saab I, et al. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simpson S, Kay F, Abbara S, et al. Radiological society of north America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020;35:219-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study [published online ahead of print April 11, 2020]. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;82:1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [published online ahead of print June 5, 2020]. Med. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Information de la Société Francophone de Néphrologie Dialyse Transplantation (SFNDT) sur l’épidémie de coronavirus (COVID-19) en date du 9 mars 2020. https://www.sfndt.org/sites/www.sfndt.org/files/medias/documents/Conseils%20SFNDT%20COVID-19%20-%20090320%20vDef.pdf. Accessed July 8 2020.
- 30. American Society of Nephrology. Information for screening and management of COVID-19 in the outpatient dialysis facility. https://www.asn-online.org/g/blast/files/DIALYSIS%20COVID%202019%20Update%2003.04.2020FINAL.pdf. Published 2020. Accessed July 8 2020.
- 31. European Centre for Disease Prevention and Control. Infection prevention and control for the care of patients with 2019-nCoV in healthcare settings. https://www.ecdc.europa.eu/sites/default/files/documents/nove-coronavirus-infection-prevention-control-patients-healthcare-settings.pdf. Published 2020. Accessed July 8 2020.
- 32. Suri R, Antonsen J, Banks C, et al. Management of outpatient hemodialysis during the COVID-19 pandemic: recommendations from the Canadian Society of Nephrology COVID-19 rapid response team. https://www.csncommunity.ca/docs/Outpatient%20Dialysis%20Document%20Revision%20April%2017%20CLEAN%20PrePrint%20FOR%20POSTING.pdf. Accessed July 8 2020. [DOI] [PMC free article] [PubMed]
- 33. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kreutz R, Algharably EAEH, Azizi M, et al. Hypertension, the renin-angiotensin system, and’ the risk of lower respiratory tract infections and lung injury: implications for COVID-19 [published online ahead of print April 2020]. Cardiovasc Res. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020;382:1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28:1195-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]