Abstract
BACKGROUND
Patient-ready duodenoscopes were designed with an assumed contamination rate of less than 0.4%; however, it has been reported that 5.4% of clinically used duodenoscopes remain contaminated with viable high-concern organisms despite following the manufacturer’s instructions. Visual inspection of working channels has been proposed as a quality control measure for endoscope reprocessing. There are few studies related to this issue.
AIM
To investigate the types, severity rate, and locations of abnormal visual inspection findings inside patient-ready duodenoscopes and their microbiological significance.
METHODS
Visual inspections of channels were performed in 19 patient-ready duodenoscopes using the SpyGlass visualization system in two endoscopy units of tertiary care teaching hospitals (Tri-Service General Hospital and National Taiwan University Hospital) in Taiwan. Inspections were recorded and reviewed to evaluate the presence of channel scratches, buckling, stains, debris, and fluids. These findings were used to analyze the relevance of microbiological surveillance.
RESULTS
Seventy-two abnormal visual inspection findings in the 19 duodenoscopes were found, including scratches (n = 10, 52.6%), buckling (n = 15, 78.9%), stains (n = 14, 73.7%), debris (n = 14, 73.7%), and fluids (n = 6, 31.6%). Duodenoscopes > 12 mo old had a significantly higher number of abnormal visual inspection findings than those ≤ 12 mo old (46 findings vs 26 findings, P < 0.001). Multivariable regression analyses demonstrated that the bending section had a significantly higher risk of being scratched, buckled, and stained, and accumulating debris than the insertion tube. Debris and fluids showed a significant positive correlation with microbiological contamination (P < 0.05). There was no significant positive Spearman’s correlation coefficient between negative bacterial cultures and debris, between that and fluids, and the concomitance of debris and fluids. This result demonstrated that the presence of fluid and debris was associated with positive cultures, but not negative cultures. Further multivariate analysis demonstrated that fluids, but not debris, is an independent factor for bacterial culture positivity.
CONCLUSION
In patient-ready duodenoscopes, scratches, buckling, stains, debris, and fluids inside the working channel are common, which increase the microbiological contamination susceptibility. The SpyGlass visualization system may be recommended to identify suboptimal reprocessing.
Keywords: Duodenoscope, Working channel, Visual inspection, Microbiological surveillance, Reprocessing, Endoscope reprocessing
Core tip: This study demonstrated that the common abnormal visual inspection findings of patient-ready duodenoscopes were scratches (52.6%), buckling (78.9%), stains (73.7%), debris (73.7%), and fluids (31.6%). The risk of duodenoscopes of being scratched, buckled, and stained, and accumulating debris was significantly higher at the bending section than at the insertion tube. The presence of debris and fluids is susceptible to microbiological contamination. Multivariate analysis demonstrated that fluids, but not debris, was an independent factor for bacterial culture positivity. Working channel inspection may be added to the current recommendations to identify suboptimal reprocessing or duodenoscopes requiring evaluation, repair, or replacement.
INTRODUCTION
Duodenoscopes undergo a multi-step cleaning and high-level disinfection (HLD) procedure, called reprocessing, so that they can be reused in patients. However, the complex design of duodenoscopes may impede effective cleaning[1-4]. Clinically, patient-ready duodenoscopes were designed with an assumed contamination rate of less than 0.4%[5]. The United States Food and Drug Administration (FDA) on April 12, 2019 reported that 5.4% of clinically used duodenoscopes remain contaminated with viable high-concern organisms despite following the manufacturer’s reprocessing instructions[5]. Higher-than-expected contamination rates have occurred despite documented adherence to all steps, which suggests that the current guidelines of endoscope reprocessing may be inadequate[1,2,6,7].
Working channels are subjected to wear and tear; the damaged channels allow bacteria to adhere and hide, and the subsequent formation of biofilms are difficult to remove[8,9]. Visual inspection of working channels of patient-ready endoscopes revealed various findings, including presence of scratches, adherent peel, stains, debris, and fluids[10-12]. Endoscopes with damaged working channels have been considered sources of microbiological contamination[8,13]. The FDA recommended to return the duodenoscopes to the manufacturer for inspection, servicing, and maintenance at least once a year[5]. Visual inspection may identify certain abnormalities and improve the quality and care of duodenoscope reprocessing.
However, many questions have been raised about the visual inspection findings on working channels in real-world situations[10,14]. Studies related to working channels in such situations are too limited to provide sufficient information. When should the visual inspections be performed? Which types of visual inspection findings hold clinical significance? Are the visual inspection findings correlated with microbiological contamination?
With the development of digital endoscopic technology, the SpyGlass visualization system has become increasingly available and easily accessible for clinical treatment in endoscopy units[15]. Video recordings of working channels can be used for communication, teaching, research, and education. An endoscopist may directly visualize the working channels with the SpyGlass visualization system to identify damaged duodenoscopes and return them to the manufacturer for evaluation, repair, or replacement.
This study aimed to investigate the type, severity, and location of the abnormal visual inspection findings inside working channels using the SpyGlass visualization system. We also aimed to assess the clinical significance of these visual inspection findings in microbiological surveillance of patient-ready duodenoscopes in endoscopy units.
MATERIALS AND METHODS
Study design
Visual inspections of patient-ready duodenoscopes (Olympus, Tokyo, Japan) were performed after HLD. The duodenoscope model, duodenoscope age, visual inspection abnormal findings, adenosine triphosphate (ATP) test results, and microbiological surveillance were collected for each duodenoscope. This study was conducted in two endoscopy units of tertiary care teaching hospitals (Tri-Service General Hospital and National Taiwan University Hospital) in Taiwan. A cross-sectional study of the findings of visual inspection of the duodenoscopes was carried out from January 2019 to December 2019. Duodenoscope culture reports were obtained for review and analysis via a longitudinal observational study. The present study was approved by the Institutional Review Board of Tri-Service General Hospital, Taipei, Taiwan.
Duodenoscope reprocessing
Patient-used duodenoscopes undergo standard pre-cleaning, manual cleaning, and HLD after each endoscopic retrograde cholangiopancreatography (ERCP) procedure[16]. HLD was undertaken using an automated endoscope reprocessor (AER) with ortho-phthalaldehyde (OPA) as the chemical disinfectant. The HLD cycle ends with alcohol flushes followed by an automated 1-min air purge within the AER. Patient-ready duodenoscopes were stored vertically in a storage cabinet equipped for humidity and temperature monitoring.
SpyGlass visualization system
Working channels were evaluated with the SpyGlass™ DS Direct Visualization System (Boston Scientific Corp, Natick, MA, United States), which consists of capital equipment and a SpyScope delivery catheter (SpyScope™ DS Catheter, SpyScope™ DS II Catheter). A SpyScope delivery catheter has a length of 214 cm, an outer diameter of 3.5 mm (10.5 Fr), a light source at its distal tip, an adjustable brightness, and a lens that enables high-resolution (24000 pixels) video recording during clinical treatment. A high-resolution monitor (1280 × 1024) is designed to be attached to the cart and to provide bright, clear images under various lighting conditions. The video was viewed on a Windows-based computer, which allows the capturing of both video and still images. The assigned SpyScope delivery catheter was reprocessed immediately before each visual inspection with alcohol wipes for a full 2 min of contact time, followed by air drying for 10 min[12]. After visual inspection, the delivery catheter was reprocessed with the OPA solution.
Location of visual inspection
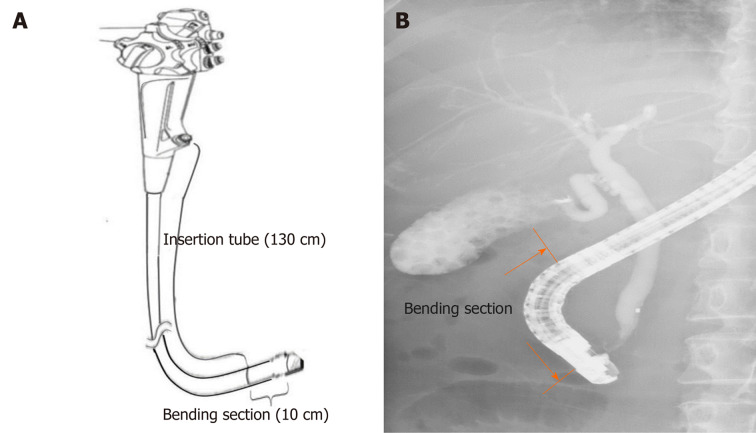
Working channels were examined by manually advancing the SpyScope delivery catheter in an anterograde fashion from the biopsy channel opening of the endoscope handle. The SpyScope delivery catheter allows visualization of the entire length of the working channel (140 cm), including the insertion tube (130 cm) starting from the biopsy channel opening at the proximal part and the bending section (10 cm) proximal to the elevator mechanism (Figure 1).
Figure 1.
A scheme depicting the working channel of a duodenoscope. Insertion tube (130 cm) starting from the biopsy channel opening at the proximal part (A) and bending section (10 cm) proximal to the elevator mechanism (B).
Type and severity of visual inspection findings
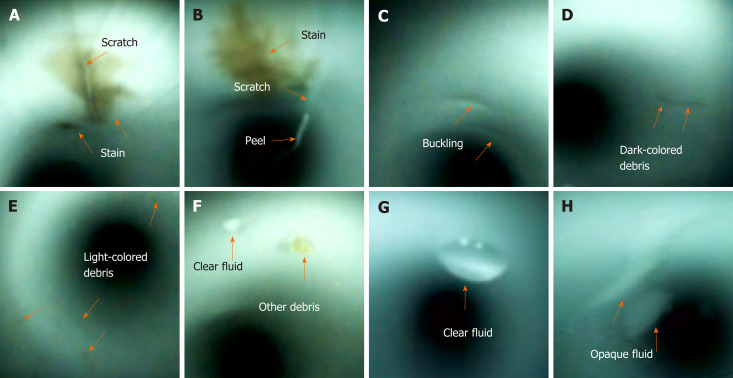
Visual inspection of the working channels of the patient-ready endoscopes revealed various abnormal findings (Figure 2), including scratches, scratches with adherent peel, stains, debris (dark-colored debris, light-colored debris, and other debris), and fluids (clear and opaque fluids)[10-12]. The severity of the visual inspection findings was evaluated using a modified form of Barakat et al[12] ’s classification system that utilized a 3-point scale with the following scores: 0 (none), 1 (mild), 2 (moderate), and 3 (severe). Visual inspection findings were recorded and reviewed by two endoscopists and two endoscopy nurses. In case of a discrepancy in the image of a given visual inspection, discussion would be held by four investigators. Repeated visual inspections were performed to validate consistency and confirm subtle findings of the duodenoscopes.
Figure 2.
Abnormal visual inspection findings (orange arrow) inside the working channels, including scratches (A and B), scratch with an adherent peel (B), buckling (C), stains (A and B), dark-colored debris (D), light-colored debris (E), other debris (F), clear fluid (F and G), and opaque fluid (H).
ATP test and microbiological surveillance
The ATP test was performed after duodenoscope manual cleaning as a routine quality control program[17]. ATP samples obtained were flushed with sterile water, and then the channel rinsate was harvested for the ATP test (Clean-Trace ATP Water, 3M, St Paul, MN, United States). ATP levels were expressed in relative light units (RLUs).
Microbiological surveillance of clinically used duodenoscopes followed the recommendation of the Digestive Endoscopy Society of Taiwan[16,18]. Ten milliliters of normal saline were injected into the working channel. The elution from the distal end was collected and mixed with 40-mL trypticase soy broth in the flask and then incubated at 37°C for 48 h. The turbid sample was chosen, and a subculture with Columbia CNA agar plate for gram-positive bacteria and MacConkey agar plate for gram-negative bacteria was performed overnight. The pure colony was picked up and underwent matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to further identify the specific bacteria.
Statistical analysis
All data were entered into an Excel software (Microsoft Corp, Redmond, WA, United States) spreadsheet. Statistical analyses were carried out using SPSS 22.0 (IBM, Armonk, NY, United States). The McNemar test was used to compare the number of the visual inspection findings among different duodenoscope ages. Multivariable logistic regression analyses were performed to calculate the adjusted odds ratios with 95% confidence intervals (CIs) of the association between the bending section and visual inspection findings. Spearman's correlation coefficients were calculated between the visual inspection findings and microbiological contamination. Statistical significance was defined as a P value < 0.05.
RESULTS
Duodenoscope characteristics
A total of 19 patient-ready duodenoscopes (JF-260V, n = 5; TJF-260V, n = 14; Olympus Medical Systems, Tokyo, Japan) were examined in the endoscopy units (Table 1). The mean age of the duodenoscopes was 35 ± 38 mo, and mean usage count was 356 ± 400. The visual inspection findings of 19 duodenoscopes included scratches (n = 10, 52.6%), buckling (n = 15, 78.9%), stains (n = 14, 73.7%), debris (n = 14, 73.7%), and fluids (n = 6, 31.6%). The mean ATP levels were 70 ± 120 RLUs. There was a total of 134 samples for microbiological surveillance; of these, 6 (4.5%) samples showed positive results.
Table 1.
Duodenoscope characteristics, inspection findings, ATP test, and microbiological surveillance
| Number | Duodenoscope (model) | Age (mo) | Usages(n) |
Inspection abnormal findings (Severity degree/location) |
ATP test (RLU) |
Microbiological surveillance |
||||||||||||
| Scratches | Peel | Buckling | Stains |
Debris |
Fluid |
|||||||||||||
| Dark color | Light color | Other | Clear | Opaque | Culture(n) | Positive(n) | Microorganism | |||||||||||
| 1 | JF-260V | 87 | 1284 | 3/B | 2/B | 2/B | 2/B | 1/B | 3/B | - | 3/I | - | 135 | 34 | 2 | P. aeruginosa | ||
| 2 | JF-260V | 2 | 18 | - | - | 1/B | 2/I | - | - | - | - | - | 185 | 2 | 1 | P. aeruginosa | ||
| 3 | JF-260V | 79 | 1267 | - | - | - | 2/B | 2/I | - | - | - | - | 117 | 27 | 0 | - | ||
| 4 | JF-260V | 64 | 825 | 3/B | 1/B | 2/B | 2/B | 1/I | - | - | 3/I | 3/I | 45 | 24 | 0 | - | ||
| 5 | JF-260V | 1 | 15 | - | - | - | - | 2/I | 1/ I&B | 1/B | 3/I | - | 19 | 2 | 1 | P. aeruginosa | ||
| 6 | TJF-260V | 126 | 649 | 3/I&B | - | 2/I&B | 2/I&B | 2/I | - | 1/B | 3/I&B | 3/I&B | 48 | 29 | 2 | P. aeruginosa | ||
| 7 | TJF-260V | 3 | 34 | - | - | 2/B | - | - | - | - | - | - | 12 | 2 | 0 | - | ||
| 8 | TJF-260V | 12 | 135 | - | - | 2/B | 2/I | 2/I | - | - | 3/I | 1/I | 108 | 2 | 0 | - | ||
| 9 | TJF-260V | 18 | 48 | - | - | 2/I&B | 2/I | - | - | - | - | - | 63 | 2 | 0 | - | ||
| 10 | TJF-260V | 93 | 164 | 1/B | 2/B | 2/B | 2/I&B | 2/I&B | - | - | - | - | 39 | 1 | 0 | - | ||
| 11 | TJF-260V | 51 | 425 | 3/B | - | 2/B | 2/I | -- | - | - | - | - | 21 | 1 | 0 | - | ||
| 12 | TJF-260V | 38 | 251 | 1/B | - | 2/B | 2/B | 3/I&B | - | - | - | - | 114 | 2 | 0 | - | ||
| 13 | TJF-260V | 37 | 612 | 3/B | 2/B | 2/B | 2/B | 3/I&B | 1/I&B | 1/I&B | - | - | 153 | 2 | 0 | - | ||
| 14 | TJF-260V | 12 | 217 | 1/B | - | 2/I&B | 1/B | 1/I | - | - | - | - | 40 | 2 | 0 | - | ||
| 15 | TJF-260V | 16 | 251 | - | - | - | 1B | 1/I | - | - | - | - | 48 | 0 | 0 | - | ||
| 16 | TJF-260V | 16 | 341 | 2/B | - | 1/B | 1/B | 1/I | - | - | - | - | 127 | 1 | 0 | - | ||
| 17 | TJF-260V | 2 | 50 | - | - | 1/B | - | 2/ I&B | - | - | - | - | 29 | 0 | 0 | - | ||
| 18 | TJF-260V | 4 | 80 | 1/B | - | 1/B | - | 2/I&B | - | - | - | - | 191 | 1 | 0 | - | ||
| 19 | TJF-260V | 4 | 67 | - | - | - | - | - | - | - | 3/I&B | - | 97 | 0 | 0 | - | ||
| Summary | 35 ± 38 | 356 ± 400 | 10 | 4 | 15 | 14 | 14 | 3 | 3 | 6 | 3 | 84 ± 57 | 134 | 6 | - | |||
Severity degree: None (0), mild (1), moderate (2), severe (3); location site: insertion tube (I) and bending section (B). Data in summary row are expressed as sum or mean ± SD. ATP: Adenosine tri-phosphate; RLU: Relative light units; P. aeruginosa: Pseudomonas aeruginosa.
Duodenoscope service life vs visual inspection findings
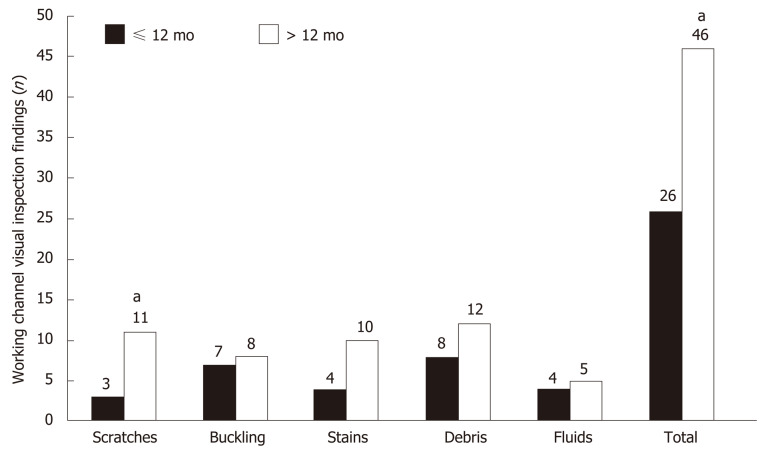
The total number of abnormal visual inspection findings (42 findings vs 26 findings, P < 0.001) and scratches (11 findings vs 3 findings, P < 0.001) were significantly higher in > 12-mo-old duodenoscopes than in ≤ 12-mo-old duodenoscopes (Figure 3). The total number of abnormal visual inspection findings (21.3 ± 11.6 findings vs 11.1 ± 6.4 findings, P = 0.043) were significantly higher in duodenoscopes that had > 200 uses than in those with < 200 uses.
Figure 3.
Comparison of visual inspection findings between > 12-mo-old and ≤ 12mo-old duodenoscopes. aIndicates statistically significant difference.
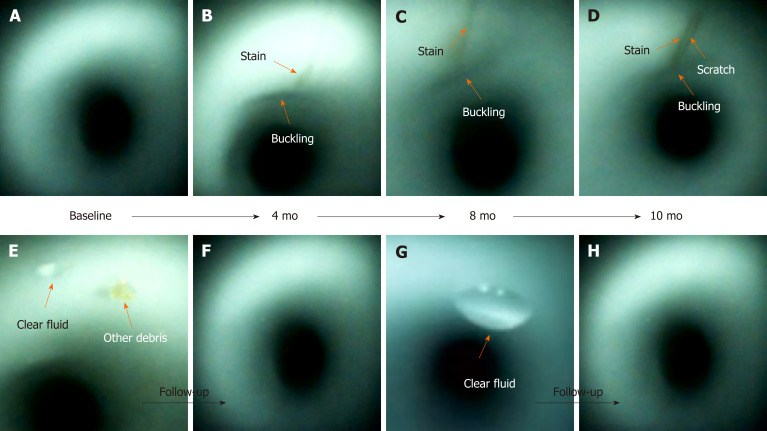
Scratches, buckling, and stains were found at the same location during the follow-up inspections (Figure 4). They may worsen and progressively increase in length and width over time. However, debris and fluids may disappear after one or more cycles of reprocessing.
Figure 4.
Scratches, buckling, and stains (orange arrow) appeared at the same location inside the working channel during the follow-up inspections (B-D). Buckling and stains progressively increased in length and width (A-D). A scratch (D) was found. Debris (E and F) and clear fluid (E-H) disappeared after one or more cycles of reprocessing.
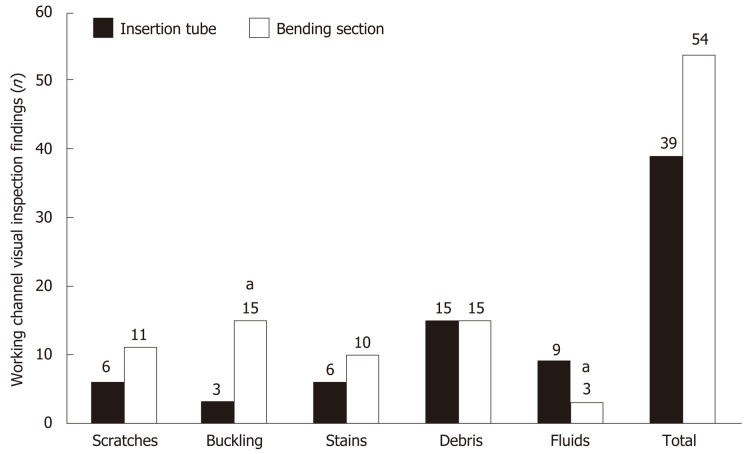
Bending section vs insertion tube
The number of abnormal visual inspection findings located at the bending section was higher than that located at the insertion tube (54 findings vs 39 findings), but it did not reach statistical significance (Figure 5). The number of buckling findings at the bending section was significantly higher than that at the insertion tube (15 findings vs 3 findings, P < 0.001). However, the number of fluid findings at the bending section was significantly lower than that at the insertion tube (3 findings vs 9 findings, P < 0.001).
Figure 5.
Comparison of visual inspection findings between the bending section and insertion tube. aIndicates statistically significant difference.
Bending section is vulnerable to damage
Multivariable logistic regression analyses (Table 2) demonstrated that the risk of abnormal visual inspection findings, including scratches (adjusted odds ratio = 2.60, 95%CI: 2.09-3.12, P < 0.001), buckling (adjusted odds ratio = 2.00, 95%CI: 1.68-2.39, P < 0.001), stains (adjusted odds ratio = 1.72, 95%CI: 1.16-2.56, P = 0.008), and debris (adjusted odds ratio = 1.88, 95%CI: 1.50-2.36, P < 0.001) , was significantly higher at the bending section, but this location had a significantly lower risk of fluid accumulation (adjusted odds ratio = 0.30, 95%CI: 0.21-0.42, P < 0.001) as compared to the insertion tube.
Table 2.
Multivariate regression analysis of abnormal visual inspection findings of the bending section and insertion tube
| Variable | Adjusted odds ratio | 95%CI | P value |
| Scratches | 2.60 | 2.09-3.12 | < 0.001 |
| Buckling | 2.00 | 1.68-2.39 | < 0.001 |
| Stains | 1.72 | 1.16-2.56 | 0.008 |
| Debris | 1.88 | 1.50-2.36 | < 0.001 |
| Fluids | 0.30 | 0.21-0.42 | < 0.001 |
Reference: Insertion tube; CI: Confidence interval.
ATP test and microbiological surveillance
The number of abnormal visual inspection findings was not significantly associated with ATP values after HLD (data not shown). Spearman's correlation coefficients were calculated between abnormal visual inspection findings and microbiological surveillance (Table 3). There was a significant positive Spearman’s correlation coefficient between microbiological surveillance and debris (correlation coefficient = 0.423, P = 0.029), between that and fluids, (correlation coefficient = 0.476, P = 0.037), and between that and concomitant debris and fluids (correlation coefficient = 0.702, P = 0.018).
Table 3.
Correlation between abnormal visual inspection findings and microbiological surveillance
| Variable |
Microbiological surveillance |
|
| Spearman's correlation coefficient | P value | |
| Scratches | 0.088 | 0.694 |
| Buckling | -0.080 | 0.746 |
| Stains | 0.133 | 0.587 |
| Debris | 0.423 | 0.029 |
| Fluids | 0.476 | 0.037 |
| Debris + fluids | 0.702 | 0.018 |
There was no significant Spearman’s correlation coefficient between negative bacterial cultures and debris (correlation coefficient = 0.512, P = 0.086), between that and fluids (correlation coefficient = 0.114, P = 0.289), and between that and concomitant debris and fluids (correlation coefficient = 0.617, P = 0.174). This result demonstrated that the presence of fluid and debris is associated with positive cultures, but not negative cultures.
Fluids is an independent factor for culture positivity
To clarify whether the debris or fluids determine bacterial culture positivity, we further performed multivariate analyses to measure the relationship between two variables whilst controlling for the effect of the other variable (Table 4). There was a significant positive partial correlation coefficient between bacterial culture positivity and fluids (correlation coefficient = 0.462, P = 0.046), but not between that and debris (partial correlation coefficient = 0.316, P = 0.187). This result demonstrated that fluids, but not debris, is an independent factor for bacterial culture positivity.
Table 4.
Multivariate analysis of abnormal visual inspection findings and microbiological surveillance
| Variable |
Microbiological surveillance |
|
| Partial correlation coefficient | P value | |
| Debris | 0.316 | 0.187 |
| Fluids | 0.462 | 0.046 |
Controlling for number of samples taken.
DISCUSSION
This study demonstrated that (1) the common abnormal visual inspection findings of patient-ready duodenoscopes were scratches (52.6%), buckling (78.9%), stains (73.7%), debris (73.7%), and fluids (31.6%); (2) the abnormal visual inspection findings, especially for scratches inside the working channels, were significantly increased in >12-mo-old duodenoscopes; (3) the risk of duodenoscopes of being scratched, buckled, and stained, and accumulating debris was significantly higher at the bending section than at the insertion tube; (4) the presence of debris and fluids makes the duodenoscope susceptible to microbiological contamination; and (5) the presence of fluids is an independent factor for bacterial culture positivity.
The results of this study are consistent with those of previous studies[11,19,20] reporting that the inside of working channels of patient-ready duodenoscopes commonly have scratches, buckling, stains, debris, and fluids (Table 5). A previous guideline only recommended to identify the wear and tear of the external surface, but it did not require the inspection of the internal surface of the working channel[7]. The recently published guidelines from several societies have recommended the performance of visual inspections of working channels during endoscope care and reprocessing[6,21,22]. This study demonstrated that abnormal visual inspection findings may accumulate during long-term endoscope use; these abnormal findings may progressively increase in length and width over time. Visual inspection may identify certain abnormalities and improve the quality of duodenoscope reprocessing in endoscopy units.
Table 5.
Summary of studies on abnormal visual inspection findings in clinically used endoscopes
| Study | Endoscopes (duodenoscopes), n (n) | Scratches (%) | Buckling (%) | Stains (%) | Debris (%) | Fluids (%) |
| Thaker et al[11], 2018 | 59 (14) | 86 | NR | 59 | 22 | 8 |
| Ofstead et al[19], 201819 | 45 (5) | NR | NR | NR | NR | 47 |
| Barakat et al[20], 2018 | 68 (7) | 99 | 3 | NR | 96 | 43 |
| This study | 19 (19) | 52.6 | 78.9 | 73.7 | 73.7 | 31.6 |
NR: Not reported.
The Spyglass visualization system is designed to interface with computer software and to record both still and video images[10,15]. This system has been widely applied to treat biliopancreatic diseases[23]. Currently, the available borescopes have short lengths of 95-110 cm[10]; therefore, borescopes do not allow for a one-step complete inspection of the 140-cm working channel of a duodenoscope. Conversely, a SpyScope delivery catheter is 214 cm in length; therefore, a one-step visual inspection of the working channel can be done with a SpyScope. Endoscopists should routinely or intermittently visualize the working channel during working hours. Early detection of these abnormal visual inspection findings may allow early reporting to the manufacturers and may promote prompt performance of quality assurance interventions before the channel lumen becomes comprised, which could impair manual cleaning. Our study is the first one to use the SpyScope delivery catheter as a tool for visual inspection, besides its original clinical role in treating biliopancreatic diseases.
Endoscopic accessories, such as biopsy forceps, biliary stent, polypectomy snare, and catheter guidewire, are frequently inserted into the working channel[24]. Forcing instrumentation through the channel can cause damages or scratches to the channel[25]. This frequently occurs at the bending section when the endoscope is extremely angulated[26]. It seems inevitable that the endoscopes undergo repeated mechanical damage at the bending section during endoscopy procedures. Multivariable logistic regression analyses (Table 3) demonstrated that the risk of developing scratches, buckling, stains, and debris is significantly higher at the bending section than at the insertion tube.
The bacteria may form biofilms in the endoscope channel, especially when the working channels are damaged, and can contribute to the failure of the decontamination process[8,27]. Colonizing microorganisms proceed with an initial attachment to the preconditioned surface. At this point, the preconditioning film and microorganisms or visible debris are loosely attached and can be easily removed by manual cleaning[28]. Bacterial biofilms or visible stains can develop inside endoscope channels if established reprocessing protocols are not met, and these biofilms can be difficult to remove[28]. Pseudomonas aeruginosa prefers a moist environment and forms biofilms that are extremely difficult to remove from endoscope channels[27].
For duodenoscopes contaminated with Pseudomonas aeruginosa, double cycles of HLD, peracetic acid high-level disinfection, and ethylene oxide sterilization[29] may not be effective; thus, endoscopists should return them to the manufacturer for replacement. With continuous use of the damaged endoscope and its accessories, organic debris may enter into different areas of the device, thereby interfering with reprocessing; this might increase the likelihood of biofilm development. Based on the results of this study, our endoscopy units developed a protocol to ensure the quality of reprocessing procedures. Duodenoscopes will be sent to the manufacturer for checking or repairing immediately under the following conditions: (1) Presence of an endoscope leak; (2) Structural or functional damage of the endoscope; (3) Repeated positive bacterial cultures despite reprocessing by well-trained personnel; and (4) Isolation of Pseudomonas aeruginosa by bacterial culture, associated with significant abnormal visual inspection findings. Duodenoscopes used for more than a year are sent to the manufacturer for annual checks.
LIMITATIONS
This study has several limitations. The number of samples taken for culture ranged from 0 to 34 (Table 1). A total of 134 samples were available for microbiological surveillance. We excluded three cases from the analysis (cases 15, 17, and 19, as shown in Table 1) where no samples were taken for culture. We analyzed the number of samples taken for culture by the service age of the duodenoscopes (> 12 mo vs ≤ 12 mo). The number of samples taken for culture surveillance was higher in duodenoscopes used for > 12 mo (11.2 ± 13.9 number of cultures, n = 11) than those used for ≤ 12 mo (1.4 ± 0.9 number of cultures, n = 8). This study showed that in real- world situations, there will be variations between the samples taken from the instruments. The duodenoscopes were observed for only a short period of time, which may have caused inconsistencies. Furthermore, this was a two-site study with a small sample size, so the findings may not be generalizable. Due to the limited number of duodenoscopes, it was difficult to conduct a subgroup analysis, which may have affected the results.
CONCLUSION
Patient-ready duodenoscopes commonly have scratches, buckling, stains, debris, and fluids inside the working channel. The presence of debris and fluids makes these devices vulnerable to microbiological contamination. We found that the presence of fluids was an independent factor for bacterial culture positivity. Routine visualization of the working channels may provide the opportunity for early quality assurance measures to be taken before the channel lumens become damaged, which may impair manual cleaning. The visual channel inspection approach in our study may be added to existing visual inspection recommendations to identify suboptimal reprocessing or endoscopes requiring repair or replacement.
ARTICLE HIGHLIGHTS
Research background
The working channels of endoscopes are subjected to wear and tear. Damaged channels allow bacteria to adhere and hide, and the biofilms that form are subsequently difficult to remove. Visual channel inspection has been proposed as a quality control measure for endoscope reprocessing.
Research motivation
Endoscopes with damaged working channels have been considered as sources of microbiological contamination. The FDA recommended returning duodenoscopes to the manufacturer for inspection, servicing, and maintenance at least once a year. Visual inspection may identify certain abnormalities and improve endoscopic quality and care of duodenoscope reprocessing. However, many questions have been raised regarding the visual inspection findings on working channels in real-world situations. Studies related to such situations are too limited to provide sufficient information.
Research objectives
We aimed to investigate the type, severity, location, and clinical significance of visual inspections inside patient-ready duodenoscopes.
Research methods
Visual inspection of channels was performed in 19 duodenoscopes. Inspections were recorded and reviewed to evaluate for channel damage (scratches, buckling, and stains), debris (dark-colored debris, light-colored debris, and other debris), and fluids (clear fluid and opaque fluid). Visual inspection findings were used to analyze the relevance of microbiological surveillance.
Research results
We found 72 abnormal visual inspection findings in the 19 duodenoscopes viewed in our study, including scratches (n = 10, 52.6%), buckling (n = 15, 78.9%), stains (n = 14, 73.7%), debris (n = 14, 73.7%), and fluids (n = 6, 31.6%). Duodenoscopes > 12 mo old had a significantly higher number of abnormal visual inspection findings than those ≤ 12 mo old (46 findings vs 26 findings, P < 0.001). Multivariable regression analyses demonstrated that the bending section had a significantly higher risk of being scratched, buckled, and stained, and accumulating debris than the insertion tube. Debris and fluids showed a significant positive correlation with microbiological contamination (P < 0.05).
Research conclusions
In patient-ready duodenoscopes, scratches, buckling, stains, debris, and fluids inside the working channel are common. Presence of debris and fluids increases the susceptibility to microbiological contamination. The presence of fluids was found to be an independent factor for bacterial culture positivity. Visual channel inspection using the SpyGlass visualization system may be added to the existing visual inspection recommendations to identify suboptimal reprocessing or endoscopes requiring repair or replacement.
Research perspectives
Endoscopists should routinely or intermittently visualize the working channel during working hours. Early detection of these abnormal visual inspection findings may allow timely reporting to the manufacturers and promote prompt performance of quality assurance interventions before the channel lumen becomes comprised, which could impair manual cleaning.
ACKNOWLEDGEMENTS
We thank Wu-Chien Chien and Chi-Hsiang Chung (Associate Professor and Assistant Professor, respectively, in the School of Public Health, National Defense Medical Center, Taiwan) for their help with the statistical analysis. We are also grateful to Ming-Chin Chan and Sun-Kang Chiu (Infection Control Office, Tri-Service General Hospital, Taiwan) for the assistance with microbiological surveillance consultation. Finally, we thank I-Hsuan Hunag (Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan) for the assistance in medical illustration.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Tri-Service General Hospital, National Defense Medical Center, Taiwan.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors declare that they have no conflicts of interest.
Peer-review started: February 16, 2020
First decision: May 1, 2020
Article in press: June 23, 2020
P-Reviewer: Kitamura K, Pavides M, Yildiz K S-Editor: Gong ZM L-Editor: MedE-Ma JY E-Editor: Zhang YL
Contributor Information
Tao-Chieh Liu, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei 114, Taiwan.
Chen-Ling Peng, Department of Integrated Diagnostics & Therapeutics, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei 10002, Taiwan.
Hsiu-Po Wang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei 114, Taiwan.
Hsin-Hung Huang, Division of Gastroenterology, Cheng Hsin General Hospital, National Defense Medical Center, Taipei 114, Taiwan.
Wei-Kuo Chang, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei 114, Taiwan. weikuohome@hotmail.com.
Data sharing statement
No additional data are available.
References
- 1.Reprocessing Guideline Task Force. Petersen BT, Cohen J, Hambrick RD 3rd, Buttar N, Greenwald DA, Buscaglia JM, Collins J, Eisen G. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85:282–294.e1. doi: 10.1016/j.gie.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Petersen BT, Chennat J, Cohen J, Cotton PB, Greenwald DA, Kowalski TE, Krinsky ML, Park WG, Pike IM, Romagnuolo J ASGE Quality Assurance in Endoscopy Committee, Rutala WA; Society for Healthcare Epidemiology of America. Multisociety guideline on reprocessing flexible GI endoscopes: 2011. Infect Control Hosp Epidemiol. 2011;32:527–537. doi: 10.1086/660676. [DOI] [PubMed] [Google Scholar]
- 3.Ross AS, Baliga C, Verma P, Duchin J, Gluck M. A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest Endosc. 2015;82:477–483. doi: 10.1016/j.gie.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Muscarella LF. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related "superbugs" during gastrointestinal endoscopy. World J Gastrointest Endosc. 2014;6:457–474. doi: 10.4253/wjge.v6.i10.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. The FDA Continues to Remind Facilities of the Importance of Following Duodenoscope Reprocessing Instructions: FDA Safety Communication. 2019. Available from: https://wwwfdagov/medical-devices/safety-communications/fda-continues-remind-facilities-importance-following-duodenoscope-reprocessing-instructions-fda. [Google Scholar]
- 6.World Gastroenterology Organisation. Endoscope disinfection update: a guide to resource-sensitive reprocessing. Available from: https://wwwworldgastroenterologyorg/guidelines/global-guidelines/endoscope-disinfection/endoscope-disinfection-english. [Google Scholar]
- 7.Beilenhoff U, Biering H, Blum R, Brljak J, Cimbro M, Dumonceau JM, Hassan C, Jung M, Neumann C, Pietsch M, Pineau L, Ponchon T, Rejchrt S, Rey JF, Schmidt V, Tillett J, van Hooft J. Prevention of multidrug-resistant infections from contaminated duodenoscopes: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) Endoscopy. 2017;49:1098–1106. doi: 10.1055/s-0043-120523. [DOI] [PubMed] [Google Scholar]
- 8.Buss AJ, Been MH, Borgers RP, Stokroos I, Melchers WJ, Peters FT, Limburg AJ, Degener JE. Endoscope disinfection and its pitfalls--requirement for retrograde surveillance cultures. Endoscopy. 2008;40:327–332. doi: 10.1055/s-2007-995477. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Sutton B, Hawes R, Varadarajulu S. Concept of disposable duodenoscope: at what cost? Gut. 2019;68:1915–1917. doi: 10.1136/gutjnl-2019-318227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visrodia K, Petersen BT. Borescope examination: Is there value in visual assessment of endoscope channels? Gastrointest Endosc. 2018;88:620–623. doi: 10.1016/j.gie.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Thaker AM, Kim S, Sedarat A, Watson RR, Muthusamy VR. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018;88:612–619. doi: 10.1016/j.gie.2018.04.2366. [DOI] [PubMed] [Google Scholar]
- 12.Barakat MT, Huang RJ, Banerjee S. Comparison of automated and manual drying in the elimination of residual endoscope working channel fluid after reprocessing (with video) Gastrointest Endosc. 2019;89:124–132.e2. doi: 10.1016/j.gie.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauwers AW, Troelstra A, Fluit AC, Wissink C, Loeve AJ, Vleggaar FP, Bruno MJ, Vos MC, Bode LG, Monkelbaan JF. Independent root-cause analysis of contributing factors, including dismantling of 2 duodenoscopes, to investigate an outbreak of multidrug-resistant Klebsiella pneumoniae. Gastrointest Endosc. 2019;90:793–804. doi: 10.1016/j.gie.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 14.The Lancet Gastroenterology Hepatology. Scoping the problem: endoscopy-associated infections. Lancet Gastroenterol Hepatol. 2018;3:445. doi: 10.1016/S2468-1253(18)30168-7. [DOI] [PubMed] [Google Scholar]
- 15.Dimas ID, Vardas E, Papastergiou V, Fragaki M, Velegraki M, Mpitouli A, Voudoukis E, Theodoropoulou A, Giannikaki E, Chlouverakis G, Paspatis GA. Comparison of digital versus fiberoptic cholangioscopy in patients requiring evaluation of bile duct disease or treatment of biliary stones. Ann Gastroenterol. 2019;32:199–204. doi: 10.20524/aog.2019.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Digestive Endoscopy Society of Taiwan (DEST) guideline on duodenoscope reprocessing: cleaning, disinfection and sterilization. 2018. Avalable from: https://wwwdestorgtw/DB/News/file/386-2pdf. 2018. Accessed December 9, 2019. [Google Scholar]
- 17.Chang WK, Liu TC, Liu TL, Peng CL, Wang HP. Enhanced manual cleaning efficacy of duodenoscope in endoscopy units: Results of a multicenter comprehensive quality control program. Am J Infect Control. 2019;47:1233–1239. doi: 10.1016/j.ajic.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Duodenoscope Surveillance Sampling and Culturing Protocols. Available from: https://wwwfdagov/media/111081/download. [Google Scholar]
- 19.Ofstead CL, Heymann OL, Quick MR, Eiland JE, Wetzler HP. Residual moisture and waterborne pathogens inside flexible endoscopes: Evidence from a multisite study of endoscope drying effectiveness. Am J Infect Control. 2018;46:689–696. doi: 10.1016/j.ajic.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Barakat MT, Girotra M, Huang RJ, Banerjee S. Scoping the scope: endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope (with video) Gastrointest Endosc. 2018;88:601–611.e1. doi: 10.1016/j.gie.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharon A, Van Wicklin RC, Cynthia Spry Association of periOperative Registered Nurses. Guideline for processing flexible endoscopes. 2018: 799-882. Available from: https://wwwaornorg/websitedata/cearticle/pdf_file/CEA16516-0001pdf. [Google Scholar]
- 22.Kaneoka A, Krisciunas GP, Walsh K, Raade AS, Langmore SE. A comparison of 2 methods of endoscopic laryngeal sensory testing: a preliminary study. Ann Otol Rhinol Laryngol. 2015;124:187–193. doi: 10.1177/0003489414550241. [DOI] [PubMed] [Google Scholar]
- 23.Karagyozov P, Boeva I, Tishkov I. Role of digital single-operator cholangioscopy in the diagnosis and treatment of biliary disorders. World J Gastrointest Endosc. 2019;11:31–40. doi: 10.4253/wjge.v11.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mammen A, Haber G. Difficult Biliary Access: Advanced Cannulation and Sphincterotomy Technique. Gastrointest Endosc Clin N Am. 2015;25:619–630. doi: 10.1016/j.giec.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Tytgat GN, Ignacio JG. Technicalities of endoscopic biopsy. Endoscopy. 1995;27:683–688. doi: 10.1055/s-2007-1005788. [DOI] [PubMed] [Google Scholar]
- 26.Rozeboom ED, Reilink R, Schwartz MP, Fockens P, Broeders IA. Evaluation of the tip-bending response in clinically used endoscopes. Endosc Int Open. 2016;4:E466–E471. doi: 10.1055/s-0042-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovaleva J, Peters FT, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26:231–254. doi: 10.1128/CMR.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts CG. The role of biofilms in reprocessing medical devices. Am J Infect Control. 2013;41:S77–S80. doi: 10.1016/j.ajic.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Rutala WA, Weber DJ. Reprocessing semicritical items: Outbreaks and current issues. Am J Infect Control. 2019;47S:A79–A89. doi: 10.1016/j.ajic.2019.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.