Abstract
Background
Chinese guidelines for the treatment of type 2 diabetes (T2D) recommend basal or premixed insulins as insulin starters after failed oral antihyperglycaemic medication (OAM). This pragmatic study compared effectiveness and safety of add‐on basal insulin analog (BI) and mid‐mixture insulin analog (MMI; 50:50 premixed insulin) as starter insulin regimens in Chinese patients with T2D in a real‐world setting.
Materials and Methods
This was a multicentre, open‐label, randomized, parallel, pragmatic trial. Patients receiving OAMs were randomized 1:1 to BI (n = 410) or MMI (n = 404) for 24 weeks. Insulin titration and OAM adjustment were determined by investigators following usual standard‐of‐care. The primary outcome was change in glycated haemoglobin (HbA1c) from baseline.
Results
Least‐squares mean changes in HbA1c from baseline to week 24 were −2.00% and −2.15% for BI and MMI groups, respectively (P = .13). The MMI group demonstrated a greater reduction in concomitant OAM therapies used than BI group (53.8% vs. 35.3%, respectively; P < .001). Very limited daily insulin dose increments were observed from baseline to week 24 in both BI and MMI groups (2.5 U/day and 1.8 U/day, respectively). Although both insulin analogs were well‐tolerated without severe hypoglycaemia, small weight gains were seen with both treatments. Higher total hypoglycaemia rates were noticed with the MMI group, while nocturnal hypoglycaemia events were comparable.
Conclusions
In real‐world settings, BI and MMI provided similar improvement in glucose control without conceding hypoglycaemia. The BI group received a greater number of OAMs in real‐world settings. Limited insulin dose titration was observed, while more adjustments occurred with OAM usage.
Keywords: China, insulin, pragmatic trials, type 2 diabetes mellitus
1. INTRODUCTION
Diabetes was estimated to be the seventh leading cause of death in 2016. The global prevalence of diabetes among adults over 18 years of age rose from 4.7% in 1980 to 8.5% in 2014, 1 with prevalence increasing rapidly in middle‐ and low‐income countries. 2 According to recent reports, the prevalence of diabetes in China is estimated to be 10.9% 3 and it is further estimated that by 2030, 140.5 million of the Chinese population will have diabetes. 4 Insulin secretion deficiency, particularly in the early phase, leads to postprandial hyperglycaemia, which is pronounced in Chinese patients compared with most other ethnic groups.5, 6 The high glycaemic index and glycaemic load of carbohydrate‐rich diets potentially cause postprandial glucose (PPG) levels and blood glucose (BG) excursions to be more pronounced in Asian patients than their Caucasian counterparts. 7 With diabetes progression and β‐cell function decrease, even with multiple oral antihyperglycaemic medication (OAM) combinations, many patients with type 2 diabetes (T2D) require insulin treatment to control glucose. Considering this pathophysiological character of Chinese patients with diabetes, the Chinese T2D guidelines 5 recommend initiating basal insulin (BI) or premixed insulin in OAM‐uncontrolled patients. This guidance differs from the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) guidelines.8, 9, 10 In China, insulin analog mixture formulations are widely used, such as low mixtures with a low ratio of rapid‐acting insulin such as insulin lispro 75/25 and insulin aspart 70/30, or mid‐mixtures with an equal ratio of rapid‐acting insulin and intermediate‐acting insulin such as insulin lispro 50/50 and insulin aspart 50/50. In clinical practice, the most commonly used BIs are long‐acting insulin analogs, glargine and detemir. Several randomized controlled trials (RCTs) have established the significance of active titration of basal, premixed or prandial insulin in achieving and maintaining glycaemic control. Despite the merits of RCTs in determining the efficacy of interventions, the overall effectiveness of interventions depends on use under real‐life conditions, which RCTs are unable to reflect. 11 Pragmatic trials are designed to evaluate the effectiveness of interventions in real‐life routine practice conditions, whereas explanatory trials aim to test whether an intervention works under optimal situations in which the background therapy is strictly defined and monitored. The main advantage of pragmatic trials is that they measure a wide spectrum of outcomes, mostly patient‐centred,11, 12 producing results that can be generalized and applied in routine practice settings. With regards to this, a substantial study comparing the effectiveness of BI and mid‐mixture insulin analog (MMI) in a real‐world setting in China is lacking. To address this gap, the current 24‐week pragmatic randomized study aimed to investigate the effectiveness and safety of add‐on BI analog or MMI analog in patients with uncontrolled BG with OAM treatment.
2. MATERIALS AND METHODS
2.1. Study design
This study (ClinicalTrials.gov NCT03018938) was a multicentre, open‐label, randomized, parallel, two‐arm pragmatic trial to study the effectiveness and safety of BI and MMI added to OAMs in adult Chinese patients with T2D uncontrolled by OAMs. The study was conducted at 32 sites in China in accordance with the Declaration of Helsinki principles 13 and the International Conference of Harmonization Good Clinical Practice, and was approved by the participating institutional review boards. All patients gave written informed consent before enrolment.
2.2. Study population
As per the study design, this pragmatic trial had minimal inclusion and exclusion criteria. Male or female patients aged ≥18 years who had been taking at least one OAM and with a glycated haemoglobin (HbA1c) value ≥7.5% were included in the trial. Patients with type 1 diabetes, patients who had received any type of insulin within 24 months of study entry, or those with a serious pre‐existing medical or other improper conditions were excluded.
2.3. Treatment
Patients who met criteria for enrolment were randomized 1:1 to receive either BI analog or MMI analog. BI analog is a once‐daily long‐acting insulin (glargine or detemir). The MMI analog is a 50/50 premixed insulin and contains an equal ratio of rapid‐ and intermediate‐acting insulin (lispro 50 or aspart 50; twice‐daily dose). Assignment to treatment groups was determined by a computer‐generated random sequence using an interactive voice‐response system or interactive web‐response system. Throughout the study period, the individual investigator adjusted the insulin dose and concomitant OAMs based on the patient condition and regular clinical practice. There was no restriction on switching or augmenting the initial insulin treatment. HbA1c, fasting plasma glucose (FPG), finger stick BG (FSBG)‐based fasting BG (FBG) and PPG data were collected at baseline and at week 24 of the study.
2.4. Outcome measurements
The primary efficacy measure was to evaluate the change in HbA1c from baseline to 24 weeks between two treatment options. Secondary efficacy measures included the proportion of patients who achieved HbA1c <7% at 24 weeks, change from baseline to 24 weeks in venous FPG, FSBG‐based FBG and PPG, and daily insulin dose. The major safety analyses were incidence and event rates of severe hypoglycaemia, nocturnal hypoglycaemia, total hypoglycaemia, and body weight change from baseline to 24 weeks. In the current study, patient follow‐up was instructed only by usual care and no additional visit was required.
2.5. Statistical analyses
A non‐inferiority margin of 0.4% was assumed considering no treatment difference between the BI group and the MMI group, with a common standard deviation (SD) of 1.5% for the change from baseline in HbA1c at week 24. A sample size of 332 was calculated to provide 99% possibility for the study to reach a conclusion for primary analysis. An estimation of 415 patients per arm (dropout rate of 20%) was calculated. Both efficacy and safety analyses were performed on randomized patients and all tests of treatment effects were conducted at a two‐sided alpha level of .05. Descriptive statistics (mean, median and SD for continuous variables) were used to summarize patient characteristics and outcomes for the study population overall and by treatment group. The comparison between treatment arms was conducted using an analysis of covariate model or mixed effects model with repeated measures. Unless specified otherwise, a two‐sample t‐test was used for continuous measurements and Fisher’s exact test was used for categorical measurements. The 95% confidence interval of least‐squares (LS) means difference was used to make conclusions for the comparison.
3. RESULTS
In total, 814 patients on OAM treatment were randomized to receive BI (n = 410) and MMI (n = 404) (Figure S1; see Supporting Information). Baseline characteristics such as age, weight, body mass index, duration of diabetes of patients and concomitant OAMs were similar between treatment groups. The total mean age of patients in the study (N = 814) was 57.6 ± 9.18 years, and most patients in both groups had poorly controlled diabetes (mean ± SD, 9.8 ± 1.61% HbA1c) (Table 1).
TABLE 1.
Demographic and baseline characteristics of patients
| Characteristics a | BI + OAM (N = 410) | MMI + OAM (N = 404) | Total (N = 814) |
|---|---|---|---|
| Age, years | 57.5 ± 9.29 | 57.8 ± 9.08 | 57.6 ± 9.18 |
| Male, n (%) | 233 (56.80) | 223 (55.20) | 456 (56.0) |
| Weight, kg | 66.9 ± 11.67 | 66.9 ± 10.97 | 66.9 ± 11.32 |
| BMI, kg/m2 | 24.5 ± 3.44 | 24.5 ± 3.11 | 24.5 ± 3.28 |
| Duration of diabetes, years | 9.3 ± 5.76 | 9.4 ± 5.79 | 9.4 ± 5.77 |
| HbA1c, % | 9.7 ± 1.56 | 9.9 ± 1.65 | 9.8 ± 1.61 |
| FPG, mmol/L | 11.3 ± 3.64 | 11.5 ± 3.30 | 11.4 ± 3.47 |
| FSBG‐based FBG, mmol/L | 10.6 ± 3.01 | 11.0 ± 2.96 | 10.8 ± 2.98 |
| FSBG‐based PPG, mmol/L | 15.7 ± 4.36 | 15.9 ± 4.64 | 15.8 ± 4.50 |
| OAM categories, n (%) | |||
| Alpha‐glucosidase inhibitors | 178 (43.4) | 182 (45.0) | 360 (44.2) |
| Biguanides | 309 (75.4) | 298 (73.8) | 607 (74.6) |
| Dipeptidyl peptide‐4 inhibitors | 35 (8.5) | 25 (6.2) | 60 (7.4) |
| Glinides | 63 (15.4) | 54 (13.4) | 117 (14.4) |
| Sodium glucose co‐transporter 2 inhibitor | 2 (0.5) | 2 (0.5) | 4 (0.5) |
| Sulfonylureas | 233 (56.8) | 226 (55.9) | 459 (56.4) |
| Thiazolidinediones | 21 (5.1) | 32 (7.9) | 53 (6.5) |
Abbreviations: BI, basal insulin analog; BMI, body mass index; FBG, fasting blood glucose; FPG, fasting plasma glucose; FSBG, finger stick blood glucose; HbA1c, glycated haemoglobin; MMI, mid‐mixture insulin analog; N, number of patients in the analyses population in specified treatment arm; OAM; oral antihyperglycaemic medication; PPG, postprandial glucose.
Values presented as mean ± SD, unless otherwise specified.
3.1. Glycaemic control
Improved glycaemic control was observed in both treatment groups. The change in LS mean (SE) from baseline to 24 weeks in HbA1c for the BI and MMI groups was similar in the real‐world setting: −2.00% (0.07) versus −2.15% (0.07), respectively; LS mean difference −0.148%, P = .13 (Figure 1). At week 24, a trend of a higher proportion of patients achieving HbA1c <7% in the MMI treatment group than the BI group was reported (34% vs. 28.5%, respectively; P = .09) (Figure 2). A higher reduction in FPG was observed in the BI group compared with the MMI group from baseline to week 24 with LS mean (SE): −2.94 (0.15) versus −2.31 (0.15), respectively (P = .002). Additionally, the reduction in FSBG‐based FBG levels from baseline was similar over the 24‐week study period in the BI and MMI groups with LS mean (SE): −2.45 (0.16) versus −2.17 (0.15), respectively (P = .20). The reduction in BG excursion and FSBG‐based PPG levels from baseline to 24 weeks was similar in the BI and MMI groups (BG excursion: −1.74 mmol/L vs. −2.28 mmol/L, P = .0523; and FSBG‐based PPG levels: −4.30 mmol/L vs. −4.35 mmol/L, respectively, P = .87) (Table 2).
FIGURE 1.

Changes from baseline in HbA1c at week 24 using analysis of covariance. †LSM difference (95% confidence interval) of MMI + OAM and BI + OAM. P = .13 (MMI + OAM group vs. BI + OAM group). LSM values and P‐values are based on analysis of covariance model. BI, basal insulin; HbA1c, glycated haemoglobin; LSM, least‐squares mean; MMI, mid‐mixture insulin; OAM, oral antihyperglycaemic medication; SE, standard error
FIGURE 2.
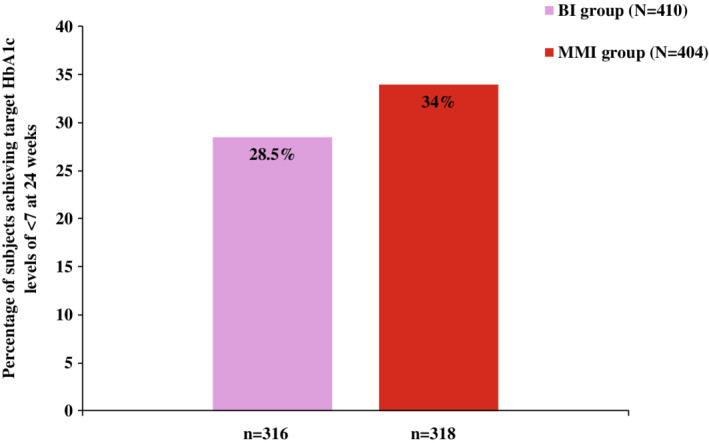
Percentage of subjects achieving target HbA1c levels of <7% at week 24. P = .09, based on logistic regression model (MMI + OAM group vs. BI + OAM group). BI, basal insulin; HbA1c, glycated haemoglobin; MMI, mid‐mixture insulin; n, patients with non‐missing HbA1c data at week 24; OAM, oral antihyperglycaemic medication
TABLE 2.
Change from baseline in HbA1c, FPG, FSBG‐based FBG and PPG, and body weight at week 24
| Variable | Treatment | Baseline, mean (SD) | Endpoint, mean (SD) | LS mean change from baseline (SE) | LS mean difference (SE) | (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| HbA1c (%) | BI + OAM | 9.70 (1.56) | 7.71 (1.19) | −2.00 (0.07) | −0.15 (0.10) | (−0.34, 0.04) | .13 |
| MMI + OAM | 9.94 (1.65) | 7.61 (1.29) | −2.15 (0.07) | ||||
| FPG (mmol/L) | BI + OAM | 11.31 (3.64) | 8.41 (2.36) | −2.94 (0.15) | 0.63 (0.21) | (0.23, 1.04) | .002 |
| MMI + OAM | 11.55 (3.30) | 9.08 (2.34) | −2.31 (0.15) | ||||
| FSBG‐based FBG (mmol/L) | BI + OAM | 10.62 (3.01) | 8.21 (2.47) | −2.45 (0.16) | 0.28 (0.22) | (−0.15, 0.71) | .20 |
| MMI + OAM | 10.99 (2.96) | 8.55 (2.15) | −2.17 (0.15) | ||||
| FSBG‐based PPG (mmol/L) | BI + OAM | 15.70 (4.36) | 11.45 (3.36) | −4.30 (0.24) | −0.06 (0.33) | (−0.71, 0.60) | .87 |
| MMI + OAM | 15.87 (4.64) | 11.39 (3.49) | −4.35 (0.23) | ||||
| Body weight (kg) | BI + OAM | 66.87 (11.67) | 67.76 (11.43) | 0.62 (0.22) | 0.67 (0.30) | (0.08, 1.27) | .03 |
| MMI + OAM | 66.89 (10.97) | 68.19 (10.90) | 1.29 (0.21) |
Abbreviations: BI, basal insulin; CI, confidence interval; FBG, fasting blood glucose; FPG, fasting plasma glucose; FSBG, finger stick blood glucose; HbA1c, glycated haemoglobin; LS, least squares; MMI, mid‐mixture insulin; OAM; oral antihyperglycaemic medications; PPG, postprandial glucose; SD, standard deviation; SE, standard error.
The mean ± SD dose of BI was 12.1 ± 4.37 U/day at baseline with minimal increments to 14.6 ± 7.07 U/day at 24 weeks. Similarly, the dose of MMI was 23 ± 7.68 U/day at baseline with minimal increments to 24.8 ± 10.25 U/day. The changes in OAMs (e.g., insulin secretagogues and alpha‐glucosidase inhibitors) between the two groups were statistically significant (P < .001); a higher proportion of patients in the MMI group decreased the usage of OAMs (53.80%) while a higher proportion of patients in the BI group increased the usage of OAMs (21.80%) (Table 3 and Table S1; see Supporting Information). Approximately 4% of patients added rapid insulin in the BI group and approximately 2% of patients in the MMI group intensified insulin treatment to basal bolus or premixed insulin three times a day.
TABLE 3.
OAM usage during week 24
| Baseline | Week 24 a | |||
|---|---|---|---|---|
| ≤2 OAMs | >2 OAMs | ≤2 OAMs | >2 OAMs | |
| BI + OAM (N = 399) | 285 (71.4) | 114 (28.6) | 286 (71.7) | 113 (28.3) |
| MMI + OAM (N = 398) | 291 (73.1) | 107 (26.9) | 347 (87.2) | 51 (12.8) |
| Decreased | Same | Increased | Overall P value | |
|---|---|---|---|---|
| BI + OAM (N = 399) | 141 (35.3) | 171 (42.9) | 87 (21.8) | <.0001 |
| MMI + OAM (N = 398) | 214 (53.8) | 136 (34.2) | 48 (12.1) | NA |
Abbreviations: BI, basal insulin; MMI, mid‐mixture insulin; N, total number of patients in specified treatment group; NA, not applicable; OAM; oral antihyperglycaemic medication.
Except for P value, all data presented as n (%).
3.2. Safety outcomes
Both BI and MMI were well tolerated throughout the 24‐week period along with OAM treatment therapies. No severe hypoglycaemia was reported in either group during the 24 weeks of treatment. The total hypoglycaemia events were higher in the MMI group in comparison with the BI group (estimated annual rate: 1.57 ± 5.07 vs. 0.61 ± 1.77, respectively; P < .0001); however, both the BI and MMI groups had a similar incidence of nocturnal hypoglycaemic events (7.1% and 8.4%, respectively; P = .12) (Table S2; see Supporting Information). The change in body weight (kg) (LS mean ± SE) from baseline to 24 weeks in the BI group was 0.62 ± 0.22 compared with 1.29 ± 0.21 in the MMI group (P = .03).
4. DISCUSSION
To the best of our knowledge, this pragmatic randomized study is the first large‐scale, real‐world study evaluating the effectiveness and safety of BI and MMI as starter insulins in patients who had inadequate glycaemic control on OAMs. The current study was conducted at 32 sites in different geographical locations across tier 1, 2 and 3 cities of China and provided an insight into the clinical situation present in China. In contrast to RCTs, pragmatic studies can provide evidence on the relative effectiveness of treatment strategies in routine clinical practice. Consequently, the results of pragmatic studies can provide maximum applicability and generalizability in diverse heterogeneous populations. Pragmatic studies, such as the current one, also avoid some of the bias usually associated with RCTs, as they present data from real‐world scenarios, in contrast to data from RCTs, which are taken from controlled environments, with accessing markers at regular intervals. 14 The results of the current study demonstrate that added BI or MMI treatment provided significant improvement in glycaemic control in patients who had inadequate glucose control with OAMs. These findings are comparable with previous study findings.15, 16, 17
American Diabetes guidelines recommend an HbA1c target of <7.0% for patients with T2D and the initiation of insulin therapy when OAMs are unable to control glucose to achieve this target. 18 However, most patients in the current study had baseline HbA1c levels of 9.8%. The delay in initiation of insulin in clinical practice in the current study is consistent with a population‐based study conducted in northern Denmark in patients with T2D receiving insulin treatment as add‐on to metformin in real‐life. Patients in that study had high baseline HbA1c values (median 9.6%). 19 Similarly, the ACTION study, conducted on retrospective data from a real‐world setting, reported mean baseline HbA1c levels from 8.8% to 10.1%. 20 In China, the real‐world prospective ORBIT study 21 reported a similar baseline HbA1c level to the current study (9.6%). The delay in insulin treatment seen in these real‐world scenarios can lead to other complications such as poor glycaemic control, reduced life expectancy, compromised quality of life and other diabetes‐related complications including blindness, organ damage and loss of circulation to limbs resulting in amputation. 22
As noticed in the current study, the MMI group had a numerically higher percentage of patients attaining the HbA1c target of < 7% than the BI group (34% vs. 28.5%). These findings were similar to the reported outcomes of a systematic review evaluating MMI and BI therapy in Asian patients. 23 Additionally, an RCT conducted in Asian patients (CLASSIFY study) 24 demonstrated greater improvement in HbA1c levels in insulin‐naive patients with higher HbA1c baseline levels receiving premixed insulins, with a higher proportion of patients who were receiving LM50 achieving an HbA1c target of < 7% (MMI: 59.7%; BI: 45.9%). However, the key reason for these results can be attributed to the controlled environment under which RCTs are conducted, with factors such as scheduled and frequent glucose tests, intense visits to clinic and dose titration, resulting in a higher proportion of patients achieving target HbA1c levels.
OAM therapy was adjusted in the current study in line with clinical practice, with OAMs adjusted based on patient condition and physician decision. In the present study, starting from a similar background of OAM use, a greater proportion of patients in the BI group concomitantly received two to three OAMs, while a greater proportion of patients in the MMI group received one or two concomitant OAMs. Likewise, the OAMs received by the BI group combined more insulin secretagogues and more alpha‐glucosidase inhibitors, glinide and dipeptidyl peptide‐4 inhibitor agents to decrease PPG and achieve similar PPG control as achieved by the MMI group with fewer OAMs.
Insulin dose adjustments were very limited in the present study, echoing the therapeutic inertia in clinical practice. During the 24‐week study, only 1‐2 units of dose were titrated from the initial dose of BI and MMI, which may have resulted in unsatisfactory glucose control. This situation is reflected in the ORBIT study where, at the 6‐month follow‐up, the mean daily dose of BI increased by only 0.03 IU/kg. 21 This lack of insulin adjustment may be due to significant concerns about weight gain, risk of hypoglycaemia, patient adherence and unwillingness for self‐monitoring of BG.25, 26
The incidence and estimated annual rates of hypoglycaemic events in the MMI group were higher compared with the BI group, a situation that has been observed in other studies.15, 17 A recent literature review has also noted that higher rates of hypoglycaemic events are observed in real‐world studies than in RCTs. 27 No severe hypoglycaemia was observed in the current study, consistent with systematic review findings.28, 29 This indicates that no significant safety concern was associated with respect to severe hypoglycaemia in either BI or MMI therapy in real‐world practice. It is worth noting that nocturnal hypoglycaemia observed in the current study in the MMI group was low and similar to the BI group (P = .12). These safety findings were consistent with findings from other studies evaluating premixed insulin analogs in insulin‐naive patients,16, 30 and with the results from systematic research evaluating premixed insulin analogs, which suggested some increase in overall hypoglycaemia, but not in nocturnal or severe hypoglycaemia. 31 Many studies have demonstrated that insulin analogs have reported less hypoglycaemia compared with human insulin;32, 33, 34 however, with faster absorption and elimination, premixed insulin analogs provide significant reduction in major hypoglycaemic events compared with premixed human insulin.
The strengths of this study were its pragmatic design, reflecting the actual use of therapies, and the large sample size, drawn from all major regions in China among a clinically relevant heterogeneous population. These factors enabled the assessment of the effectiveness and safety of BI analog and MMI regimens in Chinese clinical practice.
Limitations also need to be acknowledged. As this study was carried out in a real‐world setting with a limited number of visits, BG data and meal information could not be collected to analyse BG fluctuation during other times of the day (such as before and after lunch and dinner) or to analyse the effect of meal type on BG levels after insulin treatment. Another limitation of the study is that the analysis between groups was based on the original group (treatment at randomization). As this was a study in a real‐world setting, and insulin treatment could be changed, some patients who were initially on BI changed to premixed insulin treatment and vice versa. Thus, the results of regimens may be impacted and need further analysis.
In conclusion, in China, for patients with type 2 diabetes uncontrolled with OAMs, initiation of MMI or BI analog along with OAM adjustment, offered similar improvement in glycaemic control without any major safety issues. The total hypoglycaemia rates were higher in the MMI group compared with the BI group, while nocturnal hypoglycaemia events were comparable. We found delayed insulin initiation and inadequate insulin dose titration in Chinese clinical practice.
CONFLICT OF INTEREST
Xiaomei Zhang, Yujin Ma, Linong Ji and Lulu Chen declare that they have no affiliations with or involvement in any organization or entity with any financial interest, or non‐financial interest in the subject matter or materials discussed in this manuscript. The authors Hong Chen and Ying Lou are full‐time employees of Lilly Suzhou Pharmaceutical Co. Ltd., Shanghai, China.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
The authors would like to thank Amol Gujar from Syneos Health for medical writing in the preparation of this manuscript. This study was funded by Eli Lilly and Company.
Zhang X, Ma Y, Chen H, Lou Y, Ji L, Chen L. A pragmatic study of mid‐mixture insulin and basal insulin treatment in patients with type 2 diabetes uncontrolled with oral antihyperglycaemic medications: A lesson from real‐world experience. Diabetes Obes Metab. 2020;22:1436–1442. 10.1111/dom.14052
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14052.
Funding information Eli Lilly and Company, Grant/Award Number: NA
Contributor Information
Linong Ji, Email: jiln@bjmu.edu.cn.
Lulu Chen, Email: cheria_chen@126.com.
REFERENCES
- 1. World Health Organization . Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed July 16, 2019.
- 2. Kaiser AB, Zhang N, Pluijm WVD. Global prevalence of type 2 diabetes over the next ten years (2018–2028); 2018 Poster, Diabetes; 67.
- 3. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Diabetes Federation, IDF Diabetes Atlas, 9th edition 2019. https://www.diabetesatlas.org/en/sections/demographic-and-geographic-outline.html. Accessed November 20, 2019.
- 5. Yang W, Weng J. Early therapy for type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2:992‐1002. [DOI] [PubMed] [Google Scholar]
- 6. Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27:79‐84. [DOI] [PubMed] [Google Scholar]
- 7. Henry CJ, Lightowler HJ, Newens K, et al. Glycaemic index of common foods tested in the UKand India. Br J Nutr. 2008;99:840‐845. [DOI] [PubMed] [Google Scholar]
- 8. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2012;35(6):1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirose T, Chen CC, Ahn KJ, Kiljański J. Use of insulin glargine 100 U/mL for the treatment of type 2 diabetes mellitus in east Asians: a review. Diabetes Ther. 2019;10:805‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chinese Diabetes Society . Chinese guidelines for the management of type 2 diabetes mellitus (2013 edition). Chin J Diabetes Mellitus. 2014;6:447‐498. [Google Scholar]
- 11. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol. 2009;62:499‐505. [DOI] [PubMed] [Google Scholar]
- 12. Taljaard M, Weijer C, Grimshaw JM, et al. Developing a framework for the ethical design and conduct of pragmatic trials in healthcare: a mixed methods research protocol. Trials. 2018;19:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Medical Association Declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925‐926. [PubMed] [Google Scholar]
- 14. Attia A. Bias in RCTs: confounders, selection bias and allocation concealment. Middle East Fertil Soc J. 2005;10:258‐261. [Google Scholar]
- 15. Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH, Lispro Mixture‐Glargine Study Group . Combined therapy with insulin lispro mix 75/25 plus metformin or insulin glargine plus metformin: a 16‐week, randomized, open‐label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clin Ther. 2004;26:2034‐2044. [DOI] [PubMed] [Google Scholar]
- 16. Hedrington MS, Pulliam L, Davis SN. Basal insulin treatment in type 2 diabetes. Diabetes Technol Ther. 2001;13(suppl 1):S33‐S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24‐week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care. 2009;32:1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association . Standards of medical Care in Diabetes‐2019 abridged for primary care providers. Clin Diabetes. 2019;37:11‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomsen RW, Baggesen LM, Søgaard M, et al. Early glycaemic control in metformin users receiving their first add‐on therapy: a population‐based study of 4,734 people with type 2 diabetes. Diabetologia. 2015;58:2247‐2253. [DOI] [PubMed] [Google Scholar]
- 20. Blonde L, Raccah D, Lew E, et al. Treatment intensification in type 2 diabetes: a real‐world study of 2‐OAD regimens, GLP‐1 RAs, or basal insulin. Diabetes Ther. 2018;9:1169‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji L, Zhang P, Zhu D, et al. Observational registry of basal insulin treatment (ORBIT) in patients with type 2 diabetes uncontrolled with oral antihyperglycaemic drugs: real‐life use of basal insulin in China. Diabetes Obes Metab. 2017;19:822‐830. [DOI] [PubMed] [Google Scholar]
- 22. Kim SG, Kim NH, Ku BJ, et al. Delay of insulin initiation in patients with type 2 diabetes mellitus inadequately controlled with oral hypoglycemic agents (analysis of patient‐ and physician‐related factors): a prospective observational DIPP‐FACTOR study in Korea. J Diabetes Invest. 2017;8:346‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheu WH, Ji L, Lee WJ, Jabbar A, Han JH, Lew T. Efficacy and safety of premixed insulin analogs in Asian patients with type 2 diabetes: a systematic review. J Diabetes Invest. 2017;8:518‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watada H, Su Q, Li PF, Iwamoto N, Qian L, Yang WY. Comparison of insulin lispro mix 25 with insulin lispro mix 50 as an insulin starter in Asian patients with type 2 diabetes: a phase 4, open‐label, randomized trial (CLASSIFY study). Diabetes Metab Res Rev 2017;(1):33;1‐10. 10.1002/dmrr.2816. [DOI] [PubMed] [Google Scholar]
- 25. Spain CV, Wright JJ, Hahn RM, Wivel A, Martin AA. Self‐reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38:1653‐1664.e1. [DOI] [PubMed] [Google Scholar]
- 26. Choleau C, Albisser AM, Bar‐Hen A. A novel method for assessing insulin dose adjustments by patients with diabetes. J Diabetes Sci Technol. 2007;1:3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elliott L, Fidler C, Ditchfield A, Stissing T. Hypoglycemia event rates: a comparison between real‐world data and randomized controlled trial populations in insulin‐treated diabetes. Diabetes Ther. 2016;7:45‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lasserson DS, Glasziou P, Perera R, Holman RR, Farmer AJ. Optimal insulin regimens in type 2 diabetes mellitus: systematic review and meta‐analyses. Diabetologia. 2009;52:1990‐2000. [DOI] [PubMed] [Google Scholar]
- 29. Petrovski G, Gjergji D, Grbic A, Vukovic B, Krajnc M, Grulovic N. Switching from pre‐mixed insulin to regimens with insulin glargine in type 2 diabetes: a prospective, observational study of data from Adriatic countries. Diabetes Ther. 2018;9:1657‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in Wpe 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260‐265. [DOI] [PubMed] [Google Scholar]
- 31. Ilag LL, Kerr L, Malone JK, Tan MH. Prandial premixed insulin analog regimens versus basal insulin analog regimens in the management of type 2 diabetes: an evidence‐based comparison (review). Clin Ther. 2007;29:1254‐1270. [PubMed] [Google Scholar]
- 32. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators . The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080‐3086. [DOI] [PubMed] [Google Scholar]
- 33. Levy P. Insulin analogs or premixed insulin analogs in combination with oral agents for treatment of type 2 diabetes (review). MedGenMed. 2007;9:12. [PMC free article] [PubMed] [Google Scholar]
- 34. Little S, Shaw J, Home P. Hypoglycemia rates with basal insulin analogs. Diabetes Technol Ther. 2011;13(suppl 1):S53‐S64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


