Abstract
Primrose syndrome (PS; MIM# 259050) is characterized by intellectual disability (ID), macrocephaly, unusual facial features (frontal bossing, deeply set eyes, down‐slanting palpebral fissures), calcified external ears, sparse body hair and distal muscle wasting. The syndrome is caused by de novo heterozygous missense variants in ZBTB20. Most of the 29 published patients are adults as characteristics appear more recognizable with age. We present 13 hitherto unpublished individuals and summarize the clinical and molecular findings in all 42 patients. Several signs and symptoms of PS develop during childhood, but the cardinal features, such as calcification of the external ears, cystic bone lesions, muscle wasting, and contractures typically develop between 10 and 16 years of age. Biochemically, anemia and increased alpha‐fetoprotein levels are often present. Two adult males with PS developed a testicular tumor. Although PS should be regarded as a progressive entity, there are no indications that cognition becomes more impaired with age. No obvious genotype‐phenotype correlation is present. A subgroup of patients with ZBTB20 variants may be associated with mild, nonspecific ID. Metabolic investigations suggest a disturbed mitochondrial fatty acid oxidation. We suggest a regular surveillance in all adult males with PS until it is clear whether or not there is a truly elevated risk of testicular cancer.
Keywords: alpha‐fetoprotein, ectopic calcifications, overgrowth, Primrose syndrome, ZBTB20
1. INTRODUCTION
Primrose syndrome (PS; MIM# 259050) is an infrequently described condition characterized by increased postnatal growth in height and head circumference, unusual facial features (frontal bossing, deeply set eyes, down‐slanting palpebral fissures), cognitive deficit associated with autism spectrum disorder, and ectopic calcifications. 1 With age, distal muscle atrophy, hearing loss, cataract, sparse body hair, and a disturbed glucose metabolism can become clear.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Until recently most reported affected individuals have been adults as the phenotype may become more easily recognizable over time.
PS is mostly caused by de novo heterozygous missense variants in the N‐terminal portion of the DNA binding domain of ZBTB20 (MIM* 606025), a transcriptional repressor. 10 Two patients carrying truncating variants or small deletions have also been reported.6, 14 The protein is a member of the broad complex tramtrack bric‐a‐brac (BTB) zinc‐finger (ZnF) family and is characterized by an N‐terminal BTB domain that is involved in protein‐protein interaction, and five C2H2 zinc fingers at the C‐terminus mediating protein binding to regulatory sites within promoters of target genes.19, 20, 21, 22 ZBTB20 acts as a regulator of neurogenesis, fetal liver development, somatic growth, detoxification and glucose metabolism.23, 24, 25 Thus far, all ZBTB20 variants causing PS have been missense variants that affect amino acid residues in the first and second ZnF motifs.10, 11
Here we summarized the collective data from 42 patients with PS, 13 of whom have not been reported before, present the clinical, biochemical and molecular characteristics, and emphasize their evolution over time.
2. SUBJECTS AND METHODS
2.1. Subjects
The present series were gathered by contacting authors who have previously published on PS or because collaborators contacted one of us (RCH) because of his experience with PS. Data were collected through a table specifically designed for the study (Supplemental data Table S1). Clinical pictures, results of formal testing of cognitive development, and results of biochemical tests were also gathered. No biochemical or genetic studies were performed specifically for the present study. We gathered data from 29 patients reported previously1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and 13 hitherto unpublished patients. One stillbirth was also included. Intellectual disability (ID) was classified as mild/moderate‐severe based on neuropsychological consultations; IQ scores were included if available. The study was approved by the Medical Ethics Committee of the Amsterdam UMC (NL45451.018.13).
2.2. Molecular analyses
Molecular studies were performed either by whole‐exome sequencing (WES) using a panel aimed at detecting variants in genes known to cause ID if mutated, or by Sanger sequencing. In 32 patients, a ZBTB20 variant was detected using panel sequencing for ID, after which the clinical diagnosis was established. In four patients, the diagnosis was clinically based and the ZBTB20 variant was subsequently detected by Sanger sequencing. In one patient, the diagnosis was established based on SNP array. In five patients reported in literature, no information on methods of molecular analysis was available (all these patients showed normal karyotype).
3. RESULTS
The study included 22 males and 20 females, varying in age between 9 months and 49 years. The mean and the median age at diagnosis were 17.3 ± 15.4 years and 11.0 ± 15.4 years. The main clinical characteristics of the study participants are summarized in Tables 1 and 2 and illustrated in Figures 1 and 2 and Figure S1. The data in the tables are shown separately for children (0‐16 years) and adults (>16 years). Detailed information for each patient is available in Table S1, see Supplement. In the text, only data for which information is not reported in the tables are discussed. Single patient number is indicated between brackets if specific findings are mentioned.
TABLE 1.
Growth, development, and behavior in the 42 individuals with Primrose syndrome
| Children | Adults | All | |
|---|---|---|---|
| n = 29 | n = 13 | n = 42 | |
| Growth at birth | |||
| Length (cm) | 49.7 ± 3.60 | ||
| Length > 2SD | 1/22 | ||
| Weight (kg) | 3.19 ± 0.64 | ||
| Weight > 2SD | 3/29 | ||
| Head circumference (cm) | 35.91 ± 2.25 | ||
| Head circumference > 2SD | 9/22 | ||
| Postnatal growth | |||
| Mean age at last clinical evaluation (y) | 7.74 ± 4.22 | 37.38 ± 10.34 | 17.80 ± 15.6 |
| Height (cm) | 125.83 ± 29.33 | 177.50 ± 10.71 | |
| Height > 2SD | 3/25 (12%) | 0/9 (0%) | 3/34 (9%) |
| Weight (kg) | 31.91 ± 22.45 | 72.80 ± 16.09 | |
| Weight > 2SD | 6/25 (24%) | 0/6 (0%) | 6/31 (19%) |
| Head circumference (cm) | 54.71 ± 3.69 | 58.75 ± 2.46 | |
| Head circumference > 2SD | 21/26 (81%) | 8/12 (67%) | 29/38 (76%) |
| Development | |||
| Intellectual disability mild | 5/27 (19%) | 2/13 (15%) | 7/39 (18%) |
| Intellectual disability moderate‐severe | 22/27 (81%) | 11/13 (85%) | 33/39 (85%) |
| Behavior | |||
| Autism | 16/24 (67%) | 4/9 (44%) | 20/33 (61%) |
| Self‐injurious behavior | 7/19 (37%) | 4/7 (57%) | 11/26 (42%) |
| Sleep disturbances | 8/19 (42%) | 2/7 (29%) | 10/26 (38%) |
Note: Only data of at term born newborns (38‐42 wk) have been used.
TABLE 2.
Clinical features of the 42 individuals with Primrose syndrome
| HPO ID | Children | Adults | All | |
|---|---|---|---|---|
| Clinical sign | N = 29 | N = 13 | N = 42 | |
| Morphology | ||||
| Brachycephaly | 0000248 | 8/18 (44%) | 5/9 (56%) | 13/27 (48%) |
| Frontal bossing | 0002007 | 15/20 (75%) | 7/9 (78%) | 22/29 (76%) |
| Ptosis | 0000508 | 10/18 (56%) | 10/10 (100%) | 20/28 (71%) |
| Downslanted palpebral fissures | 0000494 | 11/22 (50%) | 7/12 (58%) | 18/34 (53%) |
| Deeply set eyes | 0000490 | 16/21 (76%) | 10/11 (91%) | 26/32 (81%) |
| Highly arched palate | 0002705 | 7/17 (41%) | 2/6 (33%) | 9/23 (39%) |
| Torus palatinus | 189 700 | 1/16 (6%) | 6/11 (55%) | 7/27 (26%) |
| Large jaw | 0040309 | 8/17 (47%) | 8/11 (73%) | 16/28 (57%) |
| Large ears | 0000400 | 14/25 (56%) | 10/11 (91%) | 24/36 (67%) |
| Calcification of ears | 0005103 | 2/17 (12%) | 12/12 (100%) | 14/28 (50%) |
| Neuromuscular findings | ||||
| Seizures | 0001250 | 2/20 (10%) | 4/9 (44%) | 6/29 (21%) |
| Ataxia | 0001251 | 6/18 (33%) | 2/5 (40%) | 8/23 (35%) |
| Hypotonia | 0001252 | 21/25 (84%) | 5/9 (56%) | 26/34 (76%) |
| Distal muscle wasting | 0003693 | 1/22 (5%) | 11/11100%) | 12/33 (36%) |
| Flexion contractures | 0001371 | 5/24 (21%) | 8/8 (100%) | 13/31 (42%) |
| Delayed myelination | 0012448 | 1/23 (4%) | 2/11 (18%) | 3/34 (9%) |
| Brain calcification | 0002514 | 3/23 (13%) | 1/11 (9%) | 4/34 (12%) |
| Corpus callosum anomaly | 0001273 | 11/23 (48%) | 4/11 (36%) | 15/34 (44%) |
| System involvement | ||||
| Cataract | 0000518 | 0/20 (0%) | 6/10 (60%) | 6/30 (20%) |
| Strabismus | 0000486 | 10/21 (48%) | 0/10 (0%) | 10/31 (32%) |
| Hearing loss | 0000365 | 21/27 (78%) | 12/13 (92%) | 33/40 (83%) |
| Scoliosis | 0002650 | 9/23 (39%) | 6/10 (60%) | 15/33 (45%) |
| Cystic bone lesions | 0012062 | 0/9 (0%) | 5/9 (56%) | 5/18 (28%) |
| Decreased BMD | 0004349 | 3/8 (38%) | 6/9 (67%) | 9/17 (53%) |
| Hip dysplasia | 0001385 | 1/17 (6%) | 4/8 (50%) | 5/25 (20%) |
| Thin nail | 0001816 | 6/20 (30%) | 4/7 (57%) | 10/27 (37%) |
| Sparse body hair | 0002231 | 11/12 (92%) | 11/12 (92%) | |
| Delayed puberty | 0000823 | 3/11 (27%) | 3/11 (27%) | |
| Cryptorchidism | 0000028 | 5/10 (50%) | 2/6 (33%) | 7/16 (44%) |
| Tumors | 0002664 | 0/15 (0%) | 2/9 (22%) | 2/24 (8%) |
| Diabetes mellitus | 0000819 | 2/16 (13%) | 6/9 (67%) | 8/25 (32%) |
| Anemia | 0001903 | 4/16 (25%) | 1/5 (20%) | 5/21 (24%) |
| Elevated serum AFP levels | 0006254 | 4/11 (36%) | 5/7 (71%) | 9/18 (50%) |
Abbreviations: AFP, alpha‐fetp protein; BMD, bone mineral density; HPO ID, human phenotype ontology identifier.
FIGURE 1.
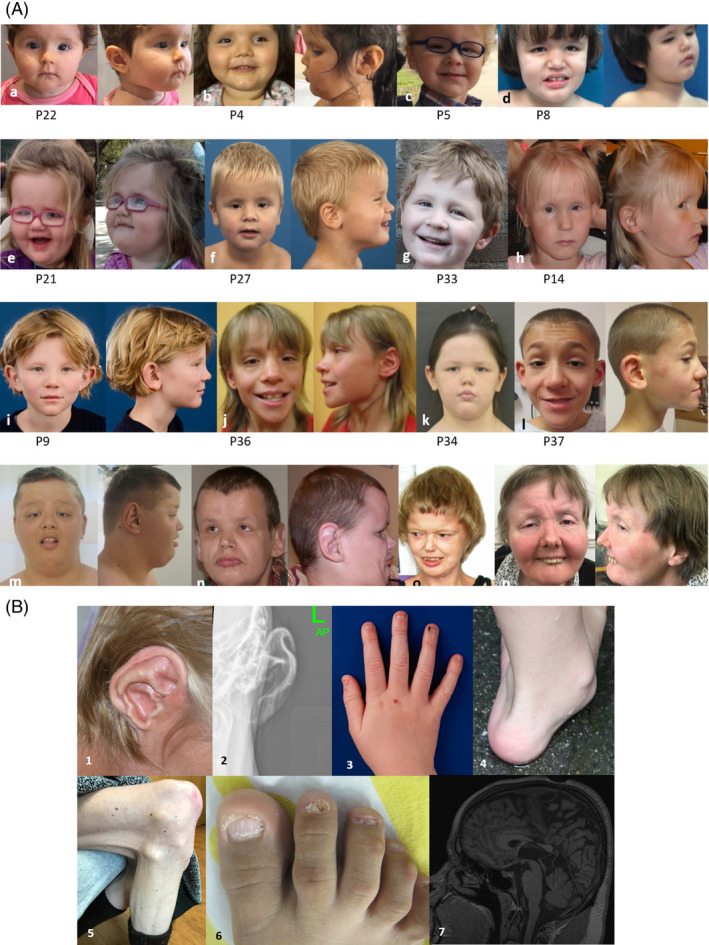
Features from selected individuals with Primrose syndrome. (A) Faces from youngest to oldest at age 1.5 years (A), 2.5 years (B), 3 years (C), 4 years (D), 4 years (E), 5 years (F), 6 years (G), 8 years (H), 9 years (I), 11 years (J), 12 years (K), 13 years (L), 18 years (M), 31 years (N), 33 years (O), and 53 years (P). The patient identification number is indicated underneath the panels. (B) Other clinical features include alobar calcified ear (1), calcified ear on X‐ray (2), incomplete extension of fingers and small nails (3), joint hypermobility (4), distal muscle wasting in an adult (5), markedly small and thin nails (6), and malformed callosal body (7) [Colour figure can be viewed at wileyonlinelibrary.com]
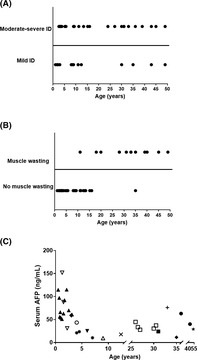
FIGURE 2.
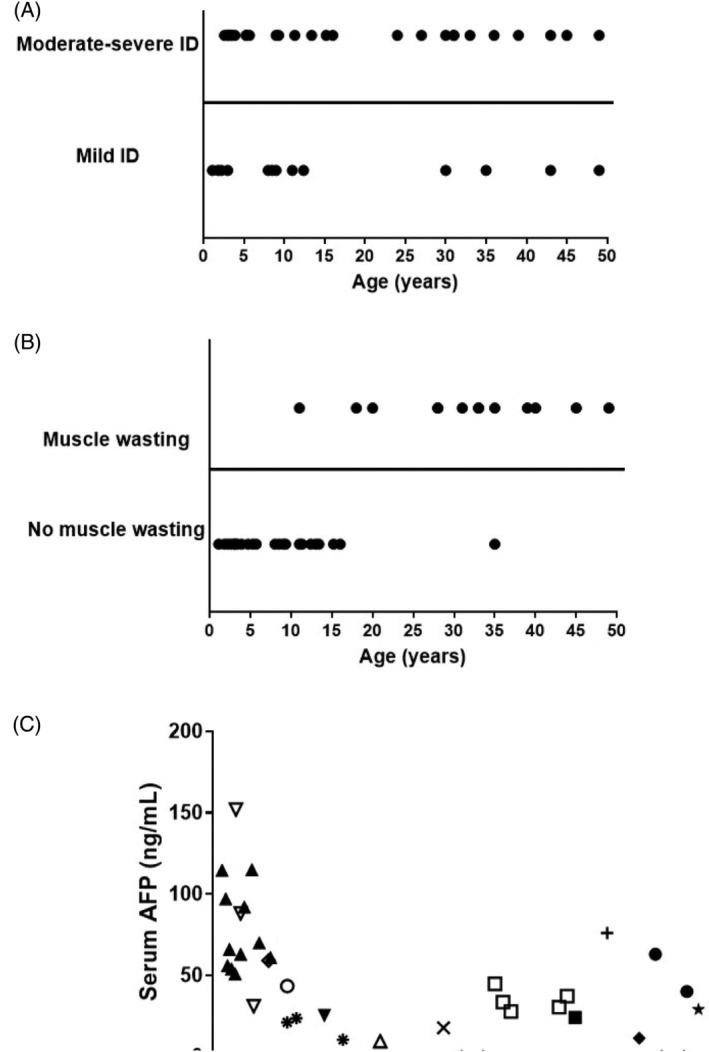
Changes with age of cognition, muscle wasting, and serum alpha‐fetoprotein (AFP) in individuals with Primrose syndrome. A, Cognition. No evident correlation. B, Muscle wasting; data are presented based on age of first appearance. Increase with age evident. C, AFP serum levels. Each symbol represents a single individual; course over time in single patients is depicted if available. Elevated levels in almost every individual; no clear change with age in a single individual
3.1. Growth
The mean duration of pregnancy was 38.8 ± 2.0 weeks. Three pregnancies (P5, P21, and P28) were complicated by oligohydramnios, one pregnancy resulted in intrauterine demise (P28). Postnatal growth in height and weight is usually between the 50th and 90th centile but in males sometimes is >98th centile (Supplemental Figure [Link], [Link]).
3.2. Development and behavior
IQ score was available in seven patients (P7, P9, P21, P33‐36; six children, IQ 25‐77, one adult, IQ 25). Infrequent findings included attention deficit hyperactivity disorder (ADHD) (P9, P36, P37) and delayed speech (P7, P8). One child showed hyperphagia (P32), one adult patient also showed schizophrenia (P42). Patients' intellectual function seems not to change over time (Figure 2A). Insufficient data were available to evaluate reliably whether behavioral problems were progressive with time or not.
3.3. Morphological signs
No morphological sign is present in all affected individuals (Table 2), and the phenotype is variable indeed (Figure 1). Infrequently reported findings included cleft palate (P37, P38) and short philtrum (P21, P22, P31, P34, and P35). Four children also showed hypertrichosis (P2, P17, P28, and P29).
3.4. Neuromuscular findings
Muscle wasting was first noticed at age 11 years and shows a clear increase with age (Table 2; Figure 2B). A muscle biopsy was available in patient 23 only, which demonstrated neurogenic atrophy. Contractures were first noticed at age 10 years and became more prominent with age as well. Hypertonia probably due to spasticity, was present in two patients (P25, P42) and was recognized first in adulthood. Infrequent findings included joint hypermobility of the upper limbs (P17, P26, P30), and Chiari malformation (P30).
3.5. Systemic findings
Someother findings show a difference in occurrence with age as well (Table 2), although some can occur at an early age as well. Dysplastic hip joint changes, cystic bone lesions, and cataract were found only in adults. Infrequent reported findings included reduced tear production (P2, P13), microphthalmia (P25, P38), unilateral blindness due to glaucoma (P2), kyphosis (P20, P32, P39), hyperlordosis (P32), pectus abnormalities (P10, P14), pulmonary artery stenosis in an adult patient (P16), small penis (P37), hypothyroidism (P3, P5, P18), and GH deficiency (P10, P37). Baseline adrenal cortex hormones were also checked in four patients, with normal results. Alpha‐fetoprotein (AFP) levels showed that levels were typically elevated but not in all affected individuals, and did not show a marked change over time (Figure 2C). One patient showed selective IgG2 deficiency (P31). 18 One patient developed a testis carcinoma at 27 years and a (fatal) seminoma in the other testis at 40 years of age 5 Another male developed a germ cell tumor at 28 years and also a seminoma at that age. 9
3.6. Metabolic investigations
Plasma amino acids were investigated in nine patients and tested normal. Plasma acylcarnitines were available in four patients showing increased C2, C4OH, C5OH, C6OH, C14, and C14:2 levels in two of them. Mild ketonuria was found in four patients, and two of these four (P14, P21) also showed mild dicarboxylic aciduria, together with increased ethylmalonic acid and glutaric acid excretion.
3.7. Molecular testing
ZBTB20 variants for all reported individuals are tabulated in Table 3, and depicted in Figure 3. None was present in the public database gnomAD (Table S2). All variants were either missense changes or insertion/deletions, acting as a frameshift, and have been classified as class 4 and class 5 according to the criteria of the American College of Medical Genetics. No variants were detected in the BTB site or in the distal part of the ZnF_C2H2 site in individuals with a phenotype‐fitting PS. No indications for mosaicism were detected in any patient. In all patients in whom one or both parents were available (n = 26), the variant was found to be de novo. No familial occurrence has been reported. Mean paternal age at birth was 33.9 ± 7.5 years; mean maternal age at birth was 30.3 ± 4.9.
TABLE 3.
Molecular characteristics of the 42 individuals with Primrose syndrome, compared to the major clinical manifestations
| Patient | Variant type | Nucleotide change | Protein change | Macrocephaly | Moderate/severe ID | Autism | Self‐injurious behavior | Distal muscle wasting | Cystic bone lesions | Cataract | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age | |||||||||||
| 1 | 0.9 y | Missense | c.626A>G | p.Gly209Arg | − | − | + | + | − | − | − | Current study |
| 2 | 32 y | Missense | c.1739G>A | p.Cys580Tyr | − | + | + | + | + | n.a. | + | Current study |
| 3 | 3 y | Missense | c.1749C>G | p.Cys583Trp | − | + | − | − | − | n.a. | n.a. | Cleaver et al 16 |
| 4 | 4.7 y | Missense | c.1760 T>C | p.Phe587Ser | + | + | − | + | − | − | − | Current study |
| 5 | 3 y | Missense | c.1766C>A | p.Ala589Asp | n.a. | + | + | − | − | − | − | Current study |
| 6 | 35 y | Missense | c.1768A>C | p.Lys590Gln | + | − | − | − | n.a. | − | − | Posmyjk et al 2011 8 |
| 7 | 31 y | Missense | c.1771C>G | p.Gln591Glu | + | + | + | + | + | + | − | Mathijssen et al 5 |
| 8 | 9 y | Missense | c.1787A>Gc.2002G>A | p.His596Argp.Gly668Arg | + | + | + | − | + | n.a. | − | Casertano et al 12 |
| 9 | 9 y | Missense | c.1794C>G | p.Phe598Leu | + | − | − | − | − | − | − | Current study |
| 10 | 15.2 y | Missense | c.1800C>G | p.His600Gln | + | + | n.a. | − | − | − | − | Grimsdottir et al 2018 15 |
| 11 | 49 y | Missense | c.1802C>T | p.Thr601Ile | − | − | − | + | + | + | − | Cordeddu et al 10 |
| 12 | 45 y | Missense | c.1805G>C | p.Gly602Ala | + | + | − | − | + | − | − | Cordeddu et al 10 |
| 13 | 2.2 y | Missense | c.1811A>C | p.Lys604Thr | + | n.a. | + | − | − | − | − | Cordeddu et al 10 |
| 14 | 5.3 y | Missense | c.1813C>T | p.Pro605Ser | − | + | n.a. | + | − | n.a. | − | Current study |
| 15 | 2.6 y | Missense | c.1822C>T | p.Cys608Arg | + | + | − | − | − | n.a. | n.a. | Ferreira et al 17 |
| 16 | 16 y | Missense | c.1832G>A | p.Cys611Tyr | + | + | + | − | − | n.a. | − | Alby et al 13 |
| 17 | 11 y | Missense | c.1837C>T | p.Arg613Cys | + | − | + | − | − | n.a. | − | Alby et al 13 |
| 18 | 5.3 y | Missense | c.1847C>Tc.2221G>A | p.Ser616Phep.Gly741Arg | + | + | + | + | − | n.a. | − | Mattioli et al 11 |
| 19 | Missense | c.1850 T>C | p.Leu617Ser | + | + | − | + | − | n.a. | n.a. | Cleaver et al 16 | |
| 20 | 30 y | Missense | c.1861C>T | p.Leu621Phe | + | n.a. | − | − | + | − | − | Carvalho et al 7 |
| 21 | 3.1 y | Missense | c.1869G>C | p.Lys623Asn | + | + | + | + | − | − | − | Casertano et al 12 |
| 22 | 1.1 y | Missense | c.1871A>C | p.His624Pro | + | n.a. | + | − | − | − | − | Current study |
| 23 | 2.5 y | Missense | c.1873A>G | p.Met625Val | + | − | n.a. | − | − | n.a. | − | Current study |
| 24 | 27 y | Missense | c.1873A>G | p.Met625Val | − | + | + | − | + | n.a. | n.a. | Ferreira et al 17 |
| 25 | 49 y | Missense | c.1876G>A | p.Val626Met | n.a. | + | n.a. | n.a. | + | + | n.a. | Battisti et al 4 |
| 26 | 8 y | Missense | c.1879A>G | p.Thr627Ala | − | n.a. | − | − | − | n.a. | − | Cleaver et al 16 |
| 27 | 9.3 y | Missense | c.1898C>T | p.Ala633Val | + | + | + | n.a. | − | n.a. | − | Current study |
| 28 | IUD | Missense | c.1906 T>C | p.Cys636Arg | − | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Alby et al 13 |
| 29 | 3.4 y | Missense | c.1931C>T | p.Thr644Ile | + | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Stellacci et al 14 |
| 30 | 11.3 y | Missense | c.1943C>T | p.Ser648Phe | + | + | − | − | − | n.a. | n.a. | Cleaver et al 16 |
| 31 | 6 y | Missense | c.1945C>T | p.Leu649Phe | − | + | + | n.a. | − | n.a. | − | Yamamoto‐Shimojima et al 25 |
| 32 | 13.4 y | Missense | c.1967A>G | p.His656Arg | + | + | − | + | − | n.a. | n.a. | Cleaver et al 16 |
| 33 | 5.7 y | Insertion/deletion | c.1203del | p.Asp401fsGlufs*26 | + | + | + | − | − | n.a. | − | Current study |
| 34 | 12.4 y | Insertion/deletion | c.1844_1846del | p.615_616del | + | − | + | − | − | − | n.a. | Current study |
| 35 | 8.5 y | Insertion/deletion | c.1024delC | p.Gln342Serfs*42 | + | − | n.a. | − | n.a. | n.a. | n.a. | Stellacci et al 14 |
| 36 | 11 y | Insertion/deletion | c.1568delC | p.Pro523fs | − | − | − | − | − | n.a. | n.a. | Current study |
| 37 | 13 y | Insertion/deletion | c.1568delC | p.Pro523fs | + | − | + | − | − | n.a. | n.a. | Current study |
| 38 | 53 y | Insertion/deletion | Del11rs12275693– rs1442927 | − | + | − | + | + | + | + |
Dalal et al 6 |
|
| 39 | 31 y | n.a. | n.a. | n.a. | + | + | n.a. | n.a. | + | n.a. | + | Liebrecht et al 9 |
| 40 | 33 y | n.a. | n.a. | n.a. | + | + | n.a. | n.a. | + | + | + | Primrose 1 |
| 41 | 39 y | n.a. | n.a. | n.a. | + | + | n.a. | n.a. | + | + | + | Collacott et al 2 |
| 42 | 43 y | n.a. | n.a. | n.a. | − | n.a. | n.a. | n.a. | + | − | + | Lindor et al 3 |
Note: + present; − absent; n.a. not available.
Abbreviation: IUD, intrauterine demise.
FIGURE 3.
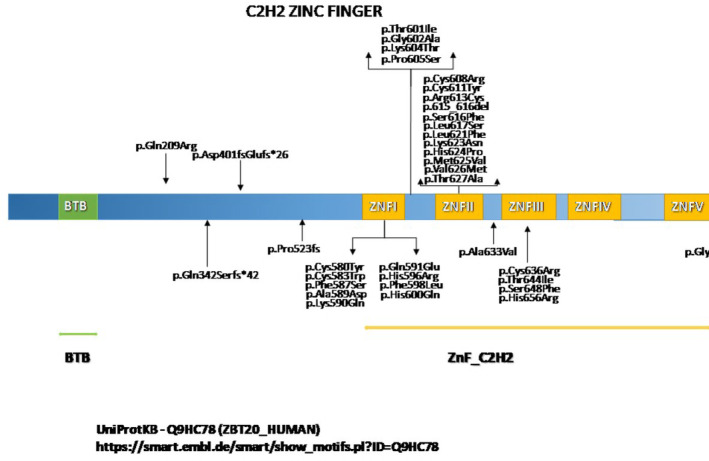
Schematic overview of the ZBTB gene and localization of mutations. It is noteworthy that patient carrying p.Gln209Arg mutation showed no macrocephaly and no ID. Autism and self‐injurious behavior were recorded [Colour figure can be viewed at wileyonlinelibrary.com]
3.8. Genotype‐phenotype correlation
The genotype was available for 38 patients (Table 3). Obviously the phenotype in the four patients reported before the causative gene was found, was more severe due to ascertainment bias. No clear genotype‐ phenotype correlation was detected. Some individuals with a variant in exon 1 (P6: c.1768A>C; P9: c.1794C>G) and in exon 5 (P34: c.1844_1846del; P19: c.1861C>T) showed a less severe ID, and some also only a limited number of the other characteristics of PS. However, other patients carrying variants in nearby base pairs showed the classical phenotype. The difference in age of the affected individuals and the progressive nature of the findings further hamper to correlate phenotype and genotype reliably.
4. DISCUSSION
We present a series of hitherto unpublished individuals with PS and summarize the findings of these individuals and those that have been reported in literature. The present study confirms that PS can present as an overgrowth syndrome with respect to brain growth (71%), but increased growth in height and weight is less marked and present in a minority of the patients (21%). Indeed, some females grow below the third centile for height and weight. The growth pattern is already present at birth and the subsequent overgrowth is non‐progressive.
The cardinal findings of PS are the ID (mild 16%, moderate‐severe 84%), mildly increased growth (height and weight between 50th and 90th centile, macrocrania 78%), and as most characteristic signs the calcified external ears, sparse body hair, bone dysplasia, and distal muscle wasting. The calcification of the ears, cataract, torus palatinus, cystic bone lesions and muscle wasting with subsequently contracture formation are clearly age‐related and become often only apparent in puberty or thereafter, so percentages differ in the various age groups. Cognition does not seem to decline with age, although sufficiently detailed studies to conclude this with certainty are missing. Hearing loss is also common both in children and adults, mostly presenting as sensorineural hearing loss.
The progression in signs and symptoms with age may point to a metabolic disturbance. Biochemically, unexplained anemia, disturbed glucose metabolism, and increased AFP levels are cardinal features of PS. Further metabolic investigations demonstrated abnormal acylcarnitine and urine organic acid profiles in some PS individuals, including increased excretion of dicarboxylic acids, ethylmalonic and glutaric acids. In one individual (P8), this pattern became more abnormal with age. Over time, this patient showed progressive lipodystrophy and developed muscle wasting with limb atrophy by 11 years of age; at that time, Oral Glucose Tolerance test also showed impaired glucose tolerance. The findings suggest disruption of the mitochondrial fatty acid oxidation. One may speculate that this is linked to pleiotropic effects of ZBTB20 on lipid and glucose metabolism.19, 25 Mitochondrial dysfunction has been reported in Zbtb20 knock‐out mouse. 26 Mitochondrial dysfunction has also been involved in the development of muscle atrophy 27 and insulin‐resistance, 28 type 2 diabetes, 29 and cataract, 30 but at the present, there is no proof that these signs can be explained in PS individuals due to mitochondrial malfunctioning. More detailed analyses of mitochondrial functioning are warranted.
Increased AFP levels constitute a remarkable sign in PS. It has been proposed that mutated ZBTB20 disrupts the AFP repression resulting in AFP increase and overgrowth. AFP levels appeared >2 SD higher than reference values by age 31 during the first months of life and progressively decreased with age. Among the presently reported males, two adults developed a testis tumor. No female developed neoplasm. Despite reports of ZBTB20 expression being associated with tumorigenesis, including gastric cancer 21 and hepatocellular carcinoma, 32 it remains unclear whether an increased risk of malignancies is part of this syndrome.
To evaluate whether ZBTB20 variants are more common in men with testicular germ cell tumor (TGCT), we interrogated WES data from lymphocyte‐derived DNA from 919 TGCT cases of Western European ancestry (comprising 306 familial and 613 unselected TGCT cases) and 1609 healthy controls of Western European ancestry from the UK 1958 Birth Cohort, all analyzed via the same pipeline.33, 34 We compared between TGCT cases and healthy controls, the frequency of high quality, rare (minor allele frequency [MAF] < 0.01) non‐synonymous variants. In the TGCT series, three rare non‐synonymous ZBTB20 variants [p.(Thr514Ala), p.(Ala693Val), and p.(Gly712Val)] were identified in the constitutional DNA of men with familial TGCT and one in a man with non‐familial TGCT [p.(Gly712Val)]. These men developed their seminoma or teratoma at ages 28, 28, 32, and 33 years, respectively. No further data regarding serum biomarkers or clinical phenotype were available for these patients. No rare non‐synonymous ZBTB20 variants were detected in 1609 healthy controls. Paired tumor germline WES data were available for an additional 179 TGCT cases: no ZBTB20 variants were detected in the constitutional or tumor DNA. 35 Thus, the frequency of germline ZBTB20 mutation in TGCT cases would appear elevated (4/1098 in cases, 0/1609 in controls, P exact < .05). Still, the absolute risk of TGCT is low (1 in 200 in Western European males, lower in other ethnicities) and TGCT typically has an excellent outcome. 36 The two males with PS who developed testicular tumors have died because of their tumors. There is no recognized protocol for TGCT surveillance established as effective for subpopulations at significant elevation of risk (such as family history, prior contralateral disease, or cryptorchidism). In addition, self‐examination is not feasible in most men with PS. Accordingly, families and other caregivers of men with PS should be alerted to the possible modest elevation in relative risk of TGCT, reassured as to the low absolute risk, and advised regarding symptom awareness and testicular examination by caregivers.
There is no evident genotype‐phenotype correlation in the present series. However, numbers are small, and it may still be that if a larger series can be evaluated this will become clear.
A dominant‐negative effect of missense variants has been previously hypothesized. 10 Cleaver et al provided very limited information on an individual with a de novo c.505G>C [p.(Glu169Gln)] variant in whom pathogenicity remained uncertain, presumably because the phenotype did not resemble PS. 16 We follow a patient (not included in the present series) with ZBTB20 variant c.1775A>G [p.(Asn519Ser)] detected by WES because of unexplained mild ID. This adult woman, age 39 years, has macrocephaly but otherwise none of the characteristic signs or symptoms of PS is present. She did show short stature and an unusual face. No other potentially pathogenic variants have been detected by WES, and the ZBTB20 variant is absent in her parents. It remains uncertain whether the variant is pathogenic. If so, it indicates that ZBTB20 variants can lead to ID and brain anomalies without the other characteristics of PS. In this respect, it may be of interest that two individuals with nearby located variants (P6: c.1768A>C; P9: c.1794C>G) show a relatively mild phenotype with less severe ID as well. Data suggest that patients with frameshift variants may show a milder phenotype. However, the small number of patients and limited data hamper reliable conclusions on genotype‐phenotype correlations.
A major limitation of the present study is its retrospective nature. Early clinical data were sometimes lacking as the clinical suspicion for PS raised later in life. Additionally, several patients came to the attention of a physician only as adults, hampering a complete early clinical history.
We conclude that PS is an established clinical entity that is recognizable in adults but more difficult to recognize in infants and children. In a clinically suspicious child checking the AFP levels can be useful. The manifestations are progressive, and repeated evaluation for anemia, diabetes, and osteoporosis are indicated. At the present, there is no clear indication that cognition shows a decline with time as well. There may be an increased risk to develop testis tumors, and regular follow‐up for this from puberty onward seems indicated.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Supplemental Table S1 Individual data of presently reported individuals with Primrose syndrome.
Supplemental Table S2 ZBTB20 new variants
Supplemental Figure 1 Growth pattern in length and weight of boys (A) and girls (B) and in head circumference in boys (C) and girls (D) of present series of individuals with Primrose syndrome.
Supplemental Figure 2 (A) Height SDS, (B) Weight SDS and (C) Head circumference SDS in children (black circle) and adults (gray square) with Primrose syndrome
ACKNOWLEDGEMENTS
This work is generated within the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability (ERN‐ITHACA). A special thanks to patients and their families.
Melis D, Carvalho D, Barbaro‐Dieber T, et al. Primrose syndrome: Characterization of the phenotype in 42 patients. Clin Genet. 2020;97:890–901. 10.1111/cge.13749
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article
REFERENCES
- 1. Primrose DA. A slowly progressive degenerative condition characterized by mental deficiency, wasting of limb musculature and bone abnormalities, including ossification of the pinnae. J Ment Defic Res. 1982;26(Pt 2):101‐106. [DOI] [PubMed] [Google Scholar]
- 2. Collacott RA, O'Malley BP, Young ID. The syndrome of mental handicap, cataracts, muscle wasting and skeletal abnormalities: report of a second case. J Ment Defic Res. 1986;30:301‐308. [DOI] [PubMed] [Google Scholar]
- 3. Lindor NM, Hoffman AD, Primrose DA. A neuropsychiatric disorder associated with dense calcification of the external ears and distal muscle wasting: Primrose syndrome. Clin Dysmorphol. 1996;5:27‐34. [DOI] [PubMed] [Google Scholar]
- 4. Battisti C, Dotti MT, Cerase A, et al. The Primrose syndrome with progressive neurological involvement and cerebral calcification. J Neurol. 2002;249:1466‐1468. [DOI] [PubMed] [Google Scholar]
- 5. Mathijssen IB, van Hasselt‐van der Velde J, Hennekam RC. Testicular cancer in a patient with Primrose syndrome. Eur J Med Genet. 2006;49:127‐133. [DOI] [PubMed] [Google Scholar]
- 6. Dalal P, Leslie ND, Lindor NM, Giulbert DL, Espay AJ. Motor tics, stereotypies, and self‐flagellation in Primrose syndrome. Neurology. 2010;75:284‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carvalho DR, Speck‐Martins CE. 2011. Additional features of unique Primrose syndrome , Additional features of unique Primrose syndrome phenotype phenotype. Am J Med Genet A 155A:1379–1383. [DOI] [PubMed] [Google Scholar]
- 8. Posmyk R, Leśniewicz R, Chorąży M, Wołczyński S. New case of Primrose syndrome with mild intellectual disability. Am J Med Genet A. 2011;155A (11):2838‐2840. [DOI] [PubMed] [Google Scholar]
- 9. Liebrecht D, Daumer‐Haas C, Braun T, Hann von Weyhern C. Primrose syndrome with testicular cancer: case report and review of the literature. Eur Soc Hum Genet Abstract. 2012;P02:209. [Google Scholar]
- 10. Cordeddu V, Redeker B, Stellacci E, et al. Mutations in ZBTB20 cause Primrose syndrome. Nat Genet. 2014;46:815‐817. [DOI] [PubMed] [Google Scholar]
- 11. Mattioli F, Piton A, Gerard B, Superti‐Fuga A, Mandel JL, Unger S. Novel de novo mutations in ZBTB20 in Primrose syndrome with congenital hypothyroidism. Am J Med Genet A. 2016;170A:1626‐1629. [DOI] [PubMed] [Google Scholar]
- 12. Casertano A, Fontana P, Hennekam RC, et al. Alterations in metabolic patterns have a key role in diagnosis and progression of Primrose syndrome. Am J Med Genet A. 2017;173A:1896‐1902. [DOI] [PubMed] [Google Scholar]
- 13. Alby C, Boutaud L, Bessières B, et al. Novel de novo ZBTB20 mutations in three cases with Primrose syndrome and constant corpus callosum anomalies. Am J Med Genet A. 2018;176A:1091‐1098. [DOI] [PubMed] [Google Scholar]
- 14. Stellacci E, Steindl K, Joset P, et al. Clinical and functional characterization of two novel ZBTB20 mutations causing Primrose syndrome. Hum Mutat. 2018;39:959‐964. [DOI] [PubMed] [Google Scholar]
- 15. Grímsdóttir S, Hove HB, Kreiborg S, et al. Novel de novo mutation in ZBTB20 in Primrose syndrome in boy with short stature. Clin Dysmorphol. 2019;28(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 16. Cleaver R, Berg J, Craft E, et al. Refining the Primrose syndrome phenotype: a study of five patients with ZBTB20 de novo variants and a review of the literature. Am J Med Genet A. 2019;179A:344‐349. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira LD, Borges‐Medeiros RL, Thies J, Schnur RE, Lam C, de Oliveira JRM. Expansion of the Primrose syndrome phenotype through the comparative analysis of two new case reports with ZBTB20 variants. Am J Med Genet A. 2019;179A:2228‐2232. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto‐Shimojima K, Imaizumi T, Akagawa H, Kanno H, Yamamoto T. Primrose syndrome associated with unclassified immunodeficiency and a novel ZBTB20 mutation. Am J Med Genet A. 2020;182(3):521‐526. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Xie Z, Zhou L, et al. The zinc finger protein ZBTB20 regulates transcription of fructose‐1,6‐bisphosphatase 1 and β cell function in mice. Gastroenterology. 2012;142:1571‐1580. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, Cao D, Zhou L, et al. ZBTB20 is a sequence‐specific transcriptional repressor of alpha‐fetoprotein gene. Sci Rep. 2015;5:11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Zhou X, Zhang M, Cheng L, Zhang Y, Wang X. ZBTB20 promotes cell migration and invasion of gastric cancer by inhibiting IκBα to induce NF‐κB activation. Artif Cells Nanomed Biotechnol. 2019;47:3862‐3872. [DOI] [PubMed] [Google Scholar]
- 22. Zhou G, Jiang X, Zhang H, et al. Zbtb20 regulates the terminal differentiation of hypertrophic chondrocytes via repression of Sox9. Development. 2015;142:385‐393. [DOI] [PubMed] [Google Scholar]
- 23. Zhang W, Mi J, Li N, et al. Identification and characterization of DPZF, a novel human BTB/POZ zinc finger protein sharing homology to BCL‐6. Biochem Biophys Res Commun. 2001;282:1067‐1073. [DOI] [PubMed] [Google Scholar]
- 24. Mitchelmore C, Kjaerulff KM, Pedersen HC, et al. Characterization of two novel nuclear BTB/POZ domain zinc finger isoforms. Association with differentiation of hippocampal neurons, cerebellar granule cells, and macroglia. J Biol Chem. 2002;277:7598‐7609. [DOI] [PubMed] [Google Scholar]
- 25. Sutherland AP, Zhang H, Zhang Y, et al. Zinc finger protein Zbtb20 is essential for postnatal survival and glucose homeostasis. Mol Cell Biol. 2009;29:2804‐2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasmussen MB, Nielsen JV, Lourenço CM, et al. Neurodevelopmental disorders associated with dosage imbalance of ZBTB20 correlate with the morbidity spectrum of ZBTB20 candidate target genes. J Med Genet. 2014;51:605‐613. [DOI] [PubMed] [Google Scholar]
- 27. Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinti MV, Fink GK, Hathaway QA, Durr AJ, Kunovac A, Hollander JM. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ‐based analysis. Am J Physiol Endocrinol Metab. 2019;316(2):E268‐E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jarrett SG, Lewin AS, Boulton ME. The importance of mitochondria in age‐related and inherited eye disorders. Ophthalmic Res. 2010;44:179‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blohm ME, Vesterling‐Hörner D, Calaminus G, Göbel U. Alpha 1‐fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol. 1998;15:135‐142. [DOI] [PubMed] [Google Scholar]
- 32. Kan H, Huang Y, Li X, Liu D, Chen J, Shu M. Zinc finger protein ZBTB20 is an independent prognostic marker and promotes tumor growth of human hepatocellular carcinoma by repressing FoxO1. Oncotarget. 2016;7:14336‐14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Litchfield K, Levy M, Dudakia D, et al. Rare disruptive mutations in ciliary function genes contribute to testicular cancer susceptibility. Nat Commun. 2016;7:13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Litchfield K, Loveday C, Levy M, et al. Large‐scale sequencing of testicular germ cell tumour (TGCT) cases excludes major TGCT predisposition gene. Eur Urol. 2018;73:828‐831. [DOI] [PubMed] [Google Scholar]
- 35. Litchfield K, Summersgill B, Yost S, et al. Whole‐exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat Commun. 2015;6:5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Litchfield K, Levy M, Huddart RA, Shipley J, Turnbull C. The genomic landscape of testicular germ cell tumours: from susceptibility to treatment. Nat Rev Urol. 2016;13:409‐419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 Individual data of presently reported individuals with Primrose syndrome.
Supplemental Table S2 ZBTB20 new variants
Supplemental Figure 1 Growth pattern in length and weight of boys (A) and girls (B) and in head circumference in boys (C) and girls (D) of present series of individuals with Primrose syndrome.
Supplemental Figure 2 (A) Height SDS, (B) Weight SDS and (C) Head circumference SDS in children (black circle) and adults (gray square) with Primrose syndrome
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article


