Abstract
Currently, there are no established pharmaceutical strategies that effectively treat social deficits in autism spectrum disorder (ASD). Oxytocin, a neurohormone that plays a role in multiple types of social behaviors, has been proposed as a possible therapeutic against social impairment and other symptoms in ASD. However, from the standpoint of pharmacotherapy, oxytocin has several liabilities as a standard clinical treatment, including rapid metabolism, low brain penetrance, and activity at the vasopressin (antidiuretic hormone) receptors. The present studies describe findings from a preclinical screening program to evaluate oxytocin receptor (OXTR) agonists and oxytocin metabolites for potential clinical use as more optimal treatments. We first investigated two synthetic oxytocin analogs, TC-OT-39 and carbetocin, using in vitro cell-based assays for pharmacological characterization and behavioral tests in the BALB/cByJ mouse model of ASD-like social deficits. Although both TC-OT-39 and carbetocin selectively activate the OXTR, neither synthetic agonist had prosocial efficacy in the BALB/cByJ model. We next evaluated two oxytocin metabolites: OT(4-9) and OT(5-9). While OT(5-9) failed to affect social deficits, the metabolite OT(4-9) led to significant social preference in the BALB/cByJ model, in a dose-dependent manner. The increased sociability was observed at both 24 hr and 12 days following the end of a subchronic regimen with OT(4-9) (2.0 mg/kg). Overall, these results suggest that the prosocial effects of oxytocin could be mediated by downstream activity of oxytocin metabolites, raising the possibility of new pathways to target for drug discovery relevant to ASD.
1. Introduction
The cyclic nonapeptide hormone oxytocin was first recognized for its role in the control of uterine contractions during parturition and was later shown to be important for mother-infant bonding (Gimpl and Fahrenholz, 2001). An ever-growing body of work demonstrates that oxytocin also has complex roles in controlling a wide range of socially relevant behaviors and that disruption of oxytocinergic systems may contribute to neuropsychiatric disorders with social impairment, including autism spectrum disorder (ASD), schizophrenia (Romano et al., 2015), and anxiety disorders (Neumann and Slattery, 2016). The roles of oxytocin in controlling neural processes, specifically those related to sociability, have led to the investigation of its clinical utility in treating mental health conditions (Bowen and Neumann, 2017; Feifel et al., 2016; Martinetz and Neumann, 2016; Neumann and Slattery, 2016; Rich and Caldwell, 2015). The strong interest in the therapeutic potential of oxytocin is highlighted by numerous clinical studies examining the effects of single use and extended exposure to oxytocin on social deficits in ASD, schizophrenia, and other disorders.
The initial reports of oxytocin’s clinical effects on ASD symptoms demonstrated that intravenous infusion of oxytocin reduced repetitive behaviors and improved social cognition in adult ASD subjects (Hollander et al., 2007; Hollander et al., 2003). More recent studies have utilized the less invasive route of intranasal administration for oxytocin treatment. An overview of randomized controlled trials of intranasal oxytocin in ASD shows the most notable benefits include increased emotion recognition, enhanced social cognition, and greater social direction of eye gaze (Preti et al., 2014). Studies with ASD subjects have correlated improvements following acute, single-dose oxytocin treatment with increased neuronal activity in the prefrontal cortex (Aoki et al., 2015; Watanabe et al., 2014) and the right anterior insular cortex (Aoki et al., 2014). Similar behavioral and functional effects have also been observed after five- and six-week oxytocin regimens (Watanabe et al., 2015; Yatawara et al., 2016). In addition to ASD, oxytocin has been shown to have therapeutic efficacy in schizophrenia (Feifel et al., 2010; Gibson et al., 2014; Guastella et al., 2015; Pedersen et al., 2011), and is being evaluated as a possible intervention in several other disorders (Cochran et al., 2013). However, across these published clinical studies, oxytocin treatment generally has small-to-moderate effect sizes and occasionally fails to exert any positive benefits (Bakermans-Kranenburg and van IJzendoorn, 2013). One explanation for the equivocal results may be the patient population. For example, in a recent report, children with ASD with the lowest pretreatment oxytocin levels had the greatest response to oxytocin treatment (Parker et al., 2017). Overall, the results from these initial clinical studies make it clear that further research is required to realize oxytocin’s unique potential as a treatment for social deficits and other symptoms associated with neuropsychiatric disorders.
One major limitation concerning the use of oxytocin as an intervention is its poor pharmacokinetic properties. Since oxytocin is not orally available, clinical studies have typically used intravenous or intranasal routes of administration. Independent of administration route, oxytocin is metabolically unstable and appears to follow a two-compartment disposition model, with the predominant component exhibiting a very short, 5-15 min half-life in humans and rodents (Gonser, 1995; Kang and Park, 2000; Mens et al., 1983; Morin et al., 2008; Nielsen et al., 2017; Seitchik et al., 1984; Tanaka et al., 2018). Although intranasal administration can lead to peak CSF and plasma oxytocin levels of two-to-five fold over basal levels, this route can have delivery-dependent effects (Dal Monte et al., 2014; Kirkpatrick et al., 2014; Modi et al., 2014), such as activation of only a limited, regionally-specific subset of oxytocin receptors (OXTRs) (Ferris et al., 2015). Thus, it is unclear if the maximum effects of targeting the oxytocinergic pathway are being realized with current strategies. Further, oxytocin can have significant activity at the arginine vasopressin receptors, indicating a potential for unwanted effects, including vasoconstriction. Small-molecule compounds and oxytocin derivatives have the potential to overcome the pharmacokinetic and selectivity limitations, and one class of non-peptide, drug-like small molecules that function as OXTR agonists has been reported (Pitt et al., 2004). It is notable that the rapid enzymatic degradation associated with oxytocin leads to biologically-active fragments of the nonapeptide (Burbach et al. 1983). Ring and colleagues (2010) have proposed that these oxytocin metabolites might also have beneficial effects, exerted through mechanisms independent from action at the OXTR, raising the intriguing possibility of an alternate mechanism of oxytocin’s effects and an additional signaling pathway to target for drug discovery.
Mouse models of ASD-like behavior can be utilized as preclinical screens to identify new pharmaceutical agents with prosocial efficacy. Previously, our research group demonstrated that peripheral administration of oxytocin reduced social deficits in two inbred mouse strains (BALB/cByJ and C58/J) and one genetically-engineered line (Grin1 knockdown) (Teng et al., 2016; Teng et al., 2013) that model ASD-like phenotypes. In these studies, a subchronic regimen of oxytocin led to increased sociability up to two weeks following the end of treatment, with effects observed in both adolescent and adult mice, and in both sexes (Teng et al., 2016; Teng et al., 2013). Interestingly, in the Cntnap2 knockout mouse model of autism, daily intranasal administration of oxytocin to preweaning mice (postnatal days 7 through 21) can lead to higher levels of sociability several days after the final treatment (Penagarikano et al., 2015). Overall, these findings show that oxytocin has prosocial efficacy in mouse models with divergent genetic and behavioral profiles, suggesting the possibility of therapeutic benefits generalizable across a spectrum of developmental disorders.
In the present study, we evaluated two synthetic OXTR agonists, TC-OT-39 (Pitt et al., 2004) and carbetocin (Gimpl et al., 2005; Manning et al., 2008), in cell-based assays for pharmacological action and specificity. These compounds were then further examined for behavioral efficacy in the BALB/cByJ mouse model. The OXTR agonists were first screened for acute effects in a marble-burying assay. Next, we determined whether these synthetic OXTR agonists enhanced social behavior following subchronic administration in the ASD mouse model. In addition, In addition, as mentioned above, there is evidence that oxytocin, a nonapeptide (i.e. OT(1-9)), is a precursor to smaller neuropeptides that are active in brain (Burbach et al., 1983; Burbach et al., 1980; de Wied et al., 1987). there is evidence that oxytocin, a nonapeptide (i.e. OT(1-9)), is a precursor to smaller neuropeptides that are active in brain (Burbach et al., 1983; Burbach et al., 1980; de Wied et al., 1987). In particular, the fragments OT(4-9) and OT(5-9) have been found following proteolytic conversion in synaptic membranes from rat forebrain and midbrain (Burbach and Lebouille, 1983). These same oxytocin fragments had greater potency than oxytocin itself on specific aspects of avoidance learning in rats (de Wied et al., 1987). Further, in a comparison of several oxytocin-derived peptides, OT(4-9) had oxytocin-like effects on social recognition in rats, dependent on dose (Popik et al., 1996). Thus, we also evaluated the OT(4-9) and OT(5-9) metabolites for efficacy against social deficits in BALB/cByJ mice.
2. Methods and materials
2.1. Materials for cell-based assays
All reagents were ACS reagent grade and used without further purification. Oxytocin (Bachem, Torrance, CA), vasopressin (Sigma), and carbetocin (Bachem) were purchased in the powder form. (2S)-N-[[4-[(4,10-Dihydro-1-methylpyrazolo[3,4-b][1,5]benzodiazepin-5(1H)-yl)carbonyl]-2-methylphenyl]methyl]-2-[(hexahydro-4-methyl-1H-1,4-diazepin-1-yl)thioxomethyl]-1-pyrrolidinecarboxamide (Pitt et al., 2004) (TC-OT-39) was synthesized by the Center for Integrative Chemical Biology and Drug Discovery at UNC-CH. Screen Quest™ Fluo-8 No Wash Calcium Assay Kit was purchased from ABD Bioquest (Sunnyvale, CA). CHO cells stably transfected with the human oxytocin receptor (CHO-OXTR), human vasopressin Avpr1a receptor (CHO-V1a), human vasopressin Avpr1b receptor (CHO-V1b), or human vasopressin Avpr2 receptor (CHO-V2), and CHO wild type cells were kindly provided by the NIMH Psychoactive Drug Screening Program at UNC-CH. Reagents used for cell culture were purchased from Gibco-Invitrogen. The PathHunter™ CHO-K1 OXTR β-Arrestin Cell Line, PathHunter™ Detection Kit, and the PathHunter™ eXpress β-Arrestin GPCR Assays were kindly provided by DiscoveRx (Fremont, CA). CHO-OXTR and CHO-V1aR were grown in Hams F-12 media supplemented with 400 μg/ml geneticin sulfate (G-418), 10% calf serum, 15 mM HEPES, and 50 U of penicillin/ 50 μg of streptomycin. CHO-V1bR and CHO-V2R cells were grown in Hams F-12, 150 μg/ml zeocine, 10% calf serum, 15 mM HEPES, and 50 U of penicillin/ 50 μg of streptomycin. Wild-type CHO cells were grown in DMEM supplemented with 10% fetal bovine serum, and 50 U of penicillin/50 μg of streptomycin.
2.2. IP3 accumulation assay
A scintillation proximity assay was used to measure [3H]-inositol phosphate accumulation. CHO-OXTR cells were plated (30,000 cells/well) in uncoated 96-well clear-bottom black-walled microplates and incubated for 24 h in 100 μl of media. On day 2, media was replaced by 100 μl of inositol-free basal medium eagle (BME) (supplemented with 5% dialyzed FBS and 50 U of penicillin/ 50 μg of streptomycin) and incubated for 1.5 h at 37 °C. Medium was removed and 100 μl of inositol-free BME containing 5% dialyzed FBS and 0.01 μCi/pl [3H]-myo-inositol was added and cells were incubated for an additional 18 h at 37 °C. The inositol-free BME was aspirated from the cells, replaced with 100 μl of varying concentrations of either TC-OT-39 or oxytocin diluted in working buffer (HBSS, 11 mM dextrose, 35 mM LiCl, and 0.2% sodium bicarbonate), and incubated for 1 h at 37 °C. The assay was terminated by aspiration of the drug solutions from the cells and addition of 30 μl of 50 mM formic acid followed by incubation for 1 h at room temperature. RNA binding YSi SPA beads (Amersham, CA) were diluted to 2.67 mg/ml in cold H2O and 75 μl of the bead slurry was dispensed to the wells of a new plate and kept on ice. The formic acid supernatant (30 μl) was added to the bead-containing plate followed by agitation at 4 °C for 30 min. The beads were allowed to settle at 4 °C for 4 h and then counted in a Wallac Micro Beta Trilux scintillation counter (Perkin Elmer, Waltham, MA). Data were analyzed as described for the calcium mobilization assay (below).
2.3. Fluorescence-based intracellular calcium mobilization assay
2.3.1. Dye preparation
Screen Quest ™ Fluo-8 No Wash Calcium Assay Kit (Fluo8-NW) dye-loading solution was prepared according to the manufacturer’s instructions. The Fluo-8 NW stock solution was made by adding 200 μl DMSO into component A (Fluo-8NW) and mixing well. The 1x assay buffer consisted of 10 ml of 10x Pluronic acid, F127 Plus (component B), 90 ml of 1X HBSS and 1ml of Tryptan red dye. The Fluo-8 NW dye-loading solution for one cell plate was made by adding 20 μl of DMSO reconstituted Fluo-8 NW stock solution into 10 ml of 1x assay buffer, mixing them well. This work solution was stable for at least 2 h at RT avoiding light.
2.3.2. Calcium mobilization assay
A FLIPRTETRA® system (Molecular Devices, Sunnyvale, CA) was used to read fluorescence (excitation wavelength: 470-495 nm, emission wavelength: 515-575 nm) in each well every 1 s for 30 s, to establish a baseline reading. After this period, the FLIPRTETRA® transferred 10 μl of the 5x compound solution from the drug plate to the cell plate. Readings were made every 1 s for 5 min. Data was collected using ScreenWorks™ 2.0.0.22 software (Molecular Devices) and analyzed using Graph Pad Prism 5 for Windows. Each kinetic trace was normalized to the initial fluorescence intensity to correct for loading of the cells, and it was reported as % normalized activation. This parameter was calculated as (sample value – negative control value) / (OT max control value – negative control value).
2.4. Chemiluminescence-based intracellular enzyme fragment complementation assay
The PathHunter CHO-K1 OXTR β-Arrestin cell line was grown in media that consisted of Hams F-12, 300 μg/ml hygromycin, 800 μg/ml geneticin sulfate (G418), 10% fetal bovine serum, and 1x penicillin/streptomycin/glutamine, following the manufacturer’s instructions. Cells were seeded at 20,000 cells/ well in 90 μl of complete medium in white-walled, clear-bottomed, 96-well plates and incubated overnight at 37 °C and 5% CO2. The cells provided in the PathHunter™ eXpress β-Arrestin GPCR Kit were handled as described in the kit instructions. For the assay, cells were incubated with 10 μl per well of the compounds for 90 min at 37 °C. Detection reagent (50 μl) was added to each well to incubate at RT for 60 min. Cell plates were read on an Envision standard luminescence plate reader (PerkinElmer, Boston, MA). For the dose-response curves, oxytocin, TC-OT-39, carbetocin, and vasopressin were dissolved in DMSO to a concentration of 1 mM. A dose response curve of 12 points was run for each compound with concentrations starting at 50 μM; each concentration was prepared as a 10x solution using 1x HBSS as vehicle. Data were analyzed using Graph Pad Prism 5 for Windows. Each kinetic trace was normalized to the initial chemiluminescent intensity to correct for loading of the cells, and reported as % normalized activation. This parameter was calculated as (sample value – negative control value) / (oxytocin max control value – negative control value).
2.5. Animals
Male BALB/cByJ mice (3–5 weeks old) were purchased from Jackson Laboratory (JAX; Bar Harbor, ME). An additional 32 C57BL/6J mice (16 males and 16 females; JAX) were used to investigate oxytocin effects on marble-burying. Mice were maintained in groups of 2-4 animals per polycarbonate mouse cage, in a room under a 12-hr light/dark cycle (lights off at 7 pm). All animal care and procedures were conducted in strict compliance with the animal welfare policies set by the National Institutes of Health and by the University of North Carolina at Chapel Hill (UNC), and were approved by the UNC Institutional Animal Care and Use Committee.
2.6. Compounds for in vivo testing
Oxytocin (Bachem, Torrance, CA) and the two oxytocin metabolites, [pGlu4,Cyt6]OT(4-9) (pGlu-Asn-Cyt-Pro-Leu-Gly-NH2) and [Cyt6]OT(5-9) (Asn-Cyt-Pro-Leu-Gly-NH2) (Biomatik, Wilmington, DE), were dissolved in saline containing 0.002% glacial acetic acid. TC-OT-39 (synthesized by the UNC Center for Integrative Chemical Biology and Drug Discovery) was dissolved in 12% DMSO and 4% Tween-20 in saline. Carbetocin (Bachem) was dissolved in saline. All injections were administered IP (intraperitoneal) in a volume of 10.0 ml/kg. Experimenters conducting the behavioral tests were blind to drug treatments.
2.7. Treatment and behavior regimens to evaluate prosocial efficacy
2.7.1. Subchronic treatment regimen
Separate sets of male BALB/cByJ mice (6-12 per treatment/dose group) were used to test each compound for effects on sociability in a 3-chamber choice test (described below). The subchronic regimen was initiated when mice were age 5-6 weeks. Mice were given 4 IP injections of vehicle or drug across 8-9 days, with at least 48 hr between each injection (i.e. mice were injected on sequential weekdays WFMW or WFTTh). Prosocial drug effects were evaluated in the 3-chamber choice test up to 14 days following the end of the subchronic regimen, with a maximum of 2 tests per mouse.
2.7.2. Three-chamber social choice test
Social approach was assessed in a 3-chamber Plexiglas box (procedure modified from (Moy et al., 2007)). The test started with a 10-min habituation phase, with free exploration of the empty test box, followed by a 10-min test for sociability. During the sociability phase, the test mouse was given a choice between an unfamiliar stranger mouse (a male C57BL/6J adult), contained in a cage placed in one side chamber, or an empty cage in the opposite side chamber. Measures were taken of the time spent in each chamber and number of entries into each chamber, either by photobeam counts or by an image tracking system (Ethovision, Noldus Information Technology, Wageningen, the Netherlands). Measures were also taken of either time spent sniffing each cage (coded by a human observer) or time spent in 5 cm proximity to each cage (by the image tracking system).
2.8. Behavioral screen for acute drug effects on marble-burying
2.8.1. Acute treatment regimen
Separate sets of mice (age 7-9 weeks at the start of testing) were used to test each compound for acute effects on marble burying. Compounds were administered 50 min before the start of each test. This pretreatment time was selected on the basis of pilot testing with oxytocin. A repeated-test design was used, with 1 test per week. Order of treatment (vehicle and dose of the selected compound) was balanced across tests.
2.8.2. Marble-burying assay
Mice were placed in individual polycarbonate mouse cages, set inside sound-attenuating chambers with a ceiling light and fan. The cages contained 5 cm deep clean corncob bedding, with 20 black glass marbles (14 mm diameter) arranged in an equidistant 5 × 4 array on top of the bedding. Measures were taken of the number of marbles covered 2/3 or more by the bedding after a 30 min test.
2.9. Open field test for activity and exploration
2.9.1. Acute treatment regimen
BALB/cByJ male mice (age 6-7 weeks at the start of testing) were used to determine the time course of oxytocin (1.0 mg/kg) and OT(4-9) (2.0 mg/kg) effects on activity in an open field. Doses were selected as having comparable efficacy on sociability in the 3-chamber choice test. Compounds were administered immediately before the start of each test, in order to examine the onset and duration of effects across 2 hr. A repeated-test design was used, with each mouse receiving 3 tests, 1 test per week. Order of treatment (vehicle, oxytocin, or OT(4-9)) was balanced across tests.
2.9.2. Open field procedure
Activity was assessed in photocell-equipped automated open fields (41 cm × 41 cm × 30 cm; Versamax System, AccuScan Instruments, Columbus, OH), located inside sound-attenuating chambers with a ceiling light and fan. Measures were taken of total distance traveled, rearing movements, and time spent in the center region of the chamber, for each 2-hr test.
2.10. Statistical analysis for in vivo assays
Data were analyzed with one-way or repeated measures analysis of variance (ANOVA), with factors treatment or dose (dependent on experiment), using Statview software (SAS, Cary, NC). Repeated measures were side of social test box or treatment (for marble-burying or open field measures). Within-treatment repeated measures ANOVAs were used to determine social preference. Fisher's protected least-significant difference (PLSD) tests were used for comparing group means only when a significant F value was determined by ANOVA. Significance was set at p<0.05.
3. Results
3.1. Comparative pharmacology of oxytocin and TC-OT-39
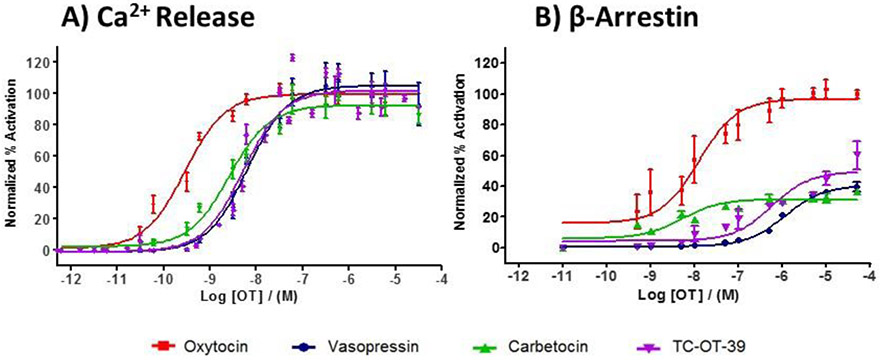
TC-OT-39 is representative of one class of small-molecule agonists of OXTR. TC-OT-39 is reported to exhibit modest selectivity for activation of OXTR (EC50 = 33 nM, full agonist in a gene reporter assay; EC50 = 667 nM, partial agonist in IP3 accumulation) when compared to other members of the oxytocin/vasopressin receptor subtypes (Pitt et al., 2004). Additionally, TC-OT-39 is reported to be an antagonist of the Avpr1a vasopressin receptor (KI = 330 nM (Frantz et al., 2010)). To confirm these previous reports, we determined that TC-OT-39 is a moderately potent full agonist at the human OXTR (EC50 180 ± 45 nM) compared to 0.4 ± 0.2 nM for OT in a fluorescence-based intracellular calcium mobilization assay (Table 1) with equivalent maximal efficacy (97 ± 3%) as oxytocin. In addition, TC-OT-39 was selective (24-fold) for the human OXTR versus the human Avpr1b vasopressin receptor (EC50 = 4300 ± 70 nM) but with reduced efficacy (74 ± 2%) compared to vasopressin. TC-OT-39 did not activate the human Avpr1a or Avpr2 receptors, and appears to be an inverse agonist at Avpr1a (Supplemental Fig. 1), consistent with reported activity. Exposure of wild-type CHO cells did not induce any response, indicating that TC-OT-39 is not a non-specific Ca2+- releasing agent (Supplemental Fig. 2).
Table 1.
Oxytocin receptor activity of TC-OT-39 and carbetocin.
| FLIPRTETRA® Ca2+ Release |
DiscoveRx β-Arrestin |
DiscoveRx Express β-Arrestin |
|
|---|---|---|---|
| EC50 (nM)a | |||
| Oxytocin | 0.30 ± 0.09 | 23 ± 22 | 44.7 |
| TC-OT-39 | 5 ± 1 | 310 ± 260 | 480 |
| Carbetocin | 3 ± 1 | 5 ± 2 | 5.3 |
| Vasopressin | 5 ± 2 | 1600 ± 900 | 2680 |
| Efficacy (% OXT Response)b | |||
| TC-OT-39 | 102 | 51 ± 6 | 48 |
| Carbetocin | 92 | 32 ± 5 | 32 |
| Vasopressin | 105 | 41 ± 6 | 38 |
Potencies and efficacies of the DiscoveRx Express cell lines were run as single points only.
Efficacies were calculated considering the oxytocin response as 100%. The DiscoveRx Express refers to the PathHunter™ eXpress β-Arrestin GPCR Kits using the assay-ready, frozen PathHunter™ eXpress cells available through the manufacturer.
To confirm activation of the human OXTR, we measured the release of IP3, which is a specific downstream second messenger of the Gq coupled pathway. Oxytocin and TC-OT-39 demonstrated EC50 values of 4 ± 1 nM and 890 ± 90 nM, respectively in IP3 accumulation assay, and the maximum efficacy of TC-OT-39 compared to oxytocin was 67 ± 4%. Further investigation of the pharmacology of TC-OT-39, involving a broad screen for receptor binding utilizing the complete set of GPCRs available through the NIMH PDSP, showed modest (Ki > 1 μM) binding to muscarinic acetylcholine receptors M2, M3, M4, and M5 and no significant binding to other human receptors.
Because GPCRs can signal through both the canonical G-protein and β-arrestin pathways (Whalen et al., 2011), we also determined the ability of OXTR ligands TC-OT-39, carbetocin and vasopressin to recruit β-arrestin to OXTR using an intracellular enzyme fragment complementation assay. Representative concentration-dependent curves were obtained using the FLIPRTETRA® platform for Gq signaling and the PathHunter™ eXpress β-Arrestin GPCR Kit for β-arrestin signaling (Fig. 1 and Table 1). The dose-response curves showed significant differences among the OXTR agonists, as only oxytocin was a full agonist of β-arrestin recruitment. Together, these data provide evidence that TC-OT-39 and carbetocin are moderately selective OXTR agonists with efficacy comparable to that of oxytocin for Gq signaling but reduced efficacy for β-arrestin recruitment. The Gq selectivity observed for carbetocin is consistent with another report (Passoni et al., 2016), though lower β-arrestin activity was observed for carbetocin in the previously reported BRET-sensor assay for β-arrestin recruitment than in the enzyme fragment complementation assay reported here.
Figure 1. Dose response curves for oxytocin, vasopressin, carbetocin, and TC-OT-39 in Ca2+ release and β-arrestin recruitment assays.
A) Activity of agonists in a Gq-coupled Ca2+ release assay using CHO cells stably expressing hOXTR. B) Activity of agonists in the PathHunter™ eXpress β-Arrestin GPCR Kit using the PathHunter™ OTR eXpress cell line.
3.2. Confirmation of persistent prosocial oxytocin effects in the BALB/cByJ model
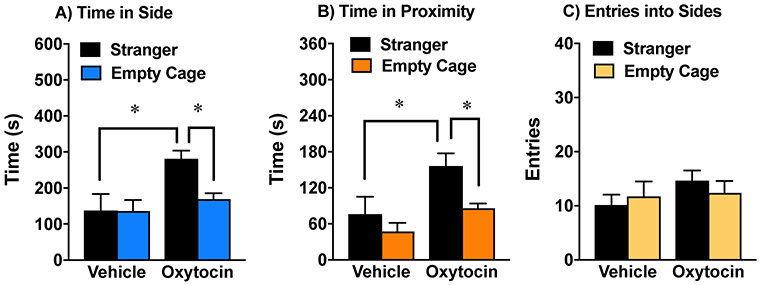
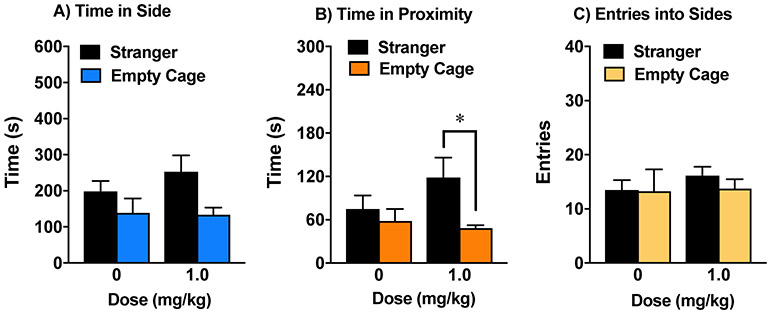
Our research group has previously shown that a subchronic regimen with oxytocin (1 mg/kg) leads to significantly enhanced sociability in BALB/cByJ mice 24 hr after the final injection (Teng et al., 2013). The present study investigated whether prosocial effects from subchronic oxytocin were still evident 48 hr post-treatment. The results indicated that vehicle-treated BALB/cByJ mice failed to demonstrate social preference in the 3-chamber test (Fig. 2A). In contrast, BALB/cByJ mice given oxytocin spent significantly more time in the side of the social test box containing the stranger mouse, versus the empty cage side. A repeated measures ANOVA on time spent in each side chamber revealed a significant main effect of treatment [F(1,14)=6.19, p=0.0261], side [F(1,14)=5.02, p=0.0418], and a treatment x side interaction [F(1,14)=4.84, p=0.0452]. In addition, the oxytocin-treated group, but not the vehicle-treated group, also showed significant preference for immediate proximity to the cage containing the stranger mouse (Fig. 2B) [within-treatment comparisons following significant main effect of treatment, F(1,14)=7.14, p=0.0182; and side, F(1,14)=8.69, p=0.0106]. Oxytocin did not alter number of entries during the test (Fig. 2C).
Figure 2. Persistent prosocial effects of oxytocin in the BALB/cByJ model.
The subchronic regimen consisted of 4 treatments with either vehicle or oxytocin (1.0 mg/kg) across an 8-9 day period. Mice were tested for sociability 48 hr after the final treatment. Data are means (+SEM) from 8 mice per group. *p<0.05.
3.3. Acute oxytocin and TC-OT-39 effects on marble burying
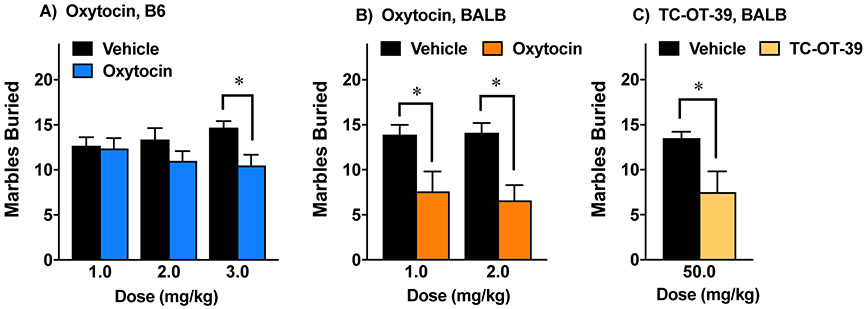
One issue with screening synthetic oxytocin agonists and other compounds for possible prosocial efficacy was the selection of appropriate dosage. We evaluated the marble-burying assay as a quick screen for OXTR agonists. Our first study used C57BL/6J mice, a strain that often serves as a background for genetic mouse models. As shown in Fig. 3A, acute treatment with oxytocin (50 min before the test) led to significant decreases in number of buried marbles in C57BL/6J mice, but only at a relatively high dose (5.0 mg/kg) [F(1,26)=9.33, p=0.0051]. BALB/cByJ proved to be more sensitive to the effects of acute oxytocin treatment (Fig. 3B), with significant decreases in marble-burying at doses of 1.0 mg/kg [F(1,14)=6.57, p=0.0225] and 2.0 mg/kg [F(1,14)=14.27, p=0.002]. TC-OT-39 led to comparable decreases in marble burying at 50 mg/kg (Fig. 3C) [F(1,14)=6.18, p=0.0261]. A higher dose of TC-OT-39 (75 mg/kg) was found to have overt sedative-like effects, and was not further tested.
Figure 3. The marble-burying assay as a screen for acute oxytocin agonist effects.
Compounds were administered 50 min before the test. C57BL/6J (B6) or BALB/cByJ (BALB) mice were given one test per week, with up to 4 tests per subject. Separate sets of BALB mice were used for oxytocin and TC-OT-39. A) Data from male and female B6 mice were combined for analysis, since overall ANOVAs at each dose did not indicate significant effects of sex. Data are means (+SEM) from 8-16 mice per group. *p<0.05.
3.4. Subchronic TC-OT-39 effects on sociability
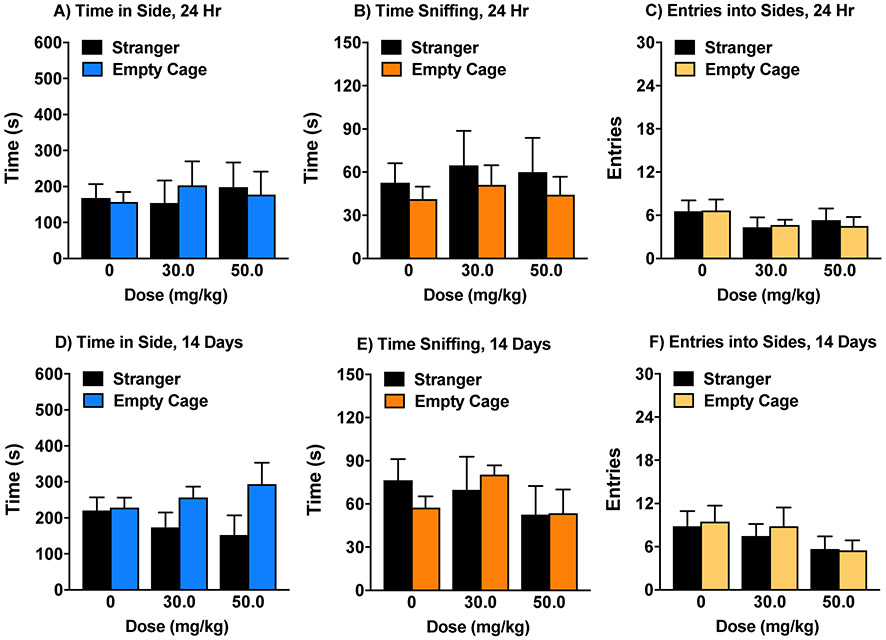
Based on the results from the marble-burying assay, a subchronic treatment regimen with TC-OT-39 (50 mg/kg) was evaluated for effects on social approach. A lower dose (30 mg/kg) was also included, to provide additional dose-response information. Mice were tested in the 3-chamber test at 2 time points, 24 hr and 14 days, after the final injection, since we had previously observed significant prosocial effects of oxytocin 2 weeks after a subchronic regimen in C58/J, another mouse model of ASD-like phenotypes (Teng et al., 2016; Teng et al., 2013). The results showed that subchronic treatment with TC-OT-39 did not have any significant effects on social approach (Fig. 4), and there was no evidence for the emergence of increased sociability at the 14-day time point.
Figure 4. Lack of TC-OT-39 effects on sociability in BALB/cByJ.
The subchronic regimen consisted of 4 treatments with either vehicle or TC-OT-39 across an 8-9 day period. Mice were tested for social approach 24 hr and 14 days following the final treatment. Data are means (+SEM) from 6-12 mice per treatment group.
3.5. Effects of carbetocin and two oxytocin metabolites on marble burying
In addition to TC-OT-39, we screened another selective OXTR agonist, carbetocin, and two oxytocin metabolites, OT(4-9) and OT(5-9), for oxytocin-like effects on digging behavior. Although carbetocin and TC-OT-39 exhibited similar activity profiles at the OXTR in cell-based assays, the two compounds had divergent effects in the marble-burying assay. We found that carbetocin, across several doses (3, 6, 10, 15, and 20 mg/kg), failed to alter the number of marbles buried (Supplemental Fig. 3A). Similarly, neither OT(4-9) nor OT(5-9) altered digging responses in the marble-burying assay (Supplemental Fig. 3B, C).
3.6. Effects of subchronic carbetocin and oxytocin metabolites on sociability
3.6.1. Lack of efficacy for carbetocin and OT(5-9)
Carbetocin resembled TC-OT-39 in the lack of effects on sociability in the 3-chamber choice test. Mice were evaluated 24 hr and 12 days following a subchronic regimen with carbetocin (6, 10, or 20 mg/kg). Even at the highest dose, carbetocin failed to have significant effects on social approach or number of side entries in the BALB/cByJ mice (Supplemental Fig. 4A-C). A similar result was found after subchronic treatment with OT(5-9), 1 mg/kg, which failed to reverse social deficits in the BALB/cByJ model (Supplemental Fig. 4D, E). However, the mice treated with OT(5-9) demonstrated a significant preference for making entries into the side with the stranger, versus the empty cage side, suggesting the possible emergence of prosocial effects [within-treatment comparisons following significant main effect of side, F(1,22)=9.29, p=0.0059] (Supplemental Fig. 4F). Further testing with a higher dose, 2.0 mg/kg, failed to reveal prosocial efficacy for the OT(5-9) metabolite (Supplemental Fig. 5).
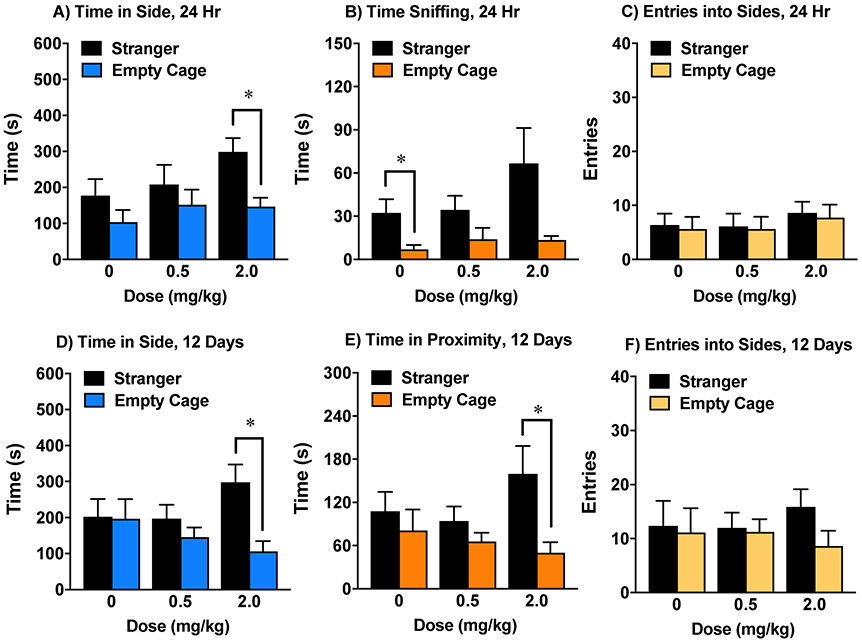
3.6.2. Dose-dependent increases in sociability following treatment with OT(4-9)
Subchronic treatment with the OT(4-9) metabolite, at a dose of 1.0 mg/kg, led to significant social preference for proximity to the stranger mouse [within-treatment comparisons following significant main effect of side, F(1,14)=6.84, p=0.0204] (Fig. 5), without changing entries during the test. Remarkably, at a higher dose (2.0 mg/kg), the prosocial effects of OT(4-9) could be observed both 24 hr and 12 days after the end of the subchronic regimen (Fig. 6). Mice given the higher dose of OT(4-9) demonstrated significant preference for spending more time in the side with the stranger mouse at both time points [within-treatment comparisons following significant main effect of side in the 24-hr test, F(1,21)=7.3, p=0.0133; and 12-day test, F(1,21)=4.85, p=0.039]. The OT(4-9) high-dose group also had significant preference for proximity to the stranger mouse 12 days following the final injection [within-treatment comparisons following significant main effect of side, F(1,21)=7.02, p=0.015].
Figure 5. Prosocial effects of OT(4-9) on time spent in proximity to each cage.
Subchronic treatment with 1.0 mg/kg led to significant preference for spending time in proximity to a stranger mouse. N=8 mice per treatment group. *p<0.05.
Figure 6. Persistent prosocial effects of OT(4-9).
The subchronic regimen consisted of 4 treatments with either vehicle or OT(4-9) across an 8-9 day period. Mice were tested 24 hr (upper panels) and 12 days (lower panels) following the final treatment. N=8 mice per treatment group. *p<0.05.
3.7. Effects of acute oxytocin and OT(4-9) on open field activity
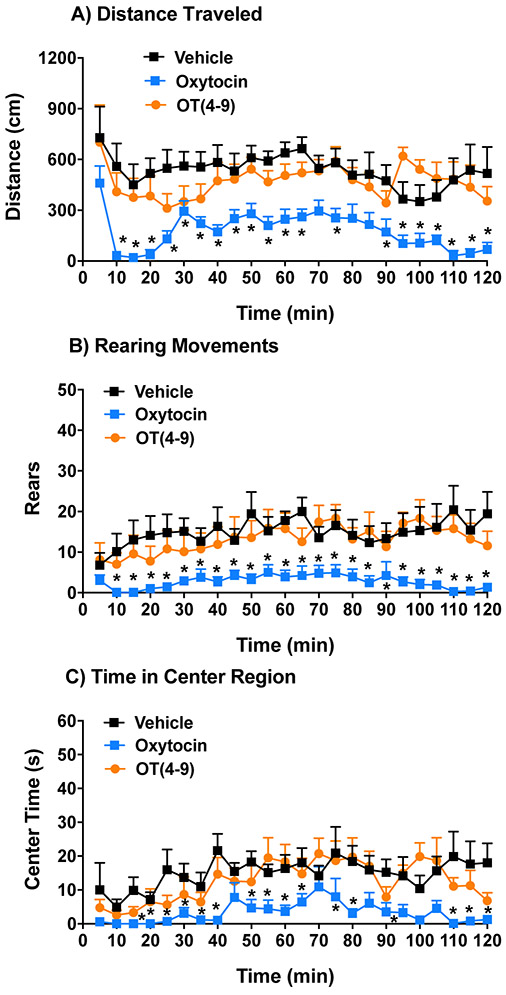
The previous results showed that a subchronic regimen of OT(4-9) (2.0 mg/kg) had prosocial efficacy similar to oxytocin (1.0 mg/kg), in contrast to the different profiles following acute administration in the marble-burying test. To further explore the divergent effects of acute treatment, we compared oxytocin and OT(4-9) in the open field test, at doses that led to increased social preference in the 3-chamber test. BALB/cByJ mice were injected immediately before placement into open field chambers, in order to determine a time course for drug effects. The results showed that acute oxytocin, but not OT(4-9), led to decreased activity across the 2-hour test (Fig. 7). The significant effects of acute oxytocin were found for measures of distance traveled [repeated measures ANOVA, main effect of treatment, F(2,16)=37.9, p<0.0001], vertical rearing movements [main effect of treatment, F(2,16)=21.0, p<0.0001], and time spent in the center region [main effect of treatment, F(2,16)=40.13, p<0.0001]. In contrast, OT(4-9), in comparison to vehicle, had no significant effects on activity or exploration in the open field, similar to the lack of effects in the marble-burying task following acute administration.
Figure 7. Decreased activity following acute treatment with oxytocin, but not OT(4-9).
Oxytocin (1.0 mg/kg), OT(4-9) (2 mg/kg), or vehicle was administered immediately before each 2-hr test in an open field. Doses were selected based on prosocial efficacy in a 3-chamber choice test. Data are means (± SEM) from 9 mice, given one test per week. *p<0.05, comparison between vehicle and oxytocin.
4. Discussion
Deficits in social behavior, a core symptom of ASD, have severe consequences for individuals with autism, their families, and society in general. Although there are no standard treatments for social impairment, oxytocin has been proposed as a therapeutic agent with unique prosocial efficacy, and is currently under evaluation in numerous clinical studies (for example, the multi-site SOARS-B clinical trial in children with ASD, http://autismcenter.duke.edu/research/study-oxytocin-autism-reciprocal-social-behaviors-soars-b). However, the pharmacological and pharmacokinetic properties of oxytocin (activity at the vasopressin receptor, limited bioavailability, and rapid metabolism) are problematic for its clinical usage. In the present study, we used a combination of cell-based and mouse phenotyping assays to study peptide and non-peptide OXTR agonists that might have prosocial efficacy. We found that TC-OT-39, a synthetic OXTR agonist (Pitt et al., 2004), had oxytocin-like effects in a marble-burying test, but did not rescue social deficits in an ASD mouse model. However, a metabolite of oxytocin, OT(4-9), had a different efficacy profile, including rescue of social deficits in a dose-dependent manner and lack of effects on marble burying or open field activity, indicating a dissociation between the acute oxytocinergic activity and effects from repeated exposures across an 8-9 day regimen. Two other compounds evaluated in these studies, the synthetic oxytocin agonist carbetocin and the metabolite OT(5-9), failed to show oxytocin-like effects in either the marble-burying assay or social approach test. Overall, these results suggest that persistent prosocial efficacy requires highly-specific pharmacological activity not broadly found across oxytocinergic compounds.
4.1. Marble-burying assay for acute oxytocin-like effects
The marble-burying assay has been used as an index of anxiety-like behavior and perseverative responses (Borsini et al., 2002; Njung'e and Handley, 1991; Thomas et al., 2009), and to evaluate drug effects in mouse models of neurodevelopmental disorders (Aguilar-Valles et al., 2015; Veeraragavan et al., 2011). A recent study (Sanathara et al., 2017) used a marble-burying test to demonstrate the effects of centrally administered oxytocin on repetitive digging responses in mice. We investigated whether the marble-burying assay would serve as a quick screen for oxytocin-like effects on behavior, informative for selecting appropriate drug doses for further testing in subchronic regimens. The initial results were promising: acute oxytocin significantly decreased marble burying in the BALB/cByJ model for ASD-like phenotypes at doses with no effects in the control C57BL/6J mouse strain. Importantly, the reduced marble burying in BALB/cByJ was observed at a dose (1.0 mg/kg) that led to enhanced sociability 24 hr (Teng et al., 2013) and 48 hr (present studies) following the end of subchronic treatment, with the caveat that this same dose had sedative-like effects in the open field test. However, our findings with the synthetic oxytocinergic compounds failed to support the utility of this assay as a screen for prosocial activity: a dose of TC-OT-39 that decreased marble burying did not have prosocial efficacy following a subchronic regimen, and carbetocin had no effects across a range of doses. Remarkably, doses of OT(4-9) that showed no activity in the marble-burying test had persistent effects, similar to oxytocin, on social approach. These results demonstrate the separate pharmacology underlying acute effects from single treatment and more long-term effects from subchronic treatment of oxytocinergic compounds.
It is notable that the lack of behavioral effects from acute carbetocin was unexpected. Previous work has shown that peripheral administration of carbetocin can have anxiolytic and antidepressant-like activity, including effects in rodent models of drug withdrawal or repeated stress exposure (Chaviaras et al., 2010; Klenerova et al., 2010; Zanos et al., 2014). At the same time, not all reports have been consistent (Feifel et al., 2012; Mak et al., 2012), and a range of different doses, pretreatment times, and behavioral tests have been utilized, making direct comparisons between studies difficult. In the present study, both oxytocin and carbetocin were administered 50 min before the start of the marble-burying assay. Since carbetocin was engineered to have an increased half-life, in comparison to oxytocin, the lack of carbetocin effects was likely not a result of the 50 min pretreatment time. However, although carbetocin has been shown to be selective for the OXTR, one study reported that carbetocin has a very different molecular profile than oxytocin, including an absence of β-arrestin recruitment to the OXTR following binding (similar to our observations), as well as action as an antagonist at the vasopressin Avpr1a and Avpr1b receptors (Passoni et al., 2016). In our studies, carbetocin functioned as a partial agonist for β-arrestin recruitment, whereas Passoni et al. did not observe any β-arrestin recruitment activity following carbetocin exposure. Possible explanations for this difference include the varying techniques for characterizing carbetocin: the present study utilized a fragment complementation strategy, while Passoni et al. employed a BRET assay. In addition, our studies had different cell lines: we used CHO cells and Passoni et al. used KEK293 cells. Despite the differences, it is clear that carbetocin has lower β-arrestin recruitment activity than oxytocin. Further studies with cells that naturally express OXTR and β-arrestin would be useful in determining the functional differences between these ligands. Overall, the divergent molecular and pharmacological characteristics found between TC-OT-39, carbetocin, and oxytocin in in vitro assays could explain the differential effects on behavior observed in the present studies, although differences in pharmacokinetics and biodistribution may also be important.
4.2. Prosocial efficacy of the OT(4-9) metabolite
Our research group has previously shown that a subchronic regimen with oxytocin can lead to persistent increases in sociability in mouse models of ASD-like behavior (Teng et al., 2016; Teng et al., 2013). This same oxytocin regimen has also been reported to increase sociability in C57BL/6J mice (Sobota et al., 2015). The present studies extended these findings by demonstrating that the oxytocin metabolite OT(4-9) had prosocial efficacy similar to oxytocin itself, with significant social preference still evident 12 days following the cessation of a subchronic treatment regimen. These effects of OT(4-9) were dose-dependent, with only the higher doses (1.0 and 2.0 mg/kg) leading to increased sociability.
The results also showed that neither oxytocin nor OT(4-9) led to alterations in entries during the 3-chamber test, indicating the prosocial effects were not due to changes in exploration or activity. In contrast, Sobota et al., 2015 found that subchronic oxytocin increased time spent exploring the center region of an open field, suggesting mice had lower levels of anxiety after oxytocin treatment. Other reports have described anxiolytic effects of acute oxytocin in the open field, light-dark preference test, and elevated zero maze (Bale et al., 2001; Blume et al., 2008; Ring et al., 2006; Yoshida et al., 2009). These findings raise the possibility that a subchronic regimen of oxytocin allows an accretion of anxiolytic effects across time, leading to adaptations in neural circuitry mediating social anxiety. Notably, long-term changes in anxiety and alcohol consumption, as well as sociability, have been reported in rats following a subchronic oxytocin regimen during adolescence (Bowen et al., 2011). However, in the present study, acute OT(4-9) lacked anxiolytic effects in the marble-burying assay and open field tests, providing evidence that increased sociability cannot simply be attributed to alterations in anxiety.
4.3. Mechanisms for oxytocin effects
OXTR is a G protein-coupled receptor (GPCR) with complex pharmacology, including signaling through different G protein and β-arrestin pathways (Busnelli et al., 2012; Grotegut et al., 2011). Our cell-based analysis of oxytocinergic compounds, combined with that of Ring et al. (2010) and Loyens et al. (2013), provides clues for possible molecular mechanisms underlying its divergent effects on behavior. In the present study, both oxytocin and TC-OT-39 led to comparable reductions in the marble-burying assay. Similarly, Ring and colleagues (2006, 2010) demonstrated that both oxytocin and WAY-267464, a non-peptide OXTR agonist similar to TC-OT-39, have comparable anxiolytic-like effects in rodent assays. These findings suggest that oxytocin, TC-OT-39, and WAY-267464 activate the same signaling pathway to modulate anxiety-like behaviors. In our cell-assays, TC-OT-39 was only a partial agonist of β-arrestin recruitment, suggesting that these acute effects were mediated by Gq/11-dependent signaling.
Neither of the OXTR agonists, TC-OT-39 or carbetocin, had prosocial efficacy, providing evidence that not all oxytocin effects are exerted through activation of the OXTR. In line with these findings, a previous study reported that a subchronic regimen of oxytocin, but not TGOT, a selective agonist of OXTR Gq and β-arrestin coupling, led to persistent increases in sociability in rats (Suraev et al., 2014). Other evidence for non-OXTR effects of oxytocin have been observed in tests for depression-like behavior. For example, Ring et al. (2010) reported that oxytocin, but not the OXTR agonist WAY-267464, could alleviate immobility during a tail-suspension test in mice (Ring et al., 2010). Further, the oxytocin effects were not blocked by pretreatment with an OXTR antagonist, leading the researchers to postulate that one of the oxytocin metabolites, working through a non-OXTR mechanism, might be the agent for the antidepressant activity. Notably, the antidepressant-like effects of oxytocin can be prevented by using pharmacological or genetic manipulations that prevent oxytocin metabolism and the subsequent formation of metabolites (Arletti and Bertolini, 1987; Gard et al., 2007; Loyens et al., 2013). An intriguing question is whether the OT(4-9) metabolite might play a role in the antidepressant action of oxytocin, in line with our findings of prosocial efficacy in the three-chamber test.
4.4. Study limitations
While our results provide evidence for differential behavioral profiles between the selected oxytocinergic probes, it is important to emphasize these are only preliminary characterizations. In particular, interpretation of these findings would be greatly enhanced by additional pharmacokinetic data on metabolism and biodistribution. In fact, a concern for these studies is whether the test compounds cross the blood-brain barrier, which is assumed to be necessary for oxytocin’s prosocial effects. Neumann et al. (2013) have provided evidence for increased oxytocin levels in brain following IP administration in mice. Further, in the present study, the observation of behavioral changes following peripheral administration of TC-OT-39, OT(4-9), and oxytocin itself suggests these agents are reaching brain. In contrast, carbetocin showed no effects in our hands, suggesting it may not cross the blood-brain barrier at high enough levels for a prosocial effect. In previous studies, intracerebroventricular administration of carbetocin was required for anxiolytic effects in a rat model (Chaviaras et al., 2010), but IP administration (6.4 mg/kg) did restore sociability in an opioid withdrawal mouse model (Zanos et al., 2014). A final limitation is that our studies were initiated with an OXTR-centric view, and there is evidence that vasopressin receptors, particularly Avpr1a and Avpr1b, also contribute to the regulation of social behavior (Bowen and McGregor, 2014; Caldwell, 2017; Ramos et al., 2013; Sala et al., 2011) and, perhaps, to the effects we observed. Overall, the studies reveal the need for further pharmacological analysis of underlying mechanisms for prosocial effects, including downstream signaling pathways following receptor activation and sites of action for the OT(4-9) metabolite, and the development of better chemical probes.
5. Conclusion
Overall, these results offer surprising insights into two pharmacodynamically distinct effects of oxytocin, allowing us to speculate that acute effects are mediated by activation of the OXTR Gq coupling, whereas separate, persistent effects on sociability require metabolic activation to OT(4-9). While future studies will help confirm these preliminary observations, the studies highlight the complexity of oxytocin effects on behavior and provide additional preclinical evidence for oxytocin-related compounds as promising new treatments for social deficits. Further investigation is needed to reveal the molecular mechanisms of oxytocin, including the OT(4-9) prosocial effect.
Supplementary Material
Highlights.
Increased sociability was observed 48 hr after a subchronic oxytocin regimen
Oxytocin and a partially Gq-selective OXTR agonist decreased marble burying
Oxytocin metabolite OT(4-9) increased sociability in an ASD mouse model
The metabolite OT(4-9) did not have oxytocin-like effects on marble burying
Two synthetic oxytocin receptor agonists failed to show prosocial effects
Acknowledgements
Support for this project was provided by the Department of Defense (AR1002312P1, AR1002312P2, and AR100231P3), the National Institute of Child Health & Human Development (U54 HD079124), the National Center for Advancing Translational Sciences (ULTR000083), and an Autism Speaks Translational Postdoctoral Fellowship to Dr. Teng (#7952). The authors wish to thank the UNC Center for Integrative Chemical Biology and Drug Discovery, the laboratory of Dr. Bryan Roth, and the NIMH Psychoactive Drug Screening Program (Contract #HHSN-271-2013-00017-C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Valles A, Matta-Camacho E, Khoutorsky A, Gkogkas C, Nader K, Lacaille JC, Sonenberg N, 2015. Inhibition of Group I Metabotropic Glutamate Receptors Reverses Autistic-Like Phenotypes Caused by Deficiency of the Translation Repressor eIF4E Binding Protein 2. J. Neurosci 35, 11125–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T, Takao H, Kawakubo Y, Kasai K, Yamasue H, 2015. Oxytocin's neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: a randomized controlled trial. Mol. Psychiatry 20, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T, Inoue H, Suga M, Takao H, Sasaki H, Gonoi W, Kunimatsu A, Kasai K, Yamasue H, 2014. Oxytocin improves behavioural and neural deficits in inferring others' social emotions in autism. Brain 137, 3073–3086. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A, 1987. Influence of protease inhibitors on the antidepressant activity of oxytocin. Neuropeptides 10, 241–248. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, 2013. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry 3, e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM, 2001. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci 21, 2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID, 2008. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci 27, 1947–1956. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D, 2002. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology 163, 121–141. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS, 2011. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PloS one 6, e27237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, McGregor IS, 2014. Oxytocin and vasopressin modulate the social response to threat: a preclinical study. The international journal of neuropsychopharmacology 17, 1621–1633. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Neumann ID, 2017. Rebalancing the Addicted Brain: Oxytocin Interference with the Neural Substrates of Addiction. Trends in neurosciences 40, 691–708. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Bohus B, Kovacs GL, Van Nispen JW, Greven HM, De Wied D, 1983. Oxytocin is a precursor of potent behaviourally active neuropeptides. Eur. J. Pharmacol 94, 125–131. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Lebouille JL, 1983. Proteolytic conversion of arginine-vasopressin and oxytocin by brain synaptic membranes. Characterization of formed peptides and mechanisms of proteolysis. J. Biol. Chem 258, 1487–1494. [PubMed] [Google Scholar]
- Burbach JP, Schotman P, de Kloet ER, 1980. Oxytocin biotransformation in the rat limbic brain: chemical characterization of two oxytocin fragments and proposed pathway for oxytocin conversion. Biochem. Biophys. Res. Commun 97, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Sauliere A, Manning M, Bouvier M, Gales C, Chini B, 2012. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J. Biol. Chem 287, 3617–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, 2017. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist 23, 517–528. [DOI] [PubMed] [Google Scholar]
- Chaviaras S, Mak P, Ralph D, Krishnan L, Broadbear JH, 2010. Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using a modification of the forced swimming test. Psychopharmacology 210, 35–43. [DOI] [PubMed] [Google Scholar]
- Cochran DM, Fallon D, Hill M, Frazier JA, 2013. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv. Rev. Psychiatry 21, 219–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB, 2014. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PloS one 9, e103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied D, Gaffori O, Burbach JP, Kovacs GL, van Ree JM, 1987. Structure activity relationship studies with C-terminal fragments of vasopressin and oxytocin on avoidance behaviors of rats. J. Pharm. Exp. Ther 241, 268–274. [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A, 2010. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol. Psychiatry 68, 678–680. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Belcher AM, 2012. The effects of oxytocin and its analog, carbetocin, on genetic deficits in sensorimotor gating. Eur. Neuropsychoph 22, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, MacDonald K, 2016. A Review of Oxytocin's Effects on the Positive, Negative, and Cognitive Domains of Schizophrenia. Biol. Psychiatry 79, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Yee JR, Kenkel WM, Dumais KM, Moore K, Veenema AH, Kulkarni P, Perkybile AM, Carter CS, 2015. Distinct BOLD Activation Profiles Following Central and Peripheral Oxytocin Administration in Awake Rats. Front. Behav. Neurosci 9, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz MC, Rodrigo J, Boudier L, Durroux T, Mouillac B, Hibert M, 2010. Subtlety of the structure-affinity and structure-efficacy relationships around a nonpeptide oxytocin receptor agonist. J. Med. Chem 53, 1546–1562. [DOI] [PubMed] [Google Scholar]
- Gard PR, Daw P, Mashhour ZS, Tran P, 2007. Interactions of angiotensin IV and oxytocin on behaviour in mice. J. Renin Angiotensin Aldosterone Syst 8, 133–138. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA, 2014. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr. Res 156, 261–265. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F, 2001. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Postina R, Fahrenholz F, Reinheimer T, 2005. Binding domains of the oxytocin receptor for the selective oxytocin receptor antagonist barusiban in comparison to the agonists oxytocin and carbetocin. Eur. J. Pharmacol 510, 9–16. [DOI] [PubMed] [Google Scholar]
- Gonser M, 1995. Labor induction and augmentation with oxytocin: pharmacokinetic considerations. Arch. Gynecol. Obstet 256, 63–66. [DOI] [PubMed] [Google Scholar]
- Grotegut CA, Feng L, Mao L, Heine RP, Murtha AP, Rockman HA, 2011. beta-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am. J. Physiol. Endocrinol. Metab 300, E468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Ward PB, Hickie IB, Shahrestani S, Hodge MA, Scott EM, Langdon R, 2015. A single dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. Schizophr. Res 168, 628–633. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S, 2007. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry 61, 498–503. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S, 2003. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology 28, 193–198. [DOI] [PubMed] [Google Scholar]
- Kang YS, Park JH, 2000. Brain uptake and the analgesic effect of oxytocin--its usefulness as an analgesic agent. Arch. Pharm. Res 23, 391–395. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Francis SM, Lee R, de Wit H, Jacob S, 2014. Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans. Psychoneuroendocrinology 46, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S, 2010. Oxytocin and carbetocin ameliorating effects on restraint stress-induced short- and long-term behavioral changes in rats. Neuro. Endocrinol. Lett 31, 622–630. [PubMed] [Google Scholar]
- Loyens E, De Bundel D, Demaegdt H, Chai SY, Vanderheyden P, Michotte Y, Gard P, Smolders I, 2013. Antidepressant-like effects of oxytocin in mice are dependent on the presence of insulin-regulated aminopeptidase. Int. J. Neuropsychopharmacol 16, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Mak P, Broussard C, Vacy K, Broadbear JH, 2012. Modulation of anxiety behavior in the elevated plus maze using peptidic oxytocin and vasopressin receptor ligands in the rat. J. Psychopharmacol 26, 532–542. [DOI] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G, 2008. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog. Brain Res 170, 473–512. [DOI] [PubMed] [Google Scholar]
- Martinetz S, Neumann ID, 2016. The potential of oxytocin as a therapeutic target for psychiatric disorders. Expert Opin. Ther. Targets 20, 515–518. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB, 1983. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 262, 143–149. [DOI] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA, 2014. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology 45, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin V, Del Castillo JR, Authier S, Ybarra N, Otis C, Gauvin D, Gutkowska J, Troncy E, 2008. Evidence for non-linear pharmacokinetics of oxytocin in anesthetizetized rat. J. Pharm. Pharm. Sci 11, 12–24. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN, 2007. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res 176, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA, 2016. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 79, 213–221. [DOI] [PubMed] [Google Scholar]
- Nielsen EI, Al-Saqi SH, Jonasson AF, Uvnas-Moberg K, 2017. Population Pharmacokinetic Analysis of Vaginally and Intravenously Administered Oxytocin in Postmenopausal Women. J. Clin. Pharmacol 57, 1573–1581. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL, 1991. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav 38, 63–67. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Garner JP, Hardan AY, 2017. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. U.S.A 114, 8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passoni I, Leonzino M, Gigliucci V, Chini B, Busnelli M, 2016. Carbetocin is a Functional Selective Gq Agonist That Does Not Promote Oxytocin Receptor Recycling After Inducing beta-Arrestin-Independent Internalisation. J. Neuroendocrinol 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL, 2011. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr. Res 132, 50–53. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH, 2015. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Science Transl. Med 7, 271ra278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt GR, Batt AR, Haigh RM, Penson AM, Robson PA, Rooker DP, Tartar AL, Trim JE, Yea CM, Roe MB, 2004. Non-peptide oxytocin agonists. Bioorg. Med. Chem. Lett 14, 4585–4589. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, Van Ree JM, 1996. Facilitation and attenuation of social recognition in rats by different oxytocin-related peptides. Eur. J. Pharmacol 308, 113–116. [DOI] [PubMed] [Google Scholar]
- Preti A, Melis M, Siddi S, Vellante M, Doneddu G, Fadda R, 2014. Oxytocin and autism: a systematic review of randomized controlled trials. J. Child. Adolesc. Psychopharmacol 24, 54–68. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS, 2013. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology 38, 2249–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich ME, Caldwell HK, 2015. A Role for Oxytocin in the Etiology and Treatment of Schizophrenia. Front. Endocrinol 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S, 2006. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 185, 218–225. [DOI] [PubMed] [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, Sukoff Rizzo SJ, Luo B, Beyer CE, Logue SF, Marquis KL, Hughes ZA, Rosenzweig-Lipson S, 2010. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology 58, 69–77. [DOI] [PubMed] [Google Scholar]
- Romano A, Tempesta B, Micioni Di Bonaventura MV, Gaetani S, 2015. From Autism to Eating Disorders and More: The Role of Oxytocin in Neuropsychiatric Disorders. Front. Neuroendocrinol 9, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B, 2011. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry 69, 875–882. [DOI] [PubMed] [Google Scholar]
- Sanathara NM, Garau C, Alachkar A, Wang L, Wang Z, Nishimori K, Xu X, Civelli O, 2017. Melanin concentrating hormone modulates oxytocin-mediated marble burying. Neuropharmacology 128, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitchik J, Amico J, Robinson AG, Castillo M, 1984. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics. Am. J. Obstet. Gynecol 150, 225–228. [DOI] [PubMed] [Google Scholar]
- Sobota R, Mihara T, Forrest A, Featherstone RE, Siegel SJ, 2015. Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav. Neurosci 129, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraev AS, Bowen MT, Ali SO, Hicks C, Ramos L, McGregor IS, 2014. Adolescent exposure to oxytocin, but not the selective oxytocin receptor agonist TGOT, increases social behavior and plasma oxytocin in adulthood. Horm. Behav 65, 488–496. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Furubayashi T, Arai M, Inoue D, Kimura S, Kiriyama A, Kusamori K, Katsumi H, Yutani R, Sakane T, Yamamoto A, 2018. Delivery of Oxytocin to the Brain for the Treatment of Autism Spectrum Disorder by Nasal Application. Mol. Pharm 15, 1105–1111. [DOI] [PubMed] [Google Scholar]
- Teng BL, Nikolova VD, Riddick NV, Agster KL, Crowley JJ, Baker LK, Koller BH, Pedersen CA, Jarstfer MB, Moy SS, 2016. Reversal of social deficits by subchronic oxytocin in two autism mouse models. Neuropharmacology 105, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS, 2013. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology 72, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R, 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 204, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R, 2011. The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behav. Neurosci 125, 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T, Aoki Y, Takao H, Kawakubo Y, Kamio Y, Kato N, Miyashita Y, Kasai K, Yamasue H, 2014. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry 71, 166–175. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, Takao H, Nippashi Y, Kawakubo Y, Kunimatsu A, Kasai K, Yamasue H, 2015. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 138, 3400–3412. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ, 2011. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol. Med 17, 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ, 2016. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol. Psychiatry 21, 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K, 2009. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci 29, 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A, 2014. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 39, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.