Abstract
Background
Helicobacter pylori infection increases the risk of stomach cancer; therefore, eradication therapy is recommended for infected individuals. Although several methods are recommended for the diagnosis and therapy of H. pylori infection, their frequency and effectiveness have not been fully investigated in Japan.
Methods
A nationwide claims database including >1.6 million patients (April 2008 – October 2016) in Japan was utilized. We analyzed the distribution of methods for H. pylori diagnosis and therapy, waiting period between eradication and diagnostic test, and success rate of primary therapy.
Results
Data for 481,041 patients were extracted. After primary eradication therapy, urea breath test was used for >80% of diagnoses, and antibody measurement for 0.7%. The success rate of primary eradication was >80% for most diagnostic methods and 69.0% for antibody measurement; inappropriately-timed antibody measurement may have contributed to this disparity. The overall success rate of eradication therapy decreased from 2011 to 2014, but increased from 2015, coinciding with launch of the potassium-competitive acid blocker vonoprazan, which showed a higher success rate of eradication than proton-pump inhibitors.
Conclusions
Diagnostic tests of H. pylori infection mostly followed Japanese Society for Helicobacter Research guidance, although some antibody measurements were timed inappropriately. Vonoprazan appears to increase the success rate of primary therapy.
Keywords: Helicobacter pylori, Claims data, Diagnostic test, Eradication therapy, Drug resistance
Introduction
Gastric cancer is the third most common cause of death of all cancers [1]. Its prevalence is especially high in East Asia. In 2012, the incidences of gastric cancer in Korea, Japan, and China were ranked as first, third, and fifth in the world, respectively [1].
Helicobacter pylori-gastritis induces chronic inflammation in the gastric mucosa and is the cause of various disorders [2, 3, 4, 5, 6], including atrophic gastritis, which potentially leads to stomach cancer [7]. The International Agency for Research on Cancer of the World Health Organization classified H. pylori as a Group 1 carcinogen in 1994 [8] and verified this in 2009 [9]. Recent randomized controlled trials [10, 11] and meta-analyses [12, 13, 14, 15, 16, 17] have indicated that the eradication of H. pylori reduces the risk of gastric cancer.
Several attempts have been made to facilitate the prevention of gastric cancer in Japan. For example, health insurance coverage was approved for H. pylori eradication therapy for gastric and duodenal ulcers in 2000. The target diseases covered by insurance have subsequently been expanded, and in 2013, H. pylori gastritis was included. Furthermore, the Japanese Society for Helicobacter Research (JSHR) revised their guidelines for diagnosis and treatment of H. pylori infection in 2016 and incorporated gastric cancer prevention by eradication and extermination of H. pylori [18]. The JSHR guidelines describe the methods of diagnosing H. pylori infection and of conducting standard eradication therapy.
According to the JSHR guidelines, one of the following 6 methods is appropriate for diagnosing H. pylori infection: a rapid urease test, direct microscopic count, microbial culture, urea breath test, antibody measurement, or stool antigen test [18]. The urea breath test and stool antigen test using monoclonal antibodies are also described as useful diagnostic methods after eradication therapy. To make a diagnosis of H. pylori infection after eradication therapy, it is recommended to wait at least 4 weeks after the termination of medication before conducting the test.
For eradication therapy, the following treatments are described in the JSHR guidelines. As the standard primary eradication therapy, 3 drugs (amoxicillin, clarithromycin [CAM], and either a proton-pump inhibitor [PPI] or potassium-competitive acid blocker [P-CAB]) are administered for 7 days [18]. In second-line regimens, metronidazole is used instead of CAM; the other drugs used and administration period are the same as those for primary therapy. Four PPIs (omeprazole, rabeprazole, lansoprazole, and esomeprazole) and one P-CAB (vonoprazan) are currently approved for eradication therapy in Japan. Of these, vonoprazan is the newest drug, being launched in February 2015. Although a direct comparison of the eradication effect between drugs using data from clinical trials is inappropriate because of the different timing and conditions in each trial, the eradication rate appears to differ among the prescribed PPIs and P-CABs, ranging from 78.8% [19] for omeprazole to 92.6% for vonoprazan [20]. A direct comparison study of the 3 different PPIs (rabeprazole, omeprazole, and lansoprazole) has not shown a difference in eradication rate [18].
H. pylori has been reported to acquire resistance to various antimicrobial drugs, and the proportion of patients with drug-resistant H. pylori has been increasing [21, 22, 23]. Between 2013 and 2014, the percentage of CAM-resistant H. pylori cases was reported to be 38.5% [21]. In parallel with this increase, the eradication success rate of H. pylori has been declining [22].
To facilitate the prevention of gastric cancer by the eradication of H. pylori infection, it is important to make an appropriate diagnosis of the infection status and provide proper eradication therapy. Although there are several methods available for diagnosis and therapy, a nationwide study has not been performed to investigate the status of diagnosis and therapy, and the extent to which the guidelines are followed at a practical level. Such information is essential for promoting the appropriate method of diagnosis and therapy. Furthermore, monitoring the success rate of H. pylori eradication using real-time data per se can be useful to detect the emergence of drug-resistant strains.
The objective of our study, therefore, was to conduct a nationwide investigation into the diagnosis and treatment of H. pylori infection. We analyzed the distribution of methods of primary eradication therapy and diagnostic testing used before and after therapy, the waiting period from the termination of medication until the diagnostic test, and the success rate of H. pylori eradication following various methods of diagnosis and therapy.
Materials and Methods
Study Design
This study was a claims-based analysis of the patients who received a diagnosis of and/or therapy for H. pylori infection from a database of hospitals that have adopted the Diagnosis Procedure Combination system (DPC hospitals) – a claims payment system which operates on a per-day payment model rather than a per-case payment model.
Data Source
A claims database from the DPC hospitals provided by Medical Data Vision Co., Ltd. (Tokyo, Japan) was used. Three investigators had access to this database. This database contains patient medical data from over 13.6 million individuals (>10% of the Japanese population) from over 200 DPC hospitals across Japan, including hospitals that treat acute or seriously ill patients on a 24-h basis. Data were cleaned by the database provider. The database covers claims from April 2008 until October 2016. This database has been widely used for similar investigations, including those that describe the actual treatment status for a particular disease in Japan [24, 25, 26, 27].
Definition of Patients, Diagnostic Tests, and Therapeutic Methods
For our analyses, we selected patients who had a record of diagnostic testing for H. pylori infection and/or a record of prescription for the 3 medications (either a combination of single drugs or a combination pack) that were used in the standard eradication therapy for H. pylori infection. Codes were used to identify patients.
Methods of diagnosis were defined by the procedure codes in the claims data (online suppl. Table S1; for all online suppl. material, see www.karger.com/doi/10.1159/000500819). Prescribed medications were defined from the anatomical therapeutic chemical codes, procedure codes, or general names in the claims data. Primary and secondary eradication therapies were defined according to the constituents of each regimen (the 3 prescribed medications) used in the standard treatment (online supp. Table S2).
Diagnostic tests for H. pylori infection were subdivided into the following 2 types. One was the diagnostic test before eradication therapy, which is the latest test within 150 days before the starting day of the treatment, and the other was the diagnostic test after eradication therapy, which is the earliest test from 7 days until 150 + 7 days after the starting date of the therapy.
The success rate of the primary eradication of H. pylori was determined in those patients who received primary eradication therapy who also had a subsequent diagnostic test after eradication therapy. Of these, patients who did not receive secondary eradication therapy were defined as being successful cases of eradication therapy.
Data and Statistical Analysis
Study size was determined by the number of eligible patients in the DPC database. Counts of patients for each diagnostic method used before and after eradication therapy and that for the interval (in days) between the therapy and diagnosis for each and all diagnostic methods were calculated for the entire time period. Monthly counts of patients who received primary eradication therapy for each medication were also calculated over the entire period of the database. Patients who were lost to follow-up were excluded.
The following success rates of primary H. pylori eradication were estimated with a 95% CIs: the success rates measured by each diagnostic method over the entire period, success rates by time interval from the eradication therapy to the subsequent diagnostic test for H. pylori infection over the entire period, monthly success rates for all types of therapy over the entire period, and success rate for therapy including each type of PPI and P-CAB between January 2015 and March 2016.
Descriptive statistics were used to analyze the data, and confidence intervals were calculated for differences. Statistical analysis was performed using Excel 2010 (Microsoft Corp., Redmond, WA, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
At the beginning of the analysis, records for 481,041 patients were extracted from the database. Among those patients, 473,011 had a history of receiving a test of H. pylori infection, and 250,197 had a history of receiving eradication therapy. Among those patients who received primary eradication therapy, 196,509 had a history of receiving a diagnostic test after eradication therapy. The estimated number of patients receiving eradication therapy for H. pylori infection in Japan was approximately 60,000 per year in 2011 and 2012 and 140,000 per year in 2013 through 2016 [28], thus, roughly 30% of all patients receiving eradication therapy in Japan from 2011 to 2016 were covered in this study.
Diagnostic Test Methods of H. pylori Infection and the Success Rate of Eradication Therapy
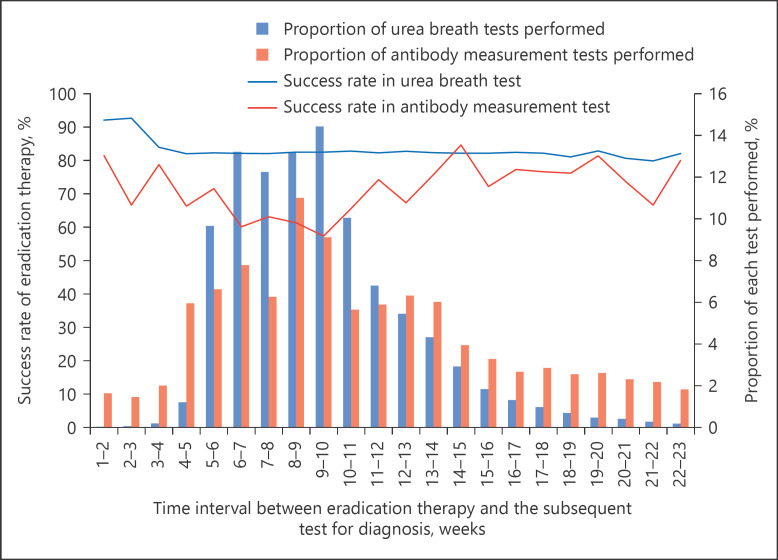
Various test methods were used for diagnosis before eradication therapy. The urea breath test was the most frequently used (27.5%), followed by the antibody measurement (25.9%) and rapid urease (21.8%) tests (Table 1). Meanwhile, the diagnostic method employed after eradication therapy was predominantly the urea breath test (89.6%), and in a few cases (0.7%), antibody measurement (Table 1). The most frequent time interval between eradication therapy and the subsequent diagnostic test was 8–9 weeks (average 9.2 weeks, median 8.9 weeks), and the frequency for a period of <4 weeks was 0.3%. The average success rates of eradication of H. pylori were >80% regardless of the period between the therapy and subsequent diagnostic test. Success rates differed as measured by various diagnostic tests after primary eradication therapy. The success rate as measured by the antibody measurement alone was the lowest (69.0%) compared with that by other methods (>80%; Table 2). As shown in Figure 1, the success rate as measured by the urea breath test was >80% for eradication of H. pylori regardless of the interval between the therapy and diagnostic test, while the success rate as measured by the antibody measurement was lower (Fig. 1); notably, the success rate was lower when there was a shorter interval between the therapy and the subsequent diagnostic test.
Table 1.
Diagnostic methods of Helicobacter pylori infection and their implementation rate between April 2008 and October 2016
| Methods of diagnosis | Number of patients who received eradication therapy | Combined proportion, % |
|---|---|---|
| Before both primary and secondary eradication therapy | ||
| Urea breath test | 60,750 | 27.5 |
| Antibody measurement1 | 57,299 | 25.9 |
| Rapid urease test | 48,250 | 21.8 |
| Antibody measurement (qualitative)2 | 22,432 | 10.1 |
| Stool antigen test | 8,585 | 3.9 |
| Microbial culture | 7,908 | 3.6 |
| Rapid urease test and direct microscopic count | 4,991 | 2.3 |
| Direct microscopic count | 4,971 | 2.2 |
| Direct microscopic count and microbial culture | 1,081 | 0.5 |
| Direct microscopic count and antibody measurement | 970 | 0.4 |
| Rapid urease test and antibody measurement | 564 | 0.3 |
| Direct microscopic count and urea breath test | 494 | 0.2 |
| Antibody measurement and urea breath test | 481 | 0.2 |
| Direct microscopic count and antibody measurement (qualitative) | 476 | 0.2 |
| Antibody measurement (qualitative) and urea breath test | 441 | 0.2 |
| Urea breath test and stool antigen test | 331 | 0.1 |
| Rapid urease test and microbial culture | 249 | 0.1 |
| Antibody measurement and stool antigen test | 141 | 0.1 |
| Microbial culture and antibody measurement | 132 | 0.1 |
| Rapid urease test and antibody measurement (qualitative) | 119 | 0.1 |
| Other tests | 507 | 0.2 |
| Total | 221,172 | 100.0 |
| After both primary and secondary eradication therapy | ||
| Urea breath test | 212,759 | 89.6 |
| Stool antigen test | 18,146 | 7.6 |
| Antibody measurement1 | 1,571 | 0.7 |
| Urea breath test and stool antigen test | 1,551 | 0.7 |
| Rapid urease test | 924 | 0.4 |
| Direct microscopic count | 755 | 0.3 |
| Antibody measurement (qualitative)2 | 479 | 0.2 |
| Microbial culture | 279 | 0.1 |
| Antibody measurement and urea breath test | 262 | 0.1 |
| Direct microscopic count and urea breath test | 234 | 0.1 |
| Rapid urease test and direct microscopic count | 181 | 0.1 |
| Other tests | 206 | 0.1 |
| Total | 237,347 | 100.0 |
Antibody measurement: it was measured with an apparatus (the latex agglutination method and enzyme immunoassay method).
Antibody measurement (qualitative): it is possible to measure by visual observation (the immunochromatography method and gold colloidal method).
Table 2.
Methods of diagnosis after primary eradication therapy and their measured success rate of eradication of Helicobacter pylori between April 2008 and October 2016
| Methods of diagnosis | Number of patients who received primary eradication therapy | Number of patients who received both primary and secondary eradication therapy | Success rate of primary eradication therapy, % | 95% CI, % |
|---|---|---|---|---|
| Urea breath test | 175,781 | 31,009 | 82.4 | 82–83 |
| Stool antigen test | 15,272 | 2,563 | 83.2 | 83–84 |
| Urea breath test and stool antigen test | 1,314 | 236 | 82.0 | 80–84 |
| Antibody measurement1 | 1,260 | 390 | 69.0 | 66–72 |
| Rapid urease test | 829 | 146 | 82.4 | 80–85 |
| Direct microscopic count | 678 | 85 | 87.5 | 85–90 |
| Antibody measurement (qualitative)2 | 385 | 124 | 67.8 | 63–72 |
| Microbial culture | 225 | 31 | 86.2 | 82–91 |
| Antibody measurement and urea breath test | 213 | 41 | 80.8 | 75–86 |
| Direct microscopic count and urea breath test | 213 | 29 | 86.4 | 82–91 |
| Rapid urease test and direct microscopic count | 161 | 33 | 79.5 | 73–86 |
| Other tests | 178 | 33 | 81.5 | 76–87 |
| Total | 196,509 | 34,720 | 82.3 | 82–83 |
Antibody measurement: it was measured with an apparatus (the latex agglutination method and enzyme immunoassay method).
Antibody measurement (qualitative): it is possible to measure by visual observation (the immunochromatography method and gold colloidal method).
Fig. 1.
Success rate of primary H. pylori eradication at different time intervals between primary eradication and the subsequent test for diagnosis.
Therapeutic Methods of H. pylori Eradication and the Success Rate
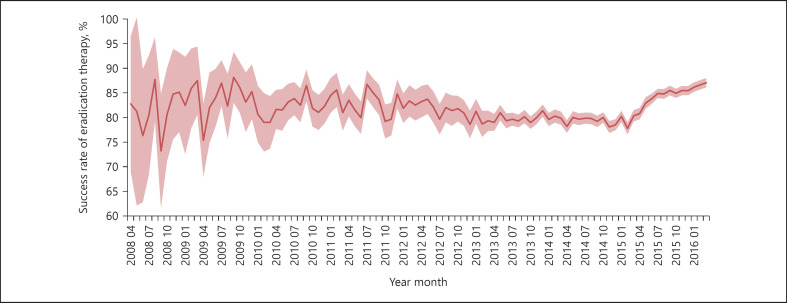
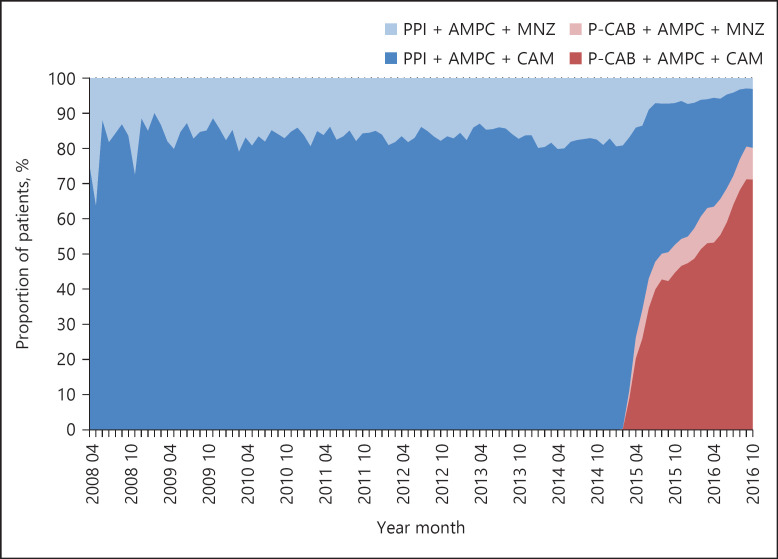
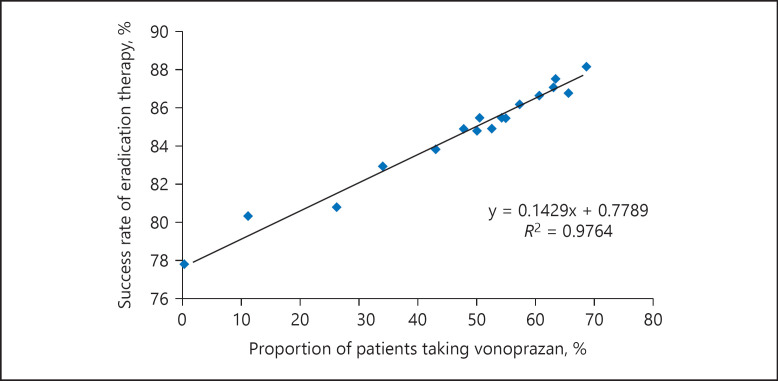
The success rate of H. pylori eradication for all types of therapy decreased from 83% to <80% between 2011 and 2014, and then increased to ≥85% in 2015 (Fig. 2). Among the medications used for eradication therapy, the proportion of regimens including a P-CAB has increased rapidly since its launch in February 2015 (Fig. 3). A positive correlation was observed between the success rate of eradication therapy and the percentage of patients treated with vonoprazan (Fig. 4).
Fig. 2.
Transition of primary H. pylori eradication success rate between April 2008 and March 2016. The red line and pink area indicate the point estimate of the success rate and 95% CI, respectively. The success rate of primary eradication of H. pylori was defined as the proportion of patients who did not receive secondary eradication therapy among the cumulative total patient population at each time point. The 95% CI was calculated as the lower and upper bounds of the success rate based on the population size at each time point.
Fig. 3.
Transition of the proportion of patients taking the standard eradication therapy regimen for H. pylori infection between April 2008 and October 2016. PPI, proton-pump inhibitor; AMPC, amoxicillin; MNZ, metronidazole; CAM, clarithromycin; P-CAB, potassium-competitive acid blocker.
Fig. 4.
Success rate and proportion of patients taking vonoprazan among all standard primary eradication therapies for H. pylori infection. The blue dots indicate actual data calculated for each month. The black line is a fitted regression line drawn by the regression equation described below the line. R2 is the coefficient of determination.
The mean success rate of eradication therapy from January 2015 to March 2016 was >90% for primary therapy including vonoprazan, which was higher than that for other therapies including PPIs (77.6–82.0%; Table 3).
Table 3.
Success rate of primary eradication therapy for Helicobacter pylori infection with PPI and P-CAB between January 2015 and March 2016
| PPIs |
P-CAB |
||||
|---|---|---|---|---|---|
| omeprazole | rabeprazole | lansoprazole | esomeprazole | vonoprazan | |
| No. of total patients | 269 | 19,236 | 18,339 | 4,984 | 31,171 |
| Proportion of each treatment, % | 0.4 | 26.0 | 24.8 | 6.7 | 42.1 |
| No. of failed patients | 52 | 04,308 | 3,740 | 899 | 2,889 |
| Success rate, % | 80.7 | 77.6 | 79.6 | 82.0 | 90.7 |
| 95% CI, % | 76.0–85.4 | 77.0–78.2 | 79.0–80.2 | 80.9–83.0 | 90.4–91.1 |
| Success rate in clinical trials1, % (n) | 78.8 (19) | 85.7–89.0 (28) | 83.7–91.1 (29) | − | 92.6 (20) |
Data in clinical trials used for the development of information written in the package insert in Japan.
P-CAB, potassium-competitive acid blocker; PPIs, proton pump inhibitors.
Discussion/Conclusion
We showed the current status of diagnostic testing and eradication therapy of H. pylori based on analyses of a nationwide claims database. The diagnostic testing of H. pylori infection before eradication therapy was performed using various methods. However, after eradication therapy, the urea breath test was used for the vast majority of diagnostic tests. The success rate of H. pylori eradication as measured by most diagnostic methods after primary eradication therapy was >80%. In contrast, the success rate measured by the antibody measurement test alone was noticeably lower (69.0%). The success rate of eradication of H. pylori after therapy decreased from 2011 until 2014, but increased from 2015. Following the launch of vonoprazan in 2015, its use has increased markedly compared with that of PPIs. The success rate of vonoprazan for the eradication of H. pylori (January 2015 to March 2016) was higher than PPIs at >90%.
The JSHR guidelines for diagnosis and treatment of H. pylori infection describe 6 methods of diagnostic testing for H. pylori infection, but do not recommend any particular method [18]. The urea breath test is a noninvasive method; it is simple, easy, and sensitive and has high specificity [29, 30, 31, 32, 33, 34]. It is also described as a useful method for determining H. pylorieradication in the JSHR guidelines [18]. Our findings support the urea breath test as the preferred diagnostic test in clinical practice.
The antibody measurement method is used to determine H. pylori infection by measuring the amount of antibodies produced as an immune response against the infection in the gastric mucosal membrane [18]. This method is useful in patients with low gastric bacterial density, such as those with atrophic gastritis, and in those where false-positive results are suspected with other methods [18]. Furthermore, this method has the advantage that drug withdrawal is not a factor as it is not affected by PPIs and defense function enhancers [18]. However, given that negative conversion of the serum antibody can take more than a year after successful H. pylori eradication [35], this method is not suitable when H. pylori eradication needs to be determined shortly after eradication therapy. Furthermore, to use this method to determine H. pylori eradication, a quantitative comparison is recommended between results before and 6 months after eradication therapy [36]. Our study revealed that even with such prerequisites, the antibody measurement is used alone in a small fraction of diagnoses (0.7%) of H. pylori infection after eradication therapy, and that the test was even conducted within 6 months of therapy. In such cases, if the test is performed before the negative conversion of the serum antibody, then patients could be misdiagnosed as having failed primary eradication therapy and receive an unnecessary second course of eradication therapy. This may account for the antibody measurement test having the lowest measured success rate of H. pylori eradication. Furthermore, receiving an unnecessary secondary eradication therapy is undesirable due to both the safety concerns of patients and use of unnecessary medical resources. Our study indicates that such inappropriate use of this diagnostic method may be taking place in relatively large hospitals. Further research is required to determine the extent of the inappropriate use of diagnostic testing at other types of facilities in Japan.
Since 2011, changes have been observed in the success rate of H. pylori eradication. Nationwide surveillance of drug-resistant strains performed by the JSHR has indicated that CAM-resistant strains increased from 18.9% in 2002 to 21.1% in 2003, 31% in 2010–2011, and 38.5% in 2013–2014 [21]. This increase may be reflected in the declining success rate of H. pylori eradication observed until 2014 in our study. Since 2015, the success rate of H. pylori eradication has been increasing. Vonoprazan was released in February 2015, and subsequently, its use among patients has been increasing. The success rate of H. pylori eradication showed a positive correlation with the percentage of patients treated with vonoprazan. The success rate for the regimen that included vonoprazan was >90%, which was higher than that for PPIs in this study. The success rate was also reported to be >90% in a randomized controlled trial [18]. Therefore, this increase in the success rate of H. pylori eradication might be attributed to the use of vonoprazan.
This present study has several limitations. The claims data used were only from large hospitals; the proportion of the hospitals with a total number of beds of <199, 200–499, and >500 were approximately 20, 60, and 20%, respectively. All hospitals included in this study were acute hospitals, covering around 16% of all acute hospitals in Japan, and thus the study population may have different characteristics compared with the general Japanese population. Another analysis using data from middle- and small-sized hospitals is required to better describe the whole picture throughout the country. This study defined successful cases of primary eradication therapy as those patients who received primary eradication therapy followed by testing for H. pylori infection and did not receive secondary eradication therapy. However, there may be patients who tested positive for H. pylori infection after the primary therapy (i.e., failure of primary therapy) and yet did not receive secondary eradication therapy, or received a second-line treatment other than those defined in this study (i.e., combination pack, PPI, amoxicillin, and metronidazole), or were lost at follow-up. If such patients exist in the database, then they would be incorrectly considered as achieving successful primary eradication therapy in our analysis. Therefore, the actual success rate of primary eradication therapy may be lower than that calculated in this study. Of note, a recent retrospective study has reported rates of patients who were lost at follow-up to be 3.5 and 4.4% for vonoprazan and PPI, respectively [37, 38]; thus, the extent of overestimation of the success rate due to the lack of follow-up may not be large in general.
In conclusion, using data from 481,041 patients, we found that in large hospitals, the diagnostic testing of H. pylori infection is performed mostly in accordance with the JSHR guidelines. However, antibody measurement testing was conducted too soon after eradication therapy in some cases, which may lead to unnecessary secondary eradication therapy. The success rate of H. pylori eradication decreased from 2011 until 2014, which was probably due to the increase of drug-resistant strains. The success rate has been gradually increasing following the clinical application of vonoprazan and its expanded patient use. Use of medications with a high eradication capacity is expected to contribute to the increased success rate of primary eradication therapy, reducing the burden to patients and the health-care system associated with secondary eradication therapy.
Statement of Ethics
This study was performed using anonymized and personally unidentifiable data; therefore, no informed consent was required, based on Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labor, and Welfare.
Disclosure Statement
H.D. and A.U. are employees of Takeda Pharmaceutical Company Limited. K.M. received lecture fees from Takeda Pharmaceutical Company Limited.
Funding Sources
This study was funded by Takeda Pharmaceutical Company Limited. The authors thank Shinzo Hiroi, PhD, at the time employed by Takeda and Kazuo Ueda, MD, employee of Takeda, for contributing to the study design and interpretation of data, respectively.
Author Contributions
H.D.: conception and design, data analysis and interpretation, drafting the manuscript, writing the manuscript, and final approval of the manuscript. A.U.: data analysis and interpretation, writing the manuscript, and final approval of the manuscript. K.M.: conception and design, data analysis and interpretation, writing the manuscript, and final approval of the manuscript.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
The authors would like to thank Kosuke Iwasaki, MBA, and Tomomi Takeshima, PhD, employees of Milliman, for performing data analysis and providing the manuscript draft and editorial support, respectively. We would also like to acknowledge Editage (www.editage.jp) for English language editing.
References
- 1.World Cancer Research Fund International Stomach cancer statistics. 2016. http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/stomach-cancer-statistics (accessed March 13, 2018)
- 2.Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan results of a large multicenter study. Helicobacter. 2001 Dec;6((4)):294–9. doi: 10.1046/j.1523-5378.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161((4)):626–33. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 4.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis Houston 1994. Am J Surg Pathol. 1996 Oct;20((10)):1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989 Dec;321((23)):1562–6. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 6.Drumm B, Sherman P, Cutz E, Karmali M. Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med. 1987 Jun;316((25)):1557–61. doi: 10.1056/NEJM198706183162501. [DOI] [PubMed] [Google Scholar]
- 7.Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, et al. Association between infection with Helicobacter pylori and risk of gastric cancerevidence from a prospective investigation. BMJ. 1991 Jun;302((6788)):1302–5. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 9.IARC Working. Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100 Pt :B1–441. [Google Scholar]
- 10.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al.; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric canceran open-label, randomised controlled trial. Lancet. 2008 Aug;372((9636)):392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 11.Li WQ, Ma JL, Zhang L, Brown LM, Li JY, Shen L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst. 2014 Jun;106((7)):106. doi: 10.1093/jnci/dju116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasiaevidence from a meta-analysis. Gastric Cancer. 2016 Jan;19((1)):166–75. doi: 10.1007/s10120-015-0462-7. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individualssystematic review and meta-analysis of randomised controlled trials. BMJ. 2014 May;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung DH, Kim JH, Chung HS, Park JC, Shin SK, Lee SK, et al. Helicobacter pylori eradication on the prevention of metachronous lesions after endoscopic resection of gastric neoplasma meta-analysis. PLoS One. 2015 Apr;10((4)):e0124725. doi: 10.1371/journal.pone.0124725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim N, Park RY, Cho SI, Lim SH, Lee KH, Lee W, et al. Helicobacter pylori infection and development of gastric cancer in Korealong-term follow-up. J Clin Gastroenterol. 2008 May-Jun;42((5)):448–54. doi: 10.1097/MCG.0b013e318046eac3. [DOI] [PubMed] [Google Scholar]
- 16.Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association between Helicobacter pylori eradication and gastric cancer incidencea systematic review and meta-analysis. Gastroenterology. 2016;150((5)):1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumorsa meta-analysis. Helicobacter. 2014 Aug;19((4)):243–8. doi: 10.1111/hel.12146. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Society for Helicobacter Research . Guidelines for the management of Helicobacter pylori infection in Japan. 2016 ed, revised. Tokyo: Sentan Igaku-Sha; 2016. [Google Scholar]
- 19.Kuwayama H, Luk G, Yoshida S, Nakamura T, Kubo M, Uemura N, et al. Efficacy of a low-dose omeprazole-based triple-therapy regimen for Helicobacter pylori eradication independent of cytochrome P450 genotypethe Japanese MACH study. Clin Drug Investig. 2005;25((5)):293–305. doi: 10.2165/00044011-200525050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan a novel potassium-competitive acid blocker as a component of first-line and second-line triple therapy for Helicobacter pylori eradicationa phase III, randomised, double-blind study. Gut. 2016 Sep;65((9)):1439–46. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashinaga M, Okimoto T, Kodama M, Mizukami K, Ogawa R, Okamoto K, et al. count report – of present conditions – 2013–2014 year resistant bacteria surveillance of drug-resistant Helicobacter pylori in our country [Japanese] Jpn J Helicobacter Res. 2016;17:45–9. [Google Scholar]
- 22.Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007 Dec;45((12)):4006–10. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi I, Azuma T, Ikeda F, Uemura T, Kato S, Kato M, et al. Count report of the present conditions 2010-2011 year resistant bacteria surveillance of drug-resistant Helicobacter pylori in our country [Japanese] Jpn J Helicobacter Res. 2013;14:102–6. [Google Scholar]
- 24.Ebata-Kogure N, Nozawa K, Murakami A, Toyoda T, Haga Y, Fujii K. Clinical and economic burdens experienced by patients with painful diabetic peripheral neuropathyan observational study using a Japanese claims database. PLoS One. 2017 Oct;12((10)):e0187250. doi: 10.1371/journal.pone.0187250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiroi S, Sugano K, Tanaka S, Kawakami K. Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japanretrospective observational study and simulation study based on real-world data. BMJ Open. 2017 Jul;7((7)):e015855. doi: 10.1136/bmjopen-2017-015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogino M, Kawachi I, Otake K, Ohta H, Otsuka Y, Iwasaki K, et al. Current treatment status and medical cost for multiple sclerosis based on analysis of a Japanese claims database. Clin Exp Neuroimmunol. 2016 May;7((2)):158–67. doi: 10.1111/cen3.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F, Mishina S, Takai S, Le TK, Ochi K, Funato K, et al. Systemic treatment patterns with advanced or recurrent non-small cell lung cancer in Japana retrospective hospital administrative database study. Clin Ther. 2017 Jun;39((6)):1146–60. doi: 10.1016/j.clinthera.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda M, Asaka M, Kato M, Matsushima R, Fujimori K, Akino K, Kikuchi S, Lin Y, Sakamoto N. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017 Oct;22((5)) doi: 10.1111/hel.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, et al. Accuracy of urea breath test in Helicobacter pylori infectionmeta-analysis. World J Gastroenterol. 2015 Jan;21((4)):1305–14. doi: 10.3748/wjg.v21.i4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisbert JP, Pajares JM. Review article13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther. 2004 Nov;20((10)):1001–17. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 31.Kato M, Asaka M, Ohara S, Toyota T. Clinical studies of 13C-urea breath test in Japan. J Gastroenterol. 1998;33(Suppl):1036–9. [PubMed] [Google Scholar]
- 32.Kato M, Saito M, Fukuda S, Kato C, Ohara S, Hamada S, et al. 13C-Urea breath test using a new compact nondispersive isotope-selective infrared spectrophotometercomparison with mass spectrometry. J Gastroenterol. 2004 Jul;39((7)):629–34. doi: 10.1007/s00535-003-1357-7. [DOI] [PubMed] [Google Scholar]
- 33.Ohara S, Kato M, Asaka M, Toyota T. The UBiT-100 13CO2 infrared analyzercomparison between infrared spectrometric analysis and mass spectrometric analysis. Helicobacter. 1998 Mar;3((1)):49–53. doi: 10.1046/j.1523-5378.1998.08046.x. [DOI] [PubMed] [Google Scholar]
- 34.Ohara S, Kato M, Asaka M, Toyota T. Studies of 13C-urea breath test for diagnosis of Helicobacter pylori infection in Japan. J Gastroenterol. 1998 Feb;33((1)):6–13. doi: 10.1007/pl00009968. [DOI] [PubMed] [Google Scholar]
- 35.Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996 Jan;91((1)):85–8. [PubMed] [Google Scholar]
- 36.Kosunen TU, Seppälä K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992 Apr;339((8798)):893–5. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 37.Shinozaki S, Nomoto H, Kondo Y, Sakamoto H, Hayashi Y, Yamamoto H, et al. Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J Med Sci. 2016 May;32((5)):255–60. doi: 10.1016/j.kjms.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol. 2016 Jul;111((7)):949–56. doi: 10.1038/ajg.2016.182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data