Abstract
Objective
More recently, attention has turned to the linkage between childhood trauma and emotional dysregulation, but the evidence in bipolar disorder (BD) is limited. To determine neurobiological relationships between childhood trauma, current anxiety, and impulsivity, we investigated cortical volumetric correlates of these clinical factors in BD.
Methods
We studied 36 patients with DSM-5 BD and 29 healthy controls. Childhood trauma, coexisting anxiety, and impulsivity were evaluated with the Korean version-Childhood Trauma Questionnaire (CTQ), the Korean version-Beck Anxiety Inventory (BAI), and the Korean version-Barratt Impulsiveness Scale (BIS). Voxel-based morphometry (VBM) was used to assess gray matter volume (GMV) alterations on the brain magnetic resonance imaging (MRI). Partial correlation analyses were conducted to examine associations between the GMV and each scale in the BD group.
Results
Childhood trauma, anxiety, and impulsivity were interrelated in BD. BD patients revealed significant inverse correlations between the GMV in the right precentral gyrus and CTQ scores (r=-0.609, p<0.0003); between the GMV in the left middle frontal gyrus and BAI scores (r=-0.363, p=0.044). Moreover, patients showed similar tendency of negative correlations between the GMV in the right precentral gyrus and BIS scores; between the GMV in the left middle frontal gyrus and CTQ scores.
Conclusion
The present study provides evidence for a neural basis between childhood trauma and affect regulations in BD. The GMV alterations in multiple frontal lobe areas may represent neurobiological markers for anticipating the course of BD.
Keywords: Bipolar disorder, Gray matter volume, Childhood trauma, Anxiety, Impulsivity
INTRODUCTION
Bipolar disorder (BD) is a chronic mental illness entailing recurrent mood episodes [1]. It has been well documented that BD is associated with high morbidity, disability, and risk of suicide [2]. A number of studies have explored risk indicators of unfavorable outcomes in BD, and several emerging literatures indicate that adverse childhood experiences, comorbid anxiety, and impulsivity often complicate the presentation and the course of BD [3-5].
BD is the psychiatric diagnosis most often associated with a history of childhood trauma [6]. Bipolar patients who experienced maltreatment in their early lives are prone to have an earlier onset, a worse clinical course, and higher suicidality [7,8]. Besides, BD patients with lifetime anxiety disorders appear to have a negative course, including higher numbers of mood episodes, more severe depressive and mixed episodes, and higher levels of suicidality [9-12]. Furthermore, coexisting impulsivity in BD seems to be a crucial determinant of clinical outcomes [13,14]. The severity of suicidal attempts in BD has been related to the level of impulsivity [15,16].
More recently, attention has been paid to the linkage among childhood trauma, current anxiety, and impulsivity in BD. A few researches reported that childhood abuse was significantly associated with affective instability in BD independent of current mood states [17], and that BD patients showed considerably higher impulsivity when anxiety was present either as a comorbidity or a symptom [18]. One possible mechanism underlying these observations is the disruption of key stress-response systems during early stages of child development, such as the hypothalamic-pituitary-adrenal axis, serotonin and catecholamine systems, and neurotrophic factors. These disturbances can substantially influence stress handling, arousal, and emotional behavior, leading to an increased allostatic load and negative consequences in brain development in the long term [19]. Despite the critical role of the childhood traumas and comorbid affective instability in BD, less is known about their relationships and neuroanatomical correlates.
The results of neuroimaging studies in BD have been inconsistent due to complicating factors. Reasons for the discrepancies include small sample sizes, heterogeneity of subject samples, and differences in methods between studies such as imaging acquisition, processing, and analyses [20]. Nonetheless, neuroimaging researches using voxel-based morphometry (VBM), a computational method to analyze gray matter volume (GMV) on the brain magnetic resonance imaging (MRI) scans, have indicated the involvement of cortical or subcortical structures in the pathophysiology of BD [20,21]. These findings would contribute to a better understanding of neurobiological mechanisms of BD, and provide evidence for future hypotheses and models about the roles of critical clinical factors in the disorder.
To date, there is a scarcity of neuroimaging studies on the role of childhood trauma, comorbid anxiety, and impulsivity in BD. Bipolar patients with adverse childhood experiences compared to healthy individuals exhibited GMV loss in the bilateral orbitofrontal cortex and the thalamus [22]. Similarly, GMV in the right dorsolateral prefrontal cortex and the right thalamus was smaller in BD patients with childhood traumas than patients without them [19]. In familial pediatric BD, there was a significant negative association between the total hippocampal volume and anxiety scores [20]. To our knowledge, no research exists integrating the structural brain correlates of various and comprehensive clinical aspects of BD.
The present study aimed to examine cortical or subcortical volumetric correlates of the three paramount clinical variables described above in bipolar patients. First, childhood trauma, comorbid anxiety, and impulsivity of BD patients were assessed via standardized clinical scales, and the relationships between each scale were explored. Second, the correlations between GMV alterations and the scores of the clinical scales were analyzed within the BD group. We hypothesized that GMV changes in the brain would be associated with childhood trauma, anxiety, and impulsivity in BD, and that the brain regions with such alterations would overlap between the patients.
METHODS
Subjects
36 patients with BD I or II and 29 healthy individuals between 18 and 49 years were recruited from the Department of Mood Disorder at the National Center for Mental Health, Seoul, Republic of Korea, from 2017 to 2018. Patients were composed of 27 inpatients and nine outpatients at the time of enrollment. All subjects were interviewed by a psychiatrist who used the Korean version of the Mini-International Neuropsychiatric Interview (MINI) [23,24]. Best-estimate lifetime diagnoses were made according to the Diagnostic and statistical manual of mental disorders (DSM-5) [25] criteria. In cases with a doubt, a diagnosis was made by at least two psychiatrists of the research team, and a consensus was reached via discussion where necessary. Healthy controls (HC) were excluded if they had a history of major Axis I psychiatric disorders, psychiatric illnesses in first-degree relatives, and/or childhood trauma. The exclusion criteria were as follows: 1) intellectual disability or any evidence of organic brain injury, 2) any physical conditions affecting mental states except treatable thyroid disorders, 3) difficulty understanding Korean, and 4) contraindications to MRI scanning. This study was performed according to the guidelines of the Helsinki Declaration, and approved by the Institutional Review Board of the National Center for Mental Health (NCMH IRB 116271-2018-04). Written informed consent was acquired from all participants after a complete study description was provided.
Assessment of clinical variables
Clinical characteristics of bipolar patients were acquired through the Korean version of the MINI. The severity of BD was evaluated based on the Clinical Global Impression for Bipolar Disorder-Overall Severity (CGI-BP-OS) [26]. The levels of childhood trauma, coexisting anxiety, and impulsivity in the patient group were assessed with standard self-reported scales; the Korean version-Childhood Trauma Questionnaire (CTQ) [27], the Korean version-Beck Anxiety Inventory (BAI) [28], and the Korean version-Barratt Impulsiveness Scale (BIS) [29], respectively. The CTQ is a widely used screening tool which aims to detect experiences of childhood abuse and neglect in adults and adolescents [30]. The reliability of the CTQ has been demonstrated in patients with BD [31]. In addition, we intended to measure the severity of current anxiety and impulsivity. The BAI is a reliable instrument for evaluating current anxiety [32]. The BIS was used to assess the severity of current impulsivity [33], and the reliability of the BIS has been proved in patients with BD [34]. All of these self-reported scales have been translated to Korean and validated in Korean subjects [35-37].
Image data acquisition
Brain images of the patients and HC were obtained using 3 Tesla MRI scanner (Ingenia CX, Philips, Erlangen, Germany) equipped with a 32-channel head coil at the National Center for Mental Health. High-resolution anatomical T1-weighted (T1W) images were acquired with a turbo field echo sequence (spin-echo TR=9.8 ms, TE=4.6 ms, matrix size: 240×240, 170 sagittal slices, FOV=240 mm, slice thickness=1 mm, and flip angle=8°).
Image data processing and analyses
T1W images of the subjects were processed through the VBM analysis pipeline of the Statistical Parametric Mappingversion 12 (SPM12) software (Wellcome Department of Imaging Neuroscience, University College, London, UK) (http://www.fil.ion.ucl.ac.uk/spm/). The T1W images were segmented into gray matter, white matter, and other including cerebrospinal fluid, using the New Segmentation option in SPM12. Segmented T1W gray matter maps were warped to the Montreal Neurological Institute (MNI) 152 template. The goal of this normalization procedure was to reflect individual characteristics of cranial morphology and improve the accuracy of the analysis. Next, modulation was conducted by multiplying gray matter images to the Jacobian determinates of the deformation fields from the normalization process. The modulation ensured that the total amount of gray matter in each voxel was preserved. Finally, modulated gray matter images were smoothed with a Gaussian kernel of 8×8×8 mm3 full width at half-maximum (FWHM).
We initially carried out voxel-wise group comparisons of GMV over the whole brain between BD patients and HC using analysis of covariance (ANCOVA) with age, sex and intracranial volume (ICV) as covariates. Clusters were reported as significantly different if they survived the false discovery rate (FDR) correction for multiple comparisons (p<0.01).
Finally, partial correlation analyses were conducted within the BD group to examine the correlations between mean values of GMV in the regions that survived the group comparisons and the scores of the CTQ, BAI, and BIS, controlling for age, sex and ICV (p<0.05). To explore the effects of other clinical variables on the correlations, further partial correlation analyses were performed with additional covariates such as onset age, illness duration, number of mood episodes, and the CGI-BP-OS.
Statistical analyses of clinical and socio-demographic data
To compare demographic data between the BD group and HC, independent-sample t-tests and chi-square tests were employed for continuous and categorical variables, respectively. A partial correlation analysis was performed to investigate the relationships between the clinical scales (CTQ, BAI, and BIS), adjusting for the CGI-BP-OS scores to minimize confounding effects of current mood states. Moreover, additional partial correlation analyses between the scales were conducted adjusting for onset age, illness duration, number of mood episodes and the CGI-BP-OS individually and concomitantly to investigate the effects of various clinical factors on the relationships.
All statistical analyses were conducted using Statistical Package for the Social Science (SPSS) (IBM Corp., Released 2012. IBM SPSS Statistics for Windows, Version 21.0. IBM Corp., Armonk, NY, USA).
RESULTS
Demographics and clinical characteristics of subjects
Demographic and clinical data of the participants are presented in Table 1. The mean age of the 36 BD patients was 30.6 years, and 55.6% of them were female. There were no significant differences in age and gender between the patients and the HC. The BD patients had significantly shorter durations of education than HC. The majority of the patients were diagnosed with BD I. Mean onset age was 22.7 years and mean illness duration was 7.9 years, with 6.6 mood episodes on average. The mean CGI-BP-OS scores of the patients was 5.0, and the mean CTQ, BAI, and BIS scores were 50.8, 13.2, and 59.9, respectively. All patients were receiving combination pharmacotherapy with 2.8 psychotropic medications on average, at least one antipsychotics or mood stabilizer.
Table 1.
Demographic and clinical characteristics of the subjects
| Patients (N=36) | Controls (N=29) | |
|---|---|---|
| Demographics | ||
| Age (years, mean±SD) | 30.6±8.3 | 29.3±5.3 |
| Female (%) | 55.6 | 65.6 |
| Duration of education (year, mean±SD) | 13.3±2.1* | 16.1±1.0 |
| Other clinical characteristics | ||
| BD type I (%) | 88.9 | N/A |
| Onset age (year, mean±SD) | 22.7±6.0 | N/A |
| Illness duration (year, mean±SD) | 7.9±8.3 | N/A |
| Number of mood episodes (mean±SD) | 6.6±8.0 | N/A |
| CGI-BP-OS (current, mean±SD) | 5.0±1.3 | N/A |
| CTQ (mean±SD) | 50.8±15.5 | N/A |
| BAI (mean±SD) | 13.2±13.1 | N/A |
| BIS (mean±SD) | 59.9±14.4 | N/A |
| Current psychotropic use | ||
| Number of psychotropics (mean±SD) | 2.8±0.7 | N/A |
| Complex pharmacotherapy (%) | 100.0 | N/A |
| Antipsychotics (%) | 88.9 | N/A |
| Mood stabilizers (%) | 88.9 | N/A |
p<0.05 bipolar patients versus healthy controls.
SD: standard deviation, BD: bipolar disorder, CGI-BP-OS: Clinical Global Impression for Bipolar Disorder-Overall Severity, CTQ: Childhood Trauma Questionnaire, BAI: Beck Anxiety Inventory, BIS: Barratt Impulsiveness Scale
Relationships between childhood trauma, anxiety, and impulsivity
The partial correlation analysis adjusted for the CGI-BP-OS scores revealed significantly positive correlations between the CTQ, BAI, and BIS scores (Table 2). The CTQ showed significantly positive associations with the BAI (r=0.439, p=0.008) and the BIS (r=0.488, p=0.003). In addition, there was a significantly positive association between the BAI and BIS (r=0.480, p=0.004). These correlations remained significantly after adjusting for the CGI-BP-OS, onset age, illness duration, and number of mood episodes individually and concomitantly. These findings suggest that the severity of childhood trauma, current anxiety, and impulsivity in BD are significantly interrelated.
Table 2.
Partial correlation analysis between CTQ, BAI, and BIS scores in BD group
p<0.01.
CTQ: Childhood Trauma Questionnaire, BAI: Beck Anxiety Inventory, BIS: Barratt Impulsiveness Scale, BD: bipolar disorder
Group comparisons of GMV between BD patients and HC
Patients with BD compared to HC showed significantly decreased GMV in multiple cortical and subcortical areas including the bilateral hippocampus, bilateral precentral gyrus, left inferior temporal gyrus, right fusiform gyrus, left thalamus, right insular lobe, and left middle frontal gyrus (Table 3). According to antecedent literatures, the temporal cortex involving the hippocampal-amygdala complex, the thalamus, and the middle frontal gyrus within the prefrontal area seem to be the main brain structures involved in the pathophysiology of BD [38-40]. No brain region exhibited significantly increased GMV in the BD group compared to HC.
Table 3.
Brain regions with significant differences in GMV between BD patients and HC
| Anatomical region | Side | t max | Peak coordinates (MNI) |
Cluster size (voxels) | p value (FDR corrected) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BD<HCs | |||||||
| Hippocampus | L | 6.135 | -36 | -4.5 | -22 | 4,843 | 0.0024 |
| Precentral gyrus | L | 5.468 | -45 | -4.5 | 28 | 685 | 0.0024 |
| Hippocampus | R | 4.858 | 28.5 | -9 | -16 | 392 | 0.0041 |
| Precentral gyrus | R | 4.633 | 34.5 | -16.5 | 39 | 297 | 0.0058 |
| Inferior temporal gyrus | L | 4.878 | -57 | -24 | -24 | 213 | 0.0040 |
| Fusiform gyrus | R | 4.650 | 21 | -48 | -15 | 159 | 0.0057 |
| Thalamus | L | 4.524 | 1.5 | -7.5 | 9 | 115 | 0.0069 |
| Insular lobe | R | 4.480 | 33 | 28.5 | -1 | 98 | 0.0073 |
| Middle frontal gyrus | L | 4.626 | -18 | 46.5 | 15 | 81 | 0.0059 |
Note that all MNI coordinates of maximum t values are selected in the significant regions. GMV: gray matter volume, BD: bipolar disorder, HC: healthy controls, MNI: Montreal Neurological Institute, FDR: false discovery rate, L: left, R: right
Associations between regional GMV alterations and clinical variables in BD patients
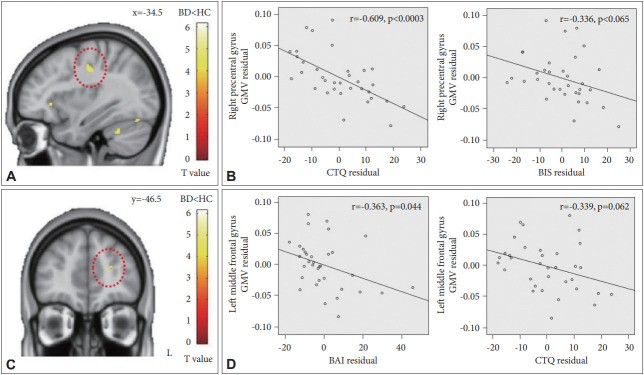
The results of the VBM analyses are presented in Figure 1. Patients with BD showed a significant inverse correlation between the mean GMV of the right precentral gyrus and the CTQ score (r=-0.609, p<0.0003), and a similar tendency of a negative association between the GMV of the right precentral gyrus and the BIS score (r=-0.336, p=0.065). Moreover, there was a significant inverse correlation between the mean GMV of the left middle frontal gyrus and the BAI score (r=-0.363, p=0.044), and a similar tendency of a negative association between the GMV of the left middle frontal gyrus and the CTQ score (r=-0.339, p=0.062).
Figure 1.
Brain regions of correlations with childhood trauma, current anxiety, and impulsivity in the bipolar group. Red circles indicate (A) the right precentral gyrus and (C) the left middle frontal gyrus. (B) Partial correlations between the clinical scale scores (CTQ and BIS) and GMV in the right precentral gyrus. (D) Partial correlations between the clinical scale scores (BAI and CTQ) and GMV in the left middle frontal gyrus. Linear regression fit lines were created using the least squares method. To determine partial correlations, variables were regressed onto covariates using a linear regression. Calculated non-standardized residuals were used to produce scatter plots. BD: bipolar disorder, HC: healthy controls, GMV: gray matter volume, CTQ: Childhood Trauma Questionnaire, BIS: Barratt Impulsiveness Scale, BAI: Beck Anxiety Inventory.
With respect to the right precentral gyrus, these correlations remained significantly after adjusting for onset age, illness duration, number of mood episodes, and the CGI-BP-OS. For the left middle frontal gyrus, the findings still appeared with adjusting for number of mood episodes and CGI-BP-OS whereas the statistical power was moderately weakened when adjusting for onset age or illness duration. The tendency of the inverse relationships between the mean GMV of the left middle frontal gyrus and the score of above-mentioned scales was still maintained.
DISCUSSION
The main results of this study are as follows: 1) the severity of childhood trauma, comorbid anxiety, and impulsivity were interrelated in patients with BD, 2) BD patients showed significant inverse correlations between the GMV in the right precentral gyrus and CTQ scores; between the GMV in the left middle frontal gyrus and BAI scores, and 3) BD patients exhibited similar tendencies of negative associations between the GMV in the right precentral gyrus and BIS scores; between the GMV in the left middle frontal gyrus and CTQ scores. Our findings support the hypothesis that childhood trauma, anxiety, and impulsivity are correlated with GMV alterations in overlapping regions within the BD group.
There is an extensive body of neuroimaging literatures which elucidate the neurobiological mechanisms underlying BD. However, the mainstream methodology in previous studies has been a group comparison between patients and normal individuals, or between BD patients with and without a certain clinical factor. It is a strength of our study that we explored correlations among various factors emerging as indicators of worse outcomes of BD and investigated the relationships between these clinical variables and neuroanatomical changes within a bipolar group.
In the recent years interest has been paid to the relationships among childhood trauma, current anxiety, and impulsivity, but the evidence for such links in BD is limited. Our findings are in agreement with the few preceding studies, which reported that childhood abuse was significantly associated with affective instability in BD regardless of current mood states [17] and that subjects with BD showed considerably higher impulsivity when anxiety was present either as a comorbidity or a symptom [18]. In bipolar patients, adverse experiences in early life may affect mood dysregulation based on a neurobiological mechanism linked to anxiety and impulsivity [17,41].
In accordance with the significant associations between childhood trauma, anxiety, and impulsivity, our neuroimaging study also indicated that the cortical volumetric correlates of the three clinical variables overlapped in the right precentral gyrus and the left middle frontal gyrus within the BD group. Previous studies pointed out that decreased GMV of the orbitofrontal cortex in BD patients compared to a healthy group was only observed in subjects with a history of childhood maltreatment [22], and that CTQ scores in a BD group were inversely correlated with GMV in the right dorsolateral prefrontal cortex and the right thalamus [19]. We found a significantly negative correlation between the CTQ scores and the GMV in the right precentral gyrus, and a similar tendency in the left middle frontal gyrus. There results may represent neural evidence of the impact of childhood abuse on emotional control in BD. In addition, a few neuroimaging studies reported that bipolar patients with anxiety and impulsivity exhibited GMV loss in the posteromedial rectal gyrus and the anterior cingulate cortex, respectively [42,43]. We observed significant associations between GMV deficits in the left middle frontal gyrus and anxiety, and a similar tendency between GMV deficits in the right precentral gyrus and impulsivity. Although these correlations in the left middle frontal gyrus might be affected by onset age or illness duration, similar tendency of the findings remained in the present study. Taken together, our results on cortical volumetric correlates of childhood trauma are partially replicated, whereas neuronal correlates of anxiety and impulsivity are inconsistent with earlier researches. This discrepancy may be due to heterogeneity of subject samples as well as dissimilarities in methods including image acquisition, processing and analysis.
The precentral gyrus, identified as a structural correlate of childhood trauma in BD in this study, includes the primary motor cortex responsible for voluntary movement. It is also believed that the precentral gyrus is involved in cognitive processing and emotional regulation [44-46]. Several structural MRI studies demonstrated abnormalities in the precentral gyrus in BD, and provided evidence that the dysfunction in the bipolar brain occurred not only in regions related to emotional processing, but also in the traditional motor cortex-precentral gyrus [47-49]. The middle frontal gyrus, discovered as a structural correlate of current anxiety in BD in the present study, is located in the prefrontal areas and plays an important role in the prefrontal-limbic system. The middle frontal gyrus includes parts of Brodmann areas (BA) 9, 10, and 46, which are related to the emotion regulation and pathophysiology of BD [50-54]. BA 9 on the left has been reported to be associated with the processing of positive and negative emotional information [55,56]. BA 10, one of the most poorly understood regions in the human brain, is possibly engaged in integrating consequences of multiple cognitive operations for achieving a higher goal in the next behavior [54]. Furthermore, BA 46 is thought to be involved in high-level cognitive processing such as planning [57]. Earlier studies have revealed that the middle frontal gyrus has dense connections with the amygdala and other limbic structures related to emotional regulation [58]. Meanwhile, a meta-study reported that associations between cortical thickness in BD and illness duration or onset age were mostly non-significant and there was the scarcity of study pointing out that cortical thinning was related to illness duration of BD in certain frontal areas such as left middle frontal cortex [59,60]. In summary, the current findings present evidence of abnormalities in multiple frontal lobe areas in BD that process nonverbal cues as well as emotional stimuli associated with childhood trauma and affect regulation.
This study has some clinical and academic implications. First, histories of childhood trauma, comorbid anxiety, and impulsivity need to be simultaneously considered and appropriately assessed in the management of BD. Literature review has emphasized these clinical factors as determinants of unfavorable outcomes of BD and risk factors for suicidality [11,13,61,62]. One previous study in particular proposed that bipolar populations with early life stress and comorbid anxiety might comprise a distinct etiological type of BD [61]. Our findings suggest that bipolar patients with a history of childhood trauma, current anxiety, and impulsivity may constitute a discrete group with a worse prognosis. Second, the GMV alterations in the precentral gyrus and middle frontal gyrus may be used as potential neurobiological markers for the categorization of patients with BD, or as a complementary tool for diagnosing and staging BD [63,64]. These implications can contribute to a more individualized assessment and treatment of BD.
We have noticed some limitations in this study. First, the relatively small sample size and the enrollment from only one institution may limit the generalizability of the present findings. The number of subjects in recent studies investigating neuronal correlates of clinical variables in mood disorders was 20–39 [19,20,65]. However, we recruited not only patients but also HC to overcome this limitation. Second, effects of psychotropic medications on brain structures may need to be considered. Nonetheless, the statistical effect of such treatment on the present findings seemed to be very weak, since our patient sample was very homogenous regarding psychotropic medications (Table 1) [66]. Third, the scales measuring clinical variables (CTQ, BAI, and BIS) were all self-reported. Especially for the CTQ, a retrospective report, biases may occur when adults recall adverse memories from their childhood [67]. Nevertheless, a longitudinal cohort study reported that CTQ scores were consistent with prospective self-reports of childhood trauma exposure [68]. Fourth, the design of this study was cross-sectional, based on an one-time assessment. Longitudinal investigations may be helpful to underpin the causal relationships among childhood trauma, structural brain changes, and clinical outcomes of BD [69].
Despite these limitations, this is the first study demonstrating that in BD, childhood trauma, anxiety, and impulsivity are interrelated and that the cortical volumetric correlates of these clinical variables overlap in the right precentral gyrus and the left middle frontal gyrus. The present findings suggest that childhood trauma, anxiety, and impulsivity may affect outcomes of BD based on neural mechanism, and that the GMV changes could be used as neurobiological markers predicting the prognosis of BD. Further studies are warranted to establish more evidence for the application of the current results in clinical practice.
Acknowledgments
This research was supported by a grant from the National Center for Mental Health (2019-01). The current data were also presented at the regional meeting of the Korean Neuropsychiatric Association in Busan, October 12-13, 2018.
Footnotes
The authors have no potential conflicts of interest to disclose.
Author contribution
Conceptulization: Vin Ryu, Dong Yeon Park. Data curation: Hyehyun Song, Rina Yu. Formal analysis: Hyeongrae Lee, Wonhye Lee. Funding acquisition: Dong Yeon Park. Investigation: Hyehyun Song, Rina Yu, Dong Yeon Park. Methodology: Dong-Kyun Lee. Project administration: Dong Yeon Park. Resources: Vin Ryu, Rina Yu, Jung Hyun Lee. Software: Dong-Kyun Lee. Supervision: Myong-Wuk Chon, Hyeongrae Lee, Dong Yeon Park. Validation: Myong-Wuk Chon, Hyeongrae Lee, Wonhye Lee, Dong Yeon Park. Visualization: Hyeongrae Lee, Wonhye Lee. Writing—original draft: Hyehyun Song, Dong Yeon Park. Writing—review & editing: Dong Yeon Park.
REFERENCES
- 1.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–1572. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- 2.Park DY, Goffin KC, Shah S, Yuen LD, Holtzman JN, Hooshmand F, et al. Differential prevalence and demographic and clinical correlates of second-generation antipsychotic use in bipolar I versus bipolar II disorder. J Psychiatr Res. 2016;76:52–58. doi: 10.1016/j.jpsychires.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134:91–103. doi: 10.1111/acps.12581. [DOI] [PubMed] [Google Scholar]
- 4.Reinares M, Papachristou E, Harvey P, Mar Bonnin C, Sanchez-Moreno J, Torrent C, et al. Towards a clinical staging for bipolar disorder: defining patient subtypes based on functional outcome. J Affect Disord. 2013;144:65–71. doi: 10.1016/j.jad.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Schaffer A, Isometsa ET, Tondo L, Moreno DH, Turecki G, Reis C, et al. International Society for Bipolar Disorders Task Force on Suicide: meta-analyses and meta-regression of correlates of suicide attempts and suicide deaths in bipolar disorder. Bipolar Disord. 2015;17:1–16. doi: 10.1111/bdi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugaya L, Hasin DS, Olfson M, Lin KH, Grant BF, Blanco C. Child physical abuse and adult mental health: a national study. J Trauma Stress. 2012;25:384–392. doi: 10.1002/jts.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnew-Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:342–349. doi: 10.1016/S2215-0366(15)00544-1. [DOI] [PubMed] [Google Scholar]
- 8.Post RM, Altshuler LL, Kupka R, McElroy SL, Frye MA, Rowe M, et al. Verbal abuse, like physical and sexual abuse, in childhood is associated with an earlier onset and more difficult course of bipolar disorder. Bipolar Disord. 2015;17:323–330. doi: 10.1111/bdi.12268. [DOI] [PubMed] [Google Scholar]
- 9.Azorin JM, Kaladjian A, Adida M, Hantouche EG, Hameg A, Lancrenon S, et al. Psychopathological correlates of lifetime anxiety comorbidity in bipolar I patients: findings from a French national cohort. Psychopathology. 2009;42:380–386. doi: 10.1159/000241193. [DOI] [PubMed] [Google Scholar]
- 10.Pavlova B, Perlis RH, Alda M, Uher R. Lifetime prevalence of anxiety disorders in people with bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:710–717. doi: 10.1016/S2215-0366(15)00112-1. [DOI] [PubMed] [Google Scholar]
- 11.Pavlova B, Perroud N, Cordera P, Uher R, Alda M, Dayer A, et al. Anxiety disorders and childhood maltreatment as predictors of outcome in bipolar disorder. J Affect Disord. 2018;225:337–341. doi: 10.1016/j.jad.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Perroud N, Baud P, Preisig M, Etain B, Bellivier F, Favre S, et al. Social phobia is associated with suicide attempt history in bipolar inpatients. Bipolar Disord. 2007;9:713–721. doi: 10.1111/j.1399-5618.2007.00471.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SL, Carver CS, Tharp JA. Suicidality in bipolar disorder: the role of emotion-triggered impulsivity. Suicide Life Threat Behav. 2017;47:177–192. doi: 10.1111/sltb.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malloy-Diniz LF, Neves FS, de Moraes PH, De Marco LA, RomanoSilva MA, Krebs MO, et al. The 5-HTTLPR polymorphism, impulsivity and suicide behavior in euthymic bipolar patients. J Affect Disord. 2011;133:221–226. doi: 10.1016/j.jad.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez E, Arias B, Mitjans M, Goikolea JM, Ruiz V, Brat M, et al. Clinical features, impulsivity, temperament and functioning and their role in suicidality in patients with bipolar disorder. Acta Psychiatr Scand. 2016;133:266–276. doi: 10.1111/acps.12548. [DOI] [PubMed] [Google Scholar]
- 17.Marwaha S, Gordon-Smith K, Broome M, Briley PM, Perry A, Forty L, et al. Affective instability, childhood trauma and major affective disorders. J Affect Disord. 2016;190:764–771. doi: 10.1016/j.jad.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Bellani M, Hatch JP, Nicoletti MA, Ertola AE, Zunta-Soares G, Swann AC, et al. Does anxiety increase impulsivity in patients with bipolar disorder or major depressive disorder? J Psychiatr Res. 2012;46:616–621. doi: 10.1016/j.jpsychires.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Duarte DG, Neves Mde C, Albuquerque MR, de Souza-Duran FL, Busatto G, Correa H. Gray matter brain volumes in childhood-maltreated patients with bipolar disorder type I: a voxel-based morphometric study. J Affect Disord. 2016;197:74–80. doi: 10.1016/j.jad.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Simeonova DI, Jackson V, Attalla A, Karchemskiy A, Howe M, Adleman N, et al. Subcortical volumetric correlates of anxiety in familial pediatric bipolar disorder: a preliminary investigation. Psychiatry Res. 2009;173:113–120. doi: 10.1016/j.pscychresns.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strakowski SM, DelBello MP, Adler C, Cecil KM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 22.Poletti S, Vai B, Smeraldi E, Cavallaro R, Colombo C, Benedetti F. Adverse childhood experiences influence the detrimental effect of bipolar disorder and schizophrenia on cortico-limbic grey matter volumes. J Affect Disord. 2016;189:290–297. doi: 10.1016/j.jad.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 24.Yoo SW, Kim YS, Noh JS, Oh KS, Kim CH, NamKoong K, et al. Validity of Korean version of the mini-international neuropsychiatric interview. Anxiety Mood. 2006;2:50–55. [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Arlington, VA: American Psychiatric Pub; 2013. [Google Scholar]
- 26.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 29.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Grassi-Oliveira R, Cogo-Moreira H, Salum GA, Brietzke E, Viola TW, Manfro GG, et al. Childhood Trauma Questionnaire (CTQ) in Brazilian samples of different age groups: findings from confirmatory factor analysis. PLoS One. 2014;9:e87118. doi: 10.1371/journal.pone.0087118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etain B, Mathieu F, Henry C, Raust A, Roy I, Germain A, et al. Preferential association between childhood emotional abuse and bipolar disorder. J Trauma Stress. 2010;23:376–383. doi: 10.1002/jts.20532. [DOI] [PubMed] [Google Scholar]
- 32.Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. J Anxiety Disord. 1992;6:55–61. [Google Scholar]
- 33.Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Individ Diff. 2009;47:385–395. [Google Scholar]
- 34.Peluso M, Hatch J, Glahn D, Monkul E, Sanches M, Najt P, et al. Trait impulsivity in patients with mood disorders. J Affect Disord. 2007;100:227–231. doi: 10.1016/j.jad.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Park SC, Yang H, Oh DH. Reliability and validity of the Korean version of the childhood trauma questionnaire-short form for psychiatric outpatients. Psychiatry Investig. 2011;8:305–311. doi: 10.4306/pi.2011.8.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yook S, Kim Z. A clinical study on the Korean version of Beck Anxiety Inventory: comparative study of patient and non-patient. Korean J Clin Psychol. 1997;16:185–197. [Google Scholar]
- 37.Lee SR, Lee WH, Park JS, Kim SM, Kim JW, Shim JH. The study on Reliability and Validity of Korean Version of the Barratt Impulsiveness Scale-11-Revised in nonclinical adult subjects. J Korean Neuropsychiatr Assoc. 2012;51:378–386. [Google Scholar]
- 38.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98. doi: 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 41.Aas M, Henry C, Andreassen OA, Bellivier F, Melle I, Etain B. The role of childhood trauma in bipolar disorders. Int J Bipolar Disord. 2016;4:2. doi: 10.1186/s40345-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida JR, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, et al. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuo K, Nicoletti MA, Peluso MA, Hatch JP, Nemoto K, Watanabe Y, et al. Anterior cingulate volumes associated with trait impulsivity in individuals with bipolar disorder. Bipolar Disord. 2009;11:628–636. doi: 10.1111/j.1399-5618.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 44.Seo D, Olman CA, Haut KM, Sinha R, MacDonald AW, 3rd, Patrick CJ. Neural correlates of preparatory and regulatory control over positive and negative emotion. Soc Cogn Affect Neurosci. 2014;9:494–504. doi: 10.1093/scan/nst115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas LA, Brotman MA, Bones BL, Chen G, Rosen BH, Pine DS, et al. Neural circuitry of masked emotional face processing in youth with bipolar disorder, severe mood dysregulation, and healthy volunteers. Dev Cogn Neurosci. 2014;8:110–120. doi: 10.1016/j.dcn.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, Wang Y, Jia Y, Zhong S, Sun Y, Qi Z, et al. Altered interhemispheric functional connectivity in remitted bipolar disorder: a resting state fMRI study. Sci Rep. 2017;7:4698. doi: 10.1038/s41598-017-04937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eker C, Simsek F, Yilmazer EE, Kitis O, Cinar C, Eker OD, et al. Brain regions associated with risk and resistance for bipolar I disorder: a voxel-based MRI study of patients with bipolar disorder and their healthy siblings. Bipolar Disord. 2014;16:249–261. doi: 10.1111/bdi.12181. [DOI] [PubMed] [Google Scholar]
- 48.Fung G, Deng Y, Zhao Q, Li Z, Qu M, Li K, et al. Distinguishing bipolar and major depressive disorders by brain structural morphometry: a pilot study. BMC Psychiatry. 2015;15:298. doi: 10.1186/s12888-015-0685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorgensen KN, Nerland S, Norbom LB, Doan NT, Nesvag R, MorchJohnsen L, et al. Increased MRI-based cortical grey/white-matter contrast in sensory and motor regions in schizophrenia and bipolar disorder. Psychol Med. 2016;46:1971–1985. doi: 10.1017/S0033291716000593. [DOI] [PubMed] [Google Scholar]
- 50.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic Definition of Prefrontal Areas in the Normal Human Cortex: I. Remapping of Areas 9 and 46 using Quantitative Criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 52.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic Definition of Prefrontal Areas in the Normal Human Cortex: II. Variability in Locations of Areas 9 and 46 and Relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 53.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833-857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 55.Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, et al. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012;42:29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- 56.Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 57.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 58.Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 59.Hanford LC, Nazarov A, Hall GB, Sassi RB. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18:4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- 60.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 61.Pavlova B, Perroud N, Cordera P, Uher R, Dayer A, Aubry JM. Childhood maltreatment and comorbid anxiety in people with bipolar disorder. J Affect Disord. 2016;192:22–27. doi: 10.1016/j.jad.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Richard-Lepouriel H, Kung AL, Hasler R, Bellivier F, Prada P, Gard S, et al. Impulsivity and its association with childhood trauma experiences across bipolar disorder, attention deficit hyperactivity disorder and borderline personality disorder. J Affect Disord. 2019;244:33–41. doi: 10.1016/j.jad.2018.07.060. [DOI] [PubMed] [Google Scholar]
- 63.Kapczinski F, Dias VV, Kauer-Sant’Anna M, Brietzke E, Vazquez GH, Vieta E, et al. The potential use of biomarkers as an adjunctive tool for staging bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1366–1371. doi: 10.1016/j.pnpbp.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 64.Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381:1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YJ, Kim S, Gwak AR, Kim SJ, Kang SG, Na KS, et al. Decreased regional gray matter volume in suicide attempters compared to suicide non-attempters with major depressive disorders. Compr Psychiatry. 2016;67:59–65. doi: 10.1016/j.comppsych.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Lyoo IK, Dager SR, Kim JE, Yoon SJ, Friedman SD, Dunner DL, et al. Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacology. 2010;35:1743–1750. doi: 10.1038/npp.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maughan B, Rutter M. Retrospective reporting of childhood adversity: issues in assessing long-term recall. J Pers Disord. 1997;11:19–33. doi: 10.1521/pedi.1997.11.1.19. [DOI] [PubMed] [Google Scholar]
- 68.Liebschutz JM, Buchanan-Howland K, Chen CA, Frank DA, Richardson MA, Heeren TC, et al. Childhood Trauma Questionnaire (CTQ) correlations with prospective violence assessment in a longitudinal cohort. Psychol Assess. 2018;30:841–845. doi: 10.1037/pas0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dusi N, De Carlo V, Delvecchio G, Bellani M, Soares JC, Brambilla P. MRI features of clinical outcome in bipolar disorder: a selected review: special section on “Translational and neuroscience studies in affective disorders.” Section Editor, Maria Nobile MD, PhD. This section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J Affect Disord. 2019;243:559–563. doi: 10.1016/j.jad.2018.05.066. [DOI] [PubMed] [Google Scholar]