Abstract
Objective:
The aim of this study is to assess the financial burden associated with treatment options for resectable pancreatic cancer.
Background:
As the volume of cancer care increases in the United States, there is growing interest among both clinicians and policy-makers to reduce its financial impact on the healthcare system. However, costs relative to the survival benefit for differing treatment modalities used in practice have not been described.
Methods:
Patients undergoing resection for pancreatic cancer were identified in the Truven Health MarketScan database. Associations between chemoradiation therapies and survival were performed using parameterized multivariable accelerated failure time models. Median payments over time were calculated for surgery, chemoradiation, and subsequent hospitalizations.
Results:
A total of 2408 patients were included. Median survival among all patients was 21.1 months [95% confidence interval (CI): 19.8–22.5 months], whereas median follow-up time was 25.1 months (95% CI: 23.5–26.5 months). After controlling for comorbidity, receipt of neoadjuvant therapy, and nodal involvement, a longer survival was associated with undergoing combination gemcitabine and nab-paclitaxel [time ratio (TR) = 1.26, 95% CI: 1.02–1.57, P = 0.035) or capecitabine and radiation (TR = 1.25, 95% CI: 1.04–1.51, P = 0.018). However, median cumulative paymentsvfor gemcitabine with nab-paclitaxel were highest overall [median $74,051, interquartile range (IQR): $38,929–$133,603).
Conclusions:
Total payments for an episode of care relative to improvement in survival vary significantly by treatment modality. These data can be used to inform management decisions about pursuing further care for pancreatic cancer. Future investigations should seek to refine estimates of the cost-effectiveness of different treatments.
Keywords: financial toxicity, pancreatic cancer, survival post-surgery
Cancer is one of the leading causes of death in the United States and incident cases are expected to double in the coming decades.1 Consequently, cancer care has grown faster than nearly all other healthcare sectors, yet the financial implications for patients, families, providers, and third-party payors remains largely unexplored.2 Pancreatic cancer, which represents the fourth leading cause of cancer-related death nationwide,3 is a cancer where the tradeoff between costs and survival is potentially steep. Despite advances in diagnostic techniques and adjuvant therapies, prognosis for pancreatic cancer remains poor with 5-year overall survival ranging from 10% to 20% even among patients amenable to treatment such as surgical resection, systemic chemotherapy, and/or radiation therapy.4 Given pancreatic cancer’s relative poor prognosis, it is important to view the potential survival benefit of different treatment modalities in light of their respective costs to the healthcare system.
As healthcare costs increase, insurers have shifted some of the cost burden to patients via higher deductibles, rising copayments, and coinsurance.2 Such maneuvers have led to rising healthcare expenses and heightened concerns regarding the “financial toxicity”; for patients receiving expensive treatments, as well as the healthcare system in general.5 Several studies have demonstrated the adverse effects of the financial burden of care on patients’ quality of life and treatment decisions.6 For example, rising costs have been associated with individuals diagnosed with cancer taking fewer medications than prescribed, replacing prescription medications with over-the-counter drugs, skipping appointments or declining procedures, and forgoing or prematurely discontinuing their treatment.6–9 Collectively, these studies have led some clinicians to argue that the growing financial burden of certain therapies should be taken into consideration in cancer management and care planning.5,10
Currently, the payments associated with cancer care can vary markedly between patients, with little information available regarding the expenses incurred for each assigned treatment regimen.11–13 In particular, data on the topic of the financial burden associated with treatment of pancreatic cancer are particularly limited. In a study using data now over a decade old, Chang et al estimated >$7000 in estimated differences in average monthly costs among commercially insured patients with pancreatic cancer compared with matched controls, with costs nearly doubling in the later stages of disease.14 In a more recent investigation using estimates of costs from Medicare fee schedules combined with estimated median survival reported in clinical trials, Goldstein et al15 estimated that monthly costs of treatments ranged from just over $1300 to approximately $12,200.
Although these 2 studies provided a basis for considering the value of care, no study to date has specifically examined patients with potentially resectable disease. The implications of both upfront payments for surgery, as well as payments for neoadjuvant or adjuvant therapy, are most salient in this group of patients. As such, the objective of the present study was to examine the total payments associated with cancer care (resection, outpatient chemotherapy and radiation treatments, and subsequent hospitalizations) relative to survival of patients with resectable pancreas cancer. Specifically, financial and clinical data from a nationally representative cohort of commercially insured patients with pancreatic cancer were analyzed to assess the financial burden associated with treatment options for resectable pancreatic cancer.
METHODS
Data Source and Patient Population
Data were obtained from the Truven Health MarketScan Commercial Claims and Encounters Database between January 1, 2010 and December 31, 2014. The Truven Health MarketScan Database is a medical and drug insurance claims database that collects data from over 138 million unique, de-identified patients including active employees, early retirees, Consolidated Omnibus Budget Reconciliation Act (COBRA) continuers, and their dependents who are insured by over 100 different employer-sponsored plans. Variables in the database include inpatient admission records, outpatient services, prescription drug claims, populations, and eligibility status. Claims include total payments, net payments, co-payments, and, where applicable, specific payments to providers and/or institutions.16
Patients ≥18 years with a primary diagnosis of pancreatic cancer [International Classification of Diseases, 9th edition (ICD-9-CM) code 157.*) who underwent a total pancreatectomy, pancreaticoduodenectomy, or partial or distal pancreatectomy (ICD-9-CM 52.5*, 52.6*, 52.7) were identified. The presence of lymph node metastases at the time of surgery was also identified using relevant ICD-9-CM diagnosis codes (ICD-9-CM 196.*–199.*). Patient comorbidity was classified according to the Charlson co-morbidity index (CCI) using a previously validated mapping algorithm.17 Similarly, surgical complications during the index admission were identified using a set of ICD-9-CM diagnosis codes as previously described and validated for use in administrative data.18 To limit confounding because of insufficient follow-up, or insufficient time under observation to account for neoadjuvant therapy, patients who did not have at least 6 months of time under observation before or after undergoing resection were excluded from the analysis. Finally, patients with missing data for any included covariates were excluded from analysis. Survival time was accrued from the date of surgery to the following endpoints: death (ascertained by patient records of inpatient mortality or enrollment dropout not due to transition to Medicare) or to the date of last follow-up as of December, 31, 2014 (Fig. 1).
FIGURE 1.
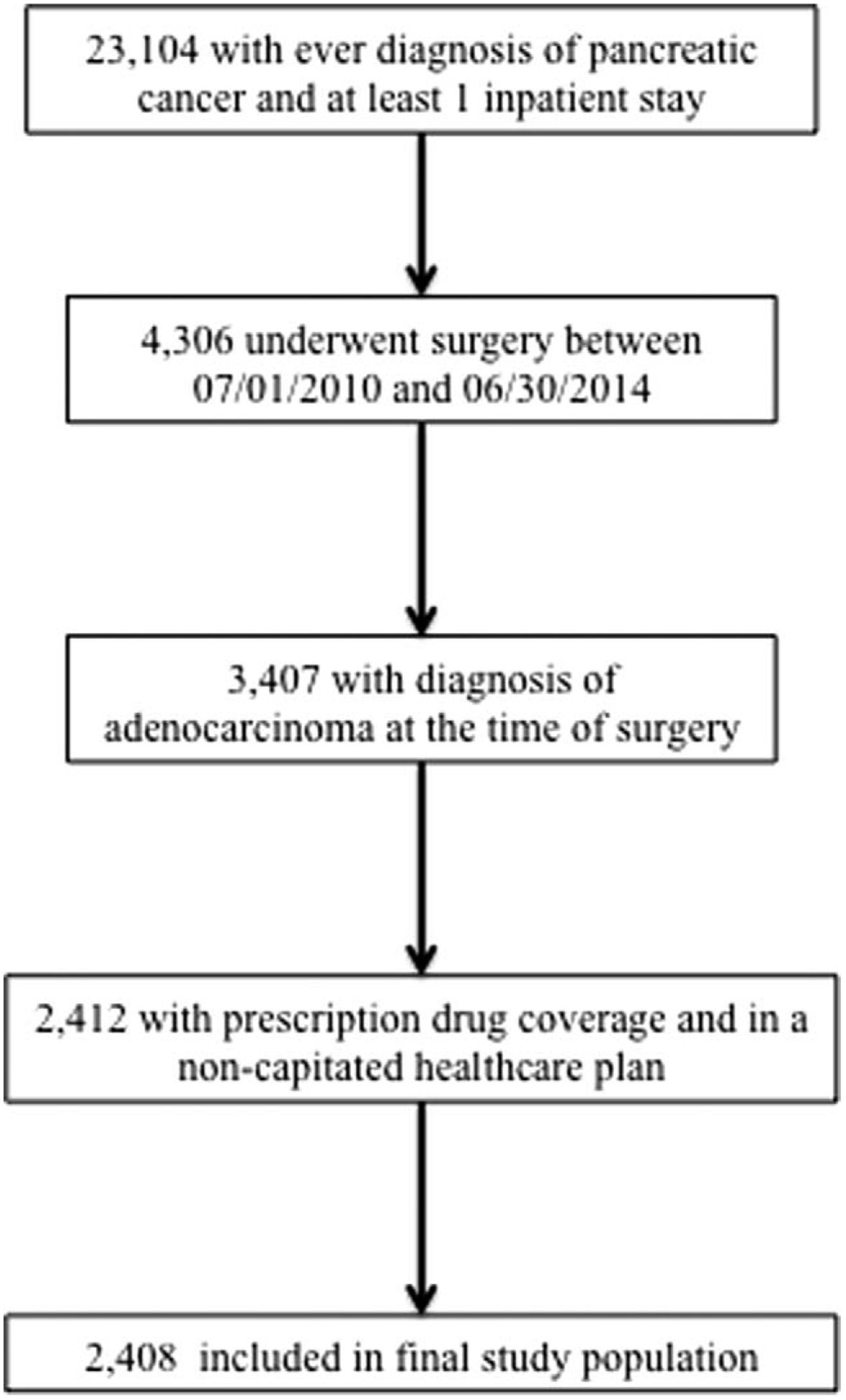
Flow diagram detailing study population selection.
Episodes of inpatient or outpatient chemo- or radiation therapy were identified via Health Care Financing Administration (HCFA) revenue codes, Healthcare Common Procedure Coding System (HCPCS) codes, or procedure group identifiers designated in the MarketScan database. Therapies were designated as adjuvant or neoadjuvant by comparing the date of service for a particular inpatient or outpatient claim (ie, the date of the visit or filling of the prescription), in relation to the date of the hospital admission for surgical resection. The specific type of chemotherapy administered during each episode of care was determined using HCPCS Current Procedural Terminology (CPT) codes. Receipt of oral chemotherapy was identified via National Drug Codes indicated in the MarketScan Pharmacy Claims file. Total payments were derived from the following possible domains of care: hospitalizations, outpatient services/ visits, and outpatient pharmaceutical prescriptions, and converted to 2014 dollars using equivalences defined by the Consumer Price Index.19
Statistical Analysis
Categorical variables were reported as total frequencies and proportions, whereas continuous data were described using means with standard deviation (SD) (if normally distributed) or median values with interquartile range (IQR) (if highly skewed). Univariable analysis was performed using the Pearson chi-squared test and Fisher exact test for categorical data; the Kruskal-Wallis test, or 1-way unpaired/paired Student’s t test was used for continuous data, as appropriate. Overall survival (OS) from the date of surgery was estimated using the Kaplan-Meier method. Median follow-up was calculated using the reverse Kaplan-Meier method of Schemper and Smith.20 The validity of the assumption of proportional hazards over time was assessed using the methods of Grambsch and Therneau (ie, a test of nonzero slope in a generalized linear regression of the scaled Schoenfeld residuals on time).21 Separate parameterized regression models of accelerated failure time under the generalized gamma distribution were constructed using all patients and subpopulations of patients by treatment regimen. Akaike information criterion (AIC) and likelihood-ratio test were used to select the most parsimonious model.22 Regression coefficients were exponentiated and presented as time ratios (TRs) with corresponding 95% confidence intervals (CIs), which denote the relative time to the event of interest, that is, a lower time ratio (<1.00) represents a shorter survival or a faster time to death. For example, a time ratio of 0.75 represents a survival time that is 25% lower than that calculated for the reference group. Linear combinations of model coefficients of clinically relevant combined therapies were exponentiated to determine calculated combined effects of oral chemotherapy with radiation therapy.
Total episode-specific payments were calculated by summing the total payments for services rendered at each outpatient or inpatient encounter. This included total payments for the index surgical hospitalization, for each episode of chemo- or radiation therapy administration, and for each subsequent hospitalization after resection. Median payments per month for chemo- or radiation therapy were calculated for the 6 months preceding resection, and in periods of 6 months following resection. Median per-month payments for hospitalizations following resection were similarly calculated. Variation in payments was reported as interquartile range. Previously reported sensitivity analyses of the effect of exclusionversus imputation of payment data in the MarketScan databases have shown similar results for both, and sensitivity analyses for overall differences in estimated survival time were conducted before excluding patients based on lack of payment data.23 Therefore, patients with capitated claims were excluded from analyses, as total payments for these patients were reported to be approximately zero per episode of care. Similarly, patients who did not have prescription drug coverage reported in the MarketScan database were also excluded, given the possibility of false-negative reporting of use of oral chemotherapy. All analyses were conducted using Stata 14.0 MP (StataCorp LP, College Station, TX). A P value of <0.05 was defined as statistically significant. All patient data were de-identified and compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA); patient consent was therefore waived. The study was approved by the Johns Hopkins University Institutional Review Board.
RESULTS
Study Population
A total of 2408 patients were identified who met inclusion criteria (Table 1). Most patients were male (n = 1230, 51.1%) with a median age of 57 years (IQR: 51–61 years). Just over half of patients had a Charlson co-morbidity index of ≥3 (n = 1332, 55.3%) and approximately one-third of patients had lymph node metastasis (n = 816, 33.9%). When assessing the entire cohort, 39.1% (n = 942) underwent surgery alone without any adjuvant or neo-adjuvant therapy; 55.4% (n = 1333) received adjuvant therapy, and 17.2% (n = 415) received neoadjuvant therapy. A majority of patients (n = 1525, 63.3%) underwent a Whipple procedure; 25.2% (n = 608) patients experienced at least 1 postoperative complication during the index hospitalization (Table 1). Of the 1466 patients who received adjuvant or neoadjuvant chemoradiation, 34.5% (n = 506) received >1 type of regimen. In this group, 72.7% (n = 1066) were treated with gemcitabine (with or without nab-paclitaxel); 24.8% (n = 364) received treatment with an oxaliplatin-based therapy (including FOLFIRINOX), 23.8% (n = 349) had oral capecitabine, and 4.2% (n = 61) received treatment with erlotinib. Among patients who had adjuvant or neoadjuvant therapy, 44.1% (n = 647) received adjuvant radiation only, 15.1% (n = 222) received neoadjuvant radiation only, and 1.5% (n = 22) received both adjuvant and neoadjuvant radiation therapy. The median number of episodes of radiation treatment was 29 (IQR: 13–31), and the median number of episodes of chemotherapy infusion was 13 (IQR: 7–19).
TABLE 1.
Demographic and Clinical Characteristics Among All Patients Stratified By Receipt of Adjuvant Therapy
| No Adjuvant Therapy (n = 1075) | Adjuvant Therapy (n = 1333) | Total (n = 2408) | |
|---|---|---|---|
| Sex | |||
| Male | 506 (47.1) | 724 (54.3) | 1230 (51.1) |
| Female | 569 (52.9) | 609 (45.7) | 1178 (48.9) |
| Age group, y | |||
| 18–34 | 68 (6.3) | 9 (0.7) | 77 (3.2) |
| 35–44 | 106 (9.9) | 82 (6.2) | 188 (7.8) |
| 45–54 | 274 (25.5) | 349 (26.2) | 623 (25.9) |
| 55–64 | 627 (58.3) | 893 (67.0) | 1520 (63.1) |
| Charlson Co-morbidity Index | |||
| ≤2 | 568 (52.8) | 508 (38.1) | 1076 (44.7) |
| >2 | 507 (47.2) | 825 (61.9) | 1332 (55.3) |
| Procedure type | |||
| Proximal pancreatectomy | 15 (1.4) | 13 (1.0) | 28 (1.2) |
| Distal pancreatectomy | 418 (38.9) | 229 (17.2) | 647 (26.9) |
| Radical antegrade pancreaticosplenectomy | 14 (1.3) | 12 (0.9) | 26 (1.1) |
| Other partial pancreatectomy | 60 (5.6) | 22 (1.7) | 82 (3.4) |
| Total pancreatectomy | 42 (3.9) | 58 (4.4) | 100 (4.2) |
| Whipple | 526 (48.9) | 999 (74.9) | 1525 (63.3) |
| Complications hospitalization | |||
| 0 | 783 (72.8) | 1017 (76.3) | 1800 (74.8) |
| 1 | 266 (24.7) | 293 (22.0) | 559 (23.2) |
| 2 or more | 26 (2.4) | 23 (1.7) | 49 (2.0) |
| Nodal involvement | |||
| No | 838 (78.0) | 754 (56.6) | 1592 (66.1) |
| Yes | 237 (22.0) | 579 (43.4) | 816 (33.9) |
| Neoadjuvant therapy | |||
| No | 942 (87.6) | 1051 (78.8) | 1993 (82.8) |
| Yes | 133 (12.4) | 282 (21.2) | 415 (17.2) |
Results from Survival Analysis
The median survival among all patients was 21.1 months (95% CI: 19.8–22.5 months), and median follow-up time was 25.1 months (95% CI: 23.5–26.5 months). On univariable analysis, a worse OS was associated with receipt of neoadjuvant therapy (neoadjuvant chemotherapy: TR = 0.70, 95% CI: 0.60–0.81; neoadjuvant radiation: TR = 0.69, 95% CI: 0.58–0.82), a higher Charlson Co-morbidity Index (TR = 0.67, 95% CI: 0.60–0.74, ref: CCI ≤2), and lymph node involvement at the time of surgery (TR = 0.63, 95% CI: 0.57–0.71, all P < 0.001). In addition, a shorter survival time was associated with receipt of several specific therapies, including radiation with and without concurrent chemotherapy infusion. In a pooled multivariable analysis, shorter survival continued to be associated with lymph node involvement (TR = 0.73, 95% CI: 0.63–0.85, P < 0.001), and a higher Charlson Co-morbidity Index (TR = 0.80, 95% CI: 0.69–0.93, P = 0.003, ref: CCI ≤2), receipt of neoadjuvant radiation (TR = 0.76, 95% CI: 0.61–0.95, P = 0.016), and specifically with radiation with concurrent 5-fluorouracil (TR = 0.83, 95% CI: 0.71–0.98; P = 0.030). In this analysis, a longer survival was associated with receipt of combination gemcitabine and nab-paclitaxel (TR = 1.26, 95% CI: 1.02–1.57, P = 0.035) (Table 2). The calculated association of combined oral capecitabine and radiation therapy was also significant. In particular, a 25% longer survival time was associated with combined oral capecitabine and radiation therapy (TR = 1.25, 95% CI: 1.04–1.51, P =0.018).
TABLE 2.
Results From Univariable and Multivariable Analysis of Factors Associated With Survival
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| TR | 95% CI | P | TR | 95% CI | P | |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.08 | (0.97–1.21) | 0.153 | 1.05 | (0.95–1.17) | 0.343 |
| Procedure type | ||||||
| Proximal pancreatectomy | Reference | Reference | ||||
| Distal pancreatectomy | 1.32 | (0.80–2.17) | 0.272 | 1.35 | (0.83–2.18) | 0.222 |
| Radical antegrade pancreaticosplenectomy | 0.81 | (0.40–1.63) | 0.553 | 0.81 | (0.41–1.59) | 0.536 |
| Other partial pancreatectomy | 1.10 | (0.62–1.92) | 0.748 | 1.16 | (0.67–1.99) | 0.598 |
| Total pancreatectomy | 1.09 | (0.63–1.89) | 0.756 | 1.17 | (0.69–1.99) | 0.567 |
| Whipple | 1.07 | (0.66–1.74) | 0.789 | 1.14 | (0.71–1.83) | 0.579 |
| Age | 1.00 | (1.00–1.01) | 0.153 | 1.01 | (1.00–1.02) | 0.006 |
| Nodal Involvement | 0.63 | (0.57–0.71) | <0.001 | 0.73 | (0.63–0.85) | <0.001 |
| Initial complications | ||||||
| None | Reference | Reference | ||||
| 1 | 0.93 | (0.81–1.06) | 0.272 | 0.93 | (0.82–1.06) | 0.288 |
| 2 or more | 0.63 | (0.44–0.90) | 0.012 | 0.62 | (0.45–0.88) | 0.006 |
| Charlson co-morbidity index | ||||||
| ≤2 | Reference | Reference | ||||
| >2 | 0.67 | (0.60–0.74) | <0.001 | 0.80 | (0.69–0.93) | 0.003 |
| Neoadjuvant chemotherapy | 0.70 | (0.60–0.81) | <0.001 | 0.82 | (0.68–1.00) | 0.055 |
| Neoadjuvant radiation | 0.69 | (0.58–0.82) | <0.001 | 0.76 | (0.61–0.95) | 0.016 |
| Chemotherapy | ||||||
| Gemcitabine | 0.89 | (0.79–1.00) | 0.055 | 1.23 | (0.93–1.64) | 0.153 |
| Gemcitabine + nab-paclitaxel | 1.09 | (0.89–1.33) | 0.399 | 1.26 | (1.02–1.57) | 0.035 |
| FOLFIRINOX | 0.87 | (0.74–1.03) | 0.108 | 1.09 | (0.89–1.33) | 0.397 |
| Oxaliplatin | 0.94 | (0.74–1.18) | 0.578 | 0.99 | (0.79–1.25) | 0.937 |
| Capecitabine | 1.00 | (0.86–1.17) | 0.968 | 1.10 | (0.94–1.29) | 0.235 |
| Erlotinib | 0.73 | (0.53–0.99) | 0.044 | 0.79 | (0.58–1.07) | 0.126 |
| Radiation therapy | ||||||
| Radiation without concurrent infusion | 0.88 | (0.78–0.99) | 0.036 | 1.14 | (0.97–1.33) | 0.103 |
| Radiation with 5-FU | 0.81 | (0.72–0.91) | 0.001 | 0.83 | (0.71–0.98) | 0.030 |
| Radiation with gemcitabine | 0.86 | (0.76–0.97) | 0.014 | 0.79 | (0.58–1.08) | 0.141 |
| Adjuvant chemotherapy | 0.89 | (0.79–1.01) | 0.068 | |||
| Adjuvant radiation therapy | 0.99 | (0.87–1.13) | 0.936 | |||
5-FU indicates 5-fluorouracil; CI, confidence interval; FOLFIRINOX, folinic acid, 5-fluorouracil, irinotecan, oxaliplatin; TR, time ratio. For variables used as indicators of ever-receipt of certain chemotherapy or radiation regimens, the reference category is nonreceipt of that chemotherapy or radiation therapy.
To account for differences in initial resectability, patients were further stratified by receipt of neoadjuvant therapy as there were marked differences observed between overall non-parametric estimates of survival. Among patients who did not receive neoadjuvant therapy, estimates of overall survival at 1, 2, and 3 years were 71.3% (95% CI: 69.1%–73.3%), 48.6% (95% CI: 46.0%–51.2%), and 38.5% (95% CI: 31.7%–37.4%), respectively. Conversely, among patients who received neoadjuvant therapy, overall survival at 1, 2, and 3 years was 60.1% (95% CI: 55.5%–65.6%), 33.0% (95% CI: 27.5%–38.5%), and 21.8% (95% CI: 16.4%–27.7%). Among patients who did not receive neoadjuvant therapy, shorter survival was associated with nodal involvement (TR = 0.73, 95% CI: 0.62–0.87, P < 0.001). In this group, longer survival was also associated with treatment with gemcitabine and nab-paclitaxel (TR = 1.32, 95% CI: 1.01–1.72, P = 0.044), and with radiation treatment without concurrent chemotherapy infusion (TR = 1.25, 95% CI: 1.05–1.50, P = 0.013). Of note, among patients who received neoadjuvant therapy, improved survival was not associated with any adjuvant therapies (Table 3).
TABLE 3.
Stratified Analysis by Receipt of Neoadjuvant Therapy
| No Neoadjuvant Therapy (n = 1993) | Neoadjuvant Therapy (n = 415) | |||||
|---|---|---|---|---|---|---|
| TR | 95% CI | P | TR | 95% CI | P | |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.05 | (0.93–1.18) | 0.448 | 1.04 | (0.81–1.33) | 0.752 |
| Procedure type | ||||||
| Proximal pancreatectomy | Reference | Reference | ||||
| Distal pancreatectomy | 1.13 | (0.65–1.97) | 0.653 | 3.53 | (1.21–10.24) | 0.021 |
| Radical antegrade pancreaticosplenectomy | 0.69 | (0.31–1.51) | 0.352 | 2.00 | (0.48–8.35) | 0.342 |
| Other partial pancreatectomy | 1.11 | (0.60–2.05) | 0.750 | 0.95 | (0.28–3.20) | 0.928 |
| Total pancreatectomy | 1.02 | (0.55–1.88) | 0.949 | 2.83 | (0.90–8.92) | 0.076 |
| Whipple | 0.99 | (0.57–1.70) | 0.961 | 2.70 | (0.95–7.65) | 0.062 |
| Age | 1.01 | (1.00–1.02) | 0.018 | 1.02 | (1.00–1.04) | 0.043 |
| Complications during index hospitalization | ||||||
| None | Reference | Reference | ||||
| 1 | 0.91 | (0.79–1.06) | 0.221 | 0.94 | (0.71–1.24) | 0.670 |
| 2 or more | 0.68 | (0.46–1.01) | 0.053 | 0.54 | (0.28–1.06) | 0.072 |
| Charlson Co-morbidity Index | ||||||
| ≤2 | Reference | Reference | ||||
| >2 | 0.74 | (0.63–0.87) | <0.001 | 1.16 | (0.80–1.68) | 0.424 |
| Nodal Involvement | 0.73 | (0.62–0.87) | <0.001 | 0.74 | (0.51–1.06) | 0.102 |
| Chemotherapy | ||||||
| Gemcitabine | 1.23 | (0.86–1.77) | 0.250 | 1.25 | (0.76–2.05) | 0.378 |
| Gemcitabine + nab-paclitaxel | 1.32 | (1.01–1.72) | 0.044 | 1.19 | (0.81–1.74) | 0.382 |
| FOLFIRINOX | 1.21 | (0.94–1.57) | 0.134 | 0.94 | (0.66–1.32) | 0.712 |
| Oxaliplatin | 1.02 | (0.76–1.38) | 0.880 | 0.90 | (0.61–1.33) | 0.587 |
| Capecitabine | 1.02 | (0.84–1.24) | 0.851 | 1.21 | (0.90–1.62) | 0.203 |
| Erlotinib | 0.90 | (0.60–1.35) | 0.619 | 0.72 | (0.45–1.15) | 0.164 |
| Radiation therapy | ||||||
| Radiation without concurrent infusion | 1.25 | (1.05–1.50) | 0.013 | 0.98 | (0.63–1.53) | 0.943 |
| Radiation with 5-FU | 0.77 | (0.64–0.94) | 0.009 | 1.00 | (0.72–1.38) | 0.993 |
| Radiation with gemcitabine | 0.74 | (0.51–1.08) | 0.115 | 0.98 | (0.57–1.69) | 0.951 |
5-FU indicates 5-fluorouracil; CI, confidence interval; FOLFIRINOX, folinic acid, 5-fluorouracil, irinotecan, oxaliplatin; TR, time ratio. For variables used as indicators of ever-receipt of certain chemotherapy or radiation regimens, the reference category is non-receipt of that chemotherapy or radiation therapy.
Total and Monthly Payments After Surgery
Variability in payments per episode of care was explored by the type of treatment regimen. Median payments for surgery ranged from $37,030 (IQR: $27,308–$54,502) for a distal pancreatectomy to $57,893 (IQR: $40,354–$82,825) for a Whipple procedure (Fig. 2). Overall, median cumulative chemotherapy payment for all patients was $39,098 (IQR: $13,304–$76,034). Median cumulative payments for gemcitabine treatment was $39,041 (IQR: $17,836–$70,295); with nab-paclitaxel, median cumulative payment for gemcitabine was nearly double (median $74,051, IQR: $38,929–$133,603), as was cumulative payment for patients who received FOLFIRINOX (median $70,419, IQR: $35,662–$123,078).
FIGURE 2.
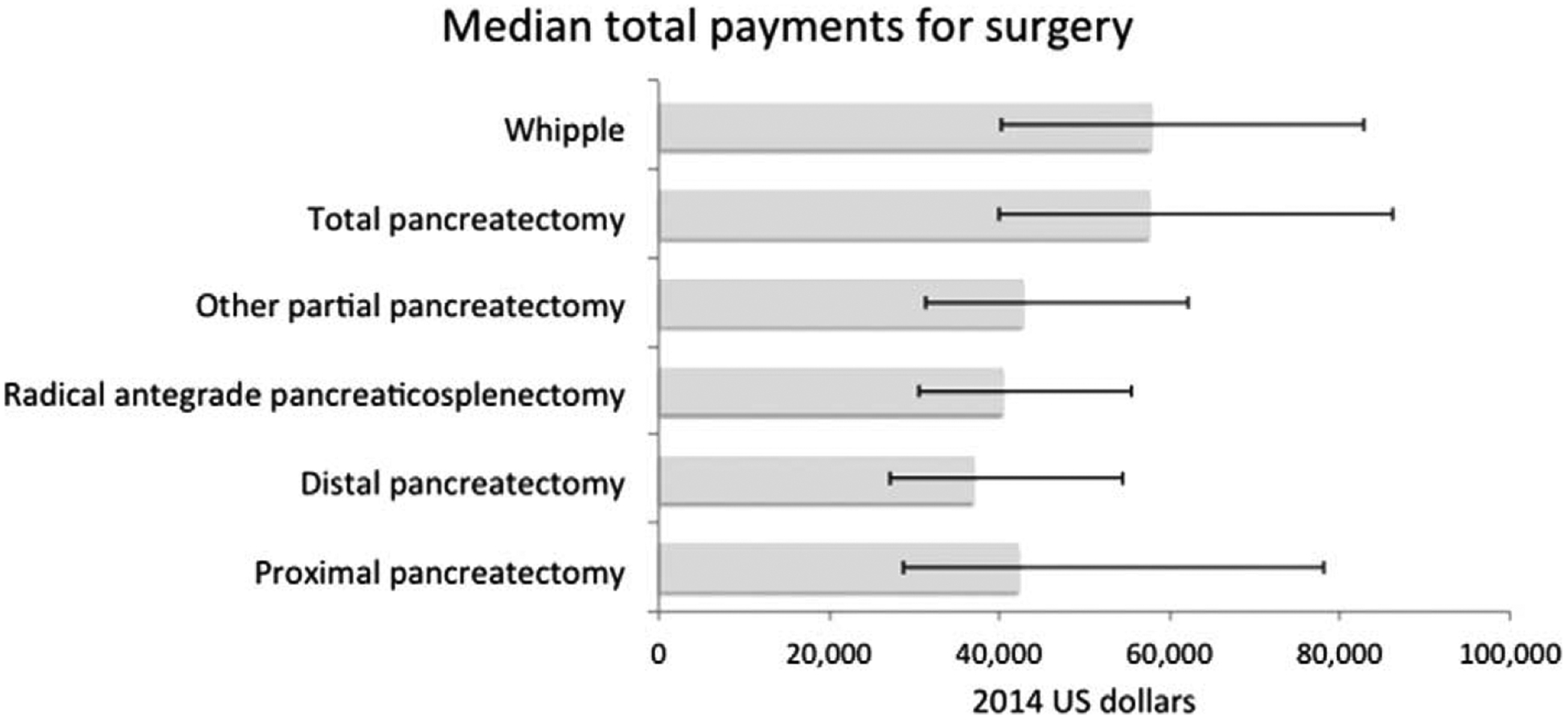
Median total payments for index surgical procedure, with interquartile range.
The variation in median cumulative payments for radiation therapy also varied widely, but was driven by the type of accompanying chemotherapy regimen undertaken. For patients who received FOLFIRINOX and also received radiation, median total payments for chemoradiation therapy was $93,336 (IQR: $57,403–$154,784), whereas for patients who received capecitabine therapy in addition to radiation, the median cumulative payment for chemoradiation was $19,294 (IQR: $4955–$54,606). For patients who received gemcitabine treatment and radiation therapy, median cumulative payment for chemoradiation was $46,074 (IQR: $21,674–$85,749) (Table 4). Median total payment for filled capecitabine prescriptions was $5538 (IQR: $3939–$8706), whereas median total payments for filled erlotinib prescriptions was $15,929 (IQR: $9264–$27,289).
TABLE 4.
Cumulative and Per-month Calculated Payments, by Chemoradiation Regimen
| Cumulative (in 2014 US Dollars) | Payments Per Month (in 2014 US Dollars) | |||||||
|---|---|---|---|---|---|---|---|---|
| Median Chemoradiation Payments (IQR) | Median Hospitalization Payments (IQR) | Median Chemoradia- tion Payments (IQR) | Median Hospitalization Payments (IQR) | |||||
| Capecitabine + radiation | 19,294 | (4955–54,606) | 39,797 | (17,182–93,487) | 1293 | (343–8916) | 2660 | (963–8916) |
| Gemcitabine + radiation | 46,074 | (21,674–85,749) | 32,335 | (14,865–69,640) | 3191 | (1595–5250) | 2363 | (1001–5250) |
| FOLFIRINOX + radiation | 95,336 | (57,403–154,784) | 39,991 | (20,408–83,575) | 6427 | (3731–10,587) | 2472 | (997–5370) |
| Gemcitabine | 39,041 | (17,836–70,295) | 32,255 | (14,098–69,582) | 2811 | (1394–5312) | 2400 | (1001–5312) |
| Gemcitabine + nab-paclitaxel | 74,051 | (38,929–133,603) | 31,751 | (15,068–71,783) | 4951 | (2276–5736) | 2268 | (997–5736) |
| FOLFIRINOX | 70,419 | (35,662–123,078) | 42,951 | (25,555–83,575) | 7149 | (3485–10,972) | 4414 | (1520–10,972) |
| Overall | 39,098 | (13,304–76,034) | 31,751 | (15,418–69,615) | 2824 | (1186–5785) | 2360 | (971–5785) |
Furthermore, when variation in median payments for chemoradiation was examined in the months preceding and following resection, an overall decline in the per-month payments was observed for all patients, though median total payments per-month was greatest for FOLFIRINOX treatment, followed by nab-paclitaxel (Fig. 3A), and this trend was consistently observed in the subset of patients who underwent radiation therapy (Fig. 3B). In examining the total payments per month after resection for subsequent hospitalizations, however, no discernable trend was observed within each category of chemoradiation treatment (Figs, 3C and D). Of note, however, total per-month payments increased most sharply for patients who received FOLFIRINOX and radiation treatment, with per-month payments of $6495 (IQR: $5668–$7321) at 24 to 30 months following resection compared to per-month payments for hospitalization of $3529 (IQR: $2079–$8094) for patients who received treatment with gemcitabine and radiation.
FIGURE 3.
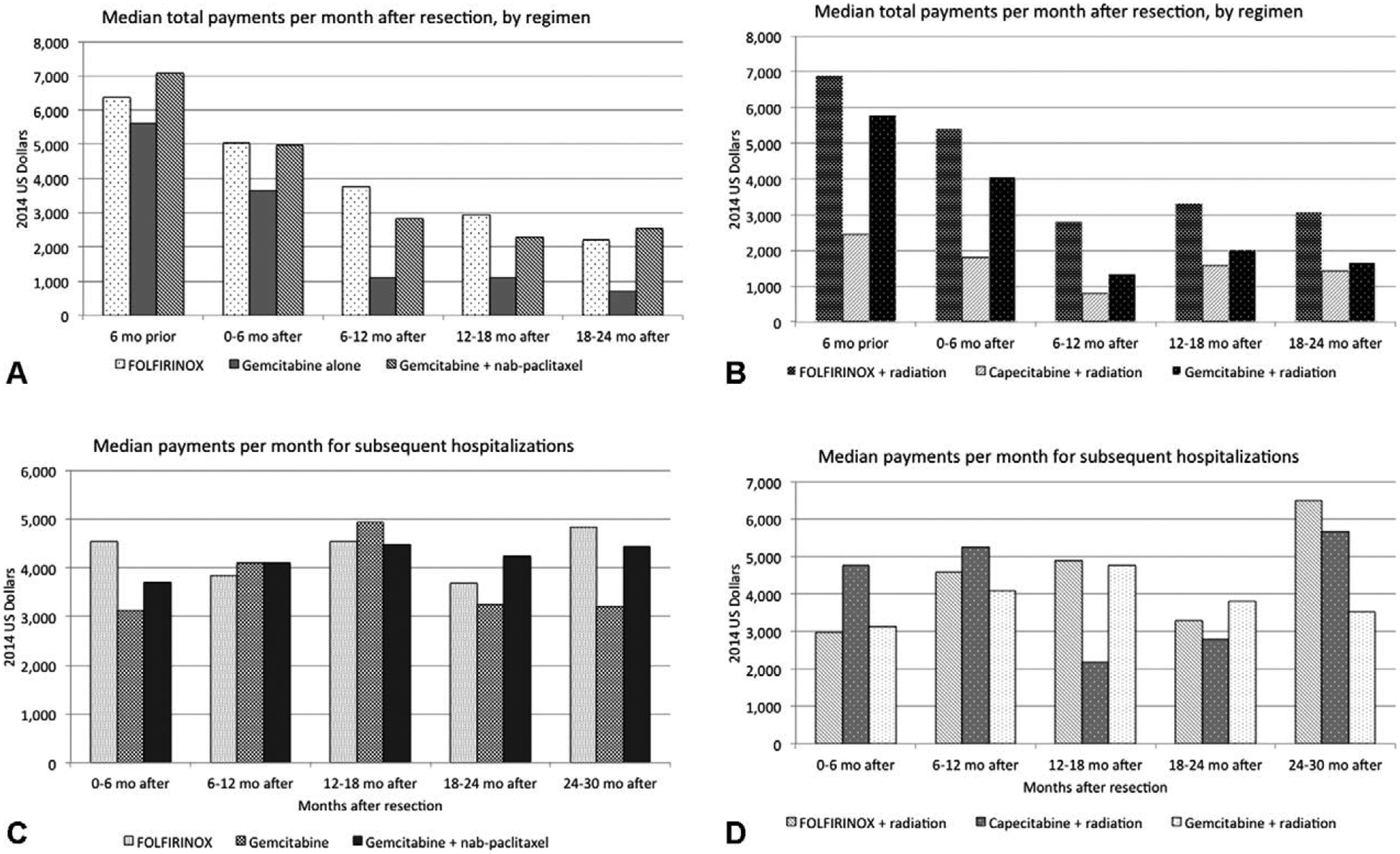
(A) Median payments (calculated per-month) for adjuvant therapy, by chemoradiation regimen, all patients, and (B) median payments (calculated per-month) monthly payments for adjuvant therapy, by chemoradiation regimen, in patients receiving radiation therapy. (C) Median payments (calculated per-month) for subsequent hospitalization, by chemoradiation regimen, all patients, and (D) median payments (calculated per-month) for subsequent hospitalization, by chemoradiation regimen, in patients receiving radiation therapy.
DISCUSSION
The cost of comprehensive cancer therapy remains a significant burden to the healthcare system.10,11 For pancreatic cancer, which carries a poor prognosis, the total financial burden of any treatment course for individual patients varies widely because of the variable effectiveness of specific therapies.13–15 In this study, we sought to describe the association between survival and specific treatment regimens for patients undergoing resection for their disease, based on particular clinical and demographic factors, and then evaluate the variation in overall payments for care. Specifically, using a large, nationally representative sample of commercially insured patients who underwent surgical resection for pancreatic cancer, we calculated the total payments for surgery, episodes of outpatient chemo- and radiation therapy, and subsequent hospitalizations. Since our study population was treated in the years following the CONKO-001 and FOLFIRINOX trials, our analysis offers insight into clinical outcomes of patients whose own treatment–and possibly prognosis–was impacted by changes in treatment approaches over this period.24–27 Of note, the association between most adjuvant therapies and improved survival was marginal, with minimal difference in overall survival time ratios regardless of the therapy delivered. And in light of this, the payments for adjuvant chemotherapy and radiation therapy as well as for subsequent hospitalization varied considerably among patients.
The present study specifically delineates the overall payments associated with a variety of adjuvant and neoadjuvant therapies used for patients who underwent an operation for their disease. Interestingly, although some adjuvant therapies such as gemcitabine combined with nab-paclitaxel were associated with a modest increased survival time (ranging from 26% to 32% in all patients and patients not undergoing neoadjuvant therapy, respectively), an increase in survival time was only associated with adjuvant radiation among patients who potentially received simultaneous oral capecitabine therapy (with a 25% longer time to death), and among patients who did not receive neoadjuvant therapy.
Our findings echo previous findings from prior randomized and observational studies that have demonstrated an inconsistent and questionable effect of adjuvant therapy among patients with pancreatic cancer. The Norwegian and ESPAC trials noted a varied effect of adjuvant chemotherapy using 5-FU-based chemotherapy combinations, with some trials showing no significant advantage of chemotherapy, whereas other data suggested a benefit.28–30 Similarly, a combined meta-analysis with data from EORTC, ESPAC-1 and ESPAC-3 trials demonstrated no survival benefit of chemoradiation plus gemcitabine or 5-fluorouracil over chemotherapy alone.31 RTOG 97–04 examined the effect of gemcitabine added to a chemoradiation regimen on overall survival, with the effects of chemoradiation difficult to differentiate from adjuvant chemotherapy.25,27 In our multivariable analyses among all patients and in patients stratified by receipt of neoadjuvant therapy, chemoradiation with either 5-FU or gemcitabine showed no association with longer survival. This variation was likely because of individual patients having different responses to therapy after resection, which ultimately was linked to as yet poorly understood tumor biology. Taken together, however, the aggregate data would suggest that adjuvant chemotherapy and radiation therapy following resection of pancreatic adenocarcinoma likely has only a modest benefit.
The calculated total payments associated with pancreatic cancer care in our study are consistent with prior published data.13,32,33 For example, in a study among Medicare enrollees, O’Neill et al13 reported that the mean total costs for patients with resectable, locoregional pancreatic cancer averaged approximately $134,000, and Vandeneede et al32 reported overall medical costs of care that ranged from €19,890 to €120,071 for patients with resectable disease. Although previous studies on the economics and cost-effectiveness of interventions for pancreatic cancer have examined the cost-savings from a health-system point of view,12 or the utility of comparable interventions for surgical or medical palliation of symptoms,34–36 assessing predictive modeling around payments of cancer care has been limited because of difficulties regarding the censoring of payments as patients with lower survival often incur fewer medical costs.37 More recent investigations, however, have attempted to more directly assess tradeoffs between payments and survival using predefined thresholds of “willingness-to-pay.”38,39 Indeed, recent investigations have concluded that radiation therapy combined with gemcitabine did not confer sufficient benefit to justify the cost.40
Similar criticisms have been leveled at certain systemic chemotherapy regimens. For example, although Moore et al33 reported an OS benefit of 0.33 months for erlotinib when added to gemcitabine, Miksad et al41 argued that the associated diminished quality of life, as well as out-of-pocket expenses, made such an approach difficult to justify. In fact, Miksad et al reported that the addition of erlotinib was associated with an average incremental cost of $15,194 per patient, which amounted to about $410,000 to $510,000 per quality-adjusted life-year owing to toxicity. Furthermore, other studies have shown that though the individual pricing of chemotherapeutic agents may suggest a lower overall financial burden associated with the FOLFIRINOX regimen, the need for supportive medication and hydration significantly increase its expense.42
The findings from the present study reflect real-world practice patterns rather than estimates derived from a clinical trial setting. Consistent with past reports, the observed total payments for treatment were highest among patients receiving FOLFIRINOX or gemcitabine and nab-paclitaxel therapy. In addition, radiation therapy was associated with observed cumulative payments ranging from just under $20,000 to over $90,000, depending on other chemotherapies received. When the payments associated with the surgical resection were included, the median total payment for cancer-related care was >$150,000 for patients who received neoadjuvant and adjuvant treatment in addition to surgery. Collectively, these data emphasize the substantial expenditures associated not only with surgery, but also the entire multimodal treatment plan often utilized in caring for patients with pancreatic cancer.
For surgeons who participate in the multidisciplinary care of patients with pancreatic cancer, these data have several implications. As compensation for comprehensive cancer care shifts from traditional fee-for-service payments to value-based payment systems with the introduction of bundled payments under the Oncology Care Model, our data provide a context in which to consider the relative gains of surgery, chemo-, and radiation therapy. For example, in the current analysis, improved survival was only associated with select adjuvant and neoadjuvant therapies and a modest survival benefit, whereas significant payments were accrued among all patient populations ranging from approximately $1300 per month to >$7000 per month. Although the goal of this study was not to explicitly determine the comparative cost-effectiveness of a specific therapy, our results serve to highlight the need for conversations regarding decisions to pursue additional care relative to the economic consequences for patients and healthcare at large. Given the recent increased emphasis on higher value and more efficient care at lower cost, providers need to be cognizant of both the clinical benefit as well as the potential financial burden of available treatment options.
Our study was subject to several limitations. Because this study was conducted using administrative claims data, we could not account for all clinical and sociodemographic factors that may have influenced pancreatic cancer risk, diagnosis, and prognosis such as cancer stage or anatomic location.43 To account for this potential bias, our analyses were conducted within sets of patients that were assigned to treatment regimens that are used for similar indications. In addition, we conducted iterative analyses to account for events that may have occurred outside previously defined clinical time-frames by performing additional subanalyses among patients with a minimum follow-up of > 6 months.44 The data were also subject to possible under-reporting of specific clinical covariates, including tumor stage and lymph node involvement at the time of surgery, which may have differed from previous estimates. However, consistent with other sensitivity analyses, the distribution of reported variables did not change at each step in the selection of the study population, and therefore any reporting bias was likely random. In addition, our study examined the total payments for treatment, rather than estimates of direct costs to patients in the form of co-payments and/or out-of-pocket costs. These payments represent the entire monetary burden placed on the healthcare system, and therefore act only as a surrogate of the potential financial burden to an individual patient. Lastly, we were unable to account for variations in contractual differences between payors and patients.45
In conclusion, the financial burden associated with care for resectable pancreatic cancer was highly variable, with considerable expenses distributed between surgery, chemotherapy and radiation, and eventual hospitalizations. Any increase in survival time was at most up to 25% to 30%, and was associated with treatment with gemcitabine and nab-paclitaxel or with combination capecitabine and radiation therapy. The results from our study underscore the degree to which potential benefit of further therapy in surgical patients might be considered in light of the financial burden it incurs.
Supplementary Material
Acknowledgments
This study investigated the financial burden of surgically managed pancreatic cancer relative to observed survival. Although associated with greater payments, adjuvant chemotherapy or radiation was not consistently associated with a survival benefit.
Footnotes
Presented at the 57th Annual Meeting of the Society for Surgery of the Alimentary Tract held on May 21–24, 2016 in San Diego, California.
Presented at the 50th Annual Pancreas Club held on May 20–21, 2016 in San Diego, California.
The authors report no conflicts of interest.
REFERENCES
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer. 2014;120:3245–3253. [DOI] [PubMed] [Google Scholar]
- 3.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1083–1093. [DOI] [PubMed] [Google Scholar]
- 4.Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar SY, McNeil RB, Thomas CM, et al. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markman M, Luce R. Impact of the cost of cancer treatment: an internet-based survey. J Oncol Pract. 2010;6:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–311. [DOI] [PubMed] [Google Scholar]
- 10.Shankaran V, Ramsey S. Addressing the financial burden of cancer treatment: from copay to can’t pay. JAMA Oncol. 2015;1:273–274. [DOI] [PubMed] [Google Scholar]
- 11.Yabroff KR, Warren JL, Banthin J, et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: what is the impact of data source? Med Care. 2009;47(7 Suppl 1):S64–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardou M, Le Ray I. Treatment of pancreatic cancer: a narrative review of cost-effectiveness studies. Best Pract Res Clin Gastroenterol. 2013;27: 881–892. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill CB, Atoria CL, O’Reilly EM, et al. Costs and trends in pancreatic cancer treatment. Cancer. 2012;118:5132–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S, Long SR, Kutikova L, et al. Burden of pancreatic cancer and disease progression: economic analysis in the US. Oncology. 2006;70:71–80. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DA, Krishna K, Flowers CR, et al. Cost description of chemotherapy regimens for the treatment of metastatic pancreas cancer. Med Oncol. 2016;33:48. [DOI] [PubMed] [Google Scholar]
- 16.Hansen L, Chang S. Health Research Data for the Real World: the Market-Scan® Databases. Truven Health Analytics Ann Arbor. 2013. [Google Scholar]
- 17.Stagg V. CHARLSON: Stata module to calculate Charlson index of comorbidity. Statistical Software Components. 2015. [Google Scholar]
- 18.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. [DOI] [PubMed] [Google Scholar]
- 19.Bureau of Labor Statistics, Consumer Price Index, U.S. Department of Labor, available at http://www.bls.gov/cpi (last visited August 18, 2016).
- 20.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 21.Therneau TM, Grambsch PM. Testing proportional hazards In Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000;127–152. [Google Scholar]
- 22.Cox C, Chu H, Schneider MF, et al. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–4374. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Interim Report of the Committee on Geographic Variation in Health Care Spending and Promotion of High-Value Health Care. Washington, D.C: National Academies Press; 2013. [PubMed] [Google Scholar]
- 24.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 25.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. [DOI] [PubMed] [Google Scholar]
- 26.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 27.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakkevold KE, Arnesjø B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater–results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A:698–703. [DOI] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. [DOI] [PubMed] [Google Scholar]
- 30.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. [DOI] [PubMed] [Google Scholar]
- 31.Liao W-C, Chien K-L, Lin Y-L, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095–1103. [DOI] [PubMed] [Google Scholar]
- 32.Vandeneede N, De Paepe A, Specenier P, et al. Cost and cost-effectiveness data on pancreatic cancer: a comprehensive review of the literature. Value in Health. 2015;18:A448–A449. [Google Scholar]
- 33.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 34.Kahaleh M, Brock A, Conaway MR, et al. Covered self-expandable metal stents in pancreatic malignancy regardless of resectability: a new concept validated by a decision analysis. Endoscopy. 2007;39:319–324. [DOI] [PubMed] [Google Scholar]
- 35.Katsinelos P, Paikos D, Kountouras J, et al. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20:1587–1593. [DOI] [PubMed] [Google Scholar]
- 36.Jeurnink SM, Polinder S, Steyerberg EW, et al. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2009;45:537–543. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y. Cost analysis with censored data. Med Care. 2009;47(7 Suppl 1): S115–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 39.Tam VC, Ko YJ, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20:e90–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung HWC, Chan ALF, Muo C-H. Cost-effectiveness of Gemcitabine plus modern radiotherapy in locally advanced pancreatic cancer. Clin Ther. 2016. doi: 10.1016/j.clinthera.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506–4507. [DOI] [PubMed] [Google Scholar]
- 42.Chiorean EG, Whiting S, Binder G, et al. Cost-Effectiveness of nab-paclitaxel plus gemcitabine versus erlotinib plus gemcitabine in metastatic pancreatic cancer. ASCO Annual Meeting Proceedings. 2014;32:353. [Google Scholar]
- 43.Haut ER, Pronovost PJ, Schneider EB. Limitations of administrative databases. JAMA. 2012;307 2589-authorreply2589–90. doi: 10.1001/jama.2012.6626. [DOI] [PubMed] [Google Scholar]
- 44.Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–512. [DOI] [PubMed] [Google Scholar]
- 45.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 Suppl 1):S51–S55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


