Abstract
Background
Conventional medications for Crohn's disease (CD) include anti‐inflammatory drugs, immunosuppressants and corticosteroids. If an individual does not respond, or loses response to first‐line treatments, then biologic therapies such as tumour necrosis factor‐alpha (TNF‐α) antagonists such as adalimumab are considered for treating CD. Maintenance of remission of CD is a clinically important goal, as disease relapse can negatively affect quality of life.
Objectives
To assess the efficacy and safety of adalimumab for maintenance of remission in people with quiescent CD.
Search methods
We searched the Cochrane IBD Group Specialized Register, CENTRAL, MEDLINE, Embase, and clinicaltrials.gov from inception to April 2019.
Selection criteria
We considered for inclusion randomized controlled trials (RCTs) comparing adalimumab to placebo or to an active comparator.
Data collection and analysis
We analyzed data on an intention‐to‐treat basis. We calculated risk ratios (RRs) and corresponding 95% confidence intervals (95% CI) for dichotomous outcomes. The primary outcome was failure to maintain clinical remission. We define clinical remission as a Crohn's Disease Activity Index (CDAI) score of < 150. Secondary outcomes were failure to maintain clinical response, endoscopic remission, endoscopic response, histological remission and adverse events (AEs). We assessed biases using the Cochrane 'Risk of bias' tool. We used GRADE to assess the overall certainty of evidence supporting the primary outcome.
Main results
We included six RCTs (1158 participants). We rated four trials at low risk of bias and two trials at unclear risk of bias. All participants had moderate‐to‐severe CD that was in clinical remission. Four studies were placebo‐controlled (1012 participants). Two studies (70 participants) compared adalimumab to active medication (azathioprine, mesalamine or 6‐mercaptopurine) in participants who had an ileocolic resection prior to study enrolment.
Adalimumab versus placebo Fifty‐nine per cent (252/430) of participants treated with adalimumab failed to maintain clinical remission at 52 to 56 weeks, compared with 86% (217/253) of participants receiving placebo (RR 0.70, 95% CI 0.64 to 0.77; 3 studies, 683 participants; high‐certainty evidence). Among those who received prior TNF‐α antagonist therapy, 69% (129/186) of adalimumab participants failed to maintain clinical or endoscopic response at 52 to 56 weeks, compared with 93% (108/116) of participants who received placebo (RR 0.76, 95% CI 0.68 to 0.85; 2 studies, 302 participants; moderate‐certainty evidence). Fifty‐one per cent (192/374) of participants who received adalimumab failed to maintain clinical remission at 24 to 26 weeks, compared with 79% (149/188) of those who received placebo (RR 0.66, 95% CI 0.52 to 0.83; 2 studies, 554 participants; moderate‐certainty evidence). Eighty‐seven per cent (561/643) of participants who received adalimumab reported an AE compared with 85% (315/369) of participants who received placebo (RR 1.01, 95% CI 0.94 to 1.09; 4 studies, 1012 participants; high‐certainty evidence). Serious adverse events were seen in 8% (52/643) of participants who received adalimumab and 14% (53/369) of participants who received placebo (RR 0.56, 95% CI 0.39 to 0.80; 4 studies, 1012 participants; moderate‐certainty evidence) and withdrawal due to AEs was reported in 7% (45/643) of adalimumab participants compared to 13% (48/369) of placebo participants (RR 0.59, 95% CI 0.38 to 0.91; 4 studies, 1012 participants; moderate‐certainty evidence). Commonly‐reported AEs included CD aggravation, arthralgia, nasopharyngitis, urinary tract infections, headache, nausea, fatigue and abdominal pain.
Adalimumab versus active comparators
No studies reported failure to maintain clinical remission. One study reported on failure to maintain clinical response and endoscopic remission at 104 weeks in ileocolic resection participants who received either adalimumab, azathioprine or mesalamine as post‐surgical maintenance therapy. Thirteen per cent (2/16) of adalimumab participants failed to maintain clinical response compared with 54% (19/35) of azathioprine or mesalamine participants (RR 0.23, 95% CI 0.06 to 0.87; 51 participants). Six per cent (1/16) of participants who received adalimumab failed to maintain endoscopic remission, compared with 57% (20/35) of participants who received azathioprine or mesalamine (RR 0.11, 95% CI 0.02 to 0.75; 51 participants; very low‐certainty evidence). One study reported on failure to maintain endoscopic response at 24 weeks in ileocolic resection participants who received either adalimumab or 6‐mercaptopurine (6‐MP) as post‐surgical maintenance therapy. Nine per cent (1/11) of adalimumab participants failed to maintain endoscopic remission compared with 50% (4/8) of 6‐MP participants (RR 0.18, 95% CI 0.02 to 1.33; 19 participants).
Authors' conclusions
Adalimumab is an effective therapy for maintenance of clinical remission in people with quiescent CD. Adalimumab is also effective in those who have previously been treated with TNF‐α antagonists. The effect of adalimumab in the post‐surgical setting is uncertain. More research is needed in people with recent bowel surgery for CD to better determine treatment plans following surgery. Future research should continue to explore factors that influence initial and subsequent biologic selection for people with moderate‐to‐severe CD. Studies comparing adalimumab to other active medications are needed, to help determine the optimal maintenance therapy for CD.
Plain language summary
Adalimumab for maintenance of remission in Crohn's disease
What is Crohn's disease?
Crohn's disease (CD) is an inflammatory disorder that can affect the whole gastrointestinal tract from the mouth to the anus. Participants can experience a wide range of symptoms related to their disease, including abdominal pain, diarrhea, weight loss, and fever. Participants commonly have periods where their disease is more active (in other words, they experience symptoms), alternating with periods where their disease is inactive (no CD symptoms). When CD is inactive it is said to be in remission. With treatment, medications can control the inflammation in the gastrointestinal tract, for long‐term control of disease activity.
What is adalimumab?
Adalimumab is a biologic drug that blocks the development of inflammation in the gastrointestinal tract. It belongs to a class of drugs know as tumour necrosis factor‐alpha (TNF‐α) antagonists. This medication is used in people who have CD, to reduce the amount of inflammation in the gastrointestinal tract or to prevent the inflammation from developing.
What is the purpose of this study?
The purpose of this study was to determine whether adalimumab is an effective medication for maintenance of remission in people with inactive CD, and whether it is associated with any harms or side effects.
How was this study performed?
We conducted a systematic review of current literature, to determine whether adalimumab therapy is effective in maintaining remission in CD. We ran electronic searches of several databases on 15 April 2019 and selected studies that met our inclusion criteria for further evaluation. We performed statistical analyses to determine whether adalimumab provides an overall benefit for maintenance of remission in CD.
What were the results?
We included six studies (1158 participants). All participants had moderate‐to‐severe CD that was in clinical remission. Four studies (1012 participants) compared adalimumab to placebo (a fake drug). Two studies (70 participants) compared adalimumab to active medication (azathioprine or mesalamine and 6‐mercaptopurine) in participants who had surgery for CD before study enrolment.
Participants who received adalimumab were more likely to maintain clinical remission (no symptoms of CD) and endoscopic remission (no inflammation in their gastrointestinal tract) than those who received placebo (the fake drug). This was also true for participants who have previously been treated with other TNF‐α drugs (for example, infliximab), The overall certainty of the evidence for these outcomes ranged from moderate to high. We did not see an increased rate of side effects in participants who received adalimumab compared to those who received placebo. The most common side effects included headache, fatigue and infections, both urinary tract infections and upper respiratory tract infections.
Two small studies looked at groups of patients who had recently required a narrowed area of their bowel to be removed by surgery. These participants were given adalimumab or azathioprine (an immunosuppressive drug), mesalamine (an anti‐inflammatory drug), or 6‐mercaptopurine (an immunosuppressive drug) within 45 days of surgery, to see if these drugs helped to prolong remission after surgery. Overall, adalimumab appeared to be more likely to maintain remission than azathioprine, mesalamine or 6‐mercaptopurine. Howvever, these were small studies with very low‐certainty evidence. These two studies did not report on side effects.
What are the conclusions from this study?
Adalimumab is an effective therapy for maintaining clinical remission in people with inactive CD. Adalimumab is also effective in those who have previously been treated with other TNF‐α drugs. The effect of adalimumab in the post‐surgical setting is uncertain. More research is needed in patients following bowel surgery for CD to better determine treatment plans after their operations. Future research should continue to explore factors that influence initial and later biologic selection for participants with moderate‐to‐severe CD. Studies comparing adalimumab to other active medications are needed, to help decide the best maintenance therapy for CD.
Summary of findings
Summary of findings 1. Adalimumab compared to placebo for maintenance of remission in Crohn's disease.
| Adalimumab compared to placebo for maintenance of remission in Crohn's disease | ||||||
| Patient or population: People with quiescent Crohn's disease Setting: Outpatient Intervention: Adalimumab (40 mg/week or 40 mg every other week) Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Adalimumab | |||||
| Failure to maintain clinical remission Follow‐up: 52 to 56 weeks |
Study population | RR 0.70 (0.64 to 0.77) | 683 (3 RCTs) | ⊕⊕⊕⊕ HIGH | Clinical remission defined as a CDAI < 150 | |
| 858 per 1000 | 600 per 1000 (549 to 660) | |||||
| Failure to maintain clinical remission Follow‐up: 24 to 26 weeks |
Study population | RR 0.66 (0.52 to 0.83) | 554 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | Clinical remission defined as a CDAI < 150 | |
| 793 per 1000 | 523 per 1000 (412 to 658) | |||||
| Failure to maintain endoscopic remission Follow‐up: 52 weeks |
969 per 1000 | 717 per 1000 (611 to 843) |
RR 0.74 (0.63 to 0.87) |
129 (1 RCT) |
⊕⊕⊕⊝ MODERATEb | Endoscopic remission defined as an absence of mucosal ulceration |
| Adverse events Follow‐up: 52 to 56 weeks |
Study population | RR 1.01 (0.94 to 1.09) | 1012 (4 RCTs) | ⊕⊕⊕⊕ HIGH | Commonly‐reported adverse events included CD aggravation, arthralgia, nasopharyngitis, urinary tract infections, headache, nausea, fatigue and abdominal pain | |
| 854 per 1000 | 862 per 1000 (802 to 930) | |||||
| Serious adverse events Follow‐up: 52 to 56 weeks |
Study population | RR 0.56 (0.39 to 0.80) | 1012 (4 RCTs) | ⊕⊕⊕⊝ MODERATEc | Reported serious adverse events included infectious complications including tuberculosis, abscess formation and wound infections, multiple sclerosis, pulmonary embolism | |
| 144 per 1000 | 80 per 1000 (56 to 115) | |||||
| Withdrawals due to adverse events Follow‐up: 52 to 56 weeks |
Study population | RR 0.59 (0.38 to 0.91) | 1012 (4 RCTs) | ⊕⊕⊕⊝ MODERATEd | Adverse events leading to withdrawal included flare of CD, infectious complications | |
| 130 per 1000 | 77 per 1000 (49 to 118) | |||||
| Failure to maintain clinical or endoscopic response with prior TNF‐α antagonist exposure Follow‐up: 52 to 56 weeks |
Study population | RR 0.76 (0.68 to 0.85) | 302 (2 RCTs) | ⊕⊕⊕⊝ MODERATEe | Clinical response defined as decrease in baseline CDAI score of ≥ 100. Endoscopic response defined as mucosal healing seen at time of endoscopy | |
| 931 per 1000 | 708 per 1000 (633 to 791) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level due to serious inconsistency (I2 = 55%). bDowngraded by one level due to sparse data (109 events). cDowngraded by one level due to sparse data (105 events). dDowngraded by one level due to sparse data (93 events). eDowngraded by one level due to sparse data (237 events).
Summary of findings 2. Adalimumab compared to active comparator for maintenance of remission in Crohn's disease.
| Adalimumab compared to active comparator for maintenance of remission in Crohn's disease | ||||||
| Patient or population: People with quiescent Crohn's disease (ileocolic resection) Setting: Outpatient Intervention: Adalimumab (40 mg every other week) Comparison: Active comparator ‐ azathioprine (2 mg/kg/day), mesalamine (3 g/day), or 6‐mercaptopurine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with active comparator | Risk with Adalimumab | |||||
| Failure to maintain clinical remission | Outcome not reported | Outcome not reported | ||||
| Failure to maintain endoscopic remission Follow‐up: 104 weeks |
Study population | RR 0.11 (0.02 to 0.75) | 51 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | Endoscopic remission defined as Rutgeerts score < i2 | |
| 571 per 1000 | 63 per 1000 (11 to 429) | |||||
| Adverse events | Outcome not reported | Outcome not reported | ||||
| Serious adverse events | Outcome not reported | Outcome not reported | ||||
| Withdrawal due to adverse events | Outcome not reported | Outcome not reported | ||||
| Failure to maintain clinical or endoscopic response with prior TNF‐α antagonist exposure | Outcome not reported | Outcome not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level due to unclear risk of bias. bDowngraded two levels due to very sparse data (21 events).
Background
Description of the condition
Crohn's disease (CD) is a chronic, episodic, inflammatory condition of the gastrointestinal system, with affected regions consisting of transmural ulceration separated by normal mucosa. The small intestine is most commonly affected, although the large intestine may also be involved. Common symptoms include abdominal pain, diarrhea, weight loss, bleeding, nausea and vomiting. Enteric complications may include bowel obstruction, perforation, abscesses, fistulas, and peri‐anal disease. Approximately 20% of people with CD experience extra‐intestinal complications that may include musculoskeletal, ocular, dermatologic, hepatobiliary, renal and hematological conditions (Doherty 2015).
The pathophysiology of CD is complex and not completely understood. However, it is known that there is a genetic component, as evidenced by concordance between monozygotic twins, familial and ethnic aggregation, and genetic anticipation (Khor 2011; Loddo 2015). Evidence also exists for strong epigenetic and environmental factors, with tobacco use being the most studied environmental factor, showing that early tobacco use increases the risk of developing CD (Cosnes 1996; Khor 2011). The disease is known to occur after an episode of infectious gastroenteritis, suggesting that an altered gut microbiota may be a factor in the pathogenesis of CD (Chassaing 2011; Matsuoka 2015). It is believed that an altered relationship between the gut microflora and the host immune system plays a major role in CD. One key potential mechanism by which this altered relationship is thought to mediate the development of CD is through increased permeability between epithelial cells lining the intestinal barrier. The increased permeability permits an increased flux of intestinal antigens to the lamina propria, causing an enhanced immune reaction. In‐vitro and animal studies have linked the increase in permeability to T cells, tumour necrosis factor‐alpha (TNF‐α), and interferon‐γ (Baumgart 2012).
Conventional medications for CD include anti‐inflammatory drugs, immunosuppressants and corticosteroids. However, if the patient does not respond, or loses response to these first‐line treatments, then biologic therapies such as TNF‐α antagonists including infliximab, certolizumab pegol and adalimumab are then considered for the treatment of CD. Top‐down approaches for CD therapy, including the early use of combination therapy with biologics and immunosuppressives, are increasingly being used and may provide benefit in people with complicated or extensive disease suggestive of an aggressive disease course, and those with poor prognostic factors (Tsui 2018).
Description of the intervention
Adalimumab is a monoclonal antibody that binds to and inactivates TNF‐α, thereby limiting the inflammatory response that occurs in CD. Since its initial approval by the FDA for treatment of rheumatoid arthritis, adalimumab has been approved for several other diseases, including hidradenitis suppurativa, iritis, ankylosing, psoriatic, and juvenile idiopathic arthritis; plaque psoriasis; ulcerative colitis; and CD (FDA 2011). Adalimumab is indicated for cases of moderate‐to‐severe CD for symptomatic control and induction and maintenance of clinical remission (Cassinotti 2008).
How the intervention might work
It has been demonstrated that people with CD have higher concentrations of TNF‐α and decreased immune cell apoptosis in their intestinal mucosa (Di Sabatino 2004). Inhibition of TNF‐α by adalimumab reduces activity of neutrophils, proliferation of T‐cells, activation of macrophages, and survival of immune cells, thereby limiting the immune response (Tracey 2008). In addition, TNF‐α inhibition has been shown to increase intestinal T‐cell and monocyte apoptosis in people with CD (Asgharpour 2013).
Why it is important to do this review
Maintenance of remission of CD is a clinically important goal, as relapses of the disease can have a profound impact on the quality of life of patients (Ghosh 2007). Commonly‐used treatment options for active CD include corticosteroids, which are effective for short‐term disease control, but are associated with several adverse events (AEs). Furthermore, corticosteroid resistance occurs in as many as 20% of cases (Munkholm 1994; Steinhart 2003). Corticosteroids are not effective for maintenance of remission in CD (Steinhart 2003). Furthermore, many patients do not respond to treatment with aminosalicylates and immunosuppressives (Hanauer 2002).
Adalimumab is a promising option for the treatment of moderate‐to‐severe CD. The CHARM trial showed that adalimumab is more effective than placebo in maintaining remission in moderate‐to‐severe CD after 56 weeks from initiation of therapy (Colombel 2007). Unfortunately, the human immune system may reduce the effectiveness of interventions over time, and the effectiveness of a monoclonal antibody for initial remission of CD may not predict its ability to maintain remission. For example, it has been shown that repeated infusions of infliximab, a chimeric monoclonal antibody to TNF‐α used to treat CD, may lead to the production of antibodies to the drug itself, thereby reducing its ability to maintain remission (Baert 2003). The development of anti‐drug antibodies with adalimumab treatment has also been reported and may have implications for long‐term treatment (Moses 2019). It is therefore necessary to conduct a review to determine the efficacy and safety of adalimumab for maintenance of remission in CD.
Objectives
To assess the efficacy and safety of adalimumab for maintenance of remission in people with quiescent CD.
Methods
Criteria for considering studies for this review
Types of studies
We include randomized controlled trials (RCTs) that assessed the efficacy and safety of adalimumab for maintenance of remission in CD.
Types of participants
This review includes participants of any age who have been diagnosed with CD using clinical, radiological, endoscopic or histological criteria. Participants must be in remission (as defined by the included studies) at study entry to be included.
Types of interventions
This review includes trials that compared adalimumab either to a placebo or to an active comparator.
Types of outcome measures
Primary outcomes
The proportion of participants with CD who failed to maintain clinical remission, as defined by the original trials. Clinical remission is often defined as a Crohn's Disease Activity Index (CDAI) score of < 150.
Secondary outcomes
The proportion of participants:
Who failed to maintain clinical response;
Who failed to maintain endoscopic remission;
Who failed to maintain endoscopic response;
Who failed to maintain clinical or endoscopic response with prior TNF‐α exposure;
Who failed to maintain histologic remission;
Who failed to maintain histologic response;
Who failed to maintain steroid withdrawal;
Who developed adverse events (AEs);
Who developed serious adverse events (SAEs);
Who withdrew due to AEs; and
Quality of life.
Search methods for identification of studies
Electronic searches
We searched the following databases for relevant studies from inception to 15 April 2019:
Cochrane IBD Group Specialized Register;
Cochrane Central Register of Controlled trials (CENTRAL);
MEDLINE (Ovid);
Embase (Ovid).
The search strategies are listed in Appendix 1.
Searching other resources
As well as searching the electronic databases, we identified additional studies by handsearching the reference lists of relevant papers. We conducted handsearches of conference proceedings from Digestive Disease Week, United European Gastroenterology Week and the European Crohn's and Colitis Organisation Congress for the last five years. We identified ongoing trials by approaching leading experts in the field, and by searching the clinicaltrials.gov database and controlled-trials.com databases.
Data collection and analysis
Selection of studies
Two review authors (CT and TN) independently assessed the titles and abstracts of studies identified by the search criteria to determine eligibility according to the inclusion criteria. We discussed disagreements until we reached a consensus among the review authors, and consulted with a third review author (JKM) when we could not reach agreement.
Data extraction and management
Two review authors (CT and TN) independently extracted data using a standardized extraction form. We discussed any disagreements over extracted data, and then brought them to a third review author (JKM) for resolution as required. We extracted the following information:
General information (type of publication, title, journal, year);
Study design features (method of randomization, concealment of allocation and blinding, power calculation, a priori and post hoc analyses, dates of enrolment and follow‐up, study duration, number of centres and location, and study withdrawals);
Eligibility (number of participants screened and their randomization);
Participant characteristics (age, sex, race, severity of disease, current and prior medications);
Intervention (dose and type of medication, and whether it was compared to placebo or active comparator);
Primary and secondary outcomes;
Follow‐up (dates of follow‐up along with withdrawals and number of participants lost to follow‐up);
Funding details and author conflicts of interest.
Assessment of risk of bias in included studies
Two review authors (CT and TN) independently assessed risks of bias using the Cochrane 'Risk of bias' tool (Higgins 2017). We assessed several study characteristics for risks of bias, including random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. Based on these criteria, we rated the studies as having a low, high or unclear risk of bias for each category. We discussed any disagreements about risks of bias and then brought them to a third review author (JKM) as necessary.
Measures of treatment effect
We used Review Manager 5 (RevMan5) to analyze the data. For dichotomous outcomes, we calculated the risk ratio (RR) with a 95% confidence interval (CI). For continuous outcomes, we calculated the mean difference (MD) with a 95% CI.
Unit of analysis issues
To ensure independent comparisons between each treatment group (e.g. dose groups) and placebo, we divided the placebo group across the number of treatment groups to deal with trials with multiple arms. For trials with an odd number of participants in the placebo group, we split the placebo group so that the placebo group for the lower‐dose arm had a larger number of participants. This helped avoid overestimating treatment effects for the higher‐dose arm. We included cross‐over trials if the data prior to the first cross‐over were available. We reported on the proportion of participants who experienced at least one event, for studies where events may recur (e.g. AEs).
Dealing with missing data
We contacted study authors to obtain missing data when necessary. According to the intention‐to‐treat (ITT) analysis, we assumed that participants with missing dichotomous outcome data were treatment failures. We performed a sensitivity analysis to determine the impact of the assumption of treatment failure for missing data on the effect estimate. We conducted an available‐case analysis in the event of missing continuous outcome data.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test and the I2 statistic (Higgins 2003). We considered an I2 value of less than 25% indicative of low heterogeneity, greater than 50% indicative of moderate heterogeneity and greater than 75% high heterogeneity. For the Chi2 test, we considered a P value of 0.10 to be statistically significant. If the I2 statistic and Chi2 test suggested heterogeneity, we visually inspected the forest plot for outliers. We used a sensitivity analysis (e.g. excluding outliers) to explore potential explanations for heterogeneity.
Assessment of reporting biases
We evaluated reporting bias by comparing outcomes listed in protocols (or in the Methods section of the published paper if no protocol is available) to those reported in the final article. If a sufficient number of studies were included (more than 10) in the pooled analysis, we planned to use a funnel plot to assess publication bias (Egger 1997). As this review only includes six studies, we did not construct a funnel plot.
Data synthesis
We combined data from individual trials for meta‐analysis when interventions, participant groups and outcomes were sufficiently similar. We determined this by discussion and consensus among the review author team. We calculated the pooled RR with a 95% CI for dichotomous outcomes, and the pooled MDs with a 95% CI for continuous outcomes that were measured with the same scale. When different scales were used to measure the same underlying construct (e.g. quality of life), we calculated the standardized mean difference (SMD) with its 95% CI. We used a random‐effects model to pool the data. If we detected a high degree of heterogeneity (I2 > 75%), we did not pool data for meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned for the following subgroup analyses:
Participant characteristics (disease duration, disease severity, disease extent, concomitant medication); and
Drug doses and routes of administration.
The data only allowed for a subgroup analysis by drug dose regimen.
Sensitivity analysis
We used sensitivity analysis to examine the impact of the following variables on the pooled effect estimate:
Random‐effects versus fixed‐effect modeling;
Low risk of bias versus unclear or high risk of bias;
Relevant loss to follow‐up (more than 10%): best‐case versus worst‐case scenario; and
Full‐text articles versus abstract or unpublished studies.
Summary of Findings Table
We used GRADE to assess the overall certainty of the evidence supporting the primary and secondary outcomes (Schünemann 2017). For the 'Summary of findings' table, we included the following outcomes: failure to maintain clinical remission (at study endpoint), failure to maintain endoscopic remission, adverse events, serious adverse events, withdrawals due to adverse events, and failure to maintain clinical or endoscopic response in patients with previous TNF‐α exposure.
RCTs begin as high‐certainty evidence, and may be downgraded according to:
Risk of bias;
Indirect evidence;
Inconsistency (unexplained heterogeneity);
Imprecision (sparse data); and
Reporting bias (publication bias).
After considering each of these elements, we classified the overall quality of the evidence supporting each outcome as high‐certainty (further research is very unlikely to change our confidence in the estimate of effect); moderate‐certainty (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low‐certainty (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); or very low‐certainty (we are very uncertain about the estimate) (Guyatt 2008).
Results
Description of studies
Results of the search
A literature search conducted on 15 April 2019 identified 1766 records. After we had removed duplicates 1298 records were left for review. Two review authors (CT and TN) independently reviewed the titles and abstracts of these records and selected 40 full‐text articles for review (see Figure 1). We excluded four studies with reasons (see Characteristics of excluded studies). Thirty‐seven reports of six studies (total of 1158 participants) met the predefined inclusion criteria and were included in this review (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Savorino 2013; Scapa 2015; Watanabe 2012). Four of these studies were randomized placebo‐controlled trials (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012) and two were active‐comparator trials (Savorino 2013; Scapa 2015; see Characteristics of included studies). We found one ongoing study, but with no available data (NCT03464136).
1.
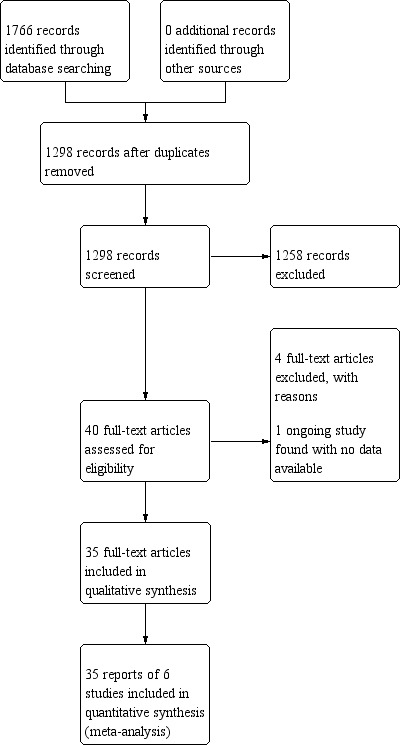
Study flow diagram.
Included studies
Colombel 2007, the CHARM trial, was a randomized, double‐blind, placebo‐controlled trial. In this study, 499 adult participants with known moderate‐to‐severe CD (CDAI 220 to 450) diagnosed either endoscopically or radiologically were considered for inclusion. Participants had to be on stable doses of azathioprine, 6‐mercapropurine, methotrexate, 5‐aminosalicylates, sulfsalazine, or oral mesalamine for at least four weeks prior to screening. CD‐related antibiotics were permitted if at stable doses for two weeks prior to screening. Prednisone (≤ 30 mg of prednisone a day) or budesonide (≤ 9 mg daily) were permitted if participants were taking a stable dose for at least two weeks before screening. Participants could have been on TNF‐α antagonists other than adalimumab previously, provided it was more than 12 weeks prior to randomization and they did not have a clinical response to the medication.
Exclusion criteria included symptomatic obstructive disease, bowel resection within six months prior to screening, an ostomy, extensive small bowel resection or short bowel syndrome, current total parenteral nutrition (TPN) therapy, previous history of cancer, HIV, demyelinating diseases, Listeria infection, positive Clostridium difficile assay, untreated tuberculosis, previous chemotherapy within 30 days prior to screening, or poorly‐controlled comorbid conditions. Women who were currently pregnant or breastfeeding were excluded. People who had a history of illicit drug use or a significant alcohol history within the last year were also excluded. Individuals were also excluded if they had previously been given adalimumab, a rectal enema two weeks prior to screening or received cyclosporine, mycophenolate mofetil or tacrolimus eight weeks prior to screening.
Participants initially entered a two‐week screening period where they received open‐label adalimumab 80 mg subcutaneously at week 0 and 40 mg at week 2. Participants were randomized at week 4 to one of three groups: adalimumab 40 mg every other week (eow); adalimumab 40 mg weekly; or placebo, and were also stratified by responder status (decrease in CDAI ≥ 70) and previous TNF‐α antagonist exposure. Participants who flared during the study timeline were moved to an open‐label study. Primary end points included the participants who responded at week 4, defined as a decrease in CDAI ≥ 70, and participants who achieved clinical remission, defined as CDAI < 150 at weeks 26 and 56.
Secondary end points included the number of participants with a clinical response (decrease in CDAI score from baseline by ≥ 70 points and by ≥ 100 points) at weeks 26 and 56; changes from baseline in Inflammatory Bowel Disease Questionnaire (IBDQ) total scores at weeks 26 and 56; number of participants in clinical remission at weeks 26 and 56 who were able to discontinue corticosteroid use; number of participants in clinical remission at weeks 26 and 56 who were able to discontinue corticosteroid use for ≥ 90 days; number of participants with fistula remission (closure of all fistulas); number of participants using immunosuppressants; prior TNF‐α antagonist use (experienced versus naïve); median time in clinical remission among randomized responders achieving remission; and AEs.
Rutgeerts 2012, the EXTEND trial, was a randomized placebo‐controlled trial including 129 adult participants. Participants were included in this trial if they had moderate‐to‐severe CD (CDAI 220 to 450) and active mucosal ulceration during screening ileocolonoscopy. Therapy with azathioprine, 6‐mercaptopurine, and methotrexate was allowed, if participants were taking these medications for at least 12 weeks and at a stable dose for at least four weeks prior to screening. Participants could remain on prescribed aminosalicylates or CD‐related antibiotics, if they were on a stable dose for at least four weeks prior to screening. Participants who had previously received infliximab or other TNF‐α antagonists, excluding adalimumab, were eligible unless they had no clinical response. Treatment with prednisone (maximum dose 40 mg/day) or budesonide (maximum dose 9 mg/day) was allowed if participants were taking a stable dose for at least two weeks before screening. Exclusion criteria included TNF‐α antagonist exposure within eight weeks of screening, use of any other study medication during the study, and prior exposure to adalimumab or natalizumab.
Participants initially received an open‐label induction of adalimumab, 160 mg subcutaneously at week 0 and 80 mg at week 4. Participants were randomized to adalimumab 40 mg eow or placebo. Participants who flared during the study timeline were moved to an open‐label study.
The primary end point was mucosal healing at week 12. Secondary end points included mucosal healing at week 52, the Crohn's Disease Endoscopic Index of Severity (CDEIS) score at weeks 12 and 52, CDAI remission and response, and AEs.
Reinisch et al. reported additional data from the EXTEND study, i.e. 199 adult participants with moderate‐to‐severe CD (CDAI 220 to 450) who had active mucosal ulceration during screening ileocolonoscopy were considered for inclusion. Study design is previously described under Rutgeerts 2012. Study end points included CDEIS score at weeks 12 and 52, Simple Endoscopic Score for CD (SES‐CD) score at weeks 12 and 52, and mucosal healing at week 12.
In Sandborn 2007, the CLASSIC II trial, 55 participants with moderate‐to‐severe CD (CDAI 220 to 450) who were in clinical remission at the end of the CLASSIC I trial were considered for inclusion. All patients deemed to have normal cardiac, renal and hepatic function were considered for inclusion. Prior to randomization, participants were given adalimumab 40 mg at week 0 and 40 mg at week 2. Participants who were in clinical remission (CDAI < 150) at week 4 were randomized to one of three groups in a 1:1:1 ratio: adalimumab 40 mg eow; adalimumab 40 mg weekly; and placebo. Participants were followed to week 56. Participants who experienced a flare of their disease during the study timeline could be moved to the open‐label study. All other medications were kept at stable doses throughout the trial, except that prednisone doses greater than 10 mg were tapered by 5 mg starting at week 8 until participants were on 10 mg/kg a day and then the dose was reduced by 2.5 mg a week until stopped.
The primary end point was the number of participants in clinical remission (CDAI < 150) at week 56. Secondary outcomes included the number of participants in clinical remission (CDAI < 150) at week 24; 100‐point clinical response (decrease in CDAI ≥ 100) at weeks 24 and 56; 70‐point clinical response (decrease in CDAI ≥ 70) at weeks 24 and 56; IBDQ score; number of participants who stopped steroid therapy without loss of remission at weeks 24 and 56; and AEs. However, authors acknowledged that the study was not adequately powered for the primary end point.
In Watanabe 2012, 50 patents aged 15 to 75 with moderate‐to‐severe ileocolonic CD (CDAI 220 to 450) diagnosed at least four months prior to study enrolment were considered for inclusion. Participants were allowed prior exposure to TNF‐α antagonists other than adalimumab, providing it was not within eight weeks of study enrolment. Exclusion criteria included no response to prior TNF‐α antagonist, a history of cancer, hematologic malignancy or lymphoproliferative disorder, active or latent tuberculosis infection, active HIV infection, active Clostridium difficile infection, infections requiring hospital admission, intravenous antibiotics in the last 28 days or oral antibiotics in the last 14 days, history of demyelinating disease, bowel resection within the last six months, body weight less than 30 kg, receiving TPN or enteral nutrition, abnormalities on electrocardiogram, uncontrolled comorbid condition putting them at risk by participating in the trial, and women of child‐bearing age who were not on an effective form of birth control.
Participants initially entered an induction trial where they were randomized to one of three groups in a 2:3:3 ratio: placebo; adalimumab 160 mg at week 0 and 80 mg at week 2; or adalimumab 80 mg at week 0 and 40 mg at week 2. Participants were eligible for enrolment in the maintenance trial if they had at least a 70‐point decrease in their CDAI score at week 4. Participants were randomized in a 1:1 ratio to receive either 40 mg eow or placebo, and were followed to week 52.
The primary end point was clinical remission (CDAI < 150) at week 52. Secondary end points included the number of participants in clinical remission every four weeks until week 52; number of participants with clinical response, defined as a decrease in CDAI score by 100 points or 70 points every four weeks until week 52; serum adalimumab levels; anti‐adalimumab antibody levels; and AEs. Investigators also reported the changes from baseline values in the CDAI, International Organization of Inflammatory Bowel Disease score (IOIBD), mental component summary and physical component summary of the Short Form‐36 Health Survey.
In Savorino 2013, 51 participants who had an ileocolic resection two weeks prior to study enrolment were randomized to receive adalimumab 160 mg at week 0, 80 mg at week 2, followed by 40 mg eow (n = 16); azathioprine 2 mg/kg daily (n = 17); or mesalamine (5‐ASA) 3 g a day (n = 18), and were followed for 104 weeks. The primary end point was endoscopic recurrence (Rutgeerts score or i2 to i4) and clinical recurrence (based on investigators' own scale rating severity of symptoms from 1 to 4). Secondary end points included IBDQ score at 104 weeks.
In Scapa 2015, 19 participants who had ileocolonic resection 45 days prior to study entry were randomized to receive adalimumab 160 mg at week 0, 80 mg at week 2, followed by 40 mg eow (n = 11); or 6‐mercaptopurine (6‐MP) 1.5 mg/kg daily (n = 8). The primary end point was the number of participants in endoscopic remission (Rutgeets i0 to i1) at week 24. Secondary outcomes included the number of participants in endoscopic remission (Rutgeets i0 to i1) at week 12; improvement in CDAI score; improvement in IBDQ score; improvement in fecal calprotectin levels; weight gain; and C‐reactive protein level.
Excluded studies
We excluded four studies with reasons (De Cruz 2015; Dewint 2014; Faubion 2017; Khanna 2015; See Characteristics of excluded studies table). Two studies compared treatment algorithms for managing participants with CD (De Cruz 2015; Khanna 2015), one study had both treatment and control groups on adalimumab therapy (Dewint 2014), and one study was an open‐label extension study with no control group (Faubion 2017). We found one ongoing study, but no data were available for this review (NCT03464136).
Risk of bias in included studies
We assessed the methodological quality of each study using the Cochrane 'Risk of bias' tool (Higgins 2017), and we summarize our findings in Figure 2. We attempted to contact study authors for additional information to clarify unclear items, but did not receive a response.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies did not report on how participants were randomized to treatment groups or how allocation concealment was achieved. We rated these studies at unclear risk of bias for these domains (Rutgeerts 2012; Savorino 2013; Scapa 2015; Watanabe 2012).
Blinding
Two studies did not report on how blinding was maintained for participants, personnel or outcome assessors throughout the study time period. We rated these studies at unclear risk of bias for these domains (Savorino 2013; Scapa 2015).
Incomplete outcome data
Scapa 2015 did not report on the number of participants who were initially randomized, so we could not determine the number of participants who had withdrawn during the study period. We rated the study at unclear risk of bias for this domain.
Selective reporting
All of the included studies reported on all expected outcomes and were rated at low risk of bias for this domain (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Savorino 2013; Scapa 2015; Watanabe 2012).
Other potential sources of bias
None of the included studies appeared to have other potential sources of bias and were rated at low risk of bias for this domain (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Savorino 2013; Scapa 2015; Watanabe 2012).
Effects of interventions
Adalimumab compared to placebo for maintenance of remission in CD
Failure to maintain clinical remission at 52 to 56 weeks
Three studies (N = 683) reported on the number of participants who failed to maintain clinical remission at 52 to 56 weeks (Colombel 2007; Rutgeerts 2012; Sandborn 2007). Two hundred and fifty‐five participants received 40 mg of adalimumab eow, 175 participants received 40 mg of adalimumab weekly and 253 participants received placebo. Overall, 59% (252/430) of participants receiving adalimumab failed to maintain clinical remission compared to 86% (217/253) in the placebo group (RR 0.70, 95% CI 0.64 to 0.77; Analysis 1.1). There was no heterogeneity detected for this outcome (I2 = 0%). The GRADE analysis indicated that the overall certainty of evidence was high (Table 1). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.69, 95% CI 0.63 to 0.76). A sensitivity analysis excluding the study at unclear risk of bias (Rutgeerts 2012) also had no impact on the overall results (RR 0.69, 95% CI 0.63 to 0.77). Due to insufficient data, we did not undertake any of the other planned sensitivity analyses for this outcome.
1.1. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 1: Failure to maintain clinical remission at 52 to 56 weeks
In participants who received adalimumab 40 mg eow, 62% (157/255) of participants failed to maintain clinical remission at 52 to 56 weeks compared to 87% (138/159) of participants who received placebo (RR 0.73, 95% CI 0.65 to 0.81). In participants who received adalimumab 40 mg weekly, 54% (95/175) of participants who received adalimumab failed to maintain clinical remission compared to 84% (79/94) who received placebo (RR 0.66, 95% CI 0.57 to 0.77). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 1.07, df = 1, P = 0.30, I2 = 6.6%; Analysis 1.1). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Failure to maintain clinical remission at 24 to 26 weeks
Two studies (N = 554) reported on the number of participants who failed to maintain clinical remission at 24 to 26 weeks (Colombel 2007; Sandborn 2007). One hundred and ninety‐one participants received 40 mg of adalimumab eow, 175 participants received 40 mg of adalimumab weekly and 188 participants received placebo. Overall, 52% (192/366) of participants on adalimumab failed to maintain clinical remission at 24 to 26 weeks compared to 79% (149/188) of participants who received placebo (RR 0.66, 95% CI 0.52 to 0.83; Analysis 1.2). We detected moderate heterogeneity for this outcome (I2 = 55%). The GRADE analysis indicates that the overall certainty of evidence was moderate, due to serious inconsistency (I2 = 55%) (Table 1). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.64, 95% CI 0.57 to 0.72).
1.2. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 2: Failure to maintain clinical remission at 24 to 26 weeks
In participants who received adalimumab 40 mg eow, 56% (107/191) failed to maintain clinical remission at 24 to 26 weeks compared to 80% (75/94) of participants who received placebo (RR 0.55, 95% CI 0.23 to 1.32). In participants who received adalimumab 40 mg weekly, 52% (85/175) failed to maintain clinical remission at 24 to 26 weeks compared to 79% (74/94) of placebo participants (RR 0.39, 95% CI 0.08 to 1.81). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 0.15, df = 1, P = 0.70, I2 = 0%; Analysis 1.2). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Failure to maintain clinical response at 52 to 56 weeks
Four studies (N = 733) reported on the number of participants who failed to maintain clinical response at 52 to 56 weeks (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012). Two hundred and eighty participants received 40 mg of adalimumab eow, 175 participants received 40 mg of adalimumab weekly and 278 participants received placebo. Overall, 54% (244/455) of participants on adalimumab failed to maintain clinical response at 52 to 56 weeks compared to 81% (226/278) of participants who received placebo (RR 0.68, 95% CI 0.62 to 0.75; Analysis 1.3). We detected no heterogeneity for this outcome (I2 = 0%), A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.67, 95% CI 0.61 to 0.74). A sensitivity analysis excluding the two studies with unclear risk of bias (Rutgeerts 2012; Watanabe 2012) also had no impact on the overall results (RR 0.66, 95% CI 0.59 to 0.74). Due to insufficient data, we did not undertake any of the other planned sensitivity analyses for this outcome.
1.3. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 3: Failure to maintain clinical response at 52 to 56 weeks
In participants who received adalimumab 40 mg eow, 57% (160/280) of participants failed to maintain clinical response at 52 to 56 weeks compared to 82% (151/184) of participants who received placebo (RR 0.71, 95% CI 0.63 to 0.80). In participants who received adalimumab 40 mg weekly, 48% (84/175) failed to maintain clinical response compared to 80% (75/94) of placebo participants (RR 0.53, 95% CI 0.27 to 1.06). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 0.65, df = 1, P = 0.42, I2 = 0%; Analysis 1.3). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Failure to maintain clinical or endoscopic response at 52 to 56 weeks with prior TNF‐α antagonist exposure
Two studies (N = 302) reported the number of participants who failed to maintain clinical or endoscopic response at 52 to 56 weeks after having received prior TNF‐α antagonist therapy (Colombel 2007; Rutgeerts 2012). Overall, 69% (129/186) of adalimumab participants failed to maintain clinical or endoscopic response compared with 93% (108/116) receiving placebo (RR 0.76, 95% CI 0.68 to 0.85; Analysis 1.4). We detected no heterogeneity for this outcome (I2 = 0%). The GRADE analysis indicated that the overall certainty of evidence was moderate due to sparse data (237 events; Table 1). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.76, 95% CI 0.68 to 0.84). A sensitivity analysis excluding the study with unclear risk of bias (Rutgeerts 2012) also had no impact on the overall results (RR 0.75, 95% CI 0.66 to 0.86). Due to insufficient data, we did not undertake any of the other planned sensitivity analyses for this outcome.
1.4. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 4: Failure to maintain clinical or endoscopic response at 52 to 56 weeks with prior TNF antagonist exposure
In participants who received adalimumab 40 mg eow, 71% (82/115) of participants failed to maintain clinical or endoscopic response at 52 to 56 weeks compared to 95% (71/75) who received placebo (RR 0.77, 95% CI 0.67 to 0.88). In participants who received adalimumab 40 mg weekly, 66% (47/71) of participants who received adalimumab failed to maintain clinical or endoscopic response compared to 90% (37/41) of placebo participants (RR 0.73, 95% CI 0.60 to 0.89). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 0.17, df = 1, P = 0.68, I2 = 0%; Analysis 1.4). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Failure to maintain clinical response at 24 to 26 weeks
Two studies (N = 554) included failure to maintain clinical response at 24 to 26 weeks (Colombel 2007; Sandborn 2007). Overall, 44% (162/366) of participants who received adalimumab failed to maintain clinical response compared with 70% (132/188) of those who received placebo (RR 0.65, 95% CI 0.56 to 0.74; Analysis 1.5). We detected no heterogeneity for this outcome (I2 = 0%). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.63, 95% CI 0.55 to 0.73).
1.5. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 5: Failure to maintain clinical response at 24 to 26 weeks
In participants who received adalimumab 40 mg eow, 45% (86/191) failed to maintain clinical response compared with 70% (66/94) who received placebo (RR 0.65, 95% CI 0.53 to 0.79). In participants who received adalimumab 40 mg weekly, 43% (76/175) failed to maintain clinical response compared to 70% (66/94) of participants who received placebo (RR 0.39, 95% CI 0.08 to 1.81). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 0.41, df = 1, P = 0.52, I2 = 0%; Analysis 1.5). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Failure to maintain endoscopic remission at 52 weeks
One study (N = 129) reported on the number of participants who failed to maintain endoscopic remission at 52 weeks (Rutgeerts 2012). Seventy‐two per cent (46/64) of participants who received adalimumab failed to maintain endoscopic remission compared to 97% (63/65) of participants who received placebo (RR 0.74, 95% CI 0.63 to 0.87; Analysis 1.6). The GRADE analysis indicated that the overall certainty of evidence was moderate, due to sparse data (109 events; Table 1).
1.6. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 6: Failure to maintain endoscopic remission at 52 weeks
Failure to maintain endoscopic response at 52 weeks
One study (N = 123) reported on the number of participants who failed to maintain endoscopic response at 52 weeks (Rutgeerts 2012). Seventy‐six per cent (47/62) of participants who received adalimumab failed to maintain endoscopic response compared to 100% (61/61) of participants who received placebo (RR 0.76, 95% CI 0.66 to 0.88; Analysis 1.7).
1.7. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 7: Failure to maintain endoscopic response at 52 weeks
Failure to maintain histological remission at 52 weeks
One study (N = 129) reported on the number of participants who failed to maintain histologic remission at 52 weeks (Rutgeerts 2012). Seventy‐six per cent (47/62) of participants who received adalimumab failed to maintain histological remission compared to 95% (62/65) of participants who received placebo (RR 0.79, 95% CI 0.68 to 0.92; Analysis 1.8).
1.8. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 8: Failure to maintain histological remission at 52 weeks
Adverse events
Four studies (N = 1012) reported on AEs (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012). Overall, 87% (561/643) of participants who received adalimumab reported an AE compared with 85% (315/369) of participants who received placebo (RR 1.01, 95% CI 0.94 to 1.09; Analysis 1.9). We detected some heterogeneity for this outcome (I2 = 40%). The GRADE analysis indicates that the overall certainty of the evidence was high (Table 1). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 1.03, 95% CI 0.97 to 1.08). A sensitivity analysis excluding the two studies with unclear risk of bias (Rutgeerts 2012; Watanabe 2012) also had no impact on the overall results (RR 0.99, 95% CI 0.90 to 1.08). Due to insufficient data, we did not undertake any of the other planned sensitivity analyses for this outcome. Some commonly‐reported AEs included CD aggravation, arthralgia, nasopharyngitis, urinary tract infections, headache, nausea, fatigue and abdominal pain.
1.9. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 9: Adverse events
In participants who received adalimumab 40 mg eow, 89% (327/368) of participants experienced an AE compared with 86% (196/229) of participants who received placebo (RR 1.03, 95% CI 0.93 to 1.14). In participants who received adalimumab 40 mg weekly, 85% (234/275) of participants experienced an AE compared with 85% (119/140) of participants who received placebo (RR 0.94, 95% CI 0.76 to 1.17). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 0.52, df = 1, P = 0.47, I² = 0%; Analysis 1.9). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Serious adverse events
Four studies (N = 1012) reported SAEs (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012). SAEs were seen in 8% (52/643) of participants who received adalimumab and 14% (53/369) of participants who received placebo (RR 0.56, 95% CI 0.39 to 0.80; Analysis 1.10). We detected no heterogeneity for this outcome (I2 = 0%). The GRADE analysis indicates that the overall certainty of evidence was moderate, due to sparse data (105 events; Table 1). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.55, 95% CI 0.38 to 0.79). A sensitivity analysis excluding the two studies at unclear risk of bias (Rutgeerts 2012; Watanabe 2012) also had no impact on the overall results (RR 0.56, 95% CI 0.38 to 0.82). Due to insufficient data, we did not undertake any of the other planned sensitivity analyses for this outcome. The reported SAEs included infectious complications including tuberculosis, abscess formation and wound infections, multiple sclerosis, and pulmonary embolism.
1.10. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 10: Serious adverse events
In participants who received adalimumab 40 mg eow, 8% (31/368) of participants experienced a SAE compared to 14% (32/229) of participants who received placebo (RR 0.59, 95% CI 0.36 to 0.94). In participants who received adalimumab 40 mg weekly, 8% (21/275) experienced a SAE compared with 15% (21/140) who received placebo (RR 0.52, 95% CI 0.29 to 0.91). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 0.11, df = 1, P = 0.73, I2 = 0%; Analysis 1.10). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Withdrawal due to adverse events
Four studies (N = 1012) reported on withdrawal due to AEs (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012). Withdrawals due to AEs were reported in 7% (45/643) of adalimumab participants compared to 13% (48/369) of placebo participants (RR 0.59, 95% CI 0.38 to 0.91; Analysis 1.11). We detected low heterogeneity for this outcome (I2 = 13%). The GRADE analysis indicates that the overall certainty of evidence was moderate due to sparse data (93 events; Table 1). A sensitivity analysis using a fixed‐effect model had no impact on the overall results (RR 0.57, 95% CI 0.39 to 0.83). A sensitivity analysis excluding the two studies with unclear risk of bias (Rutgeerts 2012; Watanabe 2012) had no major impact on the overall results (RR 0.44, 95% CI 0.28 to 0.69). Due to insufficient data, we did not undertake any of the other planned sensitivity analyses for this outcome. AEs leading to withdrawal included a flare of CD and infectious complications.
1.11. Analysis.

Comparison 1: Adalimumab versus placebo, Outcome 11: Withdrawals due to adverse events
In participants who received adalimumab 40 mg eow, 9% (32/368) of participants withdrew due to an AE compared to 13% (30/229) of participants who received placebo (RR 0.73, 95% CI 0.43 to 1.21). In participants who received adalimumab 40 mg weekly, 5% (13/275) withdrew due to an AE compared with 13% (18/140) who received placebo (RR 0.37, 95% CI 0.19 to 0.73). The test for subgroup differences by dosing regimen showed no difference between the regimens (test for subgroup differences Chi2 = 2.43, df = 1, P = 0.12, I2 = 58.9%; Analysis 1.11). We did not conduct any of the other planned subgroup analyses due to insufficient data.
Adalimumab compared to active comparator for maintenance of remission in CD
Failure to maintain clinical response at 104 weeks
One study (N = 51) reported on failure to maintain clinical response at 104 weeks in participants who received either adalimumab, azathioprine or mesalamine (Savorino 2013). Thirteen per cent (2/16) of participants who received adalimumab failed to maintain clinical response compared with 54% (19/35) of participants who received azathioprine or mesalamine (RR 0.23, 95% CI 0.06 to 0.87; Analysis 2.1).
2.1. Analysis.

Comparison 2: Adalimumab versus active comparator, Outcome 1: Failure to maintain clinical response at 104 weeks
Failure to maintain endoscopic remission at 104 weeks
One study (N = 51) reported on failure to maintain endoscopic remission at 104 weeks in participants who received either adalimumab, azathioprine or mesalamine (Savorino 2013). Six per cent (1/16) of participants who received adalimumab failed to maintain endoscopic remission compared with 57% (20/35) of participants who received azathioprine or mesalamine (RR 0.11, 95% CI 0.02 to 0.75; Analysis 2.2). The GRADE analysis indicates that the certainty of evidence was very low due to unclear risk of bias and very sparse data (Table 2).
2.2. Analysis.

Comparison 2: Adalimumab versus active comparator, Outcome 2: Failure to maintain endoscopic remission at 104 weeks
Failure to maintain endoscopic remission at 24 weeks
One study (N = 19) reported on failure to maintain endoscopic response at 24 weeks in participants who received either adalimumab or 6‐MP (Scapa 2015). Nine per cent (1/11) of participants who received adalimumab failed to maintain endoscopic remission compared with 50% (4/8) of participants who received 6‐MP (RR 0.18, 95% CI 0.02 to 1.33; Analysis 2.3).
2.3. Analysis.

Comparison 2: Adalimumab versus active comparator, Outcome 3: Failure to maintain endoscopic remission at 24 weeks
Discussion
Summary of main results
This review includes six studies (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Savorino 2013; Scapa 2015; Watanabe 2012). Four of these studies are randomized placebo‐controlled trials that examined the efficacy of adalimumab for maintaining remission in participants with quiescent CD (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012). Two of the studies are active comparator trials that examined the efficacy of adalimumab for maintaining surgically‐induced remission in CD (Savorino 2013; Scapa 2015). Savorino 2013 compared adalimumab to azathioprine or mesalamine. Savorino 2013 compared adalimumab to 6‐MP.
All studies used validated outcome measures for study end points. Clinical remission was reported using a CDAI score of 150 or less in four studies (Colombel 2007; Rutgeerts 2012; Sandborn 2007; Watanabe 2012). Histological end points were reported using both CDEIS and SES‐CD scores (Rutgeerts 2012). Two studies examined the efficacy of adalimumab in the postoperative course (Savorino 2013; Scapa 2015), using the Rutgeerts score to report clinical recurrence at the ileocolic anastomosis.
Three studies reported on the proportion of participants who failed to maintain clinical remission at 52 to 56 weeks (Colombel 2007; Rutgeerts 2012; Sandborn 2007). Overall, 59% (252/430) of participants who received adalimumab failed to maintain clinical remission compared to 86% (217/253) in the placebo group (high‐certainty evidence). Subgroup analyses based on dose showed that weekly dosing and bi‐weekly dosing of adalimumab were both effective, with minimal difference in clinical remission rates seen between the two dosing regimens. Although these relapse rates are fairly high it should be noted that these participants had moderate‐to‐severe Crohn’s disease prior to induction of remission and many of these participants had failed treatment with other biologics (e.g. infliximab) or standard maintenance therapies (e.g. azathioprine or 6‐mercaptopurine) prior to participating in the RCTs. An optimal maintenance therapy in people with CD with these characteristics has yet to be identified.
High‐certainty evidence suggests that there was no difference in adverse events (AEs) between the adalimumab and placebo groups. Eighty‐seven per cent (561/643) of participants who received adalimumab reported an AE compared with 85% (315/369) of participants who received placebo. Moderate‐certainty evidence suggests there was no difference in serious adverse event (SAE) rates between groups. Eight per cent (52/643) of participants who received adalimumab experienced SAEs compared with 14% (53/369) of participants who received placebo. Moderate‐certainty evidence suggests there was no difference between the groups in the number of participants who withdrew due to AEs. Seven per cent (45/643) of adalimumab participants withdrew due to AEs compared to 13% (48/369) of placebo participants.
Rutgeerts 2012 reported on endoscopic and histologic end points in participants who had received adalimumab compared with participants who received placebo. Rutgeerts 2012 found that 72% (46/64) of participants who received adalimumab failed to maintain endoscopic remission at 52 weeks compared to 97% (63/65) of participants who received placebo. Rutgeerts 2012 also reported that 76% (47/62) of participants who received adalimumab failed to maintain histological remission at 52 weeks compared to 95% (62/65) of participants who received placebo. We could draw minimal conclusions from these data, as no other studies reported on these important end points.
Two studies reported on the proportion of participants who failed to maintain clinical or endoscopic response at 52 to 56 weeks after having prior TNF‐α antagonist therapy (Colombel 2007; Rutgeerts 2012). Overall, 69% (129/186) of adalimumab participants failed to maintain clinical or endoscopic response compared with 93% (108/116) receiving placebo (moderate‐certainty evidence).
Two small studies reported on the proportion of participants who failed to maintain clinical and endoscopic end points, following surgical resection for CD (Savorino 2013, Scapa 2015). Adalimumab therapy was compared with either azathioprine, mesalamine or 6‐mercaptopurine. Savorino 2013 reported that 13% (2/16) of participants who received adalimumab failed to maintain clinical response at 104 weeks, compared with 54% (19/35) of participants who received azathioprine or mesalamine. Also, six per cent (1/16) of participants who received adalimumab failed to maintain endoscopic remission at 104 weeks compared with 57% (20/35) of participants who received azathioprine or mesalamine (very low‐certainty evidence). Scapa 2015 reported that 9% (1/11) of participants who received adalimumab failed to maintain endoscopic remission at 24 weeks compared with 50% (4/8) of participants who received azathioprine. Overall, data surrounding maintenance therapy in the postoperative course are of very low certainty, and further research should be done to determine the best treatment course postoperatively.
Overall completeness and applicability of evidence
The results of this Cochrane Review are applicable to people with moderate‐to‐severe CD (CDAI 220 to 450) who are in remission. Study participants included people who had previous treatment with infliximab and had lost response or had become intolerant to the drug, and people who were TNF‐α‐naïve. However, the overall evidence cannot be considered complete. The overall completeness of the data pertaining to both histological and endoscopic end points is lacking. Only one study reported on both endoscopic and histologic outcomes (Rutgeerts 2012). These outcomes are of interest to clinicians, and further evidence in this area would be required to draw definitive conclusions about the certainty of this evidence.
Two small studies reported on the course of treatment in participants who had recently had a surgical resection (Savorino 2013; Scapa 2015). These studies were small and reported on few outcomes. They compared adalimumab therapy to different active drugs, including azathioprine, mesalamine and 6‐mercaptopurine. Minimal conclusions can therefore be drawn from these data and the evidence base in the postoperative setting cannot be considered complete.
Quality of the evidence
We judged two of the included studies to be at low risk of bias (Colombel 2007; Sandborn 2007). Four studies were rated at unclear risk of bias for random sequence generation and allocation concealment (Rutgeerts 2012; Savorino 2013; Scapa 2015; Watanabe 2012). We rated two studies at unclear risk of bias for blinding (Savorino 2013; Scapa 2015), and one study at unclear risk of bias for incomplete outcome data (Scapa 2015). For the placebo‐controlled studies, the GRADE analysis for the primary outcome of failure to maintain clinical remission at 52 to 56 weeks was high, and was moderate for failure to maintain clinical remission at 24 to 26 weeks. The GRADE rating for the other secondary outcomes was moderate, except for adverse events, which we rated as high‐certainty evidence. For the studies comparing adalimumab to azathioprine, mesalamine or 6‐mercaptopurine in the postoperative setting the overall certainty of the evidence for the secondary outcome of failure to maintain endoscopic remission at 104 weeks was very low. The studies in the postoperative setting did not report on any of our other prespecified primary or secondary outcomes.
Potential biases in the review process
We conducted a comprehensive literature review to help ensure that we included all relevant studies. Two review authors independently assessed for study inclusion, extracted data and assessed for risks of bias. The main limitation of this review is the lack of data available for endoscopic and histological end points.
Agreements and disagreements with other studies or reviews
One other systematic review and meta‐analysis has recently been published that also discusses the efficacy of adalimumab for maintenance of remission in CD. Singh 2018 assessed the efficacy and safety of first‐ and second‐line TNF‐α antagonists for treatment of moderate‐to‐severe CD. The results for failure to maintain remission at 52 to 56 agree with the results of our review. However, Singh 2018 focused on identifying which TNF‐α antagonists should be considered for first‐ and second‐line therapy, a question which we did not address in this review.
Authors' conclusions
Implications for practice.
Adalimumab is an effective therapy to maintain clinical remission in participants with CD. Adalimumab is also effective in participants who have previously been treated with TNF‐α antagonists. The effect of adalimumab in the post‐surgical setting is uncertain.
Implications for research.
More high‐quality well‐powered studies are needed in this area. More research is needed in participants with recent bowel surgery for CD, to better determine treatment plans following surgery. Future research should continue to explore factors that influence initial and subsequent biologic selection for participants with moderate‐to‐severe CD. Studies comparing adalimumab to other active medications are needed, to help determine the optimal maintenance therapy for CD.
History
Protocol first published: Issue 11, 2017 Review first published: Issue 5, 2020
Appendices
Appendix 1. Search Strategies
EMBASE (1946 ‐ present)
1. random$.tw. 2. factorial$.tw. 3. (crossover$ or cross over$ or cross‐over$).tw. 4. placebo$.tw. 5. single blind.mp. 6. double blind.mp. 7. triple blind.mp. 8. (singl$ adj blind$).tw. 9. (double$ adj blind$).tw. 10. (tripl$ adj blind$).tw. 11. assign$.tw. 12. allocat$.tw. 13. crossover procedure/ 14. double blind procedure/ 15. single blind procedure/ 16. triple blind procedure/ 17. randomized controlled trial/ 18. or/1‐17 19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 20. 18 not 19 21. Exp Crohn disease/ 22. Crohn*.mp. 23. inflammatory bowel disease*.mp. 24. IBD.mp. 25. 21 or 22 or 23 or 24 26. Adalimumab.mp. 27. Humira.mp. 28. Exemptia.mp. 29. 26 or 27 or 28 30. 20 and 25 and 29
MEDLINE (1946 ‐ present)
1. random$.tw. 2. factorial$.tw. 3. (crossover$ or cross over$ or cross‐over$).tw. 4. placebo$.tw. 5. single blind.mp. 6. double blind.mp. 7. triple blind.mp. 8. (singl$ adj blind$).tw. 9. (double$ adj blind$).tw. 10. (tripl$ adj blind$).tw. 11. assign$.tw. 12. allocat$.tw. 13. randomized controlled trial/ 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 not 15 17. Exp Crohn disease/ 18. Crohn*.mp. 19. inflammatory bowel disease*.mp. 20. IBD.mp. 21. 17 or 18 or 19 or 20 22. Adalimumab.mp. 23. Humira.mp. 24. Exemptia.mp. 25. 22 or 23 or 24 26. 16 and 21 and 25
CENTRAL
#1 MeSH descriptor: [Crohn Disease] explode all trees #2 Crohn #3 Inflammatory Bowel Disease #4 IBD #5 #1 or #2 or #3 or #4 #6 Adalimumab #7 Humira #8 Exemptia #9 #6 or #7 or #8 #10 #5 and #9
SR‐IBD
Title: Adalimumab and Crohn
Humira and Crohn
Data and analyses
Comparison 1. Adalimumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Failure to maintain clinical remission at 52 to 56 weeks | 3 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.64, 0.77] |
| 1.1.1 Dose 40 mg every other week | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.65, 0.81] |
| 1.1.2 Dose 40 mg weekly | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.77] |
| 1.2 Failure to maintain clinical remission at 24 to 26 weeks | 2 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.52, 0.83] |
| 1.2.1 Dose 40 mg every other week | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.23, 1.32] |
| 1.2.2 Dose 40 mg weekly | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.81] |
| 1.3 Failure to maintain clinical response at 52 to 56 weeks | 4 | 733 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.62, 0.75] |
| 1.3.1 Dose 40 mg every other week | 4 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.63, 0.80] |
| 1.3.2 Dose 40 mg weekly | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.27, 1.06] |
| 1.4 Failure to maintain clinical or endoscopic response at 52 to 56 weeks with prior TNF antagonist exposure | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.68, 0.85] |
| 1.4.1 Dose 40 mg every other week | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 1.4.2 Dose 40 mg weekly | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.60, 0.89] |
| 1.5 Failure to maintain clinical response at 24 to 26 weeks | 2 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.56, 0.74] |
| 1.5.1 Dose 40 mg every other week | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.53, 0.79] |
| 1.5.2 Dose 40 mg weekly | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.81] |
| 1.6 Failure to maintain endoscopic remission at 52 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Failure to maintain endoscopic response at 52 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Failure to maintain histological remission at 52 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Adverse events | 4 | 1012 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 1.9.1 Dose 40 mg every other week | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.93, 1.14] |
| 1.9.2 Dose 40 mg weekly | 2 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.17] |
| 1.10 Serious adverse events | 4 | 1012 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.39, 0.80] |
| 1.10.1 Dose 40 mg every other week | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.36, 0.94] |
| 1.10.2 Dose 40 mg weekly | 2 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.91] |
| 1.11 Withdrawals due to adverse events | 4 | 1012 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 1.11.1 Dose 40 mg every other week | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.21] |
| 1.11.2 Dose 40 mg weekly | 2 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.19, 0.73] |
Comparison 2. Adalimumab versus active comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Failure to maintain clinical response at 104 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Failure to maintain endoscopic remission at 104 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Failure to maintain endoscopic remission at 24 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Colombel 2007.
| Study characteristics | ||
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial The study was conducted at 92 sites in Europe, the United States, Canada, Australia, and South Africa from July 2003 to September 2005 Intention‐to‐treat not performed Study duration 56 weeks |
|
| Participants | Included participants aged 18 ‐ 75 with CD diagnosed at least 4 months ago Diagnosis was supported by both radiological and histological evidence CDAI 220 ‐ 450 (N = 854) All participants received open‐label adalimumab induction at week 0 (adalimumab 80 mg sc) and week 2 (adalimumab 40 mg sc). Participants who responded to induction therapy (defined as decrease in CDAI ≥ 70 points compared with baseline) were randomized at week 4 to adalimumab 40 mg eow, adalimumab 40 mg weekly or placebo. This was referred to as the responder group Non‐responders (did not achieve decrease in CDAI ≥ 70 points) were also randomized to 3 treatment groups Participants in responder group who did not maintain response on or after week 12 (increase in CDAI ≥ 70 points compared with week 4 and a CDAI score > 220), were transitioned into open‐label trial Participants were then divided by responder (decrease in CDAI ≥ 70 points compared to baseline) or non‐responder status (did not attain a decrease in CDAI ≥ 70 points compared to baseline) |
|
| Interventions | Participants were randomized to 1 of 3 groups: adalimumab 40 mg weekly (n = 257, responder n = 157); adalimumab 40 mg eow (n = 260, responder n = 172); placebo (n = 261, responder n = 170) and followed for 56 weeks | |
| Outcomes | Primary end point was number of participants in clinical remission (CDAI < 150) at weeks 26 and 56. Secondary end points included: percentage of participants with a clinical response at weeks 26 and 56 (decrease in baseline CDAI ≤ 70 points and by ≤ 100 points); changes from baseline in IBDQ total scores at weeks 26 and 56; percentage of participants in clinical remission at weeks 26 and 56 who were able to discontinue corticosteroid therapy; percentage of participants in clinical remission at weeks 26 and 56 who were able to discontinue corticosteroid use for ≥ 90 days; percentage of participants with fistula remission (closure of all fistulas that were draining at screening and baseline visits); previous/concomitant use of immunosuppressants (with vs without); previous use of TNF‐α antagonists (experienced vs naïve); median time in clinical remission among randomized responders achieving remission | |
| Notes | This study was funded by Abbott Laboratories. Author conflicts of interest were not reported in the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized centrally using an interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Centralized randomization was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical exam was performed by blinded investigators bi‐weekly until week 12 and then every 4 weeks. IBDQ was completed by participants at week 0, 4, 12, 26 and 56 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who were switched into open‐label arm or discontinued study were reported. 81% (138/170) of participants withdrew from placebo group prior to 56 weeks. 55% (95/172) of participants withdrew from adalimumab eow group prior to 56 weeks and 48% (75/157) of participants withdrew from adalimumab weekly group prior to 56 weeks. Reasons for study withdrawal include disease relapse and adverse events. These participants were moved to the open‐label part of the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other apparent sources of bias |
Rutgeerts 2012.
| Study characteristics | ||
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial The study was conducted at 19 sites in Europe, the United States, and Canada from August 2006 to September 2008 Intention‐to‐treat analysis performed Study duration 52 weeks |
|
| Participants | Adults aged 18 ‐ 75 with a diagnosis of ileocolonic CD for at least 4 months; CDAI score 220 ‐ 450 (N = 129) All participants received open‐label induction therapy with adalimumab 160 mg sc at week 0 and 80 mg sc at week 2 |
|
| Interventions | Participants who responded to induction (decrease in CDAI of at least 70 points from baseline) were randomized at week 4 to adalimumab 40 mg eow (n = 64) or placebo (n = 65). Participants were followed for 52 weeks | |
| Outcomes | Primary end points included mucosal healing at week 12 Secondary end points included: mucosal healing at week 52 (defined as CDEIS ≤ 9); CDEIS remission (defined as CDEIS score ≤ 4) at weeks 12 and 52; CDAI remission and response at weeks 12 and 52; the percentages of participants achieving > 75% decrease in CDEIS score from baseline at weeks 12 and 52 | |
| Notes | This study was funded by Abbott Laboratories. Author conflicts of interest are reported in the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded to participant assignment. Study primary end points included mucosal healing as per CDEIS, and other end points based on standardized rating scales (CDAI and CDEIS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study used a modified ITT analysis. The number of dropouts was low |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Sandborn 2007.
| Study characteristics | ||
| Methods | Multicenter randomized, double‐blind, placebo‐controlled trial This study was conducted at 53 centres in Europe, the United States and Canada between August 2002 and January 2005 Intention‐to‐treat analysis performed 56‐week trial All participants had previously completed the CLASSIC I trial prior to enrolling in this study. Only those who achieved clinical remission (CDAI < 150) after induction were eligible for enrolment in this study |
|
| Participants | Adults aged 18 ‐ 75 with CD who achieved remission following 4 week induction protocol in CLASSIC I were eligible for inclusion. Participants were in remission (CDAI < 150) at time of randomization (N = 55) | |
| Interventions | Participants who responded to both induction therapy and open‐label doses (adalimumab 40 mg sc) at week 0 and 2 were randomized (1:1:1) into 3 groups: adalimumab 40 mg bi‐weekly (n = 19); adalimumab 40 mg weekly (n = 18); and placebo (n = 18) | |
| Outcomes | Primary end point was the proportion of participants in clinical remission (CDAI < 150) at week 56 Secondary end points included the percentages of participants in remission at week 24; 70‐point and 100‐point clinical responses at weeks 24 and 56; changes in IBDQ total score from baseline to weeks 24 and 56; percentages of participants who completely discontinued steroids without loss of remission at weeks 24 and 56 | |
| Notes | This study was funded by a research grant from Abbott Laboratories, Abbott Park, Illinois, USA. Author conflicts of interest were not reported in the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization was performed |
| Allocation concealment (selection bias) | Low risk | Pharmacist or designate was responsible for dispensing study drug |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measurements performed to assess end points (CDAI, IBDQ) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants withdrew from the placebo group; 1 due to adverse events, 3 withdrew consent and 1 failed to respond. 3 participants withdrew from the adalimumab bi‐weekly group; 1 due to adverse events, 1 withdrew consent and 1 was lost to follow‐up. 2 participants withdrew from the adalimumab weekly group; 1 due to adverse events and 1 withdrew consent |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias |
Savorino 2013.
| Study characteristics | ||
| Methods | Randomized active comparator trial Intention‐to‐treat not performed 104‐week trial |
|
| Participants | Adults with known CD who had ileocolic resection 2 weeks prior to study initiation (N = 51) | |
| Interventions | Participants were randomized to receive adalimumab160 mg at week 0, 80 mg at week 2, followed by 40 mg every other week (n = 16), azathioprine 2 mg/kg/day (n = 17) or mesalamine 3 g/day (n = 18) and were followed for 104 weeks | |
| Outcomes | Primary end point was the proportion of participants with endoscopic recurrence based on Rutgeerts score and clinical recurrence based on a grading scale developed by study investigators (score ≥ 2 on a scale where 1 indicates absent, 2 mild, 3 moderate and 4 severe symptoms) Secondary end point was the assessment of quality of life by means of the (IBDQ | |
| Notes | Abstract publication: location, dates the study was conducted, funding support and conflicts of interest were not reported Data for IBDQ was presented as a mean number in each treatment group We could not extract safety data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported, although with 3 different therapies that are delivered by different routes and on different days, it suggests both participants and investigators were aware of treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomized were included in the results |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias |
Scapa 2015.
| Study characteristics | ||
| Methods | Randomized active comparator trial Preliminary data at 24 weeks |
|
| Participants | Participants who had first ileocolic resection (N = 19) | |
| Interventions | Randomized within 45 days of ileocolic resection to receive adalimumab (n = 11) or 6‐mercaptopurine (n = 8) | |
| Outcomes | Primary end point was endoscopic recurrence as defined by the Rutgeert's score at 24 weeks | |
| Notes | Abstract publication: location, dates the study was conducted, funding support and conflicts of interest were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported, although considering therapies selected for comparison, unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not reported. Standardized scoring scales used for outcome assessment (CDAI, IBDQ, Rutgeerts score) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study does not mention how many participants were initially randomized |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias |
Watanabe 2012.
| Study characteristics | ||
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial This study was conducted in Japan between March 2007 and December 2008 Intention‐to‐treat not performed 52‐week trial |
|
| Participants | Participants aged 15 ‐ 75 with moderate‐to‐severely active CD (CDAI 220 ‐ 450) (N = 50) Previous exposure to TNF‐α antagonist were permitted if last dose was given 8 weeks prior to randomization |
|
| Interventions | Participants were initially randomized (3:3:2) to induction protocol of adalimumab 160 mg on week −4 and 80 mg on week −2, adalimumab 80 mg on week −4 and 40 mg on week −2 or placebo Randomized to adalimumab 40 mg eow (n = 25) or placebo (n = 25) | |
| Outcomes | Primary end point was clinical remission (CDAI < 150) at week 52
Secondary end points included: proportion of participants in clinical remission (CR ‐ 100 or CR ‐ 70) every 4 weeks; CDAI changes from baseline; IOIBD score: mental component summary (MCS) and physical component summary (PCS) of the Short Form‐36 (SF‐36) Health Survey; IBDQ |
|
| Notes | This study was sponsored by Abbott Japan Co, Ltd., Tokyo, Japan and Eisai Co, Ltd., Tokyo, Japan. Author conflicts of interest are reported in the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded to study group assignments. Standardized scoring systems used to describe study end points |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants in treatment arm withdrew from trial: 2 due to adverse events, and 1 with reasons not reported 1 participant withdrew from treatment arm (adverse event). 34 participants were moved to open‐label arm of study due to disease flare or non‐response: 20 participants in placebo group and 14 participants in adalimumab group 2 participants completed trial in the placebo arm out of original 25 and 10 participants in the adalimumab arm completed the trial out of the original 25 |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias |
CD: Crohn's disease; CDAI: Crohn's disease activity index; CDEIS: Crohn's disease endoscopic index of severity; eow: every other week; IBDQ: inflammatory bowel disease questionnaire; ITT: intention‐to‐treat; sc: subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Cruz 2015 | Study compared 2 different treatment protocols for CD patients following intestinal resection. Participants in both groups received adalimumab |
| Dewint 2014 | All participants received adalimumab, Study compared combination of adalimumab and ciprofloxacin to adalimumab. Overall comparison discusses whether antibiotic is superior to placebo for perianal fistula closure |
| Faubion 2017 | This was an open‐label extension study with no control group |
| Khanna 2015 | Cluster‐randomized controlled trial of 2 different treatment algorithms for treatment of CD. Both treatment algorithms involved the use of adalimumab |
Characteristics of ongoing studies [ordered by study ID]
NCT03464136.
| Study name | A phase 3b, mMulticenter, randomized, blinded, active‐controlled study to compare the efficacy and safety of ustekinumab to that of adalimumab in the treatment of biologic naïve subjects with moderately‐to‐severely active Crohn's disease |
| Methods | Active comparator trial studying safety of both ustekinumab and adalimumab. Both groups will receive induction doses of both medications, and will then be randomized to receive adalimumab or ustekinumab maintenance therapy |
| Participants | Participants over 18 years of age with moderately‐to‐severely active CD with a baseline CDAI score of ≥ 220 and ≤ 450 who are naïve to biologic therapy |
| Interventions | Ustekinumab 90 mg daily and adalimumab 40 mg every other week |
| Outcomes | Primary outcome will be the percentage of participants in clinical remission at week 52
Secondary outcomes include:
corticosteroid‐free remission;
clinical response;
endoscopic remission at week 52;
clinical remission at week 16;
clinical remission and clinical response at week 8 through 52: changes in baseline SES‐CD score, CRP and fecal calprotectin at week 52; normalization of CRP at week 52; adverse events; serious adverse events; infections: serious infections; percentage of participants with anti‐drug antibodies at week 52 |
| Starting date | 13 March 2018 |
| Contact information | Janssen Scientific Affairs, LLC, JNJ.CT@sylogent.com |
| Notes |
CRP: C‐reactive protein; SES‐CD: simple endoscopic score for Crohn's disease
Differences between protocol and review
We planned a subgroup analysis by prior TNF‐α antagonist exposure, but this was amended post hoc to be considered as a secondary outcome.
We included the following outcomes in the 'Summary of findings' tables: failure to maintain clinical remission at study end point, failure to maintain endoscopic remission at study end point, AEs, SAEs, withdrawals due to AEs, and failure to maintain clinical response at 52 to 56 weeks with prior TNF‐α antagonist exposure at study end point.
Based on advice from the updated Handbook, we decided to use a random‐effects model to pool data.
Contributions of authors
Cassandra Townsend: development of the protocol, screening of abstracts and titles, screening of full‐text articles, data extraction, 'Risk of bias' assessment; data entry, data analysis, reporting of results, and manuscript preparation.
Tran M Nguyen: development of the protocol, running the literature search, screening of abstracts and titles, screening of full‐text articles, data extraction, 'Risk of bias' assessment; data entry, data analysis, reporting of results, and manuscript preparation.
Jeremy Cepek: development of the protocol.
Mohamad Abbass: development of the protocol.
Claire E Parker: development of the protocol, provided methodological expertise, critical revision of the manuscript and approval of the final manuscript.
John K MacDonald: development of the protocol, adjudication of study selection, data extraction and 'Risk of bias' assessment, providing methodological expertise, checking data extraction and analysis, checking GRADE analysis, checking reporting of results; manuscript preparation, critical revision of the manuscript and approval of the final manuscript.
Reena Khanna: development of the protocol, providing clinical and methodological expertise, critical revision of the manuscript and approval of the final manuscript.
Vipul Jairath: development of the protocol, provided clinical and methodological expertise, critical revision of the manuscript and approval of the final manuscript.
Brian G Feagan: development of the protocol, provided clinical and methodological expertise, critical revision of the manuscript and approval of the final manuscript.
Declarations of interest
Cassandra Townsend: None known
Tran M Nguyen: None known
Jeremy Cepek: None known
Mohamad Abbass: None known
Claire E Parker: None known
John K MacDonald: None known
Reena Khanna: Reena Khanna has been a speaker advisory board member, and or clinical investigator for AbbVie, Encycle, Gilead, Innomar, Janssen, Lilly, Merck, Pfizer, Pendopharm, Robarts Clinical Trials, Roche/Genetec, Shire, Takeda.
Vipul Jairath: Dr Jairath has received consulting fees from Abbvie, Sandoz, Takeda, Pfizer, and Janssen; and Lecture fees from Takeda, Ferring, Janssen, and Shire. All of these activities are outside the submitted work.
Brian G Feagan has received fee(s) from Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics Inc., Bristol‐Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, JnJ/Janssen, Merck, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, Tillotts Pharma AG, UCB Pharma for Board membership; fee(s) from Abbott/AbbVie, Actogenix, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics Inc., Axcan, Baxter Healthcare Corp., Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Merck, Millennium, Nektar, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, Tillotts, UCB Pharma, Vertex Pharma, Warner‐Chilcott, Wyeth, Zealand, and Zyngenia for consultancy; and lecture fee(s) from: Abbott/AbbVie, JnJ/Janssen, Takeda, Warner‐Chilcott, and UCB Pharma. All of these activities are outside the submitted work.
New
References
References to studies included in this review
Colombel 2007 {published data only}
- *.Colombel J, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: The CHARM trial. Gastroenterology 2007;132(1):52-65. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Castillo MM, Zhou Q, Thakkar RB. Efficacy and safety of adalimumab in moderate compared with severe Crohn's disease: Pooled data from the charm and extend trials. Gut 2012;61:A229. [Google Scholar]
- Colombel JF, Sandborn WJ, Rutgeerts P, Kamm MA, Yu AP, Wu EQ, et al. Comparison of two adalimumab treatment schedule strategies for moderate-to-severe Crohn's disease: Results from the CHARM trial. American Journal of Gastroenterology 2009;104(5):1170-9. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Schwartz DA, Sandborn WJ, Kamm MA, D'Haens G, Rutgeerts P, et al. Adalimumab for the treatment of fistulas in patients with Crohn's disease. Gut 2009;58(7):940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel JF. The CHARM trial of adalimumab in Crohn's disease. Gastroenterology and Hepatology 2006;2(7):486-8. [PMC free article] [PubMed] [Google Scholar]
- Feagan BG, Panaccione R, Sandborn WJ, D'Haens GR, Schreiber S, Rutgeerts PJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn's disease: results from the CHARM study. Gastroenterology 2008;135(5):1493-9. [DOI] [PubMed] [Google Scholar]
- Loftus E, Yang M, Mulani P, Chao J. Adalimumab maintenance therapy improves patient-reported outcomes in patients with moderate Crohn's disease: Subanalysis of CHARM. American Journal of Gastroenterology 2011;106:S449-S50. [Google Scholar]
- Loftus EV, Feagan BG, Colombel JF, Rubin DT, Wu EQ, Yu AP, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn's disease: Patient-reported outcomes of the CHARM trial. American Journal of Gastroenterology 2008;103(12):3132-41. [DOI] [PubMed] [Google Scholar]
- Rubin DT, Mulani P, Chao J, Pollack PF, Bensimon AG, Yu AP, et al. Effect of adalimumab on clinical laboratory parameters in patients with Crohn's disease: results from the CHARM trial. Inflammatory Bowel Diseases 2012;18(5):818-25. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Reinisch W, Colombel JF, Sandborn W, Robinson A, Huang B, et al. Subgroup analysis of the placebo-controlled CHARM trial: Increased remission rates for adalimumab-treated patients with early Crohn's disease. Journal of Crohn's and Colitis 2012;6:S106. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Colombel J, Robinson AM, Huang B, Thakkar RB, Pollack PF. Effect of concomitant immunomodulator use on the efficacy of adalimumab in Crohn's disease patients stratified by prior anti-TNF use. Journal of Crohn's and Colitis 2011;5(1):S68-S9. [Google Scholar]
- Wasvary HJ. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: The CHARM trial. Diseases of the Colon and Rectum 2007;50(8):1291. [DOI] [PubMed] [Google Scholar]
Rutgeerts 2012 {published data only}
- Colombel J, Sandborn WJ, Louis E, Panaccione R, Yang M, Chao J, et al. Economic impact of deep remission in adalimumab-treated patients with Crohn's disease: Results from EXTEND. Journal of Crohn's and Colitis 2011;5(1):S41-S2. [Google Scholar]
- Colombel J, Schreiber S, Rutgeerts P, Sandborn W, Yang M, Lomax K, et al. Effect of disease duration on 'deep remission': Results from the EXTEND trial. Inflammatory Bowel Diseases 2011;17:S44. [Google Scholar]
- Colombel JF, Rutgeerts P, Sandborn W, Yang M, Lomax K, Pollack P, et al. Achievement of early deep remission predicts better long-term outcomes for adalimumab-treated patients with Crohn's disease: Data from extend. American Journal of Gastroenterology 2010;105:S434-S5. [Google Scholar]
- Feagan B, Sandborn WJ, Rutgeerts P, Levesque BG, Khanna R, Huang B, et al. Performance of Crohn's disease clinical trial endpoints based upon different cutoffs for patient reported outcomes or endoscopic activity: Analysis of EXTEND data. Inflammatory Bowel Diseases 2018;24(5):932-42. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Colombel J, D’Haens G, Sandborn WJ, Rutgeerts P, Geboes K, et al. Characterisation of mucosal healing with adalimumab treatment in patients with moderately to severely active Crohn’s disease: Results from the EXTEND Trial. Journal of Crohn's & Colitis 2017;11(4):425-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch W, Colombel JF, D'Haens G, Sandborn W, Rutgeerts P, Petersson J, et al. Characterization of mucosal healing with adalimumab treatment in patients with moderately to severely active Crohn's disease from extend. American Journal of Gastroenterology 2014;109:S475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch W, Colombel JF, D'Haens G, Sandborn WJ, Rutgeerts P, Petersson J, et al. Characterization of mucosal healing with adalimumab treatment in patients with moderately to severely active Crohn's disease from extend. United European Gastroenterology Journal 2014;1:A537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgeerts P, Reinisch W, Sandborn WJ, D'Haens G, Colombel JF, Petersson J, et al. Evaluation of site versus central assessment of endoscopies in Crohn's disease: Comparison using data from EXTEND. Journal of Crohn's & Colitis 2014;8:S30-S1. [Google Scholar]
- *.Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology 2012;142(5):1102-11. [DOI] [PubMed] [Google Scholar]
- Rutgeerts PJ, Reinisch W, Sandborn W, D'Haens GR, Colombel JF, Petersson J, et al. Evaluation of site versus central assessment of endoscopies in Crohn's disease: Comparison using data from extend. Gastroenterology 2014;1:S585. [Google Scholar]
- Sandborn W, Colombel JF, Lomax K, Pollack P, Thakkar R, Camez A, et al. Achievement of early deep remission is associated with lower rates of weekly dosing for adalimumab-treated patients with Crohn's disease: Data from EXTEND. American Journal of Gastroenterology 2010;105:S442-S3. [Google Scholar]
- Sandborn W, Colombel JF, Panaccione R, Louis E, Yang M, Pollack P, et al. Importance of deep remission compared with mucosal healing only: Results from EXTEND. Journal of Crohn's & Colitis 2012;6:S112. [Google Scholar]
- Sandborn W, Wolf DC, Colombel JF, Panes J, Eichner S, Iezzi A, et al. Adalimumab achieves efficacy in mucosal healing regardless of baseline disease severity in patients with Crohn's disease: Data from extend. Gastroenterology 2014;1:S589. [Google Scholar]
- Sandborn WJ, Colombel JF, Lomax KG, Pollack PF, Thakkar R, Camez A, et al. Achievement of early deep remission is associated with lower rates of weekly dosing for adalimumab-treated patients with Crohn's disease: Data from extend. Gut 2011;60:A136-A7. [Google Scholar]
- Sandborn WJ, Wolf D, Colombel JF, Panes J, Eichner S, Iezzi A, et al. Adalimumab achieves efficacy in mucosal healing regardless of baseline disease severity in patients with Crohn's disease: Data from EXTEND. Journal of Crohn's & Colitis 2014;8:S306-S7. [Google Scholar]
- Sandborn WJ, Wolf DC, Colombel JF, Panes J, Eichner S, Iezzi A, et al. Adalimumab achieves efficacy in mucosal healing regardless of baseline disease severity in patients with Crohn's disease: Data from EXTEND. Journal of Gastroenterology and Hepatology 2014;29:118. [Google Scholar]
Sandborn 2007 {published data only}
- Colombel J, Rutgeerts P, Sandborn WJ, Reinisch W, Loftus EV Jr, Tang J, et al. Impact of induction dosing on maintenance outcome with adalimumab in Crohn's disease. Journal of Crohn's & Colitis 2011;5(1):S88. [Google Scholar]
- *.Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lulcas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn's disease: Results of the CLASSIC II trial. Gut 2007;56(9):1232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savorino 2013 {published data only}
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease-a randomized trial. Digestive and Liver Disease 2013;45:S94-S5. [DOI] [PubMed] [Google Scholar]
- *.Savarino E, Bodini G, Dulbecco P, Marabotto E, Assandri L, Bruzzone L, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease-a randomized trial. Gastroenterology 2013;1:S21. [DOI] [PubMed] [Google Scholar]
- Savarino E, Bodini G, Dulbecco P, Savarino V. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease-a randomized trial. United European Gastroenterology Journal 2013;1:A17. [DOI] [PubMed] [Google Scholar]
Scapa 2015 {published data only}
- Scapa E, Fliss-Isakov N, Tulchinsky H, Yanai H, White I, Dotan I, et al. Early initiation of adalimumab significantly diminishes endoscopic post-operative Crohn's disease recurrence, and is superior to immunomodulatory therapy, regardless of risk stratification. A randomized, controlled study. United European Gastroenterology Journal 2018;6:A458. [Google Scholar]
- *.Scapa E, Maharshak N, Kariv Y, Ben-Horin S, White I, Santo E, et al. Early initiation of adalimumab significantly diminishes post-operative Crohn's Disease recurrence, and is superior to immunomodulator therapy. Preliminary results from the POPART trial. Journal of Crohn's & Colitis 2015;9:S303-S4. [Google Scholar]
- Scapa E, Maharshak N, Kariv Y, Ben-Horin S, White ID, Santo E, et al. Early initiation of adalimumab significantly diminishes post-operative Crohn's disease recurrence, and is superior to immunomodulator therapy. Preliminary results from the POPART trial. Gastroenterology 2015;1:S240-S1. [Google Scholar]
Watanabe 2012 {published data only}
- Watanabe M, Hibi T, Lomax KG, Paulson SK, Chao J, Alam MS, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn's disease. Journal of Crohn's & Colitis 2012;6(2):160-73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
De Cruz 2015 {published data only}
- De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn's disease management after intestinal resection: A randomised trial. Lancet 2014;385(9976):1406-17. [DOI] [PubMed] [Google Scholar]
Dewint 2014 {published data only}
- Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M, et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut 2014;63(2):292-9. [DOI] [PubMed] [Google Scholar]
Faubion 2017 {published data only}
- Faubion WA, Dubinsky M, Ruemmele FM, Escher J, Rosh J, Hyams JS, et al. Long-term efficacy and safety of adalimumab in paediatric patients with Crohn’s disease. Inflammatory Bowel Diseases 2017;23(3):453-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khanna 2015 {published data only}
- Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): A cluster randomised controlled trial. Lancet 2015;386(10006):1825-34. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03464136 {published data only}
- NCT03464136. Safety and efficacy of adalimumab versus ustekinumab for one year (SEAVUE). clinicaltrials.gov/ct2/show/NCT03464136 (first received 13 March 2018).
Additional references
Asgharpour 2013
- Asgharpour A, Cheng J, Bickston S. Adalimumab treatment in Crohn's disease: an overview of long-term efficacy and safety in light of the EXTEND trial. Clinical and Experimental Gastroenterology 2013;6(6):153-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baert 2003
- Baert F, Noman M, Vermeire S, Van Assche G, D’Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. New England Journal of Medicine 2003;348(7):601-8. [DOI] [PubMed] [Google Scholar]
Baumgart 2012
- Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380(9853):1590-605. [DOI] [PubMed] [Google Scholar]
Cassinotti 2008
- Cassinotti A, Ardizzone S, Porro GB. Adalimumab for the treatment of Crohn's disease. Biologics 2008;2(4):763-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chassaing 2011
- Chassaing B, Darfeuille–Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140(6):1720-8. [DOI] [PubMed] [Google Scholar]
Cosnes 1996
- Cosnes J, Carbonnel F, Beaugerie L, Quintrec YL, Gendre J. Effects of cigarette smoking on the long-term course of Crohn's disease. Gastroenterology 1996;110(2):424-31. [DOI] [PubMed] [Google Scholar]
Di Sabatino 2004
- Di Sabatino A, Ciccocioppo R, Cinque B, Millimaggi D, Morera R, Ricevuti L, et al. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut 2004;53(1):70-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doherty 2015
- Doherty GM. Current Diagnosis & Treatment. McGraw-Hill Education LLC, 2015. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2011
- FDA. HUMIRA: Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s0110lbl.pdf (accessed 15 June 2017).
Ghosh 2007
- Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. Journal of Crohn's & Colitis 2007;1(1):10-20. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2002
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359(9317):1541-9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Khor 2011
- Khor B, Gardet A, Xavier R. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474(7351):307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loddo 2015
- Loddo I, Romano C. Inflammatory bowel disease: genetics, epigenetics and pathogenesis. Frontiers in Immunology 2015;6:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuoka 2015
- Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Seminars in Immunopathology 2015;37(1):47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moses 2019
- Moses J, Lambert-Jenkins K, Momotaz H, Sattar A, Debanne SM, Splawski J, et al. Time to antibody detection and associated factors for presence of anti-drug antibodies in pediatric inflammatory bowel disease patients treated with anti-TNF therapy. European Journal of Gastroenterology & Hepatology 2019;31(10):1228-33. [DOI] [PubMed] [Google Scholar]
Munkholm 1994
- Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut 1994;35(3):360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Singh 2018
- Singh S, Fumery M, Sandborn W, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Alimentary Pharmacology & Therapeutics 2018;48(4):394–409. [DOI] [PubMed] [Google Scholar]
Steinhart 2003
- Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000301] [DOI] [PubMed] [Google Scholar]
Tracey 2008
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacology Therapy 2008;117(2):244-79. [DOI] [PubMed] [Google Scholar]
Tsui 2018
- Tsui JJ, Huynh HQ. Is top-down therapy a more effective alternative to conventional step-up therapy for Crohn's disease? Annals of Gastroenterology 2018;31(4):413-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cepek 2017
- Cepek J , Abbass M , Nguyen TM , Parker CE , MacDonald JK , Feagan BG , Jairath V, Khanna R. Adalimumab for maintenance of remission in Crohn's disease (Protocol). Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012877] [DOI] [PMC free article] [PubMed] [Google Scholar]


