Abstract
Background
Gestational diabetes mellitus (GDM) is carbohydrate intolerance first recognised during pregnancy and associated with complications for mothers and babies. Probiotics are naturally occurring micro‐organisms, which when ingested in adequate amounts, may confer health benefits. Evidence of the role of probiotics as treatment for GDM is limited.
Objectives
To evaluate the safety and effectiveness of probiotics in treating women with GDM on maternal and infant outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth’s Trials Register ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP) (24 July 2019), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) comparing the use of probiotics versus placebo/standard care for the treatment of GDM.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data, checked data accuracy, and assessed risk of bias of included trials. The certainty of evidence for selected maternal and infant/child outcomes was assessed using GRADE.
Main results
Nine RCTs (695 pregnant women with GDM) comparing probiotics versus placebo were identified. The overall risk of bias in the nine RCTs was low to unclear and the evidence was downgraded for imprecision due to the small numbers of women participating in the trials. The trials were carried out in hospitals and universities in Iran (seven trials), Thailand (one trial) and Ireland (one trial). All trials compared probiotics with placebo.
Maternal outcomes
We are uncertain if probiotics have any effect compared with placebo on hypertensive disorders of pregnancy, (risk ratio (RR) 1.50, 95% confidence interval (CI) 0.64 to 3.53; participants = 256; studies = 3; low‐certainty evidence) and mode of birth as caesareans (average RR 0.64, 95% CI 0.30 to 1.35; participants = 267; studies = 3; low‐certainty evidence) because the certainty of evidence is low and the 95% CIs span possible benefit and possible harm.
No trials reported primary outcomes of: mode of birth as vaginal/assisted and subsequent development of type 2 diabetes.
We are uncertain if probiotics have any effect compared with placebo on induction of labour (RR 1.33, 95% CI 0.74 to 2.37; participants = 127; studies = 1; very low‐certainty evidence).
For other secondary maternal outcomes, we are uncertain if there are differences between probiotics and placebo for: postpartum haemorrhage; weight gain during pregnancy intervention and total gestational weight gain; fasting plasma glucose and need for extra pharmacotherapy (insulin). Probiotics may be associated with a slight reduction in triglycerides and total cholesterol.
In probiotics compared with placebo, there was evidence of reduction in markers for insulin resistance (HOMA‐IR) and HOMA‐B; and insulin secretion. There was also an increase in quantitative insulin sensitivity check index (QUICKI).
Probiotics were associated with minor benefits in relevant bio‐markers with evidence of a reduction in inflammatory markers high‐sensitivity C‐reactive protein (hs‐CRP), interleukin 6 (IL‐6), and marker of oxidative stress malondialdehyde; and an increase in antioxidant total glutathione, but we are uncertain if there is any difference in total antioxidant capacity.
No trials reported secondary outcomes: perineal trauma, postnatal weight retention or return to pre‐pregnancy weight and postnatal depression.
Infant/child/adult outcomes
We are uncertain if probiotics have any effect, compared with placebo, on the risk of large‐for‐gestational‐age babies (RR 0.73, 95% CI 0.35 to 1.52; participants = 174; studies = 2; low‐certainty evidence) or infant hypoglycaemia (RR 0.85, 95% CI 0.39 to 1.84; participants = 177; studies = 3; low‐certainty evidence) because the certainty of evidence is low and the 95% CIs span possible benefit and possible harm.
No trials reported primary outcomes of: perinatal (fetal/neonatal) mortality; or neurosensory disability.
For other secondary outcomes, we are uncertain if there is any difference between probiotics and placebo in gestational age at birth, preterm birth, macrosomia, birthweight, head circumference, length, infant hypoglycaemia, and neonatal intensive care unit (NICU) admissions.
There was evidence of a reduction in infant hyperbilirubinaemia with probiotics compared with placebo.
No trials reported secondary outcomes: infant adiposity, and later childhood adiposity.
There were no adverse events reported by any of the trials.
Authors' conclusions
Low‐certainty evidence means we are not certain if there is any difference between probiotic and placebo groups in maternal hypertensive disorders of pregnancy, caesareans; and large‐for‐gestational‐age babies.
There were no adverse events reported by the trials.
Due to the variability of probiotics used and small sample sizes of trials, evidence from this review has limited ability to inform practice. Well‐designed adequately‐powered trials are needed to identify whether probiotics may improve maternal blood glucose levels and/or infant/child/adult outcomes; and whether they can be used to treat GDM.
Plain language summary
Probiotics as an added treatment for gestational diabetes to improve mother and baby outcomes
What is the issue?
Gestational diabetes mellitus (GDM) is carbohydrate intolerance resulting in high blood glucose levels, first recognised during pregnancy. Pregnant women with GDM are at risk of high blood pressure, labour induction, and caesareans. Their babies are at risk of being born large, birth difficulties, respiratory distress, low blood glucose at birth and jaundice that can cause brain injury. There is increased risks of having long‐term diabetes in the mother, and the baby being overweight. Probiotics are micro‐organisms naturally in food and are in fermented milk, yogurt, or capsules. There are many different probiotics; the two most used are Lactobacillus and Bifidobacterium, and if consumed in adequate amounts may confer health benefits.
Why is this important?
Probiotics need to be safe and maternal blood glucose levels carefully managed during pregnancy.
Women with GDM may receive dietary and physical activity education with monitoring blood glucose levels as initial management. When blood glucose levels are above a certain threshold, women with GDM are prescribed glucose‐lowering medications including metformin and/or insulin. This review aimed to determine the safety and effectiveness of probiotics in treating women with GDM.
What evidence did we find?
We searched for evidence for randomised controlled trials (latest July 2019). We identified nine studies, involving 695 women with GDM. All trials compared probiotics with placebo. The certainty of the evidence was assessed as very low or low. The overall risk of bias was low to unclear.
Seven trials were conducted in Iran; one in Thailand, and one in Ireland. Trials took place in hospitals and universities.
We are uncertain if there is any difference between probiotic and placebo in rates of: high blood‐pressure disorders (three studies, 256 participants, low‐certainty evidence); caesarean section (three studies, 267 women, low‐certainty evidence); and large‐for‐gestational‐age babies (two studies, 174 participants, low‐certainty evidence).
We are uncertain if there is any difference between probiotic and placebo for induction of labour (one study, 127 participants, very low‐certainty evidence) and low blood glucose levels in the newborn (three studies, 177 participants, low‐certainty evidence). We are also uncertain if there is any difference between probiotics and placebo for heavy bleeding immediately after birth, weight gain during pregnancy or total gestational weight gain.
We are uncertain if there is any difference in fasting blood glucose between probiotics and placebo (seven studies, 554 participants). Probiotics may be assoicated with a slight reduction in triglycerides and total cholesterol (four studies, 320 participants). There was reduction in insulin secretion with probiotics (seven studies, 505 participants). One trial (60 participants) showed no difference between groups in need for insulin.
Biomarkers, did show a reduction in insulin resistance (HOMA‐IR), (seven studies, 505 participants) and insulin resistance and β cell function (HOMA‐B) (two studies,130 participants) with probiotics. Quantitative insulin sensitivity check index (QUICKI) increased (four studies, 276 participants) with probiotics.
Inflammatory markers, hs‐CRP (four studies, 248 participants) and interleukin 6 (two studies, 128 participants) were reduced with probiotics. Antioxidant total glutathione was increased (two studies, 120 participants) and the oxidative stress biomarker malondialdehyde was reduced with probiotics (three studies, 176 participants). We are uncertain if there is any difference in total antioxidant capacity (four studies 266 participants).
For the newborn baby, we are uncertain if there is any difference between groups for: birthweight, gestational age at birth, preterm births, large babies, head circumference and length scores, or need for admission to the neonatal intensive care unit. The number of babies with high levels of bilirubin was reduced with probiotics.
No adverse events were reported by the trials.
What does this mean?
Based on the clinical trials available, the evidence is limited to support the use of probiotics as treatment for women with GDM to improve pregnancy outcomes for mothers and their babies. Larger well‐designed randomised controlled trials are needed to assess the effects of probiotics on management of glucose levels and when available, they can be included in the update of this review.
Summary of findings
Summary of findings 1. Probiotic compared to placebo for treating women with gestational diabetes for improving maternal and infant health and well‐being ‐ maternal outcomes.
| Probiotic compared to placebo for treating women with gestational diabetes for improving maternal and fetal health and well‐being ‐ maternal outcomes | |||||
| Patient or population: pregnant women diagnosed with gestational diabetes Setting: Iran (8), Ireland (1), Thailand (1) Intervention: probiotics (any type) administered by any route given during pregnancy to treat women with gestational diabetes Comparison: placebo (similar appearance and taste to the probiotics) or standard care | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with probiotic | ||||
| Hypertensive disorders (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia) | 256 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 | RR 1.50 (0.64 to 3.53) | Study population | |
| 63 per 1000 | 26 more per 1000 (26 fewer to 151 more) | ||||
| Subsequent development of type 2 diabetes | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| Mode of birth (caesarean) | 267 (3 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | RR 0.64 (0.30 to 1.35) | Study population | |
| 351 per 1000 | 224 fewer per 1000 (105 fewer to 474 more) | ||||
| Induction of labour | 127 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 4 | RR 1.33 (0.74 to 2.37) | Study population | |
| 231 per 1000 | 76 more per 1000 (60 fewer to 316 more) | ||||
| Perineal trauma | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| Postnatal weight retention or return to pre‐pregnancy weight | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| Postnatal depression | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded two levels due to serious concerns related to imprecision as only has 3 small studies with wide confidence intervals.
2 Downgraded one level due to serious concerns related to imprecision as only has 3 small studies with wide confidence intervals.
3 Downgraded one level due to serious concerns related to inconsistency as I2 of 69%, studies showed different findings.
4 Downgraded two levels due to serious concerns related to imprecision as only one small study with wide confidence intervals. We downgraded for indirectness as the population of one study will not reflect population of all women with GDM.
Summary of findings 2. Probiotic compared to placebo for treating women with gestational diabetes for improving maternal and infant health and well‐being‐ infant/child/adult outcome.
| Probiotic compared to placebo for treating women with gestational diabetes for improving maternal and infant health and well‐being ‐ infant/child/adult outcomes | |||||
| Patient or population: pregnant women diagnosed with gestational diabetes Setting: Iran (1), Ireland (1) Intervention: probiotic Comparison: placebo | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with probiotic | ||||
| Perinatal (fetal and neonatal) mortality | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| Large‐for‐gestational age > 90 centile | 174 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | RR 0.73 (0.35 to 1.52) | Study population | |
| 159 per 1000 | 43 fewer per 1000 (103 fewer to 83 more) | ||||
| Composite serious neonatal outcomes (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture, or nerve palsy | (0 studies) | not estimable | No data reported for composite serious neonatal outcomes (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture, or nerve palsy in any of the included studies. | ||
| Neurosensory disability | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| Neonatal hypoglycaemia requiring treatment (variously defined) | 177 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 | RR 0.85 (0.39 to 1.84) | Study population | |
| 135 per 1000 | 20 fewer per 1000 (82 fewer to 113 more) | ||||
| Adiposity (neonatal/child/child as an adult) | (0 studies) | not estimable | No outcome data reported in the included studies. | ||
| Diabetes(type1 or type2) or impaired glucose tolerance (child/adult) |
(0 studies) | not estimable | No outcome data reported in the included studies. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded two levels due to serious concerns related to imprecision as only has 2 small studies with wide confidence intervals.
Background
Description of the condition
Gestational diabetes (GDM) is defined as "carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy" (Alberti 1998). The prevalence of GDM is thought to vary from 1.5% to 14% worldwide and varies between ethnic groups (ACOG 2001; Dabelea 2005; Ekeroma 2015; Ferrara 2007; Poston 2013), and countries or institutions depending on the diagnostic criteria for GDM being used (ADA 2010; Diabetes Care 2010; Ekeroma 2015; NICE 2015). The global epidemic of obesity (a risk factor for GDM) is continuing to rise in developed and developing countries (Swinburn 2011), with the concomitant increase in rates of pregnancy complications (WHO 2016), including GDM. Health risks for women with GDM include pre‐eclampsia, induction of labour (Crowther 2005), caesarean section, and over half of women with GDM will develop type 2 diabetes within 10 years of the birth (Kim 2002). The risks for their infants include macrosomia (baby born much larger than average), respiratory distress syndrome, birth injuries such as nerve palsy, bone fracture and shoulder dystocia, jaundice, and hypoglycaemia, which if prolonged or severe can cause brain injury (Crowther 2005; Landon 2009). In addition, there is increasing recognition of the association between intrauterine fetal programming effects with adverse long‐term health consequences for the infant, creating a vicious intergenerational cycle of obesity, diabetes, and metabolic syndrome (Boney 2005; Dabelea 2005).
Description of the intervention
Probiotics are micro‐organisms that naturally occur in foods and when consumed in adequate amounts may confer health benefits for the host (FAO 2001). Probiotics are usually found in fermented milk products, yogurt or dietary supplements as well as in capsules. There are many different types of probiotics and the two most widely used genera are Lactobacillus and Bifidobacterium (Laitinen 2009).
The gut microbiota (micro‐organisms that colonise the gut) have the potential to influence obesity and type 2 diabetes through modification of energy extraction, inflammation, hunger and satiety, as well as lipid and glucose metabolism (Flint 2012; Nieuwdorp 2014; Turnbaugh 2006). Type 2 diabetes has been associated with changes in the gut microbiome (Larsen 2010). Obese women have also been identified to have a different gut microbiome compared to lean women (Nieuwdorp 2014; Turnbaugh 2006). Gut microbiota differences also exist between pregnant overweight and normal weight women (Collado 2008), as well as in the third trimester of pregnancy compared to the first trimester, with the third trimester microbiome being similar to non pregnant individuals with metabolic syndrome (Koren 2012). Supplementation with probiotics has been shown to improve glycaemic control in men and women with type 2 diabetes (Andreasen 2010; Ejtahed 2012). Probiotics have been shown to prevent GDM in a sample of pregnant women in a general population (Luoto 2010), and probiotics with dietary counselling reduced mean plasma glucose concentrations and improved insulin sensitivity in another study of healthy pregnant women both antenatally and postpartum (Laitinen 2009). Probiotic milk products reduced pre‐eclampsia in a large Norwegian cohort study (Braentsaeter 2011) and are considered safe to use in pregnancy (Allen 2010; Elias 2011). Probiotic capsules (Lactobacillus rhamnosus) in a double‐blind randomised controlled trial showed significant and sustainable weight loss in obese non pregnant women (Sanchez 2014). A larger randomised controlled trial of probiotic versus placebo in pregnant women in Australia (Nitert 2013), to determine whether probiotics can prevent GDM in overweight and obese women has recently been published (Callaway 2019). A systematic review and meta‐analysis looking at the effect of treatment of GDM on pregnancy outcomes showed that treatment significantly reduced the risks of fetal macrosomia, large‐for‐gestational‐age births, shoulder dystocia and gestational hypertension, as well as a tendency to reduction of perinatal/neonatal mortality and birth trauma (Poolsup 2014). A Cochrane Review of probiotics for prevention of GDM included one study that reported lower rates of women diagnosed with GDM and lower birthweight with probiotics (Barrett 2014). GDM treatment to date has mostly comprised of dietary and glucose‐lowering agents either insulin and or tablets (biguanides or second‐generation sulphonylureas) (Coustan 2013). The role of probiotics in treating pregnant women with GDM has yet to be clearly established.
How the intervention might work
Probiotics in the 1960s were hypothesised to have the beneficial effects of producing substances that may promote the growth of other micro‐organisms and was further defined in the 1980s as a microbial feed supplement that improves the intestinal balance of the host (FAO 2001). The discovery of the gut microbiome and its relationship to health and disease, together with DNA sequencing technology meant easier identification of the host genome and host micro‐organisms or microbiome (Solt 2015). Microbiome changes influence gut content by allowing the predominance of some organisms over others, which in turn can cause a generalised increase in inflammatory markers in the host and increasing risks of diseases (Solt 2015). Modification of the gut microbiome (Flint 2012) by probiotics may be used as an intervention to prevent or treat metabolic diseases through various complex intracellular metabolic pathways within the gut (Nieuwdorp 2014; Turpin 2010). The mechanisms are complex from probiotics actively competing with pathological bacteria to dampening their inflammatory effect possibly by producing more butyrate; to improving the bile acid pool to reduce insulin resistance; or binding to mucosal receptors in the gut altering metabolic pathways responsible for the metabolic syndrome and satiety (Nieuwdorp 2014). Furthermore, probiotics have an anti‐obesity action by influencing energy extraction in humans through increased lipolysis and reduction in lipoprotein lipase, which may reduce excess energy storage (Turpin 2010). The microbiomes of obese people have been found to have the ability to convert non digestible carbohydrates to digestible short‐chain fatty acids, with increased uptake in the gut increasing energy harvest, storage and consequently increasing adiposity (Flint 2012). High adiposity in human and animal studies has been associated with increased systemic inflammation, which impacts adversely on pregnancy outcomes especially increasing risks of pre‐eclampsia (Braentsaeter 2011), and increased insulin resistance. Probiotics have been shown to reduce the rates of severe pre‐eclampsia (Braentsaeter 2011), reduce insulin resistance (Asemi 2013) and improve insulin sensitivity (Laitinen 2009). Other beneficial effects of probiotics include reduction of psychological distress in healthy volunteers (Messaoudi 2011), and consumption of probiotic yoghurt improved mood (Benton 2007) possibly by reducing systemic inflammatory markers (Dinan 2011). Futhermore, individuals with depression have been shown to have a different microbiome to healthy individuals (Jiang 2015), as well as high levels of inflammatory cytokines (Dinan 2011), with probiotics predicted to dampen the negative effects of inflammation causing depression. Trials of probiotics in preterm neonates have demonstrated a reduction in necrotising enterocolitis and mortality (AlFaleh 2014).
Why it is important to do this review
The prevalence of GDM is increasing and the implementation of the International Association of Diabetes and Pregnancy Study Group diagnostic (IADPSG) criteria could also be contributing to the increase in women diagnosed with GDM. (Cundy 2014; Ekeroma 2015). All women with GDM may receive lifestyle advice (Metzger 2007), and for some women, this may be an effective treatment to maintain glycaemic control without the addition of pharmacotherapy (Brown 2017). The use of probiotics may prove a useful adjunct to lifestyle interventions and reduce the need for pharmacotherapy possibly by influencing metabolic pathways that lead to development of GDM (Nieuwdorp 2014). This review will establish the effectiveness of such an intervention in particular for women with GDM.
Objectives
To evaluate the safety and effectiveness of probiotics in treating pregnant women with gestational diabetes mellitus (GDM) on maternal and infant outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). Cluster‐randomised trials were eligible for inclusion but none were identified. Quasi‐randomised and cross‐over trials were not eligible for inclusion. There were no restrictions to language or year of publication.
Types of participants
Pregnant women diagnosed with gestational diabetes (diagnosis as defined by the individual trial). Trials of women with type 1 or type 2 diabetes diagnosed prior to pregnancy were excluded.
Types of interventions
Probiotics (any type) administered by any route given during pregnancy to treat women with gestational diabetes and where the control group received placebo or standard care (as defined by the trialist).
Types of outcome measures
Primary outcomes
Maternal
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Subsequent development of type 2 diabetes (as defined by trialist)
Mode of birth
Infant
Perinatal (fetal and neonatal) mortality
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual trial)
Composite of serious neonatal outcomes (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Neurosensory disability (defined by trialists)
Secondary outcomes
Maternal
Induction of labour
Perineal trauma
Placental abruption
Postpartum haemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention
Behaviour changes associated with the intervention
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free‐fatty acids, triglycerides, high‐density lipoproteins (HDL), low‐density lipoproteins (LDL), insulin)
Sense of well‐being and quality of life (any validated Well‐being and Quality of life scores)
Views of the intervention
Breastfeeding (e.g. at discharge, six weeks postpartum)
Use of additional pharmacotherapy
Glycaemic control during/end of treatment (as defined by trialists)
Maternal hypoglycaemia
Maternal mortality
Long‐term maternal outcomes
Postnatal depression (any validated postnatal depression scores e.g. Edinburgh Postnatal Depression Scale (EPDS))
Postnatal weight retention or return to pre‐pregnancy weight
Body mass index (BMI)
GDM in a subsequent pregnancy
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)
Infant
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia
Small‐for‐gestational age
Birthweight and z‐score
Head circumference and z‐score
Length and z‐score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia requiring treatment (variously defined)
Hyperbilirubinaemia
Neonatal hypocalcaemia
Polycythaemia
Relevant biomarker changes associated with the intervention (e.g. cord c peptide, cord insulin)
Later childhood
Weight and z score
Height and z score
Head circumference and z score
Adiposity (including BMI, skinfold thickness)
BP
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Educational achievement
Adulthood outcomes
Weight
Height
Adiposity (including skin folds, fat mass)
Cardiovascular health (as defined by trialists, including BP, hypertension, cardiovascular disease, metabolic syndrome)
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Employment, education and social status/achievement
Health services
Number of antenatal visits or admissions
Number of hospital or health professional visits (including midwife, obstetrician, physician, dietician, diabetic nurse)
Admission to neonatal intensive care unit/nursery
Length of antenatal stay
Length of postnatal stay (maternal)
Length of postnatal stay (baby)
Cost of maternal care
Cost of offspring care (including neonatal intensive care unit admission)
Costs associated with the intervention
Costs to families associated with the management provide
Search methods for identification of studies
The following methods section of this protocol is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (24 July 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches FSTA;
weekly searches Biosis;
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results were screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (24 July 2019) for unpublished, planned and ongoing trial reports using search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of all retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The methods was based on the standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors Karaponi OKesene‐Gafa (KOG) and Abigail Moore (AM) independently assessed for inclusion all potential studies identified as a result of the search strategy. Any disagreement was resolved through discussion with senior author Professor Caroline A Crowther (CAC).
Data extraction and management
We extracted relevant data using the Cochrane Pregnancy and Childbirth Group's data extraction form. We collected information on type of intervention, frequency and route of administration; trialists' declarations of interest and trial dates. For eligible studies, two review authors extracted the data using the agreed form. Discrepancies were resolved through discussion. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy. We contacted trial authors for the original reports to provide further details if required.
Assessment of risk of bias in included studies
Two review authors (KOG and AM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving our senior author (CAC).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); we planned to exclude studies judged to be of high risk of bias.
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants and personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Assessment of the quality of the evidence using the GRADE approach
For the main comparison or probiotic versus placebo, the quality of the evidence will be assessed using the GRADE approach, outlined in the GRADE handbook and Chapters 11 and 12 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011), for the outcomes listed below.
Maternal
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Subsequent development of type 2 diabetes (as defined by trialist)
Mode of birth
Induction of labour
Perineal trauma
Postnatal weight retention or return to pre‐pregnancy weight
Postnatal depression
Infant/child/adult
Perinatal (fetal and neonatal) mortality
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual trial)
Composite of serious neonatal outcomes (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Neurosensory disability (defined by trialists)
Neonatal hypoglycaemia
Adiposity (neonatal/child/adult)
Diabetes (type 1 or type 2) or impaired glucose tolerance (child/adult)
We used the GRADEpro Guideline Development tool to import data from Review Manager 5.3 (RevMan 2014) in order to create 'Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented the results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CIs as outcomes were measured in the same way between trials. We planned to use the standardised mean difference (SMD) with 95% CIs to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials in this review. If cluster‐randomised trials are identified in future updates of this review, we will include them in the analyses along with individually‐randomised trials. We will make adjustments using the methods described in the Handbook [Section 16.3.4 or 16.3.6] (Higgins 2011) using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will consider it reasonable to combine the results from both cluster‐randomised trials and individually‐randomised trials if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Multiple pregnancies
There were no multiple pregnancies identified in this review. In future updates of this review, if studies involving multiple pregnancies are identified, we will present maternal data as per woman randomised and neonatal data per infant.
Multiple‐arm studies
There were no studies with multiple arms identified in this review. If in future updates of this review, if studies with multiple intervention arms are identified, we will avoid 'double counting' of participants by combining groups to create a single pair‐wise comparison if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had we included more than 10 studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. We planned to assess funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Different types of probiotic (probiotic A versus probiotic B)
Mode of administration of probiotic (capsule versus yoghurt versus nutritional supplement)
Dosage (high versus low dose)
Diagnostic criteria used for GDM (IADPSG, American College of Obstetrics and Gynaecology, World Health Organization, Carpenter and Coustan, Australian Diabetes in Pregnancy Society, other criteria not specified above, diagnostic criteria not specified)
Subgroup analysis will be restricted to the review's primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
In order to examine robustness of individual decisions being made to this systematic review, we planned to carry out sensitivity analysis restricting our analyses to:
studies at a low risk of bias (for allocation concealment);
full‐text papers;
number of participants > 300;
RCTs (excluding cluster‐randomised trials in order to investigate the effect of the randomisation unit);
studies without high levels of missing data.
Results
Description of studies
Results of the search
See: Figure 1
1.
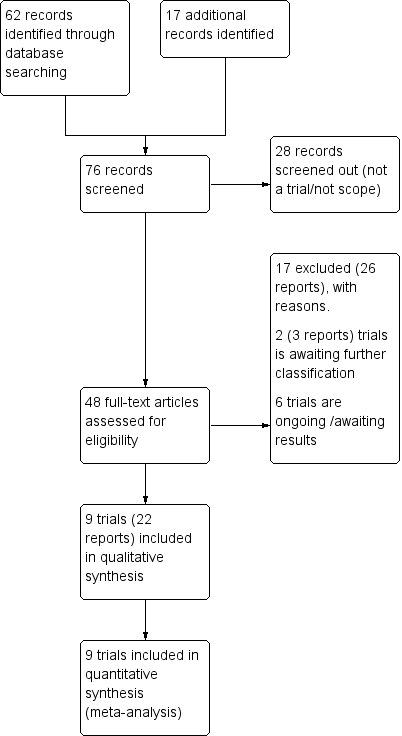
Study flow diagram.
We retrieved 31 trial reports from the Cochrane Pregnancy and Childbirth database searches plus an additional 17 from other sources. We included nine trials (22 reports) and excluded 17. Two trials (three reports) are awaiting further classification (Characteristics of studies awaiting classification), and six trials are ongoing (Characteristics of ongoing studies).
Included studies
Nine trials were selected and analysed (Ahmadi 2016; Badehnoosh 2018; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Karamali 2018; Kijmanawat 2019; Lindsay 2015; Nabhani 2018). All trials randomised women with gestational diabetes mellitus (GDM) to probiotics or placebo.
Information regarding the included trials is reported in the Characteristics of included studies tables.
Design
All studies were randomised controlled clinical trials comparing probiotics with placebo. Probiotics used in most studies were different in strengths and combinations (refer to interventions and comparisons).
Sample sizes
From the nine included trials, sample sizes ranged from 60 to 149 participants. The total number of participants were 695 randomised and 674 analysed. Total number of participants per study randomised (final analysis) were: Hajifaraji 2017; randomised 64 (analysed 56); Kijmanawat 2019 randomised 60 (analysed 57); Badehnoosh 2018, Karamali 2016, Karamali 2018 randomised 60 (analysed 60) in each trial. Ahmadi 2016 randomised 70 (analysed 70); Jafarnejad 2016 randomised 82 (analysed 72) and the trial with largest number of participants was Lindsay 2015 which randomised and analysed 149; and Nabhani 2018 randomised and analysed 90.
Setting
Seven studies were carried out in hospital or university settings in Iran (Ahmadi 2016; Badehnoosh 2018; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Karamali 2018; Nabhani 2018. One of the studies was carried out in Bangkok (Thailand) (Kijmanawat 2019), and one in Dublin (Ireland) (Lindsay 2015).
Dates of studies
Ahmadi 2016 took place between February and May 2016; Badehnoosh 2018 between April to September 2016, Hajifaraji 2017 during spring and summer 2014 (April to August); Jafarnejad 2016 between May 2014 to October 2015; Karamali 2016 between November 2015 to January 2016; Karamali 2018 between April and December 2016; Kijmanawat 2019 between July 2016 and February 2017; Lindsay 2015 between March 2012 and May 2014; Nabhani 2018 between January 2015 and September 2016.
Participants
All participants were women diagnosed with GDM according to the criteria chosen by each research team at between 24 to 28 weeks' gestation. Ahmadi 2016, Badehnoosh 2018, Hajifaraji 2017, Karamali 2016, Karamali 2018, Kijmanawat 2019, Nabhani 2018 used the American Diabetes Association (ADA) criteria after taking a 75 g oral glucose tolerance test (OGTT), and having a fasting blood glucose of ≥ 92 mg/dL, one‐hour OGTT ≥ 180 mg/dL and two‐hour OGTT ≥ 153 mg/dL. Kijmanawat 2019, as well as using the ADA diagnostic criteria also used a fasting plasma glucose ≥ 92 mg/dL at the first prenatal visit as a diagnosis for GDM. Jafarnejad 2016 used the Australasian Diabetes in Pregnancy Society (ADIPS) criteria with 75 g OGTT with results of fasting venous plasma glucose level, ≥ 5.5 mmol/L−1 or two‐hour venous plasma glucose level, ≥ 8.0 mmol/L−1. Lindsay 2015 used the Carpenter Coustan criteria results of a three‐hour 100 g OGTT with fasting ≥ 95 mg/dL, one‐hour ≥ 180 mg/dL, two‐hours ≥ 155 mg/dL, three‐hour 140 mg/dL for newly diagnosed impaired glucose tolerance (IGT) (1 raised value) or GDM (≥ 2 raised values).
Participants were between 18 to 45 years of age.
Three trials specifically reported participants as nulliparous (Badehnoosh 2018; Hajifaraji 2017; Karamali 2016). The other six trials did not clearly report baseline information related to parity (Ahmadi 2016; Jafarnejad 2016; Karamali 2018; Kijmanawat 2019; Lindsay 2015; Nabhani 2018).
Interventions and comparisons
Probiotics used in studies were of different strengths and combinations and given to participants in capsule form daily for either four, six or eight weeks. Participants in Ahmadi 2016 were given Lactobacillus casei and, Bifidobacterium bifidum (2 × 109 colony‐forming units (CFU)/g each) plus 0·8 g inulin for six weeks. Badehnoosh 2018 gave participants Lactobacillus acidophilus, L casei and B bifidum (2 x 109 CFU/g each) for six weeks. Hajifaraji 2017 used (4Biocap capsules) containing 180 mg (4 x 109 CFU) standard power including freeze‐dried cultures of Lactobacillus acidophilus LA‐5, Bifibacterium BB12, Streptococcus thermophilus STY‐31, and Lactobacillus delbrueckii bulgaricus LBY‐27 + dextrose anhydrate filler and magnesium stearate lubricant for eight weeks. Jafarnejad 2016 used VSL#3, a freeze‐dried probiotic preparation containing eight strains of lactic acid bacteria (S thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and L delbrueckii subsp. Bulgaricus (112.5 × 109 CFU/capsule), plus microcrystalline cellulose, stearic acid, magnesium stearate, and vegetable capsule (hydroxypropyl methylcellulose), silicon dioxide for eight weeks. Karamali 2016 and Karamali 2018 used three viable freeze‐dried strains: L acidophilus (2 × 109 CFU/g), L. casei (2 × 109 CFU/g), L casei (2 × 109 CFU/g) and B bifidum (2 × 109 CFU/g) for six weeks. Kijmanawat 2019 gave participants L acidophilus and B bifidum (1 x 109) CFU for four weeks. Lindsay 2015 used 100 mg Lactobacillus salivarus UCC118 (109 CFU/capsule) for four to six weeks. Nabhani 2018 used Lacidophilus, Lactobacillusplantarum, Lactobacillus fermentum, Lactobacillus gasseri (1.5–7.0 x 109–10 CFU/g) – with fructo‐oligosaccharide (38.5 mg) with lactose (300 mg), magnesium stearate, talc, colloidal silicon dioxide (each of them 5.5 mg), flavourings and sweeteners that have neutral effects for six weeks.
All studies had placebo capsules as a comparison. Placebo in studies are explained in more detail in the Blinding (performance bias and detection bias) section of the review.
To support the interventions, four trials sent daily reminder text messages to participants (Ahmadi 2016; Badehnoosh 2018; Karamali 2016; Karamali 2018). Four trials carried out weekly phone interviews ( Hajifaraji 2017; Jafarnejad 2016; Kijmanawat 2019; Nabhani 2018). One study did not use phone interviews in their processes (Lindsay 2015).
Outcomes
Three trials (Badehnoosh 2018; Karamali 2018; Lindsay 2015) reported hypertensive disorders of pregnancy including pre‐eclampsia and pregnancy‐induced hypertension. No trials reported eclampsia. Three trials (Badehnoosh 2018; Karamali 2018; Lindsay 2015) reported caesarean section rates. Two trials (Badehnoosh 2018; Lindsay 2015) reported large‐for‐gestational age > 90 centile. One trial (Lindsay 2015) reported induction of labour and postpartum haemorrhage. Six trials (Ahmadi 2016; Badehnoosh 2018; Jafarnejad 2016; Karamali 2016; Karamali 2018; Kijmanawat 2019) reported weight gain during pregnancy (during the intervention) . Three trials (Badehnoosh 2018; Kijmanawat 2019; Lindsay 2015) reported total gestational weight gain.
For relevant biomarker for oxidative stress, three trials (Badehnoosh 2018; Hajifaraji 2017; Karamali 2018) reported malondialdehyde (MDA); two trials (Badehnoosh 2018; Karamali 2018) reported total glutathione (GSH), and one trial (Hajifaraji 2017) reported uric acid.
For inflammatory bio markers: four trials (Badehnoosh 2018; Hajifaraji 2017; Jafarnejad 2016; Karamali 2018) reported high‐sensitivity C‐reactive protein (hs‐CRP); one trial (Jafarnejad 2016) reported interleukin 10 (IL‐10) and interferon c (IFN‐c); two trials (Hajifaraji 2017; Jafarnejad 2016) reported interferon 6 (IL‐6) and tumour necrosis factor alpha (TNF‐α).
For antioxidants: one trial (Karamali 2018) reported nitrous oxide; four trials (Badehnoosh 2018; Hajifaraji 2017; Karamali 2018; Nabhani 2018) reported total antioxidant capacity (TAC), and one trial (Hajifaraji 2017) reported serum GSH reductase (GSHR), erythrocyte superoxide dismutase (SOD) and erythrocyte glutathione peroxidase (GPx).
In biomarkers for insulin resistance: seven trials (Ahmadi 2016; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Kijmanawat 2019; Lindsay 2015; Nabhani 2018) reported Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR); and two trials (Ahmadi 2016; Karamali 2016) reported HOMA‐B (β‐cell function ).
The biomarker for insulin sensitivity QUICKI ( quantitative insulin‐sensitivity check index) was reported by four trials (Ahmadi 2016; Hajifaraji 2017; Karamali 2016; Nabhani 2018).
Insulin secretion was reported by seven trials (Ahmadi 2016; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Kijmanawat 2019; Lindsay 2015; Nabhani 2018).
For lipids: four trials (Ahmadi 2016; Karamali 2016; Lindsay 2015; Nabhani 2018) reported triglycerides (TAG); two trials (Ahmadi 2016; Karamali 2016) reported very low‐density lipoprotein (VLDL); and four trials (Ahmadi 2016; Karamali 2016; Lindsay 2015; Nabhani 2018) reported low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol, and total cholesterol.
Use of additional pharmacotherapy was reported by one trial (Badehnoosh 2018).
For glycaemic control: seven trials (Ahmadi 2016; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Kijmanawat 2019; Lindsay 2015; Nabhani 2018) reported fasting plasma glucose.
For neonatal outcomes: three trials (Badehnoosh 2018; Karamali 2018; Lindsay 2015) reported gestational age at birth; two trials (Badehnoosh 2018; Karamali 2018) reported preterm birth; three trials (Badehnoosh 2018; Karamali 2018; Lindsay 2015) reported macrosomia; one trial (Lindsay 2015) reported small‐for‐gestational age (SGA); four trials (Badehnoosh 2018; Karamali 2018; Kijmanawat 2019; Lindsay 2015) reported birthweight; three trials (Badehnoosh 2018; Karamali 2018; Lindsay 2015) reported head circumference, length and infant hypoglycaemia (requiring treatment, variously defined); two trials (Badehnoosh 2018; Karamali 2018) reported hyperbilirubinaemia; one trial (Lindsay 2015) reported Cord C peptide; and two trials (Badehnoosh 2018; Lindsay 2015) reported on neonatal intensive care unit (NICU) or nursery admissions.
All trials reported no significant issues or important clinical adverse effects with probiotics.
Sources of funding
Grants from: the Vice Chancellor for Research AUMS, Iran funded Ahmadi 2016; Vice Chancellor for Research, IUMS, Tehran, Iran funded Badehnoosh 2018; Tehran, Shahid Beheshti, University Medical Sciences funded Hajifaraji 2017; Vice Chancellor for Research, IUMS, Tehran, Iran funded Karamali 2016 and Karamali 2018; Thailand Research Fund (TRF) funded Kijmanawat 2019; National Maternity Hospital Medical Fund with support from the Ivo Drury Award and the European Union’s Seventh Framework Program (FP7/2007‐2013), project Early Nutrition under grant agreement number 289346 funded Lindsay 2015; and Tabriz University of Medical Sciences, Iran, and Nutrition Research Center funded Nabhani 2018.
No details of funding for one trial (Jafarnejad 2016),
Declarations of conflict of interest
A total of nine trials declared no conflict of interest except two trials that declared conflict of interest of at least one of its members (Kijmanawat 2019) (SR received grant support from Merck Sharp and Dohme, research equipment support from ResMed, and speaker honoraria from Sanofi, Novo Nordisk and Medtronic), (Lindsay 2015) (F.S. was a shareholder in Alimentary Health Ltd and has received grants from GlaxoSmithKline and the Procter and Gamble Company in the past).
Excluded studies
There were 17 articles that were excluded including one conference abstract.
In seven of the trials (Asemi 2013; Asemi 2013a; Barthow 2016; Luoto 2010; Nitert 2013; Okesene‐Gafa 2018; Wickens 2017), the participants in the randomised controlled trial (RCTs) were not women with GDM. Four articles (Al‐Dughaishi 2016; Gomez 2015; Lindsay 2013; Lindsay 2014) were not RCTs. Two of the papers (Barrett 2012; Barrett 2014) were systematic reviews; the latter a Cochrane Review. One of the papers (Muktabhant 2015) was a Cochrane Review on diet and exercise. Two of the trials (Fei 2014; Zhang 2018) used prebiotics and not probiotics.
Studies awaiting classification
Two trials (Gonai 2014; Jamilian 2019) are awaiting classification.
Risk of bias in included studies
Risk of bias is summarised in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation
We assessed all nine studies as low risk of bias for random sequence generation because they appeared to have adequate randomisation processes.
Eight trials reported that their random sequence was computer‐generated (Ahmadi 2016; Badehnoosh 2018; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Karamali 2018; Lindsay 2015; Nabhani 2018).
One of the trials reported that a statistician generated the randomisation sequence using blocks (Kijmanawat 2019).
Three trials used computer block randomisation (Hajifaraji 2017, Kijmanawat 2019, Nabhani 2018).
Two trials specified that a separate researcher assistant (counsellor/therapist/trained personnel) carried out the randomisation and allocated the capsule packages according to the random sequence generated by the computer program (Hajifaraji 2017; Jafarnejad 2016).
Allocation concealment
Of the nine trials, one was rated as low risk of bias for allocation concealment ( Lindsay 2015). The remaining eight studies were rated as unclear as we were unable to determine if researchers were aware of the allocation sequence when recruiting participants (Ahmadi 2016; Badehnoosh 2018; Jafarnejad 2016; Karamali 2016; Nabhani 2018; Hajifaraji 2017; Karamali 2018; Kijmanawat 2019).
Blinding of participants and personnel (performance bias)
All nine trials were specifically reported as double‐blind, placebo‐controlled randomised trials and were all graded as low risk of performance bias.
Six trials specified that probiotics and placebo were indistinguishable from each other (Ahmadi 2016; Hajifaraji 2017; Karamali 2016; Kijmanawat 2019; Lindsay 2015; Nabhani 2018). One trial stated their placebo capsules were identical to probiotics and contained 40 mg microcrystalline cellulose (Jafarnejad 2016).
Two trials did not offer adequate details of their placebo capsules: one trial stated that the placebo contained starch (Badehnoosh 2018); the other trial stated that their placebo contained gelatin (Kijmanawat 2019. One trial reported use of placebo with no specifics (Karamali 2018).
Four trials reported that a coder or supplier of capsules anonymously labelled the packages as A or B, whereas the contents of the packages were unknown to the researcher allocating the treatment in four trials (Hajifaraji 2017; Jafarnejad 2016; Lindsay 2015; Nabhani 2018). In one of these studies the packages (A or B) were placed in sequentially‐numbered, sealed opaque envelopes (Lindsay 2015).
Blinding of outcome assessment (detection bias)
We assessed all nine trials as low risk of detection bias since they all reported adequate blinding of outcome assessors.
Incomplete outcome data
All nine studies were graded as low risk of bias, as there were minimal dropouts and no differential attrition. Two trials also stated that they used intention‐to‐treat analysis (Ahmadi 2016; Hajifaraji 2017.
Selective reporting
We assessed all nine trials as unclear risk of reporting bias because none of them had published study protocols, nor were any of them registered prospectively in any clinical trials registry, therefore we had insufficient information to judge which outcomes were pre‐specified outcomes and if they were reported in full.
Other potential sources of bias
One trial had a significant difference in baseline cholesterol level between the probiotics and placebo groups. After adjusting for biochemical values, maternal age and body mass index (BMI) at baseline, there was no significant differences in these results (Ahmadi 2016).
One trial had significant differences in baseline levels of fasting plasma glucose (FPG) and HDL cholesterol between the two groups, but after further adjusting these variables as well as for baseline maternal age and BMI, the results were similar in both groups except for HOMA‐B (P = 0.08) (Karamali 2016).
One trial had a slightly lower rate of Caucasian ethnicity and obesity and a higher rate of primiparity in the probiotic compared to placebo group, although these differences were not significant (Lindsay 2015).
One trial showed that there was a difference in energy, protein and total fat intakes (P < 0.05); thus, final analyses were adjusted for the measures of energy intake, BMI and baseline values (Nabhani 2018).
One trial stated that the women in their trial were taking 400 µg early pregnancy and 60 mg/day of ferrous sulphate from the second trimester (Badehnoosh 2018).
We assessed all nine studies as low risk of other sources of bias.
Effects of interventions
Probiotics versus placebo
Primary outcomes
Maternal
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
We are uncertain if there is any difference in hypertensive disorders in the probiotics compared to the placebo group, due to the wide 95% confidence intervals (CIs) which span possible benefit and potential harm (risk ratio (RR) 1.50, 95% CI 0.64 to 3.53; participants = 256; studies = 3; I2 = 0%; low‐certainty evidence;Analysis 1.1).
1.1. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 1: Hypertensive disorders (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Subsequent development of type 2 diabetes
This outcome was not reported by the included trials.
Mode of birth (caesarean)
We are uncertain if there is any difference in caesarean sections as a mode of birth in the probiotics compared to the placebo group, because the quality of the evidence is low and the 95% CI is consistent with possible benefit and possible harm (average RR 0.64, 95% CI 0.30 to 1.35; participants = 267; studies = 3; I2 = 69%; low‐certainty evidence;Analysis 1.2).
1.2. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 2: Mode of birth (caesarean)
Infant
Perinatal (fetal and neonatal) mortality
This outcome was not reported by the included trials.
Large‐for‐gestational age (LGA) > 90 centile
We are uncertain if there is any difference in LGA in the probiotics compared to the placebo group because the quality of evidence is low and the 95% CI spans possible benefit and potential harm (RR 0.73, 95% CI 0.35 to 1.52; participants = 174; studies = 2; I2 = 0%; low‐certainty evidence;Analysis 1.3).
1.3. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 3: Large‐for‐gestational age > 90 centile
Composite serious neonatal outcomes (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture, or nerve palsy)
This outcome was not reported by the included trials.
Neurosensory disability
This outcome was not reported by the included trials.
Secondary outcomes
Maternal
Induction of labour
We are uncertain if there is any difference in induction of labour in probiotic versus placebo because the certainty of evidence is very low and the 95% CI is consistent with possible benefit and possible harm (RR 1.33, 95% CI 0.74 to 2.37; participants = 127; studies = 1; Analysis 1.4).
1.4. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 4: Induction of labour
Perineal trauma
This outcome was not reported by the included trials.
Placental abruption
This outcome was not reported by the included trials.
Postpartum haemorrhage
We are uncertain if there is any difference in incidence of postpartum haemorrhage (RR 0.77, 95% CI 0.36 to 1.62; participants = 126; studies = 1; Analysis 1.5).
1.5. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 5: Postpartum haemorrhage
Postpartum infection
This outcome was not reported by the included trials.
Weight gain during pregnancy
We are uncertain if there is any difference in weight gain from the beginning of intervention to the end of the intervention in the probiotics compared with placebo groups (mean difference (MD) 1.38, 95% CI ‐0.49 to 3.24; participants = 379; studies = 6; I2 = 0%; Analysis 1.6).
1.6. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 6: Weight gain during pregnancy (kg)
Total gestational weight gain (kg)
We are uncertain if there is any difference in total gestational weight gain in the probiotics compared with the placebo groups (MD 0.24, 95% CI ‐0.30 to 0.78; participants = 239; studies = 3; I2 = 0%; Analysis 1.7).
1.7. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 7: Total gestational weight gain (kg)
Adherence to intervention
This outcome was not reported by the included trials.
Behaviour changes associated with the intervention
This outcome was not reported by the included trials.
Relevant biomarker changes associated with the intervention:
Homeostatic model assessment for Insulin resistance (HOMA‐IR)
There was evidence of a reduction in marker for HOMA‐IR in the probiotics compared to the placebo group (MD ‐0.30, 95% CI ‐0.35 to ‐0.25; participants = 505; studies = 7; I2 = 70%; Analysis 1.8).
1.8. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 8: Relevant biomarker changes associated with the intervention
Homeostatic model assessment for beta cell function (HOMA‐B)
There was evidence of a reduction in HOMA‐B in the probiotic compared to the placebo group (MD ‐25.38, 95% CI ‐38.32 to ‐12.44; participants = 130; studies = 2; I2 = 0%; Analysis 1.8).
Quantitative insulin sensitivity check index (QUICKI)
There was some evidence of an increase in QUICKI levels in the probiotic compared to the placebo group (MD 0.01, 95% CI 0.00 to 0.02; participants = 276; studies = 4; I2 = 0%; Analysis 1.8).
Triglycerides (TAG) (mg/dL)
There was evidence of a reduction in triglycerides in the probiotic compared with the placebo group (MD ‐19.19, 95% CI ‐35.69 to ‐2.70; participants = 320; studies = 4; I2 = 46%) Analysis 1.8).
Very low‐density lipoprotein (VLDL) cholesterol (mg/dL)
There was evidence of a reduction in VLDL cholesterol with probiotics compared with placebo group (MD ‐7.80, 95% CI ‐12.93 to ‐2.66; participants = 130; studies = 2; I2 = 26%; Analysis 1.8).
Low‐density lipoprotein (LDL) cholesterol (mg/dL)
We are uncertain if there is any difference in LDL cholesterol with probiotics compared with placebo (MD ‐5.36, 95% CI ‐12.83 to 2.12; participants = 320; studies = 4; I2 = 0%; Analysis 1.8).
High‐density lipoprotein (HDL) cholesterol (mg/dL)
There was evidence of a reduction in HDL cholesterol with probiotics compared to placebo (MD ‐3.48, 95% CI ‐6.02 to ‐0.93; participants = 320; studies = 4; I2 = 76%; Analysis 1.8).
Total cholesterol (mg/dL)
There was evidence of a reduction in total cholesterol with probiotics compared to placebo (MD ‐10.63, 95% CI ‐19.73 to ‐1.54; participants = 320; studies = 4; I2 = 56%; Analysis 1.8).
High‐sensitivity C‐reactive protein (hs‐CRP) (µg/mL)
There was evidence of a reduction in maternal inflammatory marker hs‐CRP in probiotics compared to the placebo group (MD ‐1.29, 95% CI ‐1.72 to ‐0.86; participants = 248; studies = 4; I2 = 44%; Analysis 1.8).
Nitrous oxide (NO) (µmol/L)
There was no evidence of a clear difference in levels of NO (vasodilator) with probiotics compared to placebo groups (MD 1.70, 95% CI ‐0.94 to 4.34; participants = 120; studies = 2; I2 = 0%; Analysis 1.8).
Malondialdehyde (MDA) (µmol/L)
There was evidence of a decrease in MDA (marker of oxidative stress) levels in the probiotics compared to the placebo group (MD ‐0.85, 95% CI ‐1.20 to ‐0.50; participants = 176; studies = 3; I2 = 0%; Analysis 1.8).
Total glutathione (GSH) (µmol/L)
There was evidence of increased GSH levels (antioxidant) with probiotics compared with placebo (MD 44.95, 95% CI 13.36 to 76.55; participants = 120; studies = 2; I2 = 0%; Analysis 1.8).
Total glutathione reductase (GSHR) (ng/mL)
There was evidence of increased GSHR levels (an antioxidant) with probiotics compared with placebo (MD 5.78, 95% CI 0.30 to 11.26; participants = 56; studies = 1; I2 = 0%; Analysis 1.8).
Total antioxidant capacity (TAC) (mmol/L)
There may be little to no difference in TAC with probiotics compared to placebo (MD 0.02, 95% CI ‐0.05 to 0.10; participants = 266; studies = 4; I2 = 92%; Analysis 1.8). This meta‐analysis has a high level of heterogeneity which may be due to the different methods used to measure TAC. We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Interleukin 10 (IL‐10) (pg/mL)
Only one trial Jafarnejad 2016 reported IL‐10 levels. We are uncertain if there is a difference in IL‐10 levels between probiotics compared to placebo group (MD ‐0.27, 95% CI ‐2.93 to 2.39; participants = 72; studies = 1; Analysis 1.8).
Inteferon c (IFN‐c)
Only one trial Jafarnejad 2016 reported on IFN‐c and it is not certain if there is any difference in levels between probiotics compared to placebo (MD ‐1.90, 95% CI ‐9.38 to 5.58; participants = 72; studies = 1; Analysis 1.8).
Inter leukin 6 (IL‐6) (pg/mL)
There was evidence of a reduction in IL‐6 in the probiotic compared to the placebo group (MD ‐0.89, 95% CI ‐1.17 to ‐0.60; 128 participants; 2 trials Tau² = 0.00; I² = 0%; Analysis 1.8).
Tumour necrosis factor alpha (TNF‐α) (pg/mL)
There was evidence of reduction in TNF‐α in the probiotic compared to the placebo group: mean difference (MD ‐0.53, 95% CI ‐0.78 to ‐0.27; participants = 128; studies = 2; I2 = 80%; Analysis 1.8).
Serum uric acid (mg/dL)
Only one trial looked at serum uric acid (Hajifaraji 2017). We are uncertain if there is any difference between probiotics and placebo (MD ‐0.21, 95% CI ‐0.52 to 0.10; participants = 56; studies = 1; Analysis 1.8).
Erythrocyte superoxide dismutase (SOD) (U/gHBb)
Only one trial looked at levels of erythrocyte SOD (Hajifaraji 2017). We are uncertain if there is any difference in erythrocyte SOD with probiotics compared with placebo (MD 189.20, 95% CI ‐57.31 to 435.71; participants = 56; studies = 1; Analysis 1.8).
Erythrocyte glutathione peroxidase (GPx) (U/gHb)
One trial calculated Erythrocyte GPx Hajifaraji 2017 and there was evidence of an increase in erythrocyte GPX with probiotics compared with placebo (MD 6.93, 95% CI 1.34 to 12.52; participants = 56; studies = 1; Analysis 1.8).
Sense of well‐being and quality of life
These outcomes were not reported by the included trials.
Views of the intervention
This outcome was not reported by the included trials.
Breastfeeding at discharge or six weeks postpartum
This outcome was not reported by the included trials.
Use of additional pharmacotherapy
Use of additional pharmacotherapy was reported by one trial. We are uncertain if there is any difference between probiotics and placebo in requiring additional pharmacotherapy (insulin) (RR 0.67, 95% CI 0.12 to 3.71; participants = 60; studies = 1; Analysis 1.9).
1.9. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 9: Use of additional pharmacotherapy
Glycaemic control during/end of treatment (as defined by trialist)
This outcome was not reported by the included trials.
Fasting blood glucose(mg/dL)
We are uncertain if there is any difference in fasting plasma glucose in those in the probiotics arm compared to placebo (MD ‐0.42, 95% CI ‐1.66 to 0.82; participants = 554; studies = 7; I2 = 46%; Analysis 1.10).
1.10. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 10: Glycaemic control during/ end of treatment (as defined by trialists)
Postprandial blood glucose
This outcome was not reported by the included trials.
Maternal hypoglycaemia
This outcome was not reported by the included trials.
Maternal mortality
This outcome was not reported by the included trials.
Long‐term maternal outcomes
None of the included studies reported any of our pre‐specified long‐term maternal outcomes.
Infant outcomes
Stillbirth
This outcome was not reported by the included trials.
Neonatal mortality
This outcome was not reported by the included trials.
Gestational age at birth (days)
We are uncertain if there is any difference in gestational age at birth between probiotics and placebo (MD 1.37 days, 95% CI ‐1.33 to 4.07; participants = 267; studies = 3; I2 = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 11: Gestational age at birth (days)
Preterm birth
We are uncertain if there is any difference in rates of preterm births in the probiotics compared to placebo groups (RR) 1.00, 95% CI 0.18 to 5.59; participants = 120; studies = 2; I2 = 0%; Analysis 1.12).
1.12. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 12: Preterm birth
Apgar score less than seven in five minutes
This outcome was not reported by the included trials.
Macrosomia (> 4000 g)
We are uncertain if there is any difference in macrosomia (> 4000 g) in the probiotics compared to placebo groups (RR 0.84, 95% CI 0.50 to 1.43; participants = 267; studies = 3; I2 = 48%; Analysis 1.13).
1.13. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 13: Macrosomia (> 4000 g)
Small‐for‐gestational age (SGA)
Only one trial (Lindsay 2015) reported on SGA. We are uncertain if there is any difference in SGA in the probiotics group compared to placebo (RR 1.04, 95% CI 0.39 to 2.76; participants = 114; studies = 1; Analysis 1.14).
1.14. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 14: Small‐for‐gestational age (SGA)
Birthweight (g) and z scores
We are uncertain if there is any difference in birthweight in the probiotics groups compared to the placebo groups (MD ‐79.14 g, 95% CI ‐183.00 to 24.73; participants = 324; studies = 4; Analysis 1.15).
1.15. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 15: Birthweight (g)
Head circumference (cm) and z scores
We are uncertain if there is any difference in head circumference of infants in the probiotic group compared to the placebo group (MD ‐0.02 cm, 95% CI ‐0.52 to 0.48; participants = 249; studies = 3; I2 = 0%; Analysis 1.16).
1.16. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 16: Head circumference (cm)
Length(cm) and z scores
We are uncertain if there is any difference in length of infants in the probiotic groups compared to the placebo groups (MD ‐0.35 cm, 95% CI ‐1.03 to 0.33; participants = 248; studies = 3; I2 = 0%; Analysis 1.17).
1.17. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 17: Length (cm)
Ponderal index
This outcome was not reported by the included trials.
Adiposity
This outcome was not reported by the included trials.
Shoulder dystocia
This outcome was not reported by the included trials.
Bone fracture
This outcome was not reported by the included trials.
Nerve palsy
This outcome was not reported by the included trials.
Respiratory distress syndrome
This outcome was not reported by the included trials.
Neonatal hypoglycaemia requiring treatment (variously defined)
We are uncertain if there is any difference in neonatal hypoglycaemia in the probiotics groups compared with the placebo groups because the quality of evidence is low and the 95% CI is consistent with possible benefit and possible harm (RR 0.85, 95% CI 0.39 to 1.84; participants = 177; studies = 3; I2 = 0%; Analysis 1.18; low‐certainty evidence; Table 2).
1.18. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 18: Infant hypoglycemia requiring treatment (variously defined)
Hyperbilirubinemia
There was evidence of a reduction in infant hyperbilirubinaemia with probiotics compared to placebo (RR 0.18, 95% CI 0.05 to 0.57; participants = 120; studies = 2; I2 = 0%; Analysis 1.19).
1.19. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 19: Hyperbilirubinemia
Neonatal hypocalcaemia
This outcome was not reported by the included trials.
Polycythaemia
This outcome was not reported by the included trials.
Relevant infant biomarkers associated with intervention (cord C peptide, cord insulin)
C‐peptide
A marker of insulin secretion cord C peptide was reported by only one trial Lindsay 2015. We are uncertain if there is any difference in C‐peptide secretions, in probiotics compared to placebo (MD ‐0.05, 95% CI ‐0.44 to 0.34; participants = 100; studies = 1; Analysis 1.20).
1.20. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 20: Relevant infant biomarker's associated with intervention (cord C peptide, cord insulin)
Later childhood
None of the included studies reported any of our pre‐specified outcomes relating to later childhood.
Adulthood outcomes
None of the included studies reported any of our pre‐specified adulthood outcomes.
Health services
Number of antenatal visits or admissions
This outcome was not reported by the included trials.
Number of hospital or heath professional visits (including midwives, obstetricians, physicians, dietician, diabetic nurse)
This outcome was not reported by the included trials.
Admission to NICU/nursery
We are uncertain if there is any difference between probiotics and placebo in NICU or nursery admissions (average RR 1.71, 95% CI 0.45 to 6.53; participants = 202; studies = 2; I2 = 66%) (Analysis 1.21).
1.21. Analysis.

Comparison 1: Probiotic versus placebo, Outcome 21: Admission to NICU/nursery
One of the included trials (Badehnoosh 2018) only reported newborn hospitalisations and we made the assumption that any neonatal hospitalisations would be to the neonatal nursery and this study was added to the analysis for admission to NICU/nursery.
Length of antenatal stay
This outcome was not reported by the included trials.
Length of postnatal stay (maternal)
This outcome was not reported by the included trials.
Length of postnatal stay (baby)
This outcome was not reported by the included trials.
Cost of maternal care
This outcome was not reported by the included trials.
Cost of offspring care (including NICU admissions)
This outcome was not reported by the included trials.
Costs associated with the interventions
This outcome was not reported by the included trials.
Costs associated with the interventions
This outcome was not reported by the included trials.
Discussion
Summary of main results
Nine randomised controlled trials (RCTs) involving 695 women with gestational diabetes mellitus (GDM) and their babies met the inclusion criteria for this review. All trials compared probiotics (some used the same and others used different strengths and compositions and administered at different lengths of time) with placebo.
For mothers, we are uncertain if probiotics have any effect on the risk of hypertensive disorders, mode of birth or induction of labour compared with placebo because the evidence is low to very low certainty, and the 95% confidence intervals are consistent with possible benefit and possible harm (Table 1). We identified no evidence relating to the risk of developing type 2 diabetes, perineal trauma, postnatal weight retention or postnatal depression.
For infants, we are uncertain if probiotics lead to more or fewer large‐for‐gestational age infants, or if probiotics have any effect on the risk of neonatal hypoglycaemia, compared with placebo, because the evidence is low‐certainty and the 95% confidence intervals are consistent with possible benefit and possible harm (Table 2). We identified no evidence relating to perinatal mortality, serious neonatal outcomes, neurosensory disability, or adiposity or diabetes later in life.
In other secondary maternal outcomes, in the probiotics compared to the placebo group, there was a reduction in inflammatory markers hs‐CRP and interleukin 6. There was an increase in antioxidant total glutathione and reduction in maker of oxidative stress malondialdehyde. There may be a reduction in triglyceride and total cholesterol, but we are uncertain of a difference in fasting plasma glucose. For neonatal/infant outcomes we are uncertain whether probiotics compared to placebo have any effect on birthweight, gestational age at birth, preterm births, macrosomia, head circumference and length; or increase in NICU admissions.
Limitations of the studies at the outcome level were their small sample sizes. At the reporting level, a number of RCTs focused on probiotics to prevent development of GDM but not as a treatment for GDM and were therefore excluded.
Overall completeness and applicability of evidence
The trials in this review were conducted in women with GDM. The studies recruited pregnant women with GDM mostly from Iran (Ahmadi 2016; Badehnoosh 2018; Hajifaraji 2017; Jafarnejad 2016; Karamali 2016; Karamali 2018; Nabhani 2018), with only two studies outside Iran where one was conducted in Bangkok (Thailand) (Kijmanawat 2019), and the other in Dublin (Ireland) (Lindsay 2015). The largest trial involved 149 women (Lindsay 2015); most of the other trials had smaller numbers of women (60 to 95). All trials reported the outcomes specified in the trials.
Included studies did not report a number of our main GRADE outcomes, nor a large number of our secondary outcomes.
Quality of the evidence
Overall, the nine trials were judged as low to unclear risk of bias. The risk of selection bias was generally unclear because there was insufficient information to judge whether allocation concealment had been carried out adequately. Additionally, the risk of reporting bias was assessed as unclear due to a lack of published study protocols against which to compare the outcomes selected for reporting in the trial results.
Using GRADE methodology, the evidence was assessed as low to very low certainty. Downgrading decisions for mode of birth was mainly due to imprecision and inconsistency (low‐certainty evidence), and for induction of labour (a secondary outcome) (very low‐certainty evidence), downgraded one level due to indirectness and two levels for imprecision.
Potential biases in the review process
To reduce the potential for publication bias, the Information Specialist of the Cochrane Pregnancy and Childbirth group was asked to conduct a systematic detailed search process for this review. It is possible that additional trials assessing the use of probiotics as a treatment of GDM may be available that may have been carried out but not yet published, or have recently been published but are not included in this review. Should they be identified, they will be included in future updates of this review.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review of this topic and hence, agreements and disagreements with other reviews is not possible. Most of the Cochrane or non‐Cochrane Reviews or systematic reviews of randomised controlled trial already carried out were to determine whether probiotics compared to placebo prevented development of GDM. One meta‐analysis looked at effect of probiotics on metabolic health in pregnant women who were or were not diagnosed with GDM (Zheng 2018).
Authors' conclusions
Implications for practice.
Low‐certainty evidence showed that we were uncertain of any difference between probiotic and placebo groups in maternal hypertensive disorders of pregnancy and mode of birth by caesarean section; and neonatal/infant outcome of large for gestational age.
There were no reported adverse events reported by the trials.
Due to the variability of probiotics used and small sample sizes of the trials, the evidence from this review has limited ability to inform practice. Future studies need to consider the use of a specific probiotic type and strength, to be able to identify the impact on glucose metabolism and core clinical outcomes and to facilitate standardised reporting.
Implications for research.
Further adequately powered, well‐designed randomised controlled trials are needed to clarify the effects of probiotics on glucose metabolism in pregnant women with gestational diabetes mellitus (GDM). There are currently six ongoing trials, with over 500 participants to be recruited. Once completed, these trials will add to the evidence base. These trials have been designed to examine some of our clinical outcomes but it would appear that they are not designed to measure long‐term infant outcomes. There is still a need for trials to be designed to address this need.
There is also a need for trials to be in agreement as to what probiotics should be used for research to avoid uncertainty of benefit with variability in probiotic type and strength. Clear evidence of benefit of these specific probiotics in improving fasting, pre‐prandial and/or postprandial glucose should also theoretically translate into improvement in important maternal and neonatal/infant clinical outcomes. In future trials, it is important to consider collecting important immediate and long‐term maternal and offspring outcomes as outlined in this review, but were not available. Important bio‐markers of insulin resistance (HOMA‐IR and HOMA‐B), insulin sensitivity (QUICKI), as well as inflammatory markers, antioxidants and markers of oxidative stress can be explored further to determine their role in modifying the effect of insulin resistance during pregnancy and whether they also play a role in improving glucose metabolism and consequently improve clinical outcomes. Another important factor to be considered in future trials is agreed criteria for diagnosing GDM.
We look forward to the results of current ongoing studies. The results of these studies will be included in future updates of this review.
History
Protocol first published: Issue 2, 2018 Review first published: Issue 6, 2020
Acknowledgements
Portions of the methods section of this protocol were based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group. Outcomes may be similar to other Cochrane Reviews on treatment for gestational diabetes due to the attempt to have consistency across all protocols and reviews on this condition.
We acknowledge the support from the Cochrane Pregnancy and Childbirth Review Group editorial team in Liverpool, the Australian and New Zealand Satellite of the Cochrane Pregnancy and Childbirth Review Group and the Liggins Institute, University of Auckland, New Zealand.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Dr Mohammad Othman, Assistant Professor of Obstetrics and Gynaecology, Faculty of Medicine, Al‐Baha University, Saudi Arabia; Cristina Scifres, MD, Indiana University School of Medicine, USA.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We wish also thank Julie Brown who was instrumental during the development of the protocol for this review, and assisted with the assessment of potential relevant trials for this review.
Appendices
Appendix 1. Keywords for searching trials registries
ICTRP
probiotics AND gestational
probiotics AND diabetes AND pregnancy
ClinicalTrials.gov
Advanced search
Interventional studies | probiotics | gestational diabetes
pregnancy | Interventional Studies | Diabetes | probiotics
Data and analyses
Comparison 1. Probiotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Hypertensive disorders (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia) | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.64, 3.53] |
| 1.2 Mode of birth (caesarean) | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.30, 1.35] |
| 1.3 Large‐for‐gestational age > 90 centile | 2 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.35, 1.52] |
| 1.4 Induction of labour | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.74, 2.37] |
| 1.5 Postpartum haemorrhage | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.62] |
| 1.6 Weight gain during pregnancy (kg) | 6 | 379 | Mean Difference (IV, Fixed, 95% CI) | 1.38 [‐0.49, 3.24] |
| 1.7 Total gestational weight gain (kg) | 3 | 239 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.30, 0.78] |
| 1.8 Relevant biomarker changes associated with the intervention | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 HOMA‐IR | 7 | 505 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.35, ‐0.25] |
| 1.8.2 HOMA‐B | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐25.38 [‐38.32, ‐12.44] |
| 1.8.3 Insulin (microIU/L) | 7 | 505 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.27, ‐0.80] |
| 1.8.4 QUICKI | 4 | 276 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [0.00, 0.02] |
| 1.8.5 TAG (Triglycerides) (mg/dL) | 4 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐19.19 [‐35.69, ‐2.70] |
| 1.8.6 VLDL cholesterol (mg/dL) | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐7.80 [‐12.93, ‐2.66] |
| 1.8.7 LDL‐cholesterol (mg/dL) | 4 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐5.36 [‐12.83, 2.12] |
| 1.8.8 HDL‐cholesterol (mg/dL) | 4 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐3.48 [‐6.02, ‐0.93] |
| 1.8.9 Total cholesterol (mg/dL) | 4 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐10.63 [‐19.73, ‐1.54] |
| 1.8.10 hs‐CRP (µg/mL) | 4 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.72, ‐0.86] |
| 1.8.11 NO (nitrous oxide)µmol/L | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 1.69 [‐0.95, 4.33] |
| 1.8.12 MDA (malondialdehyde) (µmol/L) | 3 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.20, ‐0.50] |
| 1.8.13 GSH (total glutathione µmol/L) | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 44.95 [13.36, 76.55] |
| 1.8.14 Glutathione reductase (GSHR)(ng/mL) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 5.78 [0.30, 11.26] |
| 1.8.15 TAC (total antioxidant capacity)mmol/L | 4 | 266 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.10] |
| 1.8.16 IL‐10(pg/mL) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐2.93, 2.39] |
| 1.8.17 IFN‐c | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐9.38, 5.58] |
| 1.8.18 IL‐6(pg/mL) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.17, ‐0.60] |
| 1.8.19 TNF‐α(pg/mL) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 1.8.20 Serum uric acid (mg/dL) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.52, 0.10] |
| 1.8.21 Erythrocyte superoxide dismutase (SOD) (U/gHBb) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 189.20 [‐57.31, 435.71] |
| 1.8.22 Erythrocyte glutathione peroxidase (GPx) (U/gHb) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.93 [1.34, 12.52] |
| 1.9 Use of additional pharmacotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.9.1 Insulin therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Glycaemic control during/ end of treatment (as defined by trialists) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Fasting blood glucose(mg/dL) | 7 | 554 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.66, 0.82] |
| 1.10.2 Postprandial blood glucose(mg/dL) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 1.11 Gestational age at birth (days) | 3 | 267 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [‐1.33, 4.07] |
| 1.12 Preterm birth | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.18, 5.59] |
| 1.13 Macrosomia (> 4000 g) | 3 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.50, 1.43] |
| 1.14 Small‐for‐gestational age (SGA) | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.76] |
| 1.15 Birthweight (g) | 4 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐79.14 [‐183.00, 24.73] |
| 1.16 Head circumference (cm) | 3 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.52, 0.48] |
| 1.17 Length (cm) | 3 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐1.03, 0.33] |
| 1.18 Infant hypoglycemia requiring treatment (variously defined) | 3 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.39, 1.84] |
| 1.19 Hyperbilirubinemia | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.57] |
| 1.20 Relevant infant biomarker's associated with intervention (cord C peptide, cord insulin) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.44, 0.34] |
| 1.20.1 Cord C peptide (ng/mL) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.44, 0.34] |
| 1.21 Admission to NICU/nursery | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.45, 6.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadi 2016.
| Study characteristics | ||
| Methods | Parallel randomised double‐blind placebo‐controlled trial | |
| Participants | Pregnant women with GDM (n = 70) Setting Kosar Clinic in Arak, Iran Dates of study February 2016 to May 2016 Inclusion criteria Pregnant women with GDM, age 18‐40years, diagnosed with GDM by the American Diabetes Association guidelines by the 1‐step 2‐hour, 75 g OGTT, at 24‐28 weeks gestation, having a fasting blood glucose of ≥ 92 mg/dL, 1‐hour OGTT ≥ 180 mg/dLand 2‐hour OGTT ≥ 153 mg/dL. Exclusion criteria Women taking synbiotic or probiotic supplements, including probiotic yogurt, kefir and other fermented foods. Women taking insulin, with placenta abruption, pre‐eclampsia, eclampsia, hypothyroidism and hyperthyroidism, smokers, and those with kidney or liver diseases. |
|
| Interventions | Probiotic/symbiotic ‐ capsules containing Lactobacillus acidophilus, Lactobacillus casei and, Bifidobacterium bifidum (2 × 109 CFU/g each) plus 0·8 g inulin (n = 30) Placebo capsules containing starch without bacteria and inulin (n = 35) The appearance of the placebo was indistinguishable with regard to colour, shape, size and packaging, smell and taste from the probiotic capsule. |
|
| Outcomes | Primary outcomes: markers of insulin metabolism: FPG, serum insulin, quantitative insulin sensitivity check index (QUICKI), Homoeostatic model assessment for insulin resistance (HOMA‐IR), Homoeostatic model assessment for B‐cell function (HOMA‐B) Secondary outcomes: measurements of lipid concentrations: serum TAG, VLDL cholesterol, Total cholesterol, HDL cholesterol, LDL cholesterol. |
|
| Notes |
Funding: a grant from the Vice Chancellor for Research AUMS, Iran. Conflicts of interest: authors declare no conflict of interest. Participants were also sent reminder text messages daily to take their probiotics. Total time for the intervention was 6 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used to assign the participants. |
| Allocation concealment (selection bias) | Unclear risk | The randomised allocation sequence, enrolling participants and allocating them to interventions were conducted by a trained staff at the gynaecology clinic. Quote: "Randomisation and allocation were concealed from researchers and participants". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation and allocation were concealed from the researchers and participants until all the analyses were completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70 women randomised. 3 women in the placebo group withdrew for personal reasons, 1 woman in the intervention group also withdrew for personal reasons. 70 women analysed |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | This is a journal article. The groups were balanced at baseline. (mean age height, baseline weight and BMI as well as their means after 6 weeks intervention were not significant between the synbiotic group and the placebo group. Comparison of the 3‐day dietary intake throughout the study revealed no significant differences in micronutrient and macronutrient intakes, energy carbohydrates, proteins, fats, SFA, PUFA, MUFA, total dietary fibre, Fe, Mg, Zn, and Mn between the groups. (There was a significant difference in baseline cholesterol levels between the 2 groups. The baseline concentration of the biochemical values, maternal age and BMI at baseline were controlled for the analysis. After adjustments, no significant differences in the results were identified.) |
Badehnoosh 2018.
| Study characteristics | ||
| Methods | Parallel randomised control trial | |
| Participants | Pregnant women with GDM (n = 60) Setting Akbarabadi Clinic in Tehran, Iran Dates of Study April to September 2016 Inclusion criteria Pregnant women, primigravidae, age 18‐40 years, with GDM by the 1‐step 2‐hour 75 g OGTT with GDM diagnosed according to the American Diabetes Association guidelines (FPG ≥ 92 mg/dL, 1‐hour OGTT ≥ 180 mg/dL and 2‐hour OGTT ≥ 153 mg/dL). Exclusion criteria Placenta abruption, pre‐eclampsia, eclampsia, hypo‐ and hyperthyroidism, urinary tract infection, smokers, those with kidney or liver diseases and required commencing insulin therapy during the intervention. Taking any probiotic products including probiotic yogurt and kefir during the trial. |
|
| Interventions |
Probiotics: probiotic supplements 6 weeks containing Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2 x 109 CFU/g each) strains (n = 30) Placebo: capsules containing starch (n = 30). |
|
| Outcomes | hs‐CRP, FPG, NO, MDA, TAC GSH, MDA/TAC ratio, caesarean sections, preterm delivery, need for insulin intervention after intervention, pre‐eclampsia, polyhydramnios, maternal hospitalisation, macrosomia, gestational age, newborn: weight, length, head circumference, LGA, 1‐ and 5‐minute Apgar score, hyperbilirubinaemia, hospitalisations, hypoglycaemia | |
| Notes |
Funding: grant from the Vice Chancellor for Research, IUMS, Tehran, Iran. Conflicts of interest: authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation assignment was carried out using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | The randomised allocation sequence, enrolling patients and allocating them to interventions were done by a trained midwife at the gynaecology clinic. Randomisation and allocation were concealed from the researchers and participants until the final analyses were completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Researchers and participants were blinded by the use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 women randomised. Total 60 women analysed. All participants completed the study, none lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | All groups were balanced at the baseline |
Hajifaraji 2017.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled randomised trial | |
| Participants | Pregnant women with GDM (n = 64) Setting Gynecology and Endocrinology clinics at Tabriz Al‐Zahra referral University hospital, Tabriz, Iran Dates of study Spring and summer 2014 Inclusion criteria Nulliparity, newly diagnosed gestational diabetes at 24 and 28 weeks + 6 days, GDM diagnosed by a fasting BG of 92 mg/dL to125 mg/dL at time of diagnosis. Age 18 to 45 years, with BMI ≥ 18.5 kg/m2. No history of IGT in early pregnancy, T2DM, chronic diseases, smoking, alcohol, ingestion of probiotics (including yoghurt, kefir, other fermented food) within 2 weeks of intervention, or antibiotic use 1 month prior to intervention. No acute GI problems for a month prior to intervention. Exclusion criteria Needing insulin therapy or other medications (fasting BG > 105 and BG postprandial > 120 mg/dL) during intervention. Ingestion of other forms of probiotics (yoghurt, kefir, fermented foods), antibiotics, glucocorticoids, immunosuppressive drugs, having acute GI problems, and not taking adequate number of tablets. |
|
| Interventions |
Probiotic: capsules (4Biocap) containing 180 mg (> 4 x 109 CFU) standard power including freeze dried cultures of Lactobacillus acidophilusLA‐5, Bifibacterium BB‐12,Streptococcus thermophiles STY‐31, and Lactobacillus delbrueckii bulgaricus LBY‐27 + dextrose anhydrate filler and magnesium stearate lubricant (n = 32). Placebo: capsules similar design, shape and colour to the probiotics (n = 32). |
|
| Outcomes | Systolic and diastolic BP changes from baseline, to 2 weeks, 4 weeks, 6 weeks and 8 weeks after recruitment. Serum inflammatory markers: serum hs‐CRP, lnTNF‐α, Serum IL‐6. Oxidative stress markers: serum TAC, serum MDA, serum GSHR, erythrocyte GPX, serum uric acid, erythrocyte SOD. Weight changes, FPG, serum insulin levels, HOMA‐IR, QUICKI index |
|
| Notes |
Funding: Tehran, Shahid Beheshti University Medical Sciences. Tehran Darou pharmaceuticals, Tehran, Iran – provided the 4‐Biocap (probiotic supplement). Conflicts of interest: authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. To confirm double‐blinding, a coder secretly labelled the capsule packages A or B. |
| Allocation concealment (selection bias) | Unclear risk | All 64 females were allocated using block randomisation techniques to either probiotic or placebo; it is unclear whether the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 64 women who were randomised, 56 were analysed in the final analysis. Reasons for discontinuation: probiotic arm, (3 loss to follow‐up); declined to continue (1); needing drug therapy (2): n = 29 (91%). In the placebo arm: (5 loss to follow‐up); declined to continue (2), preterm pregnancy (1), needing drug therapy (2). |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | Baseline characteristics were balanced across groups. |
Jafarnejad 2016.
| Study characteristics | ||
| Methods | Parallel randomised control trial (Block randomisation) | |
| Participants | Pregnant women with GDM (n = 82) Setting: Tuba Endocrine clinic (Sari, Iran) Dates of study May 2014 to October 2015 Inclusion criteria Pregnant women diagnosed with GDM diagnosed by 2‐hour, 75 g OGTT: fasting venous plasma glucose level, 5.5mmol⋅L−1 or higher or 75‐g OGTT 2‐hour venous plasma glucose level, 8.0 mmol⋅L−1 or higher Exclusion criteria Unwillingness to follow a prescribed diet, pre‐GDM (either type 1 or type 2 DM), the need for insulin treatment, and pregnancy co‐morbidities other than obesity, hypertension, and/or dyslipidaemia |
|
| Interventions |
Probiotics: VSL#3 is a freeze‐dried pharmaceutical probiotic preparation containing 112.5 × 109 CFU/capsule of 8 strains of lactic acid bacteria (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. Bulgaricus), microcrystalline cellulose, stearic acid, magnesium stearate, and vegetable capsule (hydroxypropyl methylcellulose), silicon dioxide (n = 41). Placebo: capsules containing 40mg microcrystalline cellulose; placebo boxes had identical appearances (n = 41) |
|
| Outcomes | FPG, HbA1c, HOMA‐IR, insulin levels, IL‐6, IL‐10, TNF‐α, hs‐CRP, IFN‐ɣ | |
| Notes |
Funding: no detail Conflicts of interest: authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation performed by trained personnel at the endocrine clinic. |
| Allocation concealment (selection bias) | Unclear risk | Allocation of the intervention or control group was concealed from the researchers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the participants nor the investigators were aware of the treatment assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Allocation blinded from the researchers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82 women randomised. 4 women excluded from probiotic group (2 women needed insulin and 2 birthed prematurely < 35 weeks); 6 women in the placebo group were excluded (5 needed insulin treatment and 1 birthed prematurely). |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | Groups were balanced at the baseline |
Karamali 2016.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial | |
| Participants | Pregnant women with GDM (n = 60) Dates of study November 2015 and January 2016 Setting Kosar Clinicin Arak, Iran Inclusion criteria Pregnant women with GDM diagnosed by 1 step 75 OGTT based on ADA criteria of FPG ≥ 92 mg/dL (5.1 mmol); 1‐hour OGTT ≥ 180 mg/dL (10 mmol); or 2‐hour OGTT ≥ 153 mg/dL (8.5 mmol) Exclusion criteria Women with: preterm premature rupture of membranes, placental abruption, pre‐eclampsia, eclampsia, hypo or hyperthyroidism, a history of T2DM, a family history of GDM, smokers, and those with kidney or liver disease, or taking probiotics, antibiotics or glucocorticoids, or requiring insulin therapy. |
|
| Interventions |
Probiotics: group took a daily capsule that contained 3 viable freeze‐dried strains: Lactobacillus acidophilus (2×109 CFU/g), L. casei (2×109 CFU/g) and Bifidobacterium bifidum (2×109 CFU/g) for 6 weeks (n = 30). Placebo: group took daily capsules filled with cellulose and indistinguishable in colour, shape, size and packaging, as well as in smell and taste, from the probiotic capsules (n = 30). |
|
| Outcomes | FPG, serum insulin levels, HOMAR‐IR, and HOMAR B cell function (only for unadjusted results, when results adjusted for baseline differences between groups there was no significant different in HOMAR B), insulin sensitivity check index, serum triglycerides and VLDL cholesterol concentrations. | |
| Notes |
Funding: grant from the Vice Chancellor for Research, IUMS, Tehran, Iran. All capsules were produced by the Tak Gen Zist Pharmaceutical Company in Tehran, Iran, and approved by the Food and Drug Administration. Conflicts of interest: authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment of the participants was conducted using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocations were concealed from the researchers and participants until the final analyses were completed". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The trial was blinded with placebo capsules were indistinguishable in colour, shape, size and packaging, as well as in smell and taste, from the probiotic capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 women randomised. 3 women in the placebo group were lost to follow‐up (withdrew for personal reasons). None of the participants in the intervention arm were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | Significant differences in baseline levels of FPG and HDL cholesterol between the 2 groups, but after further adjusting these variables as well as for baseline maternal age and BMI. the results were similar in both groups except for HOMA‐B (P = 0.08). |
Karamali 2018.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | Pregnant women with GDM (n = 60) Setting Akbarabadi clinic affiliated to Iran University of Medical Sciences (IUMS), Tehran, Iran. Dates of Study April 2016 and December 2016. Inclusion criteria Pregnant women diagnosed with GDM using the ADA guidelines, aged 18‐40 years (n = 30). Exclusion criteria Pre‐eclampsia, eclampsia, hypo‐ and hyperthyroidism, smokers and those with kidney or liver diseases and required commencing insulin therapy during intervention; taking any probiotic and/or synbiotic products including probiotic yogurt and kefir during the trial (n = 30). |
|
| Interventions | Synbiotic capsule containing Lactobacillus acidophilus strain T16 (IBRC‐M10785), L. casei strain T2 (IBRC‐M10783) and Bifidobacterium bifidum strain T1 (IBRC‐M10771) (2 × 109 CFU/g each) plus 800 mg inulin (HPX) or placebo for 6 weeks. | |
| Outcomes | Primary outcomes: inflammatory markers. Secondary outcomes: biomarkers of oxidative stress and pregnancy outcomes. |
|
| Notes |
Funding: funded by a grant from the Vice Chancellor for Research, IUMS, Tehran, Iran Conflicts of interest: authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if researchers were aware of the upcoming allocation when recruiting participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial in the text |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up and all participant data were analysed. |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | None |
Kijmanawat 2019.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Pregnant women with GDM (n = 60) Setting Antenatal Care Clinic, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand Dates of Study July 2016 and February 2017. Inclusion criteria Pregnant women with GDM, between 24‐28 weeks' gestation, age 18 to 45 years. GDM by the International Association of Diabetes and Pregnancy Study Groups criteria as follows: FPG ≥ 92 mg/dL at the first prenatal visit, or an abnormal glucose tolerance test at 24–28 weeks‐of‐gestation using a 75‐g oral glucose load (defined as 1 or more of the following abnormal glucose values: FPG ≥ 92 mg/dL, 1‐hour ≥ 180 mg/dL, 2‐hour ≥ 153 mg/dL). Exclusion criteria Fetal or chromosomal abnormalities, chronic diseases (such as immunodeficiency, hypertension, pre‐gestational diabetes, kidney disease or liver disease). Consumption of probiotic food products, such as yogurt, fermented foods and bean paste during the 2 weeks before enrolment; exposure to antibiotics during the 4 weeks before enrolment. |
|
| Interventions | Probiotic capsule (Infloran) contained 1,000 million CFU of Lactobacillus acidophilus and 1,000 million CFU of Bifidobacterium bifidum (n = 30) Placebo capsule contained gelatin (n = 30) |
|
| Outcomes | Primary outcome: mean differences in FPG, insulin and insulin resistance index. Secondary outcomes: mean difference in maternal weight gain across the study period. |
|
| Notes |
Funders The Thailand Research Fund (TRF). Conflict of interest SR receives grant support from Merck Sharp and Dohme, research equipment support from ResMed, and speaker honoraria from Sanofi, Novo Nordisk and Medtronic. Other authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence was carried out in blocks of 4 by the statistician. |
| Allocation concealment (selection bias) | Unclear risk | Researchers arranged the enrolment and intervention assignment of participants. Unclear if they knew. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The capsules and their packages were unidentified to participants, researchers and primary investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 women were randomised. Discontinued from the probiotic group (2): diagnosis of systemic lupus erythematosus requiring steroid treatment (1), antibiotic use during the study period (1). Discontinued from the placebo group: (1) due to a subsequent diagnosis of systemic lupus erythematosus requiring steroid treatment. 57/60 (95%) participants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | Baseline characteristics were balanced between both groups. |
Lindsay 2015.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial | |
| Participants | Pregnant women with GDM (n = 149). Setting National Maternity Hospital, Dublin, Ireland Dates of Study March 2012 to May 2014. Final birth July 2014 Inclusion criteria Pregnant women attending the National Maternity Hospital who were newly diagnosed with either IGT (1 raised value) or GDM (2 raised values) following a 3‐hour 100‐g OGTT in the current pregnancy, age > 18 years, < 34 weeks’ gestation, singleton pregnancy and adequate English Exclusion criteria Pregestational diabetes, were aged < 18 years, were 34 weeks’ gestation, had a multiple pregnancy or fetal anomaly, were commenced on insulin or metformin therapy immediately after diagnosis, or had a poor understanding of the English language |
|
| Interventions | Probiotic: capsule contained 100 mg of Lactobacillus salivarius UCC118 at a target dose of 109 CFU (n = 74). Placebo: identical in appearance to probiotics (n = 75). |
|
| Outcomes | Maternal outcomes: FPG, C‐peptide, triglycerides; requirement for pharmacological
therapy, Total, LDL, and HDL cholesterol, insulin, and triglycerides, HOMAR‐IR, gestational weight gain, hypertension, delivery complications Neonatal/infant outcomes: birthweight, glucose, c‐peptide, lipids, 5‐minute Apgar score, and admission to NICU |
|
| Notes |
Funding: National Maternity Hospital Medical Fund with support from the Ivo Drury Award and the European Union’s Seventh Framework Program (FP7/2007‐2013), project Early Nutrition under grant agreement number 289346. Conflicts of interest: F.S. is a shareholder in Alimentary Health Ltd and has received grants from GlaxoSmithKline and the Procter and Gamble Company in the past. The remaining authors report no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated simple randomisation process in a ratio of 1:1. |
| Allocation concealment (selection bias) | Low risk | Allocation to either 1 of the capsules was conducted by an independent researcher. The allocation sequence was sequentially numbered in sealed, opaque envelopes and was concealed from the research dietitian enrolling and assessing the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probiotics and placebo identical capsules were supplied and researchers and participants were unaware of the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinical and laboratory staff who were involved with care of study participants or analysis of samples remained blinded to the allocation sequence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 149 women randomised. In the probiotic arm, 9 women were lost to follow‐up, 4 discontinued intervention when they changed their minds. In the placebo arm, 9 women were lost to follow‐up and 4 changes their minds. 79 were analysed |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | There was a slightly lower rate of Caucasian ethnicity and obesity and a higher rate of primiparity in the probiotic compared to placebo group but these differences were not significant. Otherwise the groups were balanced at the baseline |
Nabhani 2018.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants | Pregnant women with GDM (n = 95). Setting Diabetes East Health Centre, Ahwaz, Iran Dates of study January 2015 and September 2016 Inclusion criteria Pregnant women aged 18‐40 years, diagnosed with GDM according to Diabetes Association guideline criteria, by a 1‐step 2‐hour, 75 g OGT at 24‐28 weeks' gestation. (Fasting ≥ 92 mg/dL, 1 hour ≥ 180 mg/dL, 2 hour ≥ 153 mg/dL.) Exclusion criteria Use of anti‐diabetic drugs, (metformin, insulin) Current smokers, placenta abruption, pre‐eclampsia, eclampsia, hypo/hyperthyroidism, kidney, liver, inflammatory or immunodeficiency diseases, thyroid disorders, lactose intolerant. Also using any kinds of oestrogen, progesterone, cholesterol‐lowering drugs or diuretics; consuming any type of probiotics in the past 1 month prior to GDM diagnosis. Also if currently taking probiotic food or supplements during study period, taking antibiotics or glucocorticoids. |
|
| Interventions | Synbiotic capsule (LactoFem) contained 500 mg of Lactobacillus probiotic strains consisting of: L. acidophilus (5 x 1010 CFU/g), L. plantarum (1.5 x 1010 CFU/g), L. fermentum (7 x 109 CFU/g), L. gasseri (2 x 1010 CFU/g) and 38.5 mg of FOS as prebiotic substance. Other components included lactose (300 mg), magnesium stearate, talc, colloidal silicon dioxide (each of them 5.5 mg), flavourings and sweeteners that have neutral effects (n = 48). Placebo capsules contained similar as above: lactose (300 mg), magnesium stearate, talc, colloidal silicon dioxide (each of them 5.5 mg), flavourings and sweeteners that have neutral effects) without the probiotics. The appearance, texture, weight and smell of capsules and packages were identical for both synbiotics and placebo and they were only different in their codes A or B that were placed on their packs (n = 47). |
|
| Outcomes | Biochemical factors of FPG, serum insulin, HOMA‐IR, quantitative insulin sensitivity check index (QUICKI), lipid profile (LDL‐C, HDL‐C, TG) and TC, TAC and blood pressure indices. | |
| Notes |
Source of funding Tabriz University of Medical Sciences, Tabriz, Iran and Nutrition Research Center. (LactoFem) were produced by ZistTakhmir Pharmaceutical Company in Tehran, Iran, and registered at the Food and Drug Administration. Conflicts of interest: No conflicts of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence by random allocation software. Computer block randomisation |
| Allocation concealment (selection bias) | Unclear risk | All researchers were unaware of the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The appearance, texture, weight and smell of capsules and packages were identical for both synbiotics and placebo and they were only different in their codes A or B that were placed on their packs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All researchers were blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Synbiotic: discontinued n = 3 (1 preterm labour before 3 weeks, 1 change of mind, 1 did not consume supplements according to plan). Placebo group – discontinued intervention n = 2 (1 changed mind, 1 preterm labour before 35 weeks) |
| Selective reporting (reporting bias) | Unclear risk | No retrospective trial registration. Insufficient information to judge if pre‐specified outcomes are reported in full. |
| Other bias | Low risk | Analysis of dietary intakes showed that there were no significant differences between the 2 groups for the macro‐ and micronutrient intakes, except for the energy, protein and total fat intakes (P < 0.05); thus, final analyses were adjusted for the measures of energy intake, BMI and baseline values. |
BG: blood glucose;BMI: body mass index; BP: blood pressure; CFU: colony‐forming units; CRP: C‐reactive protein; erythrocyte GPX: red blood cell glutathione peroxidase; erythrocyte SOD: red blood cell superoxide dismutase; Fe: iron; FOS: Fructo‐oligosaccharides; FPG: fasting plasma glucose; GDM: gestational diabetes mellitus; GI: gastrointestinal; GSH: glutathione; HDL: high‐density lipoprotein; HOMA: Homeostasis Model Assessment; IGT: impaired glucose tolerance; IL‐6: interleukin 6;LGA: large‐for‐gestational age; MDA: malondialdehyde; MET: metabolic equivalents; MFA: monounsaturated fatty acids; Mg: magnesium; NICU: neonatal intensive care unit; NO: nitric oxide; LDL: low‐density lipoprotein; OGTT: oral glucose tolerance test; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids; TAC: total antioxidant capacity; very low‐density lipoprotein; TAG: triglycerides; TNF: tumour necrosis factor; ZN: zinc.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Dughaishi 2016 | Not an RCT |
| Asemi 2013 | RCT not in GDM women |
| Asemi 2013a | RCT in T2DM |
| Barrett 2012 | Systematic Review of probiotics for preventing GDM |
| Barrett 2014 | Cochrane review on probiotics for preventing GDM |
| Barthow 2016 | RCT not in GDM women |
| Fei 2014 | RCT using a prebiotic soybean oligosaccharide. |
| Gomez 2015 | Not an RCT |
| Hamano 2013 | Non‐blinded study comparing probiotic yoghurt and standard diet. |
| Lindsay 2013 | Observational study |
| Lindsay 2014 | RCT of probiotics in women with obesity not GDM |
| Luoto 2010 | RCT of probiotics but not in GDM women |
| Muktabhant 2015 | Cochrane Review on Diet or exercise not GDM women |
| Nitert 2013 | SPRING RCT in overweight and obese and not in women with GDM |
| Okesene‐Gafa 2018 | RCT not in women with GDM |
| Wickens 2017 | Probiotics not in women with GDM to see if they reduce prevalence of GDM |
| Zhang 2018 | RCT (3 arms) using prebiotics (Xylooligosaccharide) and not probiotics |
GDM: gestational diabetes mellitus; RCT: randomised controlled trial; T2DM: type 2 diabetes.
Characteristics of studies awaiting classification [ordered by study ID]
Gonai 2014.
| Methods | Randomised trial |
| Participants | Pregnant women with GDM Setting Japan (Kawasaki) Dates of Study Not defined Inclusion criteria Pregnant Japanese women age > 40 years, plasma glucose > 140 mg/dL, past GDM history, BMI > 30 and past delivery of macrosomia. Exclusion criteria Not stated (appears pre‐existing diabetes excluded) |
| Interventions |
Probiotics (lactobacilli GG yoghurt) plus dietary intervention Standard care: regular dietary intervention |
| Outcomes | Fasting plasma glucose and glucagon like peptide‐1 (GLP‐1) just prior to delivery and OGTT 2 months after delivery. |
| Notes | Hypothesis: dietary intervention like lactobacillus yoghurt may improve GDM patients' metabolic status or newborn outcomes through. |
Jamilian 2019.
| Methods | Randomised trial |
| Participants | Pregnant women with GDM Setting Iran Dates of Study Inclusion criteria Pregnant women, age 18‐40 years, at 24‐28 weeks of gestation, diagnosed with GDM by a 2‐hour 75g OGTT, based on the American Diabetes Association guidelines. Exclusion criteria Intake of probiotics or synbiotics, vitamin D supplements anytime during the last three months prior to the intervention; requiring insulin treatment, smoker, hyperthyroidism, pre‐eclampsia, eclampsia, hypo‐ and hyperthyroidism. |
| Interventions |
Vitamin D + probiotics: 50,000 IU vitamin D3 plus probiotics containing (8x109 CFU/g) Lactobacillus acidophilus, Bi‐dobacterium bidum, L. reuteri, and Lactobacillus fermentum Probiotics: Receive one probiotic per day containing (8 x 109 CFU/g) Lactobacillus acidophilus, Bi‐dobacterium bidum, L. reuteri, and Lactobacillus fermentum. Placebo: paraffin (as placebo for vitamin D) and starch (placebo for probiotics). These were identical in appearance (size, shape, colour), taste and smell. |
| Outcomes | Primary outcomes: markers of insulin metabolism Secondary outcomes: lipid profiles; biomarkers of inflammation and oxidative stress. (Serum hydroxyvitamin D, serum insulin concentrations, HOMA‐IR, Quantitative insulin sensitivity index (QUICKI), fasting plasma glucose, serum triglycerides, VLDV, LDL, HDL‐cholesterol, hs‐CRP, Nitric oxide (NO), total antioxidant capacity (TAC), total glutathione, malondialdehyde (MDA) concentrations). Clinical outcomes: polyhydramnios, preterm birth (birth < 37 weeks). Newborn outcome: hyperbilirubinaemia. |
| Notes | Aims: to assess effects of vitamin D together with probiotics (8 x 109 CFU/day) compared to placebo on markers of metabolism and pregnancy outcomes of women with GDM. Total treatment time of six weeks. |
CFU: colony‐forming units; BMI: body mass index; GDM: gestational diabetes mellitus; HOMA: Homeostasis Model Assessment;OGTT: oral glucose tolerance test..
Characteristics of ongoing studies [ordered by study ID]
Asemi 2019.
| Study name | The effects of selenium and probiotics co‐supplementation on pregnancy outcomes, inflammatory factors, oxidative stress biomarkers and gene expression related to inflammation in women with gestational diabetes |
| Methods | Randomised double‐blind placebo‐controlled trial. |
| Participants | Pregnant women with GDM, aged 18‐40 years, referred to Kosar Clinic affiliated to Arak University of Medical Sciences. Exclusion: overt diabetes mellitus. Taking any supplements before the intervention. Unwillingness to cooperate. |
| Interventions | Combined probiotics Lactobacillus acidophilus, Bifidobacterium Bifidum and Bifidobacterium langum (all 2 x 109) daily and selenium supplements (Webber Naturals Canada) 200 μg orally daily for 6 weeks. Other group receives placebo (Barij Essence, Kashan, Iran) daily orally for 6 weeks. |
| Outcomes | Primary outcomes: markers of insulin metabolism Secondary outcomes: lipid profiles, gene expression related to insulin and lipids. |
| Starting date | Recruitment dates: no information. |
| Contact information | Contact Zatollah Asemi, asemi_z@kaums.ac.ir |
| Notes | Title: The effects of selenium and probiotics co‐supplementation on pregnancy outcomes, inflammatory factors, oxidative stress biomarkers and gene expression related to inflammation in women with gestational diabetes. Total sample size: 60. IRCT registration number: IRCT20170513033941N55 |
CTRI/2018/08/015432.
| Study name | Effect of probiotic supplementation on blood glucose of gestational diabetic mellitus (GDM) mothers |
| Methods | Double‐blind randomised trial. |
| Participants | Pregnant women, primigravidae with a singleton pregnancy, with a normal fetal scan, at 12‐14 weeks' gestation, with an OGTT confirming GDM as per respective O&G consultants. Exclusion: pregnant GDM mothers with gestational age > 28 weeks, pre‐GDM, medications that influence insulin resistance (glucocorticoids, immunosuppressants), taking any form of probiotics < 1 month before recruitment. |
| Interventions | Receive 1 probiotic capsule a day that contains recommended strains of bacteria from the onset of GDM Routine care without probiotics receive counselling session on diet and physical activity interventions for pregnant women with GDM. Trained dietitians would give the counselling sessions on modified diet with portions and size; printed educational materials that included common diet guidelines and physical activity information on GDM. In the postnatal period, they will receive session on importance of compliance of lifestyle modifications such as diet and physical activity after delivery. |
| Outcomes | Primary outcomes: blood glucose levels (fasting and postprandial), urine analysis for pus cells. Secondary outcomes: maternal: mode of delivery, preterm delivery, perineal tear, pregnancy‐induced hypertension, hydramnios, vaginal infections. Neonatal/infant: birthweight, congenital malformations, birth injuries, shoulder dystocia, hypoglycaemia, NICU admissions. |
| Starting date | |
| Contact information | |
| Notes | Title: The effect of maternal probiotic supplementation on glycaemic control and pregnancy outcomes among GDM. Total sample size: 202. CTRI/2018/08/015432. Kavitha Ramanathan: Study not yet recruiting. Locality Chennai, India. |
IRCT20121224011862N2.
| Study name | The effect of probiotic yogurt and conventional yogurt in blood glucose control and outcomes of pregnancy in women with gestational diabetes |
| Methods | Double‐blind randomised clinical trial |
| Participants | Pregnant women with gestational diabetes 24‐28 weeks' gestation with a singleton. Exclusion criteria: history of diabetes in previous pregnancy or fetal abnormality. |
| Interventions | Intervention group will consume probiotic yoghurt containing 106colony of Lactobacillus acidophilus and Bifidobacterium lactis for 8 weeks. Control group will consume 300 g/day of conventional yoghurt. |
| Outcomes | HBA1c and fasting blood glucose before and after 8 weeks. |
| Starting date | |
| Contact information | Contact: Farnaz Sahaf email: sahaf@tbzmed.ac.ir |
| Notes | The effect of probiotic yogurt and conventional yogurt in blood glucose control and outcomes of pregnancy in women with gestational diabetes. Recruitment centres: Alzahra Hospital, South Artesh Street, East Azarbaijan, Iran and Taleghani Hospital, Rah Ahan, Tabriz, East Azerbaijan Province, Iran. Funding: Tabriz University of Medical Sciences IRCT registration number: IRCT20121224011862N2 Registration date: 2018‐05‐19, 1397/02/29 Registration timing: registered_while_recruiting Last update: 2018‐05‐19, 1397/02/29 Current status: recruiting |
IRCT2015110310089N4.
| Study name | Comparison of probiotic capsules and placebo on fasting blood sugar and metabolic parameters in pregnant woman with gestational diabetes or impaired glucose tolerance test |
| Methods | Randomised controlled double‐blind trial |
| Participants | Pregnant women with GDM 18‐49 years (GDM diagnosed by a 3‐hourOGTT based on carpenter criteria by internist). Inclusions criteria: single pregnancy; new diagnosis of IGT or GDM in the current pregnancy; < 34 weeks’ gestation; without insulin or metformin therapy. Exclusions criteria: "pre‐gestational diabetes; fetal anomaly; without follow; do not use probiotics" Locality: prenatal clinic in Al‐Zahra hospital of Guilan University of Medical Sciences. |
| Interventions | Probiotics with brand Lactofem |
| Outcomes | Primary outcomes: probiotics user, placebo user. Secondary outcomes: fasting blood glucose, triglyceride, total cholesterol, low density lipoprotein, high density lipoprotein, blood pressure. |
| Starting date | Expected starting date 22.12.2015, expected completion date 21.12.2016. Currently no information. |
| Contact information | Contact: Arezoo Fani, email: arezoo.fani@yahoo.com |
| Notes | IRCT registration number: IRCT2015110310089N4 Registration date: 2016‐02‐01, 1394/11/12 Registration timing: registered while recruiting |
IRCT20171010036697N1.
| Study name | The effect of probiotic supplementation on gene expression related to insulin, lipid and inflammation in patients with gestational diabetes |
| Methods | Double‐blind randomised placebo‐controlled clinical trial |
| Participants | Pregnant women 18 to 40 years with gestational diabetes Exclusion: unwillingness to cooperate |
| Interventions | Intervention group: probiotic supplements containing 4 strains ofLactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2×109 CFU/g) and Bifidobacterium bifidum (2 × 109 CFU/g), Lactobacillus fermentum (2 × 109 CFU/g), daily, for 6 weeks orally. Control group: placebo, daily, for 6 weeks orally. |
| Outcomes | Primary outcomes at baseline and 6 weeks after intervention, PCR measurements for: expressed levels of GLUT‐1 gene, PPAR‐? gene. Secondary outcomes at baseline and 6 weeks post intervention: expressed levels LDLR gene, IL‐1 gene, TNF‐a gene, LPa gene and IL‐8 gene. |
| Starting date | |
| Contact information | |
| Notes | Funding: Vice Chancellor for research, Kashan University of Medical Sciences |
Nachum 2019.
| Study name | The effect of oral probiotics on glycaemic control of women with gestational diabetes mellitus |
| Methods | Randomised, double‐blind, placebo‐controlled trial, phase 4. |
| Participants | Inclusion: pregnant women diagnosed with GDM from 13 to 32.6 weeks, > 18 years old, singleton pregnancy. |
| Interventions | Dietary supplement (Femina II) 2 capsules per day until delivery Placebo: 2 capsules per day until delivery. |
| Outcomes | Primary outcomes: the rate of women who will require pharmacotherapy for glycaemic control. The mean value of the daily glucose charts after 2 weeks of treatment with the study products. Secondary outcomes: the rate of women with controlled diabetes; mean daily glucose charts; mean daily pre‐prandial glucose levels; mean daily postprandial glucose levels; level of glycated molecules; rate of: women with mean pre‐prandial values ≥ 95 mg/dL, mean post‐prandial values ≥ 130 mg/dL, and mean daily glucose > 100 mg/dL; caesareans; labour inductions; birthweight > 4000 g/> 90th percentile; admission to NICU; 1‐minute and 5‐minute Apgar score; neonatal hypoglycaemia; neonatal hypomagnesaemia; cord blood PH levels; neonatal malformations and developmental disorders; birthweight; head circumference; maternal adverse effects; duration of time until pharmacotherapy for glycaemic control is indicated. |
| Starting date | Not yet recruiting |
| Contact information | Zohar Nachum; nachum_zo@clalit.org.il |
| Notes | Objectives: to examine the effect of a mixture of probiotic strains given daily on maternal glycaemic parameters, and pregnancy outcomes among women with GDM. |
CFU: colony‐forming units; GDM: gestational diabetic mellitus; IGT: impaired glucose tolerance; NICU: neonatal intensive care unit;OGTT: oral glucose tolerance test; PCR: polymerase chain reaction.
Differences between protocol and review
The following differences exist between the published protocol for this review (Okesene‐Gafa 2018) and the full review.
We changed the title from 'probiotics for treating women with gestational diabetes for improving maternal and fetal health and well‐being' to 'probiotic treatment for women with gestational diabetes to improve maternal and infant health and well‐being'.
Contributions of authors
Karaponi Okesene‐Gafa prepared the original draft of this review protocol and also carried out the analysis for this review. Abigail Moore assisted with checking data entry. Vanessa Jordan assisted with the analysis and provided technical advice. Caroline Crowther and Lesley McCowan provided input and feedback for this review.
Sources of support
Internal sources
-
Department of Obstetrics and Gynaecology, University of Auckland, New Zealand
Funding for Dr Okesene‐Gafa's PhD with this review as part of her thesis.
External sources
-
Liggins Institute, University of Auckland, New Zealand
Infrastructure support for Cochrane authors has been provided by the Liggins Institute, University of Auckland.
-
Cochrane Pregnancy and Chidbirth Australasian Satellite, New Zealand
We acknowledge support from the Pregnancy and Childbirth Australian Satellite
Declarations of interest
Karaponi Okesene‐Gafa ‐ was recently involved with the Healthy Mums and Babies (HUMBA) randomised controlled demonstration trial, which has now been completed and published. The HUMBA RCT is not be eligible for inclusion in this review. In kind we have been provided with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12) and placebo capsules free of charge from Christian Hansen Denmark (http://www.chr‐hansen.com/en) for our HUMBA trial (http://humba.ac.nz/). In this randomised controlled double‐blind trial, women were randomised to receive probiotics or placebo capsules with the main aim of reducing pregnancy weight gain and infant birthweight (ANZCTR registration number 12615000400561). In kind: Roche International‐ equipment and consumables for HBA1c for the HUMBA trial. National Heart Foundation assisted by letting us use some of their resources for the study and development of some of the content in the text messages as part of HUMBA trial. Public interest funding for the Healthy Mums and Babies (HUMBA) trial was received from Cure Kids, Counties Manukau Health, Mercia Barnes Trust Fund, University of Auckland Faculty Research Development Fund and Lottery Health Research Fund. Karaponi Okesene‐Gafa received funding from her organisation (Department of Obstetrics and Gynaecology, University of Auckland) for her PhD, with this review forming part of her thesis.
Abigail Moore is a medical student and declares no conflict of interest.
Vanessa Jordan is a Research Fellow in the University of Auckland and declares no conflict of interest.
Lesley McCowan ‐ was also involved with the Healthy Mums and Babies (HUMBA) randomised controlled demonstration trial. The HUMBA RCT is not be eligible for inclusion in this review. In kind we have been provided with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12) and placebo capsules free of charge from Christian Hansen Denmark (http://www.chr‐hansen.com/en) for our HUMBA trial (http://humba.ac.nz/). In this randomised controlled double‐blind trial, women are randomised to receive probiotics or placebo capsules with the main aim of reducing pregnancy weight gain and infant birthweight (ANZCTR registration number 12615000400561). In kind: Roche International‐ equipment and consumables for HBA1c for the HUMBA trial. National Heart Foundation assisted by letting us use some of their resources for the study and development of some of the content in the text messages as part of HUMBA trial.
Caroline Crowther ‐ was also involved with the Healthy Mums and Babies (HUMBA) randomised controlled demonstration trial. The HUMBA RCT is not be eligible for inclusion in this review. In kind we have been provided with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12) and placebo capsules free of charge from Christian Hansen Denmark (http://www.chr‐hansen.com/en) for our HUMBA trial (http://humba.ac.nz/). In this randomised controlled double‐blind trial, women are randomised to receive probiotics or placebo capsules with the main aim of reducing pregnancy weight gain and infant birthweight (ANZCTR registration number 12615000400561). In kind: Roche International‐ equipment and consumables for HBA1c for the HUMBA trial. National Heart Foundation assisted by letting us use some of their resources for the study and development of some of the content in the text messages as part of HUMBA trial.
New
References
References to studies included in this review
Ahmadi 2016 {published data only}
- Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. British Journal of Nutrition 2016;116(8):1394-401. [DOI] [PubMed] [Google Scholar]
- Anonymous. Corrigendum: the effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomized, double-blind, placebo-controlled trial (British Journal of Nutrition (2016) DOI: 10.1017/S0007114516003457). British Journal of Nutrition 2016;116(11):1998. [CENTRAL: CN-01298950] [EMBASE: 613758353] [DOI] [PubMed]
- Asemi Z, IRCT201605085623N77. Clinical trial of the effect of synbiotic supplementation compared with the placebo on metabolic profiles in patients with gestational diabetes. http://en.irct.ir/trial/6107 (first received 11 May 2016).
Badehnoosh 2018 {published data only}
- Asemi Z, IRCT201611115623N91. Clinical trial of the effect of probiotic supplementation compared with the placebo on pregnancy outcomes in women with gestational diabetes. http://en.irct.ir/trial/6121 (first received 23 November 2016).
- Badehnoosh B, Karamali M, Zarrati M, Jamilian M, Bahmani F, Tajabadi-Ebrahimi M, et al. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(9):1128-36. [DOI] [PubMed] [Google Scholar]
Hajifaraji 2017 {published data only}
- Dolatkhah N, Hajifaraji M, Abbasalizadeh F, Aghamohammadzadeh N, Mehrabi Y, Mesgari Abbasi M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. Journal of Health, Population, and Nutrition 2015;33(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajifaraji M, IRCT201405181597N3. Effect of probiotics in comparison with placebo on glucose metabolism, weight changes and inflammatory and oxidative stress indices in women with gestational diabetes mellitus. http://en.irct.ir/trial/1005 (first received 16 June 2014).
- Hajifaraji M, Jahanjou F, Abbasalizadeh F, Aghamohammadzadeh N, Abbasi MM, Dolatkhah N. Effect of probiotic supplementation on blood pressure of females with gestational diabetes mellitus: a randomized double blind controlled clinical trial. Iranian Red Crescent Medical Journal 2017;19(6):e55662. [Google Scholar]
- Hajifaraji M, Jahanjou F, Abbasalizadeh F, Aghamohammadzadeh N, Abbasi MM, Dolatkhah N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pacific Journal of Clinical Nutrition 2018;27(3):581-91. [DOI] [PubMed] [Google Scholar]
Jafarnejad 2016 {published data only}
- Jafarnejad S, Saremi S, Jafarnejad F, Arab A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: a randomized controlled clinical trial. Journal of Nutrition and Metabolism 2016;2016:5190846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karamali 2016 {published data only}
- Asemi Z, IRCT201601035623N63. Clinical trial of the effect of probiotic supplementation compared with the placebo on metabolic profiles in patients with gestational diabetes. http://en.irct.ir/trial/6093 (first received 3 January 2016).
- Karamali M, Dadkhah F, Sadrkhanlou M, Jamilian M, Ahmadi S, Tajabadi-Ebrahimi M, et al. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes & Metabolism 2016;42(4):234-41. [DOI] [PubMed] [Google Scholar]
Karamali 2018 {published data only}
- Asemi Z, IRCT201704205623N108. Clinical trial of the effect of synbiotic supplementation compared with the placebo on pregnancy outcomes in women with gestational diabetes. http://en.irct.ir/trial/6138 (first received 27 April 2017).
- Karamali M, Nasiri N, Taghavi Shavazi N, Jamilian M, Bahmani F, Tajabadi-Ebrahimi M, et al. The effects of synbiotic supplementation on pregnancy outcomes in gestational diabetes. Probiotics and Antimicrobial Proteins 2018;10(3):496-503. [DOI] [PubMed] [Google Scholar]
Kijmanawat 2019 {published data only}
- Kijmanawat A, Panburana P, Reutrakul S, Tangshewinsirikul C. The effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial. Journal of Diabetes Investigation 2019;10(1):163-70. [DOI] [PMC free article] [PubMed]
- Kijmanawat A, Panburana P, Reutrakul S, Tangshewinsirikul C. Erratum to: effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial (Journal of Diabetes Investigation, (2019), 10, 1, (163-170), 10.1111/jdi.12863) [Erratum to: Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial (Journal of Diabetes Investigation, (2019), 10, 1, (163-170), 10.1111/jdi.12863)]. Journal of Diabetes Investigation 2019;10(5):1388. [CENTRAL: CN-02003802] [EMBASE: 2002646319] [DOI] [PMC free article] [PubMed]
- Kijmanawat A, TCTR20170606002. Study of probiotic supplements to reduce insulin resistance in gestational diabetes mellitus. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2580 (first received 5 June 2017).
Lindsay 2015 {published data only}ISRCTN97241163
- Lindsay K, Brennan L, Kennelly M, Maguire O, Smith T, Curran S, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S22. [DOI] [PubMed] [Google Scholar]
- Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, Curran S, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. American Journal of Obstetrics & Gynecology 2015;212:496.e1-496.e.11. [DOI] [PubMed] [Google Scholar]
- McAuliffe F, ISRCTN97241163. A randomised controlled trial of probiotics in pregnancy to reduce maternal glucose in obese and gestational diabetic women. http://www.isrctn.com/ISRCTN97241163 (first received 5 February 2012).
Nabhani 2018 {published data only}
- Gargari BP, IRCT201511183140N16. Effect of synbiotic supplementation on lipid profile, glycemic control and hs-CRP in women with gestational diabetes mellitus. http://en.irct.ir/trial/3183 (first received 29 December 2015).
- Nabhani Z/Hezaveh SJ, Razmpoosh E, Asghari-Jafarabadi M, Gargari BP. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: a randomized double blind placebo controlled clinical trial. Diabetes Research and Clinical Practice 2018;138:149-57. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Dughaishi 2016 {published data only}
- Al-Dughaishi T, Nikolic D, Zadjali F, Al-Hashmi K, Al-Waili K, Rizzo M, et al. Nutraceuticals as lipid-lowering treatment in pregnancy and their effects on the metabolic syndrome. Current Pharmaceutical Biotechnology 2016;17(7):614-23. [DOI] [PubMed] [Google Scholar]
Asemi 2013 {published data only}
- Asemi Z, Samimi M, Tabassi Z, Naghibi Rad M, Rahimi Foroushani A, Khorammian H, et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. European Journal of Clinical Nutrition 2013;67(1):71-4. [DOI] [PubMed] [Google Scholar]
Asemi 2013a {published data only}
- Asemi Z, Zare Z, Shakeri H, Sabihi S, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Annals of Nutrition and Metabolism 2013;63(1-2):1-9. [DOI] [PubMed] [Google Scholar]
Barrett 2012 {published data only}
- Barrett H L, Callaway L K, Nitert M D. Probiotics: a potential role in the prevention of gestational diabetes? Acta Diabetologica 2012;49 Suppl 1:S1-13. [DOI: 10.1007/s00592-012-0444-8] [DOI] [PubMed] [Google Scholar]
Barrett 2014 {published data only}
- Barrett HL, Dekker Nitert M, Conwell LS, Callaway LK. Probiotics for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD009951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barthow 2016 {published data only}
- Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, et al. The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy & Childbirth 2016;16(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fei 2014 {published data only}
- Fei BB, Ling L, Hua C, Ren SY. Effects of soybean oligosaccharides on antioxidant enzyme activities and insulin resistance in pregnant women with gestational diabetes mellitus. Food Chemistry 2014;158:429-32. [DOI] [PubMed] [Google Scholar]
Gomez 2015 {published data only}
- Gomez Arango LF, Barrett HL, Callaway LK, Nitert MD. Probiotics and pregnancy. Current Diabetes Reports 2015;15(1):567. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hamano 2013 {published data only}
- Hamano K, UMIN000012416. The efficacy of the probiotics for gestational diabetes patients. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014534 (first received 1 December 2013).
Lindsay 2013 {published data only}
- Lindsay KL, Walsh CA, Brennan L, McAuliffe FM. Probiotics in pregnancy and maternal outcomes: a systematic review. Journal of Maternal-Fetal and Neonatal Medicine 2013;26(8):772-8. [DOI] [PubMed] [Google Scholar]
Lindsay 2014 {published data only}
- Lindsay K, Maguire O, Smith T, Brennan L, McAuliffe F. A randomized controlled trial of probiotics to reduce maternal glycaemia in obese pregnancy. American Journal of Obstetrics and Gynecology 2014;1:S342. [DOI] [PubMed] [Google Scholar]
Luoto 2010 {published data only}
- Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. British Journal of Nutrition 2010;103(12):1792-9. [DOI] [PubMed] [Google Scholar]
Muktabhant 2015 {published data only}
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD007145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nitert 2013 {published data only}
- Nitert MD, Barrett HL, Foxcroft K, Tremellen A, Wilkinson S, Lingwood B, et al. SPRING: an RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy & Childbirth 2013;13(11):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okesene‐Gafa 2018 {published data only}
- Okesene-Gafa K, McKinlay CJ, Li M, Taylor RS, Wall CR, Rush EC, et al. Dietary intervention and/or probiotic capsules in obese pregnant women-effect on gestational weight gain and infant birthweight: Healthy mums and babies (HUMBA) trial. Journal of Paediatrics and Child Health 2018;54 (Supplement 1):4-5. [Google Scholar]
Wickens 2017 {published data only}
- Wickens KL, Barthow CA, Murphy R, Abels PR, Maude RM, Stone PR, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. British Journal of Nutrition 2017;117(6):804-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang F, ChiCTR1800015853. Study on the effect of probiotics on the improvement of intestinal flora in gestational diabetes mellitus. http://www.chictr.org.cn/showproj.aspx?proj=25072 (first received 24 April 2018).
References to studies awaiting assessment
Gonai 2014 {published data only}
- Gonai M, Nakadaira I, Kurasaki K, Hamano K. The effects of lactobacilli on glycemic control and the secretion of glucagon-like peptide-1 in Japanese gestational diabetes mellitus patients. Diabetes 2014;63(Suppl 1):A333, Abstract no: 1278-P. [Google Scholar]
Jamilian 2019 {published data only}
- Asemi Z, IRCT201706075623N119. Clinical trial of the effect of combined probiotic and vitamin D supplementation compared with the placebo on metabolic profiles in women with gestational diabetes. http://en.irct.ir/trial/6149 (first received 9 June 2017).
- Jamilian M, Amirani E, Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2019;38(5):2098-105. [CENTRAL: CN-01667601] [EMBASE: 2001290656] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Asemi 2019 {published data only}
- Asemi Z//IRCT20170513033941N55. The effects of selenium and probiotics co-supplementation on on pregnancy outcomes, inflammatory factors, oxidative stress biomarkers and gene expression related to inflammation in women with gestational diabetes. http://en.irct.ir/trial/38429 (first received 5 April 2019).
CTRI/2018/08/015432 {published data only}
- Ramanathan K, CTRI/2018/08/015432. Effect of probiotic supplementation on blood glucose of gestational diabetic mellitus (GDM) mothers. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27682 (first received 23 August 2018).
IRCT20121224011862N2 {published data only}
- Sahaf F, IRCT20121224011862N2. The effect of probiotic yogurt and conventional yogurt in blood glucose control and outcomes of pregnancy in women with gestational diabetes. http://en.irct.ir/trial/29872 (first received 19 May 2018).
IRCT2015110310089N4 {published data only}
- Fani A, IRCT2015110310089N4. Comparison of probiotic capsules and placebo on fasting blood sugar and metabolic parameters in pregnant woman with gestational diabetes or impaired glucose tolerance test. http://en.irct.ir/trial/10568 (first received 1 February 2016).
IRCT20171010036697N1 {published data only}
- Asemi Z, IRCT20171010036697N1. The effect of probiotic supplementation on gene expression related to insulin, lipid and inflammation in patients with gestational diabetes. http://en.irct.ir/trial/27359 (first received 3 January 2018).
Nachum 2019 {published data only}
- Nachum Z//NCT03864549. The effect of oral probiotics on glycemic control of women with gestational diabetes mellitus - randomized, double blind, placebo controlled trial, phase 4. https://clinicaltrials.gov/ct2/show/NCT03864549 (first received 6 March 2019. [DOI] [PubMed]
Additional references
ACOG 2001
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational Diabetes. Obstetrics & Gynecology 2001;98(3):525-38. [PubMed]
ADA 2010
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. [Erratum appears in Diabetes Care. 2010 Apr;33(4):e57] Diabetes Care 2010;33 Suppl 1:S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alberti 1998
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539-53. [DOI] [PubMed] [Google Scholar]
AlFaleh 2014
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evidence-Based Child Health: a Cochrane Review journal 2014;9(3):584-671. [DOI] [PubMed] [Google Scholar]
Allen 2010
- Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor M, Garaiova I, et al. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. Journal of Nutrition 2010;140(3):483-8. [DOI] [PubMed] [Google Scholar]
Andreasen 2010
- Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Moller K, Svendsen KD, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. British Journal of Nutrition 2010;104(12):1831-8. [DOI] [PubMed] [Google Scholar]
Benton 2007
- Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal of Clinical Nutrition 2007;61(3):355-61. [DOI] [PubMed] [Google Scholar]
Boney 2005
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115(3):e290-6. [DOI] [PubMed] [Google Scholar]
Braentsaeter 2011
- Brantsaeter AL, Myhre R, Haugen M, Myking S, Sengpiel V, Magnus P, et al. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. American Journal of Epidemiology 2011;174(7):807-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017
- Brown J, Alwan NA, West J, Brown S, McKinlay CJD, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD011970.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Callaway 2019
- Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care Jan 18;42(3):364-71. [DOI: ] [DOI] [PubMed] [Google Scholar]
Collado 2008
- Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. American Journal Clinical Nutrition 2008;88(4):894-9. [DOI] [PubMed] [Google Scholar]
Coustan 2013
- Coustan DR. Gestational diabetes mellitus. Clinical Chemistry 2013;59(9):1310-21. [DOI] [PubMed] [Google Scholar]
Crowther 2005
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian carbohydrate intolerance study in pregnant women trial group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477-86. [DOI] [PubMed] [Google Scholar]
Cundy 2014
- Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ 2014;348:g1567. [DOI] [PubMed] [Google Scholar]
Dabelea 2005
- Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS, Kaiser Permanente of Colorado GDMSP. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005;28(3):579-84. [DOI] [PubMed] [Google Scholar]
Diabetes Care 2010
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinan 2011
- Dinan TG, Quigley EM. Probiotics in the treatment of depression: science or science fiction? Australian & New Zealand Journal of Psychiatry 2011;45(12):1023-5. [DOI] [PubMed] [Google Scholar]
Ejtahed 2012
- Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asqhari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012;28(5):539-43. [DOI] [PubMed] [Google Scholar]
Ekeroma 2015
- Ekeroma AJ, Chandran GS, McCowan L, Ansell D, Eagleton C, Kenealy T. Impact of using the international association of diabetes and pregnancy study groups criteria in South Auckland: prevalence, interventions and outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2015;55:34-41. [DOI] [PubMed] [Google Scholar]
Elias 2011
- Elias J, Bozzo P, Einarson A. Are probiotics safe for use during pregnancy and lactation? Canadian Family Physician 2011;57(3):299-301. [PMC free article] [PubMed] [Google Scholar]
FAO 2001
- FAO - Food, Agriculture Organization of the United Nations World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed 2015). 2001:1-34.
Ferrara 2007
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2):S141-6. [DOI] [PubMed] [Google Scholar]
Flint 2012
- Flint HJ. The impact of nutrition on the human microbiome. Nutrition Reviews 2012;70:Suppl 1:S10-3. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jiang 2015
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity 2015;4:186-94. [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862-8. [DOI] [PubMed] [Google Scholar]
Koren 2012
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150(3):470-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laitinen 2009
- Laitinen K, Poussa T, Isolauri E, Allergy Mucosal Immunology Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. British Journal of Nutrition 2009;101(11):1679-87. [DOI] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal Medicine 2009;361(14):1339-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 2010
- Larsen N, Vogensen FK, den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLOS One [Electronic Resource] 2010;5(2):e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Messaoudi 2011
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition 2011;105:755-64. [DOI: 10.1017/S000711451000431] [DOI] [PubMed] [Google Scholar]
Metzger 2007
- Metzger BE, Buchanan TA, Coustan DR, Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):s251-s260. [DOI] [PubMed] [Google Scholar]
NICE 2015
- The National Institute for Health and Care Excellence. New thresholds for diagnosis of diabetes in pregnancy. https://www.nice.org.uk/news/article/new-thresholds-for-diagnosis-of-diabetes-in-pregnancy 2015.
Nieuwdorp 2014
- Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014;146(6):1525-33. [DOI] [PubMed] [Google Scholar]
Poolsup 2014
- Poolsup N, Suksomboon N, Amin M. Effect of treatment of gestational diabetes mellitus: a systematic review and meta-analysis. PLOS One 2014;3:e92485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poston 2013
- Poston L, Briley A, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy and Childbirth 2013;13(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanchez 2014
- Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. British Journal of Nutrition 2014;111(8):1507-19. [DOI] [PubMed] [Google Scholar]
Solt 2015
- Solt I. The human microbiome and the great obstetrical syndromes: a new frontier in maternal-fetal medicine. Best Practice & Research in Clinical Obstetrics & Gynaecology 2015;29(2):165-75. [DOI] [PubMed] [Google Scholar]
Swinburn 2011
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378(9793):804-14. [DOI] [PubMed] [Google Scholar]
Turnbaugh 2006
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444(7122):1027-31. [DOI] [PubMed] [Google Scholar]
Turpin 2010
- Turpin W, Humblot C, Thomas M, Guyot J-P. Lactobacilli as multifaceted probiotics with poorly disclosed molecular mechanisms. Food Microbiology 2010;143(3):87-102. [DOI] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Nutrition Challenges: Overweight and Obesity. http://www.who.int/nutrition/challenges/en/ 2016.
Zheng 2018
- Zheng J, Feng Q, Zheng S, Xiao X. The effects of probiotics supplementation on metabolic health in pregnant women: An evidence based meta-analysis. PLOS One May 21, 2018;13(5):e0197771. [DOI: 10.1371/journal.pone.0197771] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Okesene‐Gafa 2018
- Okesene‐Gafa KAM, Brown J, McCowan L, Crowther CA. Probiotics for treating women with gestational diabetes for improving maternal and fetal health and well‐being. Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD012970] [DOI] [Google Scholar]


