Abstract
Background
Female breast, prostate, lung, and colorectal cancers are the leading incident cancers among American Indian and Alaska Native (AI/AN) and non-Hispanic White (NHW) persons in the United States. To understand racial differences, we assessed incidence rates, analyzed trends, and examined geographic variation in incidence by Indian Health Service regions.
Methods
To assess differences in incidence, we used age-adjusted incidence rates to calculate rate ratios (RRs) and 95% confidence intervals (CIs). Using joinpoint regression, we analyzed incidence trends over time for the four leading cancers from 1999 to 2015.
Results
For all four cancers, overall and age-specific incidence rates were lower among AI/ANs than NHWs. By Indian Health Service regions, incidence rates for lung cancer were higher among AI/ANs than NHWs in Alaska (RR: 1.46; 95% CI: 1.37, 1.56) and Northern (RR: 1.29; 95% CI: 1.25, 1.33) and Southern (RR: 1.06; 95% CI: 1.03, 1.09) Plains. Similarly, colorectal cancer incidence rates were higher in AI/ANs than NHWs in Alaska (RR: 2.29; 95% CI: 2.14, 2.45) and Northern (RR: 1.04; 95% CI: 1.00, 1.09) and Southern (RR: 1.11; 95% CI: 1.07, 1.15) Plains. Also, AI/AN women in Alaska had a higher incidence rate for breast cancer than NHW women (RR: 1.05; 95% CI: 1.05, 1.20). From 1999 to 2015, incidence rates for all four cancers decreased in NHWs, but only rates for prostate (average annual percent change: –4.70) and colorectal (average annual percent change: –1.80) cancers decreased considerably in AI/ANs.
Conclusion
Findings from this study highlight the racial and regional differences in cancer incidence.
Keywords: American Indian and Alaska Native, Breast cancer, Colorectal cancer, Lung cancer, Prostate cancer, Racial disparity
INTRODUCTION
Cancer is a major cause of morbidity and mortality in the United States. It is the second leading cause of all deaths nationally (22% of total deaths) and among American Indians and Alaska Natives (AI/ANs) (17.3%) and non-Hispanic Whites (NHWs) (21.9%).1 The leading incident cancer sites in the United States are female breast, prostate, lung and bronchus, and colon and rectum.2 The burden of cancer among AI/ANs has been historically underestimated.3–5 Cancer data in this population have been plagued by racial misclassification6,7 owing to the incorrect identification of racial and ethnic status on health records8,9 and lack of linkage between cancer registries and the Indian Health Service (IHS) records.10 Spanning over 570 federally recognized tribes across the nation, the pattern of cancers among AI/AN populations is unique, and regional variations exist. Across the United States, incidence and mortality rates for cancers differed geographically among AI/ANs and between AI/ANs and NHWs.4,11 For example, mortality rates for all cancer sites in AI/ANs were highest in the Northern Plains, and a two-fold difference in rates was reported between the Northern Plains and Southwest IHS regions.4 Furthermore, disparities in cancer mortality and survival between AI/AN and NHW persons were present.5,12 From 1990 to 2009, mortality rates for all malignant cancers combined were higher for AI/ANs than for NHWs.4 Similarly, from 1992 to 2010, the 5-year relative survival for all cancer sites combined was lowest in AI/ANs (58.1%) compared with other races and ethnicities.13
In the present study, we obtained age-adjusted incidence rates (AAIRs), calculated rate ratios (RRs), and conducted a trend analysis over time to determine the burden and pattern of cancers among AI/AN and NHW populations. The present work will provide evidence to further quantify cancer incidence and allow health agencies and professionals to evaluate progress made, or lack thereof, in cancer prevention and control. It will also supplement the current literature with an analysis and examination of incidence by race and IHS regions using the most recent cancer data available. The objectives of this study were three-fold: (1) to present and compare age-adjusted and age-specific incidence rates for the four leading cancers between AI/ANs and NHWs in the United States, (2) to present long-term cancer incidence trends from 1999 to 2015 for both races, and (3) to assess geographic variation in incidence by IHS regions.
METHODS
Cancer Sites
We analyzed the four leading incident cancers in the United States: female breast (referred to as breast henceforth), prostate, lung and bronchus (referred to as lung henceforth), and colon and rectum (referred to as colorectal henceforth) cancer. Incident cases were stratified by primary cancer site and coded according to the International Classification of Diseases for Oncology, third edition.14 Thereafter, based on the revised Surveillance, Epidemiology, and End Results (SEER) Program site recodes, cancers were defined as breast (C500-C509), prostate (C619), lung (C340–349), and colorectal (C180-C189, C199, C209–212, C218, C260), excluding histologies 9050–9055, 9140, 9590–9992.15 We restricted incidence rates for breast cancer to females and incidence rates for prostate cancer to males. We excluded data for in situ cancers from this study.
Data Source
We obtained cancer incidence data from 1999 to 2015 from the Centers for Disease Control and Prevention’s (CDC) publicly available online database, CDC WONDER (Wide-ranging Online Data for Epidemiologic Research).16 CDC WONDER houses health-related data, including the official federal statistics on cancer, US Cancer Statistics (USCS). The USCS includes high-quality cancer registry data from the CDC’s National Program of Cancer Registries and the National Cancer Institute’s SEER Program.17 From 2003 onward, all cancer registries participating in the National Program of Cancer Registries and SEER Program met the USCS publication criteria and covered 100% of the US population.18 Denominators for incidence rates were race-, ethnicity-, and sex-specific population estimates modified from the US Census intercensal (for 1999–2009) and Vintage 2016 (for 2010–2015) annual time series. The modifications for the population estimates incorporated bridged single-race estimates that were derived from the multiple race categories in the 2000 and 2010 censuses.19
Study population
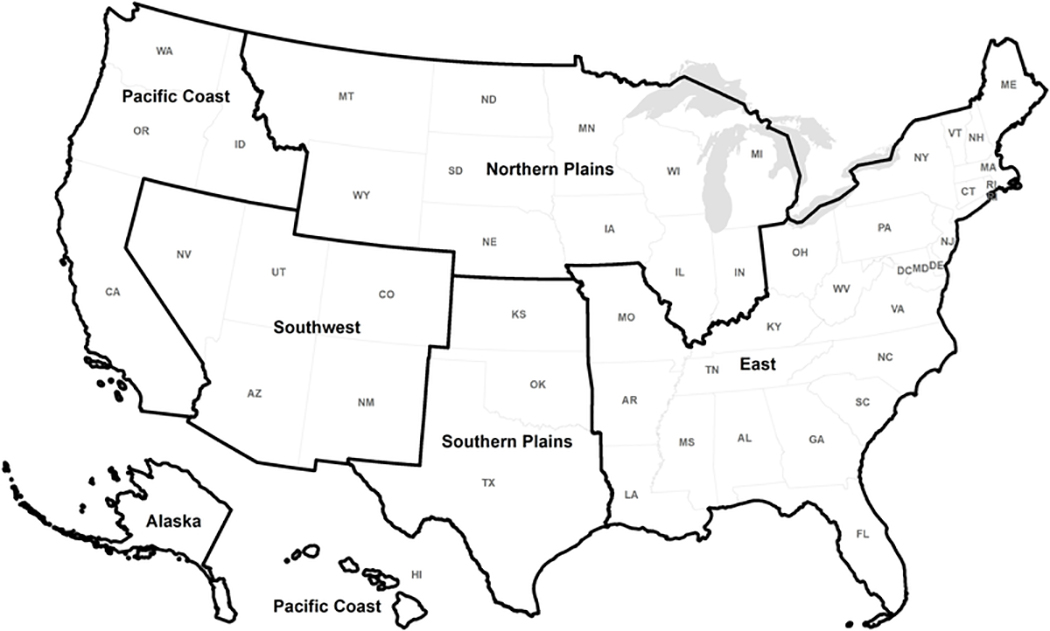
We included new cases of breast, prostate, lung, and colorectal cancer from all 50 states and the District of Columbia. We analyzed data for all states combined and by the six IHS regions individually (Figure): Northern Plains (Illinois, Indiana, Iowa, Michigan, Minnesota, Montana, Nebraska, North Dakota, South Dakota, Wisconsin, and Wyoming), Southern Plains (Kansas, Oklahoma, and Texas), Southwest (Arizona, Colorado, Nevada, New Mexico, and Utah), Pacific Coast (California, Hawaii, Idaho, Oregon, and Washington), East (Alabama, Arkansas, Connecticut, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Mississippi, Missouri, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Vermont, Virginia, and West Virginia), and Alaska (Alaska). Analyses were limited to AI/AN and NHW populations. Analyses included Hispanic AI/ANs but excluded Hispanic Whites. As these four cancers were rare in the young population (fewer than 70 incident cases reported among AI/ANs younger than 25 years for each of the cancers during 1999–2015), we excluded cases below the age of 25 years.
Figure.
States by Indian Health Service Regions, United States
Data analysis
AAIRs, expressed per 100,000 population, were obtained adjusted to the 2000 US standard million population to reduce the potential confounding effect of age.17 Based on the AAIRs, RRs and 95% confidence intervals (CIs) were calculated to assess the difference in the incidence of cancers between AI/ANs and NHWs. RRs were calculated as the ratio of the AAIR in AI/ANs divided by the AAIR in NHWs. The corresponding 95% CIs were computed using the formula:20
Trends for incidence of leading cancers were analyzed by race using the Joinpoint Regression Program (version 4.6.0.0; National Cancer Institute, Bethesda, MD). Based on 17 years of data from 1999 to 2015, up to three joinpoints were fit, which allowed up to four trend periods.21 We used a log-linear model and interpreted trends in terms of a rate change at a constant percent per year through annual percent change (APC). We also calculated the average annual percent change (AAPC) to describe the average APCs over all 17 years. If the slope of the trend was significantly different from zero at an alpha of 0.05, we considered the trend of the APC or AAPC to increase or decrease; otherwise, the trend was reported as stable.
As publicly available data were used, this study did not meet the criteria for human subjects research as determined by the Institutional Review Board of the University of Oklahoma Health Sciences Center.
RESULTS
Incidence rates for the four leading cancers are presented by sex and age in Table 1 and by IHS regions in Table 2. Trends are presented in Table 3 and eFigures 1–4; http://links.lww.com/EDE/B617. For AI/ANs and NHWs, incidence rates increased successively with each age group before peaking in the age group of 65–84 years for breast, prostate, and lung cancers. For colorectal cancer, rates increased with age and were highest in the age group of 85 years and older for both races. In all age groups for all four cancers, incidence rates for AI/ANs were significantly lower than incidence rates for NHWs.
TABLE 1.
Female Breast, Prostate, Lung and Bronchus, and Colon and Rectum Cancer Incidence Rates by Sex and Age for AI/ANs and NHWs, United States, 1999–2015.
| Female Breasta | Prostate | Lung and Bronchus | Colon and Rectum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI/AN | NHW | AI/AN:NHW | AI/AN | NHW | AI/AN:NHW | AI/AN | NHW | AI/AN:NHW | AI/AN | NHW | AI/AN:NHW | |
| Count (Rate; 95% CI)b | Count (Rate; 95% CI) | Rate Ratio (95% CI) | Count (Rate; 95% CI) | Count (Rate; 95% CI) | Rate Ratio (95% CI) | Count (Rate; 95% CI) | Count (Rate; 95% CI) | Rate Ratio (95% CI) | Count (Rate; 95% CI) | Count (Rate; 95% CI) | Rate Ratio (95% CI) | |
| Overall | 17,503 (72.7; 71.6, 73.8) | 2,851,031 (130.4; 130.3, 130.6) | 0.56 (0.55, 0.57) | 13,450 (78.0; 76.5, 79.5) | 2,669,324 (134.7; 134.5, 134.9) | 0.58 (0.57, 0.59) | 17,135 (46.2; 45.5, 46.9) | 2,967,679 (69.3; 69.3, 69.4) | 0.67 (0.66, 0.68) | 13,373 (33.8; 33.2, 34.4) | 1,948,914 (46.0; 45.9, 46.1) | 0.74 (0.72, 0.75) |
| Sex | ||||||||||||
| Male | – | – | – | – | – | – | 8,926 (54.6; 53.4, 55.9) | 1,592,154 (83.5; 83.4, 83.7) | 0.65 (0.64, 0.67) | 6,925 (38.4; 37.4, 39.5) | 1,003,753 (53.4; 53.3, 53.5) | 0.72 (0.70, 0.74) |
| Female | – | – | – | – | – | – | 8,209 (40.0; 39.1, 40.9) | 1,375,525 (58.7; 58.6, 58.8) | 0.68 (0.67, 0.70) | 6,448 (30.2; 29.4, 31.0) | 945,161 (39.9; 39.8, 40.0) | 0.76 (0.74, 0.77) |
| Age groupc | ||||||||||||
| 25–44 years | 2,618 (30.6; 29.5, 31.8) | 263,436 (60.2; 60.0, 60.5) | 0.51 (0.49, 0.53) | 75 (0.9; 0.7, 1.2) | 11,227 (2.5; 2.5, 2.6) | 0.37 (0.28, 0.45) | 413 (2.5; 2.2, 2.7) | 44,831 (5.1; 5.0, 5.1) | 0.48 (0.44, 0.53) | 1,113 (6.4; 6.0, 6.8) | 78,509 (8.9; 8.9, 9.0) | 0.72 (0.67, 0.76) |
| 45–64 years | 9,144 (138.2; 135.4, 141.1) | 1,248,250 (255.8; 255.3, 256.2) | 0.54 (0.53, 0.55) | 5,675 (92.2; 89.8, 94.6) | 991,398 (197.2; 196.8, 197.6) | 0.47 (0.46, 0.48) | 6,454 (50.5; 49.3, 51.8) | 862,009 (85.4; 85.2, 85.6) | 0.59 (0.58, 0.61) | 5,853 (45.6; 44.5, 46.8) | 578,405 (58.8; 58.6, 58.9) | 0.78 (0.76, 0.80) |
| 65–84 years | 5,252 (266.7; 259.4, 274.2) | 1,153,353 (450.2; 449.3, 451.0) | 0.59 (0.58, 0.61) | 7,215 (456.1; 445.2, 467.3) | 1,543,310 (739.6; 738.5, 740.8) | 0.62 (0.60, 0.63) | 9,448 (274.8; 269.1, 280.5) | 1,825,681 (395.9; 395.4, 396.5) | 0.69 (0.68, 0.71) | 5,625 (165.7; 161.2, 170.2) | 1,030,724 (223.2; 222.7, 223.6) | 0.74 (0.72, 0.76) |
| ≥85 years | 460 (208.5; 189.9, 228.5) | 184,452 (366.3; 364.7, 368.0) | 0.57 (0.52, 0.62) | 482 (429.6; 392.1, 469.7) | 123,237 (524.0; 521.0, 526.9) | 0.82 (0.75, 0.89) | 806 (242.2; 225.8, 259.5) | 233,868 (316.6; 315.3, 317.9) | 0.77 (0.71, 0.82) | 714 (214.6; 199.1, 230.9) | 257,663 (348.8; 347.5, 350.1) | 0.62 (0.57, 0.66) |
Notes:
Data for in situ breast cancers were not included for female breast cancer.
Rates are per 100,000 persons and standardized to the 2000 U.S. standard population.
The number of incident cases across the age groups do not total the overall number of cases, as cases under the age of 25 years were excluded.
Abbreviations: AI/AN, American Indian and Alaska Native; CI, confidence interval; NHW, non-Hispanic White.
TABLE 2.
Female Breast, Prostate, Lung and Bronchus, and Colon and Rectum Cancer Incidence Rates by IHS Regions for AI/ANs and NHWs, United States, 1999–2015.
| Female Breasta | Prostate | Lung and Bronchus | Colon and Rectum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI/AN | NHW | AI/AN:NHW | AI/AN | NHW | AI/AN:NHW | AI/AN | NHW | AI/AN:NHW | AI/AN | NHW | AI/AN:NHW | |
| Count (Rate; 95% CI)b | Count (Rate; 95% CI) | Rate Ratio (95% CI) | Count (Rate; 95% CI) | Count (Rate; 95% CI) | Rate Ratio (95% CI) | Count (Rate; 95% CI) | Count (Rate; 95% CI) | Rate Ratio (95% CI) | Count (Rate; 95% CI) | Count (Rate; 95% CI) | Rate Ratio (95% CI) | |
| IHS Regionsd | ||||||||||||
| Northern Plains | 3,048 (96.4; 92.8, 100.1) | 510,875 (128.7; 128.4, 129.1) | 0.75 (0.72, 0.78) | 2,466 (113.8; 108.7, 119.0) | 495,273 (140.2; 139.8, 140.6) | 0.81 (0.78, 0.84) | 3,986 (87.2; 84.3, 90.2) | 518,718 (67.7; 67.5, 67.9) | 1.29 (1.25, 1.33) | 2,504 (49.7; 47.6, 51.9) | 364,891 (47.6; 47.4, 47.8) | 1.04 (1.00, 1.09) |
| Alaska | 1,083 (146.3; 137.4, 155.5) | 4,727 (130.5; 126.6, 134.5) | 1.12 (1.05, 1.20) | 407 (73.3; 65.6, 81.5) | 4,665 (132.8; 128.5, 137.2) | 0.55 (0.50, 0.61) | 1,097 (95.7; 89.8, 101.9) | 4,047 (65.4; 63.2, 67.6) | 1.46 (1.37, 1.56) | 1,172 (96.8; 90.9, 102.8) | 2,695 (42.2; 40.5, 44.0) | 2.29 (2.14, 2.45) |
| Southern Plains | 4,347 (108.9; 105.5, 112.3) | 222,041 (126.7; 126.2, 127.3) | 0.86 (0.83, 0.88) | 3,346 (112.8; 108.6, 117.0) | 210,162 (133.3; 132.7, 133.8) | 0.85 (0.82, 0.88) | 4,887 (74.4; 72.2, 76.7) | 239,106 (70.3; 70.0, 70.6) | 1.06 (1.03, 1.09) | 3,316 (49.4; 47.7, 51.3) | 149,628 (44.6; 44.3, 44.8) | 1.11 (1.07, 1.15) |
| Southwest | 2,636 (58.4; 56.2, 60.8) | 150,324 (124.2; 123.6, 124.8) | 0.47 (0.45, 0.49) | 1,975 (69.4; 66.2, 72.8) | 145,860 (125.7; 125.0, 126.3) | 0.55 (0.53, 0.58) | 1,198 (18.3; 17.2, 19.4) | 129,502 (54.2; 53.9, 54.5) | 0.34 (0.32, 0.36) | 2,107 (28.0; 26.8, 29.3) | 91,564 (39.1; 38.8, 39.3) | 0.72 (0.69, 0.75) |
| Pacific Coast | 3,512 (65.8; 63.5, 68.2) | 396,841 (140.5; 140.1, 140.9) | 0.47 (0.45, 0.48) | 2,418 (61.1; 58.4, 63.9) | 361,766 (138.5; 138.1, 139.0) | 0.44 (0.42, 0.46) | 3,040 (37.9; 36.5, 39.4) | 335,054 (60.4; 60.2, 60.6) | 0.63 (0.60, 0.65) | 2,371 (27.5; 26.3, 28.7) | 237,462 (43.0; 42.8, 43.2) | 0.64 (0.61, 0.67) |
| East | 2,877 (45.4; 43.7, 47.2) | 1,566,223 (129.9; 129.7, 130.1) | 0.35 (0.34, 0.36) | 2,838 (58.4; 56.0, 60.8) | 1,451,598 (133.2; 133.0, 133.5) | 0.44 (0.42, 0.45) | 2,927 (29.0; 27.9, 30.2) | 1,741,252 (73.4; 73.3, 73.5) | 0.40 (0.38, 0.41) | 1,903 (18.2; 17.3, 19.1) | 1,102,674 (47.1; 47.0, 47.2) | 0.39 (0.37, 0.40) |
Data for in situ breast cancers were not included for female breast cancer.
Rates are per 100,000 persons and standardized to the 2000 U.S. standard population.
Northern Plains (Illinois, Indiana, Iowa, Michigan, Minnesota, Montana, Nebraska, North Dakota, South Dakota, Wisconsin, and Wyoming), Southern Plains (Kansas, Oklahoma, and Texas), Southwest (Arizona, Colorado, Nevada, New Mexico, and Utah), Pacific Coast (California, Hawaii, Idaho, Oregon, and Washington), East (Alabama, Arkansas, Connecticut, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Mississippi, Missouri, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Vermont, Virginia, and West Virginia), and Alaska (Alaska).
Abbreviations: AI/AN, American Indian and Alaska Native; CI, confidence interval; IHS, Indian Health Service; NHW, non-Hispanic White.
TABLE 3.
Trends in Incidence of Leading Cancers by AI/AN and NHW Race, United States, 1999–2015.
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | Average APC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | Years | APC | 95% CI | Years | APC | 95% CI | Years | APC | 95% CI | Years | APC | 95% CI | Years | AAPC | 95% CI |
| Female Breast | |||||||||||||||
| AI/AN | 1999–2015 | −0.05 | −0.49, 0.39 | 1999–2015 | −0.05 | −0.49, 0.39 | |||||||||
| NHW | 1999–2004 | −2.34* | −3.19, −1.49 | 2004–2015 | 0.31* | 0.05, 0.57 | 1999–2015 | −0.53* | −0.82, −0.24 | ||||||
| Prostate | |||||||||||||||
| AI/AN | 1999–2009 | −2.01* | −3.19, −0.81 | 2009–2015 | −9.02* | −11.20, −6.80 | 1999–2015 | −4.70* | −5.71, −3.68 | ||||||
| NHW | 1999–2008 | −1.30 | −2.64, 0.05 | 2008–2015 | −7.13* | −9.13, −5.10 | 1999–2015 | −3.90* | −4.95, −2.84 | ||||||
| Lung and Bronchus | |||||||||||||||
| AI/AN | 1999–2005 | 1.48 | −0.40, 3.41 | 2005–2015 | −1.62* | −2.32, −0.92 | 1999–2015 | −0.47 | −1.21, 0.28 | ||||||
| NHW | 1999–2007 | −0.14 | −0.29, 0.02 | 2007–2015 | −1.94* | −2.10, −1.79 | 1999–2015 | −1.04* | −1.14, −0.95 | ||||||
| Colon and Rectum | |||||||||||||||
| AI/AN | 1999–2015 | −1.80* | −2.12, −1.49 | 1999–2015 | −1.80* | −2.12, −1.49 | |||||||||
| NHW | 1999–2001 | −0.95 | −2.17, 0.29 | 2001–2008 | −2.84* | −3.05, −2.63 | 2008–2011 | −4.07* | −5.36, −2.77 | 2011–2015 | −1.22* | −1.65, −0.78 | 1999–2015 | −2.43* | −2.69, −2.18 |
Notes:
APC or AAPC has increased or decreased considerably.
Abbreviations:
AI/AN, American Indian and Alaska Native; APC, annual percent change; AAPC, average annual percent change; CI, confidence interval; NHW, non-Hispanic White.
Breast Cancer
From 1999 to 2015, 17,503 new cases of breast cancer were reported in AI/ANs with an AAIR of 72.7 per 100,000 women. During the same period, 2,851,031 cases were reported among NHWs with an AAIR of 130.5 per 100,000 women. Overall, AI/ANs had lower incidence rates than did NHWs (RR: 0.56; 95% CI: 0.55, 0.57). By IHS region, rates were higher for AI/ANs than for NHWs in Alaska only (RR: 1.12; 95% CI: 1.05, 1.20). For all remaining IHS regions, the RRs were lower among AI/ANs than among NHWs. Among AI/ANs, rates were highest in Alaska (146.3 per 100,000 women), followed by Southern Plains (108.9 per 100,000 women), and Northern Plains (96.4 per 100,000 women).
The incidence trend for AI/ANs was stable (APC and AAPC: –0.05) from 1999 to 2015. For NHWs, incidence decreased overall (AAPC: –0.53; 95% CI: –0.82, –0.24) and annually from 1999 to 2004 (APC: –2.34; 95% CI: –3.19, –1.49), but increased from 2004 to 2015 (APC: 0.31; 95% CI: 0.05, 0.57).
Prostate Cancer
A total of 13,450 cases with an AAIR of 78.0 per 100,000 men and 2,669,324 cases with an AAIR of 134.7 per 100,000 men were reported in AI/ANs and NHWs, respectively. Prostate cancer incidence rates were lower for AI/ANs overall (RR: 0.58; 95% CI: 0.57, 0.59) and lower in all age groups and IHS regions compared with NHWs. Between IHS regions, rates for AI/ANs were highest for the Plains (Northern Plains: 113.8 per 100,000 men; Southern Plains: 112.8 per 100,000 men) compared with other regions.
For AI/ANs, incidence decreased overall from 1999 to 2015 (AAPC: –4.70; 95% CI: –5.71, –3.68), including decreases of 2.01% per year from 1999 to 2009 and 9.02% per year from 2009 to 2015. Among NHWs, the incidence trend also decreased over the 17-year time period (AAPC: –3.90; 95% CI: –4.95, –2.84). Furthermore, incidence rates in NHWs decreased by 7.1% annually from 2008 to 2015, but rates were stable from 1999 to 2008 (APC: –1.30; 95% CI: –2.64, 0.05)
Lung Cancer
New lung cancer cases were reported in 17,135 AI/ANs with an AAIR of 46.2 per 100,000 persons and in 2,967,679 NHWs with an AAIR of 69.3 per 100,000 persons. Rates were lower for AI/ANs overall (RR: 0.67; 95% CI: 0.66, 0.68) and in all age groups compared with NHWs. However, incidence rates were higher for AI/ANs than for NHWs in Alaska (RR: 1.46; 95% CI: 1.37, 1.56), Northern Plains (RR: 1.29; 95% CI: 1.25, 1.33), and Southern Plains (RR: 1.06; 95% CI: 1.03, 1.09), but were lower in the Southwest, Pacific Coast, and East regions. Both AI/AN (54.6 per 100,000 men) and NHW (83.5 per 100,000 men) males had higher incidence rates than did AI/AN (40 per 100,000 women) and NHW (58.7 per 100,000 women) females.
Lung cancer incidence overall among AI/ANs was stable from 1999 to 2015 (AAPC: –0.47), but decreased from 2005 to 2015 (APC: –1.62; 95% CI: –2.32, –0.92). For NHWs, lung cancer incidence decreased overall from 1999 to 2015 (AAPC: –1.04; 95% CI: –1.14, –0.95) and during 2007–2015 (APC: –1.94; 95% CI: –2.10, –1.79), but was stable from 1999 to 2007 (APC: –0.14; 95% CI: –0.29, 0.02).
Colorectal cancer
For colorectal cancer, 13,373 cases with an AAIR of 33.8 per 100,000 persons and 1,948,914 cases with an AAIR of 46.0 per 100,000 persons were reported in AI/ANs and NHWs, respectively. Incidence was lower among AI/ANs than among NHWs overall and for each age group. Incidence rates were higher for AI/ANs than for NHWs in Alaska (RR: 2.29; 95% CI: 2.14, 2.45) and in the Northern Plains (RR: 1.04; 95% CI: 1.00, 1.09) and Southern Plains (RR: 1.11; 95% CI: 1.07, 1.15), but lower in the Southwest, Pacific Coast, and East regions. Incidence rates for colorectal cancer were higher among AI/AN and NHW males (38.4 and 53.4 per 100,000 men, respectively) than among females (30.2 and 39.9 per 100,000 women, respectively).
From 1999 to 2015, colorectal cancer incidence decreased for AI/ANs (APC and AAPC: –1.80; 95% CI: –2.12, –1.49). Among NHWs, incidence decreased over the entire time period (AAPC: –2.43; 95% CI: –2.69, –2.18) and specifically from 2001 to 2008 (APC: –2.84; 95% CI: –3.05, –2.63), from 2008 to 2011 (APC: –4.07; 95% CI: –5.36, –2.77), and from 2011 to 2015 (APC: –1.22; 95% CI: –1.65, –0.78). However, the trend was stable from 1999 to 2001 (APC: –0.95; 95% CI: –2.17, 0.29).
DISCUSSION
Overall incidence rates of all four leading cancers were lower in AI/ANs than in NHWs. Over the course of 17 years from 1999 to 2015, incidence rates for all four cancers decreased considerably in NHWs, but only prostate and colorectal cancer incidence rates decreased in AI/AN persons. In more recent years, incidence rates decreased considerably for lung cancer in AI/ANs.
Similar to prior studies,12,22,23 we observed lower rates of breast cancer incidence in AI/ANs than in NHWs. This result may be owing to lower mammography use among AI/AN women compared with other races.12,24 An analysis of 11 years of data beginning in 2000 from the Behavioral Risk Factor Surveillance System found that AI/AN women were less likely to have had a mammogram in the past 2 years than were White women (67.8% vs. 76%).25 Some reasons for lower use among AI/AN women include lack of provider recommendation,26 transportation issues,27 location (rural vs. urban),28 and lower education and income levels.29 In line with previous reports,4,23 a sharp geographic variation in breast cancer incidence was observed in AI/ANs across IHS regions, but not among NHWs. AI/AN women from Alaska had a three-fold higher incidence than did women in the East and a two-fold higher incidence than did women in the Southwest and Pacific Coast regions. Some of this observed variation may be explained through health-care access, sociodemographic characteristics, and health-related behaviors.23 Long-term breast cancer incidence rates were stable for AI/AN women. For NHWs, the increase in the incidence trend after a decrease until 2004 may be linked with a drop in the use of hormone replacement therapy among postmenopausal women, which may delay diagnosis and compromise diagnostic performance of mammograms.30–32 Patterns of age-specific incidence rates for female breast cancer were consistent with previous studies.33,34 A decline in the incidence for the oldest age group may stem from lower screening rates (based on screening recommendations) or from incomplete detection.35
Consistent with prior studies,36,37 the overall prostate cancer incidence rate was lower in AI/AN men than in NHW men. Differences in rates between the two races could stem from lower prostate-specific antigen (PSA) testing rates in AI/AN men.37,38 In an analysis of the Health and Retirement Survey from 1996 to 2008, prostate screening rates were lower among AI/ANs (57.0% in 1996 and 55.7% in 2008) than among Whites (68.6% in 1996 and 71.3% in 2008).38 Among AI/AN men, incidence rates for prostate cancer varied markedly by IHS regions, with the two Plain regions having rates that were double than those observed in the Pacific Coast and East regions. These differences in geographic variation among AI/ANs are not completely understood.37 An overall decrease in the incidence of prostate cancer was observed for both races; however, the decrease was more pronounced after 2008 and 2009 for AI/AN and NHW men, respectively. This decrease in incidence mirrors the reduction in the use of PSA testing, which resulted from the recommendations of the US Preventive Services Task Force against screening in 2008 and 2012.39,40 In addition, incidence rates increased with age before declining in the oldest age group, possibly owing to recommendations against screening men over 70 years with PSA.41
Compared with incidence in NHWs, lung cancer incidence in AI/ANs was lower overall and in all age groups, but higher in both Plain regions and Alaska. Incidence among AI/AN persons varied more than five-fold across IHS regions. This variation may stem from the substantial regional differences in tobacco use, which is a major risk factor for lung cancer. For instance, smoking prevalence rates among AI/ANs were highest in the Plains and Alaska and lowest in the Southwest and Pacific Coast.42–44 Despite the high smoking prevalence among AI/ANs in some IHS regions, there was an overall decrease in lung cancer incidence among AI/ANs each year from 2005 to 2015. There was also an annual decrease for NHWs from 2007 to 2015. A possible reason for the decline is the implementation of tobacco control strategies and policies; however, declines in lung cancer owing to tobacco control are observed decades after increased tobacco control efforts, owing to the long latency period between smoking and cancer.45 Despite progress made toward reducing tobacco use and tobacco-related cancers in the general population during the past several decades, smoking remains disproportionately high for AI/ANs. The National Survey on Drug Use and Health found that the prevalence of cigarette smoking for AI/ANs was considerably higher than that in NHWs (38.9% vs. 24.3%) in 2010–2013 and that there was no decline in smoking for AI/ANs between the 2002–2006 and 2010–2013 periods assessed, although this survey did not ascertain uses of tobacco for ceremonial purposes.46 More research is needed to identify ways of strengthening and sustaining tobacco control efforts that will reduce tobacco-related cancer disparities.
Overall colorectal cancer incidence was found to be lower for AI/ANs than for NHWs. The incidence of colorectal cancer among AI/ANs previously has been reported to vary.47–49 Our study found a five-fold difference in incidence between the IHS regions with the lowest and highest incidence. Incidence rates were highest in Alaska for AI/ANs, followed by the Southern Plains; rates in both regions were also higher than incidence rates in NHWs. A decline in overall incidence was observed for both races during the 17-year period. Much of the decline in incidence could be attributed to screening.49–51 However, screening disparities between the two races exist, with AI/ANs being less likely to be up-to-date with screening recommendations.25,47,52 Despite declines in incidence among adults 55 years of age and older,49,53 age-specific rates were highest in the oldest age groups. Geographic variation in access and utilization of screening for AI/ANs, which may explain some of the variation in incidence by IHS regions, has also been reported.54
The present study has several limitations. Racial misclassification is a widespread problem among AI/ANs7 and may still exist despite the IHS linkage process. To reduce misclassification, cancer registries link case data with IHS administrative records to reclassify individuals as AI/AN.55 However, not all AI/ANs have registered with IHS, and misclassification may continue to lead to the underestimation of cancer incidence in AI/ANs relative to Whites, especially in states with only a few or no IHS facilities.6
In the 2010 census, an unexpected increase of 19% in the AI/AN population was observed, possibly owing to differences in self-identification or emphasis on addressing underenumeration. This increase in population may result in relatively lower incidence rates among AI/ANs than among Whites.56 State or metropolitan cancer registries may have some incomplete incidence data for any given year; however, the data used in this report met the quality standards and the USCS publication criteria.55
Previous publications4,7,36,42,48,57 have used Purchased/Referred Care Delivery Areas (PRCDA) (formerly Contract Health Service Delivery Area) county classification for AI/ANs to limit racial misclassification. We did not use these classifications for two reasons. First, cancer incidence data by PRCDA counties are not provided by CDC WONDER. Second, PRCDA counties in certain regions are less representative of the total AI/AN population. For example, PRCDA counties in the East only account for 13.1% of the total AI/AN population for the region.10 In addition, only 56% of the AI/AN population and 20% of the NHW population reside in counties designated as PRCDA.10
In summary, incidence rates for AI/ANs were lower than incidence rates for NHWs for cancers overall and in all age groups. We also observed wide geographic variation in incidence among AI/ANs and in comparison with NHWs. During 1999–2015, overall incidence rates decreased for all cancers in NHWs, but only for prostate and colorectal cancers in AI/ANs. Findings from the present study highlight the racial and regional differences in cancer incidence. Future studies should consider evaluating differences in stage at diagnosis and survival by region, age, and race and ethnicity to further understand disparities and provide data to support interventions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors appreciate the helpful feedback from the reviewers in strengthening this manuscript. The authors are also grateful for the efforts of Kathy J. Kyler (Staff Editor, Office of the Vice President for Research, University of Oklahoma Health Sciences Center) in preparing this manuscript for publication.
FUNDING
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number S06GM123546 and National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R25MD011564. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
We used publicly available data from the Centers for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) database.
REFERENCES
- 1.Heron M Deaths: leading causes for 2016. Natl Vital Stat Rep. 2018;67:1–77. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Puukka E, Stehr-Green P, Becker TM. Measuring the health status gap for American Indians/Alaska Natives: getting closer to the truth. Am J Public Health. 2005;95:838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104(suppl 3):S377–S387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiggins CL, Espey DK, Wingo PA, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113(5 suppl):1142–1152. [DOI] [PubMed] [Google Scholar]
- 6.Frost F, Taylor V, Fries E. Racial misclassification of Native Americans in a surveillance, epidemiology, and end results cancer registry. J Natl Cancer Inst. 1992;84:957–962. [DOI] [PubMed] [Google Scholar]
- 7.Jim MA, Arias E, Seneca DS, et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health. 2014;104(suppl 3):S295–S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias E, Heron M, Hakes J. The validity of race and Hispanic-origin reporting on death certificates in the United States: an update. Vital Health Stat 2. 2016;172:1–21. [PubMed] [Google Scholar]
- 9.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008;148:1–23. [PubMed] [Google Scholar]
- 10.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(5 suppl): 1120–1130. [DOI] [PubMed] [Google Scholar]
- 11.Haverkamp D, Espey D, Paisano R, Cobb N. Cancer Mortality Among American Indians and Alaska Natives: Regional Differences, 1999–2003. Rockville, MD: Indian Health Service; 2008. [Google Scholar]
- 12.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Online Summary of Trends in US Cancer Control Measures. https://progressreport.cancer.gov/after/survival. Published February 2019. Accessed 1 March 2019.
- 14.World Health Organization. International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 15.National Cancer Institute Surveillance E, and End Result Program. Site Recode. https://seer.cancer.gov/siterecode/. Accessed 12 January 2019.
- 16.Centers for Disease Control and Prevention. CDC WONDER. https://wonder.cdc.gov/. Accessed 10 January 2019.
- 17.Centers for Disease Control and Prevention. United States Cancer Statistics Data. https://wonder.cdc.gov/wonder/help/cancer-v2015.html. Accessed 10 January 2019.
- 18.Centers for Disease Control and Prevention. Registries that met U.S. Cancer Statistics publication criteria; https://www.cdc.gov/cancer/uscs/technical_notes/criteria/registries.htm. Published 2018, June 4 Accessed 18 May 2019. [Google Scholar]
- 19.National Cancer Institute. U.S. Population Data - 1969–2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed 18 May 2019. [Google Scholar]
- 20.Agresti A An Introduction to Categorical Data Analysis. 2nd ed. Hoboken, NJ: Wiley-Interscience; 2007. [Google Scholar]
- 21.National Cancer Institute. Surveillance Research Program. Number of Joinpoints. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/number-of-joinpoints. Accessed 16 January 2019.
- 22.Roen EL, Copeland GE, Pinagtore NL, Meza R, Soliman AS. Disparities of cancer incidence in Michigan’s American Indians: spotlight on breast cancer. Cancer. 2014;120:1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingo PA, King J, Swan J, et al. Breast cancer incidence among American Indian and Alaska Native women: US, 1999–2004. Cancer. 2008;113(5suppl):1191–1202. [DOI] [PubMed] [Google Scholar]
- 24.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- 25.Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104(suppl 3):S481–S489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandhi N, Guadagnolo BA, Kanekar S, Petereit DG, Smith MA. Cancer screening in Native Americans from the Northern Plains. Am J Prev Med. 2010;38:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley CM, Filippi M, James AS, et al. American Indian community leader and provider views of needs and barriers to mammography. J Community Health. 2012;37:307–315. [DOI] [PubMed] [Google Scholar]
- 28.Nuño T, Gerald JK, Harris R, Martinez ME, Estrada A, García F. Comparison of breast and cervical cancer screening utilization among rural and urban Hispanic and American Indian women in the Southwestern United States. Cancer Causes Control. 2012;23:1333–1341. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher MC, Slattery ML, Lanier AP, et al. Prevalence and predictors of cancer screening among American Indian and Alaska native people: the EARTH study. Cancer Causes Control. 2008;19:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. [DOI] [PubMed] [Google Scholar]
- 31.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20:733–739. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Anderson G, Pettinger M, et al. ; Women’s Health Initiative Investigators. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–7; quiz 345. [DOI] [PubMed] [Google Scholar]
- 33.Harding C, Pompei F, Wilson R. Peak and decline in cancer incidence, mortality, and prevalence at old ages. Cancer. 2012;118:1371–1386. [DOI] [PubMed] [Google Scholar]
- 34.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Atlanta: American Cancer Society, Inc; 2017. [Google Scholar]
- 36.Hoffman RM, Li J, Henderson JA, Ajani UA, Wiggins C. Prostate cancer deaths and incident cases among American Indian/Alaska Native men, 1999–2009. Am J Public Health. 2014;104(suppl 3):S439–S445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson JA, Espey DK, Jim MA, German RR, Shaw KM, Hoffman RM. Prostate cancer incidence among American Indian and Alaska Native men, US, 1999–2004. Cancer. 2008;113(5 suppl):1203–1212. [DOI] [PubMed] [Google Scholar]
- 38.Goins RT, Schure MB, Noonan C, Buchwald D. Prostate cancer screening among American Indians and Alaska Natives: the health and retirement survey, 1996–2008. Prev Chronic Dis. 2015;12:E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Berkowitz Z, Hall IJ. Decrease in prostate cancer testing following the US Preventive Services Task Force (USPSTF) recommendations. J Am Board Fam Med. 2015;28:491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol. 2015;194:1587–1593. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman RM, Meisner AL, Arap W, et al. Trends in United States prostate cancer incidence rates by age and stage, 1995–2012. Cancer Epidemiol Biomarkers Prev. 2016;25:259–263. [DOI] [PubMed] [Google Scholar]
- 42.Plescia M, Henley SJ, Pate A, Underwood JM, Rhodes K. Lung cancer deaths among American Indians and Alaska Natives, 1990–2009. Am J Public Health. 2014;104(suppl 3):S388–S395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bliss A, Cobb N, Solomon T, et al. Lung cancer incidence among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113(5 suppl):1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska Natives-findings from the behavioral risk factor surveillance system, 2000–2006. Cancer. 2008;113(5 suppl):1131–1141. [DOI] [PubMed] [Google Scholar]
- 45.de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res. 2018;7:220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martell BN, Garrett BE, Caraballo RS. Disparities in adult cigarette smoking - United States, 2002–2005 and 2010–2013. MMWR Morb Mortal Wkly Rep. 2016;65:753–758. [DOI] [PubMed] [Google Scholar]
- 47.Day LW, Espey DK, Madden E, Segal M, Terdiman JP. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011;56:2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perdue DG, Haverkamp D, Perkins C, Daley CM, Provost E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am J Public Health. 2014;104(Suppl 3):S404–S414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. [DOI] [PubMed] [Google Scholar]
- 50.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46:228–236. [DOI] [PubMed] [Google Scholar]
- 53.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towne SD Jr, Smith ML, Ory MG. Geographic variations in access and utilization of cancer screening services: examining disparities among American Indian and Alaska Native Elders. Int J Health Geogr. 2014;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.United States Cancer Statistics. 2013. Technical Notes. https://www.cdc.gov/cancer/npcr/uscs/pdf/uscs-2013-technical-notes.pdf. Accessed 21 March 2018.
- 56.Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.United States Cancer Statistics. Cancers Associated with Human Papillomavirus in the American Indian and Alaska Native Population, United States—1999–2015 (Purchased/Referred Care Delivery Areas-PRCDA). https://www.cdc.gov/cancer/uscs/pdf/USCS-DataBrief-No6-December2018-508.pdf. Published 2018. Accessed 1 March 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.