Abstract
Background
Hyperkalaemia is a common electrolyte abnormality caused by reduced renal potassium excretion in patients with chronic kidney diseases (CKD). Potassium binders, such as sodium polystyrene sulfonate and calcium polystyrene sulfonate, are widely used but may lead to constipation and other adverse gastrointestinal (GI) symptoms, reducing their tolerability. Patiromer and sodium zirconium cyclosilicate are newer ion exchange resins for treatment of hyperkalaemia which may cause fewer GI side‐effects. Although more recent studies are focusing on clinically‐relevant endpoints such as cardiac complications or death, the evidence on safety is still limited. Given the recent expansion in the available treatment options, it is appropriate to review the evidence of effectiveness and tolerability of all potassium exchange resins among people with CKD, with the aim to provide guidance to consumers, practitioners, and policy‐makers.
Objectives
To assess the benefits and harms of potassium binders for treating chronic hyperkalaemia among adults and children with CKD.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 10 March 2020 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐randomised controlled studies (quasi‐RCTs) evaluating potassium binders for chronic hyperkalaemia administered in adults and children with CKD.
Data collection and analysis
Two authors independently assessed risks of bias and extracted data. Treatment estimates were summarised by random effects meta‐analysis and expressed as relative risk (RR) or mean difference (MD), with 95% confidence interval (CI). Evidence certainty was assessed using GRADE processes.
Main results
Fifteen studies, randomising 1849 adult participants were eligible for inclusion. Twelve studies involved participants with CKD (stages 1 to 5) not requiring dialysis and three studies were among participants treated with haemodialysis. Potassium binders included calcium polystyrene sulfonate, sodium polystyrene sulfonate, patiromer, and sodium zirconium cyclosilicate. A range of routes, doses, and timing of drug administration were used. Study duration varied from 12 hours to 52 weeks (median 4 weeks). Three were cross‐over studies. The mean study age ranged from 53.1 years to 73 years. No studies evaluated treatment in children.
Some studies had methodological domains that were at high or unclear risks of bias, leading to low certainty in the results. Studies were not designed to measure treatment effects on cardiac arrhythmias or major GI symptoms.
Ten studies (1367 randomised participants) compared a potassium binder to placebo. The certainty of the evidence was low for all outcomes. We categorised treatments in newer agents (patiromer or sodium zirconium cyclosilicate) and older agents (calcium polystyrene sulfonate and sodium polystyrene sulfonate). Patiromer or sodium zirconium cyclosilicate may make little or no difference to death (any cause) (4 studies, 688 participants: RR 0.69, 95% CI 0.11, 4.32; I2 = 0%; low certainty evidence) in CKD. The treatment effect of older potassium binders on death (any cause) was unknown. One cardiovascular death was reported with potassium binder in one study, showing that there was no difference between patiromer or sodium zirconium cyclosilicate and placebo for cardiovascular death in CKD and HD. There was no evidence of a difference between patiromer or sodium zirconium cyclosilicate and placebo for health‐related quality of life (HRQoL) at the end of treatment (one study) in CKD or HD. Potassium binders had uncertain effects on nausea (3 studies, 229 participants: RR 2.10, 95% CI 0.65, 6.78; I2 = 0%; low certainty evidence), diarrhoea (5 studies, 720 participants: RR 0.84, 95% CI 0.47, 1.48; I2 = 0%; low certainty evidence), and vomiting (2 studies, 122 participants: RR 1.72, 95% CI 0.35 to 8.51; I2 = 0%; low certainty evidence) in CKD. Potassium binders may lower serum potassium levels (at the end of treatment) (3 studies, 277 participants: MD ‐0.62 mEq/L, 95% CI ‐0.97, ‐0.27; I2 = 92%; low certainty evidence) in CKD and HD. Potassium binders had uncertain effects on constipation (4 studies, 425 participants: RR 1.58, 95% CI 0.71, 3.52; I2 = 0%; low certainty evidence) in CKD. Potassium binders may decrease systolic blood pressure (BP) (2 studies, 369 participants: MD ‐3.73 mmHg, 95%CI ‐6.64 to ‐0.83; I2 = 79%; low certainty evidence) and diastolic BP (one study) at the end of the treatment. No study reported outcome data for cardiac arrhythmias or major GI events.
Calcium polystyrene sulfonate may make little or no difference to serum potassium levels at end of treatment, compared to sodium polystyrene sulfonate (2 studies, 117 participants: MD 0.38 mEq/L, 95% CI ‐0.03 to 0.79; I2 = 42%, low certainty evidence). There was no evidence of a difference in systolic BP (one study), diastolic BP (one study), or constipation (one study) between calcium polystyrene sulfonate and sodium polystyrene sulfonate.
There was no difference between high‐dose and low‐dose patiromer for death (sudden death) (one study), stroke (one study), myocardial infarction (one study), or constipation (one study).
The comparative effects whether potassium binders were administered with or without food, laxatives, or sorbitol, were very uncertain with insufficient data to perform meta‐analysis.
Authors' conclusions
Evidence supporting clinical decision‐making for different potassium binders to treat chronic hyperkalaemia in adults with CKD is of low certainty; no studies were identified in children. Available studies have not been designed to measure treatment effects on clinical outcomes such as cardiac arrhythmias or major GI symptoms. This review suggests the need for a large, adequately powered study of potassium binders versus placebo that assesses clinical outcomes of relevance to patients, clinicians and policy‐makers. This data could be used to assess cost‐effectiveness, given the lack of definitive studies and the clinical importance of potassium binders for chronic hyperkalaemia in people with CKD.
Plain language summary
Are potassium treatments effective for reduce the excess of potassium among people with chronic kidney disease?
What is the issue?
High levels of potassium (a body salt) can build up with chronic kidney disease. This can lead to changes in muscle function including the heart muscle, and cause problems with heart rhythms that can be dangerous. Dialysis can remove potassium from the blood, but for some patients levels can still be high. Patients with severe kidney failure who have not yet started dialysis may have high potassium levels. Treatments have been available for many years but can cause constipation and abdominal discomfort, which make them intolerable for many patients. Newer treatments have been developed including patiromer and sodium zirconium cyclosilicate. These may be more tolerable but it is uncertain whether they help to prevent heart complications.
What did we do?
We searched for all the research trials that have assessed the potassium‐lowering treatments for children and adults with chronic kidney diseases. We evaluated how certain we could be about the overall findings using a system called "GRADE".
What did we find?
There are 15 studies involving 1849 randomised adults. Patients in the studies were given a potassium binder or a dummy pill (placebo) or standard care. The treatment they got was decided by random chance. The studies were generally short‐term over days to weeks and focused on potassium levels. Heart related complications could not be measured in this short time frame. Based on the existing research, we can't be sure whether potassium binders improve well‐being or prevent complications in people with chronic kidney disease. There were no studies in children.
Conclusions
We can't be certain about the best treatments to reduce body potassium levels for people with chronic kidney disease. We need more information from clinical studies that involve a larger number of patients who have the treatment over several months or years.
Summary of findings
Background
Description of the condition
Hyperkalaemia, defined as a serum potassium > 5.5 mmol/L is one of the most common laboratory electrolyte abnormalities (Betts 2018). Hyperkalaemia may result from various acute and chronic conditions that affect renal potassium excretion including a decreased glomerular filtration rate (GFR), and impaired adaptive responses to hyperkalaemia, such as decreased aldosterone release or extrarenal excretion across the gut. In addition, to impaired potassium excretion in chronic kidney diseases (CKD), hyperkalaemia can be incurred by renin‐angiotensin‐aldosterone system (RAAS) inhibitors as first‐line therapy to lower blood pressure (James 2014). Hyperkalaemia frequently results in down‐titration or discontinuation of such guideline‐directed therapy (Collins 2017) and may be associated with poorer clinical outcomes as a result, especially in patients with structural cardiac disease (Montford 2017). Because of the key role of the kidney in maintaining potassium homeostasis, CKD (GFR category 3 to 5) and acute kidney injury are the most important risk factors associated with hyperkalaemia, and as the estimated (e) GFR decreases, the rates of major cardiovascular events and death increase (Tamargo 2018), especially in low‐income countries (Chowdhury 2018).
Hyperkalaemia occurs at a rate of approximately 8/100 person‐months for people with CKD (GFR < 60 mL/min/1.73 m2) and commonly occurs among people who have other comorbidities such as heart failure, diabetes mellitus, and hypertension (Alvarez 2017; Einhorn 2009). The prevalence of hyperkalaemia in the general population has been estimated at 2% to 3% (Kumar 2017). When the eGFR decreases from between 60 to 90 to below 20 mL/min/1.73 m2, the prevalence of hyperkalaemia (> 5 mEq/L) increases from 2% to 42% and to 56.7% in patients with CKD GFR category 5 (Tamargo 2018). Hyperkalaemia has been reported to develop in 44% to 73% of transplant recipients who receive immunosuppressive therapy with cyclosporin or tacrolimus (Palmer 2004).
For many individuals, hyperkalaemia can be asymptomatic or present with nonspecific signs and symptoms (e.g. weakness, fatigue, or gastrointestinal (GI) hypermotility) (Ng 2017) and it has been suggested that the incidence and prevalence of hyperkalaemia in the general population is underestimated (Davidson 2017; Rafique 2017). In the setting of CKD or heart failure, the overall medical costs can be higher for people with hyperkalaemia compared with patients without hyperkalaemia (Alvarez 2017).
Description of the intervention
Until recently, treatment strategies for chronic hyperkalaemia have been limited to dietary potassium restriction, reducing or eliminating exacerbating factors including RAAS inhibitor treatment, mineralocorticoid administration or introducing loop diuretics with or without sodium bicarbonate (Dunn 2015; Fried 2017; Rafique 2017). These treatment options can be unsustainable due to poor tolerability or may be relatively ineffective (dietary restriction).
Potassium binders are artificial resins that exchange cations (e.g. sodium or calcium) bound to a resin for potassium ions in the gut. This exchange increase potassium excretion in the stool.
The potassium exchange resins may be administered orally or per rectum. The first US Food and Drug Administration (FDA)‐approved potassium binder for the treatment of hyperkalaemia was sodium polystyrene sulfonate, approved for use in 1958. Sodium polystyrene sulfonate exchanges sodium for potassium, increasing faecal potassium excretion. Despite the absence of high‐quality randomised controlled trials (RCTs) confirming benefits on patient‐level outcomes, sodium polystyrene sulfonate became widely used. Documented adverse effects of treatment included nausea, vomiting, and constipation. Co‐administration of sorbitol to increase potassium excretion may infrequently have caused fatal intestinal necrosis (Cowan 2017; Davidson 2017).
A second potassium‐binding agent, calcium polystyrene sulfonate, exchanges calcium for potassium in the distal colon, thus potentially limiting the sodium retention associated with sodium polystyrene sulfonate and providing calcium supplementation. Calcium polystyrene sulfonate may also cause GI adverse events, including colonic necrosis and perforation, which has led to warnings in the prescribing information (Das 2018; Yu 2017).
Newer ion exchange resins have been developed for treatment of hyperkalaemia. These include patiromer and sodium zirconium cyclosilicate (also known as ZS‐9) (Cowan 2017). Patiromer is a potassium binder consisting of a non absorbed cation exchange polymer together with a calcium‐sorbitol counter‐ion complex that increases stability. Patiromer exchanges potassium ions for calcium ions, which may limit exposure to sodium for patients who are sensitive to sodium delivery including those with severe kidney, heart, or liver failure. ZS‐9 is an insoluble, non‐absorbed compound that has a lattice structure with octahedral‐ and tetrahedral‐coordinated zirconium and silicon atoms bridged by oxygen that exchange sodium and hydrogen for potassium and ammonium as it moves through the GI tract (Kumar 2017). The zirconium octahedral units confer a negative charge to favour cation exchange and entrapment of potassium ions.
Patiromer and ZS‐9 are available as a powder for oral suspension, and do not expand within the GI tract, which may lead to lower GI adverse effects, such as diarrhoea, constipation, nausea, and vomiting.
Other adverse events are rarely reported. ZS‐9 also binds and excretes ammonium ions which may lead to increased plasma bicarbonate levels at higher doses. These changes might have potential benefit for patients with CKD, who often present with metabolic acidosis (Tamargo 2018).
How the intervention might work
Patiromer lowers and maintains serum potassium levels among people with CKD who are prescribed RAAS inhibitors (AMETHYST‐DN 2015; Weir 2015). ZS‐9 normalises potassium levels in patients receiving RAAS inhibitors for the treatment of cardiovascular or kidney disease (HARMONIZE 2014; Packham 2015). Newer potassium binders may have greater selectivity for potassium than other cations such as calcium and magnesium and may therefore result in lower risk of clinical electrolyte abnormalities.
Through reductions in serum potassium, potassium exchange resins are hypothesised to improve clinically‐relevant endpoints associated with hyperkalaemia such as death and cardiovascular events. However, while potassium exchange resins have been shown to lower serum potassium levels and reduce recurrence of hyperkalaemia compared to placebo (AMETHYST‐DN 2015; HARMONIZE 2014; Packham 2015; PEARL‐HF 2011; Weir 2015) in proof‐of‐concept, short‐term studies, there is limited evidence regarding the efficacy and safety of these interventions on clinically‐relevant endpoints such as hospitalisation and cardiovascular complications (AMETHYST‐DN 2015; Tamargo 2018).
The effectiveness and safety of treatments may differ in people with CKD compared with other populations, due to the increased prevalence of metabolic derangements (e.g. hypocalcaemia, acidosis, and elevated uraemic solutes), bowel dysfunction, structural heart disease, and the altered metabolism of commonly‐used medications that may lead to drug‐drug interactions.
Why it is important to do this review
Newer potassium exchange resins have recently been approved by the FDA (patiromer; FDA 2015 and ZS‐9; FDA 2018) and older agents have been widely adopted into clinical practice. In light of the emergence of new pharmacological agents for hyperkalaemia and recent RCTs, it is necessary to review the evidence of effectiveness and tolerability of all potassium exchange resins among people with CKD.
As current studies of potassium exchange resins principally use hyperkalaemia and serum potassium levels as the primary efficacy outcomes, it is important to accumulate data from all existing studies to evaluate the evidence for patient‐centred endpoints including adverse events and death. A comprehensive systematic review is required to provide guidance to practitioners and policy‐makers interested in using potassium exchange resins for hyperkalaemia and to enable greater use of therapies that may also incur hyperkalaemia, such as RAAS inhibitors. We did not examine studies evaluating treatment of acute hyperkalaemia or emergency interventions for hyperkalaemia as these have been summarised in previous Cochrane reviews (Batterink 2015; Mahoney 2005).
Objectives
To assess the benefits and harms of potassium binders for treating chronic hyperkalaemia among adults and children with CKD.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs of any design (e.g. parallel, cross‐over, factorial) and controlled clinical studies using a quasi‐randomised method of allocation (such as alternation, use of alternate medical records, date of birth, or other predictable methods). Reports of studies were eligible regardless of the language used or date of publication. We included studies published as full articles or available as a full study report, studies in which results were published on a trial registry, or studies published as conference proceedings.
Types of participants
Inclusion criteria
We included studies that enrolled adults and children with CKD and with chronic hyperkalaemia or at risk of chronic hyperkalaemia (including people with progressive kidney decline, comorbidities, the use of medications that affect the RAAS, and a diet high potassium) (Bozkurt 2003; Einhorn 2009; Moranne 2009; RALES 1996; Shah 2005; Weiner 2010). We also included studies involving participants with tubular renal disorders associated with hyperkalaemia, including renal tubular acidosis disorders and pseudo‐hypoaldosteronism types 1 and 2. Chronic hyperkalaemia was defined as serum potassium levels > 5.5 mmol/L (> 5.5 mEq/L). CKD was defined by Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for evaluation and management of CKD (KDIGO 2013) and included all stages of CKD. We included people treated with dialysis (CKD stage 5D), those who had end‐stage kidney disease (ESKD) treated with conservative care, recipients of a kidney transplant (CKD 5T), and those with earlier stages (1 to 4) of CKD. We included studies of potassium binders for hyperkalaemia in people with several medical conditions (such as heart failure) if the study included people with CKD. We obtained all study characteristics and outcome data pertaining to people with CKD, if these data were not available within study reports.
Exclusion criteria
We excluded studies that evaluated acute management of hyperkalaemia using interventions such as salbutamol, calcium gluconate, insulin‐dextrose, or sodium bicarbonate. We excluded studies of interventions for hyperkalaemia within the hospital setting.
Types of interventions
We included studies that evaluated potassium binders defined as potassium exchange resins that act to bind potassium within the GI tract including calcium polystyrene sulfonate, sodium polystyrene sulfonate with or without sorbitol, patiromer sorbitex calcium, and ZS‐9. Control of serum potassium levels could be achieved regardless of the route of administration, duration, frequency, or dose of the potassium binders. We included interventions administered orally or rectally.
Comparator treatments could be any of the following.
Placebo
Usual care (best supportive care)
Second potassium binder
Dietary restriction
Withdrawal of RAAS inhibition
Different doses of a potassium binder
Different routes of administration
Different frequency of administration.
Types of outcome measures
The outcomes selected included the relevant SONG core outcome sets as specified by the Standardised Outcomes in Nephrology initiative (SONG 2017).
Primary outcomes
Death (any cause and cardiovascular death)
Health‐related quality of life (HRQoL) (using any validated HRQoL measure)
GI symptoms (major or minor) (see Table 4 for definitions)
1. List of the most important gastrointestinal side effects.
| Gastrointestinal side effects | |
| Major | Minor |
| Haematemesis | Nausea |
| GI bleeding | Vomiting |
| GI haemorrhage | Gastroenteritis |
| Gastric ulceration | Abdominal pain |
| Gastric cancer | Indigestion |
| Pyloric stenosis | Diarrhoea |
| Melena | Constipation |
| Peptic ulcer | Flatulence |
| Bowel perforation | Heart‐burn |
| Bowel ischemias/necrosis | Dyspepsia |
| Irritable bowel syndrome | ‐ |
| Gastroesophageal reflux disease | ‐ |
| Crohn's disease | ‐ |
| Ulcerative colitis | ‐ |
| Haemorrhoids | ‐ |
| Diverticulitis | ‐ |
| Appendicitis | ‐ |
| Colitis | ‐ |
| Blood in stool (visible/occult) | ‐ |
| Vomiting blood | ‐ |
| GI event leading to abdominal surgery, including for bowel resection | ‐ |
| Gallstones | ‐ |
| Pancreatitis | ‐ |
GI ‐ gastrointestinal
Secondary outcomes
Serum potassium level
Cardiovascular disease (including cardiac arrhythmia)
Hospitalisation (any cause)
Life participation (any validated measure)
Vascular access
Graft health
Transplant graft loss, acute rejection, function
Fatigue
Cancer
Infection
Blood pressure (BP)
Withdrawal of blood pressure lowering therapy
Adverse events
We included studies that measure outcomes using standardised questionnaires with established reliability and validity (e.g. PIPER Fatigue Scale, Beck Depression Inventory (BDI), Short‐Form 36 (SF‐36)). We extracted endpoints as post‐intervention mean or change scores, together with standard deviations (SD), or the number of participants experiencing one or more events. We considered the study period and follow‐up as described in the included studies.
We categorised treatments in newer agents (patiromer or ZS‐9) and older agents (calcium polystyrene sulfonate and sodium polystyrene sulfonate). We reported outcomes as newer or older agents. Outcomes were assessed at the end of the follow‐up or as a change during follow‐up. When assessing outcomes in relation to time points, we grouped the data as: immediate post‐intervention, short‐term (post‐intervention to one month), medium‐term (between one and three months follow‐up), and long‐term (more than three months follow‐up) effects. We reported all primary outcomes in a table of Summary of findings for the main comparisons. To maximize clinical utility, we separated studies according to clinical setting (CKD/dialysis/transplant and for adults and children). Where necessary, we showed the overview of our results structure using a diagram to explain to the reader how the information was presented.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 10 March 2020 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations, and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were searched.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. Studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary, the full text, of these studies to determine which studies satisfy the predetermined inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a third author.
Data extraction and management
Data extraction was carried out independently by two authors using a standard data extraction form developed for this review. The review authors resolved discrepancies through discussion or adjudication by a third author. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions was highlighted.
For each included study, we recorded the following:
Study characteristics including type (e.g. parallel, cross‐over, factorial), country, source of funding, and trial registration status (with registration number recorded if available)
Participant characteristics including age, sex, stage of CKD, inclusion criteria, exclusion criteria
Intervention characteristics for each treatment group, and use of co‐interventions
Outcomes reports including the measurement instrument used and timing of outcome assessment
To prevent/minimise selective inclusion of data based on the results, we used the following a priori defined decision rules to select data from studies.
Where trial lists report both final values and change from baseline for the same outcome, we extracted final values
Where trial lists report both unadjusted and adjusted values for the same outcome, we extracted unadjusted values
Where trial lists report both data analysed on the intention‐to‐treat principle and another sample (e.g. per protocol, as treated), we extracted intention‐to‐treat data
For cross‐over studies, we extracted data from the first period only.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (death (any cause), myocardial infarction, stroke, cardiac arrhythmia, hospitalisation, dialysis vascular access complications, kidney transplant graft outcomes, cancer, infection, withdrawal of blood pressure lowering therapy, adverse events), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (HRQoL, serum potassium, life participation scales, depression, kidney transplant function) the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales have been used.
Meta‐analysis of change scores
We considered both change‐from‐baseline and final value scores for continuous outcomes. We combined change‐from‐baseline outcomes with final measurement outcomes using the MD method.
Time‐to‐event outcomes
We did not perform meta‐analysis of time‐to‐event outcomes.
Unit of analysis issues
Cluster‐randomised studies
We anticipated that studies using clustered randomisation were controlled for clustering effects. In case of doubt, we contacted the authors to ask for individual participant data to calculate an estimate of the intra‐cluster correlation coefficient (ICC). If this was not possible, we obtained external estimates of the ICC from a similar study or from a study of a similar population as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If ICCs from other sources were used, we reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC.
Cross‐over studies
Cross‐over studies were analysed using data from the first study period before cross‐over.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author/s) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
If the number of patients analysed was not presented for each time point, we considered the number of randomised patients in each group at baseline. For continuous outcomes with no SD reported, we calculated SDs from standard errors (SEs), 95% CIs or P‐values using the calculator tool in RevMan. If no measures of variation were reported and SDs could not be calculated, we imputed SDs from other studies in the same meta‐analysis, using the median of the other SDs available. For continuous outcomes presented only graphically, we extracted the mean and 95% CIs from the graphs using plotdigitizer (http://plotdigitizer.sourceforge.net/). For dichotomous outcomes, we used percentages to estimate the number of events or the number of people assessed for an outcome. Where data were imputed or calculated (e.g. SDs calculated from SEs, 95% CIs or P‐values, or imputed from graphs or from SDs in other studies), we reported this in the Characteristics of included studies.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures, and timing of outcome measurement are similar across studies. We assessed the heterogeneity by visual inspection of the forest plot. We quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003).
We interpreted the I2 statistic using the following as an approximate guide:
0% to 40%: may not be important heterogeneity
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
In the case of considerable heterogeneity, we explored the data further by comparing the characteristics of individual studies and any subgroup analyses.
Assessment of reporting biases
To assess publication bias, we planned to generate funnel plots if at least 10 studies examining the same treatment comparison were included in the review, and comment on whether any asymmetry in the funnel plot was due to publication bias, or methodological or clinical heterogeneity of the studies. To assess for potential small‐study effects in meta‐analysis (i.e. the intervention effect is more beneficial in smaller studies), we planned to compare effect estimates derived from a random‐effects model and a fixed‐effect model of meta‐analysis. In the presence of small‐study effects, the random‐effects model could give a more beneficial estimate of the intervention than the fixed‐effect estimate.
To assess outcome reporting bias, we compared the outcomes specified in study protocols with the outcomes reported in the corresponding study publications.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis to explore possible sources of heterogeneity (where sufficient data are available).
Stage of CKD
Co‐prescribing of RAAS inhibitors
However, subgroup analysis could not be done for heterogeneity owing to insufficient data.
Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. Where possible, the risk difference (RD) with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country
However, sensitivity analysis could not be done for heterogeneity owing to insufficient data.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b).
We presented the following outcomes in the 'Summary of findings' tables.
Death (any cause)
Cardiovascular death
Cardiac arrhythmia
HRQoL
Serum potassium
Major GI adverse events or minor GI adverse events (constipation)
Results
Description of studies
Results of the search
The electronic search strategy of the Cochrane Kidney and Transplant Specialised Register (10 March 2020) identified 105 records and handsearching identified one record (Figure 1). After initial title and abstract screening and examination of the full text, none of the retrieved records were excluded. Three studies are ongoing (DIALIZE China 2020; DIAMOND 2019; NCT03781089) and will be assessed in a future update of this review. This review therefore includes 15 studies (103 reports) randomising 1849 adults.
1.
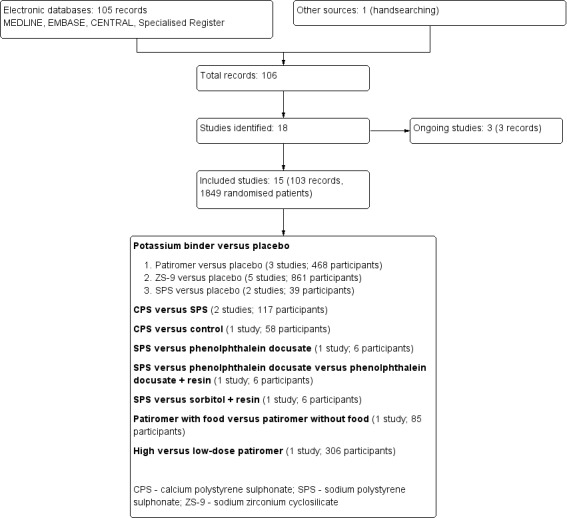
Study flow diagram
Included studies
The characteristics of the participants and the interventions in included studies are detailed in the Characteristics of included studies table.
Study design, setting and characteristics
Study duration varied from 12 hours to 52 weeks, with a median of 4 weeks. Three studies (Gruy‐Kapral 1998; Nakayama 2018; Wang 2018a) had a cross‐over study design in which participants were administered each of the study interventions sequentially, with or without a washout period. Studies were conducted from 1998 to 2019 in Canada (Lepage 2015), China (Wang 2018a), Japan (Kashihara 2018; Nakayama 2018), Pakistan (Nasir 2014), Europe (AMETHYST‐DN 2015), Europe and the USA (OPAL‐HK 2015), Europe, the USA, Ukraine, Russia, and Georgia (PEARL‐HF 2011), Europe, the USA, South Africa, Ukraine and Georgia (AMBER 2018), the USA (Ash 2015; Gruy‐Kapral 1998; TOURMALINE 2017), the USA, Japan, Russia and the UK (DIALIZE 2019), and the USA, Australia and South Africa (HARMONIZE 2014; Packham 2015). Fourteen studies received at least some funding from companies that manufacture potassium binders. Nasir 2014 provided no specific details about funding sources.
Study participants
Twelve studies involved participants with CKD (stages 1 to 5) not requiring dialysis. Three studies (DIALIZE 2019; Gruy‐Kapral 1998; Wang 2018a) involved participants treated with haemodialysis (HD). The sample size varied from six participants (Gruy‐Kapral 1998) to 320 participants (Packham 2015) (median of 39 participants). No studies evaluated treatment in children with CKD. The mean study age ranged from 53.1 years (Nasir 2014) to 73 years (Kashihara 2018) (median 66.8 years). Four studies (HARMONIZE 2014; Packham 2015; PEARL‐HF 2011; TOURMALINE 2017) evaluated treatment in people with CKD and heart failure, diabetes mellitus (type 1 or 2), and/or hypertension.
Interventions
Eight studies compared the more recently developed potassium binders (patiromer and ZS‐9) to placebo (AMBER 2018; Ash 2015; DIALIZE 2019; HARMONIZE 2014; Kashihara 2018; OPAL‐HK 2015; Packham 2015; PEARL‐HF 2011). Two studies compared an older potassium binder (sodium polystyrene sulphonate) to placebo (Gruy‐Kapral 1998; Lepage 2015). No studies compared calcium polystyrene sulphonate to placebo and in one study (Wang 2018a), calcium polystyrene sulphonate was compared to control. Two studies (Nakayama 2018; Nasir 2014) compared calcium polystyrene sulphonate to sodium polystyrene sulphonate. Gruy‐Kapral 1998 compared sodium polystyrene sulphonate either to laxatives or placebo. TOURMALINE 2017 evaluated administration of patiromer with compared to without food, and AMETHYST‐DN 2015 compared high‐dose to low‐dose patiromer.
There were no studies comparing newer (patiromer and ZS‐9) to older (calcium polystyrene sulphonate and sodium polystyrene sulphonate) potassium binders.
Excluded studies
No studies were excluded in this review.
Risk of bias in included studies
The risk of bias for studies overall are summarised in Figure 2 and the risk of bias in each individual study is reported in Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Methods for generating the random sequence were deemed to be at low risk of bias in four studies (AMETHYST‐DN 2015; Ash 2015; Lepage 2015; Wang 2018a). In the remaining 11 studies, the method for generating the random sequence was unclear.
Allocation concealment was judged to be at low risk of bias in three studies (AMBER 2018; AMETHYST‐DN 2015; DIALIZE 2019). The risk of bias for allocation concealment was unclear in the remaining 12 studies.
Blinding
Eight studies (AMBER 2018; Ash 2015; DIALIZE 2019; HARMONIZE 2014; Kashihara 2018; Lepage 2015; Packham 2015; PEARL‐HF 2011) were blinded and considered to be at low risk of bias for performance bias. The remaining seven studies were not blinded and were considered at high risk of performance bias.
As most studies were based on laboratory assessment or patient‐centred outcomes including death, 13 studies were considered at low risk of bias and two studies (Lepage 2015; Nasir 2014) were considered as high risk of bias for blinding of outcome assessment.
Incomplete outcome data
Nine studies (AMBER 2018; AMETHYST‐DN 2015; Ash 2015; DIALIZE 2019; Lepage 2015; Nakayama 2018; Nasir 2014; PEARL‐HF 2011; Wang 2018a) met criteria for low risk of attrition bias. Two studies (Kashihara 2018; OPAL‐HK 2015) were considered at high risk of attrition bias as there was differential loss to follow‐up between treatment groups and high attrition rates was substantially higher in both treatment groups. In the remaining four studies, attrition bias was considered unclear. Loss to follow‐up was commonly due to withdrawal from the study, lack of available central laboratory results or adverse events.
Selective reporting
Twelve studies (AMBER 2018; AMETHYST‐DN 2015; Ash 2015; DIALIZE 2019; HARMONIZE 2014; Lepage 2015; Nakayama 2018; Nasir 2014; OPAL‐HK 2015; Packham 2015; PEARL‐HF 2011; TOURMALINE 2017) reported expected and clinically‐relevant outcomes and were deemed to be at low risk of bias. The remaining three studies did not report patient‐centred outcomes of death or adverse events.
Other potential sources of bias
One study (Lepage 2015) appeared to be free from other sources of bias. Ten studies (AMBER 2018; AMETHYST‐DN 2015; Ash 2015; DIALIZE 2019; HARMONIZE 2014; Kashihara 2018; OPAL‐HK 2015; Packham 2015; PEARL‐HF 2011; TOURMALINE 2017) were considered at high risk of bias due to the potential role of funding and for the remaining four studies the assessment of other source of bias was unclear.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Potassium binder versus placebo for chronic hyperkalaemia in people with chronic kidney disease (CKD).
| Potassium binder versus placebo for chronic hyperkalaemia in people with CKD | ||||||
|
Patient or population: people with CKD (including haemodialysis) Settings: majority of studies involved people with CKD not requiring dialysis. Only one study involved people undergoing haemodialysis Intervention: newer agents (patiromer, ZS‐9, RLY5016) or older agents (SPS, CPS) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Potassium binder | |||||
|
Death (any cause) Follow‐up: 0.29 to 14 weeks (median 9 weeks) |
6 per 1000 | 2 fewer per 1000 (5 fewer to 20 more) | RR 0.69 (0.11 to 4.32) | 688 (4) | ⊕⊕⊝⊝
low 1, 2 (CKD, 3 studies) ⊕⊝⊝⊝ very low 1,2,3 (HD, 1 study) |
Potassium binder may make little or no difference to death (any cause) compared to placebo in CKD The effect of potassium binder on death (any cause) in HD is very uncertain |
|
Cardiovascular death (newer agents) Follow‐up: 10 weeks |
No events | 1/97** | RR 3.06 (0.13 to 74.24) | 196 (1) | ⊕⊝⊝⊝ very low 4 | Studies were not designed to measure effects of potassium binder on cardiovascular death in HD |
| Cardiac arrhythmia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
HRQoL Follow‐up: 14 weeks |
The mean change in HRQoL in the potassium binder group was 2 points higher (0.22 to 3.78 points higher) than the placebo group | ‐‐ | 289 (1) | ⊕⊝⊝⊝ very low 5 | Studies were not designed to measure effects of potassium binder on HRQoL in CKD | |
|
Serum potassium Follow‐up: 1 to 10 weeks (median 5 weeks) |
The mean serum potassium level in the placebo group ranged from 4.85 to 5.70 mg/dL The mean serum potassium level in the phosphate binder group was 0.62 mg/dL lower (0.97 to 0.27 mg/dL lower) |
‐‐ | 277 (3) | ⊕⊕⊝⊝ low 1, 6 (CKD and HD, 2 studies and 1 study, respectively) | Potassium binders may lower serum potassium levels compared to placebo or no treatment in CKD and HD Additional comments 1) Serum potassium ‐ newer agents (patiromer, ZS‐9, RLY5016) Follow‐up: 8 to 10 weeks (median 9 weeks) The mean serum potassium level ranged across control groups from 4.85 to 5.70 mg/dL The mean serum potassium level with newer agents was 0.45 mg/dL lower (0.71 to 0.19 mg/dL lower) 2) Serum potassium ‐ older agents (SPS, CPS) Follow‐up: 1 week The mean serum potassium level in the control arm was 5.03mg/dL The mean serum potassium level with the older agents was 1.04 mg/dL lower (1.37 to 0.71 mg/dL lower) |
|
|
Constipation Follow‐up: 0.29 to 10 weeks (median 4.5 weeks) |
36 per 1000 | 21 more per 1000 (10 fewer to 90 more) |
RR 1.58 (0.71 to 3.52) |
425 (4) | ⊕⊕⊝⊝
low 1,2 (CKD, 3 studies) ⊕⊝⊝⊝ very low 1,2,3 (HD, 1 study) |
Potassium binder may make little or no difference to constipation compared to placebo in CKD It is very uncertain the effect of potassium binder on constipation in HD |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CKD: chronic kidney disease; CPS: calcium polystyrene sulphonate; HRQoL: health‐related quality of life; RR: risk ratio; SPS: sodium polystyrene sulphonate | ||||||
| GRADE Working Group grades of evidence ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. Most studies had unclear risks for sequence generation and allocation concealment and some studies were not blinded (participants and/or investigators)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to indirectness population
4 Cardiovascular death was reported by as a single study
5 HRQoL was reported by as a single study
6 Evidence certainty was downgraded by one level due to substantial between‐study heterogeneity
Summary of findings 2. Calcium polystyrene sulphonate (CPS) versus sodium polystyrene sulphonate (SPS) for chronic hyperkalaemia in people with chronic kidney disease (CKD).
| CPS versus SPS for chronic hyperkalaemia in people with CKD | ||||||
|
Patient or population: people with CKD Settings: all studies involved people with CKD not requiring dialysis Intervention: CPS Comparison: SPS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sodium polystyrene sulphonate (SPS) | Calcium polystyrene sulphonate (CPS) | |||||
| Death (any cause) | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Cardiovascular death | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Cardiac arrhythmia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| HRQoL | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
Serum potassium Follow‐up: 0.43 to 4 weeks (median 2.2 weeks) |
The mean serum potassium level in the SPS group ranged from 4.12 to 4.30 mg/dL The mean serum potassium level in the CPS group was 0.38 mg/dL higher (0.03 lower to 0.79 mg/dL higher) |
‐‐ | 117 (2) | ⊕⊕⊝⊝ low 1, 2 | CPS may make little or no difference to serum potassium level compared to SPS | |
|
Constipation Follow‐up: 0.43 weeks |
170 per 1000 | 51 fewer per 1000 (126 fewer to 150 more) |
RR 0.70 (0.26 to 1.88) | 97 (1) | ⊕⊕⊝⊝ low 3 | Studies were not designed to measure effects of CPS or SPS on constipation |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CKD: chronic kidney disease; CPS: calcium polystyrene sulphonate; HRQoL: health‐related quality of life; RR: risk ratio; SPS: sodium polystyrene sulphonate | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations. All studies had unclear risks for random sequence generation and allocation concealment and were not blinded (participants and/or investigators)
2 Evidence certainty was downgraded by one level due to moderate between‐study heterogeneity
3 Constipation was reported by as a single study
Summary of findings 3. High versus with low‐dose potassium binder for chronic hyperkalaemia in people with chronic kidney disease (CKD).
| High versus with low‐dose potassium binder for chronic hyperkalaemia in people with CKD | ||||||
|
Patient or population: people with CKD Settings: all studies involved people with CKD not requiring dialysis Intervention: high‐dose potassium binder Comparison: low‐dose potassium binder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low‐dose potassium binder | High‐dose potassium binder | |||||
| Death (any cause; sudden death) | 10 per 1000 | 9 more per 1000 (8 fewer to 201 more) | RR 1.94 (0.18 to 21.08) | 203 (1) | ⊕⊕⊝⊝ low 1 | Studies were not designed to measure effects of high‐dose potassium binder on death (any cause) |
| Cardiovascular death | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Cardiac arrhythmia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| HRQoL | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Serum potassium | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
Constipation Follow‐up: 53 weeks |
60 per 1000 | 28 more per 1000 (28 fewer to 176 more) | RR 1.46 (0.54 to 3.94) | 203 (1 study) |
⊕⊕⊝⊝ low 2 | Studies were not designed to measure effects of high dose potassium binder on constipation |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; CKD: chronic kidney disease; HRQoL: health‐related quality of life; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Death (any cause; sudden death) was reported by as a single study
2 Constipation was reported by a single study
Potassium binder versus placebo
Ten studies (AMBER 2018; Ash 2015; DIALIZE 2019; Gruy‐Kapral 1998; HARMONIZE 2014; Kashihara 2018; Lepage 2015; OPAL‐HK 2015; Packham 2015; PEARL‐HF 2011) involving 1367 randomised participants compared a potassium binder to placebo. The median follow‐up was 3.4 weeks. The certainty of the evidence was low for all outcomes (Table 1) in CKD and low or very low in HD.
Newer potassium binders (patiromer or ZS‐9) may make little or no difference to all‐cause death (any cause) in CKD (Analysis 1.1 (4 studies, 688 participants): RR 0.69, 95% CI 0.11 to 4.32; I2 = 0%; low certainty evidence), and DIALIZE 2019 (196 participants) reported no difference between potassium binders and placebo for cardiovascular death in people requiring HD (Analysis 1.2.1 (1 study, 196 participants): RR 3.06, 95% CI 0.13, 74.24). The treatment effect of older potassium binders (calcium polystyrene sulfonate or sodium polystyrene sulfonate) on death (any cause) and cardiovascular death was uncertain, as these outcomes were not reported.
1.1. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 1: Death (any cause)
1.2. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 2: Cardiovascular death
Potassium binders had uncertain effects on nausea (Analysis 1.3 (3 studies, 229 participants): RR 2.10, 95% CI 0.65 to 6.78; I2 = 0%; low certainty evidence), diarrhoea (Analysis 1.4 (5 studies, 720 participants): RR 0.84, 95% CI 0.47 to 1.48; I2 = 0%; low certainty evidence), vomiting (Analysis 1.5 (2 studies, 122 participants): RR 1.72, 95% CI 0.35 to 8.51; I2 = 0%; low certainty evidence) and constipation (Analysis 1.6 (4 studies, 425 participants): RR 1.58, 95% CI 0.71 to 3.52; I2 = 0%; low certainty evidence) in CKD. Ash 2015 reported no difference between potassium binders and placebo for abdominal pain (Analysis 1.7 (1 study, 90 participants): RR 1.52, 95% CI 0.06 to 36.34) in CKD.
1.3. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 3: Nausea
1.4. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 4: Diarrhoea
1.5. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 5: Vomiting
1.6. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 6: Constipation
1.7. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 7: Abdominal pain
Potassium binders may lower serum potassium levels at the end of treatment (Analysis 1.8 (3 studies, 277 participants): MD ‐0.62 mEq/L, 95% CI ‐0.97 to ‐0.27; I2 = 92%; low certainty evidence) in CKD and HD, and may favourably change serum potassium levels during treatment (Analysis 1.9 (2 studies, 105 participants): MD ‐0.75 mEq/L, 95% CI ‐1.27 to ‐0.23; I2 = 90%; low certainty evidence) in CKD. There was substantial statistical heterogeneity in the treatment effects between studies.
1.8. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 8: Serum potassium
1.9. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 9: Change in serum potassium
Potassium binders may make little or no difference to hypokalaemia (Analysis 1.10 (2 studies, 228 participants): RR 1.71, 95% CI 0.31 to 9.47; I2 = 34%; low certainty evidence), and hospitalisation (Analysis 1.11 (3 studies, 522 participants): RR 0.26, 95% CI 0.03 to 2.32; I2 = 0%; low certainty evidence) in CKD and HD. DIALIZE 2019 reported no difference between potassium binders and placebo for angina pectoris (Analysis 1.12 (1 study, 196 participants): RR 5.10, 95% CI 0.25 to 104.92) and infection (Analysis 1.13 (1 study, 196 participants): RR 1.36, 95% CI 0.60 to 3.08) in HD.
1.10. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 10: Hypokalaemia
1.11. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 11: Hospitalisation
1.12. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 12: Angina pectoris
1.13. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 13: Infection
Potassium binders may decrease systolic BP (Analysis 1.14 (2 studies, 369 participants): MD ‐3.73 mmHg, 95% CI ‐6.64 to ‐0.83; I2 = 79%; low certainty evidence) (Analysis 1.15 (1 study, 74 participants): MD ‐5.49 mmHg, 95% CI ‐6.32 to ‐4.66), with substantial statistical heterogeneity in the treatment effects between studies, and diastolic BP (Analysis 1.16 (1 study. 74 participants): MD ‐2.65 mmHg, 95% CI ‐3.44 to ‐1.86) (Analysis 1.17 (1 study, 74 participants): MD ‐3.87 mmHg, 95% CI ‐4.42 to ‐3.32) in CKD.
1.14. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 14: Systolic blood pressure
1.15. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 15: Change in systolic blood pressure
1.16. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 16: Diastolic blood pressure
1.17. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 17: Change in diastolic blood pressure
AMBER 2018 reported no difference between potassium binders and placebo for HRQoL at end of treatment (Analysis 1.18 (1 study, 189 participants): EuroQoL score MD 2.60, 95% CI 0.04, 5.16) and change HRQoL (Analysis 1.19 (1 study, 189 participants): EuroQoL score MD 2.00, 95% CI 0.22 to 3.78) in CKD.
1.18. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 18: HRQoL
1.19. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 19: Change in Health‐related QoL
DIALIZE 2019 reported no difference between potassium binders and placebo for shunt stenosis (Analysis 1.20 (1 study, 196 participants): RR 0.34 (95% CI 0.04 to 3.21) and kidney transplantation (Analysis 1.21 (1 study, 196 participants): RR 0.34, 95% CI 0.01 to 8.25) in HD.
1.20. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 20: Shunt stenosis
1.21. Analysis.

Comparison 1: Potassium binder versus placebo, Outcome 21: Kidney transplantation
There were no available data for the outcomes of cardiac arrhythmia or major GI events.
Calcium polystyrene sulfonate versus sodium polystyrene sulfonate
Two studies (Nakayama 2018; Nasir 2014) involving 117 participants compared calcium polystyrene sulfonate with sodium polystyrene sulfonate in CKD. Studies were not designed to evaluate the outcomes specified in this systematic review. The certainty of the evidence was low for all reported outcomes (Table 2).
Nasir 2014 reported calcium polystyrene sulfonate may increase nausea (Analysis 2.1 (1 study, 97 participants): RR 0.42, 95% CI 0.21 to 0.83) compared to sodium polystyrene sulfonate, while reported no difference between calcium polystyrene sulfonate and sodium polystyrene sulfonate for diarrhoea (Analysis 2.2 (1 study, 97 participants): RR 2.82, 95% CI 0.12 to 67.64), vomiting (Analysis 2.3 (1 study, 97 participants): RR 0.19, 95% CI 0.01 to 3.82), constipation (Analysis 2.4 (1 study, 97 participants): RR 0.70, 95% CI 0.26 to 1.88), and abdominal pain (Analysis 2.5 (1 study, 97 participants): RR 0.31, 95% CI 0.03 to 2.91).
2.1. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 1: Nausea
2.2. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 2: Diarrhoea
2.3. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 3: Vomiting
2.4. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 4: Constipation
2.5. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 5: Abdominal pain
Calcium polystyrene sulfonate may make little or no difference to serum potassium levels at the end of treatment compared to sodium polystyrene sulfonate (Analysis 2.6 (2 studies, 117 participants): MD 0.38 mEq/L, 95% CI ‐0.03 to 0.79; I2 = 42%; low certainty evidence). There was moderate statistical heterogeneity in the treatment effects between studies.
2.6. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 6: Serum potassium
Nasir 2014 reported no difference in systolic BP (Analysis 2.7 (1 study, 97 participants): MD 2.65 mmHg, 95% CI ‐4.79 to 10.09) and diastolic BP (Analysis 2.8 (1 study, 97 participants): MD ‐4.30 mmHg, 95% CI ‐9.32 to 0.72) at the end of treatment, between calcium polystyrene sulfonate and sodium polystyrene sulfonate.
2.7. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 7: Systolic blood pressure
2.8. Analysis.

Comparison 2: Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS), Outcome 8: Diastolic blood pressure
Data were not available for death (any cause or cardiovascular), cardiac arrhythmias, and HRQoL.
Calcium polystyrene sulfonate versus control
Wang 2018a (58 participants) evaluated calcium polystyrene sulfonate versus control for three weeks. No data were available for any of our review outcomes.
Sodium polystyrene sulfonate versus phenolphthalein docusate
Gruy‐Kapral 1998 (6 participants) evaluated sodium polystyrene sulfonate versus phenolphthalein docusate during a 12‐hour experiment. No data were available for any of our review outcomes.
Sodium polystyrene sulfonate versus phenolphthalein docusate versus phenolphthalein docusate + resin
Gruy‐Kapral 1998 (6 participants) evaluated sodium polystyrene sulfonate versus phenolphthalein docusate plus resin during a 12‐hour experiment. No data were available for any of our review outcomes.
Sodium polystyrene sulfonate versus sorbitol + resin
Gruy‐Kapral 1998 (6 participants) evaluated sodium polystyrene sulfonate versus sorbitol plus resin during a 12‐hour experiment. No data were available for any of our review outcomes.
Patiromer with food versus patiromer without food
TOURMALINE 2017 (85 participants) compared patiromer with food with patiromer without food for 4 weeks. No data were available for any of our review outcomes.
High‐dose versus low‐dose patiromer
AMETHYST‐DN 2015 compared high‐dose with low‐dose of patiromer for 52 weeks (Table 3) in CKD. They reported no difference between high‐ and low‐dose patiromer for death (any cause; sudden death) (Analysis 3.1 (1 study, 203 participants): RR 1.94, 95% CI 0.18 to 21.08) sudden death, while high‐dose patiromer may increase diarrhoea (Analysis 3.2 (1 study, 203 participants): RR 0.22, 95% CI 0.05 to 0.97) compared to low‐dose patiromer. AMETHYST‐DN 2015 reported no difference between high‐dose and low‐dose patiromer for constipation (Analysis 3.3 (1 study, 203 participants): RR 1.46, 95% CI 0.54 to 3.94), hypokalaemia (Analysis 3.4 (1 study, 203 participants): RR 0.97, 95% CI 0.20 to 4.70), stroke (Analysis 3.5 (1 study, 203 participants): RR 0.97, 95% CI 0.06 to 15.31) and myocardial infarction (Analysis 3.6 (1 study, 203 participants): RR 2.91, 95% CI 0.12 to 70.68).
3.1. Analysis.

Comparison 3: High dose potassium binder versus low dose potassium binder, Outcome 1: Death (any cause)
3.2. Analysis.

Comparison 3: High dose potassium binder versus low dose potassium binder, Outcome 2: Diarrhoea
3.3. Analysis.

Comparison 3: High dose potassium binder versus low dose potassium binder, Outcome 3: Constipation
3.4. Analysis.

Comparison 3: High dose potassium binder versus low dose potassium binder, Outcome 4: Hypokalaemia
3.5. Analysis.

Comparison 3: High dose potassium binder versus low dose potassium binder, Outcome 5: Stroke
3.6. Analysis.

Comparison 3: High dose potassium binder versus low dose potassium binder, Outcome 6: Myocardial infarction
Data were not available for cardiovascular death, cardiac arrhythmias, HRQoL, and serum potassium level.
Discussion
Summary of main results
We identified 15 studies randomising 1849 adult participants with CKD evaluating potassium binders for chronic hyperkalaemia (defined either as > 5 or > 5.5 mmol/L). Most studies (10 studies randomising 1367 participants) compared a potassium binder with placebo for a median of 3.4 weeks. Data for clinical outcomes included in this review were available in five of these 10 studies. Potassium binders for chronic hyperkalaemia reduce serum potassium levels compared with placebo in patients with CKD. In low or very low certainty evidence, it is uncertain whether potassium binders have effects on death (any cause or cardiovascular) and GI events. Potassium binders were also compared to no treatment, laxatives, sorbitol, a second binder, and the administration with and without food. Meta‐analysis was not possible for any of our review outcomes for these compared treatments, since single studies available did not address outcomes of our interest. The comparative effects of different doses of potassium binder is very uncertain.
Overall completeness and applicability of evidence
For this review, we identified 15 studies comparing different potassium binders approaches for patients with CKD, and the information from these studies is of insufficient certainty to inform clinical care or policy. Very few studies were in dialysis setting or compared different treatment doses and often the appropriateness of the dosage were not clearly reported. High‐dose patiromer was associated with more adverse events, although it decreased the potassium concentration. Most studies compared potassium binders with placebo, and outcome data were rarely reported or missing. All studies had small sample sizes (6 to 320 participants), were often short‐term, had methodological limitations, were a cross‐over design, or were primarily designed to evaluate surrogate measures of effect. No study reported outcome data for cardiac arrhythmias and withdrawal from BP‐lowering therapy. The short duration of the majority of included studies precluded the assessment of cardiovascular outcomes, including those potentially deriving from discontinuation or under dosing of demonstrably beneficial RAAS inhibitors, especially in proteinuric patients with CKD or concomitant heart failure. No study was designed to assess key patient outcomes, and potential adverse events related to treatment are not well understood or systematically reported. Standardisation of outcome reporting in future potassium binders studies as prioritised by the Standardised Outcomes in Nephrology (SONG 2017) by patients, caregivers and health professionals may assist to improve the evidence base for potassium binder therapy studies. Consistent measures for study outcomes would improve our confidence in the results of available studies.
Quality of the evidence
We used standard risks of bias domains within the Cochrane tool together with GRADE methodology (GRADE 2008) to assess the quality of study evidence. Since confidence in the evidence for death (any cause and cardiovascular), cardiac arrhythmias, and HRQoL were uncertain or could not be estimated, further studies might provide different results. Some studies were at high or unclear risks of bias for most of risk domains assessment, limiting the certainty of the evidence. We noted that four studies were at low risk of random sequence generation and three studies were at low risk of allocation concealment. Blinding of participants and investigators and outcome assessment were at low risk of bias in eight and 13 studies, respectively. Nine studies were at low risk methods for attrition, 12 studies were at low risk of selective reporting, and one study was at low risk of other potential sources of bias. The overall certainty of the evidence was assessed as low or very low certainty for all outcomes for which there were extractable data.
In this review, clinical outcomes were rarely available for many of the treatments. The variabilities of reporting methods in the individual studies hamper the data summary. Moderate or substantial heterogeneity in definitions and methods of reporting serum potassium levels and systolic BP were particularly relevant. The limited number of studies prevented exploration of other potential sources of heterogeneity in the analyses. Subgroup and sensitivity analysis could not be done for heterogeneity owing to insufficient data. Due to the limited number of studies and participants, it was not clear if there was a difference between the evidence from older to newer potassium binders. Since data were sparse, the assessment of adverse events in both treatments categories was not possible. All studies reported SD or SE as estimate of variance and some of them provided data in descriptive or figure format only.
Potential biases in the review process
This review was carried out using standard Cochrane methods. Each step was completed independently by at least two authors including selection of studies, data management, and risk of bias assessment, thus reducing the risks of errors in identification of eligible studies and adjudication of evidence certainty. A highly sensitive search of the Cochrane Kidney Transplant specialised register was last undertaken without language restriction in March 2020. The registry contains hand‐searched literature and conference proceedings, maximising the inclusion of grey literature in this review. We additionally requested data from authors. Many studies did not report key outcomes in a format available for meta‐analysis.
In this review the data availability in the individual studies were a potential source of bias. First, there was heterogeneity between treatment interventions and robust statistical estimates could not be estimated, due to the small number of included studies. Second, most studies were at high risks of bias, but poorer quality studies could not be excluded due to the small number of data observations. The limited number of studies was a constraint on our ability to assess for potential reporting bias and selective outcome reporting. Third, the definition of kidney disease varied across the eligible studies, although most participants had CKD (stage 1 to 5) not requiring dialysis. Fourth, the effects of potassium binder interventions on longer‐term outcomes is uncertain and the treatment endpoints were principally surrogate markers of health (BP, serum potassium). Finally, adverse event reporting was rarely provided.
Formal assessment for publication bias through visualisation of asymmetry in funnel plots was precluded for all treatments and outcomes because of few studies.
Agreements and disagreements with other studies or reviews
Few studies have examined the efficacy of potassium binders for people with CKD and the number of meta‐analysis published in this field is limited. The current Cochrane review is consistent with the findings of systematic review and meta‐analysis of published RCTs evaluating the efficacy and safety of patiromer for treating hyperkalaemia in patients with CKD or heart failure (Das 2018). In that review that included three studies, the authors found that there was a reduction of serum potassium with patiromer compared to placebo. Patiromer compared with placebo did not show a significant reduction in death risk (any cause) and serious cardiovascular events. In a second meta‐analysis of both RCTs and observational studies, dietary education significantly reduced the prevalence of hyperkalaemia and serum potassium, compared with control (Palaka 2018). In that analysis, the population of interest was not restricted to chronic hyperkalaemia in CKD, the interventions were both pharmacological and non‐pharmacological, observational studies were included, and GRADE was not used to evaluate evidence certainty.
A single large RCT, evaluating the effect of ZS‐9 in 320 patients with hyperkalaemia, reported a significant reduction in potassium levels compared with placebo (Packham 2015). In a previous Cochrane review of pharmacological interventions for the acute management of hyperkalaemia in adults (7 studies, 241 participants), salbutamol and other medications were safe and well‐tolerated, and may decrease serum potassium levels. There was very low certainty about whether medications made any difference to reduce death and cardiac arrhythmias compared to placebo (Batterink 2015). Potassium binders decreased serum potassium but did not improve death, cardiovascular events, or HRQoL.
Authors' conclusions
Implications for practice.
Potassium binders reduce serum potassium level compared to placebo among people with CKD, although there is low certainty evidence on all‐cause and cardiovascular death, cardiac arrhythmias and HRQoL. No data for treatment effects in children were identified. There is scant evidence to inform decision‐making about newer potassium binders or the comparative effectiveness and safety between older and newer treatments. Evidence is largely lacking in the setting of peritoneal dialysis, HD, home‐based HD, or transplantation. The potential adverse effects of treatment are largely unknown ‐ in particular, major GI events.
Implications for research.
Future adequately powered, rigorous RCTs are needed to assess the benefits and potential harms of potassium binders, including determining if their use will lead to maximizing the concomitant administration of RAAS inhibitors to improve clinical outcomes in people with CKD treated for chronic hyperkalaemia. More recent studies seem to be more promising and could show sufficient statistical power to detect treatment effects on important patient outcomes (including death, cardiovascular, and major GI adverse events). Further research is likely to change the estimated effects of treatments for chronic hyperkalaemia. Future potassium binder studies compared with placebo will increase our certainty of the evidence based on limitations in existing studies and a paucity of evidence in the CKD setting.
Evaluation of cost‐effectiveness for potassium binders approaches in CKD setting would assist decision‐making by policy‐makers and health care providers.
History
Protocol first published: Issue 11, 2018 Review first published: Issue 6, 2020
Acknowledgements
We would like to thank the editorial team of Cochrane Kidney Transplant for guidance and support throughout this protocol process. We would like to thank the editor and reviewers of this protocol and review.
The authors are grateful to the following peer reviewers for their time and comments: Kwek Jia Liang (Consultant, Department of Renal Medicine, Singapore General Hospital, Singapore), George L Bakris MD (Professor of Medicine, Director, Am. Heart Assoc. Comprehensive Hypertension Center, Department of Medicine, University of Chicago Medicine, Chicago, IL, USA), Stephen Walsh (Associate Professor in Experimental Medicine and Honorary Consultant Nephrologist, UCL Department of Renal Medicine, University College London, London, UK), Michael Emmett MD (Department of Internal Medicine, Baylor University Medical Center, Dallas, Texas, USA).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Potassium binder versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death (any cause) | 4 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.11, 4.32] |
| 1.1.1 Newer agents (patiromer, ZS‐9, RLY5016) | 4 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.11, 4.32] |
| 1.1.2 Older agents (SPS, CPS) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2.2 Older agents (SPS, CPS) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Nausea | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.65, 6.78] |
| 1.3.1 Newer agents (patiromer, ZS‐9, RLY5016) | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.38, 13.43] |
| 1.3.2 Older agents (SPS, CPS) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.42, 9.42] |
| 1.4 Diarrhoea | 5 | 720 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.47, 1.48] |
| 1.4.1 Newer agents (patiromer, ZS‐9, RLY5016) | 4 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.54, 2.18] |
| 1.4.2 Older agents (SPS, CPS) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.19, 1.33] |
| 1.5 Vomiting | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.35, 8.51] |
| 1.5.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.16, 13.82] |
| 1.5.2 Older agents (SPS, CPS) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.20, 19.91] |
| 1.6 Constipation | 4 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.71, 3.52] |
| 1.6.1 Newer agents (patiromer, ZS‐9, RLY5016) | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.50, 5.75] |
| 1.6.2 Older agents (SPS, CPS) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.52, 4.32] |
| 1.7 Abdominal pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Older agents (SPS, CPS) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Serum potassium | 3 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.97, ‐0.27] |
| 1.8.1 Newer agents (patiromer, ZS‐9, RLY5016) | 2 | 246 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.71, ‐0.19] |
| 1.8.2 Older agents (SPS, CPS) | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.37, ‐0.71] |
| 1.9 Change in serum potassium | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.27, ‐0.23] |
| 1.9.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | 74 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.55, ‐0.47] |
| 1.9.2 Older agents (SPS, CPS) | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.36, ‐0.72] |
| 1.10 Hypokalaemia | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.31, 9.47] |
| 1.10.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.31, 3.41] |
| 1.10.2 Older agents (SPS, CPS) | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.39, 125.44] |
| 1.11 Hospitalisation | 3 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.32] |
| 1.11.1 Newer agents (patiromer, ZS‐9, RLY5016) | 2 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.32] |
| 1.11.2 Older agents (SPS, CPS) | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.12 Angina pectoris | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.12.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.12.2 Older agents (SPS, CPS) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.13 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.13.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.13.2 Older agents (SPS, CPS) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.14 Systolic blood pressure | 2 | 369 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐6.64, ‐0.83] |
| 1.14.1 Newer agents (patiromer, ZS‐9, RLY5016) | 2 | 369 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐6.64, ‐0.83] |
| 1.14.2 Older agents (SPS, CPS) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.15 Change in systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.15.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.15.2 Older agents (SPS, CPS) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.16 Diastolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.16.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.16.2 Older agents (SPS, CPS) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.17 Change in diastolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.17.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.17.2 Older agents (SPS, CPS) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18 HRQoL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18.2 Older agents (SPS, CPS) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19 Change in Health‐related QoL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19.2 Older agents (SPS, CPS) | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.20 Shunt stenosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.20.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.20.2 Older agents (SPS, CPS) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.21 Kidney transplantation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.21.1 Newer agents (patiromer, ZS‐9, RLY5016) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.21.2 Older agents (SPS, CPS) | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Calcium polystyrene sulfonate (CPS) versus sodium polystyrene sulfonate (SPS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Vomiting | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Constipation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5 Abdominal pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6 Serum potassium | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.03, 0.79] |
| 2.7 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.8 Diastolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. High dose potassium binder versus low dose potassium binder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1.1 Sudden death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Constipation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4 Hypokalaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5 Stroke | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.6 Myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMBER 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned (1:1)." Comment: Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomly assigned (1:1) with an interactive web response system to receive either placebo or patiromer (8.4 g once daily), in addition to open‐label spironolactone (starting at 25 mg once daily) and their baseline blood pressure medications." Comment: An interactive web response system is considered as a low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This phase 2, multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study of patiromer to enable spironolactone use for blood pressure control in patients with resistant hypertension and chronic kidney disease. [...] Participants, the study team that administered treatments and measured blood pressure, and the investigators were masked to assigned treatment groups." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At weeks 1, 4, 8, and 12, medication adherence was evaluated via the measurement of spironolactone in plasma (validated liquid chromatography–tandem mass spectrometry; minimum level of detection 1 ng/mL) and qualitative assessment of associated chromatograms for peaks corresponding to spironolactone metabolites 7α‐thiomethylspironolactone and canrenone. A single 24‐h urine collection was done, beginning at least 24 h before the baseline visit, which was used to determine urine sodium, K+, and the albumin to creatinine ratio. [...] Blood pressure was measured using an oscillometric monitoring device. [...] The study was overseen by an independent Data Safety and Monitoring Committee, which was responsible for reviewing and evaluating all relevant information that may have had an impact on the safety of the study participants, assessing risks and benefits to study participants, providing recommendations to the study sponsor concerning continuation, termination or amendments to the study and reviewing safety, dosing and pharmacodynamic data of both spironolactone and patiromer throughout the study." Comment: An independent Data Safety and Monitoring Committee assessed the outcomes. Outcomes were at low risk of detection bias due to the nature of other outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Overall, 141 (95%) of 148 patients in the placebo group and 144 (98%) of 147 patients in the patiromer group completed the study. The most common reasons for premature study discontinuation were adverse events (three patients in the placebo group and one patient in the patiromer group) and consent withdrawal (three patients in the placebo group and one patient in the patiromer group). [...] Efficacy endpoints and safety were assessed in all randomised patients (intention to treat)." Comment: As reported in figure 1, all participants were analysed |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
AMETHYST‐DN 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Stratum 1
Stratum 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A validated interactive web response system was used to assign patients to cohorts and starting doses using computer‐generated randomisation lists stratified by cohort, with a block size of 3." Comment: A computer generation model is considered as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "A validated interactive web response system was used to assign patients to cohorts." Comment: A validated interactive web response system is consider as a low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The AMETHYST‐DN phase 2 multicenter, open‐label, dose‐ranging randomised clinical trial was conducted to inform dose selection." Comment: An open‐label study is considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Central laboratory measurements were used for assessments of baseline values and efficacy and safety analyses. [...] Prior to database lock and after the last patient’s follow‐up visits were completed, a safety review board was convened to independently review all deaths occurring within the study. Adjudication was performed for cause of death and whether the death was related to hypokalaemia or hyperkalaemia." Comment: Outcomes were at low risk of detection bias due to independent outcome adjudication and the nature of other outcome measures. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 324 patients were enrolled; 222 patients entered stratum 1 (mild hyperkalaemia) and 84 entered stratum 2 (moderate hyperkalaemia), for a total of 306 randomised patients (Figure 1). Two patients with mild hyperkalaemia did not receive patiromer and therefore were not included in the efficacy or safety analyses. Of the 304 patients evaluated at week 4 or time of first dose titration, 300 were analysed for the primary efficacy endpoint. One patient each with mild hyperkalaemia in the patiromer 8.4 g/d and 25.2 g/d groups and 1 patient with moderate hyperkalaemia in the patiromer 25.2 g/d group were excluded from the analysis because of missing baseline values. A patient with mild hyperkalaemia in the patiromer 33.6 g/d group was excluded because of a lack of available central laboratory results. Disposition for the entire study is presented in Figure 1." Comment: As reported in figure 1, 300/306 participants were analysed |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
Ash 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote; "A centralized computer‐generated, block randomisation scheme was used (random block size, three or six per site for each cohort)." Comment: A computer generation model is considered as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Here we conducted a phase 2 randomised, double‐blind, placebo‐controlled dose‐escalation study to assess safety and efficacy of sodium zirconium cyclosilicate (ZS‐9)." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Serum samples were analysed locally and by the central laboratory; study eligibility used local laboratory values." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "None withdrew and sodium zirconium cyclosilicate (ZS‐9) dose dependently reduced serum potassium." Comment: As reported in figure 1, all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
DIALIZE 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In the treatment period, patients were randomised 1:1 to receive orally a starting dose of sodium zirconium cyclosilicate 5 g or placebo once daily on non dialysis days. [...] Randomization codes were generated in blocks of 4 (2 + 2 for each treatment arm) to ensure balance (1:1) between the two treatment arms. Randomization was stratified by country." Comment: Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed using randomisation codes and an interactive voice/interactive web response system." Comment: An interactive voice/interactive web response system is considered as low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study site staff and patients were blinded to treatment assignment. To ensure blinding, sachets were enclosed in a carton with a tamper evident seal intended to be broken exclusively by patients immediately before taking the study drug." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All adverse events were classified using the Medical Dictionary for Regulatory Activities. [...] Serum K+ concentrations were measured using central laboratory assessment and a point‐of‐care i‐STAT device." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All randomised patients except for one patient in the sodium zirconium cyclosilicate group received treatment (99.5% [n=195 of 196]). In total, 95.9% of patients (n=188 of 196) completed the study. Rates of study completion were balanced between treatment groups (sodium zirconium cyclosilicate group, 94.8% [n=92 of 97]; placebo group, 97.0% [n=96 of 99])." Comment: As reported in figure 1, all participants were analysed |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
Gruy‐Kapral 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Total stool output of cations was calculated as the product of stool weight and cation concentration of an acid‐digested stool sample. Insoluble cation concentration is the difference between total and soluble cation output. Plasma concentration of chloride, bicarbonate, and glucose were measured by automated analysis. Stool resin concentration was estimated by measuring stool cation binding capacity." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Major clinical and adverse event outcomes were not systematically reported. Data were not reported appropriately for a cross‐over trial design in a format that was extractable for meta‐analysis |
| Other bias | Unclear risk | Co‐interventions, adherence, similarity of treatment groups at baseline, intention‐to‐treat analysis and timing of outcome assessments were not reported sufficiently to adjudicate risk. Funder was unlikely to influence data analysis and study reporting or interpretation |
HARMONIZE 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "HARMONIZE was a phase 3, multicenter, randomised, double‐blind, placebo‐controlled trial evaluating zirconium cyclosilicate in outpatients with hyperkalaemia (serum potassium >= 5.1 mEq/L)." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All potassium levels were measured (after an 8‐hour fast) in whole blood with a point‐of‐care device. All samples were then centrifuged on site and serum sent to a central laboratory." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication for people with CKD |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Baseline characteristics were not reported for patients with CKD. Funder was likely to influence data analysis and study reporting or interpretation |
Kashihara 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We evaluated sodium zirconium cyclosilicate doses for correcting serum potassium in Japanese patients with hyperkalaemia in a multicenter, double‐blind,
placebo‐controlled phase 2/3 trial." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All patients (34 on sodium zirconium cyclosilicate 5 g; 36 on sodium zirconium cyclosilicate 10 g; 33 on placebo) completed the study except 2 placebo patients discontinued due to hyperkalaemia." Comment: 101/103 participants completed the study. However, all patients who discontinued the treatment were assigned in the placebo group, meaning that there were differences between groups |
| Selective reporting (reporting bias) | High risk | Major clinical and adverse event outcomes were not systematically reported |
| Other bias | High risk | Co‐interventions, adherence, similarity of treatment groups at baseline, intention‐to‐treat analysis were not reported sufficiently to adjudicate risk. Funder was likely to influence data analysis and study reporting or interpretation |
Lepage 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised in a 1:1 ratio in blocks of four to receive a fixed dose of 30 g sodium polystyrene sulfonate (SPS) or placebo orally one time per day for 7 days. The random treatment arm sequence was generated by the web site www.randomization.com and managed by independent pharmacists of the research sector of the pharmacy department." Comment: This sequence generation (using the web site www.randomization.com) is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "33 outpatients with CKD and mild hyperkalaemia (5.0–5.9 mEq/L) in a single teaching hospital were included in this double–blind randomised clinical trial. [...] Patients, investigators, care providers, and statisticians were blinded for the duration of the trial and until all statistical analyses were completed." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Seven days after randomisation, patients returned for blood tests and completed the same side effects questionnaire as on day 3." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, subjective measures were included in outcomes, and were possibly influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients had no measure of serum potassium levels at the end of the study period (day 7) and therefore, could not be included in the analysis. One patient allocated to the sodium polystyrene sulfonate (SPS) group left the study on day 3 for important gastrointestinal side effects and refused to come to the follow‐up visit on day 7. One patient receiving placebo had unplanned blood work done through a different laboratory, which revealed an elevated serum potassium level of 6.2 mEq/L. On the basis of the commendation of the patient’s regular physician, the patient left the study to receive unblinded treatment." Comment: 31/33 participants completed the study, without differences between groups |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | Low risk | Similar participants baseline characteristics and co‐interventions. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Nakayama 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Twenty hyperkalaemic patients were randomly assigned to calcium polystyrene sulfonate (CPS) group (n = 10) or sodium polystyrene sulfonate (SPS) group (n = 10) by an envelope method." Comment: Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was designed as a prospective, open‐labelled, randomised, and cross‐over study." Comment: An open‐label study is considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blood and urine chemistries were measured at a commercially available laboratory. [...] We calculated corrected calcium levels using the calcium correction formula. Venous blood gas was taken to analyse the plasma bicarbonate (HCO3‐) levels." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | Unclear risk | There was no similarity between treatment groups at baseline. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
Nasir 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This single‐blind randomised control trial was done from 15th January 2010 till 31st December 2010." Comment: A single‐blind study is considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Adverse events were recorded in an event reporting form." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. However, subjective measures were included in outcomes, and were possibly influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As reported in figure 1, all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | Unclear risk | There were similarities between treatment groups at baseline. Supporting source and timing of outcome assessments were not reported sufficiently to adjudicate risk |
OPAL‐HK 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study had two phases (a 4‐week single group, single‐blind initial treatment phase and an 8‐week) placebo‐controlled, single‐blind, randomised withdrawal phase." Comment: A single‐blind study is considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All electrocardiograms were read at a core electrocardiographic laboratory." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Ten patients (18%) in the patiromer group and 22 (42%) in the placebo group discontinued the randomised withdrawal phase prematurely; the most common reasons for discontinuation were elevated potassium levels that met the prespecified withdrawal criteria (2 patients (4%) in the patiromer group and 16 (31%) in the placebo group) and potassium levels of less than 3.8 mmol per litre (3 patients (5%) in the patiromer group and 1 (2%) in the placebo group)." Comment: 75/107 participants completed the study, with differences between groups |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | There were similarities between treatment groups at baseline. Funder was likely to influence data analysis and study reporting or interpretation |
Packham 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In this multicenter, two‐stage, double‐blind, phase 3 trial, we randomly assigned 753 patients with hyperkalaemia to receive either sodium zirconium cyclosilicate (at a dose of 1.25 g, 2.5 g, 5 g, or 10 g) or placebo three times daily for 48 hours." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All samples were centrifuged on site, and serum samples were sent to a central laboratory for analysis and verification." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform an adjudication for people with CKD |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Baseline characteristics were not reported for patients with CKD. Funder was likely to influence data analysis and study reporting or interpretation |
PEARL‐HF 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This 4‐week, double‐blind, randomised, placebo‐controlled, parallel‐group study was conducted at 38 centres. [...] Following baseline assessments, patients who continued to meet eligibility criteria were randomised 1:1 to RLY5016 or placebo treatment in a blinded fashion." Comment: A double‐blind study is considered as a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Key outcomes were laboratory measures and were unlikely to be influenced by knowledge of treatment assignment. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy and safety analyses were performed on the modified intent‐to‐treat population, defined as all randomised patients who received study medication and had available efficacy data. All modified intent‐to‐treat patients were included in the primary and secondary efficacy analyses." Comment: All participants were included into the analysis |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Similar participants baseline characteristics and co‐interventions. Funder was likely to influence data analysis and study reporting or interpretation |
TOURMALINE 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Adults with hyperkalaemia (potassium ≥ 5.0 mEq/L) were randomised (1:1) to receive patiromer once daily without food or with food for 4 weeks." Comment: Methods for generation of the random sequence were not reported in sufficient detail to assess risk |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "TOURMALINE was an open‐label study of patients with hyperkalaemia. Patients and site study staff were not blinded to treatment assignment (i.e., with or without food groups); sponsor, contractors, and personnel supporting the study who did not require access to patient source documents or to the electronic data capture system were blinded to treatment assignment." Comment: An open‐label study is considered as a high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Overall, 114 patients were randomised to patiromer without food (n= 57) or with food (n= 57). Of these, 103 patients (90%) completed the study (figure 2); reasons for early study termination included adverse events (n= 3), investigator’s decision (n= 3), withdrawal by patient (n= 3), lost to follow‐up (n= 1), and other reason (n= 1; patient did not disclose taking prohibited medications). One patient in the with‐food group did not receive any patiromer dose and was excluded from the efficacy and safety analyses. A second patient in the with‐food group had an important protocol deviation, had no post‐baseline serum potassium, and was excluded from the efficacy analyses." Comment: Not reported in sufficient detail to perform an adjudication for people with CKD |
| Selective reporting (reporting bias) | Low risk | Clinically‐relevant outcomes that would be expected for this type of intervention were reported |
| Other bias | High risk | Treatment groups were quite similar at baseline. Funder was likely to influence data analysis and study reporting or interpretation |
Wang 2018a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1 ratio into two groups, the calcium–polystyrene sulfonate group and the blank control group, using computer‐generated randomised sequences." Comment: Computer generation is considered as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation were not reported in sufficient detail to perform adjudication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Participants and investigators were unlikely to be blinded to treatment allocation due to physical differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Before and after treatments, patients underwent conventional 12‐lead electrocardiography after a 5‐minute resting period. Under the guidance of standardised operation procedures, two specialists who were blinded to the serum potassium concentration independently evaluated the electrocardiography changes." Comment: Outcomes were principally laboratory measures and were at low risk of detection bias regardless of whether blinding of investigators or outcome assessors occurred. The specialists who assessed electrocardiography changes were blind to the treatment assigned. It was not stated whether GI adverse events were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As reported in figure 1, all participant completed the study |
| Selective reporting (reporting bias) | High risk | Major clinical and adverse event outcomes were not systematically reported. Data were not reported appropriately for a cross‐over trial design in a format that was extractable for meta‐analysis |
| Other bias | Unclear risk | There was no similarity between treatment groups at baseline. Funder was unlikely to influence data analysis and study reporting or interpretation. No other apparent sources of bias |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ACR ‐ albumin:creatinine ratio; ARB ‐ angiotensin receptor blocker; AKI ‐ acute kidney injury; AV ‐ arteriovenous; BMI ‐ body mass index; BP ‐ blood pressure; BPM ‐ beats per minute; CKD ‐ chronic kidney disease; DBP ‐ diastolic blood pressure; (e)GFR ‐ (estimated) glomerular filtration rate; ESKD ‐ end‐stage kidney disease; GI ‐ gastrointestinal; Hb ‐ haemoglobin; HD ‐ haemodialysis; HDF ‐ haemodiafiltration; HIV ‐ human immunodeficiency virus; Kt/V ‐ dialysis adequacy; MI ‐ myocardial infarction; NSAID ‐ nonsteroidal anti‐inflammatory drug; (i)PTH ‐ (intact) parathyroid hormone; RCT ‐ randomised controlled trial; RAAS(i) ‐ renin‐angiotensin‐aldosterone system (inhibitor); SBP ‐ systolic blood pressure; SCr ‐ serum creatinine; SD ‐ standard deviation; URR ‐ urea reduction ratio; ZS‐9 ‐ sodium zirconium cyclosilicate
Characteristics of ongoing studies [ordered by study ID]
DIALIZE China 2020.
| Study name | A phase 3b, multicentre, prospective, randomised, double‐blind, placebo‐controlled study to reduce incidence of pre‐dialysis hyperkalaemia with sodium zirconium cyclosilicate in Chinese subjects |
| Methods | Randomised, double‐blind, placebo‐controlled study to determine the safety and efficacy of ZS‐9 in ESKD subjects with hyperkalaemia and on stable HD. This study consists of a screening period, an 8‐week randomised treatment period, and a follow‐up period. Approximately 134 stable HD subjects with persistent pre‐dialysis hyperkalaemia will be enrolled in the study across research sites in China |
| Participants | Country: China Setting: multicentre Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 5 June 2020 |
| Contact information | AstraZeneca Email: information.center@astrazeneca.com |
| Notes | Study stage: not yet recruiting |
DIAMOND 2019.
| Study name | Patiromer for the Management of Hyperkalemia in Subjects Receiving RAASi Medications for the Treatment of Heart Failure (DIAMOND) ‐ the official title should be: "A Multicenter, Double‐blind, Placebo‐controlled, randomised Withdrawal, Parallel Group Study of Patiromer for the Management of Hyperkalemia in Subjects Receiving Renin Angiotensin Aldosterone System Inhibitor (RAASi) Medications for the Treatment of Heart Failure (DIAMOND)" |
| Methods | Study design: phase 3b multinational, multicentre, double‐blind, placebo‐controlled, randomised withdrawal, parallel group study Study duration: not reported Duration of follow‐up: approximately 2.5 years |
| Participants | Country: multinational Setting: multicentre Inclusion criteria
Exclusion criteria
Number estimated randomised: 2388 |
| Interventions | Treatment group
Control group
C0‐interventions
|
| Outcomes |
|
| Starting date | April 2019 |
| Contact information | Study director: Goehring UM Phone number: 650‐421‐9500 Email: Diamond_Study@viforpharma.com |
| Notes | Funding: Relypsa, Inc. and Vifor Pharma Trials registration: NCT03888066 |
NCT03781089.
| Study name | Patiromer Efficacy to Reduce Episodic Hyperkalemia in End Stage Renal Disease Patients Treated With Hemodialysis (PEARL‐HD) |
| Methods | A prospective, randomised, open‐label trial. Eligible ESRD patients who are on thrice weekly HD schedule will be screened from retrospective review of clinical and laboratory parameters from our clinical practice group. A total of 40 patients (randomised 1:1 study drug: usual care) will be enrolled. Duration of study medication exposure will be 4 weeks. The total duration of study, from enrolment until the end of the washout period will be 7 weeks |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 20 June 2019 |
| Contact information | Robin Gilliam, MSW Email: robin.gilliam@duke.edu |
| Notes |
AV ‐ arteriovenous; ESKD ‐ end‐stage kidney disease; Hb ‐ haemoglobin; HD ‐ haemodialysis; HDF ‐ haemodiafiltration; MI ‐ myocardial infarction; PTH ‐ parathyroid hormone; Qb ‐ blood flow; RAAS(i) ‐ renin‐angiotensin‐aldosterone system (inhibitor); URR ‐ urea reduction ratio; ZS‐9 ‐ sodium zirconium cyclosilicate
Differences between protocol and review
Since major GI adverse events were not reported, we included minor GI events (constipation) in Summary of findings tables. We categorised treatments in newer agents (patiromer or ZS‐9) and older agents (calcium polystyrene sulfonate and sodium polystyrene sulfonate). We reported outcomes as newer or older agents.
Contributions of authors
Draft the protocol: PN, SP
Study selection: PN, MR
Extract data from studies: PN, MR
Enter data into RevMan: PN, MR
Carry out the analysis: PN, SP
Interpret the analysis: PN, SP, MR, VS, GFMS
Draft the final review: PN, SP
Disagreement resolution: SP
Update the review: SP, GFMS
Declarations of interest
Patrizia Natale: none known
Suetonia C Palmer: none known
Marinella Ruospo: none known
Valeria M Saglimbene: none known
Giovanni FM Strippoli: none known
New
References
References to studies included in this review
AMBER 2018 {published data only}
- Agarwal R, Rossignol P, Garza D, Mayo MR, Warren S, Arthur S, et al. Patiromer to enable spironolactone use in the treatment of patients with resistant hypertension and chronic kidney disease: rationale and design of the AMBER study. American Journal of Nephrology 2018;48(3):172-80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Rossignol P, Garza D, Mayo MR, Warren S, Ma J, et al. Patiromer to enable spironolactone in patients with resistant hypertension and CKD: primary results of amber [abstract]. American Journal of Kidney Diseases 2019;73(6):899. [EMBASE: 2001959391] [Google Scholar]
- Agarwal R, Rossignol P, Mayo M, Warren S, Ma J, Conrad A, et al. Patiromer vs. placebo to enable spironolactone in patients with resistant hypertension and CKD according to baseline kidney function (AMBER trial) [abstract no: TH-OR052]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):14. [Google Scholar]
- Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019;394(10208):1540-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Williams B, Agarwal R, Rossignol P, Ackourey G, Garza D, Mayo MR, et al. Patiromer vs. placebo to enable spironolactone in patients with resistant hypertension and chronic kidney disease (AMBER): results in prespecified subgroups [abstract]. Journal of Cardiac Failure 2019;25(11):939. [EMBASE: 2003883004] [Google Scholar]
AMETHYST‐DN 2015 {published data only}
- Bakris G, Pitt B, Mayo M, Garza D, Stasiv Y, Zawadzki R, et al. Reduction of serum K+ with patiromer for 1 year in patients with diabetic CKD who developed hyperkalemia while on maximal losartan and/or spironolactone [abstract]. Journal of the American Society of Hypertension 2015;9(4 Suppl 1):e75-6. [EMBASE: 72244084] [Google Scholar]
- Bakris G, Pitt B, Weir M, Mayo M, Garza D, Stasiv Y, et al. Patiromer lowered serum potassium for up to 1 year in hyperkalemic patients with diabetes and advanced kidney disease on RAAS inhibitors [abstract NO: SuO019]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii51-2. [EMBASE: 72206394] [Google Scholar]
- Bakris GL, Pitt B, Mayo M, Garza D, Stasiv Y, Berman L, et al. Effect of patiromer on hyperkalemia in patients with diabetic nephropathy: results of a 1-year randomized trial [abstract no: SA-PO1099]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):B5. [Google Scholar]
- Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial [Erratum appears in JAMA. 2015 Aug 18;314(7):731 Note: Dosage error in article text; PMID: 26284731]. JAMA 2015;314(2):151-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bushinsky D, Romero A, Du Mond C, Mayo M, Ketteler M. Effect of patiromer on serum potassium in hyperkalemic patients with severe CKD on RAAS inhibitors: results from OPAL-HK and amethyst-DN [abstract no: TO028]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii89-90. [EMBASE: 617290254] [Google Scholar]
- Cope J, Bushinsky DA, Berman L. High rates of hyperkalemia in patients with chronic kidney disease stage 3 or 4, diabetes, and hypertension [abstract no: PUB455]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):996A. [Google Scholar]
- Pitt B, Bakris GL, Weir MR, Freeman MW, Lainscak M, Mayo MR, et al. Long-term effects of patiromer for hyperkalaemia treatment in patients with mild heart failure and diabetic nephropathy on angiotensin-converting enzymes/angiotensin receptor blockers: results from AMETHYST-DN. ESC Heart Failure 2018;5(4):592-602. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B, Bushinksy DA, Garza D, Stasiv Y, Mond CD, Berman L, et al. 1-year safety and efficacy of patiromer for hyperkalemia in heart failure patients with chronic kidney disease on renin angiotensin-aldosterone system inhibitors [abstract]. Heart & Lung 2015;44(6):549-50. [EMBASE: 623564656] [Google Scholar]
- Pitt B, Bushinsky D, Garza D, Stasiv Y, Du Mond C, Berman L, et al. 1-year safety and efficacy of patiromer for hyperkalemia in heart failure patients with chronic kidney disease on renin-angiotensin-aldosterone system inhibitors [abstract]. Journal of the American College of Cardiology 2015;65(10 Suppl 1):A855. [EMBASE: 71833912] [Google Scholar]
- Pitt B, Garza D, Zawadzki R, Romero A, Conrad A, Lainscak M. Long-term effects of patiromer for hyperkalaemia treatment in patients with HFrEF and diabetic nephropathy on RAASi [abstract]. European Journal of Heart Failure 2017;19(Suppl 1):55. [EMBASE: 616173131] [Google Scholar]
- Pitt B, Mayo M, Garza D, Arthur S, Lainscak M. Long-term effect of patiromer for hyperkalaemia treatment in patients with HFmrEF and diabetic nephropathy on RAAS inhibitors [abstract no: P2206]. European Journal of Heart Failure 2018;20(Suppl 1):591-2. [EMBASE: 622652230] [Google Scholar]
- Pitt B, Weir M, Bushinsky D, Mayo M, Garza D, Stasiv Y, et al. Patiromer reduces serum K+ in hyperkalemic patients with HF and CKD on RAAS inhibitors: results from OPAL-HK and AMETHYST-DN [abstract]. Journal of Cardiac Failure 2015;21(8 Suppl 1):S107-8. [EMBASE: 72169334] [Google Scholar]
- Pitt B, Weir M, Bushinsky DA, Mayo M, Garza D, Stasiv Y, et al. Patiromer reduced serum K+ in hyperkalaemic patients with HF and advanced CKD on RAAS inhibitors: results from OPAL-HK and AMETHYST-DN [abstract no: P1799]. European Heart Journal 2015;36(Suppl 1):318-9. [EMBASE: 72019966] [Google Scholar]
- Rossignol P, Gross C, Weir M, Arthur S, Labonte E, Bakris G. Insulin therapy for diabetes does not modify the effect of patiromer on serum potassium in hyperkalaemic patients with type 2 diabetes on RAAS inhibitors [abstract no: 1050]. Diabetologia 2016;59(1 Suppl 1):S503-4. [EMBASE: 612314294] [Google Scholar]
- Weir M, Bushinsky D, Mayo M, Garza D, Stasiv Y, Arthur S, et al. Patiromer lowers serum potassium in patients with elevated potassium and diabetes and advanced CKD on RAAS inhibitors: results from OPAL-HK and AMETHYST-DN [abstract no: 1110]. Diabetologia 2015;58(1 Suppl 1):S533-4. [EMBASE: 72031353] [Google Scholar]
- Weir MR, Mayo M, Garza D, Budden JJ, Arthur S, Bakris GL. Patiromer controls serum potassium for up to 1 year in hyperkalemic patients with diabetes and advanced kidney disease on RAAS inhibitors regardless of age [abstract no: FR-PO255]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):501. [Google Scholar]
Ash 2015 {published data only}
- Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney International 2015;88(2):404-11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. Safety and efficacy of ZS 9, a novel selective cation trap, for treatment of hyperkalemia in CKD patients [abstract no: HI-OR05]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):2B. [Google Scholar]
- Singh B, Ash SA, Lavin PT, Yang A, Rasmussen HS. ZS-9, a novel selective cation trap, leads to a rapid and predictable rate of decline in serum potassium in patients with chronic kidney disease and hyperkalemia [abstract]. Journal of the American College of Cardiology 2014;63(12 Suppl 1):A369. [EMBASE: 71406393] [Google Scholar]
- Singh B, Ash SR, Lavin P, Yang A, Rasmussen HS. Change from baseline in serum K+ with ZS-9 in patients with chronic kidney disease treated for hyperkalemia [abstract]. Journal of the American Society of Hypertension 2014;8(4 Suppl 1):e68. [EMBASE: 71500582] [Google Scholar]
- Singh B, Ash SR, Lavin P, Yang A, Rasmussen HS. Effect of ZS-9, a novel selective cation trap, on urinary potassium and sodium excretion when used for the treatment of hyperkalemia in patients with chronic kidney disease [abstract]. Journal of the American Society of Hypertension 2014;8(4 Suppl 1):e15. [EMBASE: 71500462] [Google Scholar]
- Singh B, Ash SR, Lavin P, Yang A, Rasmussen HS. ZS-9, a novel selective cation trap, does not significantly increase sodium excretion when used for the treatment of hyperkalemia in patients with CKD [abstract no: 337]. American Journal of Kidney Diseases 2014;63(5):A103. [EMBASE: 71448604] [Google Scholar]
- Singh B, Ash SR, Lavin PT, Yang A, Rasmussen HS. Acid-base balance in a phase 2 trial of ZS-9 for hyperkalaemia in patients with chronic kidney disease [abstract no: SP251]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii155. [EMBASE: 71491879] [Google Scholar]
DIALIZE 2019 {published data only}
- Fishbane S, Ford M, Fukagawa M, McCafferty K, Rastogi A, Spinowitz B, et al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. Journal of the American Society of Nephrology 2019;30(9):1723-33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane S, Ford ML, Fukagawa M, McCafferty KRA, Spinowitz B, Staroselskiy K, et al. Sodium zirconium cyclosilicate (SZC) improves potassium balance in hyperkalemic hemodialysis patients: results from the phase 3b, randomized, placebo-controlled DIALIZE study [abstract no: SA-OR061]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):95. [Google Scholar]
Gruy‐Kapral 1998 {published data only}
- Gruy-Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. Journal of the American Society of Nephrology 1998;9(10):1924-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HARMONIZE 2014 {published data only}
- Alpert JS, Singh B, Menoyo J, Rasmussen H, Kosiborod M. Rapid onset of potassium: Lowering with sodium zirconium cyclosilicate (ZS-9) across patients stratified by race, age, and comorbidities in the randomized, double-blind, placebo-controlled phase 3 HARMONIZE study [abstract]. Journal of the American College of Cardiology 2016;67(13 Suppl 1):1345. [EMBASE: 72242848] [Google Scholar]
- De Francisco A, Rasmussen H, Lavin P, Singh B, Yang A, Jadoul M, et al. Achievement and maintenance of normokalaemia in patients with non-dialysis stage 5 chronic kidney disease [abstract no: FP003]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii66-7. [EMBASE: 72206424] [Google Scholar]
- De Francisco A, Rasmussen H, Lavin P, Singh B, Yang A, Mann J, et al. Normalization of serum bicarbonate with sodium zirconium cyclosilicate (ZS-9) in the phase 3 randomized, double-blind, placebo-controlled HARMONIZE study [abstract no: FO012]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii6. [EMBASE: 72206293] [Google Scholar]
- El-Shahawy MA, Rasmussen HS, Lavin PT, Yang A, Qunibi W. Treatment of hyperkalemia with ZS-9, a selective nonabsorbed cation exchange resin, does not lead to hypomagnesemia: results from two multicenter, randomized, double blind, placebo-controlled phase 3 trials [abstract]. Journal of the American College of Cardiology 2015;65(10 Suppl 1):A842. [EMBASE: 71833899] [Google Scholar]
- Jadoul M, Rasmussen H, Lavin P, Singh B, Yang A, Mann J, et al. Effect of sodium zirconium cyclosilicate (ZS-9) on urinary potassium and sodium: an analysis of the phase 3 randomized, double-blind, placebo-controlled HARMONIZE study [abstract no: FP001]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii66. [EMBASE: 72206422] [Google Scholar]
- Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial.[Erratum in: JAMA. 2015 Feb 3;313(5):526. Dosage error in text]. JAMA 2014;312(21):2223-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lerma E, Kosiborod M, Rasmussen HS, Lavin PT, Yang A, Qunibi W. Sodium zirconium cyclosilicate (ZS-9) for hyperkalemia in stage 4/5 CKD subgroup of the phase 3 HARMONIZE study [abstract no: 157]. American Journal of Kidney Diseases 2015;65(4):A54. [EMBASE: 71875137] [Google Scholar]
- Levy P, Rasmussen HS, Lavin PT, Singh B, Yang A, Peacock WF. Sodium zirconium cyclosilicate for patients with severe hyperkalemia: Subgroup analysis of the phase 3, international, multicenter, randomized, double-blind, placebo-controlled HARMONIZE trial [abstract no: 257]. Annals of Emergency Medicine 2015;66(4 Suppl 1):S93. [EMBASE: 72032787] [Google Scholar]
- Mann J, Rasmussen H, Lavin P, Yang A, Spinowitz B. Efficiency of sodium zirconium cyclosilicate (ZS-9) increases with severity of hyperkalaemia: subgroup analysis of patients stratified by baseline potassium in the phase 3 HARMONIZE study [abstract no: FP007]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii68. [EMBASE: 72206428] [Google Scholar]
- Packham D, Rasmussen H, Lavin P, Singh B, Yang A, Roger S. Maintenance of normal serum potassium with sodium zirconium cyclosilicate (ZS-9) in subgroup of patients with history of chronic kidney disease and on RAAS inhibitors from the phase 3 randomized, double-blind, placebo-controlled harmonize study [abstract no: FP010]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii69. [EMBASE: 72206431] [Google Scholar]
- Peacock W, Rafique Z, Rasmussen H, Lavin P, Yang A, Levy P, et al. Sodium zirconium cyclosilicate (ZS-9) for severe hyperkalemia: a post-HOC analysis of the phase 3 HARMONIZE trial [abstract no: 143]. Academic Emergency Medicine 2015;22(5 Suppl 1):S69. [EMBASE: 71878784] [Google Scholar]
- Qunibi W, Kosiborod M, Rasmussen HS, Lavin PT, Yang A, Lerma E. Safety of sodium zirconium cyclosilicate (ZS-9) in hyperkalemia: analysis of GI effects in the phase 3 HARMONIZE study [abstract no: 220]. American Journal of Kidney Diseases 2015;65(4):A69. [EMBASE: 71875193] [Google Scholar]
- Qunibi W, Kosiborod M, Rasmussen HS, Lavin PT, Yang A, Lerma E. Sodium zirconium cyclosilicate (ZS-9) in hyperkalemic patients with CKD on RAAS inhibitors: subgroup analysis of phase 3 HARMONIZE study [abstract no: 221]. American Journal of Kidney Diseases 2015;65(4):A70. [EMBASE: 71875194] [Google Scholar]
- Roger SD, De Francisco A, Rasmussen H, Lavin P, Singh B, Yang A, et al. Achievement of normal serum potassium with sodium zirconium cyclosilicate (ZS-9) in a subgroup of patients with stage 4/5 chronic kidney disease and baseline potassium >=5.5 mmol/L from the phase 3 randomized, double-blind, placebo-controlled HARMONIZE study [abstract no: FP008]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii68. [EMBASE: 72206429] [Google Scholar]
- Spinowitz B, Rasmussen H, Lavin P, Yang A, Kosiborod M. The effectiveness of ZS-9 in achieving normokalemia during the open-label phase of the HARMONIZE trial [abstract]. Journal of the American Society of Hypertension 2015;9(4 Suppl 1):e56-7. [EMBASE: 72244045] [Google Scholar]
- Spinowitz B, Rasmussen H, Lavin P, Yang A, Kosiborod M. ZS-9 for treatment of hyperkalemia in black patients: subgroup analysis from the phase 3 HARMONIZE study [abstract]. Journal of the American Society of Hypertension 2015;9(4 Suppl 1):e56. [EMBASE: 72244044] [Google Scholar]
- Zannad F, Hsu B, Maeda Y, Shin SK, Vishneva EM, Rensfeldt M, et al. Sodium zirconium cyclosilicate for hyperkalemia: results of the randomized, placebo-controlled, multi-dose HARMONIZE-GLOBAL study [abstract no: TH-PO1158]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):B7. [Google Scholar]
- Zannad F, Rasmussen HS, Lavin PT, Singh B, Yang A, Anker SD. Sodium zirconium cyclosilicate for treatment of hyperkalaemia: analysis of patients on renin-angiotensin-aldosterone inhibitors (RAASi) from the phase 3 HARMONIZE study [abstract no: PP.35.03]. Journal of Hypertension 2015;33(Suppl 1):e450-1. [EMBASE: 71936068] [Google Scholar]
- Zannad F, Rasmussen HS, Lavin PT, Yang A, Singh B, Anker SD. Effect of sodium zirconium cyclosilicate (ZS-9) on aldosterone from the phase 3 randomized, double-blind, placebo-controlled HARMONIZE study [abstract no: P1592]. European Journal of Heart Failure 2015;17(Suppl 1):342. [EMBASE: 71903670] [Google Scholar]
Kashihara 2018 {published data only}
- Kashihara N, Nishio T, Osonoi T, Saka Y, Imasawa T, Ohtake T, et al. Correction of serum potassium with sodium zirconium cyclosilicate in Japanese patients with hyperkalemia: a dose-finding study [abstract no: TH-PO1157]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):B7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lepage 2015 {published data only}
- Desforges K, Lepage L, Dufour A, Doiron J, Handfield K, Bell RZ, et al. Sodium polystyrene sulfonate for the treatment of mild hyperkalemia in chronic kidney disease: a randomized clinical trial [abstract no: SA-PO926]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):846a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage L, Dufour AC, Doiron J, Handfield K, Desforges K, Bell R, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(12):2136-42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakayama 2018 {published data only}
- Nakayama Y, Ueda K, Yamagishi SI, Sugiyama M, Yoshida C, Kurokawa Y, et al. Compared effects of calcium and sodium polystyrene sulfonate on mineral and bone metabolism and volume overload in pre-dialysis patients with hyperkalemia. Clinical & Experimental Nephrology 2018;22(1):35-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nasir 2014 {published data only}
- Nasir K, Ahmad A. Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. Journal of Ayub Medical College, Abbottabad: JAMC 2014;26(4):455-8. [MEDLINE: ] [PubMed] [Google Scholar]
OPAL‐HK 2015 {published data only}
- Bushinsky D, Romero A, Du Mond C, Mayo M, Ketteler M. Effect of patiromer on serum potassium in hyperkalemic patients with severe CKD on RAAS inhibitors: Results from OPAL-HK and amethyst-DN [abstract no: TO028]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii89-90. [EMBASE: 617290254] [Google Scholar]
- Pitt B, Bakris GL, Bushinsky DA, Garza D, Mayo MR, Stasiv Y, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. European Journal of Heart Failure 2015;17(10):1057-65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B, Weir M, Bushinsky D, Mayo M, Garza D, Stasiv Y, et al. Patiromer reduces serum K+ in hyperkalemic patients with HF and CKD on RAAS inhibitors: results from OPAL-HK and AMETHYST-DN [abstract]. Journal of Cardiac Failure 2015;21(8 Suppl 1):S107-8. [EMBASE: 72169334] [Google Scholar]
- Pitt B, Weir M, Bushinsky DA, Mayo M, Garza D, Stasiv Y, et al. Patiromer reduced serum K+ in hyperkalaemic patients with HF and advanced CKD on RAAS inhibitors: results from OPAL-HK and AMETHYST-DN [abstract no: P1799]. European Heart Journal 2015;36(Suppl 1):318-9. [EMBASE: 72019966] [Google Scholar]
- Pitt B, Weir M, Mayo M, Garza D, Christ-Schmidt H, Wittes J, et al. Patiromer lowers serum potassium and prevents recurrent hyperkalemia in patients with heart failure and CKD when treated with RAAS inhibitors: results from OPAL-HK [abstract no: 9]. Heart & Lung 2015;44(6):550. [EMBASE: 623564660] [Google Scholar]
- Pitt B, Weir MR, Mayo MR, Garza D, Christ-Schmidt H, Wittes J, et al. Patiromer lowers serum potassium and prevents recurrent hyperkalemia in patients with heart failure and CKD when treated with RAAS inhibitors: results from OPAL-HK [abstract no: P397]. European Journal of Heart Failure 2015;17(Suppl 1):90. [EMBASE: 71902792] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol P, Gross C, Weir M, Arthur S, Labonte E, Bakris G. Insulin therapy for diabetes does not modify the effect of patiromer on serum potassium in hyperkalaemic patients with type 2 diabetes on RAAS inhibitors [abstract no: 1050]. Diabetologia 2016;59(1 Suppl 1):S503-4. [EMBASE: 612314294] [Google Scholar]
- Walter E, Eichhober G, Voit M. A cost-effectiveness analysis of RAASI - enabling patiromer for the treatment of hyperkalemia in Austria [abstract no: PSY115]. Value in Health 2018;21(Suppl 3):S456. [EMBASE: 2001401837] [Google Scholar]
- Weir M, Bakris G, Gross C, Mayo M, Garza D, Stasiv Y, et al. Patiromer decreased aldosterone, urine albumin/creatinine ratio, and blood pressure in patients with chronic kidney disease and hyperkalemia on RAAS inhibitors: results from OPAL-HK [abstract no: P602]. Hypertension 2015;66(Suppl 1):na. [EMBASE: 72202804] [Google Scholar]
- Weir M, Bakris G, Mayo M, Garza D, Stasiv Y, Arthur S, et al. Patiromer increased time to RAAS inhibitor discontinuation compared with placebo in advanced CKD patients with hyperkalemia [abstract]. Journal of the American Society of Hypertension 2015;9(4 Suppl 1):e57-8. [EMBASE: 72244047] [Google Scholar]
- Weir M, Bushinsky D, Mayo M, Garza D, Stasiv Y, Arthur S, et al. Patiromer reduced recurrent hyperkalemia in advanced CKD patients on RAASI [abstract no: 301]. American Journal of Kidney Diseases 2015;65(4):A90. [EMBASE: 71875267] [Google Scholar]
- Weir MR, Bakris GL, Bushinsky DA, Mayo M, Garza D, Stasiv Y, et al. Patiromer lowers serum K+ and prevents recurrent hyperkalemia in patients with diabetes and CKD on RAAS inhibitors:subgroup results of a phase 3 trial [abstract no: FR-PO792]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):550A. [Google Scholar]
- Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. New England Journal of Medicine 2015;372(3):211-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Bakris GL, Bushinsky DA, Pitt B, Mayo M, Garza D, et al. Patiromer reduced RAASi dose discontinuations in CKD patients with moderate-to-severe hyperkalemia [abstract no: FR-PO810]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):555A. [Google Scholar]
- Weir MR, Bakris GL, Gross C, Mayo MR, Garza D, Stasiv Y, et al. Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin-angiotensin system inhibitors. Kidney International 2016;90(3):696-704. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Bakris GL, Mayo M, Stasiv Y, Christ-Schmidt H, Wittes J, et al. A two-part trial of patiromer for the treatment of hyperkalemia in chronic kidney disease subjects on renin angiotensin aldosterone system inhibition [abstract no: SA-PO1085]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):4B. [Google Scholar]
- Weir MR, Bushinsky DA, Benton WW, Woods SD, Mayo MR, Arthur SP, et al. Effect of patiromer on hyperkalemia recurrence in older chronic kidney disease patients taking RAAS inhibitors. American Journal of Medicine 2018;131(5):555-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weir MR, Bushinsky DA, Mayo M, Garza D, Stasiv Y, Wilson DJ, et al. Patiromer lowers serum K+ and prevents recurrent hyperkalemia in CKD patients >65 years of age on RAAS inhibitors.[abstract no: TH-OR035]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):10A. [Google Scholar]
- Weir MR, Mayo MR, Garza D, Arthur SA, Berman L, Bushinsky D, et al. Effectiveness of patiromer in the treatment of hyperkalemia in chronic kidney disease patients with hypertension on diuretics. Journal of Hypertension 2017;35(Suppl 1):S57-63. [EMBASE: 614243668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Packham 2015 {published data only}
- El-Shahawy M, Rasmussen HS, Lavin PT, Yang A, Packham DK. Acute-phase efficacy of ZS-9 in patients with baseline serum potassium levels above 5.5 MEQ/l: analysis from a phase 3, multicenter, randomized, double blind, placebo-controlled trial [abstract]. Journal of the American College of Cardiology 2015;65(10 Suppl 1):A878. [EMBASE: 71833935] [Google Scholar]
- El-Shahawy MA, Rasmussen HS, Lavin PT, Yang A, Packham DK. Acute-phase efficacy in a phase 3 multicenter, randomised, double-blind, placebo-controlled trial of ZS-9 for hyperkalaemia [abstract no: SP320]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii178. [EMBASE: 71491947] [Google Scholar]
- El-Shahawy MA, Rasmussen HS, Lavin PT, Yang A, Qunibi WY. Once daily ZS-9 for treatment of hyperkalemia: achievement and maintenance of normokalemia in black patients in a phase 3, multicenter, randomized, double-blind, placebo-controlled trial [abstract no: SA-PO152]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):669A. [Google Scholar]
- El-Shahawy MA, Singh B, Rasmussen HS, Lavin PT, Yang A, Qunibi W. Maintenance of normal serum K+ with ZS-9 once daily in patients with CHF: subgroup analysis of a phase 3 multicenter, randomised, double-blind placebo-controlled trial of patients with hyperkalaemia [abstract no: 4140]. European Heart Journal 2014;35(Suppl 1):722. [EMBASE: 71649437] [Google Scholar]
- El-Shahawy MA, Singh B, Rasmussen HS, Lavin PT, Yang A, Qunibi W. Maintenance of normokalemia with ZS-9 once daily in CHF patients on RAAS inhibitors: subgroup analysis of a phase 3 multicenter, randomized, double-blind, placebo-controlled trial of patients with hyperkalemia [abstract no: 503]. Hypertension 2014;64(Suppl 1):na. [EMBASE: 71746082] [Google Scholar]
- Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. New England Journal of Medicine 2015;372(3):222-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Packham DK, Rasmussen HS, Lavin PT, Yang A, Qunibi WY. Safety and tolerability of ZS-9 in a multicenter, randomised, double-blind, placebo-controlled trial in patients with hyperkalaemia [abstract no: SP337]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii184. [EMBASE: 71491964] [Google Scholar]
- Packham DK, Roger SD, El-Shahawy MA, Qunibi WY, Rasmussen HS, Singh B, et al. ZS-9 effectively reduces potassium levels in hyperkalemic diabetic patients who have both renal impairment and a history of heart failure [abstract no: SA-PO1100]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):B5. [Google Scholar]
- Qunibi W, Rasmussen HS, Lavin PT, Yang A, Singh B. ZS-9 maintains normokalemia in hyperkalemic patients despite continuing RAAS inhibitors: a subgroup analysis from a phase 3 multicenter, randomized, double blind, placebo-controlled trial [abstract]. Journal of the American College of Cardiology 2015;65(10 Suppl 1):A792. [EMBASE: 71833849] [Google Scholar]
- Rasmussen HS, Block GA, Lavin PT, El-Shahawy MA, Roger SD, Packham DK, et al. Once-daily ZS-9, a novel selective potassium trap, maintained normokalemia in patients with diabetes after acute therapy for hyperkalemia [abstract no: 553-P]. Diabetes 2014;63(Suppl 1):A142. [EMBASE: 71558950] [Google Scholar]
- Rasmussen HS, Block GA, Lavin PT, El-Shahawy MA, Roger SD, Packham DK, et al. ZS-9, a novel selective potassium trap, rapidly reduced serum potassium in patients with diabetes treated for hyperkalemia [abstract no: 1139-P]. Diabetes 2014;63(Suppl 1):A296. [EMBASE: 71559522] [Google Scholar]
- Roger SD, Rasmussen HS, Lavin PT, Yang A, El-Shahawy MA. Efficacy of ZS-9 in patients receiving RAAS therapy: a subgroup analysis of a phase 3 multicenter, randomised, double-blind, placebo-controlled trial [abstract no: SP324]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii180. [EMBASE: 71491951] [Google Scholar]
- Roger SD, Rasmussen HS, Lavin PT, Yang A, Qunibi WY. Extended efficacy of ZS-9 once-daily in a phase 3 multicenter, randomised, double-blind, placebo-controlled trial of patients with hyperkalaemia [abstract no: SP331]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii182. [EMBASE: 71491958] [Google Scholar]
- Roger SD, Rasmussen HS, Lavin PT, Yang A, Singh B. Effect of ZS-9 on serum bicarbonate and BUN in a phase 3 randomized, double-blind, placebo-controlled trial [abstract no: SA-PO147]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):667A. [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, El-Shahawy MA. Achievement of normokalemia with ZS-9 across subgroups in a phase 3 multicenter, randomized, double-blind, placebo-controlled trial of patients with hyperkalemia [abstract no: 635]. Hypertension 2014;64(Suppl 1):na. [EMBASE: 71746192] [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, Packham DK. Phase 3, multicenter, randomised, double-blind, placebo-controlled trial of once-daily ZS-9 for treatment of hyperkalemia: achievement and maintenance of k+ in subgroup analysis of patients with significant renal impairment [abstract no: FR-PO896]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):576A. [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, Packham DK. Phase 3, multicenter, randomized, double-blind, placebo-controlled trial of once-daily ZS-9 for treatment of hyperkalemia: achievement and maintenance of K+ in subgroup analysis of patients with heart failure not on RAAS inhibitors [abstract no: A14686]. Circulation 2014;130(Suppl 2):A17489. [EMBASE: 71712359] [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, Qunibi WY. Phase 3, multicenter, randomized, double-blind, placebo-controlled trial of once-daily ZS-9 for treatment of hyperkalemia: achievement and maintenance of K+ in subgroup analysis of patients with significant renal impairment and diabetes [abstract no: FR-OR123]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):75A. [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, Roger SD. Phase 3, multicenter, randomised, double-blind, placebo-controlled trial of ZS-9: subgroup analysis stratified by baseline serum potassium levels [abstract no: SP321]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii178-9. [EMBASE: 71491948] [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, Roger SD. Phase 3, multicenter, randomized, double-blind, placebo-controlled trial of once-daily ZS-9 for treatment of hyperkalemia: maintenance of serum K+ in subgroup analysis of patients on RAAS therapy [abstract no: 508]. Hypertension 2014;64(Suppl 1):na. [EMBASE: 71746087] [Google Scholar]
- Singh B, Rasmussen HS, Lavin PT, Yang A, Roger SD. ZS-9 once-daily maintained serum K+ in hyperkalemic subjects on RAAS therapy: Subgroup analysis from a phase 3, multicenter, randomized, double-blind, placebo-controlled trial [abstract]. Journal of the American Society of Hypertension 2014;8(Suppl 1):e4-5. [EMBASE: 72308398] [Google Scholar]
- Yang A, Singh B, Lavin PT, Chang A, Rasmussen HS. Placebo effect in clinical studies in hyperkalemia: a double-blind, randomized, placebo-controlled phase 3 study of sodium zirconium cyclosilicate (ZS-9) [abstract no: 307]. American Journal of Kidney Diseases 2015;65(4):A91. [EMBASE: 71875272] [Google Scholar]
PEARL‐HF 2011 {published data only}
- Bushinsky DA, Huang IZ, Anker SD, Zannad F, Pitt B. RLY5016: A novel, effective, non-absorbed, oral polymer for the control of serum potassium in heart failure and chronic kidney disease [abstract no: F-PO1617]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):483A. [Google Scholar]
- Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. European Heart Journal 2011;32(7):820-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TOURMALINE 2017 {published data only}
- Bushinsky DA, Spiegel DM, Yuan J, Warren S, Fogli J, Pergola PE. Effects of patiromer on markers of mineral metabolism in patients with hyperkalemia and hyperphosphatemia [abstract no: TH-PO199]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):163. [Google Scholar]
- Bushinsky DA, Spiegel DM, Yuan J, Warren S, Fogli J, Pergola PE. Effects of the potassium-binding polymer patiromer on markers of mineral metabolism. Clinical Journal of the American Society of Nephrology: CJASN 2019;14(1):103-10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Gross C, Yuan J, Conrad A, Pergola PE. Effect of patiromer in hyperkalemic patients taking and not taking RAAS inhibitors. Journal of Cardiovascular Pharmacology & Therapeutics 2018;23(6):524-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Gross C, Yuan J, Conrad A, Pergola PE. Effect of patiromer in hyperkalemic patients with or without RAASi [abstract no: 14680]. Circulation 2017;136(Suppl 1):na. [EMBASE: 619984014] [Google Scholar]
- Pergola PE, Spiegel DM, Warren S, Yuan J, Weir MR. Patiromer lowers serum potassium when taken without food: comparison to dosing with food from an open-label, randomized, parallel group hyperkalemia study. American Journal of Nephrology 2017;46(4):323-32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol P, Gross C, Spiegel DM, Yuan J, Pergola PE. Serum potassium control and patiromer taken without or with food in hyperkalaemic patients with diabetes [abstract no: 1214]. Diabetologia 2017;60(1 Suppl 1):S559. [EMBASE: 618051788] [Google Scholar]
- Weir M, Mayo M, Yuan J, Budden J, Romero A, Pergola PE. Efficacy and safety of once-daily patiromer by baseline serum potassium level: post-hoc results from the TOURMALINE trial [abstract no: SUN-288]. Kidney International Reports 2019;4(7 Suppl):S279-80. [EMBASE: 2002179808] [Google Scholar]
Wang 2018a {published data only}
- Wang J, Lv MM, Zach O, Wang LY, Zhou MY, Song GR, et al. Calcium-polystyrene sulfonate decreases inter-dialytic hyperkalemia in patients undergoing maintenance hemodialysis: a prospective, randomized, crossover study. Therapeutic Apheresis & Dialysis 2018;22(6):609-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
DIALIZE China 2020 {published data only}
- Ni Z. Reduce incidence of pre-dialysis hyperkalaemia with sodium zirconium cyclosilicate in Chinese subjects (DIALIZE China). www.clinicaltrials.gov/ct2/show/NCT04217590 (first received 3 January 2020).
DIAMOND 2019 {published data only}
- Goehring UM. Patiromer for the management of hyperkalemia in subjects receiving RAASi medications for the treatment of heart failure (DIAMOND). www.clinicaltrials.gov/ct2/show/NCT03888066 (first received 25 March 2019).
NCT03781089 {published data only}
- Middleton JP. Patiromer efficacy to reduce episodic hyperkalemia in end stage renal disease patients. www.clinicaltrials.gov/ct2/show/NCT03781089 (first received 19 December 2018).
Additional references
Alvarez 2017
- Alvarez P, Brenner M, Butler J, Farnum C, Kangethe A, Lafayette R, et al. Focus on hyperkalemia management: expert consensus and economic impacts. Journal of Managed Care & Specialty Pharmacy 2017;23(4-a Suppl):S2-20. [DOI: 10.18553/jmcp.2017.23.4-a.s2] [DOI] [Google Scholar]
Batterink 2015
- Batterink J, Cessford TA, Taylor RA. Pharmacological interventions for the acute management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD010344.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Betts 2018
- Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Current Medical Research & Opinion 2018;34(6):971-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bozkurt 2003
- Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. Journal of the American College of Cardiology 2003;41(2):211-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chowdhury 2018
- Chowdhury F, Ghosh PK, Shahunja KM, Shahid AS, Shahrin L, Sarmin M, et al. Hyperkalemia was an independent risk factor for death while under mechanical ventilation among children hospitalized with diarrhea in Bangladesh. Global Pediatric Health 2018;5:2333794X17754005. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2017
- Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. American Journal of Nephrology 2017;46(3):213-21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cowan 2017
- Cowan AC, Ghariba EG, Weir MA. Advances in the management of hyperkalemia in chronic kidney disease. Current Opinion in Nephrology & Hypertension 2017;26(3):235-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Das 2018
- Das S, Dey JK, Sen S, Mukherjee R. Efficacy and safety of patiromer in hyperkalemia: a systematic review and meta-analysis. Journal of Pharmacy Practice 2018;31(1):6-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davidson 2017
- Davidson JP, King AJ, Kumaraswamy P, Caldwell JS, Korner P, Blanks RC, et al. Evaluation of the pharmacodynamic effects of the potassium binder RDX7675 in mice. Journal of Cardiovascular Pharmacology & Therapeutics 2017;23(3):244-53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2015
- Dunn JD, Benton WW, Orozco-Torrentera E, Adamson RT. The burden of hyperkalemia in patients with cardiovascular and renal disease. American Journal of Managed Care 2015;21(15 Suppl):S307-15. [MEDLINE: ] [PubMed] [Google Scholar]
Einhorn 2009
- Einhorn L, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Archives of Internal Medicine 2009;169(12):1156-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2015
- Food Drug Administration. Drug approval. Veltassa (Patiromer) powder for oral suspension. Application No: 205739. Approved October 21, 2015. www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205739Orig1s000Approv.pdf (accessed 10 June 2020).
FDA 2018
- Food Drug Administration. Drug approval. Lokelma (sodium zirconium cyclosilicate) for oral suspension. Application No: 207078. Approved May 18, 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/207078s000lbl.pdf (accessed 10 June 2020).
Fried 2017
- Fried L, Kovesdy CP, Palmer BF. New options for the management of chronic hyperkalemia. Kidney International Supplements 2017;7(3):164-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
James 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8).[Erratum in JAMA. 2014 May 7;311(17):1809]. JAMA 2014;311(5):507-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2013
- Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements 2013;3(1):1-150. [EMBASE: 369856107] [Google Scholar]
Kumar 2017
- Kumar R, Kanev L, Woods SD, Brenner M, Smith B. Managing hyperkalemia in high-risk patients in long-term care. American Journal of Managed Care 2017;23(2 Suppl):S27-36. [MEDLINE: ] [PubMed] [Google Scholar]
Mahoney 2005
- Mahoney BA, Smith WAD, Lo D, Tsoi K, Tonelli M, Clase C. Emergency interventions for hyperkalaemia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003235.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montford 2017
- Montford JR, Linas S. How dangerous is hyperkalemia? Journal of the American Society of Nephrology 2017;28(11):3155-65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moranne 2009
- Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, et al. Timing of onset of CKD-related metabolic complications. Journal of the American Society of Nephrology 2009;20(1):164-71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2017
- Ng KE, Lee CS. Updated treatment options in the management of hyperkalemia. U.S. Pharmacist 2017;42(2):HS15-8. [EMBASE: 614599844] [Google Scholar]
Palaka 2018
- Palaka E, Leonard S, Buchanan-Hughes A, Bobrowska A, Langford B, Grandy S. Evidence in support of hyperkalaemia management strategies: A systematic literature review. International Journal of Clinical Practice 2018;72(2). [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2004
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. New England Journal of Medicine 2004;351(6):585-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rafique 2017
- Rafique Z, Weir MR, Onuigbo M, Pitt B, Lafayette R, Butler J, et al. Expert panel recommendations for the identification and management of hyperkalemia and role of patiromer in patients with chronic kidney disease and heart failure. Journal of Managed Care & Specialty Pharmacy 2017;23(4-a Suppl):S10-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RALES 1996
- Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized ALdactone Evaluation Study [RALES]). American Journal of Cardiology 1996;78(8):902-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schünemann 2011b
- Schunemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Shah 2005
- Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. Journal of the American College of Cardiology 2005;46(5):845-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SONG 2017
- SONG Initiative. The SONG Handbook Version 1.0. www.songinitiative.org/reports-and-publications/ (accessed 10 June 2020).
Tamargo 2018
- Tamargo J, Caballero R, Delpón E. New therapeutic approaches for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors. Cardiovascular Drugs & Therapy 2018;32(1):99-119. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weiner 2010
- Weiner ID, Linas SL, Wingo CS. Disorders of potassium metabolism. In: Floege J, Johnson RJ, Feehally J, editors(s). Comprehensive Clinical Nephrology. 4th edition. Philadelphia: Saunders/Elsevier, 2010:118-29. [Google Scholar]
Weir 2015
- Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. New England Journal of Medicine 2015;372(3):211-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yu 2017
- Yu MY, Yeo JH, Park JS, Lee CH, Kim GH. Long-term efficacy of oral calcium polystyrene sulfonate for hyperkalemia in CKD patients. PLoS ONE [Electronic Resource] 2017;12(3):e0173542. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Natale 2018
- Natale P, Palmer SC, Ruospo M, Saglimbene VM, Strippoli GF. Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD013165] [DOI] [PMC free article] [PubMed] [Google Scholar]


