Abstract
Background:
The objective of this study is to examine the effectiveness and safety of electroacupuncture (EA) in the treatment of urinary incontinence (UI) in patients with spinal cord injury (SCI).
Methods:
All potential studies will be retrieved from the electronic databases of MEDLINE, EMBASE, Cochrane Library, PsycINFO, Web of Science, CBM, and China National Knowledge Infrastructure from origin of each database up to January 31, 2020. Additionally, we will check other resources, such as Google scholar, dissertations, conference proceedings, and reference lists of included studies. No language and publication date limitations will be considered in the literature resources search. All randomized controlled trials using EA for the treatment of UI in patients with SCI will be included. Two independent investigators will perform study selection, data extraction and study quality assessment. If any conflicts occur, we will invite a third investigator to solve them. Cochrane risk of bias will be used for study quality assessment, and RevMan 5.3 software will be employed for statistical analysis.
Results:
This study will summarize the most recent evidence to assess the effectiveness and safety of EA for the treatment of UI in patients with SCI.
Conclusion:
The results of this study will provide helpful evidence to determine whether EA is effective and safety for the treatment of UI in patients with SCI or not.
PROSPERO registration number:
PROSPERO CRD42020165562.
Keywords: effectiveness, electroacupuncture, spinal cord injury, urinary incontinence
1. Introduction
Spinal cord injury (SCI) is a serious and debilitating central nervous system neurological disorder.[1–3] It is reported that about 250,000 and 500,000 new cases annually and most of them are traumatic, with male-to-female of 2:1.[4,5] Several factors result in SCI, such as traffic accidents, violence, sports, and falls.[6–11] Patients with SCI often experience a variety of complications, including pain, spasticity, pressure ulcers, respiratory, cardiovascular, and urinary and bowel disorders, especially urinary incontinence (UI), which leads to very poor quality of life.[12–16]
Electroacupuncture (EA) has been widely used to treat numerous diseases around the world.[17–22] Studies suggested that it can effectively manage UI in patients with SCI.[23–30] However, no systematic review has been conducted on this subject. Thus, this study aims to supply sufficient evidence for the clinical application of EA for the treatment of UI following SCI.
2. Methods
2.1. Study registration
This study was funded and registered on PROSPERO (CRD42020165562). It is reported according to the guidelines of the preferred reporting items for systematic reviews and meta-analysis protocol statement.[31,32]
2.2. Criteria for including studies
2.2.1. Types of studies
This study will only consider randomized controlled trials (RCTs) assessing the effectiveness and safety of EA for the treatment of UI in patients with SCI for inclusion. We will not limit their language and publication date to all included RCTs.
2.2.2. Types of interventions
The intervention of the trial group only used EA for the treatment of UI in patients with SCI.
The intervention of the control group could use any treatments, such as conventional therapy, medication, and any others. However, we will exclude EA or EA combined with other therapies as comparators.
2.2.3. Types of patients
Regardless of ethnicity, age, sex, educational background, any SCI patients who were diagnosed as UI will be included in this study.
2.2.4. Types of outcome measurements
The primary outcome is the change from baseline in the amount of urine leakage, as measured by the pad-weighing test or other tests.
The secondary outcomes are urination diary, bladder capacity, severity of UI, a 72-hour incontinence episode frequency, clinical symptom scores, the number of participants healed completely within study period and adverse events.
2.3. Data sources and search
We will comprehensively search the electronic databases of MEDLINE, EMBASE, Cochrane Library, PsycINFO, Web of Science, CBM, and China National Knowledge Infrastructure from origin of each database up to January 31, 2020. All literature resources will be searched regardless language and publication date. The detailed search strategy of MEDLINE is built (Table 1). Similar search strategies of other electronic databases will be modified.
Table 1.
Search strategy for MEDLINE.
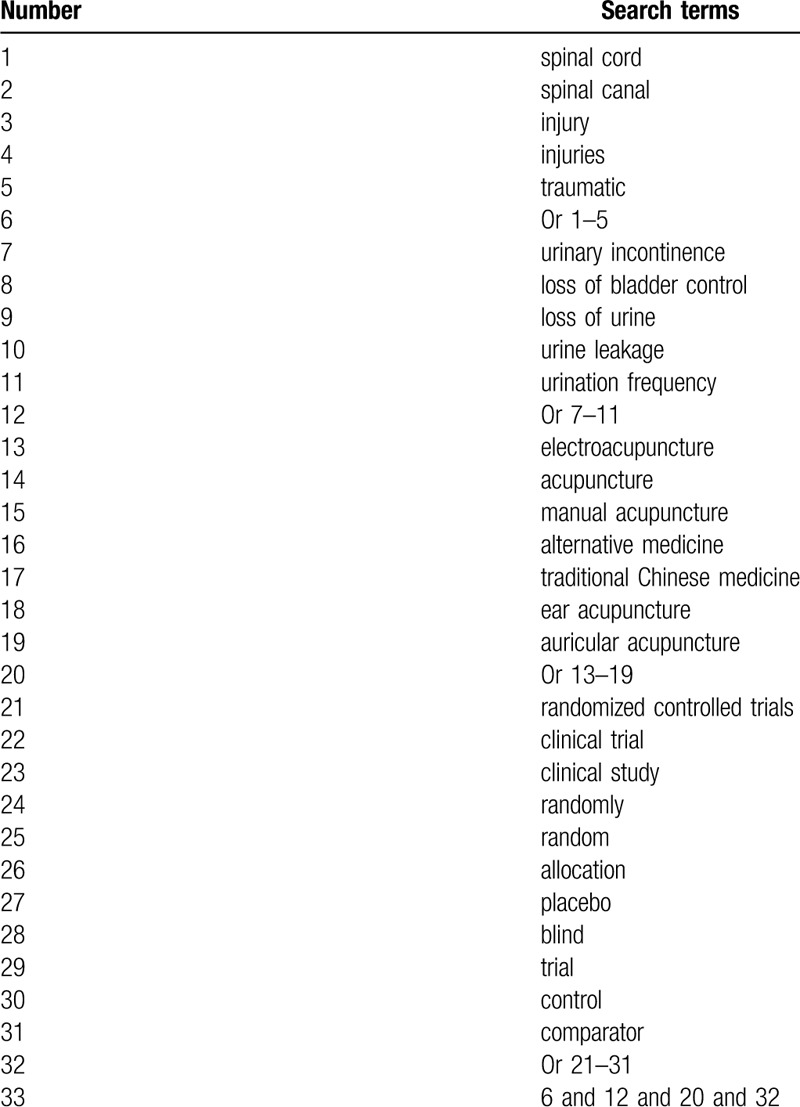
In addition, we will search other sources, such as Google scholar, dissertations, conference proceedings, and reference lists of eligible trials.
2.4. Data collection and analysis
2.4.1. Study selection
All retrieved literatures will be imported into Endnote X7 and all duplicates will be removed. Two investigators will independently screen the titles and abstracts of all searched literatures, and any unconnected studies will be excluded. Then, full-texts of the remaining studies will be obtained and read cautiously against all inclusion criteria. Any uncertainty between two investigators will be resolved through consulting a third investigator. The procedure of study selection will be exerted in a flow diagram.
2.4.2. Data extraction
Two investigators will independently extract all essential data from each included trial using a predefined data extraction form. Any divergences will be figured out with the help of a third investigator through discussion. We will extract the following information:
Study information: first author, publication year, country, et al.
Patient information: gender, age, race, diagnostic criteria, inclusion and exclusion criteria, et al.
Trial methods: details of randomization, concealment, blind, et al.
Specifics of intervention and controls: treatment duration, dosage, frequency, et al.
Outcome details: primary and secondary outcomes, adverse events, et al.
2.4.3. Missing data dealing with
If there is unclear or insufficient data, we will contact primary authors to request it. If we cannot receive those data, we will only analyze available data. If necessary, we will discuss its potential effects on the study findings.
2.4.4. Study quality assessment
Two investigators will independently appraise study quality using Cochrane risk of bias tool through 7 fields. Each item is graded as high, unclear or low risk of bias. Any differences will be solved by a third investigator through discussion and a consensus will be reached after discussion.
2.4.5. Subgroup analysis
We will carry out subgroup analysis to identify the possible factors that may result in significant heterogeneity based on the different interventions, controls and outcome indicators.
2.4.6. Sensitivity analysis
We will perform sensitivity analysis to test the robustness and stability of study findings by excluding low quality trials.
2.4.7. Reporting bias
We will examine the reporting bias using funnel plot and Egger regression test if more than 10 included trials are included.[33,34]
2.5. Data synthesis
RevMan 5.3 software will be used to undertake statistical analysis. To assess the extracted data, mean difference or standardized mean difference and 95% confidence intervals will be used for continuous data. For dichotomous data, we will use risk ratio and 95% confidence intervals. Statistical heterogeneity across eligible trials will be inspected using I2 statistics. I2 ≤ 50% means fair heterogeneity, and a fixed-effect model will be examined. If sufficient outcome data is extracted, we will conduct a meta-analysis. I2 > 50% suggests apparent heterogeneity, and a random-effect model will be used. We will carry out subgroup analysis to investigate the sources of heterogeneity. If the sources of heterogeneity cannot be identified, synthetic analysis will not be performed and descriptive analysis will be adopted.
2.5.1. Quality of evidence
The quality of evidence for major outcomes will be appraised by 2 independent investigators using grading of recommendations assessment, development and evaluation.[35] Any disagreements will be disentangled by another investigator through consultation.
2.6. Dissemination and ethics
We expect to publish this study on a peer-reviewed journal. This study will not inquire ethic approval because it will not collect individual patient data.
3. Discussion
UI is a progressive disorder in patients with SCI.[23–30] EA is currently used in the treatment of UI after SCI, relieving clinical symptoms, frequency and severity of UI. Although previous clinical studies have reported that EA has positive therapeutic effects on UI following SCI, all conclusions drawn are based on the individual trial. Thus, this study is designed to systematically and comprehensively assess the effectiveness and safety of EA for the treatment of UI in patients with SCI. The results of this study will provide evidence to determine whether EA is an effective and safety treatment for UI following SCI, which may benefit clinical practice and patients.
3.1. Uncited references
Author contributions
Conceptualization: Tian-Shu Wang, Wei-Dong Song, Zhao-Chen Tang, Yu Zhao, Guan-kai Wang.
Data curation: Zeng-Mian Wang, Yu Zhao.
Formal analysis: Tian-Shu Wang, Zeng-Mian Wang, Wei-Dong Song, Zhao-Chen Tang.
Investigation: Yu Zhao.
Methodology: Tian-Shu Wang, Zeng-Mian Wang, Wei-Dong Song, Zhao-Chen Tang, Guan-Kai Wang.
Project administration: Yu Zhao.
Resources: Tian-Shu Wang, Zeng-Mian Wang, Wei-Dong Song, Zhao-Chen Tang, Guan-Kai Wang.
Software: Tian-Shu Wang, Zeng-Mian Wang, Wei-Dong Song, Zhao-Chen Tang, Guan-Kai Wang.
Supervision: Yu Zhao.
Validation: Tian-Shu Wang, Zeng-Mian Wang, Zhao-Chen Tang, Yu Zhao, Guan-Kai Wang.
Visualization: Tian-shu Wang, Wei-dong Song, Zhao-chen Tang, Yu Zhao.
Writing – original draft: Tian-Shu Wang, Zeng-Mian Wang, Wei-Dong Song, Zhao-Chen Tang, Yu Zhao, Guan-Kai Wang.
Writing – review & editing: Tian-Shu Wang, Zeng-Mian Wang, Wei-Dong Song, Yu Zhao, Guan-Kai Wang.
Footnotes
Abbreviations: EA = electroacupuncture, RCTs = randomized controlled trials, SCI = spinal cord injury, UI = urinary incontinence.
How to cite this article: Wang TS, Wang ZM, Zhao Y, Tang ZC, Song WD, Wang GK. Effectiveness of electroacupuncture (EA) for the treatment of urinary incontinence (UI) in patients with spinal cord injury (SCI): a protocol of systematic review of randomized controlled trials. Medicine. 2020;99:30(e21077).
TSW and ZMW contributed equally to this study.
This study was supported by Scientific Research Project of Heilongjiang Health and Family Planning Commission (2017–387). The supporters had no role in this study.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Ning GZ, Wu Q, Li YL, et al. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med 2012;35:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lu Y, Yang J, Wang X, et al. Research progress in use of traditional Chinese medicine for treatment of spinal cord injury. Biomed Pharmacother 2020;127:110136. [DOI] [PubMed] [Google Scholar]
- [3].Rabinstein AA. Traumatic spinal cord injury. Continuum (Minneap Minn) 2018;24:551–66. [DOI] [PubMed] [Google Scholar]
- [4].Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med 2017;40:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].WHO-Spinal Cord Injury. WHO, Fact sheet N°384 (2013). Available online at: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury [accessed date March 1, 2020]. [Google Scholar]
- [6].Eckert MJ, Martin MJ. Trauma: spinal cord injury. Surg Clin North Am 2017;97:1031–45. [DOI] [PubMed] [Google Scholar]
- [7].Suriá Martínez R. Factors associated with empowerment in people with a spinal cord injury due to traffic accidents. Gac Sanit 2015;29: Suppl 1: 49–54. [DOI] [PubMed] [Google Scholar]
- [8].Moslavac S, Dzidić I, Kejla Z. Neurological outcome in road traffic accidents with spinal cord injury. Coll Antropol 2008;32:583–6. [PubMed] [Google Scholar]
- [9].Kroll T. Rehabilitative needs of individuals with spinal cord injury resulting from gun violence: the perspective of nursing and rehabilitation professionals. Appl Nurs Res 2008;21:45–9. [DOI] [PubMed] [Google Scholar]
- [10].Franz M, Richner L, Wirz M, et al. Physical therapy is targeted and adjusted over time for the rehabilitation of locomotor function in acute spinal cord injury interventions in physical and sports therapy. Spinal Cord 2018;56:158–67. [DOI] [PubMed] [Google Scholar]
- [11].Wilson A, Kurban D, Noonan VK, et al. Falls during inpatient rehabilitation in spinal cord injury, acquired brain injury, and neurologmusculoskeletal disease programs. Spinal Cord 2020;58:334–40. [DOI] [PubMed] [Google Scholar]
- [12].Qi Z, Middleton JW, Malcolm A. Bowel dysfunction in spinal cord injury. Curr Gastroenterol Rep 2018;20:47. [DOI] [PubMed] [Google Scholar]
- [13].Dinh A, Bouchand F, Davido B, et al. Management of established pressure ulcer infections in spinal cord injury patients. Med Mal Infect 2019;49:9–16. [DOI] [PubMed] [Google Scholar]
- [14].Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018;15:635–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Finnerup NB. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord 2017;55:1046–50. [DOI] [PubMed] [Google Scholar]
- [16].Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop 2015;6:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu SY, Lin CH, Chang NJ, et al. Combined effect of laser acupuncture and electroacupuncture in knee osteoarthritis patients: A protocol for a randomized controlled trial. Medicine (Baltimore) 2020;99:e19541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Z, Dong H, Wang Q, et al. Effects of electroacupuncture on anxiety and depression in unmarried patients with polycystic ovarian syndrome: secondary analysis of a pilot randomised controlled trial. Acupunct Med 2019;37:40–6. [DOI] [PubMed] [Google Scholar]
- [19].Ning Y, Jia H, Chen P, et al. Efficacy and safety of electroacupuncture on metabolic syndrome due to olanzapine and risperidone: Study protocol for a randomized controlled pilot trial. Medicine (Baltimore) 2019;98:e17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu T, Lu Y, Yu J, et al. Effect of auricular electroacupuncture combined with body acupuncture in improving the consciousness of patients after traumatic brain injury: Study protocol for a randomized controlled trial. Medicine (Baltimore) 2019;98:e16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhong S, Huang H, Xie J, et al. Application of electroacupuncture for postoperative pain management after total knee arthroplasty: a study protocol for a single-blinded, randomised placebo-controlled trial. BMJ Open 2019;9:e026084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang W, Liu Y, Zhao J, et al. Electroacupuncture versus manual acupuncture in the treatment of plantar heel pain syndrome: study protocol for an upcoming randomised controlled trial. BMJ Open 2019;9:e026147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xian DL, Wang XB, Lin R, et al. Fuyuan Huoxue Decoction combined with electroacupuncture for neurological function rehabilitation after spinal cord injury. Chin J Exp Pharmacol 2020;20:1–0. [Google Scholar]
- [24].Cheng XK, Sun YC. Clinical study on electroacupuncture for treatment of neurogenic bladder incontinence of spinal cord injury. Shanghai J Acupunct Moxibust 2019;38:646–9. [Google Scholar]
- [25].Zhu N, Ma Q, Zhang J, et al. Clinical observation of acupuncture combined with pelvic-sacral muscle stimulation on neurogenic bladder with spinal cord injury. Ningxia Med J 2017;39:553–5. [Google Scholar]
- [26].Lu CJ, Li X, Zhang HR. Observation on the therapeutic effect of different electroacupuncture waveforms on neurogenic bladder with spinal cord injury. Shanghai J Acupunct Moxibust 2016;35:1442–4. [Google Scholar]
- [27].Meng ZX, Wang T, Yin ZL, et al. Clinical study on electroacupuncture combined with transperineal BTX-A injection for neurogenic bladder after spinal cord injury. Chin Acupunc 2015;35:17–20. [PubMed] [Google Scholar]
- [28].Feng XJ, Wei XC, Wu JX, et al. Treatment of 23 cases of neurogenic bladder with incomplete spinal cord injury by electroacupuncture. J Anhui Univ Trad Chin Med 2014;33:43–6. [Google Scholar]
- [29].Ma L, Chen XY. Clinical observation of electroacupuncture at Ba li points combined with warm moxibustion for the treatment of 30 cases of spinal cord-induced urinary incontinence. Hunan J Trad Chin Med 2012;28:81–3. [Google Scholar]
- [30].Cong HL, Liao LM, Si T, et al. The effect of electroacupuncture regulating sacral 3 nerve on neurogenic detrusor overactivity. Chin J Urol 2010;11:741–4. [Google Scholar]
- [31].Shamseer L, Moher D, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- [32].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


