Supplemental Digital Content is available in the text
Keywords: AliveCor, arrhythmia, atrial fibrillation, detection, electrocardiogram, screening
Abstract
Background:
Increasing prevalence of atrial fibrillation has a significant impact on health, society, and healthcare resource utilization, due to increased morbidity, mortality, risk of stroke, and reduction in quality of life. Early diagnosis allows for treatment initiation, a reduction in complications and associated costs, and so innovation to improve screening and enable easy access are needed Developments in digital technology have significantly contributed to the availability of screening tools. The single-lead electrocardiogram AliveCor (Mountainview, CA) device offers the opportunity to provide heart rhythm screening and has been used extensively in clinical practice and research studies.
Methods:
This review investigates the feasibility, validity, and utility of the AliveCor device as a tool for atrial fibrillation detection in clinical practice and in wider research. Databases searched included PUBMED, CINAHL, MEDLINE, and World of Science, plus grey literature search. Search terms related to atrial fibrillation, screening, and AliveCor with adults >18 years. Feasibility metrics were applied including process, resource, management, and scientific outcomes. Studies not written in the English language were excluded. Validity of AliveCor was explored by extracting sensitivity and specificity data from eligible studies and overall effectiveness analyzed by incorporating the above, with wider issues surrounding screening approaches, cost effectiveness and appropriateness of AliveCor as a screening tool.
Results:
The AliveCor device screening was reviewed in 11 studies matching inclusion criteria. Atrial fibrillation detection rates ranged from 0.8% to 36% and this largely correlated to the study population, where wider age inclusion and mass/population screening represented lower atrial fibrillation detection. Recruitment from higher-risk groups (older age, targeted localities, chronic disease) identified higher numbers with atrial fibrillation. Feasibility metrics demonstrated AliveCor as an effective tool of choice in terms of process, resources, and management. Duration of screening time had an impact on rates of atrial fibrillation detection. There was however significant heterogeneity between studies reviewed.
Conclusion:
The AliveCor device offers a convenient, valid, and feasible means of monitoring for atrial fibrillation. Further analysis of electrocardiograms produced by AliveCor may be necessary in some circumstances. The AliveCor electrocardiogram device can be successfully implemented into both opportunistic and systematic screening strategies for atrial fibrillation.
1. Introduction
1.1. Background
Atrial fibrillation (AF) is increasing in prevalence with 1 in 4 people developing this common arrhythmia in later life.[1] AF is a leading cause of stroke, with as many as 15% to 20% of strokes being related to the arrhythmia.[2] In addition, AF can often be asymptomatic and therefore the diagnosis may not be detected before a debilitating stroke. However, AF can be detected through various screening tools and approaches and the development of digital health technologies to assist with AF screening has led to further advances in this area. Screening for AF has received significant focus with a dedicated international and multidisciplinary collaboration established in 2016 (AF Screen), whose aim it is to promote discussion and research about unknown or untreated AF, as a way to reduce stroke and death.[3]
The AliveCor (Mountainview, CA) heart monitor provides a portable electrocardiogram (ECG) recorder and works with a compatible mobile device such as a smart phone. More recently, the device has been rebranded as Kardia but for the purpose of this paper we will continue to use the term “AliveCor device,” as the traditional name is what many recognize the device as. To use the device, 2 fingers are placed on the pocket-sized metal pad and an instant ECG recording is displayed. The ECG reading is enabled by the wireless transmission to the AliveCor app. and like other devices, uses a single ECG lead, normally analogous to lead I. The AliveCor device is an event-type monitor recommended for use in England when episodes are more than 24 hours apart.[4] It has been studied extensively and offers a convenient and practical approach to portable ECG event monitoring or screening. The AliveCor device can be used as a single-point-in time screening tool, obtaining individual brief recordings, or used repeatedly for intermittent screening. (Throughout this review when ECG is mentioned, this will refer to the ECG reading from the AliveCor device. Where this relates to a more traditional 12 lead ECG, this will be documented as 12 lead ECG).
1.2. Why is this review needed?
There is growing evidence relating to the use of digital apps and tools for the detection of AF and arrhythmias. The AliveCor device has been used in clinical practice since 2011 and there is a plethora of research from across the world, where it has been the tool of choice. The device has demonstrated high sensitivity and specificity in screening studies.[5,6] Targeted screening in chronic disease groups such as diabetes, has been suggested as an optimal approach, rather than mass screening of the population due to time and cost efficiency.[7,8] Utilizing an appropriate screening tool for the purpose is equally as important as the approach and this review, therefore, seeks to explore and review evidence relating to the utilization and effectiveness of the AliveCor device in AF screening studies to date.
There has been 1 recent systematic review with meta-analysis on screening tools for AF detection, but this incorporated an array of handheld and Holter style ECG monitoring.[9] The AliveCor device did feature within this review, being the tool utilized in 5 of 54 eligible studies. This previous review concluded that portable ECG devices offer an efficient screening option for AF compared with 24-hour Holter monitoring.
2. Objectives
2.1. Objectives
The objective of this review is to explore and analyze the clinical effectiveness of the AliveCor device in AF screening studies:
The utility of the device in the eligible studies is evaluated in terms of appropriateness and feasibility as a tool in practice.
The validity of the device as a screening tool in terms of sensitivity and specificity is also explored and where relevant, compared to the evidence of other screening tools used in the reviewed research.
Finally, the screening approach undertaken within the research is examined with regards to the strategy e.g. is it single-point-in-time, intermittent or continuous. This is considered in the discussion section of this review. The objectives of this review will be achieved by answering the following primary questions:
How useful and beneficial (utility) is the AliveCor device in AF screening studies and how can this be related to the wider clinical effectiveness through implementation?
How easy and convenient is the tool to use and is it feasible to consider widespread application of the device as a tool of choice in further research studies and clinical practice, also considering cost implications as a resource of choice?
How valid is the device in the eligible studies and how does this compare to other methods of screening in comparison studies analyzed in this review?
2.2. Inclusion and exclusion criteria
Inclusion criteria are set out in Table 1. The AliveCor device was only available for use from 2011, hence the reason for this date inclusion. An initial brief scoping review exercise identified a propensity towards observational studies and therefore it was important to include all methodologies and not just experimental designs. Furthermore, screening studies are often conducted within a cross-sectional design and this was demonstrated in the located studies, hence an important consideration when designing eligibility criteria. Whilst AF is the arrhythmia of interest here, atrial flutter and atrial arrhythmia were included as this difference in arrhythmia is not always clearly defined in searches.
Table 1.
Inclusion and exclusion criteria.

3. Methods
3.1. Search strategy
The search strategy aimed to find both published and unpublished studies. The search strategy was undertaken in 3 stages. Initially a search for published studies was undertaken using databases including MEDLINE, CINAHL, PUBMED, World of Science, Cochrane Library, Clinical Trials database, European Union Clinical Trials register, the National Institute for Health Research and Evidence Based Medicine. The exploratory stage of searching for relevant studies was facilitated through the use of keywords, Boolean operators and associated Medical Subject Headings (MeSH) terms. Results were then screened as per eligibility criteria at title and abstract until a final selection was obtained for full text review. Then, the reference lists of the final full text studies were reviewed for eligibility. Only texts in the English language were included in the search process, and dates were set from January 1st, 2011, to December 28th, 2018.
Formalizing the search question was aided by contextualizing using the PICO framework, which offers the contextual components of population/problem, intervention, comparison/control, and outcome.[10] The review question was combined using PICO headings. Keywords were entered into the search databases utilizing Boolean operators. Results were further refined by including only human adults aged over 18 years. When entering search criteria, “all adults 19+ years” was offered as the adult years option in the MEDLINE and PUBMED database and “all adults” in CINAHL.
Unpublished studies were located by searching databases for grey literature including eThoS, ERIC, WorldCat, Google, Google Scholar, and keyword internet searching. All search strategies are detailed in full, in Appendix 1.
3.2. Search results
The PRISMA flow diagram offers diagrammatic representation of identification, screening, eligibility, and inclusion of studies for this review (Fig. 1). Combined search results are demonstrated, totaling an initial 1120 studies. There were 811 studies remaining after removal of duplicates and this number was screened at title and abstract. The majority of search results was excluded at this stage as they failed to match even the primary study requirements e.g. not screening for AF (n = 761 excluded). Where there was discrepancy or uncertainty over the applicability of located studies, the clinical supervisor for this research acted as the second reviewer, where he performed a blind review of the studies. Principally, some of the papers inaccurately represented inclusion criteria, instead incorporating an array of screening methods and other chronic disease. For example, screening for cancer arose as did methods of screening such as radiological imaging. Despite utilizing the AND Boolean operator to combine searches, further studies of relevance which truly incorporated screening exclusively within this patient group (people with AF) were not located. The search was therefore widened (using the Boolean operator OR) to consider alternative terms for screening as per MeSH suggested terms. MeSH alternatives were not always applicable and alongside “screening,” produced irrelevant terms including “cancer screening” and “bowel screening.” This failed to expose supplementary papers despite adopting a comprehensive and methodical approach (Supplementary Digital Content - Appendix 1).
Figure 1.
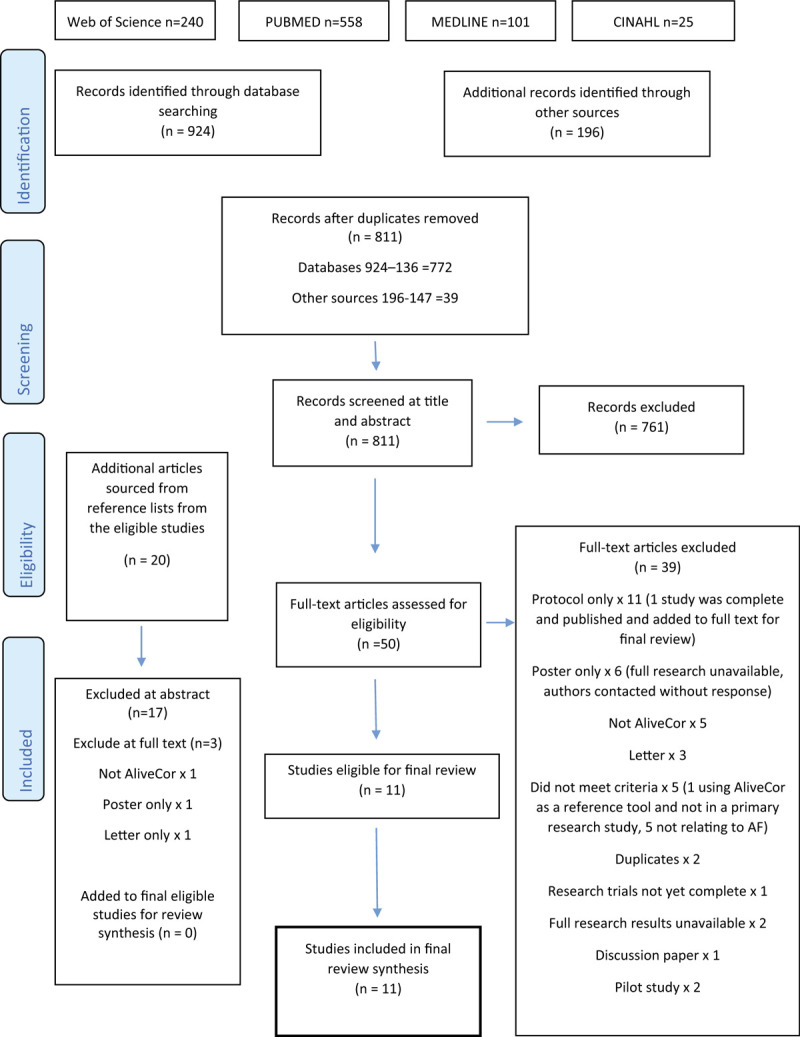
PRISMA flow diagram.
Various results were returned via Google basic search, Google scholar, WorldCat, EThOS, and ERIC. Of these results, none were relevant for full text inclusion. Additional databases searched included the Cochrane Library, Clinical Trials database, the European Union Clinical Trials Register, the Center for Evidence Based Medicine, National Institute for Health Research and the AliveCor Clinical Research pages. Of these, 6 results reached the full text review.
3.3. Study selection
Combined results, removal of duplicates, and citation screening resulted in 50 full-text articles accessed and screened against selection criteria, independently by 2 reviewers. Of these 50 full-text articles, 39 were excluded (Fig. 1). Reasons for non-selection included research not using the relevant screening tool, results not incorporating the full research (eg, conference paper or poster only and lack of response when authors were contacted for the research), trials not yet complete or published and research not looking for AF exclusively. One author of a protocol did respond to explain their full study would be published later in 2019. The authors of all excluded pilot studies were contacted without response. From the final 11 papers, reference lists were hand searched, identifying 20 further reports for consideration. Of these, 17 were excluded at abstract and the remaining 3 were accessed for full text review, with one being included in the final review. Therefore, a total of 11 research papers were eligible for inclusion into this review.
Authors of the final 11 eligible studies were contacted by email for further information regarding similar ongoing or completed studies or unpublished literature but no further studies were identified. Finally, local professionals in the fields of diabetology and cardiology were consulted to validate sources of enquiry. Feedback failed to suggest additional literature sources from the diabetes specialists, but 3 screening studies were suggested from cardiology with 2 being duplicates and one not meeting full criteria. Characteristics of the final included studies are presented in Table 2 . It is worthy of mention that some of the eligible studies are from similar author groups (eg, Soni et al[18,19] wrote 2 of the papers and there are some similarities between their study designs. Lowres et al also features as the lead author in 2 studies but there are more differences between their protocols.[6,17]
Table 2.
Characteristics of included studies.

4. Quality appraisal
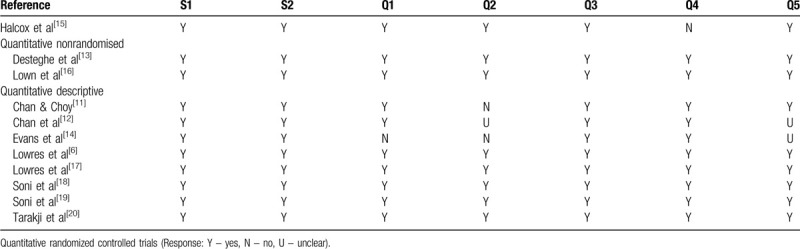
Appraisal of methodological quality of the eligible studies was assessed using the Mixed Methods Appraisal Tool, version 2018 (MMAT)[21] (Supplementary Digital Content - Appendix 2). Whilst the research studies included here are quantitative, they adopt different designs and therefore a tool that incorporates experimental and non-experimental appraisal, offers ease of application and comparability. The algorithm incorporated within the MMAT assists the reviewer in selecting the most appropriate checklist to appraise the methodological quality of the research. The MMAT offers 5 categories of study design and this review applies 3 of these – quantitative randomized controlled trials, quantitative nonrandomized trials and quantitative descriptive. Table 3 demonstrates to what extent the methodological quality criteria is evident in the research study.
Table 2 (Continued).
Characteristics of included studies.

The majority of the eligible studies that were appraised had a quantitative descriptive design, with 2 quantitative nonrandomized and just 1 study as a randomized controlled trial. This was unsurprising when considering the nature of the eligible studies and focus of this systematic review. Overall, the methodological quality was high. Where criteria were uncertain, this related more to the study design and the quality criteria not being directly applicable, rather than inaccurate representation or error.
Halcox et al provided limited details relating to randomization, only in that a simple 1:1 allocation was applied.[15] Baseline characteristics were compared between the standard care and intervention group using statistical testing and were highly comparable. The outcome assessors were not blinded to the intervention. The study team identified closer contact with the intervention participants, raising the possibility that relevant events may have been missed in routine care patients.
In the quantitative descriptive studies, sampling strategies varied. Soni et al used random selection in terms of location and participants recruited.[18,19] The remaining studies in this group employed consecutive recruitment, whereby eligible participants were approached for enrolment. These studies were of a cross-sectional, observational design, and whilst sampling did not adopt the probability method, the study design outlined their criteria. For example, Chan et al screened 11,574 people in their community screening program with minimal exclusion criteria (53.6% participation rate from the members in the screened communities).[12] Whilst large numbers of participants were screened in their research study, it is unclear how representative the sample was. There was no identification of specific disease groups, only that people over 50 years were recruited. Chan and Choy provided their recruitment numbers and total population for the city, but no further breakdown in terms of statistical representation of this population.[11] They did however highlight that their research was not that of a targeted nature, and overall prevalence and incidence with numbers needing treatment was provided. Statistical tests used were outlined but these were not presented in their publication.
Whilst there were no significant concerns in terms of methodological quality with the research by Lowres et al they reported that 24% of participants approached, declined to take part in the study.[17] This was explained as many people feeling overwhelmed post-surgery, reflecting lower numbers recruited (n = 44). An earlier study by the group provided fewer results and discussion relating to the feasibility aspect of their study in relation to cost-effectiveness, with the latter predominating the outcomes of their publication.[6]
In the quantitative nonrandomized studies, confounders were not clearly outlined. Confounding aspects relating to utilization of the AliveCor device may include tremor, ability to operate, experience or previous use, dexterity, artifact and clarity of transmission. Confounding bias was generally low as anticipated confounding factors were accounted.
5. Results
5.1. Feasibility considerations
The feasibility of utilizing the AliveCor device in clinical practice relates to the overarching concept of whether employing the use of this heart rhythm screening tool in clinical practice is possible. Whilst we know the device is already utilized in clinical environments and by patients, the components necessary to make this practical and achievable whilst providing value in terms of accuracy, is important when reviewing overall effectiveness.
The feasibility of implementation of the AliveCor device as a heart rhythm screening aid in AF screening studies was high. Implementation feasibility is defined as a high proportion of people invited for screening taking it up, along with sufficiently low barriers and resource drain.[22] Processes relating to recruitment and retention of participants were favorable in the eligible studies, with lower numbers of recruitment evident in some studies and this can be seen in Table 4 , which also highlights the ability to screen all participants involved.[14,16,17,20] Drop-out rates were low in all eligible studies, perhaps reflecting the study design, compliance and ease of use with the AliveCor device. The minimal number (n = 5/60) who did drop-out of the research studies were for reasons including moving away from the geographical study location (n = 1), purchasing a replacement mobile phone of an alternative brand (n = 1) and withdrawal of consent (n = 3).[16] In a postoperative study of research of AF recurrence, 2 participants failed to complete the study (reasons unexplained).[17] These 2 studies reflected a design requiring self-recording of ECGs once away from the research team at either specified times or frequencies throughout the follow-up period.
Table 2 (Continued).
Characteristics of included studies.
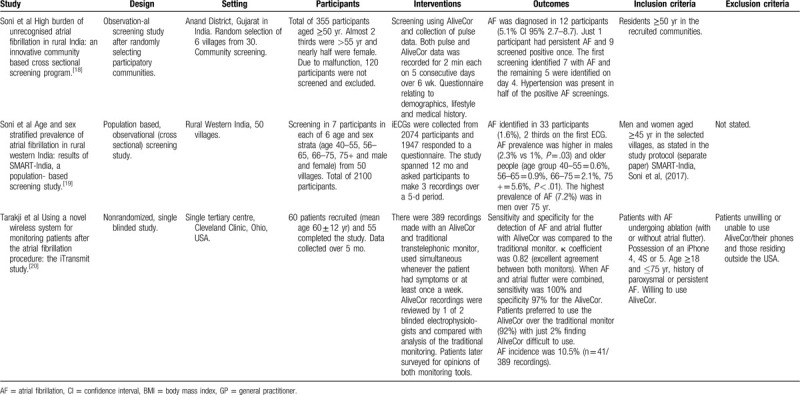
Resources were problematic in the study by Soni et al whereby a proportion of participants had to be excluded due to malfunction of the device.[18] Acceptability from users was encouraging, retention rates were high, with all those recruited remaining engaged with the study, and when compared to other devices for rhythm monitoring, the AliveCor device was rated favorably[13,16] (Table 4 ). In a comparison study using 4 screening devices, the AliveCor device was rated the most comfortable device to use.[16] Desteghe et al did not formally evaluate user-friendliness but no patients objected to using either AliveCor or MyDiagnostick.[13] They offered justification for acceptability of both devices such as immediate visualization of the ECG recording where diagnosis and judgement can be made regarding quality and clarity with the AliveCor device. Training requirements for physicians were minimal (eg, pharmacists in the study by Lowres et al but tuition for patients was more time consuming).[6] Some patients needed further tuition following the initial guidance.[17] However, Evans et al identified the AliveCor device to be a feasible service in low-resource settings when used in a hospital in Kenya.[14] Availability of mobile devices and internet received an affirmative response when health professionals were asked about their access.[14]
Data processing, time, and resource intensiveness are metrics in the management aspects of feasibility studies (detailed in Table 4 ). This varied according to the study design and method of ECG analysis. In the studies by Desteghe et al and Lowres et al, manual interpretation was implemented, and the remaining studies relied upon the automated algorithm for ECG interpretation, with professional overview of the abnormal or non-diagnostic ECGs.[13,17] Furthermore, the number of ECG recordings made impacted upon time for data gathering and processing. Single screening episodes would pose less demand on the study team compared to those for whom protocol demanded repeated ECG recordings.
Scientific components incorporated qualities relating to factors that interfered with obtaining diagnostic ECGs and these were referred to in most studies e.g. tremor and inability to hold the device (see Table 4 ). Statistical testing was generally as outlined in the study design but not all the tests were displayed in the published article.
5.1.1. Cost effectiveness
Cost effectiveness deserves attention due to the constant pressures enforced upon the current economic climate in healthcare. The cost effectiveness analysis by Lowres et al incorporated costs of AliveCor ECG recordings, treatment, and outcome data according to the numbers incidentally detected as having AF.[6] They concluded that the approach adopted in their study, whereby pharmacy customers aged ≥65 years were screened using the AliveCor device, was cost effective. The estimated incremental cost-effectiveness ratio of screening to prevent 1 stroke or to increase 1 quality adjusted life year, was well within the range fundable on a population basis.[6,23] Their analysis incorporated calculations of anticoagulant prescription and adherence and identified improved cost-effectiveness with direct oral anticoagulants compared to vitamin K antagonists. This is significant, given the high risk of stroke and premature death identified in people with asymptomatic AF, the salutary effect of anticoagulants in reducing adverse outcomes and the cost effectiveness in stroke and thromboembolism prevention through the appropriate use of anticoagulants.[24,25,26] Halcox et al completed health economic evaluation in part, calculating a cost per AF diagnosis of £8255, according to UK NHS tariffs at their time of writing.[15] They did not complete analysis of cost effectiveness to stroke prevention in the community but suggest their conclusions align with other health economic studies.[6,27,28]
In summary, feasibility metrics demonstrated that the AliveCor device is an effective tool of choice in terms of process (response rate, ability to screen), resources (retention, compliance, suitability for the intention, minimal training), and management (adherence, equipment). Staffing impact was more intensive where further analysis of ECGs was required (management and scientific metric). Cost effectiveness analysis whilst not a primary focus, forms part of the objectives of this review in terms of overall considerations around the feasibility of implementation. AF is costly in terms of healthcare consumption and the associated burden on wider society and utilizing the AliveCor device proved cost effective in the analysis in this review.
5.2. Validity of the AliveCor device
The sensitivity for AF detection varied across the included studies, ranging from 54.5% in the study by Desteghe et al, to 100% in the research by Tarajki et al[13,20] (Table 5). Lowres et al, reported a 98.5% sensitivity for AF detection and 91.4% specificity with a further study indicating a sensitivity of 94.6% (95% confidence interval [CI], 85.1–98.9) and 92.9% specificity (95% CI, 92.0–93.8).[6,17] The majority of false-positive ECGs were associated with low-voltage p-waves and QRS complexes, atrial ectopy, and left bundle branch block.
Table 3.
Assessment of methodological quality of included reviews.
Evans et al reported 84% of ECGs as “normal” by the automated diagnosis, 8% “unclassifiable” (all of which when later analyzed manually by a cardiologist were deemed normal) and 8% as “possible AF,” which were later confirmed as AF by the cardiologist.[14] In the AF screening study by Chan and Choy of 13,122 AliveCor ECGs, 56 (0.4%) were uninterpretable and it is unclear in their publication if these were later reviewed and classified as normal or not.[11] They did apply an age cut-off threshold of 60 years or above and when this was used, there was a 98% sensitivity and unexplained low specificity of 29.2% when detecting newly diagnosed AF. This poor ability to accurately identify patients who did not have AF potentially threatened the validity of the device in this study. In a separate study, the sensitivity using the automated algorithm was 75% (95% CI 70%–80%) and specificity 98.2% (95% CI 59.3–70.5).[12] The positive predictive value was 99.5% (95% CI 99.4%–99.6%). Of 11,574 AliveCor ECGs, 839 (7.2%, 95% CI 6.7%–7.7%) were uninterpretable and it is unclear whether these underwent subsequent review. In the study by Halcox et al, 76% of AliveCor ECGs were reported as normal (out of a total of 60,440 ECGs over 12 months that were recorded in the intervention group) and none were later reclassified as AF by the cardiologist or physiologist checking the transmitted ECGs.[15] Only 6 of the 21% of ECGs reported as “undetermined,” were finally confirmed to be AF. Soni et al identified 4.2% (n = 88) of recordings to have “possible AF” according to the automated algorithm and after clinical adjudication, 32 participants were confirmed to have AF.[19] One participant had feedback of “unclassified” and this was later reviewed as being AF. The initial interpretation of AliveCor ECGs in the study by Soni et al, identified 25 inconclusive transmissions (of 823 total screenings), later resulting in 20 negative screenings and 5 positives for AF.[18] All AF diagnoses from the automated interpretation were confirmed as AF by the adjudication board. This data is summarised in Table 5, which also shows those studies where specific information relating to tool validity is omitted.
Desteghe et al compared the use of the AliveCor device to MyDiagnostick (Mydiagnostick Medical B.V), an alternative handheld rhythm screening device, demonstrating lower sensitivity from the AliveCor device and slightly superior specificity when compared with MyDiagnostick (sensitivity 54.5–78.9 and specificity 97.5–97.9 with AliveCor and sensitivity 81.8–89.5 and specificity 94.2–95.7 with MyDiagnostick).[13] Device patients (pacemakers or implantable cardioverter defibrillator) were included in the analysis and may have affected the results, as the AliveCor device only had a 36.8% sensitivity in these patients. It is, however, widely accepted that these types of device are not appropriate in these patients due to inaccuracies with interpretation and detection of pacing spikes on the ECG.[29] After the exclusion of device patients, the sensitivity and specificity for both devices improved with automated interpretation and physiologist analysis. Algorithm analysis of the AliveCor device was 54.5% whilst manual interpretation by electrophysiologists reached 90.9% of AF patient recordings.
In the study by Tarakji et al, just 7 of 831 recordings were uninterpretable.[20] A normal rhythm was correctly identified in 97% of cases and AF 100% of the time, with 3% false-positive results. The AliveCor device had a 97% specificity and 100% sensitivity. When the false-positives were more closely examined, they were related to difficulty in assessing p waves making it problematic when detecting a normal rhythm in patients with pacemakers. Lown et al also ran a comparative study between the AliveCor device and 3 other portable devices, and when the automated algorithm was used, AF was accurately detected with sensitivity of 87.8% and specificity 98.8%, and an overall accuracy of 96.65% (95% CI 94.4–98.1).[16] The AliveCor device yielded unreadable recordings from 6 participants with an average 3.3 attempts to obtain a diagnostic result. Low voltage ECG transmission accounted for 2 of the 6 unreadable recordings.
In summary, AF detection rates ranged from 0.8% to 36% and this largely correlated to the study population with a wide age inclusion and mass/population screening representing lower AF detection. Recruitment from higher-risk groups (older age, targeted localities, presence of chronic disease) demonstrated higher numbers of people with AF. Further interpretation of ECGs was required with 0.4% to 4.2% of ECGs where a differentiation between AF and normal could not be made. Different durations of screening time resulted in varying rates of AF detection. AF was detected in 0.8% to 36% of the population during single-point-in-time screening and 1.6% to 24% AF detected through repeated intermittent AliveCor ECG recordings.
5.3. Grading of evidence
The grading of recommendations assessment, development, and evaluation (GRADE) quality of evidence assessment tool was used with a second reviewer assisting with the quality assessment of eligible studies.[30,31] Assessing the quality of evidence is important and supplements the appraisal of methodological quality, facilitated in this review by employing the MMAT assessment tool. Historical grading of evidence would impose a lower ranking on many of the eligible studies in this review, due to their observational design. Strengths in methodological approach and study design, however, enhance reliability in nonexperimental studies. A summary of the quality analysis is displayed in Tables 6 and 7. Overall quality reporting was moderate. All studies described the primary objective of the research and included a summary of the main findings. Detailed comorbidities of the study participants were only adequately reported in some studies, but lack of this data was not always representative of a criticism and may simply not have been the focus of the research, for example, if feasibility was the study focus. Limitations were discussed in varying detail and there were no missing outcome data in any of the studies. Inclusion criteria including a nonselective sample of the population (eg, all adults over 18 years of age) were evident in 6 of the eligible studies. The remaining research recruited a more selective sample, restricting age eligibility along with some other criteria (eg, the CHA2DS2-VASc stroke risk stratification tool for patients with AF, where the risk score is ≥2 - Congestive heart failure, Hypertension, Age >75 years, Diabetes, prior Stroke/TIA/thromboembolism, Vascular disease, age 65 to 74 years, female sex) within their inclusion criteria.[15]
Table 4.
Metrics of feasibility in the eligible studies.

Table 4 (Continued).
Metrics of feasibility in the eligible studies.
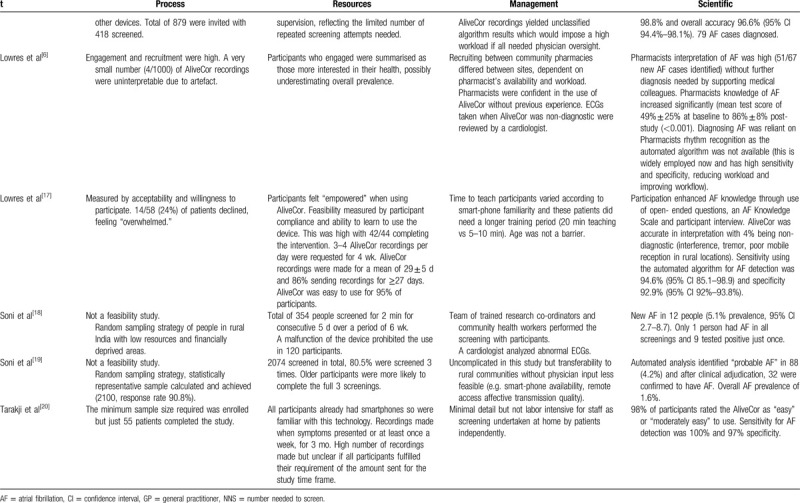
Table 6.
Quality assessment.

Table 7.
Grading and quality of evidence assessment.
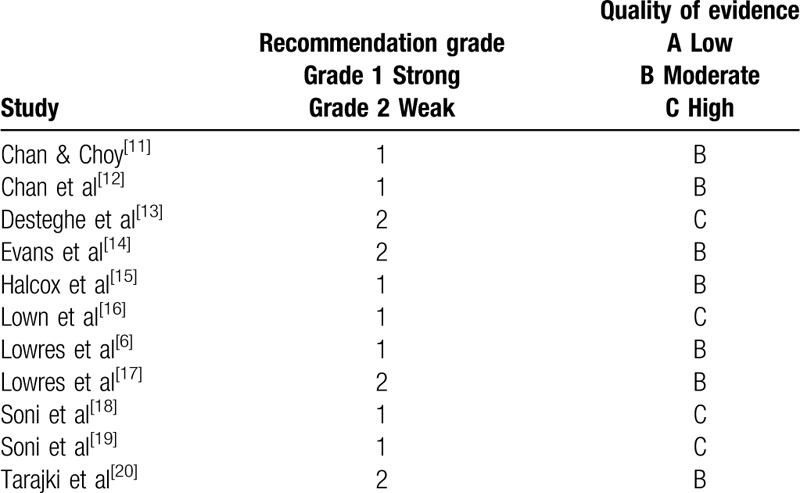
When this quality of evidence assessment is matched against the MMAT quality appraisal, it is evident that those scoring highest in terms of GRADE assessment, rate similarly well in the MMAT quality appraisal. Four studies were awarded a 1B grading with Lowres et al also meeting all the criteria in the MMAT assessment.[6] Chan et al[12] also scored highly with only 1 criterion from the MMAT being marked as “unsure.” This was followed by Chan and Choy[11] and Halcox et al[15] who missed 1 criterion each on the MMAT assessment (“is the sample representative of the population?” and “are outcome assessors blinded to the intervention?” respectively). Two studies both rated 1C on the GRADE assessment whilst meeting all the criteria on the MMAT appraisal.[18,19] Two of the remaining studies rated slightly lower at 2B, meeting all the MMAT assessment details.[17,20] Evans et al also rated 2B but there were concerns with the small sample and sampling strategy when analyzed using the MMAT.[14] The study by Desteghe et al was graded lowest with GRADE at 2C whilst meeting all the MMAT criteria.[13] Overall quality reporting was moderate and appraising the grading of evidence is important when examining research from a range of methodological designs.
5.4. Ethical considerations
Limited ethical detail was provided throughout the eligible studies. Chan and Choy, Evans et al and Lowres et al explained that consent had been sought from participants and that the research had been granted ethical approval.[6,11,14,17] Only 1 study reported that all data was anonymized.[14] Evans et al provided a statement of ethical compliance stating consent proceedings, regulations of medical ethics and anonymity.[14] Ethical considerations were incorporated within some checklists used for reporting systematic reviews, yet this detail was omitted from many publications. The complexities around systematic reviews includes the use and reporting of data intended for the primary research only. If the use of data is for a similar purpose, this poses less of a threat (eg, if the primary study was concerning a screening tool and the objective of the systematic review was to explore types of screening tools). But if authors are contacted for additional unpublished detail (eg, further details from the study participants that may not have been the primary objectives), caution must be applied so this does not affect anonymity assured to participants.
6. Discussion
This study is the only systematic review that we are aware of that has specifically focused on the AliveCor device as the screening tool for AF detection. This has enabled critique of the device in terms of effectiveness, utility, feasibility, and accuracy. As AF is the most common arrhythmia, this selectivity also enables further clarity by removing alternative arrhythmias and preventing confusion over accuracy of findings. Synthesis of the findings support the AliveCor device as a convenient, valid, and effective tool for AF screening.
6.1. AF screening using the AliveCor (the utility of the device for AF screening and clinical effectiveness)
Early diagnosis of AF provides the opportunity for early initiation of treatment, anticoagulation to reduce stroke risk and to reduce complications and hospital admissions associated with AF, and so an early screening tool could have a significant impact on both healthcare costs and quality of life. Screening tests should be low-risk, cost-effective, and use accurate methodology to be worthwhile. The success of a screening strategy depends on prevalence and incidence of the condition in the screened population and accuracy of testing but use of known risk factors in identifying people who would benefit from screening is suggested to be effective and has also been demonstrated in this review. AF is multifactorial but aging, prevalence of obesity, and sedentariness are highly contributory,[32,33] with age demonstrated as the strongest predictor of AF. A screening cut-off of ≥65 years has been recommended, on the basis of expert consensus,[34,35] and this is supported by the prevalence of AF in the reviewed papers that specify older age in their screening studies.
Since its inception, the AliveCor device has been used widely in clinical research and practice by health professionals and patients. Digital health technologies have changed health screening practices, not least within cardiology.[36] The AliveCor device provides opportunities to be used as a single-point-in time screening tool or used repeatedly for intermittent screening, demonstrating the utility of the device. The latter can be initiated during times of symptoms experienced by the user, or at regular intervals as instructed by the researcher or health professional, as demonstrated in this review. The duration of monitoring has shown congruence with AF diagnosis and studies have previously demonstrated the effectiveness of single screening episodes in detecting AF.[36,37] The largest systematic review combining data from thirty cross-sectional studies identified undiagnosed incident AF in low numbers, with identification being marginally higher in those aged ≥65 years, using single point in time ECGs via the AliveCor device.[37] Hence, this study has demonstrated that whilst using only brief singular recordings, AF can still be detected in significant numbers, most convincingly in older aged cohorts or those screened from higher-risk populations. However, the cost-effectiveness and appropriateness of screening people aged ≥18 years would be questionable in terms of low numbers and the value this would bring when resources, time and workload is considered (and further evidenced by the lower numbers of AF detection in corresponding studies in this review where age was not an exclusion to screened participants).
Previous studies have demonstrated enhanced AF detection by intermittent or continuous monitoring, suggesting that paroxysmal AF may be missed by single recordings. However, a systematic review of single point in time screening to identify unknown AF, demonstrated this still as an effective approach with slightly higher numbers of AF diagnosed in the older age groups (>65 years), supporting the evidence extracted from the papers within this review, where AF was seen in older populations.[37] In the AF screening study by Svennberg et al,[27] twice-daily ECG recordings were made for 2 weeks and this proved slightly more effective in terms of AF detection rates. Their approach also highlighted the relevance of repeated recordings, evidenced by more AF being diagnosed on subsequent ECGs and this is also supported by findings within this review.
In this review, 5 studies adopted a protocol of intermittent monitoring using the AliveCor device. Soni et al identified AF in low numbers but the majority were diagnosed on their first ECG.[19] A similar study also implemented repeated screening over consecutive days and there were higher numbers of AF diagnosed.[18] Repeated, intermittent recordings were also requested in Halcox et al[15] and Lowres et al[17] studies, the latter also requesting symptomatic activation. This, therefore, supports the use of intermittent ECGs where paroxysmal AF may be missed by single recordings, yet this approach relies upon the compliance of the individual to independently activate the device without supervision and make clear ECG rhythm recordings for analysis.
6.2. Screening approaches
Screening approaches continue to be debated with strategies generally aligning with opportunistic or systematic screening.[38–42] Both opportunistic and systematic screening increases the rate of detection compared to routine practice, but systematic screening is more expensive.[38] Screening approaches varied across the studies within this review, including population-based screening akin to mass screening[12] and more focused screening, identifying higher-risk participants according to age[6,9,15,16,18,19] and the existence of co-morbidities.[15] A correlation was seen with higher numbers of people having AF in the groups where the screening protocol was more targeted, for example, when the screening took place in hospital wards housing cardiology and geriatric patients[13] and where recruited participants had undergone cardiac surgery.[6] Age was not always a factor as AF detection and prevalence rates varied across the studies where participants were recruited from older age categories. Furthermore, a systematic approach whereby studies incorporated intermittent or repeated screening, produced mixed results. However, the lack of homogeneity across reviewed studies here makes further comparisons more difficult as study locations, participants and eligible criteria varied.
This review has demonstrated a targeted screening approach to be more effective in AF screening studies. Screening approaches have been further explored in The Screening for AF in the Elderly (SAFE) study, this being landmark research comparing 3 strategies of AF screening in the over 65-year age group in primary care.[39] Systematic screening of the target population with 12 lead ECGs was compared to opportunistic screening using pulse palpation in a target population by general practitioners (GPs) and routine care. Opportunistic screening was more effective than routine care and more cost effective than systematic screening. Improvements in detection and subsequent care in the opportunistic screening group were also noted.[39] However, Moran et al added that systematic screening had higher uptake with a third of those screened opportunistically not attending for follow up.[38] The Cochrane Collaboration analyzed randomized controlled trials focusing on AF detection in over 65-year olds, drawing similar conclusions to the SAFE study.[38] The number needed to screen in systematic screening was compared to routine practice and was marginally higher for systematic screening compared to opportunistic screening. There is further evidence showing an equivocal number of patients identified with either systematic or opportunistic screening over routine care,[39–41] again supporting the findings from this review whereby screening approaches revealed more AF when screening was targeted to specific patient groups (older age, co-morbidities, inpatient and cardiology localities).
In this review, evidence from screening cost-effectiveness modeling highlighted that screening strategies are less cost-effective in under 65-year olds and those over 80 years, but still remain within acceptable limits.[6,27,28] The studies within this review, whilst not selected for their cost effectiveness analysis, did provide details within 2 reports. They supported screening using the AliveCor device, demonstrating cost effectiveness, but it is accepted that this was only critiqued in detail in 1 study.[15] Furthermore, the cost of an AF related stroke is estimated to be significantly greater than a non-AF related stroke from a health outcome, economical, and societal perspective.[43] Background evidence has illustrated that AF related strokes are associated with an increase in inpatient costs compared to strokes unattributable to AF.[44–49] Studies incorporating rehabilitation periods of recovery represented a significant increase in costs in AF stroke patients compared to non-AF related strokes.[44,48] Ali et al estimated an adjusted independent effect of having AF on costs as an additional £2173.[43] Longitudinal studies estimated the costs of an AF related stroke to be considerably more at 1 year and similar findings were evident in the Berlin Acute Stroke Study.[50,51] This is supported by a study focusing on the economic impact of AF-related stroke as well as a Swedish study whereby AF-stroke patients were followed for 3 years.[52,53] These findings also demonstrated cost increases compared to non-AF related strokes.
6.3. Screening acceptability (considering the feasibility of the tool in wider research and clinical practice)
The feasibility of the AliveCor device as a tool of choice in wider research and clinical practice is an important consideration when contemplating optimal screening approaches. The ease of use, immediate visualization of the ECG and comfort have been rated favorably in this review and associated research. Within this review, the AliveCor device was also rated the tool of choice and easier to access when compared to a transtelephonic monitor for making symptomatic recordings.[29] Feeling empowered and having peace of mind and reassurance through self-initiated monitoring and feedback was also reported and supports the users’ acceptance and willingness to comply with remote mobile monitoring devices.[17] Patient education on how to use the AliveCor device varied in the studies reviewed, from simple instruction incorporating up to 10 minutes of tuition and practice to twenty minutes of guidance for those less familiar with smartphone or mobile technology. Importantly though, the less comfortable people were not deterred from using the device nor did it impede their ability to self-monitor. The mode of transmission, unlimited time of use, control of activation, societal adaptation to smartphone technologies and compliance, even when unsupervised, further supports the AliveCor device as a feasible tool of choice in AF screening.[16,17,19]
Patient perceptions were predominantly discussed in terms of device feasibility, but physician assessment was also shown to be important. Evans et al surveyed physician opinion relating to device access and internet connections in a remote setting and summarised this as a feasible tool for AF screening in a low-resource setting.[14] Outside of this review, Godin et al screened participants in Canadian Primary Care clinics and surveyed physicians relating to the clinical value, implementation, satisfaction, confidence, diagnostic ability, and accuracy of the AliveCor device.[54] Clinical value, ease of integration and likely acceptability from patients were rated most highly, further supporting the findings within this review.
Furthermore, the AliveCor device has been used in disparate research designs including large community screening programs and more focused high-risk groups. Populations have therefore been heterogenous with varied clinical, anthropometric, gender, age, and geographical
Characteristics, thus, demonstrating the utility, feasibility, and wide applicability of AliveCor as a screening tool, be it via an opportunistic or systematic approach.
6.4. Screening accuracy (how valid is the tool for AF screening)
The AliveCor device incorporates an automated algorithm for the detection of normal or abnormal rhythms and accuracy of this has been analyzed widely. The AliveCor device has been awarded the accolade of being the most clinically validated screening tool.[55] The studies critiqued within this review, demonstrate high sensitivity of the device at >98% with similarly high sensitivities in research outside of this review of >90%.[6,20,56,57] Lower sensitivities appeared related to the automated algorithm interpretation and once checked by a specialist, improved. Furthermore, sensitivities may be less favorable when trouble-shooting is not optimized, for example, patients with a tremor or who are unable to hold the device securely can produce a less clear ECG recording. The AliveCor device can be applied to the bare chest if this is a problem, but this does not always appear to be stipulated in the research. The exclusion of patients with a cardiac pacing device should be applied due to inaccuracies affecting automated interpretation. Specificity has also been reported highly with figures representing >99%[5,56,57,58] although this review did also uncover lower specificity in 1 study,[11] and specificity was unreported in 4 of the reviewed studies.[14,15,18,19]
Further evidence continues to support the accuracy of the AliveCor device as a screening tool both from research within and outside of this review, demonstrating favorable validity most notably after the exclusion of “unclassified” recordings. Findings from this review are further illustrated by a supporting accuracy study by Koshy et al, where enhanced sensitivity and specificity (>95%) were demonstrated after removing uninterpretable ECGs.[59] Similarly, William et al calculated comparable sensitivity and specificity but note a quarter of ECGs recorded by the AliveCor device were classified as “uninterpretable.”[60] Brasier et al also report a number of “unclassified” ECGs and once removed, resulted in optimal sensitivity and specificity.[61] Diagnostic accuracy improved when AliveCor ECGs were reviewed by practitioners experienced in rhythm analysis, compared to relying on the automated interpretation, in the studies examined in this review. This emphasizes the relevance of having practitioner oversight when patients use such devices but should not deter patients from initiating use of the AliveCor, but ensure they seek clarification over unclassified recordings. The interpretation of accuracy statistics must be appraised with caution and considered in terms of how this is presented. The frequency of unclassified or uninterpretable ECGs is significant when considering usability, as the necessity for additional adjudication when automated analysis has been non-diagnostic, imposes an additional workload on skilled health professionals required to further analyze the ECGs.
6.5. Limitations of included studies
Limitations of the included studies include the lack of homogeneity between study protocols and the differences, therefore, between screening methods. Some focused on obtaining a single-point-in time ECG where other studies required repeated screening and over a varied length of time. This had an impact on the different rates of detection of AF and the likelihood of accurate identification. The AliveCor device was operated by participants in some studies, with supervision or fully operated by a research teams in others. Experienced practitioners would have more insight in terms of trouble-shooting poor transmissions and may be able to produce enhanced recordings. Populations also differed in terms of geography and clinical groups. India, Africa, Hong Kong, Australia, and the United Kingdom encompassed the countries within which AF screening studies were undertaken, all with diverse epidemiology and health status. Whilst this is not a limitation as such, it is noteworthy that the different locations and populations within these studies contributed to the heterogeneity between the research, leading to some differences in findings.
The coexistence of chronic disease, age, and gender also differed. For example, some patient groups were targeted because of their co-morbidities (including older age), whilst other studies with fewer exclusion criteria, included younger participants who might have been less likely to have associated chronic disease. Eligibility criteria was set for older age groups in some studies but again this was not consistent among all eligible studies in this review. Some research was undertaken in the community, primary or secondary care. Community screening programs operated in pharmacies, community halls, and GPs practices. Hospital based recruitment took place in cardiology wards, general wards, geriatrics, and outpatient clinics, leading to higher numbers with a diagnosis of AF. Overall, these variabilities influence the patient groups recruited and the varying health status of participants may have impacted on outcomes e.g. where chronic disease and older age predominated, higher incidence of AF could result and this may not be truly representative of the population.
The analysis of ECGs from the AliveCor device was diverse with some studies relying on the automated algorithm and others employing interpretation by the study team. There was however consistency between further analysis of abnormal ECGs by specialists within the teams.
A final limitation is that the populations within which the AliveCor device was used may not always reflect the general population for which the device is intended and must be considered when applying results to the real-world. The context within which the devices were used for monitoring purposes must be considered when evaluating overall validity and suitability for the screening purpose.
6.6. Assessment of bias
Assessment of bias in the reviewed studies (Table 8) has been guided by the Cochrane Handbook of Systematic Reviews of Interventions.[62] Selection bias relates to studies that incorporated smaller numbers and the omission of accurate power calculations failing to offer statistical representation. The only studies that provided sample size calculations were Halcox et al,[15] Lowres et al,[6] Soni et al,[19] and Tarakji et al.[20] The speciality outpatient clinics and cardiology and geriatric wards will likely have involved patients with confounding risk factors, that could lead to imprecision over results. Patients who attended health screening days required voluntary participation and this could, therefore, bias outcomes according to the demographics of patients attending, localities, timings, and publicity.
Table 5.
Summary of validity, representing sensitivity and specificity of eligible studies.
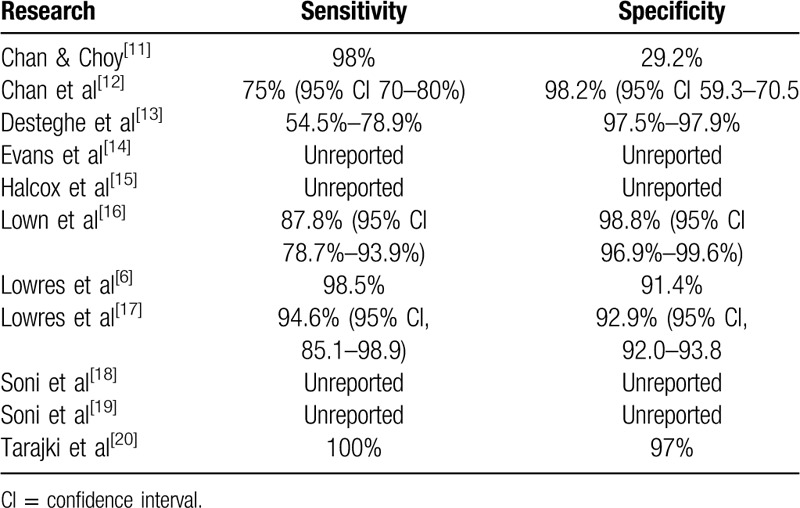
Table 8.
Risk of bias summary.
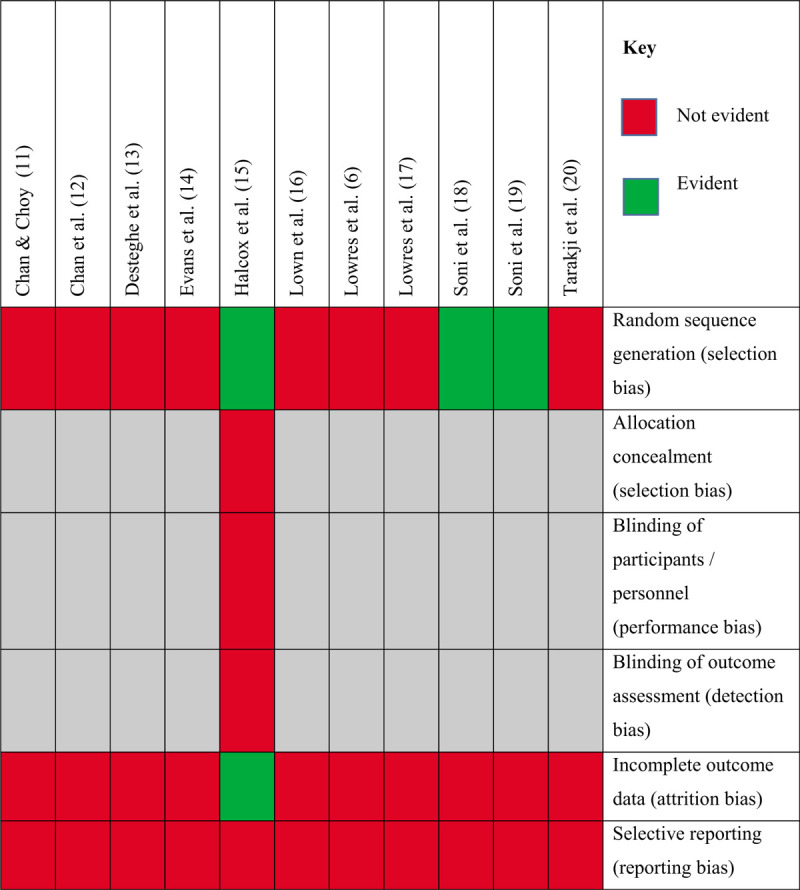
Sequence generation and randomization also relates to selection bias and some studies incorporated randomization within their studies.[15,18,19] Simple 1:1 randomization was performed in the study by Halcox et al for those who fulfilled inclusion criteria.[15] Soni et al strengthened the external validity of their studies by employing probability sampling, encouraging representative sampling, and enhanced generalisability to the target population.[18,19] Bias is more likely in the study by Chan and Choy whereby people volunteered to participate, and in the hospital or clinic-based research whereby patients were simply recruited if they fulfilled eligibility criteria.[11]
Information bias was less of a threat due to the validation of ECG applications. Ensuring studies are conceptually well planned can be evident through the use of pilot studies or detailed protocols, some of which were available in earlier publications and therefore limiting information bias.[15,63,64,65,66]
Unmeasured confounders may also impact on results, for example, in the study by Evans et al, where the co-existence of additional comorbidities could have influenced outcomes.[14] Although the medical history of the patient was taken, this was not factored into the analysis. This risk can be minimized by restricting inclusion criteria. Furthermore, regression models were not used: potential confounders could have been incorporated into such the models as explanatory risk factors. This was however evident in the studies of Chan and Choy and Halcox et al but represents bias within the results of the remaining screenings for AF.[11,15] Some disease groups infer an increased risk of AF such as hypertension, heart failure, diabetes, and stroke.[35,67] Older age groups, for example, over 65 years and men also represent higher prevalence of AF and this was not always factored into the analysis of results.[27,68,69]
As the only randomized controlled trial within this review, the study by Halcox et al was assessed for additional risk of bias in accordance with experimental trials.[15] The importance of blinding of participants and outcome assessments is highlighted and whilst the study team employed randomization via an external tool, non-blinding was evident. Indeed, the study team comment that close contact was maintained with participants and this was more so in the intervention group, inferring a higher risk of bias. Furthermore, the authors recognize that their inclusion of allowing only people who could access the internet, and those who could use the device, likely excluding a proportion of those at high risk, and therefore selection bias.
7. Limitations and recommendations
7.1. Limitations of this review
The PRISMA statement (www.prisma-statement.org/) and The Cochrane Handbook for Systematic Reviews of Interventions have been used as reference throughout this review, to ensure a methodical and rigorous approach.[62] Cochrane suggest an international collaborative approach, not restricted by nationality or language and this was reflected in the inclusion criteria of this review. It is however accepted, that additional studies may exist that did not fulfil eligibility criteria. Results were presented through addressing the primary objectives and secondary questions, with overall outcomes summarised in accordance with effectiveness of the AliveCor device as a screening tool for AF detection in screening studies.
The limitations to overall findings from this review center around the lack of homogeneity between study protocols and methods. Whilst the overall theoretical principles and study objectives have similarities (eg, the studies are looking for AF using the AliveCor device), the disparity between geographies, localities and populations and screening protocols, results in difficulties when summarising such heterogenous studies. This does however demonstrate that the AliveCor device is a tool of choice amongst diverse communities.
7.2. Recommendations
National guidelines on AF screening suggest pulse palpation followed by an ECG when the pulse is irregular.[4] National Institute of Health and Care Excellence have also produced focused guidance on using the AliveCor device as a tool of choice for AF screening.[70] The European Society of Cardiology 2016 guidelines and recommendations for AF screening suggest AF screening be undertaken opportunistically in >65-year olds via pulse palpation followed by an ECG rhythm strip if indicated.[33] The current UK National Screening Committee recommendation on AF screening in adults does not recommend systematic population screening despite acknowledging the benefits from doing so. They state there is a lack of evidence relating to the effect of treating people with AF identified through screening, so report no benefit.[71] Conversely, a report by the AF-SCREEN collaboration, promotes world-wide implementation of screening for AF in all >65-year olds.[3] This review has shown that the AliveCor device is an effective tool, evidenced widely through the findings within research undertaken utilizing this mobile ECG device. Further research would be advantageous whereby methods of screening and protocols are more homogenous. Screening matched participants as in the randomized controlled trial by Halcox et al provides the opportunity to identify the effectiveness of the AliveCor device compared to either standard care or alternative screening devices.[15] The majority of research involving the AliveCor device has adopted an observational focus using cross-sectional design and this is not dissimilar to the design often implemented in arrhythmia screening studies.
It would seem appropriate following the findings from this review, to support age group screening where AF is more likely to be detected. Targeted screening of higher risk patient groups would also seem sensible, yet we must acknowledge that AF can still occur despite the absence of high risk co-morbidities. Whilst repeated monitoring using the AliveCor device has demonstrated favorable outcomes in terms of AF being diagnosed on subsequent monitoring (eg, not on the first AliveCor ECG recording), this is more resource intensive and not always as feasible. Single-point-in-time use of the AliveCor is still advantageous when screening opportunities present. Healthcare practitioner oversight is advantageous but the AliveCor device is designed to be used by patients independently and offers the ability to self-record ECGs without professional involvement. Ensuring the patient knows how to refer on when unclassified ECGs are displayed, is important, and this can be through the availability of the interpretation service within the AliveCor device or through external sources.
8. Conclusion
In the growing digital health technology era, revolutionary tools allow new methods for screening including within cardiology for rhythm analysis. AF is growing in prevalence with a worldwide burden impacting on our increasing ageing population, further affecting health outcomes, morbidity, and mortality. This impact is not only health related but has economical and societal bearing. The AliveCor device offers a mobile, validated and secure option for heart rhythm screening and is feasible for both patients and health professionals to use in hospital and the community. Evidence demonstrates effectiveness of the AliveCor device as a screening tool in terms of validity and accuracy. This brings wider benefits in relation to early identification of AF, such as protection against thromboembolism when anticoagulation is initiated. Advancements continue within this field, with AliveCor developing enhanced algorithms and modified wearable devices, with different lead configurations, offering the consumer more options in terms of suitability and selection.
AF is a condition that can benefit from screening and should remain a key focus within national screening programs due to the significant burden this brings to patients, society, and healthcare. There are a number of tools designed to assist with AF detection, with the AliveCor device offering a convenient and effective option. A mobile device that provides a platform for both the health care provider and patient initiation supports screening programs through its accessibility. This should however be considered alongside appropriate patient selection to optimize acceptability and accuracy, particularly if used independently, without healthcare practitioner involvement. Further analysis of ECGs may be required and contemplated when selecting the most appropriate tool. Furthermore, the AliveCor device can be used in low-resource and diverse locations, demonstrated through the heterogenous studies included within this review.
Author contributions
Supervision: Andrew Mitchell, Lisa Wood, Carol Holland.
Writing – review & editing: Andrew Mitchell, Lisa Wood, Carol Holland.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CHA2DS2-VASc = congestive cardiac failure, hypertension, age, diabetes, stroke, vascular disease, age, sex - stroke risk stratification scoring system (2 represents a score of 2 being assigned to the patients risk for that category), ECG = electrocardiogram, GRADE = grading of recommendations assessment, development, and evaluation, MMAT = mixed methods appraisal tool.
How to cite this article: Hall A, Mitchell AR, Wood L, Holland C. Effectiveness of a single lead AliveCor electrocardiogram application for the screening of atrial fibrillation: a systematic review. Medicine. 2020;99:30(e21388).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Lloyd-Jones DM, Wang T, Leip EB, et al. Lifetime risk for development of atrial fibrillation. The Framingham Heart Study. Circulation 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- [2].Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med 2014;127:e15–6. [DOI] [PubMed] [Google Scholar]
- [3].Freedman SB, Camm JA, Calkins H, et al. Screening for atrial fibrillation. A report of the AF-SCREEN International Collaboration. Circulation 2017;19:1851–67. [DOI] [PubMed] [Google Scholar]
- [4].National Institute for Health and Care Excellence. Atrial Fibrillation: The Management of Atrial Fibrillation. Clinical Guideline 180. 2014. Available at: https://www.nice.org.uk/guidance/cg180?unlid=9816744662016120133316 [access March 12, 2019]. [PubMed] [Google Scholar]
- [5].Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in primary care. J Am Heart Assoc 2016;6:5e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thrombo Haemost 2014;111:1067–76. [DOI] [PubMed] [Google Scholar]
- [7].Manolis AJ, Rosei EA, Coca R, et al. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the working group ‘Hypertension, arrhythmias and thrombosis’ of the European Society of hypertension. J Hypertens 2012;30:239–52. [DOI] [PubMed] [Google Scholar]
- [8].Nichols GA, Reinier K, Chugh S. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care 2009;32:1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramkumar S, Nerlekar N, Souza S, et al. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ Open 2018;8:e024178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Richardson WS, Wilson MC, Nishikawa JN, et al. The well-built clinical question; a key to evidence-based decisions. Am J Physic 1995;123:12–3. [PubMed] [Google Scholar]
- [11].Chan NY, Choy CC. Screening for atrial fibrillation in 13,122 Hong Kong citizens with smartphone electrocardiogram. Heart 2016;103:24–31. [DOI] [PubMed] [Google Scholar]
- [12].Chan NY, Choy CC, Chan CK, et al. Effectiveness of a nongovernmental organisation led large scale community atrial fibrillation screening program using the smartphone electrocardiogram: an observational cohort study. Heart Rhythm Soc 2018;15:1306–11. [DOI] [PubMed] [Google Scholar]
- [13].Desteghe L, Raymaekers Z, Lutin M, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2016;19:29–39. [DOI] [PubMed] [Google Scholar]
- [14].Evans GF, Shirk A, Muturi P, et al. Feasibility of using mobile ECG recording technology to detect atrial fibrillation in low-resource settings. Glob Heart 2017;12:285–9. [DOI] [PubMed] [Google Scholar]
- [15].Halcox JP, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation. The RHEARSE-AF Study. Circulation 2017;136:1784–94. [DOI] [PubMed] [Google Scholar]
- [16].Lown M, Yue AM, Shah BN, et al. Screening for atrial fibrillation using economical and accurate technology. Am J Cardiol 2018;122:1339–44. [DOI] [PubMed] [Google Scholar]
- [17].Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016;50:44–51. [DOI] [PubMed] [Google Scholar]
- [18].Soni A, Earon A, Handorf A, et al. High burden of unrecognised atrial fibrillation in rural India: an innovative community based cross-sectional screening program. JMIR Public Health Surveill 2016;2:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Soni A, Karna S, Fahey N, et al. Age and sex stratified prevalence of atrial fibrillation in rural western India: results of SMART-India, a population-based screening study. Int J Cardiol 2018;280:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tarakji KG, Wazni O, Callahan TM, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation procedure: the iTransmit study. Heart Rhythm Society 2015;12:554–9. [DOI] [PubMed] [Google Scholar]
- [21].Hong Q, Pluye P, Fabregues S, et al. Mixed Methods Appraisal Tool version 2018. Canadian Intellectual Property Office, Industry Canada; 1–10. [Google Scholar]
- [22].Bradshaw EA, Cuzzi S, Kiernan SC, et al. Feasibility of implementing pulse oximetry screening for congenital heart disease in a community hospital. J Perinatol 2012;32:710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rawlins MD, Culyer AJ. National institute for clinical excellence and its value judgements. BMJ 2004;329:224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Freedman SB, Katholing A, Martinez C. Adverse prognosis of asymptomatic atrial fibrillation detected incidentally: a case for screening. J Am Coll Cardiol 2013;61:E371. [Google Scholar]
- [25].Kansal AR, Sharma M, Bradley-Kennedy C, et al. Dabigatran versus rivaroxaban for the prevention of stroke and systemic embolism in atrial fibrillation in Canada. Comparative efficacy and cost-effectiveness. Thrombosis and Heamostat 2012;108:672–82. [DOI] [PubMed] [Google Scholar]
- [26].Pink J, Lane S, Pirmohamed M, et al. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 2011;343:d6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation the STROKESTOP study. Circ Arrhythm Electrophysiol 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- [28].Welton NJ, McAleenan A, Thorn HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- [29].AliveCor. Press Release - AliveCor Heart Monitor for iPhone Receives FDA Clearance. 2012. Available at: https://www.alivecor.com/en/press/press_release/clinical-research-from-hr-validates [access September 2, 2019]. [Google Scholar]
- [30].Atkins D, Best D, Briss PA, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Br Med J 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2010;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kirchoff P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Euro Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [34].Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- [35].Stewart S, Hart CL, Hole DJ, et al. Population prevalence, incidence and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the screening study for undiagnosed atrial fibrillation (STUDY-AF). Clin Cardiol 2015;38:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lowres N, Neubeck L, Redfern J, et al. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Heamost 2013;110:213–22. [DOI] [PubMed] [Google Scholar]
- [38].Moran PS, Telijeur C, Ryan M, et al. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev 2016;3:CD009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hobbs FD, Mitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2015;9:1–74. [DOI] [PubMed] [Google Scholar]
- [40].Fitzmaurice DA, Hobbs FD, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 years or over: cluster randomised controlled trial. BMJ 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Swancutt D, Hobs R, Fitzmaurice D, et al. A randomised controlled trial and cost effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in the over 65 s: (SAFE). BMC Cardiovasc Disord 2004;4: doi:10.1186/1471-2261-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zungsontiporn N, Link M. Newer technologies for detection of atrial fibrillation. BMJ 2018;363:k3946. [DOI] [PubMed] [Google Scholar]
- [43].Ali N, Abdelhafix A. Clinical and economical implications of AF related stroke. J Atr Fibrillation 2016;8:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ali N, Howe JN, Abdel-Hafix A. Cost of acute stroke care for patients with atrial fibrillation compared with those in sinus rhythm. Pharmacoecon 2015;33:511–20. [DOI] [PubMed] [Google Scholar]
- [45].Diringer MN, Edwards DF, Mattson DT, et al. Predictors of acute hospital costs for treatment of ischemic stroke in an academic centre. Stroke 1999;30:724–8. [DOI] [PubMed] [Google Scholar]
- [46].Hannon N, Sheehan O, Kelly L, et al. Stroke associated with atrial fibrillation – incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Disease 2010;29:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang G, Joo H, Tong X, et al. Hospital costs associated with atrial fibrillation for patients with ischemic stroke aged 18–64 years in the United States. Stroke 2015;46:1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Winter Y, Wolfram C, Schaeg M, et al. Evaluation of costs and outcome in cardioembolic stroke or TIA. J Neurol 2009;256:954–63. [DOI] [PubMed] [Google Scholar]
- [49].Yoneda Y, Uehara T, Yamasaki H, et al. Hospital-based study of the care and cost of acute ischemic stroke in Japan. Stroke 2003;34:718–24. [DOI] [PubMed] [Google Scholar]
- [50].Luengo-Fernandez R, Gray AM, Rothwell PM. Population-based study of determinants of initial secondary care costs of acute stroke in the United Kingdom. Stroke 2006;37:2579–87. [DOI] [PubMed] [Google Scholar]
- [51].Brüggenjürgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin acute stroke study. Value Health 2007;10:137–43. [DOI] [PubMed] [Google Scholar]
- [52].Ghatnekar O, Glader EL. The effect of atrial fibrillation on stroke-related inpatient costs in Sweden: a 3-year analysis of registry incidence data from 2001. Value Health 2008;11:862–8. [DOI] [PubMed] [Google Scholar]
- [53].Sussman M, Menzin J, Lin L. Impact of atrial fibrillation in stroke related healthcare costs. J Am Heart Assoc 2013;2:e000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Godin R, Yeung C, Baranchuk A, et al. Screening for atrial fibrillation using a mobile single lead ECG in Canadian primary care. Can J Cardiol 2019;35:840–5. [DOI] [PubMed] [Google Scholar]
- [55].AliveCor. Three New Studies Confirm Clinical Utility of AliveCor's KardiaMobile Device and AI Algorithms. Available at: https://www.alivecor.com/press/press_release/three-new-studies-confirm-clinical-utility-of-alivecors-kardiamobile-device-and-ai-algorithms/2016 [access April 12, 2019]. [Google Scholar]
- [56].Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165:193–4. [DOI] [PubMed] [Google Scholar]
- [57].Orchard J, Lowres N, Freedman SB, et al. Screening for atrial fibrillation during influenza vaccinations by primary care nurses using a smartphone electrocardiograph (iECG): a feasibility study. Eur J Prev Cardiol 2016;23:13–20. [DOI] [PubMed] [Google Scholar]
- [58].Chan PH, Wong CK, Pun L, et al. Head-to-head comparison of the AliveCor heart monitor and Microlife WatchBP Office AFIB for atrial fibrillation screening in a primary care setting. Circulation 2017;135:110–2. [DOI] [PubMed] [Google Scholar]
- [59].Koshy AN, Sajeev JK, Negishi K, et al. Accuracy of blinded clinician interpretation of single-lead smartphone electrocardiograms and a proposed clinical workflow. Am Heart J 2018;205:149–53. [DOI] [PubMed] [Google Scholar]
- [60].William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018;15:1561–5. [DOI] [PubMed] [Google Scholar]
- [61].Brasier N, Raichle CJ, Dorr M, et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). EP Europace 2018;21:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. Available at: http://www.cochrane-handbook.org [access April 4, 2019]. [Google Scholar]
- [63].Lown M, Yue AM, Lewith G, et al. Screening for atrial fibrillation using economical and accurate technology (SAFETY) – a pilot study. BMJ Open 2017;7:e013535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lowres N, Freedman SB, Gallagher R, et al. Identifying post-operative atrial fibrillation in cardiac surgical patients post-hospital discharge, using iPhone ECG: a study protocol. BMJ Open 2015;5:e006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lowres N, Freedman SB, Redfern J, et al. Screening education and recognition in community pharmacies of atrial fibrillation to prevent stroke in an ambulant population aged ≥65 years (SEARCH-AF stroke prevention study): a cross sectional study protocol. BMJ Open 2012;2:e001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Soni A, Karna S, Patel H, et al. Study protocol for smartphone monitoring for atrial fibrillation in real-time in India (SMART-India): a community-based screening and referral programme. BMJ Open 2017;7:e017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Davis RC, Hobbs FD, Kenkr JE, et al. Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace 2012;14:1553–9. [DOI] [PubMed] [Google Scholar]
- [68].Salvatori V, Becattini C, Laureti S, et al. Holter monitoring to detect silent atrial fibrillation in high-risk subjects: the Perugia General Practitioner Study. Intern Emerg Med 2015;10:595–601. [DOI] [PubMed] [Google Scholar]
- [69].Stewart S, Murphy NF, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].National Institute for Health and Care Excellence. Alivecor Heart Monitor and AliveECG app (Kardid mobile) for Detecting Atrial Fibrillation. 2015. Available at: https://www.nice.org.uk/advice/mib35 [access July 25, 2019]. [Google Scholar]
- [71].King S, Fitzgerald A, Bartlett C, et al. Evidence Summary for Screening for Atrial Fibrillation in Adults. 2018;London, United Kingdom: The UK National Screening Committee, Available at: file:///C:/Users/angel/AppData/Local/Packages/Microsoft.MicrosoftEdge_8wekyb3d8bbwe/TempSta te/Downloads/Screening_for_atrial_fibrillation_(CONSULTATION_VERSION_2019)%20(3).pdf [access June 24, 2019]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


