Abstract
Rationale & Objective:
Traditional and nontraditional cardiovascular disease (CVD) risk factors are highly prevalent in children with chronic kidney disease (CKD). We examined the longitudinal association of adiposity with cardiac damage among children with CKD and explored whether this association was modified by sex.
Study Design:
Prospective cohort study.
Setting & Participants:
Children with mild-to-moderate CKD enrolled in the Chronic Kidney Disease in Children (CKiD) study at 49 pediatric nephrology centers across North America.
Exposure:
Age- and sex-specific body mass index (BMI) z-score.
Outcome:
Age- and sex-specific left ventricular mass index (LVMI) z-score and left ventricular hypertrophy (LVH).
Analytical Approach:
Longitudinal analyses using mixed effects models to estimate sex-specific associations of BMI z-scores with LVMI z-score and with LVH, accounting for repeated measurements over time.
Results:
Among 725 children with 2829 person-years of follow-up, median age was 11.0 years and median estimated glomerular filtration rate was 52.6 ml/min/1.73m2. Nearly one-third of both boys and girls were overweight or obese, median LVMI z-score was 0.18 (IQR: −0.67, 1.08), and 11% had LVH. Greater BMI z-scores were independently associated with greater LVMI z-scores and greater odds of LVH. For each 1-unit higher BMI z-score, LVMI z-score was 0.24 (95% CI: 0.17, 0.31) SD higher in boys and 0.38 (95% CI: 0.29, 0.47) SD higher in girls [pinteraction=0.01]. For each 1-unit higher BMI z-score, the odds of LVH was 1.5-fold (95% CI: 1.1, 2.1) higher in boys and 3.1-fold (95% CI: 1.8, 4.4) higher in girls [pinteraction= 0.005].
Limitations:
Not all children had repeated measurements. LVH is a surrogate and not a hard cardiac outcome. The observational design limits causal inference.
Conclusions:
In children, adiposity is independently associated with the markers of cardiac damage, LVMI z-score, and LVH. This association is stronger among girls than boys. Pediatric overweight and obesity may therefore have a substantial impact on CV risk among children with CKD.
Index Words: cardiovascular disease (CVD), chronic kidney disease (CKD), cardiac target organ damage, hypertension, left ventricular hypertrophy (LVH), left ventricular mass index (LVMI), adiposity, obesity, overweight, body mass index (BMI), pediatrics, children, adolescents, youth, sex differences
Introduction
Chronic kidney disease (CKD) remains a leading risk factor for cardiovascular disease (CVD) among children and young adults1. Both traditional and nontraditional CVD risk factors are highly prevalent among youth with CKD, leading to a significant burden of intermediate cardiovascular (CV) outcomes such as left ventricular hypertrophy (LVH), increased arterial stiffness, and increased carotid intima media thickness2–4. These pathological findings precede hard CV outcomes in adults and, when present in children with CKD, likely contribute to their elevated rates of CV morbidity and mortality5, 6. Determining the modifiable CVD risk factors most associated with these intermediate outcomes in youth with CKD could therefore significantly aid prevention and treatment efforts for these at-risk children.
While overweight and obesity are also associated with increasing numbers of traditional CVD risk factors among children with mild-to-moderate CKD7, the impact of adiposity on target organ damage among children with CKD has not been well described. Previous studies within the Chronic Kidney Disease in Children (CKiD) population have described the association of various assessments of adiposity with left ventricular mass index (LVMI)8, 9, but the independent association of adiposity as determined by the clinically used marker of BMI on LVH, a clinically defined form of target organ damage, has not been previously described in this population. Further, due to the expected change in LVM over time by age and height10, 11, it can be challenging to interpret associations of risk factors with LVMI without accounting for these expected normative variations in LVM. We therefore sought to quantify the longitudinal association of adiposity, as determined by age-sex-specific BMI z-score, with both LVMI z-score and LVH among children with CKD. In addition, because of the known differential increase in BPs among adolescent boys compared to girls related to body size12 and because earlier work within the CKiD study described a greater prevalence of LVH among girls compared to boys13 we a priori aimed to determine if the independent association of adiposity with target organ damage was modified by sex.
Methods
Study Population.
Between 2005 and 2014, a total of 891 children with mild to moderate CKD were enrolled in the CKiD study, a multicenter, prospective cohort study conducted at 49 pediatric nephrology centers across North America. The study design and conduct were approved by the institutional review boards of each participating center as well as an observational study monitoring board appointed by the National Institute of Diabetes and Digestive and Kidney Diseases. Informed consent was obtained from each participating family. After an initial baseline visit, study participants attended annual follow up visits with echocardiography obtained biennially. Our analysis included participants with echocardiography data and complete data on the exposure of interest and all covariates for at least one CKiD visit.
Echocardiography.
At the first annual follow-up visit and at every other subsequent follow-up visit (i.e., once every two years), participants underwent standardized protocol echocardiography at their local sites. The recorded images were read centrally by a study cardiologist. Triplicate measurements were taken and averaged to obtain left ventricular mass (LVM) according to American Society of Echocardiography criteria14, 15 using 2D M-mode.
Primary Outcomes.
The LVMI was calculated as LVM/height2.7, where LVM is in grams and height is in meters14, 16; then age-sex specific LVMI z-scores based on published norms10 were calculated and used in analyses. Left ventricular hypertrophy (LVH) was defined as LVMI ≥ age-sex specific 95th percentile10.
Primary Exposure.
Weight and height were measured twice at each annual visit, with the values averaged. These average weight and height measurements were used to calculate body mass index (BMI) and to calculate age- and sex-specific BMI z-scores17 for those ≤ 20 years of age. The age- and sex-specific BMI z-score from the current visit (visit with echocardiography) and previous visit (visit immediately prior to echocardiography) were averaged. This average BMI z-score, time-updated at each visit, was used in all analyses as the primary exposure.
Covariates.
The following variables were included in the multivariable longitudinal analyses: years from baseline visit to current visit with echocardiography (time-updated at each visit); baseline age; years with CKD at the time of the baseline visit; sex; race (African American vs. non-African American); CKD etiology (glomerular disease vs. non-glomerular disease); average age-, sex-, and height-specific systolic (SBP) and diastolic blood pressure (DBP) z-scores from current and previous visit (measured via manual auscultation and time-updated at each visit); average serum calcium-phosphorous product from current and previous visit (time-updated at each visit); and average estimated glomerular filtration rate (GFR) from current and previous visit18 (time-updated at each visit).
Statistical Analyses.
We conducted two longitudinal analyses using mixed effects models to determine the association of LVMI z-score and LVH with age- and sex- specific BMI z-scores over time, while taking into account repeated measurements over follow-up. A linear mixed effects model was used for continuous LVMI z-scores and a logistic mixed effects model was used for the dichotomous outcome of LVH. The linear model included a random intercept and a correlated random coefficient for years from baseline, while the logistic model had only a random intercept. These mixed effects models were used to estimate the relationship between current LVMI z-score and current LVH with the average BMI z-score from the current visit (visit with LVMI z-score and LVH) and previous visit (visit immediately prior to LVMI z-score and LVH). Both regression models were adjusted for years from baseline visit to current visit with echocardiography and the other previously defined covariates either at baseline or time-updated average measures from the current and previous visits as noted above. Because we hypothesized that sex would modify the association of adiposity on cardiac structure (LVMI z-score and LVH), an interaction term of sex with BMI z-score was included in each model.
Finally, to allow for informative drop out, i.e., initiation of kidney replacement therapy (KRT), we conducted a joint analysis of the LVMI z-score and time to KRT as a sensitivity analysis19. In addition to the linear mixed model described previously, the joint analysis model included a Weibull survival submodel. The covariates for the survival submodel were CKD etiology (glomerular vs non-glomerular), urinary protein-creatinine ratio (UPCR), and baseline eGFR. The survival submodel shared the random intercept and slope of the mixed model, which had their own regression coefficients in the survival submodel. The stjm command in Stata 15 was used for the joint analysis model. A two- sided p-value <0.05 was considered statistically significant.
Results
Our analysis included 725 children who contributed a total of 1483 study visits over 2829 person-years (median follow-up from study entry was 3.3 [interquartile range: 1.2, 5.2] years). Specifically, 286 (40%) of the 725 children contributed one visit, 228 (31%) contributed two visits, 127 (18%) contributed three visits, 60 (8%) contributed four visits, and 24 (3%) contributed five visits.
Table 1 describes the clinical and demographic characteristics of the 725 children (38% female, 21% African American) with mild to moderate CKD who were included in the analyses. At the first visit with echocardiography and BMI measured concurrently, the median age was 11.0 years, the median length of time with CKD was 8.0 years, and the median eGFR was 52.6 (IQR: 39.5, 67.2) ml/min/1.73m2. Ten percent of the study population had an average BP in the hypertensive range. Nearly one third of the study population was overweight or obese: 13% (n=93) had a BMI in the overweight category (defined as a BMI in the 85th-<95th age- and sex-specific percentile range) and 16% (n=119) had a BMI in the obese category (BMI ≥ 95th age- and sex-specific percentile). There was no significant difference in the prevalence of overweight or obesity by sex (32% of females were overweight/obese compared to 28% of males, p=0.2). Median LVMI was 30.5 (IQR: 25.6, 36.8) g/m2.7 and median LVMI z-score was 0.18 (IQR: −0.67, 1.08). Eleven percent of the study population had LVH.
Table 1.
Characteristics of the 725 Participants of the CKiD Study at the First Visit with Echocardiography and BMI Measured Concurrently.
| Characteristic | Value |
|---|---|
| Age- and sex-specific BMI z-scorea | 0.44 (−0.33, 1.23) |
| Overweight: BMI in 85th–<95th percentile rangea | 13% |
| Obese: BMIa ≥ 95th percentile | 16% |
| Age, years | 11.0 (7.5, 14.7) |
| CKD Duration, years | 8.0 (3.8, 12.3) |
| Female sex | 38% |
| African American race | 21% |
| Glomerular CKD diagnosis | 29% |
| Age-, sex-, and height-specific SBP z-scorea | 0.25 (−0.42, 0.85) |
| Age-, sex-, and height-specific DBP z-scorea | 0.40 (−0.14, 0.94) |
| Hypertensiona,b | 10% |
| Calcium-Phosphorus producta, mg2/dL2 | 42.8 (38.5, 47.0) |
| Estimated glomerular filtration ratea,c, ml/min/1.73m2 | 52.6 (39.5, 67.2) |
| LVMI, g/m2.7 | 30.5 (25.6, 36.8) |
| Age- and sex-specific LVMI z-score | 0.18 (−0.67, 1.08) |
| Left Ventricular Hypertrophy (LVMI ≥ 95th percentile) | 11% |
| Initiated KRT | 31% |
Values for continuous variables given as median (interquartile range).
Average of current visit and previous visits.
Systolic blood pressure (SBP) or diastolic blood pressure (DBP) ≥ 95th percentile for children < 13 years old; SBP > 130 or DBP ≥ 80 for children > 13 years old.
Based on estimating equations from Schwartz et al.18
Figure 1 shows the relationship of LVMI z-score and age-sex-specific BMI z-score by sex across 1483 study visits, with data contributed by 725 participants. Each standard deviation (SD) increase in BMI was associated with a significantly greater SD increase in LVMI for females versus males (0.15, p=0.01). Specifically, each SD increase in BMI among females was associated with a 0.40 SD increase in LVMI compared to the 0.25 SD increase in LVMI for a SD increase in BMI in males.
Figure 1.
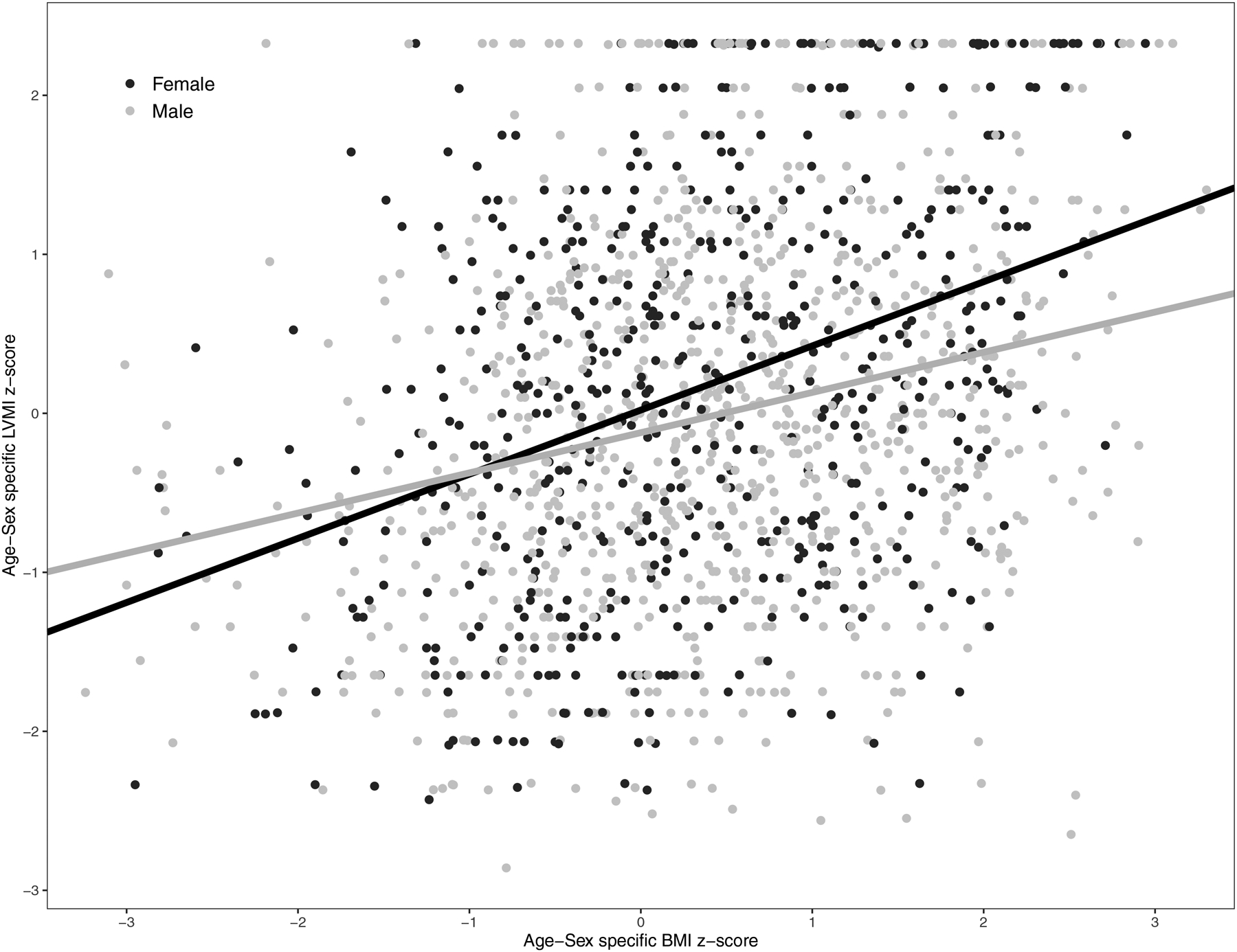
Scatter plot of age-sex specific Left Ventricular Mass Index (LVMI) z-score and age-sex specific BMI z-score at 1483 visits contributed by 725 participants of the Chronic Kidney Disease in Children (CKiD) study. The average BMI z-score of the current visit and previous visit are shown. Regression lines are shown for males and females and were obtained from a linear mixed model with LVMI z-score as the dependent variable and male sex, BMI z-score, and male sex x BMI z-score interaction term as the independent variables.
Figure 2 presents the prevalence of LVH across 1483 study visits by sex in a total of 725 participants for three BMI categories: normal (BMI < 85th age- and sex-specific percentile), overweight, and obese. In girls, the prevalence of LVH was 8.4% among those with a normal BMI, 16.0% among those who were overweight, and 33.7% among those who were obese. Among boys, the relationship between BMI and LVH was not nearly as pronounced, with the prevalence in each category of BMI being close to 7.0%, which was similar to the overall prevalence of LVH across all visits among boys.
Figure 2.

Prevalence of LVH by sex and category of Body Mass Index at 1483 visits contributed by 725 participants of the Chronic Kidney Disease in Children (CKiD) study. LVH was defined as LVMI ≥ age-sex specific 95th percentile. The average BMI of the current visit and previous visit were used.
Each additional year from baseline was associated with both a decrease in LVMI z-score (−0.10 [95% CI: −0.12, −0.08]; unadjusted analysis) and a lower odds of LVH (−20% [95% CI: −11%, −30%] per year).
The results of the multivariable longitudinal analyses are presented in Table 2. The association of BMI z-score with LVMI z-score was significantly different by sex (p=0.01). Specifically, each 1-SD greater BMI z-score was associated with a 0.38 (95% CI: 0.29, 0.47) SD greater LVMI among girls, which was significantly greater (p=0.02) than the 0.24 (95% CI: 0.17, 0.31) SD greater LVMI among boys. The association of BMI z-score with LVH was also significantly different (p=0.005) in girls and boys. Specifically, for each 1-SD greater BMI z-score, girls had a 3.1 (95% CI: 1.8, 4.4) times greater odds of LVH, while boys had a 1.5 (95% CI: 1.1, 2.1) times greater odds of LVH.
Table 2.
Longitudinal Association of Body Mass Index with measures of Cardiac Structure among 725 Children with Mild-Moderate Chronic Kidney Disease at a total of 1,483 visits.
| LVMI z-score | LVH* | |||
| Exposure |
Estimate (95% CI) |
P | Odds ratio 95% CI | P |
| Age-Sex specific BMI z-scorea, per 1 SD increase for Males | 0.24 (0.17, 0.31) | p<0.001 | 1.5 (1.1, 2.1) | p=0.01 |
| Age-Sex specific BMI z-scorea, per 1 SD increase for Females | 0.38 (0.29, 0.47) | p<0.001 | 3.1 (1.8, 4.4) | p<0.001 |
| Covariates | ||||
| Years from baseline, per 1 year increase | −0.08 (−0.11, −0.06) | p<0.001 | 0.8 (0.7, 0.9) | p=0.001 |
| Baseline Age, per 1 year increase | −0.002 (−0.02, 0.02) | p=0.8 | 1.0 (0.9, 1.1) | p=0.8 |
| Baseline CKD Duration, per 5 year increase | 0.001 (−0.0004, 0.002) | p=0.2 | 1.002 (0.998, 1.007) | p=0.3 |
| Female (vs. Male) | 0.14 (0.004, 0.28) | p=0.04 | 1.4 (0.7, 2.8) | p=0.3 |
| African American (vs. non-African American) | 0.29 (0.12, 0.45) | p=0.001 | 2.2 (1.2, 4.2) | p=0.01 |
| Glomerular diagnosis (vs. non-Glomerular) | 0.16 (0.001, 0.33) | p=0.05 | 1.8 (0.9, 3.4) | p=0.09 |
| Age-Sex-Height specific SBP z-scorea, per 1 SD increase | 0.18 (0.11, 0.25) | p<0.001 | 2.0 (1.4, 2.8) | p<0.001 |
| Age-Sex-Height specific DBP z-scorea, per 1 SD increase | 0.03 (−0.05, 0.11) | p=0.5 | 1.1 (0.7, 1.5) | p=0.8 |
| log(Calcium* Phosphorous)a, mg2/dL2 | 0.38 (−0.02, 0.79) | p=0.06 | 2.1 (0.3, 13.2) | p=0.4 |
| Estimated glomerular filtration ratea, b, ml/min/1.73m2, per 10 unit decrease | 0.12 (0.08, 0.15) | p<0.001 | 1.5 (1.3, 1.8) | p<0.001 |
LVMI ≥ 95th percentile
Average of current visit and previous visits.
Based on estimating equations from Schwartz GJ et al., Kidney Int 2012; 82:445–453.
BMI – body mass index; CKD – chronic kidney disease; DBP – diastolic blood pressure; SBP – systolic blood pressure; LVH – left ventricular hypertrophy; LVMI – left ventricular mass index; SD – standard deviation
For the LVMI z-score outcome, we conducted a joint analysis with time to KRT as a sensitivity analysis. The results of this joint model did not differ from the longitudinal model that did not take into account informative censoring. Therefore, we report the simpler longitudinal model in Table 2 and the joint analysis model in the supplementary material (Table S1). We did not conduct the joint analysis for the categorical variable LVH.
Discussion
In this longitudinal analysis of children with mild-to-moderate CKD, we demonstrate that adiposity is more greatly associated with cardiac target organ damage among girls than boys, independent of other known traditional and non-traditional co-morbid CVD risk factors. Specifically, compared to boys, we found that girls had a greater increase in LVMI z-score and a greater odds of LVH for the same degree of BMI z-score increase.
Adiposity is a known CVD risk factor20, and is independently associated with LVH among children with non-CKD-related hypertension21 and among children without hypertension or CKD22, 23. Among children with primary hypertension, adiposity is more strongly associated with both LVH and LVH regression than blood pressure (BP)22, 24–26. In a pre-post study of morbidly obese adolescents who underwent bariatric surgery, an LVMI decrease of 12 g/m2.7 was observed.27
Knowing that 1) adiposity is as prevalent among children with CKD as within the general US population28, 29; 2) CVD risk factors track from childhood to adulthood30, 31, with childhood BMI a strong predictor of adult LVH32; 3) LVH is a known risk factor for cardiac arrhythmias, diastolic dysfunction, and heart failure33; 4) co-morbid obesity not only impairs the ability to effectively treat hypertensive LVH, but can also lead to a greater likelihood of CV events34–36; and 5) CVD statistics in adults also differ by sex37, our findings suggest both an urgent need for enhanced obesity prevention and treatment in pediatric CKD and a potential role for targeted interventions.
Adiposity contributes to LVH both directly and indirectly via its effect on BP, and sex-differences in BP trends among children and adults has been well-described. Rosner et al. has shown that beginning in adolescence, the observed age related increase in BP is significantly greater in boys compared to girls, regardless of race12. Adult studies have shown that female sex has a protective effect on hypertension prevalence and CVD morbidity and mortality until menopause. After menopause, female sex is associated with a greater risk of hypertension and a similar risk for CV events as males38. These peri-pubertal and peri-menopausal differences suggest a hormonal cause for CVD risk.
In fact, the existing literature does support a causal role of endogenous sex-hormones on BP and cardiac target organ damage. Estrogen has been shown to have antiproliferative effects on cardiac fibroblasts and vascular smooth muscle cells39–41 and to inhibit the development of LVH42. Testosterone, on the other hand, increases vascular smooth muscle cell proliferation43 and promotes LVH42, 44. Notably, these hormonal levels are affected by obesity: in males, obesity lowers free testosterone (effectively decreasing its pro-CVD promoting effects45) and in pre-menopausal women, obesity decreases estrogen levels (effectively decreasing its CVD-protective effects46).
While compelling, other more traditional BP-related hormones that are likewise influenced by degree of adiposity may also explain our findings. Under normal circumstances, adipose cells secrete a multitude of adipokines and hormones, including angiotensinogen47. With obesity these hormones are upregulated, and with progressive obesity there is increasing inflammation and progressive loss of metabolic control leading to a dysregulated state48. When adipose tissue becomes dysfunctional, the RAAS system is activated49 leading to an increase in angiotensin II and aldosterone. Animal models suggest that these BP-related hormones may exert sex-dependent effects on CVD risk factors. Specifically, angiotensin II is not only related to increased cardiac hypertrophy in male rats, but angiotensin-converting enzyme levels are also greater in male mice compared to their female counterparts50. When one considers that long-term angiotensin II blockade has been shown to prevent cardiac remodeling in these same animal models51, one must consider the potential for a sex-based targeted therapeutic approach to CVD, particularly when there is co-morbid obesity.
The differential impact of obesity on LVH by sex that we describe in this work has also been described in the adult literature. Over the last several decades, it has been increasingly evident that women have different risks for CVD than men and present with less “classic” symptoms when experiencing a CV event. Adult women, particularly obese adult women who are hypertensive, are also more likely to have LVH34. It is thought that the increased prevalence of LVH among obese adult women is related to an increase in overall fat mass (vs. lean mass) compared to men of the same BMI52, and to an increase in visceral adiposity versus subcutaneous adiposity compared to men52, 53. In fact, in a recent study of Italian adults, while the overall CVD risk profiles between men and women with LVH were not different, women with obesity had a much greater likelihood of having LVH than men with obesity (comparing obese to non-obese individuals, ORs for LVH were 4.23 [95% CI: 3.60, 4.97] and 2.87 [95% CI: 2.49, 3.29] in women and men, respectively)54. Perhaps the most striking finding from the Italian study was that the “protective” effect of female sex on CV events (HR, 0.65; 95% CI: 0.44, 0.96; p=0.03) disappeared when LVH was present (HR, 0.94; 95% Cl: 0.69, 1.30; p = 0.7).
Interestingly, despite the work in this area among adults there is not much published regarding the potential differential impact of obesity on LVH by sex among children. In a school-based study of 676 healthy Korean adolescents 12–16 years of age55, the authors found that adipose mass as assessed by bioelectric impedance analysis correlated with LVM in females whereas fat free mass associated with LVM in males, consistent with findings among adults in the Strong Heart Study52.
Our study suggests that pediatric overweight and obesity may have a substantial impact on CV risk among children with CKD. The impact of intervening to improve and normalize BMI among youth, particularly females, on future risk of CVD deserves study. Pediatric nephrologists may benefit from partnering with renal dieticians and other members of their multidisciplinary teams to target weight loss/maintenance more aggressively when presented with seemingly resistant CKD-related pediatric LVH.
There are several limitations to our study which deserve mention. In our study, many children had repeated measurements; however 40% of participants only contributed data from one visit, raising the possibility for bias related to differential loss to follow up. Fortunately, sensitivity analysis allowing for informative drop out suggests this is likely minimal. It is also worth noting that while LVH is a clinical biomarker for CVD risk, itis a surrogate and not a hard cardiac outcome. In addition, our study was observational which does not allow us to infer causality.
We also note multiple strengths. The large population of children studied with mild-to-moderate CKD had both a variety of etiologies for their kidney disease and a wide range of BMIs. These children were followed longitudinally, in a national cohort study, with standardized anthropometrics, and BP and echocardiographic measurements. As a pediatric population, those studied were free of many of the usual co-morbidities that confound studies in adults (smoking, alcohol use, and diabetes as examples) and did not have an extended period of time exposed to other traditional CV risk factors (including dyslipidemia, hypertension, and kidney failure). Despite the lower burden of CVD risk compared to adults, we present adjusted results to describe the independent association of adiposity on cardiac target organ damage, specifically examining the effect of sex on this relationship, which has not been previously done.
Future studies that focus on enhanced risk stratification strategies and targeted treatment approaches among this at-risk population are needed. Determining the effect of tailored interventions on children with CKD and co-morbid obesity will provide more insight into these sex-related differences and establish the effect of weight loss on CV risk.
Supplementary Material
Table S1: Longitudinal association of BMI and LVMI z-scores among 714 children with mild-to-moderate CKD at a total of 1,426 visits, accounting for informative censoring.
Support:
The CKiD study is funded by the NIH/NIDDK with additional funding from the NICHD and the NHLBI (U01-DK-66143, U01-DK-66174, U24-DK-082194, U24-DK-066116). TMB received funding from the NIH/NHLBI (K23-HL-119622; R56-HL-139620). None of these funding sources had a role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References:
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3S1): A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh J, Wunsch R, Turzer M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106(1): 100–105. [DOI] [PubMed] [Google Scholar]
- 3.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23(4): 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady TM, Schneider MF, Flynn JT, et al. Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol. 2012;7(12): 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groothoff JW, Gruppen MP, Offringa M, et al. Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61(2): 621–629. [DOI] [PubMed] [Google Scholar]
- 6.McDonald SP, Craig JC, Australian, New Zealand Paediatric Nephrology A. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350(26): 2654–2662. [DOI] [PubMed] [Google Scholar]
- 7.Wilson AC, Schneider MF, Cox C, et al. Prevalence and correlates of multiple cardiovascular risk factors in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(12): 2759–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel HP, Saland JM, Ng DK, et al. Waist Circumference and Body Mass Index in Children with Chronic Kidney Disease and Metabolic, Cardiovascular, and Renal Outcomes. J Pediatr. 2017;191: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sgambat K, Roem J, Mitsnefes M, et al. Waist-to-height ratio, body mass index, and cardiovascular risk profile in children with chronic kidney disease. Pediatr Nephrol. 2018;33(9): 1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22(6): 709–714. [DOI] [PubMed] [Google Scholar]
- 11.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25(5): 1056–1062. [DOI] [PubMed] [Google Scholar]
- 12.Rosner B, Prineas R, Daniels SR, Loggie J. Blood pressure differences between blacks and whites in relation to body size among US children and adolescents. Am J Epidemiol. 2000;151(10): 1007–1019. [DOI] [PubMed] [Google Scholar]
- 13.Ruebner RL, Ng D, Mitsnefes M, et al. Cardiovascular Disease Risk Factors and Left Ventricular Hypertrophy in Girls and Boys With CKD. Clin J Am Soc Nephrol. 2016;11(11): 1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12): 1440–1463. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6): 450–458. [DOI] [PubMed] [Google Scholar]
- 16.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 17.Prevention CfDCa. Data Table of BMI-for-age Charts2000.
- 18.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4): 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elashoff RM, Li G, Li N. Joint Modeling of Longitudinal and Time-to-Event Data. Boca Raton, FL: CRC Press; 2017. [Google Scholar]
- 20.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. 2012;345: e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanevold C, Waller J, Daniels S, Portman R, Sorof J, International Pediatric Hypertension A. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113(2): 328–333. [DOI] [PubMed] [Google Scholar]
- 22.Crowley DI, Khoury PR, Urbina EM, Ippisch HM, Kimball TR. Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr. 2011;158(5): 709–714 e701. [DOI] [PubMed] [Google Scholar]
- 23.Sivanandam S, Sinaiko AR, Jacobs DR Jr., Steffen L, Moran A, Steinberger J. Relation of increase in adiposity to increase in left ventricular mass from childhood to young adulthood. Am J Cardiol. 2006;98(3): 411–415. [DOI] [PubMed] [Google Scholar]
- 24.Brady TM, Appel LJ, Holmes KW, Fivush B, Miller ER 3rd. Association Between Adiposity and Left Ventricular Mass in Children With Hypertension. J Clin Hypertens (Greenwich). 2016;18(7): 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady TM, Fivush B, Flynn JT, Parekh R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. 2008;152(1): 73–78, 78 e71. [DOI] [PubMed] [Google Scholar]
- 26.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91(9): 2400–2406. [DOI] [PubMed] [Google Scholar]
- 27.Ippisch HM, Inge TH, Daniels SR, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51(14): 1342–1348. [DOI] [PubMed] [Google Scholar]
- 28.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodig NM, McDermott KC, Schneider MF, et al. Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol. 2014;29(10): 1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8(7): 657–665. [DOI] [PubMed] [Google Scholar]
- 31.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84(4): 633–641. [PubMed] [Google Scholar]
- 32.Lai CC, Sun D, Cen R, et al. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64(15): 1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13): 1370–1380. [DOI] [PubMed] [Google Scholar]
- 34.Gerdts E, Okin PM, de Simone G, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51(4): 1109–1114. [DOI] [PubMed] [Google Scholar]
- 35.Lonnebakken MT, Izzo R, Mancusi C, et al. Left Ventricular Hypertrophy Regression During Antihypertensive Treatment in an Outpatient Clinic (the Campania Salute Network). J Am Heart Assoc. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerdts E, de Simone G, Lund BP, et al. Impact of overweight and obesity on cardiac benefit of antihypertensive treatment. Nutr Metab Cardiovasc Dis. 2013;23(2): 122–129. [DOI] [PubMed] [Google Scholar]
- 37.Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2(2): e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shlyakhto E Gendered Innovations in the Study of Cardiovascular Diseases In: Kerkhof PLM, Miller VM, eds. Sex-Specific Analysis of Cardiovascular Function. Advances in Experimental Medicine and Biology Vol 1065: Springer; 2018. [DOI] [PubMed] [Google Scholar]
- 39.Dubey RK, Gillespie DG, Jackson EK, Keller PJ. 17Beta-estradiol, its metabolites, and progesterone inhibit cardiac fibroblast growth. Hypertension. 1998;31(1 Pt 2): 522–528. [DOI] [PubMed] [Google Scholar]
- 40.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93(3): 577–584. [DOI] [PubMed] [Google Scholar]
- 41.Somjen D, Kohen F, Jaffe A, Amir-Zaltsman Y, Knoll E, Stern N. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension. 1998;32(1): 39–45. [DOI] [PubMed] [Google Scholar]
- 42.Hayward CS, Webb CM, Collins P. Effect of sex hormones on cardiac mass. Lancet. 2001;357(9265): 1354–1356. [DOI] [PubMed] [Google Scholar]
- 43.Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50(3–4): 169–174. [DOI] [PubMed] [Google Scholar]
- 44.Cabral AM, Vasquez EC, Moyses MR, Antonio A. Sex hormone modulation of ventricular hypertrophy in sinoaortic denervated rats. Hypertension. 1988;11(2 Pt 2): I93–97. [DOI] [PubMed] [Google Scholar]
- 45.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6): 1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicennati V, Ceroni L, Genghini S, Patton L, Pagotto U, Pasquali R. Sex difference in the relationship between the hypothalamic-pituitary-adrenal axis and sex hormones in obesity. Obesity (Silver Spring). 2006;14(2): 235–243. [DOI] [PubMed] [Google Scholar]
- 47.Carruthers M, Trinick TR, Jankowska E, Traish AM. Are the adverse effects of glitazones linked to induced testosterone deficiency? Cardiovasc Diabetol. 2008;7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2): 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obes Rev. 2012;13(1): 17–26. [DOI] [PubMed] [Google Scholar]
- 50.Freshour JR, Chase SE, Vikstrom KL. Gender differences in cardiac ACE expression are normalized in androgen-deprived male mice. Am J Physiol Heart Circ Physiol. 2002;283(5): H1997–2003. [DOI] [PubMed] [Google Scholar]
- 51.du Toit EF, Nabben M, Lochner A. A potential role for angiotensin II in obesity induced cardiac hypertrophy and ischaemic/reperfusion injury. Basic Res Cardiol. 2005;100(4): 346–354. [DOI] [PubMed] [Google Scholar]
- 52.De Simone G, Devereux RB, Chinali M, et al. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens. 2011;29(7): 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rider OJ, Francis JM, Ali MK, et al. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2009;11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerdts E, Izzo R, Mancusi C, et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network). Int J Cardiol. 2018;258: 257–261. [DOI] [PubMed] [Google Scholar]
- 55.Song YH, Kim HS, Park HS, et al. Sex differences in the relation of body composition to cardiovascular parameters and functions in Korean adolescents: a school-based study. Obes Facts. 2014;7(3): 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Longitudinal association of BMI and LVMI z-scores among 714 children with mild-to-moderate CKD at a total of 1,426 visits, accounting for informative censoring.


