Abstract
Background:
Obesity is a malnourishment epidemic worldwide. A meta-analysis of prospective human studies across the world demonstrated a consistent positive association between maternal exposure to the pesticide dichlorodiphenyltrichloroethane (DDT) and its metabolite dichlorodiphenyldichloroethylene (DDE) and children with obesity. The present study evaluates the association of maternal exposure to DDT and DDE with the risk of obesity in daughters during their mid-life in a prospective birth cohort with up to 53 years of follow-up.
Methods:
Gravidas’ blood was collected during their 1959–1967 enrollment into the prospective Child Health and Development Studies birth cohort in California. Their daughters aged 44–53 years had their height, weight, and waist circumference measured during a home visit to evaluate associations of daughters’ adiposity and relative risk of overweight and obesity with their mothers’ prenatal serum levels of DDT and DDE quantified by gas chromatograph-tandem mass spectrometer (n=511).
Results:
Maternal o,p’-DDT was positively associated with body mass index (beta=0.59 kg/m2 per ln ng/ml (95th percentile confidence interval, 95CI: 0.17, 1.00)) and waist circumference (beta=1.19 cm per ln ng/ml (95CI: 0.26, 2.13)) in multivariable models. Maternal o,p’-DDT was positively associated with a 26% (95CI: 6–49%) to 31% (95CI: 6–62%) higher risk of overweight and the same magnitude of additional risk for obesity, based on waist circumference and BMI definitions respectively, in multivariable models.
Conclusions:
These data indicate maternal DDT exposure is significantly associated with increased obesity risk among middle-aged women independent of the obesity definition, confounding, and obesity risk factors. Our findings suggest that policies supporting the use of DDT for malaria vector abatement need to consider the obesity risk as a health cost when weighing the benefits of using DDT in malaria vector control.
Introduction
Obesity is a malnourishment disease that is a major risk factor for other noncommunicable diseases, such as cardiovascular diseases, diabetes, and certain cancers.1 Because so much of the world lives in developing countries, 62% of individuals with obesity reside in developing countries.2 To combat this worldwide pandemic, the World Health Organization (WHO) set a global noncommunicable disease target set for 2025 to halt the obesity prevalence levels to those observed in 2010.3 If post-2000 trends of increasing obesity continue, this WHO target for restraining obesity will not be met.4 Hence overcoming the risk of obesity is a largely unmet need.
Given that the majority of calories burned are utilized through heat-generating metabolic processes and not physical activity,5 attention to possible obesity risk factors that disrupt metabolism is warranted.6,7 As an illustration, undernutrition of pregnant women leads to lower birth weight of offspring, and both in utero undernutrition and low birth weight have been associated with increased risk of becoming an adult with obesity.8–11 These observations support the developmental origins of health and disease (DOHAD) hypothesis which has been supported by observations that metabolic adaptations to developmental malnutrition causes susceptibility to cardiometabolic noncommunicable diseases, including obesity, in middle-age.9,12 Further, extensive experimental studies demonstrate that disruption of fetal metabolism through changes in the maternal environment is on the causal pathway to adult obesity.13–15
The role of contaminants of the maternal environment contributing to adult obesity has not yet been tested in humans.16,17 The pesticide DDT is a strong candidate obesogen for such testing for numerous reasons. First, DDT exposure continues among people residing in developing nations where DDT remains in manufacture and use on the order of 3,500 metric tons annually due to the WHO’s continued recommendation of its use in vector control.18,19 Next, two (Spain, South Africa) out of three studies of prenatal DDT exposure which prospectively followed children reported a positive association between prenatal DDT exposure and children with increased adiposity.20–22 Additionally, maternal DDT exposure increased adiposity of subsequent generations of two rodent species, e.g. rats and mice.14,23 Furthermore, early-life exposure to the primary metabolite of DDT, namely DDE, had a positive association with obesity in a meta-analysis of seven prospective human studies reporting original data among individuals up to 20 years old (ß = 0.13 body mass index (BMI)-z score per log increase in p,p’-DDE [95% confidence interval (CI) 0.01, 0.25 BMI-z score].24 In order to assess whether maternal DDT or DDE are associated with mid-life adiposity and risk of obesity, we took advantage of a prospective birth cohort that began more than 50 years ago when use of DDT in the United States was at its peak.
Methods
Data collection and participants
The Childhood Health and Development Studies (CHDS) is a birth cohort that began at Oakland Kaiser Permanente hospital with the enrollment of pregnant women who were members of the Kaiser Foundation Health Plan seeking obstetric care from 1959 until 1967.25 Over 98% of eligible women enrolled in CHDS. These women were voluntarily interviewed in-person, and gave researchers permission to access medical records of the CHDS mothers and children. Blood specimens were collected from CHDS mothers of subjects at several times through pregnancy and one post-partum sample usually within three days of delivery.
This study involved daughters who were part of the Three Generations (3Gs) study, an adult follow-up of the CHDS conducted from 2010 to 2013. Female offspring born into the CHDS who were surviving at the time of 3Gs study recruitment, not known to be institutionalized, not known to have a severe mental illness, not known to have requested “not to be contacted”, who were not already eligible for another adult follow-up study in progress and who were phone locatable were eligible to participate in the telephone interview phase of the 3Gs Study (n = 5003). Sixty percent of eligible subjects (adult daughters) completed the phone interview (n = 3003). Due to budget constraints, a subset were targeted for participation in a home visit from the following three groups: daughters of mothers with breast cancer, daughters who had participated in an earlier breast density study, and a random sample of daughters.26 Of the 1879 daughters who were eligible, 1194 completed a home visit (64%). In order to address the primary study question, subjects were required to have available organochlorines (n = 801). Women who were pregnant (n = 1) or diabetic (n = 96) at the home visit were also excluded from this analysis. The analysis of waist circumference included 511 subjects with complete data for all study variables: daughter’s height, weight, waist circumference, education, exercise level, menopause status, thyroid medication, maternal pre-pregnancy BMI, ethnicity, education, coffee consumption, and place of birth. Three of these women did not have their height measured. The institutional review board at Public Health Institute approved the 3Gs protocol.
Outcomes
Waist circumference, weight, and height were measured during the 3Gs home visit using a detailed, standardized protocol. Examiners were trained to strictly implement the protocol. Standing height was measured using a Seca 213 Stadiometer with a fixed vertical backboard and an adjustable head piece. The subject was instructed to remove any hair ornaments and to stand erect against the backboard with both feet flat and weight evenly distributed. The head piece was lowered to rest firmly on top of the subject’s head, aligned in the “Frankfort horizontal plane”, to capture the vertical distance from foot to head. Weight was measured using a Seca 813 High-Capacity floor scale. The subject was asked to stand in the center of the scale platform with hands at their sides and head straight ahead, allowing the digital readout to stabilize before the examiner recorded the weight. Abdominal circumference was measured using a Seca 201 (soft) measuring tape. The subject was asked to gather her shirt above the waist, cross her arms, and place her hands on opposite shoulders. The examiner snugly placed the measuring tape above the ilia of the pelvis without compressing the skin. The circumference was taken at the end of the subject’s normal expiration and recorded to the nearest 0.1 cm. BMI was calculated from height and weight measured during the home visit, or their highest weight and height since age 18 as self-reported during the 3Gs interview. Obesity was defined as BMI ≥ 30 kg/m2 or waist circumference of ≥ 88 cm and overweight was defined as 30 > BMI ≥ 25 kg/m2 and 88 cm > waist circumference of ≥ 80 cm.27 Abdominal obesity was regarded as the primary outcome due to its use in the metabolic syndrome definition as an unhealthy obesity that increases risk of cardiovascular disease.28 However, BMI- based assessment was also made due to the recognition of the World Health Organization that using BMI ≥ 30 kg/m2 to classify obesity is particularly valuable for use in global comparisons, and the primary use of BMI in clinically defined obesity in the United States.27
Exposures
We measured o,p’-DDT, p,p’-DDT, p,p’-DDE, and lipids in nonfasting maternal perinatal serum samples of the index participants as previously described.29–31 Previous research has demonstrated high correlation between sample periods for these chemicals and concluded that specimen collection timing during the perinatal period is inconsequential with respect to exposure assessment.32 Samples were measured using gas chromatography with electron capture detection (GC/ECD) fit with a capillary column (18%), GC/ECD fit with dual columns (45%), or GC triple quadrupole mass spectrometry (37%).
Covariables
During the in-person interview of gravidas at the CHDS inception, mothers of subjects provided information about their age, parity, ethnicity, education, coffee consumption, tobacco use, height, pre-pregnancy weight, and country of origin during their pregnancy carrying subjects. These pregnant women indicated the height and weight of subjects’ fathers. The same maternal sera from which chemical exposures were measured was used to quantify total cholesterol and triglycerides enzymatically by labs certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program.29,33 During the 3Gs in-person interview, subjects provided information on their current exercise levels, as well as their history of education, tobacco use, medicated thyroid condition, lactation, and surgical or natural menopause status. Their age was determined from medical records.
Statistical analysis
We considered possible confounders and obesity risk factors among maternal factors, e.g. pre-pregnancy BMI, age at delivery, foreign born, African American, parity, education, coffee intake, tobacco use, weight gain, blood lipids and organochlorines during pregnancy. All organochlorines had skewed distributions and were either presented as quartiles or natural log transformed whether expressed per serum volume or lipid weight. We also considered possible confounders and obesity risk factors among study subjects’ birthweight, education attainment, exercise, lactation history, tobacco use, age, menopause status, and self-report of a medicated thyroid condition. A potential confounder of an organochlorine – waist circumference relationship was those possible confounders that were significantly correlated with the organochlorine and the outcome (p < 0.2, using Spearman or Pearson options for categorical or continuous possible confounders respectively, PROC CORR, SAS 7.11). Mixed effects of mothers were modeled due to infrequent sib-ships among daughters. The standard error of these least squares means can be considered a standard deviation which is conditional on the random effect of mother and marginal over differences in sample size.
Associations of individual organochlorines with continuous waist circumference were evaluated in our first linear regression models while adjusting for the mixed effect of mother (PROC MIXED, presented as model 1 below). Second, all potential confounding variables were added to waist circumference models while adjusting for mixed effects of mother and we retained covariates causing a 10% or greater change in the estimated beta coefficient of the exposure (PROC MIXED, presented as model 2 below). We note that subjects’ age was only significant in models without subjects’ menopause and only retained menopause. Third, known obesity risk factors that were not confounders were added (PROC MIXED, presented as model 3 below). The independent variables of these three models were then applied to models of continuous BMI and of risk ratios for categorical outcomes defined by elevated waist circumference (obese ≥ 88 cm, overweight = 80–87 cm) or elevated BMI (obese ≥ 30 kg/m2, overweight= 25–29 kg/m2) using generalized log-linear multinomial models with mother as the repeated subject (PROC GENMOD).34 Similar models were used to demonstrate significance of Table 1 characteristics with waist categories (PROC GENMOD). These generalized log-linear multinomial models treat the outcome categories as counts, such that the risk of overweight relative to lean is equivalent to the risk of obesity relative to overweight. Collinearity was ruled out for linear multivariable models of outcomes using the variance inflation and condition index (VIF and COLLINOINT in PROC REG). Data are presented to hundredth digit unless rounding obscured readers’ assessment of significance.
Table 1.
Descriptive variables for 511 middle-aged daughters of the CHDS cohort across categories of waist circumference.
| Characteristic | Value | Waist circumference categories | ||
|---|---|---|---|---|
| < 80 cm | 80 – 88 cm | ≥ 88 cm | ||
| CHDS mothers | ||||
| Age1 | Years | |||
| <20 | 4 (14.8) | 3 (11.1) | 20 (74.1) | |
| 20–29* | 90 (30.3) | 60 (20.2) | 147 (49.5) | |
| 30–39 | 37 (22.2) | 37 (22.2) | 93 (55.7) | |
| >40 | 4 (20.0) | 3 (15.0) | 13 (65.0) | |
| Parity1 | # Viable Births | |||
| 0 | 47 (27.2) | 32 (18.5) | 94 (54.3) | |
| 1 | 48 (30.6) | 36 (22.9) | 73 (46.5) | |
| 2 | 19 (21.6) | 21 (23.9) | 48 (54.6) | |
| >2 | 21 (22.6) | 14 (15.0) | 58 (62.4) | |
| Ethnicity1 | ||||
| African American | 17 (17.7) | 12 (12.5) | 67 (69.8) | |
| Other* | 118 (28.4) | 91 (21.9) | 206 (49.6) | |
| Education1 | ||||
| <HS | 120 (27.4) | 94 (21.5) | 224 (51.1) | |
| ≥HS* | 15 (20.6) | 9 (12.3) | 49 (67.1) | |
| Foreign Born1 | ||||
| No | 116 (25.1) | 87 (18.8) | 259 (56.1) | |
| Yes* | 19 (38.8) | 16 (32.6) | 14 (28.6) | |
| Gravida smoking1,3 | ||||
| Never | 66 (26.1) | 48 (19.0) | 139 (54.9) | |
| Ever | 69 (26.8) | 55 (21.4) | 133 (51.8) | |
| Coffee Intake1 | Cups/day | |||
| <1 | 26 (18.7) | 27 (19.4) | 86 (61.9) | |
| 1–3* | 68 (30.0) | 52 (22.9) | 107 (47.1) | |
| ≥4 | 41 (28.3) | 24 (16.6) | 80 (55.2) | |
| Pre-pregnancy BMI1 | kg/m2 | |||
| <25 | 120 (28.6) | 86 (20.5) | 213 (50.8) | |
| 25–29* | 12 (17.9) | 12 (17.9) | 43 (64.2) | |
| ≥30* | 3 (12.0) | 5 (20.0) | 17 (68.0) | |
| Total Cholesterol2 | mg/dl | 261.1 (6.8) | 264.0 (7.7) | 250.0 (4.8) |
| Triglycerides2 | mg/dl | 207.2 (7.0) | 190.3 (8.0) | 195.9 (5.0) |
| CHDS Daughters | ||||
| Birthweight2 | G | 3284 (39) | 3237 (44) | 3349 (28) |
| Education1 | ||||
| <HS | 8 (12.7) | 14 (22.2) | 41 (65.1) | |
| ≥HS* | 127 (28.4) | 89 (19.9) | 232 (51.8) | |
| Exercise1 | Hours/week | |||
| <2.5 | 52 (22.8) | 50 (21.9) | 126 (55.3) | |
| 2.5–5 | 55 (27.0) | 35 (17.2) | 114 (55.9) | |
| >5* | 28 (35.4) | 18 (22.8) | 33 (41.8) | |
| Lactation History1 | ||||
| Never | 44 (26.0) | 32 (18.9) | 93 (55.0) | |
| Ever | 91 (26.6) | 71 (20.8) | 180 (52.6) | |
| Age2 | Years | 48.6 (0.2) | 48.8 (0.2) | 48.8 (0.1) |
| Menopause1 | ||||
| None | 96 (30.2) | 63 (19.8) | 159 (50.0) | |
| Any (natural/ surgical)* | 39 (20.2) | 40 (20.7) | 114 (59.1) | |
| Medicated Thyroid Condition1 | ||||
| No | 126 (27.9) | 90 (19.9) | 236 (52.2) | |
| Yes* | 9 (15.2) | 13 (22.0) | 37 (62.7) | |
Data are n (%) or
mean (SE).
n=510.
p-value<0.05.
We conducted several secondary analyses. First we evaluated highest self-reported BMI in multivariable mixed models accounting for repeated mothers (PROC GENMOD). Second, we examined whether exposure was significantly associated with adiposity through birth weight mediation using the delta method to estimate confidence intervals (PROC REG).35 Third, we examined whether exposure was significantly associated with adiposity when the sample was restricted to those participants selected randomly (PROC MIXED). We next performed several alternative analyses to evaluate sensitivity to sibling covariance and exposure classification. We re-ran all three multivariable models of abdominal overweight and obesity using only the oldest sister, only the youngest sister, or a randomly selected sister (PROC GENMOD). We additionally re-ran the third multivariable model for BMI-based overweight and obesity using only the oldest sister, only the youngest sister, or a randomly selected sister (PROC GENMOD). Finally, we evaluated the effect of various models of DDT lipophilicity on associations with outcomes by comparing multivariable models when 1) o,p’-DDT was “adjusted” by maternal serum lipids with the commonly used Phillips equation (which essentially divides the mass of DDT per serum volume by the mass of triglycerides and cholesterol in the serum),36 and ln transformed before modeling, 2) ln transformed volumetric o,p’-DDT (mass of DDT per serum volume) was evaluated with maternal lipids included in the model,37 or 3) maternal lipids were excluded as a covariable in the model.
Role of the Funding Source
The funding source had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Results
Of the 1000 subjects who participated in the home visit, 508 subjects had available organochlorine assays and complete data for all study variables; an additional three (n=511) had measured waist circumference. Median age of subjects at the home visit was 49 years (range 44–53 years). We examined associations between both maternal characteristics at the time of subjects’ birth and subjects’ characteristics during their lifetime, with categories of waist circumference, Table 1. There was a significant association between having a smaller waist circumference and having a mother who was born outside of the US. Having a mother with a higher pre-pregnancy BMI was associated with higher waist circumference during middle-age. Waist circumference categories during middle-age were not associated with maternal blood lipids, parity, or smoking. Indeed, there was no correlation between maternal smoking during pregnancy and either waist circumference categories in middle-age (Pearson r = −0.023, p=0.6) or DDT categories (Pearson r = −0.04, p=0.3).
We next evaluated the association between log transformed organochlorine chemicals found in maternal serum from 1959–1967 and subjects’ mid-life waist circumference. There was a positive linear association between subjects’ measured mid-life waist circumference and their maternal o,p’-DDT levels (ß = 1.17 cm per log ng/ml, 95CI = 0.18, 2.15; Table 2; Figure S1). There was a similarly increased association between their measured mid-life waist circumference with their maternal p,p’-DDT levels (ß = 2.07 cm per log ng/ml, 95CI= −0.02, 4.16), but not with p,p’-DDE levels (ß = 0.22 cm per log ng/ml, 95CI = −2.57, 3.01). Maternal exposure to o,p’-DDT remained significantly associated with mid-life waist circumference when simultaneously adjusted by p,p’-DDT and p,p’-DDE, which remained non-significant. The positive association between maternal o,p’-DDT exposure and measured waist circumference was upheld when the model was adjusted for confounders (Model 2, Table 2). Indeed this association between maternal o,p’-DDT and waist circumference of middle-aged women was independent of several obesity risk factors, e.g. levels of physical activity and medicated thyroid conditions (Model 3, Table 2; Figure 1A). Maternal exposure to o,p’-DDT alone also had a positive association with measured BMI (Model 1, Table 2) and this remained significant when confounding and risk factors were included in linear models (Figure 1B; Models 2–3, Table 2). To additionally demonstrate the magnitude of these associations, we also examined maternal o,p’-DDT levels as quartiles and noted that each quartile of maternal o,p’-DDT exposure was significantly associated with an additional 4–5 cm increase in waist circumference during middle-age in multivariable models (Models 2–3, Table 3).
Table 2.
Associations of maternal o,p’-DDT (ln ng/ml) with continuous measures of adiposity in middle-aged daughters of the CHDS cohort. Associations with waist circumference (cm) and body mass index (BMI, kg/m2) were estimated using a linear mixed model.
| Parameters | Waist circumference | BMI |
|---|---|---|
| N subjects | 511 | 508 |
| Exposure distribution | Continuous | Continuous |
| Outcome distribution | Continuous | Continuous |
| Beta (95% CI) estimate units | cm per ln ng/ml | kg/m2 per ln ng/ml |
| Model 11 | 1.17 (0.18, 2.15) | 0.57 (0.14, 1.01) |
| Model 22 | 1.25 (0.30, 2.20) | 0.61 (0.18, 1.04) |
| Model 33 | 1.19 (0.26, 2.13) | 0.59 (0.17, 1.00) |
Model 1 includes o,p’-DDT coded as 3 dummy variables representing quartiles 2, 3 and 4 vs. quartile 1 as the reference.
Model 2 includes o,p’-DDT coded as 3 dummy variables representing quartiles 2, 3 and 4 vs. quartile 1 as the reference and is adjusted for mothers of subjects’ characteristics: pre-pregnancy BMI (<25, 25–29, ≥30 kg/m2), education (<high school, ≥high school), foreign birthplace (yes, no); and, study subjects’ characteristic: menopause (none, any).
Model 3 includes o,p’-DDT coded as 3 dummy variables representing quartiles 2, 3 and 4 vs. quartile 1 as the reference and is adjusted for mothers of subjects’ characteristics: pre-pregnancy BMI (<25, 25–29, ≥30 kg/m2), education (<high school, ≥high school), foreign birthplace (yes, no); and, study subjects’ characteristics: menopause (none, any), exercise (continuous hours/week) and physician-diagnosed and medicated thyroid condition (yes, no).
Figure 1.
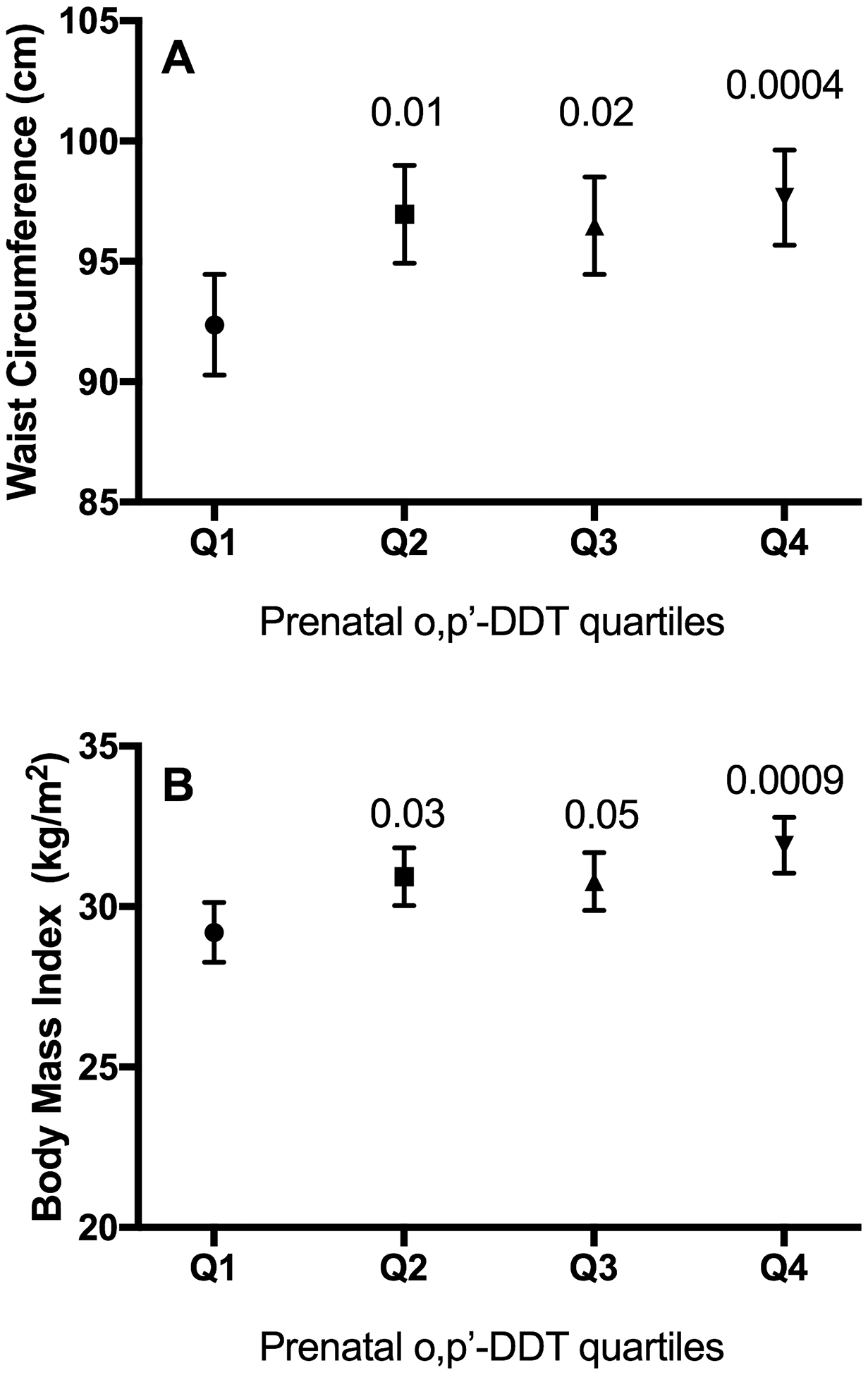
Estimates of mean and standard error of A) waist circumference and B) body mass index per quartile of maternal o,p’-DDT exposure with adiposity in middle-aged daughters of the CHDS cohort. Mixed linear model includes o,p’-DDT coded as 3 dummy variables representing quartiles 2 (square), 3 (upward triangle), and 4 (downward triangle) vs. quartile 1 as the reference (circle) and is adjusted for mothers of subjects’ characteristics: pre-pregnancy BMI (<25, 25–29, ≥30 kg/m2), education (<high school, ≥high school), foreign birthplace (yes, no); and, study subjects’ characteristics: menopause (none, any), exercise (continuous hours/week) and physician-diagnosed and medicated thyroid condition (yes, no). p-values compared to the lower quartile (Q1) are presented above the comparator quartiles (2–4).
Table 3.
Summary of associations of maternal o,p’-DDT quartiles with multiple adiposity measures in middle-aged daughters of the CHDS cohort.
| Parameters | Waist Circumference (WC)1 | Body Mass Index (BMI)2 | Overweight/Obesity from measured WC3 | Overweight/Obesity from measured BMI4 |
|---|---|---|---|---|
| N Subjects | 313 | 311 | 313 | 311 |
| Exposure distribution | Quartile categories | Quartile categories | Quartile categories | Quartile categories |
| Outcome distribution | Continuous | Continuous | Categorical | Categorical |
| Estimate | Beta (95% CI) | Beta (95% CI) | RR (95% CI) | RR (95% CI) |
| Model 15 | ||||
| Ref | Ref | Ref | Ref | |
| 5.36 (0.65, 10.1) | 1.83 (−0.27, 3.92) | 1.00 (0.997, 1.006) | 1.41 (1.002, 1.98) | |
| 4.95 (0.14, 9.76) | 1.82 (−0.32, 3.96) | 1.11 (0.95, 1.30) | 1.30 (0.94, 1.80) | |
| 5.58 (0.97, 10.2) | 3.02 (0.98, 5.06) | 1.11 (0.95, 1.30) | 1.54 (1.13, 2.09) | |
| Model 26 | ||||
| Ref | Ref | Ref | Convergence issue | |
| 5.84 (1.29, 10.4) | 2.03 (−0.001, 4.06) | 1.00 (0.997, 1.007) | ||
| 5.56 (0.91, 10.21) | 2.09 (0.02, 4.16) | 1.11 (0.96, 1.30) | ||
| 4.67 (0.16, 9.17) | 2.69 (0.69, 4.69) | 1.11 (0.96, 1.29) | ||
| Model 37 | ||||
| Ref | Ref | Ref | Ref | |
| 5.32 (0.92, 9.73) | 1.74 (−0.20, 3.68) | 0.99 (0.89, 1.09) | 1.25 (0.95, 1.66) | |
| 5.12 (0.59, 9.65) | 1.93 (−0.06, 3.93) | 1.14 (0.98, 1.33) | 1.22 (0.90, 1.65) | |
| 4.73 (0.35, 9.11) | 2.77 (0.86, 4.69) | 1.12 (0.93, 1.36) | 1.42 (1.08, 1.87) |
Beta=model coefficient, RR=Relative Risk, 95% CI=95% Confidence Interval
Association of waist circumference (cm, continuous) with o,p’-DDT (quartiles 2, 3 and 4 vs. quartile 1) was estimated from a mixed linear regression model.
Association of BMI (kg/m2, continuous) with o,p’-DDT (quartiles 2,3 and 4 vs. quartile 1) was estimated from a mixed linear regression model.
Waist circumference was coded as a 3-category outcome variable representing waist circumference ≤79 cm, 80–87 cm and ≥88 cm. Associations with waist circumference were estimated using a generalized log-linear multinomial model.
Body mass index reported in column 6 was based on measured height and weight and in column 7 was based on self-reported heaviest weight. Both BMI measures were coded as a 3-category outcome variables representing BMI <25, 25–29, and ≥30 kg/m2. Associations with BMI were estimated using a generalized log-linear multinomial model.
Model 1 includes o,p’-DDT coded as 3 dummy variables representing quartiles 2, 3 and 4 vs. quartile 1 as the reference.
Model 2 includes o,p’-DDT coded as 3 dummy variables representing quartiles 2, 3 and 4 vs. quartile 1 as the reference and is adjusted for mothers of subjects’ characteristics: pre-pregnancy BMI (<25, 25–29, ≥30 kg/m2), education (<high school, ≥high school), foreign birthplace (yes, no); and, study subjects’ characteristic: menopause (none, any).
Model 3 includes o,p’-DDT coded as 3 dummy variables representing quartiles 2, 3 and 4 vs. quartile 1 as the reference and is adjusted for mothers of subjects’ characteristics: pre-pregnancy BMI (<25, 25–29, ≥30 kg/m2), education (<high school, ≥high school), foreign birthplace (yes, no); and, study subjects’ characteristics: menopause (none, any), exercise (continuous hours/week) and physician-diagnosed and medicated thyroid condition (yes, no). This model not provided for historically highest BMI since that outcome precedes several of these characteristics.
We further examined whether associations between quartiles of maternal o,p’-DDT levels and adult adiposity extended to increased risk of being a middle-aged women with overweight and obesity. When obesity and overweight were defined by measured abdominal adiposity (≥ 88 cm and 80–87 cm waist circumference respectively), the relative risk of being with overweight compared to being with healthy weight was 27% (95CI: 7–52%) higher among women from the highest quartile of maternal o,p’-DDT exposure relative to the lowest exposure quartile (Model 2 adjusted for confounding, Table 3). Similarly, when obesity and overweight were instead defined by elevated measured BMI (≥ 30 and 25–29 kg/m2 respectively), the relative risk of being with overweight compared to being with healthy weight was 35% (95CI: 9–60%) higher among women whose maternal o,p’-DDT levels were in the highest exposure quartile relative to the lowest quartile (Model 2 adjusted for confounding, Table 3). When we calculated BMI based on self-reported heaviest body weights, the relative risk of being with overweight compared to being with healthy weight was increased by 30% (95CI: 9–54%) if their mothers had the highest o,p’-DDT quartiles relative to the lowest quartile (Model 2 adjusted for confounding, Table 3). We remind the reader that the same magnitude of increased relative risk (e.g. 27% based on measured waist circumference, 35% based on measured BMI, and 30% based on reported highest BMI) extends to being with obesity compared to being with overweight. Risk of being with overweight or obesity in association with maternal o,p’-DDT exposure was only slightly attenuated with further adjustment for obesity risk factors (Model 3, Table 3). Although birthweight has previously been associated with maternal DDT exposure in this cohort38 and with adult obesity in other studies,8,10 there was no evidence that birthweight mediated the consistent associations between maternal o,p’-DDT exposure and adiposity (Table 4), overweight, or obesity (data not shown).
Table 4.
Examination of whether birth weight (g) mediates the association of maternal o,p’-DDT and the adiposity levels of middle-aged daughters of the CHDS cohort using linear regression models. Mediation was determined using the method of Valeri and Vanderweele.35
| Waist Circumference, cm (n=511 daughters) |
Body Mass Index, kg/m2 (n=508 daughters) |
|||
|---|---|---|---|---|
| Direct effect beta1 (95% CI) | Indirect effect beta2 (95% CI) | Direct effect beta1 (95% CI) | Indirect effect beta2 (95% CI) | |
| Unadjusted Model3 | 2.28 (0.43, 4.12) |
−0.12 (−0.33, 0.08) |
1.14 (0.31, 1.96) |
−0.04 (−0.12, 0.03) |
| Adjusted Multivariable Model4 | 2.02 (0.07, 3.99) |
−0.10 (−2.06, 1.85) |
0.97 (−0.98, 2.93) |
−0.02 (−1.99, 1.94) |
Direct effect beta is the estimate of the ln o,p’-DDT (ng/ml) effect
Indirect effect beta is the estimate of the mediation effect of birth weight (g)
Unadjusted model includes ln o,p’-DDT (ng/ml) with birth weight (g) as a mediator. Interaction was not significant thus not presented.
Adjusted model (equivalent to Model 3 in Table 2) log o,p’-DDT with birth weight (g) as a mediator, adjusting for Maternal BMI, Maternal education (less than high school or other), Maternal country of birth (USA or other), daughters’ menopause presence, daughters’ weekly exercise, and daughters’ presence of thyroid conditions. Interaction was not significant thus not presented.
We next performed sensitivity analyses to demonstrate that the risk of obesity associated with maternal o,p’-DDT in subjects was not an artifact of sampling strategy. Among the 313 daughters selected randomly, maternal levels of o,p’-DDT above the bottom quartile were significantly associated with increased waist circumference in all three models (Table S1). Because there were 17 sibling pairs among the 511 daughters in the study, we also revisited the categorical exposure models of overweight and obesity based on waist circumference (Table 3), while sampling only one sister randomly (Table S2), the oldest sister (Table S3), or the youngest sister (Table S4). Regardless of which sister was sampled, daughters had 26% increased risk of waist circumference-based overweight if their mothers had o,p’-DDT in the highest quartile compared to the lowest quartile (n = 494; Tables S2–4). This association was similar across these three sister samples when overweight and obesity were instead based on measured BMI (RR = 1.29–1.32, n = 491; Table S5).
We performed additional sensitivity analyses related to the lipophilicity of DDT, given that researchers routinely consider how coincident lipids are modeled when evaluating health effects of DDT. The association of maternal o,p’-DDT with both waist circumference and BMI remained significant when o,p’-DDT was “adjusted” by maternal serum lipids with the commonly used Phillips equation,36 and ln transformed before modeling (Model B). The association of o,p’-DDT and both waist circumference and BMI also remained significant when ln transformed volumetric o,p’-DDT was evaluated with maternal lipids either included37 (Model C) or excluded (Model A) as a covariable in the models (Table S5). We noted that maternal serum lipids were not a significant variable in either model of adiposity (Model C). We conservatively elected to not adjust for lipids (Model A) throughout the presented analyses given this decision produced the smallest ß estimates for o,p’-DDT among these three models, and produced 95CI that encompassed the ß estimates from the alternative two models (Models B-C).
Discussion
To our knowledge, this is the first study to evaluate and confirm positive associations between maternal DDT exposure and middle-aged adult adiposity, as well as risk of middle-age risk of overweight and obesity. We found that maternal DDT exposure was associated with increased the risk of being with overweight and obesity among middle-aged women consistently across various approaches to modeling exposure and defining overweight and obesity, even when we accounted for confounders and other risk factors for obesity, such as thyroid disease and physical activity. These data support extending the DOHAD hypothesis to early-life environmental exposures.
There are three studies of children with obesity that support our reported observations of associations between maternal DDT and obesity in middle-aged women. The first but smallest study found no association between maternal DDT and the adiposity of adolescent boys.22 In contrast, the second study revealed a positive association between maternal DDT exposure from 1997 to 2005 and 6.5 year old children with increased overweight in Spain.21 The most recent and largest study found a positive association between maternal exposure to DDT from 2012–2013 and South African toddlers with increased weight-for-age and BMI-for-age.20 Although we know of no other studies of maternal DDT and adiposity in adult humans, a systematic review identified two rodent studies where maternal exposure to DDT during pregnancy increased adiposity in adults of subsequent generations.24
There is mechanistic evidence supporting a causal role of DDT in impaired metabolism that leads to decreased energy expenditure and increased obesity. For example, several experimental studies have shown that DDT applied to isolated mitochondrion (the power plants of the cell) can inhibit ATPase activity and resulting ATP synthesis (the production of charged batteries).39 As one would expect from such impaired mitochondrial metabolism,40 adult mice whose mothers were treated with DDT had decreased energy expenditure and increased adipose mass despite no change in physical activity.14 Although unmeasured increased calorie consumption among CHDS daughters with elevated maternal DDT levels could also explain their elevated risk of obesity, we know of no experimental evidence that maternal DDT exposure has perturbed this canonical obesity risk factor.24 Further, although persistent organic pollutants are believed to be more slowly eliminated from bodies with excess adipose mass, there was no correlation between o,p’-DDT and weight gain in pregnancy here. Whether the reported experimental relationships between DDT, impaired metabolism, and obesity could be explained by differences in metabolism or elimination of DDT remains to be investigated.
This study has several strengths. First, the study uses quantitative analytical chemistry to assess exposures. Second, the study relies on clinically evaluated measures of adiposity, a measurement which when instead is subjected to self-report, is greatly vulnerable to reporting bias.41,42 Third, our study design allows for the examination of a relatively high exposure during a narrow window of developmental susceptibility; it evaluates maternal exposure during the peak window of population exposure to a substance banned soon after exposure assessment. Fourth, we examined adiposity during middle-age when it is at its maximum.43 Fifth, we used prospectively collected data after long individual follow-up.
Our study also had limitations. We could only examine effects in women due to a parent grant to research females in a breast cancer study. Although sex effects were not identified in the recent meta-analysis of prospective studies of DDE and obesity, some sex- specificity in the adiposity of offspring was identified in the integrated in vivo studies reviewed.24 The potential sensitivity of the obesity risk in men exposed to DDT in the womb remains an uncharacterized possibility. Although our study design with such extended follow-up is very rare, any birth cohorts with an opportunity to repeat our findings and extend them to men should consider doing so.
The present research extends the association of maternal DDT exposure with obesity into middle age and supports the integrated systematic review and meta-analysis that recently categorized DDT and DDE as “presumed to be obesogenic in humans”.24 Since DDT remains in use and is manufactured in a number of low- and middle-income countries, our results suggest high current and past DDT exposure may increase the prevalence of overweight and obesity in these countries.2,44 Further, high exposure to DDT in early life in a country still using DDT or one that recently banned it may increase the risk of obesity among migrant populations living in high-income countries such as the United Kingdom and the United States.44 This may result in a great loss of disability-adjusted life years and high societal cost.6,45 The current evidence adds obesity risk to the recent categorization of DDT as “probably carcinogenic to humans” (Group 2A) by the International Agency for Research on Cancer of the World Health Organization.46 The present findings therefore support the inclusion of obesity, as well as cancer, in the World Health Organization’s evaluation of the health costs of continuing their current recommendations for using DDT in vector control.
Supplementary Material
Acknowledgements
National Institute of Health: R01ES019919, R01ES013736, P30ES023513, U01ES019471; California Breast Cancer Research Program: 15ZB-0186; USDA National Institute of Food and Agriculture, Hatch project: 1002182; University of California Davis Office of Research Perinatal Origins of Disparities Center.
Footnotes
Declaration of competing financial interests
The authors declare they have no actual or potential competing financial interests.
References
- 1.Black RE et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451, doi: 10.1016/S0140-6736(13)60937-X (2013). [DOI] [PubMed] [Google Scholar]
- 2.Ng M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, doi: 10.1016/S0140-6736(14)60460-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontis V et al. Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modelling study. Lancet 384, 427–437, doi: 10.1016/S0140-6736(14)60616-4 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Collaboration, N. C. D. R. F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396, doi: 10.1016/S0140-6736(16)30054-X (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landsberg L Core temperature: a forgotten variable in energy expenditure and obesity? Obes Rev 13 Suppl 2, 97–104, doi: 10.1111/j.1467-789X.2012.01040.x (2012). [DOI] [PubMed] [Google Scholar]
- 6.Ford ND, Patel SA & Narayan KM Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu Rev Public Health 38, 145–164, doi: 10.1146/annurev-publhealth-031816-044604 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Heindel JJ et al. Parma consensus statement on metabolic disruptors. Environmental health : a global access science source 14, 54, doi: 10.1186/s12940-015-0042-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law CM, Barker DJ, Osmond C, Fall CH & Simmonds SJ Early growth and abdominal fatness in adult life. Journal of epidemiology and community health 46, 184–186, doi: 10.1136/jech.46.3.184 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ & Bleker OP Obesity at the age of 50 y in men and women exposed to famine prenatally. American Journal of Clinical Nutrition 70, 811–816 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Eriksson J, Forsen T, Osmond C & Barker D Obesity from cradle to grave. International Journal of Obesity and Related Metabolic Disorders 27, 722–727, doi: 10.1038/sj.ijo.0802278 0802278 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 11.Han C & Hong YC Fetal and childhood malnutrition during the Korean War and metabolic syndrome in adulthood. Nutrition 62, 186–193, doi: 10.1016/j.nut.2019.01.003 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ & Osmond C Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Martin-Gronert MS & Ozanne SE Mechanisms underlying the developmental origins of disease. Rev Endocr Metab Disord 13, 85–92, doi: 10.1007/s11154-012-9210-z (2012). [DOI] [PubMed] [Google Scholar]
- 14.La Merrill M et al. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PloS one 9, e103337, doi: 10.1371/journal.pone.0103337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamorro-Garcia R et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun 8, 2012, doi: 10.1038/s41467-017-01944-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baillie-Hamilton PF Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 8, 185–192 (2002). [DOI] [PubMed] [Google Scholar]
- 17.La Merrill M & Birnbaum LS Childhood obesity and environmental chemicals. Mount Sinai Journal of Medicine 78, 22–48, doi: 10.1002/msj.20229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Berg H, Manuweera G & Konradsen F Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar J 16, 401, doi: 10.1186/s12936-017-2050-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouwman H, Becker PJ, Cooppan RM & Reinecke AJ Transfer of DDT used in malaria control to infants via breast milk. Bull World Health Organ 70, 241–250 (1992). [PMC free article] [PubMed] [Google Scholar]
- 20.Coker E et al. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environment international 113, 122–132, doi: 10.1016/j.envint.2018.01.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valvi D et al. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environmental health perspectives 120, 451–457, doi: 10.1289/ehp.1103862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladen BC et al. Prenatal DDT exposure in relation to anthropometric and pubertal measures in adolescent males. Environmental health perspectives 112, 1761–1767 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner MK et al. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Medicine 11, 228–244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cano-Sancho G, Salmon AG & La Merrill MA Association between Exposure to p,p’-DDT and Its Metabolite p,p’-DDE with Obesity: Integrated Systematic Review and Meta-Analysis. Environmental health perspectives 125, 096002, doi: 10.1289/EHP527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Berg BJ, Christianson RE & Oechsli FW The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatric and Perinatal Epidemiology 2, 265–282 (1988). [DOI] [PubMed] [Google Scholar]
- 26.La Merrill MA, Cirillo PM, Krigbaum NY & Cohn BA The impact of prenatal parental tobacco smoking on risk of diabetes mellitus in middle-aged women. Journal of developmental origins of health and disease 6, 242–249, doi: 10.1017/S2040174415000045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garvey WT et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract 22 Suppl 3, 1–203, doi: 10.4158/EP161365.GL (2016). [DOI] [PubMed] [Google Scholar]
- 28.Alberti KG et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645, doi:CIRCULATIONAHA.109.192644 [pii] 10.1161/CIRCULATIONAHA.109.192644 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Cohn BA et al. DDT Exposure in Utero and Breast Cancer. The Journal of clinical endocrinology and metabolism, jc20151841, doi: 10.1210/jc.2015-1841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Merrill M et al. Prenatal exposure to the pesticide DDT and hypertension diagnosed in women before age 50: a longitudinal birth cohort study. Environmental health perspectives 121, 594–599, doi: 10.1289/ehp.1205921 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sholtz RI et al. Assaying organochlorines in archived serum for a large, long-term cohort: implications of combining assay results from multiple laboratories over time. Environment international 37, 709–714, doi:S0160-4120(11)00015-8 [pii] 10.1016/j.envint.2011.01.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longnecker MP, Klebanoff MA, Gladen BC & Berendes HW Serial levels of serum organochlorines during pregnancy and postpartum. Archives of environmental health 54, 110–114 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Cohn BA, Wolff MS, Cirillo PM & Sholtz RI DDT and breast cancer in young women: new data on the significance of age at exposure. Environmental health perspectives 115, 1406–1414, doi: 10.1289/ehp.10260 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegelman D & Hertzmark E Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology 162, 199–200 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Valeri L & Vanderweele TJ Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 18, 137–150, doi: 10.1037/a0031034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips DL et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 18, 495–500 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Li D, Longnecker MP & Dunson DB Lipid adjustment for chemical exposures: accounting for concomitant variables. Epidemiology 24, 921–928, doi: 10.1097/EDE.0b013e3182a671e4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kezios KL et al. Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reproductive toxicology 35, 156–164, doi: 10.1016/j.reprotox.2012.10.013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elmore SE & La Merrill MA Oxidative Phosphorylation Impairment by DDT and DDE. Front Endocrinol (Lausanne) 10, 122, doi: 10.3389/fendo.2019.00122 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran L, Langlais PR, Hoffman N, Roust L & Katsanos CS Mitochondrial ATP synthase beta-subunit production rate and ATP synthase specific activity are reduced in skeletal muscle of humans with obesity. Exp Physiol 104, 126–135, doi: 10.1113/EP087278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maukonen M, S M & H T A comparison of measured versus self-reported anthropometrics for assessing obesity in adults: a literature review. Scand J Public Health. 46, 565–579, doi: 10.1177/1403494818761971 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Preston SH, Fishman E & Stokes A Effects of categorization and self-report bias on estimates of the association between obesity and mortality. Ann Epidemiol 25, 907–911 e901–902, doi: 10.1016/j.annepidem.2015.07.012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garn SM & Clark DC Trends in fatness and the origins of obesity Ad Hoc Committee to Review the Ten-State Nutrition Survey. Pediatrics 57, 443–456 (1976). [PubMed] [Google Scholar]
- 44.Daniels SI et al. Elevated Levels of Organochlorine Pesticides in South Asian Immigrants Are Associated With an Increased Risk of Diabetes. J Endocr Soc 2, 832–841, doi: 10.1210/js.2017-00480 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Legler J et al. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. The Journal of clinical endocrinology and metabolism 100, 1278–1288, doi: 10.1210/jc.2014-4326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loomis D et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. The Lancet. Oncology, doi: 10.1016/S1470-2045(15)00081-9 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


