Summary:
We found that HBEC expressed the IL-5 receptor, which triggered intracellular signaling and changed gene expression. This data indicate that in addition to targeting eosinophils, IL-5 and anti-IL-5 biologics may have a direct role on AEC.
Keywords: Airway epithelial cells, IL-5 receptor, intracellular signaling, gene expression, RNA-sequencing
To the Editor:
Airway epithelial cells (AEC) form the continuous barrier and primary defense mechanism in respiratory tract and the lungs against inhaled pathogens and allergens. Consisting of columnar, ciliated, basal, and secretory cells, AEC play a vital role in modulating innate and adaptive immune responses(1). This regulatory role occurs in the pathogenesis of asthma, specifically, through AEC involvement in mucus production, airway inflammation and remodeling(1). AEC drive allergic inflammation and airway remodeling in response to inhaled stimuli by promotion of the type 2 immune response, which includes inflammatory cytokines such as interleukin-5 (IL-5)(1). IL-5 has long been implicated in asthma due to its function as a growth and differentiation factor for eosinophils(2). Eosinophil numbers in peripheral blood and in the airways are associated with asthma severity(3). Therefore, several therapeutic monoclonal antibodies targeting the IL-5 pathway have been developed for treatment of eosinophilic asthma(4). These anti-IL-5 biologics target either IL-5 itself or its receptor. The specific α-chain of the IL-5 receptor is reported to be exclusively produced by eosinophils and to a lesser extent by basophils and some B-lymphocytes(5). Therefore, while anti-IL-5 therapy is believed to modulate inflammation through inhibition of eosinophil recruitment, its effects may extend to other cell types that express its receptor. We have found that IL5RA mRNA is expressed in differentiated primary AEC and whole sinus tissue samples using gene expression microarrays(6). Therefore, in the present study, we tested whether both α- and β-chains of the IL-5 receptor are expressed in differentiated human bronchial epithelial cells (HBEC) and bronchial tissue. In addition, we explored the functionality of the IL-5 receptor in HBEC, investigating IL-5-induced downstream signaling events and gene expression changes in IL-5-activated HBEC.
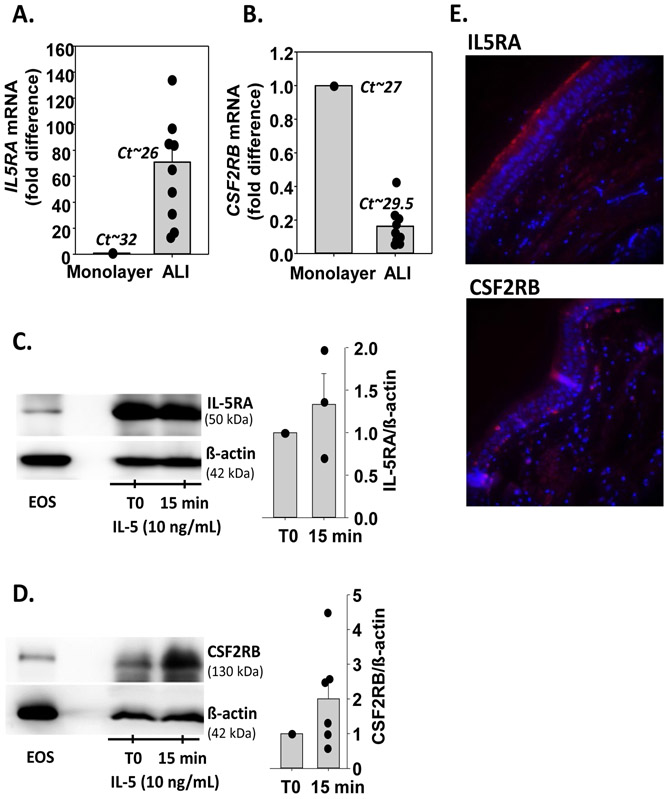
First, we assessed the mRNA expression level of the IL-5 receptor α-subunit (IL5RA) and ß-subunit (CSF2RB) in HBEC cultured at an air-liquid interface (ALI). In accordance with our previous microarray analyses(6) (Table S1), fully-differentiated HBEC in ALI cultures displayed 70-fold more IL5RA mRNA (Cycle threshold (Ct)~27), compared to undifferentiated monolayer cultures (Ct~32) (Figure 1A). The expression of CSF2RB mRNA was lower in differentiated cells compared to monolayer cultures but remained detectable (Ct ~29.5) (Figure 1B). We then evaluated IL-5 receptor protein expression in differentiated HBEC by western-blot. We detected a high levels of the IL5RA protein in differentiated cells, which was stable for at least 15 min after addition of a recombinant human (rh)-IL-5 basally (Figure 1C). CSF2RB protein was also detected in HBEC and its presence tended to increase after 15 min with rh-IL-5 (Figure 1D). We next analyzed the presence of both protein subunits of the IL-5 receptor on airway epithelial cells of bronchial tissue samples by immunofluorescence. We found that IL5RA staining was abundant along the ciliated apical surface of HBEC (Figure 1E). Of note, the presence of the IL5RA protein specifically on ciliated HBEC was also confirmed by immunofluorescence and single-cell RNA-sequencing of differentiated HBEC cultured at ALI (unpublished observations and Figure S1). Staining for CSF2RB was present but faint and dispersed along the ciliated epithelium (Figure 1E).
Figure 1. Human bronchial epithelial cells (HBEC) express the two subunits of the IL-5 receptor.
A and B. HBEC were either cultured undifferentiated as a monolayer (1 donor) or fully differentiated at an air liquid interface (ALI; 9 donors). A. IL5RA mRNA levels were determined by RT-qPCR, normalized to GUSB, and expressed as a fold difference compared to the monolayer. Dots identify fold difference for each individual culture. Mean Ct values are shown. B. CSF2RB mRNA levels. Analysis is identical to that for IL5RA mRNA in A. C and D. Differentiated HBEC in ALI cultures were treated with rh-IL-5 (10ng/mL) for 15 minutes. Western blots were performed to visualize IL5RA (C) and CSF2RB (D) proteins. Eosinophils were used as a positive control. Graphs represent ratios of IL5RA (n=3) and CSF2RB (n=6) to β-actin normalized to untreated HBEC (T0). E. In red, protein expression of the IL-5 receptor α-(IL5RA, upper panel) and β- (CSF2RB, lower panel) subunits in bronchial tissue from one lung donor as determined by immunofluorescence. In blue, DAPI (nuclei) staining.
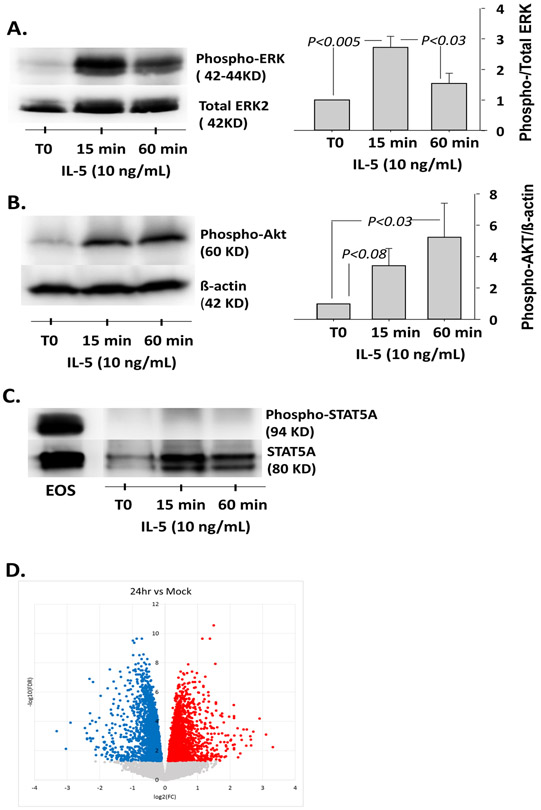
Consequently, we sought to demonstrate the functionality of the IL-5 receptor expressed on HBEC. IL-5 binds to its surface receptor and then triggers three main intracellular signaling pathways in eosinophils including the JAK-STAT5, PI3K-Akt, and Ras-ERK pathways (7, 8). We found that phosphorylation of ERK increased significantly after 15 min of stimulation with IL-5 (p<0.003, n=4) (Figure 2A). This IL-5-induced ERK phosphorylation was inhibited by a neutralizing anti-IL5RA antibody (Figure S2). Phosphorylation of Akt was also significantly increased after 60 min of rh-IL-5 activation (p<0.03, n=4) (Figure 2B). Finally, although STAT5A was present in differentiated HBEC (Figure 2C), it was not phosphorylated following rh-IL-5 stimulation. This is in contrast to eosinophils activated with rh-IL-5 (2 ng/ml) for 15 min (Figure 2C), indicating some differential response to IL-5 between eosinophils and HBEC.
Figure 2. IL-5 receptor expressed by HEBC is functional.
A. B. and C. Differentiated HBEC at ALI were treated with rh-IL-5 (10ng/mL) for 15 and 60 minutes. Analyses of ERK, Akt and STAT5A phosphorylation in HBEC lysates from four different donors were performed by western blotting. For ERK and Akt, graphs indicate means ± SEM from four cultures. Data were analyzed by ANOVA followed by Holm-Sidak method (A) and ANOVA followed by Turkey test (B). C. Eosinophils (EOS) activated with rh-IL-5 were used as a positive control. D. HBEC from three different donors, were cultured at ALI and remained untreated (Mock) or were activated with rh-IL-5 (10ng/ml) for 24 h. RNA-sequencing was performed and a volcano plot demonstrating transcript levels increased (red) and decreased (blue) is shown.
To more broadly assess the effects of the IL-5 receptor on HBEC, cells were either untreated or stimulated with rh-IL-5 for 6 or 24 h and then RNA-sequencing was performed. After filtering for protein coding genes with counts ≥ 1 in ≥ 20% of libraries and computation of mean-variance relationships and observational-level weights, 14,370 genes were analyzed with untreated (Mock) cells used as the reference. Figure 1D shows a volcano plot, illustrating transcript levels significantly increased and decreased by rh-IL-5 at 24 h compared to untreated HBEC. No statistically significant changes in gene expression were found after 6 hours of rh-IL-5 stimulation when compared to no treatment control. After 24 h of stimulation, 1403 genes displayed increased expression while 1851 genes had decreased expression (False discovery rate (FDR)<0.01) (Table S2 and Supplemental Spreadsheet 1). The main functional pathway upregulated by rh-IL-5 at 24 h was a set of genes involved in protein translation (GO_BP translation initiation, count=54 genes, FCR=1.9E-22; KEGG ribosome, count=51 genes, FDR=4.9E-20; Table S3). Genes with decreased expression after 24 hours of rh-IL-5 stimulation were most highly functionally enriched for cell adhesion molecules (GO_BP, cell-cell adhesion, count=85 genes, FDR=6.4E-19; KEGG focal adhesion, count=45 genes, FDR=5.8E-4; Table S4), including CDH1 (E-cadherin), Caveolin-1 and −2 (CAV1, CAV2), epidermal growth factor receptor (EGFR), and six integrin subunits. Interestingly, this group also showed enrichment for immune genes (GO_BP, positive regulation of immune system process, count=128 genes, FDR=6.1E-3), including CXCL8 (IL-8), CXCL1, IL10RA, IL1RL1 (ST2), and IL6R. Downregulation of these functional pathways suggests that IL-5 may reduce strength of the epithelial barrier through weakening cell-to-cell and cell-matrix adhesions, and also reduce HBEC ability to enhance components of the immune response.
In conclusion, in this study, we demonstrated the presence and functionality of the IL-5 receptor on differentiated HBEC. Considering that the concentration of IL-5 in bronchoalveolar lavage fluids from patients with symptomatic asthma can reach several hundred pg/ml(9), it is likely that IL-5 concentrations in the airways are high enough to affect HBEC function through the functional cognate receptor. The apical expression of the receptor on airway epithelial cells also suggests that it senses IL-5 secreted by luminal cells. These findings also suggest that the efficacy of anti-IL-5 therapeutics in asthma might include direct effects on airway epithelial cells to improve the integrity of the epithelial barrier. In addition, future studies are warranted to determine whether benralizumab, an antibody specific for IL-5RA that can mediate antibody-dependent cell-mediated cytotoxicity against leukocytes, might have similar effects on IL-5R-expressing airway epithelial cells in vivo.
Supplementary Material
Acknowledgement:
We would like to thank Elizabeth McKernan, B.S. and Paul Fichtinger, B.S. of the UW Eosinophil Core facility (P.I., Sameer Mathur, M.D., Ph.D.) for blood eosinophil purification and the research nurse coordinators in the UW Clinical Core facility (P.I., Loren L. Denlinger, M.D., Ph.D.) for subject recruitment and screening. Funding for this research was provided by NIH HL088594 (N.J.), NIH U19 AI104317 and NIH-NIAID UM1 AI114271 (YAB, RAB, MCA and JEG).
Funding for this research was provided by NIH HL088594 (N.J.), NIH U19 AI104317 and NIH-NIAID UM1 AI114271 (YAB, RAB, MCA and JEG).
Abbreviations:
- AEC
airway epithelial cells
- Ct
cycle threshold
- FDR
false discovery rate
- GUSB
glucuronidase beta
- HBEC
human bronchial epithelial cells
- CSF2RB
ß-subunit of the receptor for IL-5, IL-3 and GM-CSF
- IL-5
interleukin 5
- IL-5RA
IL-5 receptor α-subunit
- rh
recombinant human
- RT-qPCR
reverse transcription followed by quantitative polymerase chain reaction
Footnotes
None of the authors have any relevant conflict of interest with this study.
References
- 1.Loxham M, Davies DE. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J Allergy Clin Immunol 2017;139(6):1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esnault S, Kelly EA. Essential mechanisms of differential activation of eosinophils by IL-3 compared to GM-CSF and IL-5. Crit Rev Immunol 2016;36(5):429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBrien CN, Menzies-Gow A. The Biology of Eosinophils and Their Role in Asthma Front Med (Lausanne: ) 2017;4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J Allergy Clin Immunol 2019;143(1):190–200 e120. [DOI] [PubMed] [Google Scholar]
- 5.Takatsu K Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci 2011;87(8):463–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A 2015;112(17):5485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall DJ, Cui J, Bates ME, Stout BA, Koenderman L, Coffer PJ, et al. Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal-regulated kinase activation and interleukin-5-mediated cell viability. Blood 2001;98(7):2014–2021. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Bertics PJ. Chemoattractant-induced signaling via the Ras-ERK and PI3K-Akt networks, along with leukotriene C4 release, is dependent on the tyrosine kinase Lyn in IL-5- and IL-3-primed human blood eosinophils. J Immunol 2011;186(1):516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sur S, Gleich GJ, Swanson MC, Bartemes KR, Broide DH. Eosinophilic inflammation is associated with elevation of interleukin-5 in the airways of patients with spontaneous symptomatic asthma. Journal of Allergy and Clinical Immunology 1995;96:661–668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.