Abstract
Introduction
Age related neurodegenerative disorders affect millions of people around the world. The role of the gut microbiome (GM) in neurodegenerative disorders has been elucidated over the past few years. Dysbiosis of the gut microbiome ultimately results in neurodegeneration. However, the gut microbiome can be modulated to promote neuro-resilience.
Areas covered
This review is focused on demonstrating the role of the gut microbiome in host physiology in Parkinson’s disease (PD) and other neurodegenerative disorders. We will discuss how the microbiome will impact neurodegeneration in PD, Alzheimer’s Disease (AD), Multiple sclerosis (MS), Amyotrophic Lateral Sclerosis (ALS), and finally discuss how the gut microbiome can be influenced through diet and lifestyle.
Expert opinion
Currently, much of the focus has been to study the mechanisms by which the microbiome induces neuroinflammation and neurodegeneration in PD, AD, MS, ALS. In particular, the role of certain dietary flavonoids in regulation of gut microbiome to promote neuro-resilience. Polyphenol prebiotics delivered in combination with probiotics (synbiotics) present an exciting new avenue to harness the microbiome to attenuate immune inflammatory responses which ultimately may influence brain cascades associated with promotion of neurodegeneration across the lifespan.
Keywords: gut microbiome, neuro-resilience, polyphenols, gut dysbiosis, neurodegenerative disorders, Parkinson’s disease, Alzheimer’s disease, probiotics, synbiotics
1. Introduction
The gut microbiome (GM) consists of symbiotic organisms such as bacteria, fungi, viruses, protozoa and fungi found in the human gut [1,2]. The GM is made up of trillions of cells and outnumbers human cells 10:1 [3]. The GM plays a very important role in the human body; it is responsible for immune regulation, host metabolism, digestion, vitamin synthesis, neurological development, and energy homeostasis [4–9]. The gut microbiota is made up of a thousand different species of bacteria with much of the gut microbiota belonging to the Firmicutes and Bacteroidetes phyla. There is high variability in microbial populations between individuals. Interestingly, despite such variability between individuals, the overall function and role of the microbiome remains consistent [10–13]. The GM has been implicated in many neurodegenerative disorders in part due the bidirectional relationship between the gut and the central nervous system known as the gut-brain axis. The central nervous system uses the afferent and efferent autonomic pathways to communicate with muscles and the mucosal layer of the gut, thus influencing gut motility, secretion, and mucosal immunity [14,15]. In turn, the gut microbiota influences the central nervous system by producing neuromodulators such as choline, tryptophan, and short chain fatty acids (SCFAs) [16,17]. There is increasing evidence indicating the important role of the GM in maintaining human physiology. The microbial populations of the gut are always in a delicate balance. Many factors influence the composition of the GM resulting in the imbalance (dysbiosis) of the GM that can cause gastrointestinal disorders, low grade inflammation, increased oxidative stress, and cellular degeneration. Gut dysbiosis has also been linked to neurodegenerative disorders [18–20]. The composition of the GM is affected by many factors, such as lifestyle, hormones, diet, genetics, metabolic activity, infection, and immune responses [16]. The GM can be harnessed to combat the underlying mechanisms of neurodegenerative disorders through the use of dietary polyphenols, probiotics, and other GM mediated factors.
Millions of people in the United States suffer from age related neurodegenerative disorders [21]. As the lifespan of Americans gets longer the prevalence of age related neurodegenerative disorders is expected to rise [22]. Neurodegenerative disorders are caused by the loss of neuron function in the central nervous system resulting in the degeneration and death of selective neuronal cells [16]. Oxidative damage, inflammation, and disrupted energy metabolism are considered common pathways of neurodegeneration [20,23–25]. The role of the microbiome in both the pathology and treatment of neurodegenerative disorders such as PD, PD with Dementia (PDD), AD, ALS, and MS among others is of considerable interest. While the pathophysiology of each disease varies, the basic underlying mechanisms such as gut dysbiosis and neuroinflammation remain consistent. The GM and associated factors regulate many of these pathways, suggesting that the GM can be of therapeutic use if it is regulated. In this review, we explore the role of the GM in the host, external factors that influence the GM composition, the impact of GM dysfunction on the pathogenesis of neurodegenerative disorders, and lastly how the GM can be manipulated as a biotherapeutic to promote neuro-resilience and prevent neurodegenerative disorders.
2. The gut microbiome and its function
The GM regulates host physiology by regulating host metabolism, immune response, and homeostasis. In fact, germ-free animals without gut microbiota suffer from immature immune systems and impaired gastrointestinal development and function [26]. The GM regulates intestinal motility and maintains the intestinal epithelium. In the gut, the microbiome is also involved with fortifying the mucosal barrier, promoting angiogenesis, and combating pathogenic organisms via the secretion of antimicrobial peptides [27]. The GM influences the acquired immune response by regulating the differentiation and function of anti-inflammatory regulatory T cells and B cells [28,29]. It protects the epithelial barrier, which is the host’s first line of physical defense against pathogens by the activation of Toll-like receptors (TLR) signaling [30]. TLR2 signaling via production of bacterial ligands such as lipoteichoic acid has been specifically demonstrated to protect the intestinal epithelium and are necessary for the formation of tight junctions [31].
The gut microbiota assists in modulating the immune response to specific pathogen-associated molecular patterns. Excessive immune responses to antigens have been linked to gut pathologies such as inflammatory bowel syndrome, Crohn’s disease, and ulcerative colitis, as well as food intolerances associated with Celiac’s disease [32,33]. The GM modulates brain and peripheral inflammation by influencing the cytokine profile of dendritic cells, which in turn determines CD4+ T helper differentiation into Th1, Th2, Th17, or regulatory T cells (Treg) in secondary lymphoid tissues [32,34]. The ratio between the Firmicutes and Bacteroidetes phyla regulates the ratio of Th17 to Treg cells [35]. The role of individual GM species in immune regulation is not known. However, the bacterium Bacteroides fragilis produces a polysaccharide that is found to attenuate T cell deficiencies and Th1/Th2 imbalances in animal models [36]. Studies have also indicated the role of the GM in neurodevelopment. Germ-free animals suffer from behavioral changes such as reduced anxiety-like behavior, impaired cognition and sociability stemming from altered functional and morphological development of the brain [37,38]. Germ-free animals have been shown to have decreased expression of the brain derived neurotrophic factor in the cortex, hippocampus, and amygdala, impaired neurogenesis in the hippocampus, and altered neural morphology in the amygdala [38–40]. Additionally, the GM plays an important role in providing the host with nutrients and metabolites such as bioactive compounds, dietary fibers, and vitamins necessary to maintain gut homeostasis [8,41]. The GM is essential in metabolizing all foreign substances, including environmental toxins, pollutants, and drugs [42]. The GM plays a vital role in regulating the enteric nervous system. Interestingly, the central and enteric nervous systems share many key features indicating that bacteria and associated factors may also influence the central nervous system and by extension neurodegenerative disorders [43,44].
2.1. Gut microbiome composition and dysbiosis
Bacteria are the most prevalent component of the GM. They are found to be most abundant in the colon. Bacteria from the Firmicutes and Bacteroidetes phyla are the most abundant, comprising 99% of gut bacteria. Bacteria from the phyla Proteobacteria, Actinobacteria, Fusobacteria, Spirochaetes, Verrucomicrobia and Lentisphaerae makeup the remaining 1% [45]. At least 1000 species and more than 7000 strains of bacteria have been identified as part of the microbiome [11]. Some refer to the gut microbiota as an organ, considering its physiological activities are necessary for the host to function [46]. Despite the beneficial role of the GM to the host, dysbiosis of the gut can result in negative outcomes. GM dysbiosis has been specifically linked to the initiation and exacerbation of neurological diseases. GM dysbiosis stems from the reduction in microbiota diversity. While many factors can affect microbial composition in the gut, the most common cause of reduced diversity is aging. Recent reports also indicate the presence of GM bacteria in the brain. Bacterial phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria normally abundant in the GM were detected in the brain via 16S rRNA gene sequencing of postmortem brain samples [47]. Dysbiotic GM activation of microglia and microglia mediated inflammation is key mechanism of neurodegenerative disorders [24,48,49]. Indeed, the impact of GM dysbiosis on neurodegenerative disorders is supported by the fact that the initial symptoms of these disorders are gut related issues such as constipation and diarrhea. These findings indicate a strong relationship between the gut and brain via the gut-brain axis in neurodegeneration [24].
2.2. Microbiota-gut-brain axis
The enteric nervous system, often referred to as the “little brain”, innervates the gut and facilitates interaction between the GM and the brain [27,50]. The gut-brain axis is the communication pathway between the enteric and central nervous systems. In its entirety, the gut-brain axis involves the GM, enteric nervous system, central nervous system, parasympathetic and sympathetic nervous systems, neuroendocrine connections, signaling molecules, humoral pathways, cytokines, and neuropeptides [51]. This pathway is linked by neurons, circulating hormones and other neuroregulatory factors. The gut-brain axis facilitates communication between the gut and the brain in three ways: neuronal messages are transported via the vagal afferents, gut hormones transmit endocrine messages, and cytokines carry immune system messages [48]. The GM can send signals to the brain either directly or via the enteric nervous system. Bacteria in the gut produce many neurotransmitters and neuromodulators such as GABA, norepinephrine, serotonin, dopamine, acetylcholine, and histamine that can affect the brain directly. SCFAs, long chain fatty acids, propionate, and conjugated linoleic acid are some metabolites produced by the GM known to have neuroactivity, influencing the brain indirectly by regulating immune function, inflammation, and neurogenesis [52]. The neurochemicals secreted by the GM can also potentially induce epithelial cells to modulate neuronal signaling in the enteric nervous system. Conversely, the brain communicates directly with the GM by releasing signaling molecules into the gut lumen and indirectly by altering gastric motility, secretion, and intestinal permeability [53]. Dysfunction of the gut-brain axis has been implicated in metabolic, psychiatric, and neurodegenerative disorders.
3. Pathways of GM mediated neurodegeneration
The GM and its associated factors can initiate neurodegeneration through various pathways. In the leaky gut mechanism, bacterial components contribute to inflammation by influencing intestinal barrier integrity. GM derived inflammasome and microglial activation result in mitochondrial dysfunction and endotoxemia. Other pathways include Type 1 interferon mechanism, NF-κB signaling cascade, and glucagon like peptide 1 (GLP-1). As discussed below, these pathways drive neurodegeneration by disrupting immune function.
3.1. Leaky gut mechanism
The intestinal barrier is critical in preventing pro-inflammatory luminal contents from entering intestinal and systemic circulation. The GM is responsible for regulating intestinal barrier integrity [54]. GM produced SCFAs influence barrier integrity by positively modulating immunity and anti-inflammatory signaling as well as mitochondrial metabolism [55]. GM dysbiosis is characterized by a loss of SCFAs which inadvertently leads to leaky gut, neuroinflammation, and neurodegeneration [54]. Intestinal barrier disruption has been observed in PD and AD patients and is associated with lipopolysaccharide (LPS) induced inflammation [56]. GM dysbiosis is also linked with increased LPS production which is thought to activate Toll-like receptor 4 (TLR4) on brain microglia. TLR4 is essential in regulating immune responses to bacteria [57,58]. Endotoxins released into the blood as a result of a leaky gut affect the brain directly. All in all, GM dysbiosis influences endotoxemia, LPS involved TLR4 activation, and SCFAs associated leaky gut, resulting in systemic inflammation, neuroinflammation, and neurodegeneration [54,59].
3.2. Inflammasome pathway
Inflammasomes are large protein complexes that play a major role in innate immune responses. Inflammasomes are generally made up of a sensor protein, an adaptor protein, and Caspase-1. When activated, inflammasomes induce production of interleukin-1B (IL-1B) and IL-18 proteins, which are associated with maintaining gut homeostasis [60–62]. NLRP6 inflammasome deficiency has been observed to alter GM composition [63]. Conversely, GM mediated inflammasome dysfunction has been linked to many diseases. The NLRP3 inflammasome has been specifically linked to neurodegenerative disorders such as PD, AD, MS, and ALS [64]. It is thought that increased levels of GM derived SCFAs activate the NLRP3 inflammasome [65]. In AD pathology, NLRP3 is involved in amyloid-beta aggregation. Knockdown of NLRP3 in animal models resulted in lowered amyloid-beta accumulation [66]. In PD, NLRP3 has been implicated as a driver of α-syn mediated neuroinflammation [67]. The role of NLRP3 in MS pathology has been demonstrated in animal models, where demyelination and microglial infiltration was influenced by NLRP3 [64]. The exact mechanism of inflammasome action in ALS pathology has not been demonstrated, however, inflammasome mediated inflammation is likely given that NLRP3 and Il-18 are found to be upregulated in ALS patients [68]. Taken together, the NLRP3 inflammasome plays a key role in neuroinflammation and neurodegeneration.
3.3. Type 1 interferon pathway
Type 1 interferon (IFN-1) is a cytokine that is critical to both innate and adaptive immunity as well as the maintenance of host homeostasis. The GM has been demonstrated to influence IFN-1, as two strains of Lactobacillus acidophilus can induce anti-viral responses by modulating IFN signaling in dendritic cells [69]. Another gut bacterium Clostridium orbiscindens produces a metabolite which interferes with IFN-1 signaling [70]. Some GM bacteria have been shown to induce IFN production [71]. Interestingly, IFN-1 has also been demonstrated to influence GM composition, indicating a two-way interaction between the GM and IFN-1 [72]. Aggregation of IFN in the brain has been implicated in neurodegeneration. Neurons respond to IFN-1 by initiating pro-apoptotic signaling pathways that lead to neurodegeneration. The influence of the GM on IFN-1 signaling and the role of IFN-1 in host immunity demonstrates the impact of IFN-1 in neuroinflammation and neurodegeneration [73].
3.4. NF-κB signaling pathway
NF-κB is a family of transcription factors that affect immune responses. NF-κB activation in glial and immune cells influences pathological inflammatory responses. Indeed, NF-κB has been linked to many neurodegenerative disorders. These pathologies are associated with increased activation of NF-κB in the brain [74]. GM dysbiosis mediated inflammation involves NF-κB signaling. In the gut, LPS and other bacterial molecules bind to TLR4 thus activating NF-κB signaling resulting in a pro-inflammatory cascade which in turn activates microglia and promotes neurodegeneration [75]. Additionally, GM dysbiosis stemming from Campylobacter jejuni incursion also initiated activation of NF-κB [76]. NF-κB and increased gut inflammation induce Reg3γ (regenerating islet-derived protein III-gamma) and CRAMP (cathelin-related antimicrobial peptides) expression resulting in altered GM composition [77]. In contrast, other bacteria have been demonstrated to reduce NF-κB and associated inflammasome activation [77]. In summary, various studies have characterized the interaction between GM and NF-κB signaling in neuroinflammation and neurodegeneration [61].
3.5. Peptides & GLP-1
The intestine produces factors in response to bacterial metabolites that can directly and indirectly affect the brain. GM associated factors can induce production of incretin hormones like glucagon-like peptide-1 (GLP-1) and glucose dependent insulinotropic polypeptide in the gut. These peptides are known to affect neuroinflammation and neurodegeneration. GLP-1 is beneficial to the host in a variety of ways [59]. GLP-1 can correct insulin resistance by recruiting pancreatic cells to produce insulin, thereby regulating insulin signaling and mitochondrial function in the brain. This effect also reduces reactive oxygen species (ROS) production and neuroinflammation. GLP-1 can directly act on the brain as it can cross the blood brain barrier [78]. In animal models, GLP-1 has been demonstrated to play a role in memory, prevention of neuronal apoptosis, and the production of brain derived neurotrophic factor, a critical factor in the maintenance of healthy dopaminergic neurons [78–80]. Despite its positive effect on host neurobiology, a dysfunctional GLP-1 can cause neurodegeneration. GM dysbiosis has been observed to interfere with GLP-1 signaling. GLP-1 dysfunction has been associated with PD pathology, metabolic syndrome, and reduced brain derived neurotrophic factor. It is thought that GLP-1 mediated inhibition of the NLRP3 inflammasome results in insulin resistance, mitochondrial dysfunction, and cellular stress [59,81–83].
4. Gut microbiome and neurodegenerative disorders
Each of the neurological diseases discussed herein, have their own pathologies. However, they are all characterized by the loss of neurons, neuroinflammation, and intestinal barrier disruption. The GM plays an important role in the pathogenesis of neurodegenerative disorders. Here we discuss the role of the GM in neurodegenerative disorder pathogenesis.
4.1. Lewy body PD and PD with dementia
PD is a neurodegenerative disorder that affects a million people in the United States [84]. It is the second-most prevalent neurodegenerative disorder in the world [85]. Approximately 75% of individuals suffering from PD for at least 10 years eventually develop PDD [86]. Those affected by PD and PDD often suffer from impaired olfaction, constipation, depression, excessive daytime sleepiness, bradykinesia, muscular rigidity, rest tremor, cognitive decline as well as postural and gait impairment [87,88]. The most important feature of PD pathology is the loss of dopaminergic neurons in the substantia nigra. Both of these disorders stem from aggregation of misfolded α-syn into neuronal inclusions known as Lewy bodies. These Lewy bodies are found in the substantia nigra in the brain [89]. Numerous studies have demonstrated the role of the GM in PD and α-syn pathogenesis, however little information exists about the effect of the GM on PDD pathogenesis specifically. The progression of PD has been linked to gut dysbiosis. In a study involving PD patients, the relative abundance of the genus Lactobacillus and Bifidobacterium, as well as the families Verrucomicrobiaceae, Christensenellaceae, and Oscillospira was increased. In contrast, a lower relative abundance of genus Faecalibacterium, Coprococcus, Blautia, and the family Prevotellaceae was observed [27]. GM bacteria of the Prevotellaceae family are involved in the production of mucin and SCFAs. Reduced abundance of this family results in leaky gut, as well as increased expression and misfolding of α-syn. Introduction of bacterial toxins and LPS into the circulatory system via a leaky gut induces systemic inflammation. LPS and inflammatory cytokines have been demonstrated to disrupt the blood brain barrier and promote α-syn aggregation in the brain [89]. Another consequence of blood brain barrier disruption is the loss of dopaminergic neurons in the substantia nigra [90]. Neurodegeneration and α-syn deposition are further exacerbated due to the increased abundance of pro-inflammatory cytokine producing bacteria and reduced numbers of bacteria with anti-inflammatory properties in the GM [91]. Small intestinal bacterial overgrowth has also been associated with PD. Interestingly, transplantation of fecal microbiota from PD patients into mice resulted in the mice developing PD like symptoms [92]. Indeed, the role of the GM in PD pathogenesis is undeniable. While the explicit impact of the GM on PDD pathogenesis remains undefined, the effects of the GM on α-syn and neuroinflammation characterized in PD may potentially also be involved in PDD pathology.
4.2. Alzheimer’s disease
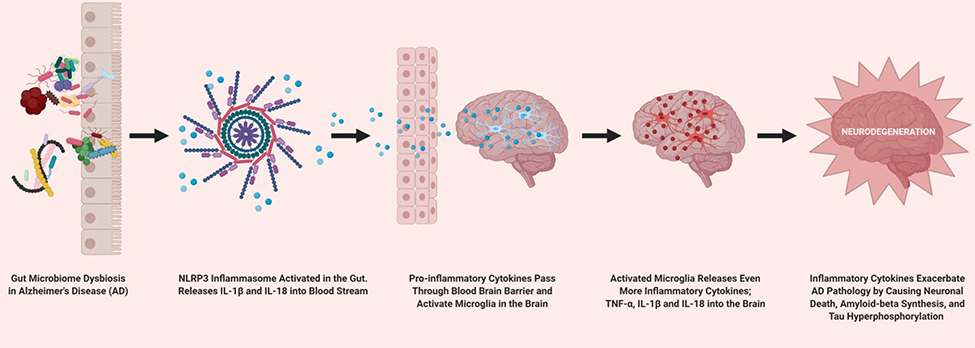
AD is a neurodegenerative disease which results in cognitive decline. AD is also considered to be the most common form of dementia in the elderly. AD pathology is characterized by neuroinflammation, accumulation of misfolded amyloid-beta peptide as well as tau hyperphosphorylation and associated neurofibrillary tangles in the brain. Amyloid-beta and tau aggregation in the brain has been demonstrated to cause neuronal apoptosis [16,61,89]. GM dysbiosis has been implicated with increasing inflammation and leaky gut in AD pathogenesis. Inflammatory responses caused by GM dysbiosis result in the disruption of the gut epithelial barriers. This has been implicated in the incursion of gut bacteria and fungi as well as their products into the brain. Individuals with AD have been found to have higher bacterial loads in the brain than those without AD. It is thought that the invasion of GM elements into the brain may play a role in peripheral and brain innate immune dysfunction, which is a characteristic of AD pathology [47]. GM derived products such as LPS, microbial amyloid, and neurotoxins have been linked to neurodegeneration, amyloid-beta aggregation, neurofibrillary tangle buildup, and neuroinflammation in the brain [93,94]. Altered GM composition is also linked to AD pathology. The relative abundance of the genus Verrucomicrobia and Proteobacteria was increased, while butyrate producing bacteria such as Ruminococcus and Butyricicoccus were decreased leading to increased pro-inflammatory cytokines in the blood [61,89,95]. Recently, a novel mechanism of neuroinflammation in AD involving GM mediated NLRP3 activation has been described (Figure 1) [96]. Fecal microbiota transplantation from AD individuals into mice resulted in increased expression of NLRP3 and other inflammatory factors in the gut. Additionally, neuroinflammation stemming from activated microglia in the hippocampus was also observed. Essentially, GM dysbiosis activates the NLRP3 inflammasome in the intestine which in turn releases pro-inflammatory cytokines into the blood stream. Large amounts of circulating inflammatory cytokines pass through the blood brain barrier and activate microglia which then releases even more pro-inflammatory cytokines, specifically, TNF-α (tumor necrosis actor-alpha), IL-1β and IL-18 into the brain exacerbating neurodegeneration [96]. TNF-α, IL-1β and IL-18 released by the microglia specifically exacerbate AD pathology by causing neuronal death, synaptic excitotoxicity, reduced synaptic plasticity, reduced long term synaptic potentiation, increased amyloid-beta synthesis, and increased tau hyperphosphorylation [97]. Intriguingly, GM modulation and resulting downstream effects via long-term antibiotic treatment was shown to be sex-specific [98]. Male mice given high doses of antibiotics demonstrated lowered GM diversity. Interestingly, antibiotics resulted in decreased amyloid-beta deposition but, increased soluble amyloid-beta [99]. Antibiotic associated modulation of the GM and amyloid-beta also altered microglial morphology in male mice only. Taken together, these studies highlight the role of the GM in AD pathogenesis.
Figure 1.
Demonstrates the NLRP3 inflammasome activation by gut microbiome dysbiosis and subsequent pathway resulting in pro-inflammatory cytokine mediated neurodegeneration.
4.3. Amyotrophic lateral sclerosis
ALS is a fatal neurodegenerative disorder that involves neurons in the brain and spinal cord. ALS results in muscle weakness and stiffness. ALS eventually leads to difficulty in speech, swallowing, and breathing, ultimately resulting in death due to respiratory paralysis. The cause of Sporadic ALS, the most common type of ALS, remains elusive. In animal models of ALS, leaky gut and disrupted blood brain barrier have been observed [16,89]. Though its etiology is unknown, ALS has been associated with reduced levels of anti-inflammatory bacteria in the gut. Specifically, butyrate producing bacteria, including Butyrivibrio fibrisolvens and Escherichia coli, as well as those of the genus, Oscillibacter, Anaerostipes, and Lachnospira were found to be reduced. Reduction of these butyrate producing bacteria has been linked to increased levels of pro-inflammatory cytokines in the intestine and serum. Additionally, the ratio of the phyla Bacteroidetes to Firmicutes is disrupted in ALS patients when compared to healthy individuals, providing further evidence of GM dysbiosis in ALS pathology [100,101]. GM derived products such as LPS have been found in the plasma of ALS patients and can cross the blood brain barrier to cause neuroinflammation, potentially contributing to ALS pathogenesis [94].
4.4. Multiple sclerosis
MS is a neurodegenerative disorder induced by immune dysfunction. MS is characterized by axonal damage, demyelination, and neurodegeneration. MS pathogenesis results in depression, cognitive impairment, and bowel dysfunction. Disrupted T cell-associated immune responses, such as pro-inflammatory cytokines, initiate demyelination. While MS pathology is generally associated with immune dysfunction, the GM regulates both innate and adaptive immunity as well as some physiological processes. Therefore, the GM can be considered to play a critical role in MS pathogenesis [61,102]. SCFAs produced by the GM are essential in regulating immune cells responsible for anti-inflammatory response. The relative abundance of GM bacteria involved in SCFA and butyrate production was found to be lowered in MS patients [89,103]. In MS patients, a GM derived epsilon toxin has been found in increased levels, resulting in a disrupted blood brain barrier, neuronal damage, and demyelination [104]. GM dysbiosis due to altered microbial composition and ensuing pro-inflammatory immune response has been linked to a leaky gut and increased circulation of GM microbiota, microbial products, and inflammatory factors [89]. LPS and other GM products have been demonstrated to cause neuroinflammation, neurodegeneration, and neuronal apoptosis. As evidenced by the studies mentioned here, the GM’s impact on host immunity contributes to MS pathogenesis.
5. External factors that affect the gut microbiome
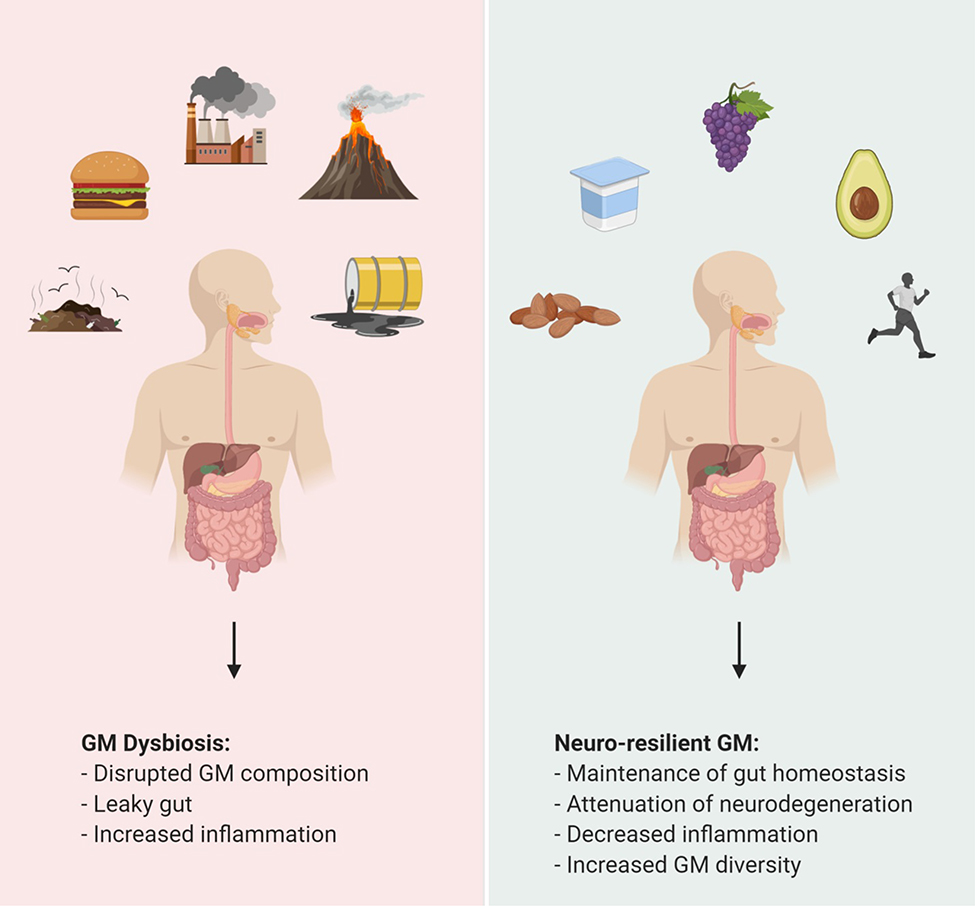
The GM and its function is affected by numerous factors, including environmental chemicals, diet, and lifestyle (Figure 2). These factors can all induce GM dysbiosis leading to the pathologies mentioned previously. Environmental chemicals such as heavy metals, air pollution, nanoparticles, and endocrine disrupting chemicals are encountered in daily life. They can be found in basic household items, plastics, and antimicrobials. These chemicals target the gut microbiota and lead to GM dysbiosis and dysfunction of host systems. Mice exposed to 10 ppm of arsenic for 4 weeks had a disrupted relative abundance of bacteria of the order Streptophyta, Clostridiales, Bacillales and Erysipelotrichales. Exposure to arsenic resulted in altered microbial metabolites which were negatively associated with gut microbiota [105]. Arsenic also induced changes in metabolic pathways involved in metal resistance and the cell transport system. Lead, another heavy metal, has been found to alter GM composition and can potentially affect metabolism of vitamin E, bile acids, nitrogen, energy, oxidative stress, and detoxification [106]. Exposure to nanoparticles of silver, copper, and zinc has been correlated with reduced GM diversity in various animal models [107]. Particulate matter of pollutants found in air affects the GM by altering immune gene expression, increased pro-inflammatory cytokine production, aggravated leaky gut, GM associated SCFAs production, and altered GM composition [108]. Endocrine disrupting chemicals such as bisphenol A, estradiol or ethinyl estradiol have been demonstrated to affect the GM in many animal models. Introduction of bisphenol A and ethinyl estradiol via diet in mice resulted in increased abundance of the bacteria Bacteroides spp., Mollicutes, Prevotellaceae, Erysipelotrichaceae, Akkermansia, Methanobrevibacter, Sutterella spp., which are associated with gut and metabolic disorders [109]. Chronic exposure to diethyl phthalate, methyl paraben, and triclosan resulted in changes to the GM composition in various animal models [107].
Figure 2.
Represents factors alter the gut microbiome. Factors, such as western diet and environmental toxins that cause gut microbiome dysbiosis. In contrast, consumption of polyphenols, probiotics, and synbiotics as well as an active lifestyle result in a healthy gut.
Diet is a major factor in regulating GM composition and function. Acute changes in diet can change GM composition within 24 hours resulting in altered GM gene expression and metabolic pathways [110,111]. Diets typically consisting of higher amounts of animal fat and protein, such as the Gluten-free diet and Western diet, have been associated with reduced overall GM abundance [112]. Conversely, the Mediterranean diet is largely considered to promote GM health. The Mediterranean diet is characterized by the intake of beneficial fatty acids, high amounts of polyphenols and other antioxidants, fiber, low glycemic carbohydrates, and vegetables as well as a moderate intake of fish, red wine, and poultry, with a limited intake of dairy products, red meat, processed meat, and sweets. Further evidence has linked the Mediterranean diet to improved lipid profile and inflammation resulting from diet associated increases in GM bacterial genera, Lactobacillus, Bifidobacterium, and Prevotella and decreases in Clostridium [112]. A high-fiber and low-fat diet decreased populations of GM phylum Firmicutes. Diets with large amounts of animal fat and protein are correlated with an abundance of bacterial phylum Bacteroides. High-fiber diets have been associated with an increased abundance of the phylum Prevotella, while a plant-based diet has been linked to a higher relative abundance of both Bacteroides and the Firmicutes in addition to increased relative GM diversity.
The individual components that make up a diet, protein, fat, carbohydrates, polyphenols, and pre/probiotics, each impact the GM. Protein, regardless of source, has been associated with increased GM diversity. However, excessive amounts of animal protein greatly increases chances of inflammatory bowel diseases [112,113]. Intake of whey and pea protein is linked to the increase in commensal bacteria and decrease in pathogenic bacteria in the GM [114,115]. Pea protein attenuates inflammation and maintains mucosal barrier by increasing gut SCFA levels [116]. High-fat diets modulate GM composition by increasing abundance of propionate and acetate producing bacterial species. Low-fat diets have been correlated with an increased abundance of Bifidobacterium and reduced fasting glucose and total cholesterol levels. Interestingly, high monosaturated fat intake did not affect relatively any GM bacterial populations. However, total bacterial load, plasma, and LDL cholesterol levels were reduced [117]. Digestible carbohydrates such as starches and sugars have been found to alter GM composition by increasing abundance of Bifidobacteria and decreasing Bacteroides. Interestingly, artificial sweeteners such as saccharin, sucralose, and aspartame have an inverse effect to natural sugars and starches. They have been found to cause GM dysbiosis by altering GM composition by decreasing abundance of Bifidobacteria and increasing Bacteroides [118,119]. Non-digestible carbohydrates such as fibers cannot be degraded by the host, instead these carbohydrates are fermented by the GM. Diets high in non-digestible carbohydrates increase GM diversity and the abundance of gut microbiota. Non-digestible components of the diet are considered prebiotics in that they benefit the host indirectly by promoting the growth of the GM. Prebiotics have been linked to reductions in IL-6, total cholesterol, serum triglycerides and insulin resistance [120,121]. Probiotics are fermented foods that contain bacteria that can be beneficial to overall GM health. Probiotics such as yogurt are known to reduce inflammation, cholesterol, triglycerides, and abundance of pathogenic bacteria [122,123]. Propionic acid, a bacterial metabolite produced from fiber rich foods has been shown to ameliorate MS pathology in an experimental model by increasing anti-inflammatory Treg in the gut [124]. Antioxidants such as dietary polyphenols are found in common foods such as fruits, vegetables, tea, and wine. Consumption of polyphenols has been linked to reduced abundance of pathogenic bacteria. Additionally, polyphenols are beneficial for increasing plasma HDL, immune regulation, and general gut health [125–127].
Daily lifestyle can also impact the GM. Smoking, for example, has been found to significantly alter GM populations [128]. A sedentary lifestyle and stress also affect the large bowel and colonic motor activity respectively, resulting in shifts in microbial composition and decreased abundance of beneficial bacteria. Stress is also correlated with functional bowel disorders [129,130]. A study involving professional athletes demonstrated that exercise coupled with proper diet resulted in an increase in GM diversity [131]. Travel and geographic location also play a major role in determining GM composition. Children from rural Africa had a more diverse GM than children from developed areas in the EU. An international study involving individuals from the USA and rural regions of Venezuela and Malawi revealed that fecal bacteria composition and associated gene expression was altered based on geography [132,133].
6. Harnessing the GM to promote neuro-resilience
The GM composition is influenced by many factors and changes throughout an individual’s lifespan. However, drastic changes in the GM result in dysbiosis leading to harmful effects on the overall health of the host. Unsurprisingly, the GM can be modulated to promote neuro-resilience and attenuate neurodegeneration briefly summarized in (Table 1). A healthy gut microbiome reinforced with proper diet and prebiotics/probiotics is essential in maintaining host health. Herein we discuss how the GM can be influenced to prevent and combat neurodegenerative disorders.
Table 1.
GM Modulators That Promote Neuro-resilience
| Theraputic Agent | Effect | Reference | |
|---|---|---|---|
| Prebiotics | Fructooligosaccharides | Increase GM diversity, reduced inflammation | [127] |
| Isolichenan | Improves cognitive function | [134] | |
| Catechin | Stimulates the growth of beneficial probiotic bacteria while inhibiting pathogenic bacteria | [136] | |
| Galactooligosaccharides | Increase GM diversity | [127] | |
| Probiotics | Lactobacillus casei shirota | Decreases pathogentic bacteria in PD | [138], [139] |
| Bacillus subtilis PXN21 | Clears α-syn aggregates | [143] | |
| Bifidobacterium lactis HN019 | Improved innate immune response in elderly individuals | [142] | |
| Lactobacillus spp. | Improved cognitive function, reduced inflammation | [89], [144] | |
| Bifidobacterium spp. | Lowered levels of pro-inflammatory cytokines, TNF-α, IL-5, IL-6, IL-1β and IL-8 | [89] | |
| Synbiotics | Lactobacillus plantarum, Lactobacillus fermentum, Bifidobacteria longum spp. infantis and Triphala | Increased survivability, motility, and reduced amyloid-beta deposition | [147] |
| Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose | Increase abundance and diversity of the GM in elderly individuals | [148] | |
| Bifidobacteria longum and a inulin based prebiotic Synergy1 | Reduced inflammation | [149] | |
| Metabolites | Propionic acid | Ameliorates MS pathology by increasing anti-inflammatory Tregs in the gut. Inhibits amyloid-beta aggregates. | [124], [135] |
| Dihydrocaffeic acid | Decreases inflammation | [153] | |
| Gallic acid | Inhibits amyloid-beta aggregation and amyloid fibrils. Scavenges free radicals. | [147] | |
| Malvidin-3′-O-glucoside | Improves cognitive resilience and neuronal plasticity | [153] | |
| Vanillic acid | Reduces neuroinflammation and oxidative damage | [147] | |
| Resveratrol | Attenuates LPS induced microglial activation and immune responses. Regulates inflammasome. | [156] | |
| Grape derived proanthocyanidins, stilbenes, anthocyanins, and flavonols | Inhibit pathogen growth, promote GM diversity and abundance. Act as antioxidants. | [136], [147] | |
| Epigallocatechin-3-gallate | Improves cognition, regulates processing of amyloid precursor proteins | [134] | |
| Curcumin | Reduces LPS induced inflammation | [134] | |
| Ferluic acid | Protects against oxidative stress, stimulates neurogenesis. | [48] | |
| Valeric acid and Butyric acid | Inhibits amyloid-beta aggregates. | [135] | |
| 3-hydroxybenzoic acid | Inhibit both α-syn and amyloid-beta aggregation | [155] | |
6.1. Prebiotics/probiotics and synbiotics
Prebiotics in the form of non-digestible fibers have been demonstrated to be beneficial to GM bacteria. Prebiotics can positively alter GM composition by increasing the abundance of bacteria that produce anti-inflammatory factors. Prebiotics have been linked to the maintenance of GM homeostasis, decreased abundance in pathogens, and improved metabolism [121]. Prebiotics are found in soybean, inulins, unrefined wheat, unrefined barley, raw oats, and other oligosaccharides. Fructooligosaccharides (FOS) and galactooligosaccharides (GOS) are some of the most common prebiotics. Fermentation of prebiotics by the GM produces SCFAs and peptidoglycans that regulate the host immune system. Prebiotics such as FOS and GOS have been found to increase neuro-resilience by increasing GM diversity and reducing inflammation [112,127]. Isolichenan, a prebiotic derived from the lichen Cetrariella islandica has been demonstrated to improve cognitive function in health adults [134]. GM derived SCFAs have also been demonstrated to specifically attenuate AD pathology by interfering with amyloid-beta aggregation [135]. While polyphenols are mainly of interest due to the neuro-protective properties of their metabolites, they can also act as prebiotics. Catechin, epicatechin, and quercetin the main polyphenols found in tea promote gut health and homeostasis by selectively stimulating the growth of beneficial probiotic bacteria while inhibiting pathogenic bacteria. Polyphenols derived from grape seeds and cocoa have also been demonstrated to act as prebiotics [136]. Alteration of the GM via the consumption of probiotics has been shown to be beneficial in combating neurodegenerative disorders. Dietary supplementation with probiotics is the consumption of live bacteria for therapeutic purposes. Probiotics can modulate immune responses, regulate hormonal and neurochemical mechanisms, maintain intestinal barrier integrity, and improve nutrient absorption [137]. Probiotics improve neuro-resilience and fight neurodegeneration via both direct and indirect mechanisms. Probiotic bacteria from the genus Bifidobacterium and Lactobacillus have been demonstrated to reduce PD like conditions. The probiotic Lactobacillus casei shirota attenuates constipation, a common PD symptom, by decreasing levels of pathogenic bacteria in PD patients [138,139]. A clinical trial administering a probiotic cocktail of bacteria, Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri and Lactobacillus fermentum improved motor function, insulin metabolism, inflammation, and oxidative stress in PD patients though the long term effectiveness of these therapeutics remains to be seen [140,141]. Another clinical trial involving Bifidobacterium lactis HN019 resulted in improved innate immune response in elderly individuals [142]. Recently, a probiotic strain of Bacillus subtilis has been found to inhibit and clear α-syn aggregates [143]. In AD patients, probiotic Lactobacillus was demonstrated to improve cognitive function [89,144]. Probiotic Bifidobacterium species have been associated to improved memory and learning as well as lowered levels of serum pro-inflammatory cytokines such as TNF-α, IL-5, IL-6, IL-1β and IL-8. In animal models of MS, probiotic Bifidobacterium and Lactobacillus have been linked to lower levels of inflammation [89]. The combination of both prebiotics and probiotics are known as synbiotics [48]. Synbiotics given to humanized transgenic Drosophila melanogaster resulted in rescue of the AD phenotype [145]. Synbiotics and their derived metabolites can also attenuate NLRP3 inflammasome activation which plays a key role in AD pathogenesis [146]. While synbiotic have been demonstrated to be viable therapeutic options for AD, they can be effective against many age related neurodegenerative disorders because synbiotic contain prebiotics which can be metabolized into bioactive molecules that attenuate inflammation and oxidative stress as well as probiotic bacteria which alter GM composition to further produce beneficial metabolites [146]. In a humanized Drosophila melanogaster model of AD a synbiotic consisting of probiotic strains of Lactobacillus plantarum, Lactobacillus fermentum, and Bifidobacteria longum spp. infantis coupled with a prebiotic polyphenol plant extract derived from Triphala resulted in increased survivability, motility, and reduced amyloid-beta deposition [147]. Clinical trials involving synbiotics have been promising. A symbiotic formulation containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose was able to restore the GM of elderly individuals which is generally lacking in abundance and diversity [148]. Another clinical trial involving a synbiotic composed of Bifidobacteria longum and a inulin based prebiotic Synergy1 significantly reduced pro-inflammatory response in the periphery [149]. Potential synbiotics are of interest as innate immune responses have been implicated in the onset and progression of the experimental autoimmune encephalomyelitis (EAE) animal model of MS and have been correlated to dysbiotic microbiota [150,151]. In essence, synbiotics and their individual components positively alter GM composition and function to promote neuro-resilience and act against neurodegeneration.
6.2. Polyphenols and GM derived products
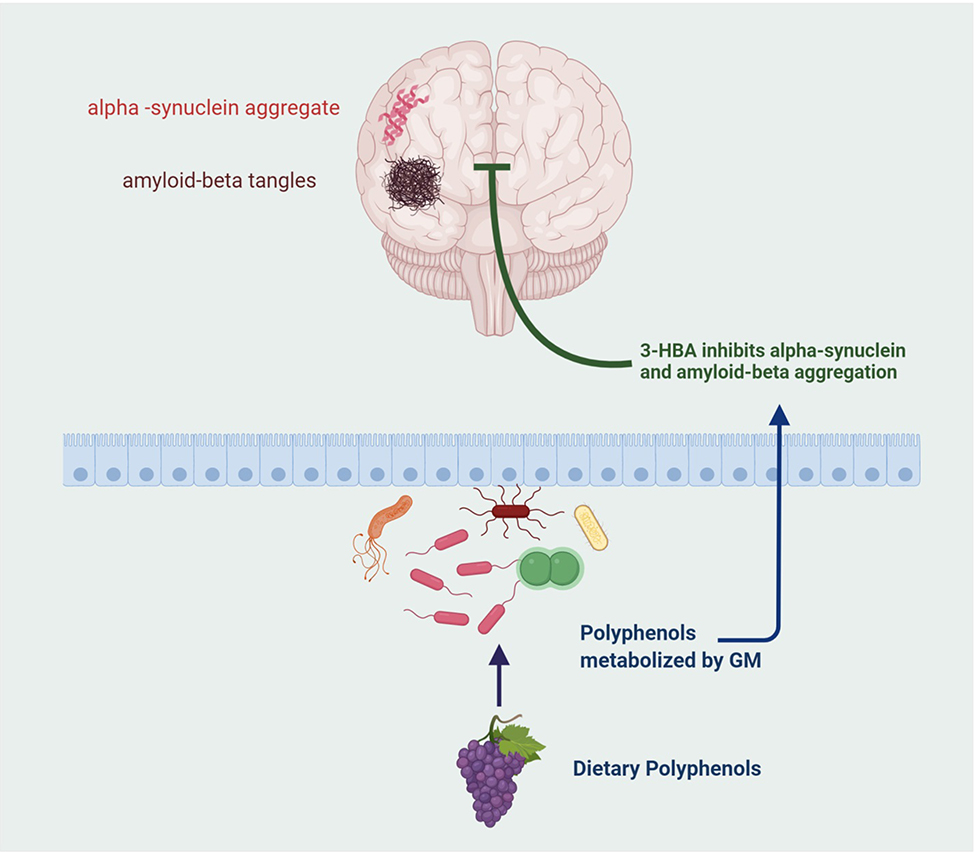
Polyphenols are a broad range of botanicals including, catechins, flavonols, flavones, anthocyanins, and phenolic acids. Polyphenols can be obtained from common foods such as fruits, seeds, vegetables, tea, cocoa, and wine. Polyphenols are metabolized by the GM into various bioactive molecules that positively affect the overall health of the host. Dietary polyphenols are metabolized via gastrointestinal absorption and xenobiotic metabolism that result in bioavailable phenolic compounds. Polyphenols promote neuro-resilience by inhibiting protein misfolding, inflammation, and other key mechanisms involved in neurodegenerative disorders. In addition to providing protection against neurodegeneration, polyphenols also reduce levels of pathogenic bacteria, positively alter GM composition, and reduce serum triacylglycerol [34,112]. Grape derived polyphenols have been shown to regulate inflammation through a variety of pathways resulting improved synaptic plasticity [152]. The GM metabolites dihydrocaffeic acid and malvidin-3′-O-glucoside, obtained from the metabolism of grape derived polyphenols can improve cognitive resilience in animal models of depression by regulating inflammation and neuronal plasticity [153]. Through their anti-inflammatory and antioxidant properties polyphenols have been demonstrated to be viable therapeutics for AD pathology [154]. Gallic acid a polyphenol metabolite derived from Triphala inhibits amyloid-beta aggregation and amyloid fibrils. It has also been shown to reduce neural damage by increasing the scavenging of free radicals. Another metabolite, vanillic acid also reduces neuroinflammation and oxidative damage [147]. Numerous polyphenol derived GM metabolites have been shown to inhibit α-syn protein misfolding, which plays a key role in PD and LBD. The polyphenol metabolite, 3-hydroxybenzoic acid, is unique in its ability to inhibit both α-syn and amyloid-beta aggregation (Figure 3) [155]. Oxidative stress and neuroinflammation are common factors in neurodegeneration. A few polyphenols have been identified for their neuroprotective properties. Microglial activation is a key driver of neuroinflammation. Resveratrol, a grape derived polyphenol known for its antioxidant and anti-cancer properties, has been shown to attenuate LPS induced microglial activation and associated immune responses through inflammasome regulation [156]. Additionally, resveratrol decreases oxidative damage in synapses and protects neurons against amyloid-beta associated toxicity. Other grape derived polyphenols such as proanthocyanidins, stilbenes, anthocyanins, and flavonols have also demonstrated similar properties as resveratrol [136,147]. The polyphenol curcumin has been demonstrated to reduce LPS induced inflammation. Epigallocatechin-3-gallate (EGCG) regulates processing of amyloid precursor proteins and has been demonstrated to improve cognition [134]. Another polyphenol metabolite, ferulic acid, directly stimulates proliferation of neural stem cells indicating its importance in neurogenesis and also protects against oxidative stress [48]. In addition to metabolizing polyphenols, the gut bacteria produce various other compounds that both directly or indirectly protect against neurodegeneration. The GM metabolites, valeric acid, butyric acid, and propionic acid, produced from dietary fiber can interfere with protein-protein interactions necessary for the formation of neurotoxic amyloid-beta aggregates in AD pathology [135]. Some bacteria of the Prevotellaceae family produce mucin and help maintain the integrity of the intestinal barrier. Roseburia spp. and Faecalibacterium spp. found in the GM produce butyrate an anti-inflammatory compound [134]. Certain Lactobacillus spp. metabolize dietary L-histidine to produce histamine, an immunomodulatory neurotransmitter with a high density of receptors in the brain [48]. Altogether, polyphenols and their associated GM metabolized products can effectively render neuroprotection to the host. Polyphenols promote neuro-resilience by acting directly on mechanisms involved in neurodegenerative disorders such as neuroinflammation, protein misfolding and aggregation. The widespread availability of polyphenols as well as their safety and efficacy make them ideal candidates for biotherapeutics for maintaining a healthy GM and combating neurodegenerative disorders.
Figure 3.
Demonstrates how a dietary polyphenol metabolite, 3-hydroxybenzoic acid (3-HBA) inhibits alpha synuclein and amyloid-beta accumulation.
7. Expert opinion
There has been a considerable amount of interest in the role of the gut microbiome in neurodegenerative disorders recently. However, further work is necessary to understand the underlying mechanisms by which the microbiome affects host physiology. It is estimated that 40% of the gut microbiome is made up of unclassified bacteria that are present in very low abundance [27]. The impact of these bacteria on the microbiome and host physiology still needs to be explored. A standardized method for studying the microbiome is also necessary. Currently, there is much variance between culturing and metagenomics protocols across studies. While much work on the modulation of the gut microbiome by external factors has been done, the effect of internal factors such as host genetics on the microbiome requires attention. This review focused on highlighting the role of the microbiome in host physiology, factors that affect the microbiome, consequences of GM dysbiosis on neurodegenerative disorders, and finally how the microbiome can be modulated to protect against neurodegeneration. Indeed, while the role of the microbiome is well characterized in disorders such as PD, AD, and MS, much work is necessary in understanding its impact on PDD and ALS. Considering the fact that these neurodegenerative diseases have no cure, prophylactic treatment through diet, especially polyphenols and synbiotics, should be explored. Dietary polyphenols metabolized by the GM produce bioactive compounds that promote neuro-resilience and in some cases directly attenuate key pathologies of many neursodegenerative disorders. Polyphenols, in conjunction with probiotics, provide an inexpensive and sustainable treatment for neurodegenerative disorders. Polyphenols and probiotics should be studied in further detail for their potential as biotherapeutics.
Article highlights.
The gut microbiome is responsible for host immune regulation, host metabolism, digestion, vitamin synthesis, neurological development, and energy homeostasis.
The composition of the microbiome is affected by many factors, such as lifestyle, diet, and environmental toxins.
Alteration in gut microbiota leads to inflammation, increased oxidative stress, and cellular degeneration and has also been linked to PD, AD, MS, and ALS. The microbiome initiates neurodegeneration through pathways such as the leaky gut mechanism, inflammasome activation, and NF-κB signaling.
In PD gut microbiome dysbiosis mediated associated to inflammatory cascades of release of inflammatory cytokines may be responsible for disruption of the blood brain barrier allowing inflammatory cytokines to penetrate the brain. The role of symbiotic metabolites (polyphenols) in the attenuation of proteostasis associated with abnormal misfolding in PD such as protein misfolding of alpha-synuclein (α-syn).
The gut microbiome plays a role in activating NLRP3 inflammasome, a mediator of innate immunity in microglia in the brain has been associated destructive neuroinflammatory cascade in AD.
Altered microbiome with reduced levels of anti-inflammatory bacteria in the gut has been associated with MS and ALS pathogenesis.
Modulation of the gut microbiome via consumption of prebiotics, probiotics, and synbiotics is associated with improved cognitive abilities and lowered levels of pro-inflammatory cytokines as well as attenuation neurodegenerative symptoms.
Acknowledgement
Figures were created with BioRender.com. The authors thank Kyle J. Trageser for his assistance in editing this manuscript.
Funding
This study was supported by Grant Number P50 AT008661-01 from the NCCIH and the ODS. Dr. Pasinetti holds a Senior VA Career Scientist Award. We acknowledge that the contents of this study do not represent the views of the NCCIH, the ODS, the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Footnotes
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- [1].Guarner F, Malagelada J-R. Gut flora in health and disease. The Lancet. 2003;361:512–519. [DOI] [PubMed] [Google Scholar]
- [2].Turnbaugh PJ, Ley RE, Hamady M, et al. The Human Microbiome Project . Nature. 2007;449:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rajilić-Stojanović M Function of the microbiota. Best Practice & Research Clinical Gastroenterology. 2013;27:5–16. [DOI] [PubMed] [Google Scholar]
- [5].Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. [DOI] [PubMed] [Google Scholar]
- [6].Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. [DOI] [PubMed] [Google Scholar]
- [7].Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].LeBlanc JG, Milani C, de Giori GS, et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Current Opinion in Biotechnology. 2013;24:160–168. [DOI] [PubMed] [Google Scholar]
- [9].Flint HJ, Scott KP, Louis P, et al. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589. [DOI] [PubMed] [Google Scholar]
- [10].Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. The Lancet Neurology. 2020;19:179–194.** Provides an excellent summary of neurodegenerative disorders and microbiome.
- [11].The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mandal RS, Saha S, Das S. Metagenomic Surveys of Gut Microbiota. Genomics, Proteomics & Bioinformatics. 2015;13:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin Ther. 2015;37:984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Neunlist M, Van Landeghem L, Mahé MM, et al. The digestive neuronal–glial–epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- [16].Zhu S, Jiang Y, Xu K, et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carmody RN, Turnbaugh PJ. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest. 2014;124:4173–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Noble EE, Hsu TM, Kanoski SE. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front Behav Neurosci [Internet]. 2017. [cited 2020 Feb 10];11 Available from: http://journal.frontiersin.org/article/10.3389/fnbeh.2017.00009/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mulak A Brain-gut-microbiota axis in Parkinson’s disease. WJG. 2015;21:10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Friedland RP. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. JAD. 2015;45:349–362. [DOI] [PubMed] [Google Scholar]
- [21].Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: A summary report and call to action: Burden of Neurological Disease. Ann Neurol. 2017;81:479–484. [DOI] [PubMed] [Google Scholar]
- [22].Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s & Dementia. 2019;15:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu X, Raina AK, Lee H-G, et al. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004;1000:32–39. [DOI] [PubMed] [Google Scholar]
- [24].Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration: Microbiota-gut-brain axis across the lifespan. The Journal of Physiology. 2017;595:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014;69:S4–S9. [DOI] [PubMed] [Google Scholar]
- [26].Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in Immunology. 2007;19:59–69. [DOI] [PubMed] [Google Scholar]
- [27].Miraglia F, Colla E. Microbiome, Parkinson’s Disease and Molecular Mimicry. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. [DOI] [PubMed] [Google Scholar]
- [29].Wesemann DR, Portuguese AJ, Meyers RM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sabroe I, Parker L, Dower S, et al. The role of TLR activation in inflammation. J Pathol. 2008;214:126–135. [DOI] [PubMed] [Google Scholar]
- [31].Podolsky DK, Gerken G, Eyking A, et al. Colitis-Associated Variant of TLR2 Causes Impaired Mucosal Repair Because of TFF3 Deficiency. Gastroenterology. 2009;137:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barberi C, Campana S, De Pasquale C, et al. T cell polarizing properties of probiotic bacteria. Immunology Letters. 2015;168:337–342. [DOI] [PubMed] [Google Scholar]
- [33].Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. [DOI] [PubMed] [Google Scholar]
- [34].Westfall S, Pasinetti GM. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front Neurosci. 2019;13:1196.* Demonstrates the role of polyphenols in modulating the microbiome.
- [35].Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences. 2010;107:12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mazmanian SK, Liu CH, Tzianabos AO, et al. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell. 2005;122:107–118. [DOI] [PubMed] [Google Scholar]
- [37].Luczynski P, McVey Neufeld K-A, Oriach CS, et al. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. International Journal of Neuropsychopharmacology. 2016;19–pyw020.** Demonstrates the necessity of the gut microbiome on host behavior and physiology.
- [38].Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ogbonnaya ES, Clarke G, Shanahan F, et al. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015;78:e7–9. [DOI] [PubMed] [Google Scholar]
- [40].Luczynski P, Whelan SO, O’Sullivan C, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Gaspar P, editor. Eur J Neurosci. 2016;44:2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- [42].Hossain A, Menezes G, Al M, et al. Role of Gut Microbiome in the Modulation of Environmental Toxicants and Therapeutic Agents In: Debasis B, Anand S, Stohs S, editors. Food Toxicology [Internet]. Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487–2742: CRC Press; 2016. [cited 2020 Feb 11]. p. 491–518. Available from: http://www.crcnetbase.com/doi/10.1201/9781315371443-25. [Google Scholar]
- [43].Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Obata Y, Pachnis V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology. 2016;151:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rajilić-Stojanović M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9:2125–2136. [DOI] [PubMed] [Google Scholar]
- [46].Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15:189–196. [DOI] [PubMed] [Google Scholar]
- [47].Westfall S, Dinh DM, Pasinetti GM. Investigation of Potential Brain Microbiome in Alzheimer’s Disease: Implications of Study Bias. Journal of Alzheimer’s Disease. 2020;1–12. [DOI] [PubMed] [Google Scholar]
- [48].Westfall S, Lomis N, Kahouli I, et al. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74:3769–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Erny D, Hrabě de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience. 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Deretzi G, Kountouras J, Grigoriadis N, et al. From the “little brain” gastrointestinal infection to the “big brain” neuroinflammation: A proposed fast axonal transport pathway involved in multiple sclerosis. Medical Hypotheses. 2009;73:781–787. [DOI] [PubMed] [Google Scholar]
- [51].O’Mahony SM, Clarke G, Borre YE, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- [52].Wall R, Cryan JF, Ross RP, et al. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. [DOI] [PubMed] [Google Scholar]
- [53].Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Obrenovich M Leaky Gut, Leaky Brain? Microorganisms. 2018;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gruber J, Kennedy BK. Microbiome and Longevity: Gut Microbes Send Signals to Host Mitochondria. Cell. 2017;169:1168–1169. [DOI] [PubMed] [Google Scholar]
- [56].Forsyth CB, Shannon KM, Kordower JH, et al. Increased Intestinal Permeability Correlates with Sigmoid Mucosa alpha-Synuclein Staining and Endotoxin Exposure Markers in Early Parkinson’s Disease. Oreja-Guevara C, editor. PLoS ONE. 2011;6:e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Caputi V, Giron M. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. IJMS. 2018;19:1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang J, Gu X, Yang J, et al. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Frontiers in Cellular and Infection Microbiology [Internet]. 2019. [cited 2020 Feb 18];9 Available from: https://www.frontiersin.org/article/10.3389/fcimb.2019.00409/full. [DOI] [PMC free article] [PubMed]
- [59].Jackson A, Forsyth CB, Shaikh M, et al. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front Neurol. 2019;10:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gagliani N, Palm NW, de Zoete MR, et al. Inflammasomes and intestinal homeostasis: regulating and connecting infection, inflammation and the microbiota. International Immunology. 2014;26:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ma Q, Xing C, Long W, et al. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Herman FJ, Pasinetti GM. Principles of inflammasome priming and inhibition: Implications for psychiatric disorders. Brain, Behavior, and Immunity. 2018;73:66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Levy M, Thaiss CA, Zeevi D, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163:1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Park JY, Kang YW, Cho WG. Inflammasome-Mediated Inflammation in Neurodegenerative Diseases. The Open Neurology Journal. 2019;13:55–62. [Google Scholar]
- [65].Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. [DOI] [PubMed] [Google Scholar]
- [66].Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Codolo G, Plotegher N, Pozzobon T, et al. Triggering of Inflammasome by Aggregated α–Synuclein, an Inflammatory Response in Synucleinopathies. Mosley RL, editor. PLoS ONE. 2013;8:e55375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Johann S, Heitzer M, Kanagaratnam M, et al. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia. 2015;63:2260–2273. [DOI] [PubMed] [Google Scholar]
- [69].Weiss G, Rasmussen S, Zeuthen LH, et al. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism: Induction of virus defence in dendritic cells by Lactobacillus acidophilus. Immunology. 2010;131:268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Steed AL, Christophi GP, Kaiko GE, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kawashima T, Kosaka A, Yan H, et al. Double-Stranded RNA of Intestinal Commensal but Not Pathogenic Bacteria Triggers Production of Protective Interferon-β. Immunity. 2013;38:1187–1197. [DOI] [PubMed] [Google Scholar]
- [72].Giles EM, Stagg AJ. Type 1 Interferon in the Human Intestine—A Co-ordinator of the Immune Response to the Microbiota: Inflammatory Bowel Diseases. 2017;23:524–533. [DOI] [PubMed] [Google Scholar]
- [73].Taylor JM, Moore Z, Minter MR, et al. Type-I interferon pathway in neuroinflammation and neurodegeneration: focus on Alzheimer’s disease. Journal of Neural Transmission. 2018;125:797–807. [DOI] [PubMed] [Google Scholar]
- [74].Camandola S, Mattson MP. NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opinion on Therapeutic Targets. 2007;11:123–132. [DOI] [PubMed] [Google Scholar]
- [75].Kouli A, Horne CB, Williams-Gray CH. Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain, Behavior, and Immunity. 2019;81:41–51. [DOI] [PubMed] [Google Scholar]
- [76].Masanta WO, Heimesaat MM, Bereswill S, et al. Modification of Intestinal Microbiota and Its Consequences for Innate Immune Response in the Pathogenesis of Campylobacteriosis. Clinical and Developmental Immunology. 2013;2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Truax AD, Chen L, Tam JW, et al. The Inhibitory Innate Immune Sensor NLRP12 Maintains a Threshold against Obesity by Regulating Gut Microbiota Homeostasis. Cell Host & Microbe. 2018;24:364–378.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hölscher C Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. Journal of Endocrinology. 2014;221:T31–T41. [DOI] [PubMed] [Google Scholar]
- [79].Kim DS, Choi H-I, Wang Y, et al. A New Treatment Strategy for Parkinson’s Disease through the Gut–Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell Transplant. 2017;26:1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mercado NM, Collier TJ, Sortwell CE, et al. BDNF in the Aged Brain: Translational Implications for Parkinson’s Disease. Austin Neurol Neurosci. 2017;2. [PMC free article] [PubMed] [Google Scholar]
- [81].Yamane S, Inagaki N. Regulation of glucagon-like peptide-1 sensitivity by gut microbiota dysbiosis. J Diabetes Investig. 2018;9:262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kappe C, Tracy LM, Patrone C, et al. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation. 2012;9:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bercik P, Denou E, Collins J, et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology. 2011;141:599–609.e3. [DOI] [PubMed] [Google Scholar]
- [84].Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. npj Parkinson’s Disease [Internet]. 2018. [cited 2020 Feb 28];4 Available from: http://www.nature.com/articles/s41531-018-0058-0. [DOI] [PMC free article] [PubMed]
- [85].Dorsey ER, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2018;17:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Medicine [Internet]. 2018. [cited 2020 Feb 28];16 Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Annals of Neurology. 2012;72:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yang D, Zhao D, Ali Shah SZ, et al. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front Neurol. 2019;10:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Roy Sarkar S, Banerjee S. Gut microbiota in neurodegenerative disorders. Journal of Neuroimmunology. 2019;328:98–104. [DOI] [PubMed] [Google Scholar]
- [90].Rite I, Machado A, Cano J, et al. Blood brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem. 2007;101:1567–1582. [DOI] [PubMed] [Google Scholar]
- [91].Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360. [DOI] [PubMed] [Google Scholar]
- [92].Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480.e12.* Indicates role of microbiome in PD pathogenesis.
- [93].Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lukiw WJ. Bacteroides fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front Microbiol [Internet]. 2016. [cited 2020 Feb 21];7 Available from: http://journal.frontiersin.org/Article/10.3389/fmicb.2016.01544/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang L, Wang Y, Xiayu X, et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2017;60:1241–1257. [DOI] [PubMed] [Google Scholar]
- [96].Shen H, Guan Q, Zhang X, et al. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2020;100:109884.* Implicates role of microbiome in AD pathogenesis and demonstrrastes role of NLRP3 in neuroinflammation.
- [97].Wang W-Y, Tan M-S, Yu J-T, et al. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dodiya HB, Kuntz T, Shaik SM, et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. The Journal of Experimental Medicine. 2019;216:1542–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Brenner D, Hiergeist A, Adis C, et al. The fecal microbiome of ALS patients. Neurobiology of Aging. 2018;61:132–137. [DOI] [PubMed] [Google Scholar]
- [101].Fang X, Wang X, Yang S, et al. Evaluation of the Microbial Diversity in Amyotrophic Lateral Sclerosis Using High-Throughput Sequencing. Front Microbiol. 2016;7:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. [DOI] [PubMed] [Google Scholar]
- [103].Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mete A, Garcia J, Ortega J, et al. Brain Lesions Associated With Clostridium perfringens Type D Epsilon Toxin in a Holstein Heifer Calf. Vet Pathol. 2013;50:765–768. [DOI] [PubMed] [Google Scholar]
- [105].Lu K, Abo RP, Schlieper KA, et al. Arsenic Exposure Perturbs the Gut Microbiome and Its Metabolic Profile in Mice: An Integrated Metagenomics and Metabolomics Analysis. Environmental Health Perspectives. 2014;122:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Gao B, Chi L, Mahbub R, et al. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chemical Research in Toxicology. 2017;30:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rosenfeld CS. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front Cell Infect Microbiol. 2017;7:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kish L, Hotte N, Kaplan GG, et al. Environmental Particulate Matter Induces Murine Intestinal Inflammatory Responses and Alters the Gut Microbiome. Bereswill S, editor. PLoS ONE. 2013;8:e62220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Javurek AB, Spollen WG, Johnson SA, et al. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Science Translational Medicine. 2009;1:6ra14–6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Singh RK, Chang H-W, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jantchou P, Morois S, Clavel-Chapelon F, et al. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105:2195–2201. [DOI] [PubMed] [Google Scholar]
- [114].Romond MB, Ais A, Guillemot F, et al. Cell-free whey from milk fermented with Bifidobacterium breve C50 used to modify the colonic microflora of healthy subjects. J Dairy Sci. 1998;81:1229–1235. [DOI] [PubMed] [Google Scholar]
- [115].Świątecka D, Dominika Ś, Narbad A, et al. The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol. 2011;145:267–272. [DOI] [PubMed] [Google Scholar]
- [116].Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw 2014;14:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fava F, Gitau R, Griffin BA, et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int J Obes (Lond). 2013;37:216–223. [DOI] [PubMed] [Google Scholar]
- [118].Eid N, Enani S, Walton G, et al. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J Nutr Sci. 2014;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. [DOI] [PubMed] [Google Scholar]
- [120].Martínez I, Lattimer JM, Hubach KL, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kim M-S, Hwang S-S, Park E-J, et al. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep. 2013;5:765–775. [DOI] [PubMed] [Google Scholar]
- [122].Rajkumar H, Mahmood N, Kumar M, et al. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu J-E, Zhang Y, Zhang J, et al. Probiotic yogurt effects on intestinal flora of patients with chronic liver disease. Nurs Res. 2010;59:426–432. [DOI] [PubMed] [Google Scholar]
- [124].Hirschberg S, Gisevius B, Duscha A, et al. Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. International Journal of Molecular Sciences. 2019;20:3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Pérez-Jiménez J, Neveu V, Vos F, et al. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur J Clin Nutr. 2010;64 Suppl 3:S112–120. [DOI] [PubMed] [Google Scholar]
- [126].Cuervo A, Valdés L, Salazar N, et al. Pilot study of diet and microbiota: interactive associations of fibers and polyphenols with human intestinal bacteria. J Agric Food Chem. 2014;62:5330–5336. [DOI] [PubMed] [Google Scholar]
- [127].Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52:7577–7587.* Excellent review demonstrating role of Synbiotics.
- [128].Benjamin JL, Hedin CRH, Koutsoumpas A, et al. Smokers with active Crohnʼs disease have a clinically relevant dysbiosis of the gastrointestinal microbiota*: Inflammatory Bowel Diseases. 2012;18:1092–1100. [DOI] [PubMed] [Google Scholar]
- [129].Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lutgendorff F, Akkermans LMA, Söderholm JD. The role of microbiota and probiotics in stress-induced gastro-intestinal damage. Curr Mol Med. 2008;8:282–298. [DOI] [PubMed] [Google Scholar]
- [131].Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. [DOI] [PubMed] [Google Scholar]
- [132].Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Di Meo F, Donato S, Di Pardo A, et al. New Therapeutic Drugs from Bioactive Natural Molecules: The Role of Gut Microbiota Metabolism in Neurodegenerative Diseases. CDM. 2018;19:478–489. [DOI] [PubMed] [Google Scholar]
- [135].Ho L, Ono K, Tsuji M, et al. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Review of Neurotherapeutics. 2018;18:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wiciński M, Gębalski J, Mazurek E, et al. The Influence of Polyphenol Compounds on Human Gastrointestinal Tract Microbiota. Nutrients. 2020;12:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Jäger R, Mohr AE, Carpenter KC, et al. International Society of Sports Nutrition Position Stand: Probiotics. J Int Soc Sports Nutr 2019;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lievin-Le Moal V, Servin AL. Anti-Infective Activities of Lactobacillus Strains in the Human Intestinal Microbiota: from Probiotics to Gastrointestinal Anti-Infectious Biotherapeutic Agents. Clinical Microbiology Reviews. 2014;27:167–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Cassani E, Privitera G, Pezzoli G, et al. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol. 2011;57:117–121. [PubMed] [Google Scholar]
- [140].Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr 2019;38:1031–1035. [DOI] [PubMed] [Google Scholar]
- [141].Gorecki AM, Dunlop SA, Rodger J, et al. The gut-brain axis and gut inflammation in Parkinson’s disease: stopping neurodegeneration at the toll gate. Expert Opinion on Therapeutic Targets. 2020;1–4. [DOI] [PubMed] [Google Scholar]
- [142].Gill HS, Rutherfurd KJ, Cross ML, et al. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. The American Journal of Clinical Nutrition. 2001;74:833–839. [DOI] [PubMed] [Google Scholar]
- [143].Goya ME, Xue F, Sampedro-Torres-Quevedo C, et al. Probiotic Bacillus subtilis Protects against α-Synuclein Aggregation in C. elegans. Cell Reports. 2020;30:367–380.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Frontiers in Aging Neuroscience [Internet]. 2016. [cited 2020 Feb 24];8 Available from: http://journal.frontiersin.org/article/10.3389/fnagi.2016.00256/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Westfall S, Lomis N, Prakash S. Longevity extension in Drosophila through gut-brain communication. Sci Rep. 2018;8:8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Westfall S, Iqbal U, Sebastian M, et al. Gut microbiota mediated allostasis prevents stress-induced neuroinflammatory risk factors of Alzheimer’s disease Progress in Molecular Biology and Translational Science [Internet]. Elsevier; 2019. [cited 2020 Feb 27]. p. 147–181. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1877117319301036. [DOI] [PubMed] [Google Scholar]
- [147].Westfall S, Lomis N, Prakash S. A novel synbiotic delays Alzheimer’s disease onset via combinatorial gut-brain-axis signaling in Drosophila melanogaster. Broughton SJ, editor. PLOS ONE. 2019;14:e0214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bartosch S, Woodmansey EJ, Paterson JCM, et al. Microbiological Effects of Consuming a Synbiotic Containing Bifidobacterium bifidum, Bifidobacterium lactis, and Oligofructose in Elderly Persons, Determined by Real-Time Polymerase Chain Reaction and Counting of Viable Bacteria. Clinical Infectious Diseases. 2005;40:28–37. [DOI] [PubMed] [Google Scholar]
- [149].Macfarlane S, Cleary S, Bahrami B, et al. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Alimentary Pharmacology & Therapeutics. 2013;38:804–816. [DOI] [PubMed] [Google Scholar]
- [150].Tankou SK, Regev K, Healy BC, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Annals of Neurology. 2018;83:1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fasching P, Stradner M, Graninger W, et al. Therapeutic Potential of Targeting the Th17/Treg Axis in Autoimmune Disorders. Molecules. 2017;22:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Caracci F, Harary J, Simkovic S, et al. Grape-Derived Polyphenols Ameliorate Stress-Induced Depression by Regulating Synaptic Plasticity. J Agric Food Chem. 2020;68:1808–1815. [DOI] [PubMed] [Google Scholar]
- [153].Wang J, Hodes GE, Zhang H, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9:477.* Demonstrates mechanisms of polyphenol metabolites in promoting neuro-resilience.
- [154].Pasinetti GM, Wang J, Ho L, et al. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2015;1852:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Ho L, Zhao D, Ono K, et al. Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity. The Journal of Nutritional Biochemistry. 2019;64:170–181.** Demonstrates polyphenol metabolite capable of inhibiting alpha-synuclein and amyloid-beta aggregation.
- [156].Zhang F, Wang H, Wu Q, et al. Resveratrol Protects Cortical Neurons against Microglia-mediated Neuroinflammation: RESVERATROL PROTECTED AGAINST MICROGLIA-MEDIATED NEUROINFLAMMATION. Phytother Res. 2013;27:344–349. [DOI] [PubMed] [Google Scholar]