Abstract
In CML, treatment-free remission (TFR) refers to having a stable deep molecular response without the need for ongoing tyrosine kinase inhibitor treatment. Whilst recommendations exist about the technical management of stopping and re-starting therapy, much is still unknown about the experiences of those considering and undertaking TFR. This study sought to obtain the patient perspective, identify areas of unmet needs and create recommendations for improvements. Fifty-six percent of patients reported fear or anxiety during treatment discontinuation, whereas only 7% of patients were asked if they needed psychological support during this period. Where patients re-initiated treatment; 59% felt scared or anxious, and 56% felt depressed. Twenty-six percent of re-initiated patients received psychological and/or emotional support at this time. Sixty percent of patients experienced withdrawal symptoms whilst discontinuing treatment, however, 40% of patients who experienced withdrawal symptoms reported that they were not fully supported by their doctor in managing all the symptoms. Healthcare professionals should further consider how they monitor the psychological well-being of patients who are discontinuing or re-initiating treatment, and review what support is offered in response to identified concerns. Surveillance of withdrawal symptoms should be a priority during treatment discontinuation, along with how healthcare professionals assist in the management of these.
Subject terms: Chronic myeloid leukaemia, Quality of life
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder that is most commonly characterized by the presence of a Philadelphia chromosome, caused by the genetic translocation t(9;22)(q34;q11). This genetic abnormality juxtaposes two genes (BCR and ABL1), whose fusion codes for the constitutively active tyrosine kinase is BCR-ABL1. Targeting this protein with tyrosine kinase inhibitors (TKIs) such as imatinib mesylate revolutionized treatment of this disorder [1]. The use of TKIs has fundamentally improved survival rates for the majority of patients with CML and many patients can expect a near normal life expectancy [2–5]. However, patients can experience TKI-related adverse side effects [6–9], toxicities [7, 10, 11], impact on quality of life (QoL) [6], and for some, the price of the drugs [12, 13]. Furthermore, TKIs are not recommended for female patients who are trying to conceive, during pregnancy or are breastfeeding [14].
In recent years, a number of studies have been conducted to demonstrate the possibility and safety of TKI cessation in well responding patients. The results of such studies have seen born the concept of treatment-free remission (TFR). Following a pilot study [15], two pioneer studies confirmed that treatment discontinuation was feasible and safe [16, 17], and numerous clinical trials and observational studies have been reported, not only after imatinib treatment [18–31], but also after second generation TKI (2GTKI) whether used in 1st or 2nd line [23–27, 32–37]. Recently, second attempts to discontinue TKI in patients who relapsed have been reported [32, 38, 39]. Recommendations on selection of patients with a higher probability of successful discontinuation have been proposed by global experts [40–43]. However, the point of view of the patients undergoing discontinuation of treatment is less frequently reported on.
The CML Advocates Network (CMLAN) is an active international network for leaders of Chronic Myeloid Leukemia (CML) patient groups. Its aim is to facilitate and support best practice sharing among patient advocates across the world. CMLAN’s objectives in this research were to inform the development of a range of guides for patients and healthcare professionals, that consider the patient experience of discontinuing treatment. The research did not seek to replicate the formal collection of scientific data such as prognosis, or clinical requirements for stopping treatment, as this has been expertly collected through existing medical studies and clinical trials. The CMLAN study was explicitly designed to focus on the patient perspective. Therefore, the areas of investigation include subjects less frequently covered in existing scientific research, but have importance to patients. These include, but are not limited to: the patient’s reasons for discontinuing treatment, the emotional impact within the TFR journey, and what information and support was provided. Through analysis of the survey data, it was identified that there were areas within the TFR journey where opportunities exist to improve patient care and experience. This manuscript summarizes and evidences these findings.
Methods
Questionnaire design
The questionnaire was designed by an expert panel of eight CML patients and went through two rounds of testing. Information was collected across five sections: about you (demographics); considerations around stopping treatment; experiences during the first six months of stopping treatment; restarting treatment; and, ongoing long-term TFR. Patients only completed the sections that were relevant to their experiences of stopping treatment. The demographic section collected data on relevant CML patient characteristics, including gender, age and country of residence. Consideration of stopping treatment was classified as “Phase I” of the stopping treatment journey. The questions collected information about the considerations respondents took before attempting, or rejecting TFR. Patients’ experiences during the first 6 months of discontinuing treatment were classified as “Phase II”. Most molecular recurrences (relapses) happen within the first 6 months after stopping treatment, therefore this stage of the stopping process is also known as the probation phase. This section collected information on respondents’ experiences during the probation phase. The restarting treatment section was classified as “Phase IIIA” and collected information from the respondents who had stopped treatment, but subsequently had to restart. The experiences of those in long-term remission beyond the 6-month probation phase were classified as “Phase IIIB”. Questions here included information on the concerns respondents still have and what they would change about their experience of discontinuing treatment.
Across all sections of the questionnaire, the aim was to ask questions that allowed insight and understanding into what the patient had experienced, rather than the clinical perspective. Due to this focus on patient perspective, it was the authors’ opinion that the views of patients discontinuing treatment within the context of a clinical trial, were no more or less important than patients who discontinue in “real life”. Consequently, information around if respondents had participated in TFR clinical trials was not collected as part of this study. It is not believed that the conclusions are biased or weaker due to the lack of this data.
Administration
Administration of the survey was through a web-based questionnaire, between 14th March 2018 and 1st August 2018. The questionnaire was made available in 11 languages: Arabic, Danish, English, Finnish, French, German, Hebrew, Italian, Japanese, Russian and Spanish. The survey was promoted by the CMLAN, across 12 CML patient organization websites; CMLAN social media channels (Facebook, Twitter, Instagram, LinkedIn,); 64 CMLAN members’ Facebook pages; six emails to CMLAN members; three newsletters to CMLAN members; at the European Hematology Association Congress 2018; and, at the CML Horizons 2018 Conference. The project elicited 1016 responses from patients across 68 countries.
Anecdotal evidence from other survey programmes suggests that patients who are already engaged in wider discussions about treatment options (for example, through patient advocacy organizations), are more likely to complete questionnaires; and that because of this engagement (for which we can find no scientific evidence) they may report either better (because they are better informed) or worse (because poor treatment led them to seek help) experiences. In the survey design for this study, there is an explicit assumption that the experience of patients stopping treatment is not statistically associated with their involvement with patient advocacy organizations, or their desire to engage in completing a questionnaire—and therefore that there is no inherent bias in the methodology or results.
Analysis of responses
For all questions (except for those asked in the form of “tick all that apply”) the percentage responses are calculated after excluding those respondents that did not answer that question. All percentages are rounded to the nearest whole number. When added together, the answers to a particular question may not total 100% because of this rounding. Figures have been calculated excluding responses where the question was not applicable to the respondent’s circumstances, or they felt unable to give a definite answer. The base size for questions that have been asked as “tick all that apply” is determined by the number of eligible respondents. As such, the missing count for a “tick all that apply” response option represents any eligible respondents who have chosen not to select that particular option.
Limitations
The survey has several limitations. The survey was only available online. Whilst there were benefits to this methodology, it will have introduced limitations to accessibility by factors such as region and socioeconomic status. Respondents were self-selected, participated on a voluntary basis and were recruited through patient organizations, therefore cannot reflect the perspectives of all CML patients.
Results
Demographics
1016 responses were collected across 68 countries. Fifty-five percent (n = 550) of respondents were female, the median age was 53 (with a range of 16–92); education levels were varied. Respondents were grouped into the designated World Health Organization (WHO) regions; 56% (n = 563) were from countries assigned to the European region. Sixty percent (n = 608) of respondents reported that their main place of treatment was a hospital, and 39% (n = 389) of respondents had been living with CML for 10 years or more. Forty-nine percent (n = 494) of respondents proceeded to stop treatment. Full demographics of respondents at each phase are shown in Table 1. Fifty-one percent (n = 242) of respondents who stopped treatment reported that the medication they were taking before stopping was imatinib. Full range of responses are shown in Table 2.
Table 1.
Demographics of the respondents.
| Variables | Phase I | Phase II | Phase IIIA | Phase IIIB |
|---|---|---|---|---|
| N % | N % | N % | N % | |
| Gender | ||||
| Male | 453 (45) | 196 (40) | 51 (32) | 98 (49) |
| Female | 550 (55) | 293 (60) | 106 (68) | 103 (51) |
| Age (at the time when the survey was completed) | ||||
| Median, range (years) | 53 (16–92) | 56 (21–92) | 56 (26–82) | 57 (21–85) |
| Education | ||||
| No formal qualifications | 50 (5) | 19 (4) | 10 (6) | 2 (1) |
| High school qualifications or High school diploma | 228 (23) | 107 (22) | 30 (19) | 48 (24) |
| University—Bachelors or Undergraduate degree | 361 (36) | 160 (33) | 44 (28) | 62 (31) |
| University—Masters or PHD | 208 (21) | 112 (23) | 41 (26) | 45 (22) |
| Career or technical qualifications | 160 (16) | 92 (19) | 32 (20) | 44 (22) |
| WHO Region (grouped from country of residence when the survey was completed) | ||||
| Africa (AFRO) | 13 (1) | 2 (0) | 0 (0) | 1 (0) |
| Eastern Mediterranean (EMRO) | 27 (3) | 9 (2) | 3 (2) | 3 (1) |
| Europe (EURO) | 563 (56) | 330 (67) | 111 (70) | 132 (66) |
| Pan Americas (PAHO) | 171 (17) | 79 (16) | 23 (15) | 36 (18) |
| South-East Asia (SEARO) | 33 (3) | 3 (1) | 0 (0) | 2 (1) |
| Western Pacific (WPRO) | 198 (20) | 67 (14) | 21 (13) | 27 (13) |
| Place of treatment (tick all that apply) | ||||
| Community/family doctor | 52 (5) | 26 (5) | 6 (4) | 15 (7) |
| Hospital | 608 (60) | 255 (52) | 85 (53) | 87 (43) |
| Specialist all cancers center | 221 (22) | 120 (24) | 43 (27) | 49 (24) |
| CML centre of excellence | 214 (21) | 135 (27) | 43 (27) | 65 (32) |
| Other | 53 (5) | 26 (5) | 8 (5) | 12 (6) |
| Time after diagnosis of CML | ||||
| Less than 1 year | 34 (3) | 3 (1) | 1 (1) | 1 (1) |
| 1–3 years | 120 (12) | 8 (3) | 2 (1) | 2 (1) |
| 3–5 years | 140 (14) | 49 (10) | 14 (9) | 13 (7) |
| 5–10 years | 308 (31) | 183 (38) | 66 (43) | 62 (32) |
| 10 years or more | 389 (39) | 237 (49) | 72 (46) | 115 (60) |
| Discontinued treatment | ||||
| Yes | 494 (49) | |||
| No | 522 (51) | |||
Table 2.
CML treatment.
| Variables | Not discontinued | Discontinued |
|---|---|---|
| N % | N % | |
| Medication | ||
| Imatinib (Glivec/Gleevec or generic Imatinib) | 263 (56) | 242 (51) |
| Nilotinib (Tasigna) | 100 (21) | 141 (29) |
| Dasatinib (Sprycel) | 88 (19) | 75 (16) |
| Bosutinib (Bosulif) | 9 (2) | 5 (1) |
| Ponatinib (Iclusig) | 3 (1) | 1 (0) |
| Interferon Alpha | 0 (0) | 14 (3) |
| ABL001/Asciminib | 0 (0) | 0 (0) |
| Other | 9 (2) | 1 (0) |
Key findings in Phase I—the consideration phase
All 1016 respondents were asked to complete the Phase I section of the questionnaire.
Most respondents reported first hearing about the possibility of stopping treatment through a healthcare professional 49% (n = 476), followed by 21% (n = 209) who heard through a patient organization. When asked to select the main reasons that made them consider attempting TFR, 51% (n = 516) said they wanted to get rid of current treatment side effects, and 48% (n = 488) wanted to see if they could be free of CML without therapy. Seventeen percent (n = 168) reported they considered stopping because their doctor proposed they join a “stopping treatment” clinical trial. The full range of responses is shown in Table 3.
Table 3.
Motivation for discontinuing treatment.
| Variables | N % |
|---|---|
| Where first heard about the possibility of stopping treatment | |
| Healthcare professional | 476 (49) |
| Patient organization | 209 (21) |
| Family | 4 (0) |
| Printed materials (e.g. brochures or leaflet) | 7 (1) |
| Media (e.g. scientific articles or lay press) | 28 (3) |
| Internet | 120 (12) |
| Social media (e.g. Facebook or web based group) | 81 (8) |
| Other | 51 (5) |
| Main reasons for considering stopping treatment (tick all that apply) | |
| To get rid of current treatment side effects | 516 (51) |
| The fear of side effects caused by long-term treatment | 429 (42) |
| Not needing to take medication everyday | 378 (37) |
| To see if can be free of CML without therapy | 488 (48) |
| Doctor proposed joining a “stopping treatment” clinical trial | 168 (17) |
| Financial reasons—reduction of costs | 98 (10) |
| Planned or unplanned pregnancy | 105 (10) |
| Other | 72 (7) |
Respondents were asked which topics they discussed with their doctor whilst making their decision to try stopping treatment: 60% (n = 606) of respondents discussed risks of stopping; 50% (n = 513) discussed the requirements to be met in order to stop; 48% (n = 490) discussed the benefits of stopping; 34% (n = 346) discussed the timing of when to stop; 21% (n = 215) discussed the drug withdrawal symptoms. Fourteen percent (n = 143) did not have a discussion with their doctor. Following the discussion with their doctor, 55% (n = 555) of respondents said they still had concerns about TFR being unsuccessful, and their disease reoccurring.
Respondents were asked to indicate the reasons that made them worry about stopping treatment. Fifty-seven percent (n = 579) reported recurrence of CML; 20% (n = 203) said they would not feel safe going off treatment; 16% (n = 158) did not have enough information about stopping treatment; 12% (n = 120) had a fear of withdrawal symptoms; 7% (n = 70) said there is a lack of proper quality PCR monitoring. Twenty-six percent (n = 261) reported that they were not worried about stopping treatment.
Seventy-three percent (n = 591) of respondents said their doctor supported their decision to try stopping treatment. Sixty-one percent (n = 624) of respondents reported they received support and information about stopping treatment from their doctor or another healthcare professional; 32% (n = 327) said they received it from a patient organization; 30% (n = 303) from other CML patients who have stopped treatment; and, 29% (n = 299) from the internet.
When asked what information about stopping treatment and TFR they would have liked to have received: 52% (n = 533) of respondents would have liked information on results from clinical trials; 49% (n = 499) general information on all the steps of the stopping treatment journey; 44% (n = 452) the expectations of the risks and opportunities of stopping treatment; 40% (n = 407) the withdrawal effects after stopping; 36% (n = 368) the side effects on restarting therapy; 32% (n = 330) the required PCR monitoring; and, 22% (n = 227) the psychological effects.
Key findings in Phase II—the probation phase
At the time of the study, no (published) consensus concerning the minimal requirements for TFR had been reached. Subsequent recommendations published by European LeukemiaNet [43] advise minimal requirements for TFR that include duration of TKI therapy >5 years (>4 years for 2GTKI) and duration of deep molecular response (DMR) (MR4 or better) >2 years. Seventy-seven percent (n = 359) of respondents reported that they had been on CML medication for ≥5 years, the median was 7 years (with a range of 1–27). Seventy-eight percent (n = 382) said they had been in DMR of at least MR4, or BCR-ABL transcript levels below 0.01%, for over 2 years before stopping. Table 4 illustrates the full range of responses.
Table 4.
Duration of treatment and deep molecular response reported by respondents to Phase II.
| Variables | N % |
|---|---|
| Time on CML treatment (years) | |
| 1 | 8 (2) |
| 2 | 15 (3) |
| 3 | 44 (9) |
| 4 | 40 (9) |
| 5 | 61 (13) |
| 6 | 33 (7) |
| 7 | 42 (9) |
| 8 | 47 (10) |
| 9 | 35 (8) |
| 10 | 34 (7) |
| 11 | 19 (4) |
| 12 | 25 (5) |
| 13 | 22 (5) |
| 14 | 21 (5) |
| 15 | 9 (2) |
| 16 | 2 (0.4) |
| 17 | 2 (0.4) |
| 18 | 1 (0.2) |
| 19 | 1 (0.2) |
| 24 | 2 (0.4) |
| 25 | 1 (0.2) |
| 26 | 1 (0.2) |
| 27 | 1 (0.2) |
| Time in deep molecular response (DMR) in years | |
| DMR defined as at least MR4.0 | |
| Not in DMR | 7 (2) |
| < 1 | 16 (3) |
| 1–2 | 55 (12) |
| 2–3 | 90 (20) |
| 3–4 | 84 (18) |
| 4–8 | 124 (27) |
| >8 | 84 (18) |
Fifty-six percent (n = 266) of respondents said that they “completely” had all the information they wanted when they stopped treatment. When asked what topics they discussed with their doctor during Phase II, 39% (n = 168) of respondents reported having a discussion on how to deal with withdrawal symptoms. Forty-seven percent (n = 222) reported that their doctor or another healthcare professional asked them if they were experiencing any physical withdrawal symptoms whilst stopping treatment; 27% (n = 128) were not asked, but would have liked to have been; and 26% (n = 121) were not asked but did not feel it was necessary. Sixty percent (n = 288) of respondents reported experiencing withdrawal symptoms; of these, 32% (n = 91) said they continued for a few months after stopping. Of those experiencing withdrawal symptoms: 89% (n = 257) reported withdrawal pain in muscles, joints or bones; 51% (n = 147) tiredness; 26% (n = 76) depressive episodes, fear or bad mood; 26% (n = 74) sweating or skin problems; and, 11% (n = 31) weight loss. Forty percent (n = 112) of the respondents who experienced side effects, reported that their doctor did not support them in managing all their physical withdrawal effects during discontinuation of treatment.
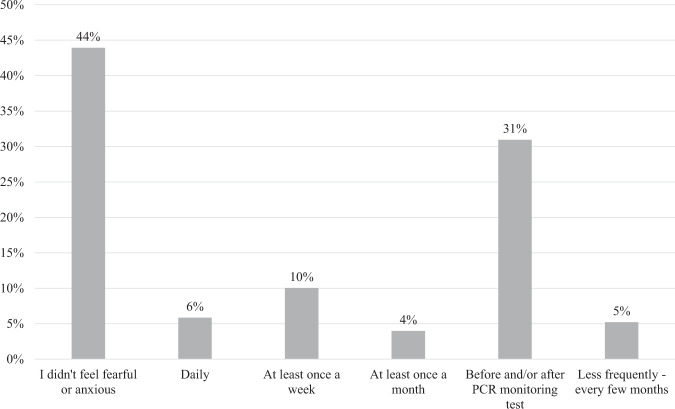
When asked what topics they discussed with their doctor during the stopping phase, 18% (n = 69) of respondents reported having a discussion on how to deal with psychological aspects. Seventy-four percent (n = 268) reported that one of the benefits of stopping treatment was that it had a positive impact on their emotional wellbeing, however, 56% (n = 268) of respondents said that they felt fear or anxiety at some point during the stopping phase, and 55% (n = 148) of these reported this happened around the time of their PCR monitoring tests. Figure 1 illustrates the full range of responses.
Fig. 1. Frequency of fear or anxiety during Phase II.
This chart illustrates how patients responded when asked how frequently they experienced fear or anxiety during Phase II.
Seven percent (n = 32) of respondents reported that their doctor asked them if they needed psychological support whilst stopping treatment; 26% (n = 120) were not asked but would have liked to have been; 67% (n = 312) were not asked, but did not feel it was necessary. Twenty percent (n = 93) of respondents reported that they did receive psychological and/or emotional support during discontinuation of treatment; 23% (n = 110) did not receive any, but would have liked to; and 57% (n = 269) did not receive any, but felt it was not necessary. Of the respondents who said they had received psychological and/or emotional support: 71% (n = 66) reported receiving support from friends and family; 26% (n = 24) from patient organization/s; 25% (n = 23) from a social media group; and, 20% (n = 19) received counseling.
During Phase II, 92% (n = 426) of respondents reported having a discussion on how often to monitor their BCR-ABL transcript levels, 76% (n = 339) discussed response levels and when/if to restart treatment and 70% (n = 294) discussed the time taken to receive the results of the last PCR test. Sixty-four percent (n = 308) of respondents reported that on average they were monitored monthly by their doctor through PCR tests during the probation phase, and 34% (n = 165) were monitored less frequently.
Key findings in Phase IIIA—the restarting treatment phase
Of the 494 respondents who reported having stopped treatment, 32% (n = 159) reported that their disease had reoccurred, and treatment had to restart. Seventy-seven percent (n = 119) of patients reported that their disease reoccurred within the first 6 months of stopping treatment.
When they were first told their disease had reoccurred: 59% (n = 81) of respondents strongly agreed/agreed that they felt scared/anxious; 91% (n = 138) strongly agreed/agreed that they felt disappointed; 56% (n = 74) strongly agreed/agreed that they felt depressed; 20% (n = 25) strongly agreed/agreed that they felt confused; and, 4% (n = 5) strongly agreed/agreed that they felt relieved.
When asked how they felt emotionally in the first few weeks after restarting treatment, 35% (n = 55) of respondents reported feeling emotionally worse at this point compared with before stopping, and 30% (n = 48) reported feeling emotionally worse than during stopping treatment.
Respondents were asked to rate side effects by the extent they affected their everyday life in the weeks before stopping treatment, and then again when they restarted treatment. A score of 1 indicated that the side effect mildly affected their everyday life, and 5 indicated that the side effect completely affected their everyday life. No response indicated they did not experience the side effect. A mean score was calculated for each side effect. The side effects that showed the biggest mean score increase in impact between before stopping and restarting were feeling sad, feeling distressed, and anxiety (Table 5).
Table 5.
Severity of side effects reported by Phase IIIA respondents, before stopping and after restarting treatment.
| Variables | N experienced before stopping treatment | % experienced before stopping treatment | severity score before stopping treatment | N experienced after restarting treatment | % experienced after restarting treatment | severity score after restarting treatment | Difference of severity score after restarting treatment | % difference of severity score after restarting treatment |
|---|---|---|---|---|---|---|---|---|
| Side effect | ||||||||
| Feeling sad | 85 | (53) | 2.14 | 86 | (54) | 2.73 | 0.59 | (28) |
| Feeling distressed (upset) | 83 | (52) | 2.34 | 84 | (53) | 2.90 | 0.57 | (24) |
| Anxiety | 84 | (53) | 2.31 | 91 | (57) | 2.80 | 0.49 | (21) |
| Headaches | 81 | (51) | 2.14 | 77 | (48) | 2.48 | 0.34 | (16) |
| Lack of appetite | 70 | (44) | 1.53 | 66 | (42) | 1.77 | 0.24 | (16) |
| Disturbed sleep | 98 | (62) | 2.80 | 96 | (60) | 3.03 | 0.24 | (08) |
| Diarrhoea | 89 | (56) | 2.12 | 81 | (51) | 2.31 | 0.19 | (09) |
| Feeling of malaise (not feeling well) | 88 | (55) | 2.63 | 86 | (54) | 2.79 | 0.17 | (06) |
| Hair loss | 89 | (56) | 2.31 | 76 | (48) | 2.46 | 0.15 | (06) |
| Bruising | 77 | (48) | 1.57 | 74 | (47) | 1.69 | 0.12 | (07) |
| Difficulty thinking clearly | 89 | (56) | 2.44 | 92 | (58) | 2.53 | 0.09 | (04) |
| Vomiting | 73 | (46) | 1.48 | 63 | (40) | 1.57 | 0.09 | (06) |
| Nausea | 88 | (55) | 2.38 | 82 | (52) | 2.46 | 0.09 | (04) |
| Menstrual cycle issues | 69 | (43) | 1.83 | 55 | (35) | 1.85 | 0.03 | (02) |
| Shortness of breath | 95 | (60) | 2.44 | 80 | (50) | 2.46 | 0.02 | (01) |
| Pain | 89 | (56) | 2.72 | 79 | (50) | 2.72 | 0.00 | (0) |
| Fatigue (tiredness) | 125 | (79) | 3.30 | 121 | (76) | 3.31 | 0.00 | (0) |
| Dry mouth | 81 | (51) | 2.22 | 78 | (49) | 2.22 | 0.00 | (0) |
| Rash or skin change | 95 | (60) | 2.69 | 82 | (52) | 2.67 | −0.02 | −(01) |
| Numbness or tingling | 90 | (57) | 2.34 | 74 | (47) | 2.31 | −0.03 | −(01) |
| Remembering things | 94 | (59) | 2.54 | 83 | (52) | 2.48 | −0.06 | −(02) |
| Eye bleeds | 90 | (57) | 1.86 | 77 | (48) | 1.77 | −0.09 | −(05) |
| Swelling of hands, feet, abdomen, and around eyes | 101 | (64) | 2.86 | 91 | (57) | 2.65 | −0.21 | −(07) |
| Skin pigment changes | 84 | (53) | 2.52 | 70 | (44) | 2.29 | −0.24 | −(09) |
| Muscle soreness or cramping | 113 | (71) | 3.25 | 103 | (65) | 2.97 | −0.28 | −(09) |
| Other | 16 | (10) | 2.63 | 14 | (09) | 1.50 | −1.13 | −(43) |
Twenty-six percent (n = 40) of respondents reported that they did receive psychological and/or emotional support during the restarting of treatment; 25% (n = 39) did not receive any, but would have liked to; and, 48% (n = 74) did not receive any, but felt it was not necessary. Of the respondents who said they had received psychological and/or emotional support: 85% (n = 34) reported receiving support from friends and family; 25% (n = 10) received counseling; 20% (n = 8) reported receiving support from a social media group; and, 18% (n = 7) received support from patient organization/s.
Key findings in Phase IIIB—the long-term remission phase
Sixty-seven percent (n = 132) of respondents reported feeling that overall, they receive adequate care in the long-term TFR phase; 30% (n = 60) reported that this happens to some extent; and, 3% (n = 6) said that they do not receive adequate care.
When asked how they felt their doctor could improve their experience of stopping treatment, 47% (n = 95) of respondents reported they felt no improvement was necessary; 32% (n = 65) wanted more information on current data in easy to understand language; 11% (n = 23) wanted better PCR monitoring; 10% (n = 21) wanted a better doctor/patient relationship; and, 10% (n = 21) wanted better psychological support.
Respondents reported the major concerns they had while in Phase IIIB. Fifty-eight percent (n = 118) reported that late reoccurrence of CML was a major concern; 34% (n = 69) had uncertainty about the future in terms of CML; 29% (n = 58) were concerned over the misunderstanding of people thinking that they are cured; 26% (n = 52) had concerns over late detection of a reoccurrence; and, 4% (n = 9) were concerned that they have more frequent PCR tests than before stopping. Twenty-eight percent (n = 57) reported not having any concerns.
Twenty-five percent (n = 49) of respondents reported that they do receive psychological and/or emotional support during the long-term remission phase; 16% (n = 32) do not receive any, but would like to; and, 59% (n = 116) do not receive any, but feel it is not necessary.
Of the respondents who said they receive psychological and/or emotional support: 76% (n = 37) reported receiving support from friends and family; 33% (n = 16) from a social media group; 31% (n = 15) from patient organization/s; and, 16% (n = 8) receive counseling.
Advice from patients
All patients (n = 494) who attempted stopping treatment (whether successful or not) were asked what their advice would be to other CML patients who are considering stopping treatment. Seventy-nine percent (n = 392) advised to always be well informed about your PCR results and treatment options; 69% (n = 342) advised to look for the best doctor with experience in stopping treatment; 59% (n = 290) advised to talk with other patients who have stopped, or are considering stopping; 51% (n = 254) advised to receive information from patient organizations about stopping treatment; 50% (n = 248) advised to look for simple and good information about each step of stopping treatment; 33% (n = 162) advised to get emotional support; and, 25% (n = 125) advised to get psychological support.
Discussion and conclusion
To our knowledge this is the largest study conducted to gain knowledge about undertaking TFR that is taken purely from the perspective of the patient. There is existing research on patient perceptions of TFR [44–47], however, these focus on motivation and concerns about undertaking TFR. We believe this study is one of a limited number that investigates the experience of patients throughout all stages, and in particular looks at the support and management of the process beyond that of the management of PCR monitoring.
Psychological and emotional impact of TFR
Our results evidence that respondents in the probation phase particularly felt fear or anxiety around the time of their PCR monitoring tests, an occurrence that is often dubbed “PCR-itis” (or scanxiety, in solid tumors) and is known to affect patients with cancer [48]. The implications of test results for TFR patients are perhaps magnified when they are not on treatment, as not only is there the potential to “fail” at TFR, but to “lose” a level of major molecular response that had previously been maintained for many years. With recommendations stating that monitoring should take place monthly in this phase [41–43], there is the potential for fear and anxiety to be frequent at this point in the TFR journey. However, only a small proportion of respondents said they discussed how to deal with psychological aspects with their doctor, and even fewer said that their doctor asked them if they needed psychological support during this phase.
The data also suggests that the requirement to restart treatment can have a strong negative psychological impact on patients, and that the desire for psychological support will be greater in the restarting treatment phase. In Phase IIIA, when they were first told they had molecular reoccurrence, many respondents reported feeling scared/anxious, and depressed. In addition, there were respondents who reported feeling emotionally worse during this phase than before discontinuation, and during discontinuation of treatment. There was also an increase in the reported severity of the impact of the emotional side effects of anxiety, feeling distressed and feeling upset in this phase, compared with before discontinuation of treatment. These results echo those of Sogawa et al. [49], who reported significantly higher anxiety and depression on the Hospital Anxiety and Depression Scale (HADS), at reintroduction of TKI than at the point of TKI discontinuation.
Despite the reported negative impact on psychological and emotional wellbeing, the data shows there were patients who wanted emotional support during the probation and restarting phases but did not receive it.
Whilst psychological support seems to be less of an issue in Phase IIIB (long-term TFR, ongoing past the probation period), late reoccurrence was reported as a major concern by a majority of respondents.
The survey did not collect information on what specifically caused concerns during the probation and restarting phases, and further investigation into this would be beneficial for the healthcare and wider CML communities.
This study’s findings support existing recommendations [50] that psychological wellbeing of CML patients attempting TFR should be a consideration of healthcare professionals and form part of routine monitoring.
TKI withdrawal
Many of the respondents who stopped treatment reported that they experienced withdrawal symptoms. However, results indicate that patients did not always discuss the possibility of drug withdrawal symptoms with their doctor before making their decision to stop treatment. During the probation phase there were patients who did not have a discussion with their doctor about how to deal with withdrawal symptoms. Furthermore, during the discontinuation of treatment not all patients were asked if they were experiencing withdrawal effects, and many of these would have liked to have been. Where respondents experienced physical withdrawal symptoms during the discontinuation of treatment, results indicate there are instances where there could be further doctor support in the management of these. With clinical study data suggesting that up to 30% of patients who stop treatment will experience withdrawal symptoms [35, 51], there is a strong indication that importance needs to be placed on the issues of support and management of TKI withdrawal.
Conclusion
Our research indicates that there are points in the TFR journey where patients do not always get all the advice, information or support they want around psychological issues and TKI withdrawal.
Results show that patients in the probation and restarting phases are likely to experience a negative impact on their mental well-being in the form of fear, worry, depression or anxiety. However, doctors do not always discuss the potential impact discontinuing treatment may have on a patient’s mental well-being, or ask them if they need psychological support. It is our recommendation that doctors should discuss mental health with patients early in the consideration and/or probation phases, so they are aware of and prepared for changes. Doctors should decide with the patient if it is appropriate to monitor their mental health, particularly during the probation period and treatment re-initiation. Further investigation into how mental health can be monitored during the TFR journey should be a consideration of future studies.
In addition, it is our recommendation that doctors should discuss what psychological support may be suitable and/or available for patients. While formal medical psychological intervention may not be necessary, signposting to other sources of support should be considered.
Research shows that TKI withdrawal is a possibility for patients who discontinue treatment. However, our results show that this is not always discussed when patients are making their decision to attempt TFR. In addition, once patients have discontinued treatment they are not always asked if they are experiencing withdrawal symptoms or offered support to manage them. It is our recommendation that healthcare professionals should address this by informing patients of the potential for withdrawal symptoms early in the TFR decision process. Once treatment has been discontinued, doctors should monitor for known withdrawal symptoms, encourage patients to report any symptoms they experience, and offer advice and support to on how to manage these.
Supplementary information
TFR Patient Experience Questionnaire - English
Acknowledgements
The authors would like to thank all member organizations of the CML Advocates Network for their assistance in promoting the survey, and the CML patients who participated in the research.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Compliance with ethical standards
Conflict of interest
GS reports: consultancy for Leukemia Patient Advocates Foundation, Israeli CML patient organization, Novartis, Incyte, AbbVie, Takeda, Bristol-Myers Squibb, Celgene and Pfizer; advisory board membership for CML Advocates Network, Leukemia Patient Advocates Foundation and Israeli CML patient organization; organizational grant funding from Novartis, Incyte, Amgen, Bristol-Myers Squibb, Celgene, AOP Orphan, CTI, Medison, Roche, Janssen, Pfizer, Takeda and Gilead. CM reports: employment for Leukemia Patient Advocates Foundation; consultancy for Psoriasis en Red and AbbVie; advisory board membership for Psoriasis en Red and FFpaciente; organizational grant funding from AbbVie, Leo Pharma, UCB, Celgene, Pfizer, Novartis Oncology, Incyte, Takeda, Bristol-Myers Squibb, The Leukemia & Lymphoma Society and International CML Foundation. JB reports employment for Quality Health Ltd. ZPW reports: employment for Leukemia Care; equity ownership of Patient Evidence; consultancy for Acute Leukemia Advocates Network, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Novartis and Pfizer; advisory board membership for Acute Leukemia Advocates Network and CML Advocates Network; speakers bureau for Amgen, Bristol-Myers Squibb, Gilead, Jazz, Novartis and Pfizer; organizational grant funding from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Incyte, Jazz, Janssen, Kyowa Kirin, Novartis and Takeda. FB reports: consultancy for Abbvie, Bristol-Myers Squibb, Celgene, Jazz, Novartis, Takeda; board membership of AIL Torino onlus, CML Advocates Network, CLL Advocates Network, MPN Advocates Network; organizational grant funding from Abbvie, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Janssen, Novartis, Pfizer, Roche, Takeda. ROC reports: consultancy for LYLE (patient organization for Lymphoma, Leukemia & MDS), Autisme 4700 DK, Bristol-Myers Squibb, Incyte, Janssen, Novartis Nordic, Roche and Takeda; board membership of LYLE and Autisme 4700 DK; organizational grant funding from Abbvie, Gilead, Incyte, Janssen, Novartis Nordic, Pfizer, Roche and Takeda. BG reports: consultancy for Bristol-Myers Squibb, Incyte, Novartis, Takeda and Pfizer; board membership of AMAL (Association des malades atteints de leucémies); organizational grant funding from Novartis. ND reports Honoraria from Novartis. MD reports no competing interests. JG reports: consultancy for AMGEN, Bristol-Myers Squibb, Bayer, Biomarin, Grünenthal, Janssen, Novartis, Pfizer, Roche, Servier, Takeda and UCB; board membership of CML Advocates Network, Leukemia Patient Advocates Foundation and LeukaNET; contributions to IMI-funded consortium projects HARMONY and EUPATI by EFPIA companies; organizational grant funding from Incyte, Novartis, Pfizer, Bristol-Myers Squibb and Takeda.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Celia Marin, Email: celia@cmladvocates.net.
Jennifer A. Bradley, Email: jennie.bradley@quality-health.co.uk
Supplementary information
The online version of this article (10.1038/s41375-020-0867-0) contains supplementary material, which is available to authorized users.
References
- 1.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–53. [DOI] [PubMed]
- 2.Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103:553–61. doi: 10.1093/jnci/djr060. [DOI] [PubMed] [Google Scholar]
- 3.Björkholm M, Ohm L, Eloranta S, Derolf Å, Hultcrantz M, Sjöberg J, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29:2514–20. doi: 10.1200/JCO.2011.34.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hehlmann R, Müller M, Lauseker M, Hanfstein B, Fabarius A, Schreiber, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose Imatinib: results from the randomized CML-study IV. J Clin Oncol. 2013;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 5.Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29:1823–31. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 6.Efficace F, Baccaran M, Breccia M, Alimena G, Rosti G, Cottone F, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118:4554–60. doi: 10.1182/blood-2011-04-347575. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in philadelphia chromosome–positive leukemias. N. Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 9.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 10.Kalmanti L, Saussele S, Lauseker M, Müller MC, DietzC T, Heinrich L, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. 5. Leukemia. 2015;29:1123–32. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 11.Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–21. doi: 10.1038/leu.2013.70. [DOI] [PubMed] [Google Scholar]
- 12.Hochhaus A. Educational session: managing chronic myeloid leukemia as a chronic disease. Hematol Am Soc Hematol Educ Program. 2011;2011:128–35. doi: 10.1182/asheducation-2011.1.128. [DOI] [PubMed] [Google Scholar]
- 13.Kanavos P, Vandoros S, Garcia-Gonzale P. Benefits of global partnerships to facilitate access to medicines in developing countries: a multi-country analysis of patients and patient outcomes in GIPAP. Glob Health. 2009;5:19. doi: 10.1186/1744-8603-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Chronic Myelogenous Leukemia. NCCN Clinical Practice Guidelines in Oncology Version 2.2020. National Comprehensive Cancer Network, 2020:45–6.
- 15.Rousselot P, Huguet F, Rea D, Legros L, Cayuel JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2006;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 16.Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 17.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–22. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi N, Taiichi K, Maeda Y, Sugihara T, Usuki K, Kawaguchi T, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97:903–6. doi: 10.3324/haematol.2011.056853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet BR, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. Blood. 2013;122:381. doi: 10.1200/JCO.2012.48.5797. [DOI] [PubMed] [Google Scholar]
- 20.Mori S, Vagge E, le Coutre P, Abruzzese E, Martino B, Pungolino E, et al. Age and PCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am J Hematol. 2015;90:910–4. doi: 10.1002/ajh.24120. [DOI] [PubMed] [Google Scholar]
- 21.Lee SE, Choi SY, Song HY, Kim SH, Choi MY, Park JS, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101:717–23. doi: 10.3324/haematol.2015.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrero D, Cerrano M, Crisà E, Aguzzi C, Giai V, Boccadoro M. How many patients can proceed from chronic myeloid leukaemia diagnosis to deep molecular response and long-lasting imatinib discontinuation? A real life experience. Br J Haematol. 2017;176:669–71. doi: 10.1111/bjh.13983. [DOI] [PubMed] [Google Scholar]
- 23.Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57. doi: 10.1016/S1470-2045(18)30192-X. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Boluda JC, Pereira A, Pastor-Galán I, Alvarez-Larrán A, Savchuk A, Puerta JM, et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J. 2018;8:91. doi: 10.1038/s41408-018-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerveira N, Loureiro B, Bizarro S, Correia C, Torres L, Lisboa S, et al. Discontinuation of tyrosine kinase inhibitors in CML patients in real-world clinical practice at a single institution. BMC Cancer. 2018;18:1245. doi: 10.1186/s12885-018-5167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi N, Tauchi T, Kitamura K, Miyamura K, Saburi Y, Hatta Y, et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int J Hematol. 2018;107:185–93. doi: 10.1007/s12185-017-2334-x. [DOI] [PubMed] [Google Scholar]
- 27.Iino M, Yamamoto T, Sakamoto Y. Outcomes of unplanned tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia: retrospective analysis of real-world experience in a single institution. Hematology. 2019;24:355–61. doi: 10.1080/16078454.2019.1590964. [DOI] [PubMed] [Google Scholar]
- 28.Fava C, Rege-Cambrin G, Dogliotti I, Cerrano M, Berchialla P, Dragani M, et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;104:1589–96. doi: 10.3324/haematol.2018.205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamoun K, Kantarjian H, Atallah R, Gonzalez GN, Issa GC, Rios MB, et al. Tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia: a single-institution experience. J Hematol Onco. 2019;12:1. doi: 10.1186/s13045-018-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujisawa S, Ueda Y, Usuki K, Kobayashi H, Kondo E, Doki N, et al. Feasibility of the imatinib stop study in the Japanese clinical setting: delightedly overcome CML expert stop TKI trial (DOMEST Trial) Int J Clin Onco. 2019;24:445–53. doi: 10.1007/s10147-018-1368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolini FE, Dulucq S, Boureau L, Cony-Makhoul P, Charbonnier A, Escoffre-Barbe M, et al. Evaluation of residual disease and TKI duration are critical predictive factors for molecular recurrence after stopping imatinib first-line in chronic Phase CML patients. Clin Cancer Res. 2019;25:6606–13. doi: 10.1158/1078-0432.CCR-18-3373. [DOI] [PubMed] [Google Scholar]
- 32.Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. 7. Blood. 2017;129:846–54. doi: 10.1182/blood-2016-09-742205. [DOI] [PubMed] [Google Scholar]
- 33.Okada M, Imagawa J, Tanaka H, Nakamae H, Hino M, Murai K, et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk. 2018;18:353–60. doi: 10.1016/j.clml.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Mahon FX, Boquimpani C, Kim DW, Benyamini N, Clementino NCD, Shuvaev V, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461–70. doi: 10.7326/M17-1094. [DOI] [PubMed] [Google Scholar]
- 35.Ross DM, Masszi T, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–54. doi: 10.1007/s00432-018-2604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumagai T, Nakaseko C, Nishiwaki K, Yoshida C, Ohashi K, Takezako N, et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018;109:182–92. doi: 10.1111/cas.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi N, Nishiwaki K, Nakaseko C, Aotsuka N, Sano K, Ohwada C, et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018;103:1835–42. doi: 10.3324/haematol.2018.194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legros L, Rousselot P, Giraudier S, Tulliez M, Huguet F, Nicolini F, et al. Second attempt to discontinue imatinib in CP-CML patients with a second sustained complete molecular response. Blood. 2012;120:1959–60. doi: 10.1182/blood-2012-02-408229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123:4403–10. doi: 10.1002/cncr.30885. [DOI] [PubMed] [Google Scholar]
- 40.Saussele S, Richter J, Guilhot J, Hjorth-Hansen H, Medina de Almeida A, Janssen JJWM, et al. “Duration of deep molecular response” has most impact on the success of cessation of tyrosine kinase inhibitor treatment in chronic myeloid leukemia - results from the EURO-SKI trial. Blood. 2017;130:313–313. [Google Scholar]
- 41.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23. doi: 10.1182/blood-2016-01-694265. [DOI] [PubMed] [Google Scholar]
- 42.Rea D, Ame S, Berger M, Cayuela JM, Charbonnier A, Coiteux V, et al. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: Recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer. 2018;124:2956–63. doi: 10.1002/cncr.31411. [DOI] [PubMed] [Google Scholar]
- 43.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanford D, Kyle R, Lazo-Langner A, Xenocostas A, Chin-Yee I, Howson-Jan K, et al. Patient preferences for stopping tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Onco. 2014;21:e241–e249. doi: 10.3747/co.21.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boquimpani CM, Szczudlo T, Mendelson E, Benjamin K, Masszi T. Attitudes and perceptions of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) toward treatment-free remission (TFR) Blood. 2014;124:4547–4547. [Google Scholar]
- 46.Villemagne Sanchez LA, O’Callaghan C, Gough K, Hal K, Kashima Y, Seymour JF, et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59:406–15. doi: 10.1080/10428194.2017.1337114. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg S, Hamarman S. Patients with chronic myelogenous leukemia may not want to discontinue tyrosine kinase inhibitor therapy. Blood. 2015;126:1584–1584. [Google Scholar]
- 48.Cho J, Kang D, Park S, Jeong Eon L, Seok Jin N, Hak Min L, et al. Scanxiety and quality of life among breast cancer survivors. J Clin Oncol. 2017;33:e20569–e20569. [Google Scholar]
- 49.Sogawa R, Kimura S, Yakabe R, Mizokami Y, Tasaki M, Sueoka-Aragane N, et al. Anxiety and depression associated with tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. Int J Clin Onco. 2018;23:974–9. doi: 10.1007/s10147-018-1275-6. [DOI] [PubMed] [Google Scholar]
- 50.Saglio G, Sharf G, Almeida A, Bogdanovic A, Bombaci F, Čugurović J, et al. Considerations for treatment-free remission in patients with chronic myeloid leukemia: a joint patient–physician perspective. Clin Lymphoma Myeloma Leuk. 2018;18:375–9. doi: 10.1016/j.clml.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Richter J, Söderlund S, Lübking A, Dreimane A, Lotfi K, Markevärn B, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32:2821–3. doi: 10.1200/JCO.2014.55.6910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TFR Patient Experience Questionnaire - English
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.