Abstract
Obesity results in a variety of metabolic alterations that may contribute to abnormalities in cardiac structure and function. Although metformin (Met) has been previously reported to exhibit beneficial effects against cardiomyopathy associated obesity, the mechanism underlying this observation remains unclear. The aim of the present study was to investigate the status of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/kelch-like ECH-associated protein 1 (Keap1) system underlying the protective effects of Met against cardiac remodeling. High-fat diet-induced obesity mouse models were first generated, which were subsequently treated with Met. Metabolic parameters, heart weight index and degree of cardiac fibrosis were examined. The expression levels of genes and proteins associated with the Nrf2/Keap1 signaling pathway were assessed using reverse transcription-quantitative PCR and western blotting. In obese mice, Met treatment significantly ameliorated the obesity phenotype, improved metabolic disorders, reduced the heart weight index and attenuated cardiac fibrosis. The cardioprotective effects of Met may be mediated through the promotion of Keap1 degradation whilst increasing the expression of Nrf2 and associated downstream antioxidant factors.
Keywords: metformin, high-fat diet, cardiac remodeling, nuclear factor (erythroid-derived 2)-like 2, kelch-like ECH-associated protein 1
Introduction
Obesity, originating from a combination of genetic and environmental factors, including lifestyle, culture, physiology and behavior, has reached pandemic proportions in the 21st century (1,2). It presents substantial health challenges and economic burden worldwide (1,2). Obesity is capable of producing a variety of alterations in the body that may predispose individuals to changes in cardiac morphology and ventricular function (3,4), in a process known as cardiac remodeling. Cardiac remodeling refers to the structural and functional dysfunction caused by molecular and genetic changes in cardiomyocytes under the influence of neurohumoral factors (5). A number of potential mechanisms have been hypothesized to underlie obesity-associated cardiomyopathy, including inflammation, neurohumoral and metabolic abnormalities (3). In addition, oxidative stress has also been reported to serve a significant role in myocardial abnormalities associated with obesity (6).
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a major regulator of redox signaling. Under physiological conditions, kelch-like ECH-associated protein 1 (Keap1) binds to and target Nrf2 for proteasomal degradation. However, during oxidative stress, Keap1 becomes inactivated by oxidation of its several cysteine residues or autophagic elimination in a sequestosome-1-dependent manner (7). Nrf2 is then released from this complex, where it enters the nucleus to promote the expression of genes associated with antioxidant activities. In the nucleus, Nrf2 can upregulate the expression of a wide range of antioxidant genes, including glutathione-S-transferase, heme oxygenase (HO-1), NADPH and quinone 1, by binding with the antioxidant response element (8). Although the Nrf2/Keap1 pathway is physiologically important for the antioxidant defense against a variety of cardiovascular diseases, its role in obesity-associated cardiac remodeling remains to be elucidated.
Metformin (Met) is one of the most commonly prescribed drugs for the treatment of type 2 diabetes (9) that has been previously demonstrated to exhibit protective effects against cardiovascular disease. Met has been reported to protect the myocardium against isoproterenol-induced infarction (10,11), attenuate cardiac remodeling in monosodium glutamate-induced obesity in mouse models and inhibit isoproterenol- and pressure overload-induced remodeling, in a manner that was either dependent or independent of adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) activation (12-14). The aim of the present study was to investigate the status of the Nrf2/Keap1 signaling pathway underlying the protective effects of Met against cardiac remodeling in mouse models of obesity.
Materials and methods
Experimental animals and groups
C57BL/6J male mice (age, 4-5 weeks, 15-17g, n=24) were purchased from the Medical Experimental Animal Center of Henan University of Science and Technology (Luoyang, China; License number: SCXK 2018-0007). After adaptive feeding for 1 week, the animals were randomly divided into the following three groups (n=8 per group): i) Control; ii) high-fat diet (HFD); and iii) HFD+Met (300 mg/kg). All mice except for mice in the control group were fed on a HFD for 24 weeks consecutively. The composition of HFD was 60% fat, 20% carbohydrate and 20% protein, while the normal chow diet consisted of 4.5% fat. In addition, mice in the HFD+Met group were administered Met in drinking water daily from week 8 onwards following the commencement of feeding on HFD. All mice were housed in a temperature and humidity regulated room (temperature, 22±2˚C; humidity, 50±5%) with controlled lighting (12-h light/dark cycle). Water and food was freely available to the mice.
The study was approved by the Animal Care and Ethics Committee of Henan University of Science and Technology (Luoyang, China) and was performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (15).
Metabolic measurements
On week 22 of the experiment, an oral glucose tolerance test (OGTT; 0.2 g/kg) was performed. Blood samples (a drop of blood) were collected through the tail artery at 5, 15, 30, 60 and 120 min after the administration of glucose by oral gavage, following which plasma glucose concentrations were measured using a blood glucose monitor (Roche Diagnostics). AUC was calculated according to the approximate trapezoidal area formula (16), which could better reflect the changes in the trend and time accumulation effect of blood glucose levels. After OGTT, animals in each group continued to live in their corresponding conditions as aforementioned until week 24. Body weight, body length, waistline and food/water consumption were monitored weekly, whilst Lee index (17) was calculated [(body weight)1/3/body length] at the end of the experiment.
Serum analysis and heart weight index calculation
After feeding on HFD for 24 weeks, all mice were anaesthetized by an intraperitoneal injection of sodium pentobarbital (45 mg/kg), following which blood (~0.5 ml) was collected from the orbital venous sinus. Fasting serum glucose (cat. no. 133011, Zhongsheng North Control Biotechnology Co., Ltd.) and insulin levels (cat. no. XY-E20353, Shanghai Biological Technology Co., Ltd.) were then measured in the blood samples collect in each group using corresponding commercial kits. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated for mice in each group according to protocols described previously (18). Following retroorbital exsanguination, all mice were immediately euthanized by cervical dislocation. The mouse hearts were then removed rapidly, washed in ice-cold 0.9% saline and weighed. Heart weight/tibial length (HW/TL) and the left ventricular weight/tibial length (LHW/TL) were calculated. ~1/3 of the left ventricular samples were fixed in 4% paraformaldehyde at room temperature (RT) for 24 h and then embedded in paraffin for subsequent morphological analysis. The remaining left ventricle samples were flash-frozen in liquid nitrogen and stored at -80˚C until further use.
Histological examination
Interstitial fibrosis of the left ventricle was determined using Masson's trichrome staining (19). The sections were deparaffinized using xylene and washed with various levels of ethanol, dried and stained for 10 min at RT with Masson composite solution. A total of 2 samples from each group were taken for preliminary morphology and molecular biological experiments, with the remaining 6 samples in each group for subsequent experiments. There were 6 heart tissues in each group, 3 sections of each tissue and 10 randomized visual fields in light microscope (Olympus; magnification, x200) were selected for statistics. To assess the degree of fibrosis, the images were quantitatively analyzed by morphometry using the Image-Pro Plus software (version 1.61; Media Cybernetics, Inc.).
Reverse transcription quantitative-PCR (RT-qPCR)
Total RNA was extracted from the left ventricular tissue (20) using the TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) on ice and reverse-transcribed using a high-capacity complementary DNA reverse transcription kit (cat. no. 4368814; Applied Biosystems) according to the manufacturer's protocol. The temperature protocol was as follows: Denaturation at 70˚C for 10 min, then extension at 37˚C for 60 min and a final inactivation at 94˚C for 10 min. qPCR was performed using SYBR™ Green PCR Master Mix kit (cat. no. CW2623; CWBio) according to manufacturer's protocol. mRNA levels of cardiac fibrosis markers, including transforming growth factor-β1 (TGF-β1), collagen I (Col I) and collagen III (Col III), in addition those of genes associated with oxidative stress, including keap1, Nrf2 and HO-1, were quantified. Primer sequences are shown in Table SI. qPCR was performed in a Step One Plus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) for 40 cycles, specifically, pre-denaturation at 94˚C for 20 sec, and then entering the cycle of 94˚C for 15 sec, and 60˚C for 1 min. The 2-ΔΔCq method was used to quantify the genes (20), and GAPDH gene was used as the internal control.
Western blot analysis
Total proteins were extracted from the left ventricle using ice-cold RIPA buffer (cat. no. CW2333; CWBio). Denatured protein samples were quantified using the BCA method, and 30 µg protein was subjected to 10% SDS-PAGE analysis. After electrophoresis, proteins were transferred to PVDF membranes (EMD Millipore), which were then blocked at RT for 1 h with 5% non-fat dry milk in TBS supplemented with 0.1% Tween-20 (TBST) and subsequently incubated with the respective primary antibodies diluted in 5% non-fat dry milk at 4˚C overnight. Dilutions used for each primary antibody was 1:500 for Nrf2, 1:500 for Keap-1, 1:500 for HO-1 and 1:8,000 for GAPDH. Rabbit and mouse polyclonal antibodies specific for Nrf2 (cat. no. 16396-1-AP), Keap1 (cat. no. 10503-2-AP), HO-1 (cat. no. 10701-1-AP) were purchased from Proteintech; and GAPDH (cat. no. BM3896) were purchased from Boster Biological Technology. After washing with TBST three times, membranes were incubated with anti-rabbit (cat. no. BA1054; Boster Biological Technology) or anti-mouse (cat. no. BA1050; Boster Biological Technology) horseradish peroxidase conjugated secondary antibodies (1:5,000) for 1 h at RT. Protein bands were visualized using chemiluminescence using the ECL chemiluminescent substrate (cat. no. BL523A; Biosharp). GAPDH was used as the loading control for total protein. Quantification of bands was performed using the ImageJ Software 1.50 (National Institutes of Health).
Determination of nuclear Nrf2 using the immunofluorescence staining method
Paraffin-embedded samples were cut into 5-µm thick sections, which were deparaffinized with xylene followed by rehydration using a descending alcohol series, subjected to antigen retrieval in EDTA buffer (pH 8.0) using a microwave (at medium strength for 10-15 min) and then placed in 3% BSA (cat. no. 9048-46-8; Sigma-Aldrich; Merck KGaA) to block non-specific staining for 30 min at room temperature. Sections were then incubated with anti-Nrf2 antibodies (1:100; cat. no. 16396-1-AP; Proteintech) at 4˚C overnight, followed by incubation with the fluorescent-labeled secondary antibody (1:300; cat. no. BA1032; Boster Biological Technology) in darkness and room temperature for 50 min. After counterstaining with 1 µg/ml DAPI for 5 min at RT, the sections were dehydrated and viewed under a fluorescence microscope (x400; Nikon Corporation).
Statistical analysis
In all experiments, values were expressed as the mean ± SEM (n=8). Differences between groups were evaluated using the Student-Newman-Keuls test after One-Way ANOVA (GraphPad Prism 5.0; GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Met treatment alleviates obesity and glucose metabolic disorder in mice
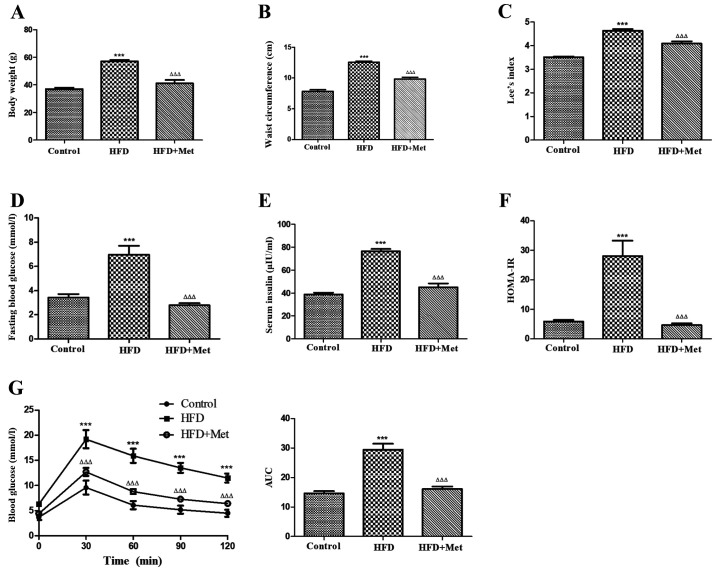
The effects of Met on the metabolic parameters of obese mice were first examined. Met treatment significantly reduced the body weight, waist circumference (Fig. 1A and B) and Lee index (Fig. 1C) in obese mice, whilst significantly reducing the fasting blood glucose (Fig. 1D) and serum insulin levels (Fig. 1E) in addition to ameliorating insulin resistance (Fig. 1F) when compared with untreated mice in the HFD group. Met also significantly improved oral glucose tolerance compared with that in the HFD only group (Fig. 1G). These results suggest that Met treatment alleviated metabolic disorder and obesity syndrome in mice with HFD-induced obesity.
Figure 1.
Met ameliorates obesity and metabolic disorders in HFD-induced obese mice after 24 weeks. (A) Met decreased the body weight, (B) waist circumference and (C) the Lee index in mice from the HFD+Met group compared with those in the HFD group. (D) Between the two HFD groups of mice, hyperglycemia, (E) hyperinsulinaemia and (F) HOMA-IR were significantly improved after Met treatment compared with those in untreated mice. (G) The effect of Met on impaired glucose tolerance, as measured by the oral glucose tolerance test. Right: AUC. Data are expressed as mean ± SEM, n=8 per group. ***P<0.001 vs. control; ΔΔΔP<0.001 vs. HFD. AUC, area under the curve; HFD, high fat diet; HOMA-IR, homeostasis model assessment of insulin resistance; OGTT, oral glucose tolerance test; Met, metformin.
Met prevents cardiac remodeling in HFD-induced obese mice
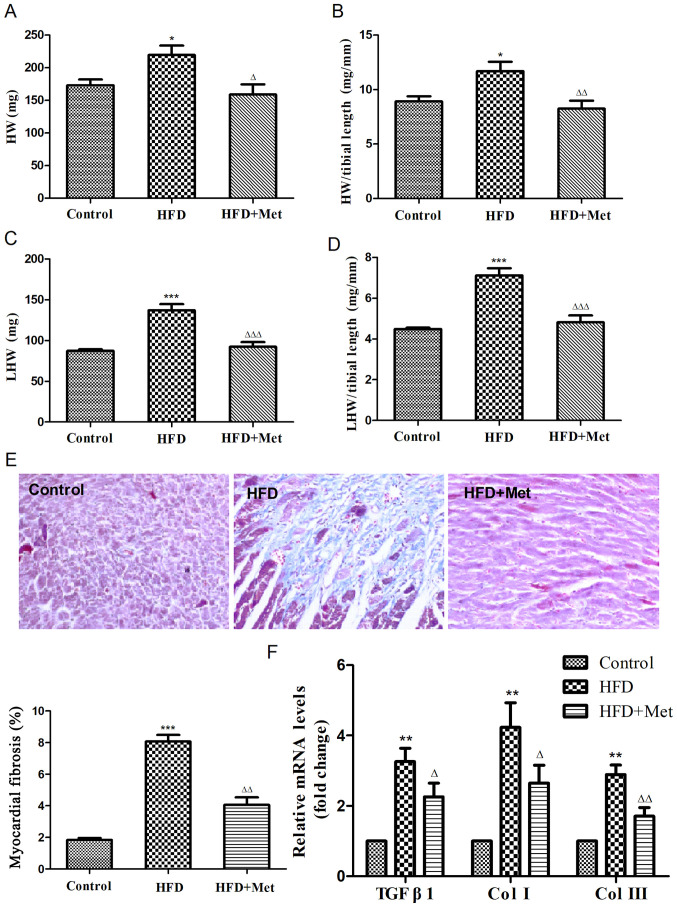
To investigate the effects of Met on cardiac remodeling associated with obesity, heart weight index and degree of cardiac fibrosis were evaluated in obese mice. HW (Fig. 2A and B) and LHW indices (Fig. 2C and D) were both found to be significantly increased in the HFD group compared with those in the control group. Masson's staining revealed that the degree of cardiac fibrosis was significantly increased in the HFD group compared with that in the control group (Fig. 2E), which was also indicated by the RT-qPCR results (Fig. 2F), suggesting the development of cardiac remodeling in obese mice. Met treatment significantly reversed this cardiac remodeling, as indicated by significant reductions in the HW index and levels of cardiac fibrosis compared with those in the HFD group (Fig. 2).
Figure 2.
Met reduces the heart weight index and ameliorates myocardial fibrosis in mice fed on HFD after 24 weeks. (A) Heart weight, (B) heart weight index, (C) left ventricular weight and (D) left ventricular weight index were calculated. (E) Representative figures of myocardial interstitial fibrosis using Masson's trichrome staining (magnification, x200) and the quantified results of Masson's staining. (F) Reverse transcription-quantitative PCR analysis of TGFB1, COL1A1 and COL3A1 expression. Data are expressed as mean ± SEM, n=6 per group. *P<0.05, **P<0.01 and ***P<0.001 vs. Control; ΔP<0.05, ΔΔP<0.01 and ΔΔΔP<0.001 vs. HFD. COL, collagen gene; HFD, high fat diet; HW, heart weight; LHW, left ventricular weight; Met, metformin; TGFB1, transforming growth factor β1 gene.
Met enhances the endogenous antioxidant system in the heart
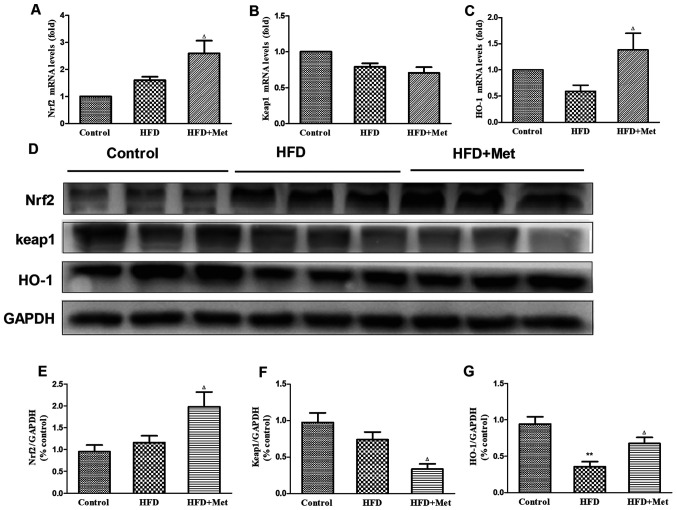
To elucidate the mechanism underlying the protective effects of Met against cardiac remodeling, the expression of genes and proteins associated with Nrf2/Keap1 signaling were analyzed using RT-qPCR and western blotting. Gene and protein expression levels of Nrf2 exhibited slight increases in the heart tissues of mice in the HFD group compared with those in the control group (Fig. 3A, D and E), whilst an opposite trend was observed in Keap1 expression (Fig. 3B, D and F). Expression of HO-1 mRNA, a downstream target gene of Nrf2, was found to be slightly reduced in the heart tissue of mice in the HFD group compared with that in the control group (Fig. 3C). Significant reductions were observed in the protein levels of HO-1 in the heart tissues of mice in the HFD group compared with mice in the control group (Fig. 3D and G). It was found that whilst Met treatment significantly reduced Keap1 protein expression (Fig. 3D and F), no effect was observed on KEAP1 gene expression between the HFD and HFD+Met groups (Fig. 3B). At the same time, Met treatment significantly upregulated Nrf2 and HO-1 expression on both mRNA and protein levels in heart tissues compared with those in untreated mice in the HFD group (Fig. 3A, C, E and G).
Figure 3.
Effect of Met on the expression level of genes and proteins associated with the Nrf2/Keap1 signaling pathway in the cardiac tissue of the three experimental groups. (A) mRNA levels of NFE2L2, (B) KEAP1 and (C) HMOX-1. (D) Representative western blot images of proteins associated with the Nrf2/Keap1 signaling pathway. (E) Densitometry analysis of Nrf2, (F) Keap1 and (G) HO-1 expression. The relative densitometry is expressed as the ratio of Nrf2, Keap1 or HO-1 to GAPDH. Data are presented as mean ± SEM; n=6 per group. **P<0.01 vs. Control; ΔP<0.05 vs. HFD. HFD, high fat diet; HO-1, heme oxygenase 1; Keap1, kelch-like ECH-associated protein 1; HMOX-1, HO-1 gene; Met, metformin; Nrf2, nuclear factor (erythroid-derived 2)-like 2; NFE2L2, Nrf2 gene.
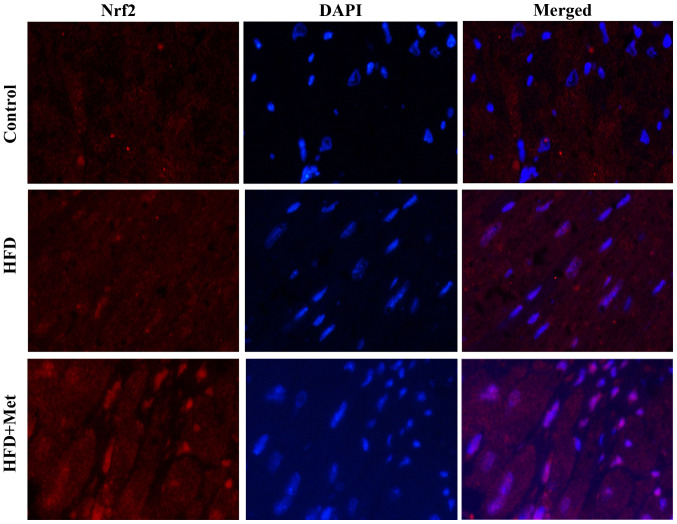
Met promotes Nrf2 translocation into the nucleus in the heart tissue
Fig. 4 shows representative immunofluorescence staining images of Nrf2 expression in the heart tissues isolated from mice from the three experimental groups. Nrf2 staining showed slight increases in the heart tissue of mice from the HFD group compared with that in control mice. By contrast, Met induced a marked increase in nuclear Nrf2 staining, suggesting that Met treatment may activate Nrf2 signaling by inducing the nuclear translocation of Nrf2.
Figure 4.
Met promotes Nrf2 nuclear translocation in the myocardial tissues. Representative images of immunofluorescent staining of Nrf2 in myocardial tissues isolated from mice from the three experimental groups. Magnification, x400. HFD, high fat diet; Met, metformin; Nrf2, nuclear factor (erythroid-derived 2)-like 2.
Discussion
The results of the present study indicated that long-term Met treatment provided protection against obesity-associated cardiac remodeling in HFD-induced obese mice. This may be mediated though the reduction of metabolic disorder and enhancement of the Nrf2/Keap1 signaling pathway of the endogenous antioxidant system. These findings suggest the potential of Met as a therapeutic agent for patients of obesity at risk of cardiac remodeling.
It is estimated that there are 671 million individuals with obesity in the world, with 62% occurring in developing countries (21). Morbid obesity has been associated with insulin resistance, diabetes mellitus and organ damage, including atherosclerosis and left ventricular hypertrophy (LVH). LVH is known has been previously found to be independently associated with adiposity (22). HFD is associated with the manifestation of obesity, metabolic disturbances, cardiac hypertrophy and interstitial fibrosis (23). Results of the present study indicated that mice fed on HFD for 24 weeks exhibited apparent metabolic syndrome, with symptoms including increases in body weight, waist circumference, Lee index, fasting blood glucose, serum insulin levels and in the HOMA-IR index. All of the aforementioned pathological changes could be mitigated by long-term Met treatment, which also improved glucose tolerance. In addition to the reported beneficial effects of Met on the metabolism, interest has also been garnered in the effects of Met on cardiovascular diseases (13,24,25), cancer (26) and aging (27). However, the underlying mechanism of Met action remain elusive. Although Met-induced activation of the energy-sensor AMPK has been well documented (28), AMPK-independent mechanisms have also been reported, including suppression of TGF-β1 expression (29), inhibition of reactive oxygen species generation by blocking the NADPH oxidase pathway (30) and the activation of endothelial nitric oxide synthase (31). Pre-treatment with Met has been previously demonstrated to activate the Nrf2 antioxidant signaling pathways in the hippocampus of rats with global cerebral ischemia (32). In the present study, the HW and the LHW indices were found to be significantly increased in mice from the HFD group compared with the control group. Masson's staining of the myocardial tissue confirmed the existence of myocardial fibrosis in mice from the HFD group. In addition, gene expression levels of TGF-β1, Col I and Col III in the myocardial tissue, markers of cardiac remodeling, were revealed to be markedly elevated in mice from the HFD group. All of these aforementioned observations support the notion that cardiac remodeling occurred in mice from the HFD group in the present study. Long-term treatment with Met significantly ameliorated cardiac remodeling, which was demonstrated by reduced HW index, LHW index and myocardial fibrosis. Amelioration of cardiac remodeling was also indicated by the observed reductions in TGF-β1, Col I and Col III gene expression in myocardial tissues in HFD mice treated with Met.
The Nrf2/Keap1 pathway is physiologically important for defense against oxidative stress (33). The present study suggested that Nrf2 in the heart may serve as a compensatory mechanism in response to oxidative stress in mice fed with HFD. Met reduced Keap1 protein levels, resulting in the activation of Nrf2 and subsequent translocation into the nucleus, leading to the upregulation of downstream antioxidative enzymes such as HO-1. Together, these data suggested that Met has protective effects against obesity-associated cardiac remodeling, which may potentially be due to its effect on alleviating metabolic disorders and enhancing endogenous antioxidant function. However, further studies are required to elucidate the direct targets of Met in the regulation of Nrf2/Keap1 signaling.
In summary, metabolic disorders and adverse cardiac remodeling were found to be evident in mice with HFD-induced obesity in the present study. Met exerted potent protective effects against the development of metabolic disorders and cardiac remodeling, which were associated with its effect on enhancing endogenous antioxidant activities by activating the Nrf2/Keap1 signaling pathway. The present study suggested that Met serve as an effective treatment option for obesity-associated cardiac remodeling, where the Nrf2/Keap1 pathway may be another potential therapeutic target.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by the Henan Key Scientific Research Projects (grant no. 19A350003).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JD designed the study and wrote the manuscript. MZ, HL, GL and YL performed the experiments. SF helped to analyze the data and revise the manuscript. All of the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Animal Care and Ethics Committee of Henan University of Science and Technology (Luoyang, China; grant no. 19A350003) and was in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Scheen AJ, Van Gaal LF. Combating the dual burden: Therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:911–922. doi: 10.1016/S2213-8587(14)70004-X. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari S, Ndisang JF. The role of obesity in cardiomyopathy and nephropathy. Curr Pharm Des. 2014;20:1409–1417. doi: 10.2174/13816128113199990562. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. New Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SG, Bae SH. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic Biol Med. 2015;88:205–211. doi: 10.1016/j.freeradbiomed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 11.Soraya H, Khorrami A, Garjani A, Maleki-Dizaji N, Garjani A. Acute treatment with metformin improves cardiac function following isoproterenol induced myocardial infarction in rats. Pharmacol Rep. 2012;64:1476–1484. doi: 10.1016/s1734-1140(12)70945-3. [DOI] [PubMed] [Google Scholar]
- 12.Burlá AK, Lobato NS, Fortes ZB, Oigman W, Neves MF. Cardiac fibrosis and vascular remodeling are attenuated by metformin in obese rats. Int J Cardiol. 2011;165:483–487. doi: 10.1016/j.ijcard.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Fu YN, Xiao H, Ma XW, Jiang SY, Xu M, Zhang YY. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol Sin. 2011;32:879–887. doi: 10.1038/aps.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha HN, Choi JH, Kim YW, Kim JY, Ahn MW, Park SY. Metformin inhibits isoproterenol-induced cardiac hypertrophy in mice. Korean J Physiol Pharmacol. 2010;14:377–384. doi: 10.4196/kjpp.2010.14.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Canis R, Demirkok SS, Osar Z, Balci H, Can G. Effects of inhaled budesonide on insulin sensitivity in nondiabetic patients with asthma and chronic obstructive pulmonary disease. Adv Ther. 2007;24:560–570. doi: 10.1007/BF02848778. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Wu JZ, Shen JZ, Chen L, He T, Jin MW, Liu H. Pentamethylquercetin induces adipose browning and exerts beneficial effects in 3T3-L1 adipocytes and high-fat diet-fed mice. Sci Rep. 2017;7(1123) doi: 10.1038/s41598-017-01206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Simon J, Nemeth E, Nemes A, Husveth-Toth M, Radovits T, Foldes G, Kiss L, Bagyura Z, Skopal J, Merkely B, Gara E. Circulating relaxin-1 level is a surrogate marker of myocardial fibrosis in HFrEF. Front Physiol. 2019;10(690) doi: 10.3389/fphys.2019.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methods. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady TM. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr Hypertens Rep. 2016;18(3) doi: 10.1007/s11906-015-0608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Fang X, Dai M, Cao Q, Tan T, He W, Huang Y, Chu L, Bao M. Cardiac-specific down-regulation of carnitine palmitoyltransferase-1b (CPT-1b) prevents cardiac remodeling in obese mice. Obesity (Silver Spring) 2016;24:2533–2543. doi: 10.1002/oby.21665. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, et al. Metformin prevents progression of heart failure in dogs: Role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daugan M, Dufaÿ Wojcicki A, d Hayer B, Boudy V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol Res. 2016;113:675–685. doi: 10.1016/j.phrs.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo T, Nocon A, Fry J, Sherban A, Rui X, Jiang B, Xu XJ, Han J, Yan Y, Yang Q, et al. AMPK Activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes. 2016;65:2295–2310. doi: 10.2337/db15-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010;87:504–513. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 30.Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, Miura D, Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232:156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Kawabata H, Ishikawa K. Cardioprotection by metformin is abolished by a nitric oxide synthase inhibitor in ischemic rabbit hearts. Hypertens Res. 2003;26:107–110. doi: 10.1291/hypres.26.107. [DOI] [PubMed] [Google Scholar]
- 32.Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Ichikawa T, Jin Y, Hofseth LJ, Nagarkatti P, Nagarkatti M, Windust A, Cui T. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J Ethnopharmacol. 2010;130:222–230. doi: 10.1016/j.jep.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.