Abstract
Multiple sclerosis (MS) is a genetically mediated autoimmune disease characterized by inflammation in the central nervous system (CNS). Disease onset is thought to occur when autoreactive T cells orchestrate a cascade of events in the CNS resulting in white and grey matter inflammation and axonal degeneration. It is unclear what triggers the activation of CNS-reactive T cells and their polarization into inflammatory subsets. Mounting evidence from animal and human studies supports the hypothesis that the gut microbiome affects MS pathogenesis. We investigated the association between the gut microbiome and inflammatory T cell subsets in relapsing-remitting MS patients and healthy controls. Gut microbiome composition was characterized by sequencing the V4 region of the 16S rRNA gene from fecal DNA, and inflammatory T cell subsets were characterized by flow cytometry. We identified an altered gut microbiome in MS patients, including decreased abundance of Coprococcus, Clostridium, and an unidentified Ruminococcaceae genus. Among circulating immune cells, patients had increased expression of CXCR3 in both CD4 and CD8 T cells, and both CD4+CXCR3+ and CD8+CXCR3+ populations expressing the gut-homing α4β7 integrin receptor were increased. Finally, we show that alpha diversity inversely correlated with a CXCR3+ Th1 phenotype in MS. These findings indicate the presence of an aberrant gut-immune axis in patients with MS.
Keywords: Microbiome, Multiple sclerosis, Autoimmunity, CXCR3, Th1
Highlights
-
•
Patients with MS exhibited decreased alpha diversity in the composition of their gut bacteria compared to healthy controls.
-
•
Circulating lymphocytes from MS patients were enriched for Th1 and Th1-17 memory CD4+ cells and for CXCR5+ memory CD4+ cells.
-
•
A larger proportion of Th1 and Th1–Th17 cells expressed the gut homing receptor α4β7 compared to Th17 cells.
-
•
The frequency of Th1, Th1-17 and CD8 T cells co-expressing α4β7 and CXCR3 wereincreased in patients compared to controls.
-
•
Reduced alpha diversity correlated significantly with expression of CXCR3+ on CD8+ T-cells and CXCR5— memory T-cells.
1. Introduction
Multiple sclerosis (MS) is a genetically mediated autoimmune disease characterized by inflammation in the central nervous system (CNS). Disease onset is hypothesized to occur when autoreactive T cells cross the blood brain barrier and orchestrate a cascade of events resulting in white and grey matter inflammation and axonal degeneration [[1], [2], [3]]. It is unclear what triggers the activation of CNS-reactive T cells or drives their polarization into inflammatory subsets [4]. Genome-wide association studies have identified over 230 common and rare genetic variants in MS, with the strongest risk residing in the MHC class II variant, HLA-DRB*1501. Further risk loci implicate CD4 T cells [5,6], but genetic contributions are only part of MS pathophysiology. Interactions between environmental factors and genetics appear to drive disease [[7], [8], [9], [10]].
Emerging evidence implicates the gut microbiome as a potential trigger for polarizing autoreactive T cells towards an inflammatory phenotype in MS [11]. Among MS patients, CNS-autoreactive T cells secrete the inflammatory cytokines IL-17 and GM-CSF. Similar autoreactive T cells in healthy individuals secrete anti-inflammatory IL-10 [[12], [13], [14], [15], [16], [17], [18]]. The functional differences between autoreactive T-cells from healthy individuals and MS patients may relate to the differences in the peripheral stimuli activating the cells. The bacterial microbiome is one potential source for these stimuli [4]. Gut bacterial communities regulate local and systemic immune responses, and microbial imbalance or maladaptation (dysbiosis) characterizes many autoimmune diseases [[19], [20], [21]]. Moderate gut dysbiosis has been identified in patients with MS [[22], [23], [24]], and recent animal studies demonstrate that MS fecal microbiomes may contribute significantly to disease [[25], [26], [27]].
This study sought to simultaneously examine the gut microbiome and adaptive immune cells implicated in MS including CD8+ T cells, CD4+ T helper 1 cells (Th1, CXCR3+), CD4+ T helper 17 (Th17, CCR6+) cells, and circulating follicular helper T cells (cTfh, CXCR5+), which can be further divided into Th1-like and Th17-like subsets (cTfh1, cTfh17) [[28], [29], [30], [31], [32]]. Further, we included the gut-homing integrin α4β7 [33] to gain insight into the potential gut-homing capacities of these T cell populations. Despite their importance in MS, circulating T-cell subsets have not been characterized ex vivo in relation to the gut microbiome.
We found that untreated, relapsing-remitting MS (RRMS) patients had reduced gut microbiome diversity, suggestive of dysbiosis, globally characterized by increased Bacteroides and decreased abundance of Firmicutes taxa. Microbial changes were associated with increased CXCR3 expression on CD8 and CD4 T cells, most strikingly on Th1 memory CD4+ cells. Co-expression of CXCR3 and α4β7 was also increased on total CD8 and CXCR5— memory CD4 T cells. We provide new evidence associating decreased diversity in the gut microbiome with circulating CXCR3+ Th1 cells, which may contribute to MS pathology.
2. Materials & methods
2.1. Subjects
This study consisted of a cohort of MS patients (n = 26) and healthy controls (n = 39). MS patients had no use of disease modifying drugs for ≥3 months prior to enrolment. Participants had no known antibiotic use for ≥3 months prior to enrolment and no autoimmune diseases other than MS. All participants donated stool samples. A subgroup of patients (n = 20) and healthy controls (n = 21) also donated peripheral blood. No significant differences in age, BMI and sex were observed between groups (Wilcoxon’s signed-sum test or Fishers exact test). Detailed cohort information is provided in Table 1.
Table 1.
16s rRNA sequencing and flow cytometry demographics. Fecal and blood sample donors from control and MS patients. All MS patients had relapse-remitting disease and were free of disease modifying treatment for 3 months prior to donation. Age and BMI are represented by the mean and standard deviation. No significant difference between groups was observed for age and BMI (Wilcoxon’s signed-sum test) or sex (Fishers exact test).
| 16S sequencing (n = 65) | Flow cytometry (chemokines) (n = 41) | Flow cytometry (integrins) (n = 33) | 16S sequencing + flow (n = 35) | ||
|---|---|---|---|---|---|
| Female, n (%) | Control | 27 (69) | 16 (76) | 15 (75) | 15 (75) |
| MS | 22 (85) | 19 (95) | 13 (100) | 14 (93) | |
| Age, mean (SD) | Control | 45 (12) | 47 (11) | 46 (11) | 46 (11) |
| MS | 42 (13) | 43 (14) | 43 (14) | 44 (14) | |
| BMI, mean (SD) | Control | 27 (5) | 27 (5) | 27 (5) | 27 (5) |
| MS | 29 (7) | 29 (7) | 30 (5) | 29 (6) | |
| Years since diagnosis, median (range) | MS | 3 (0–29) | 4.5 (0–34) | 8 (0–29) | 6 (0–29) |
| Prior history of immunomodulation, n (%) | MS | 12 (46) | 11 (55) | 7 (54) | 8 (53) |
| Months since prior immunomodulation, median (range) | MS | 10.5 (3–144) | 12 (3–144) | 12 (5–144) | 12 (5–144) |
2.2. Fecal sample collection and DNA preparation
Donors collected stool at home using a provided kit. Samples were collected at room temperature in RNAlater® solution (Ambion, cat. AM7021) and shipped overnight. Samples were then aliquoted and frozen at −70 °C. All batches had equal numbers of controls and MS patients. At analysis, RNAlater® was removed by centrifugation and bacterial DNA was isolated using the Mobio PowerSoil DNA isolation kit (cat. 12888–100). DNA was eluted in PCR grade water and stored at −20 °C for sequencing.
2.3. 16S rRNA sequencing and analysis
Bacterial DNA was purified using the E-Z 96 Cycle-Pure Kit (Omega Biotek). Clean DNA was quantified using Quant IT Picogreen (Invitrogen). The V4 region of 16S ribosomal RNA was PCR amplified (30 cycles; primer pair F515/R806, AccuPrime Pfx DNA Polymerase) [[34], [35], [36]]. After amplification, PCR amplicons were cleaned and normalized (SequalPrep plate normalization kit, Invitrogen). Samples were sequenced on a miSeq sequencer (Illumina, 2 × 250bp paired-end reads). Sequences were uploaded to the European Nucleotide Archive (accession number PRJEB34168).
Microbial diversity was analysed with Quantitative Insights Into Microbial Ecology (QIIME, version 1.9) [37]. Dual indexed paired end reads were assembled with a minimum overlap of 175 bp. Of note, this method allows for almost complete overlap of the ~253bp V4 region and provides very low error rates. Reads were demultiplexed and quality filtered with a Q-score cut-off of 30. The open-reference Operational Taxonomic Unit (OTU) picking workflow in QIIME, with UCLUST, and the Greengenes database (gg_13_5) were used to cluster the reads into 97% identity OTUs. These were filtered to retain OTUs present in at least 5% of donors and at a minimum of 0.01% total reads. After quality filtering, 5,788,546 reads were observed and 518 OTU’s identified. Filtered OTU tables were rarefied to the sample depth of 10,391 sequences for further analyses. QIIME and R (version 3.5.1) were used for microbial ecology analyses (alpha diversity, beta diversity, PCoA, ANOSIM) [37]. Linear Discriminant Analysis Effect Size (LEfSe) was used for differential abundance tests (http://huttenhower.sph.harvard.edu/galaxy/) [38]. LEfSe is an algorithm designed for high-dimensional biomarker discovery and explanation that identifies genomic features that are different between two or more biological conditions.
2.4. Peripheral blood mononuclear cell isolation and flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from the blood of donors using a Ficoll gradient, aliquoted at 1 × 107/ml in 90% human serum containing 10% DMSO and cryopreserved in liquid nitrogen. PBMCs were thawed in batches for flow cytometry analysis of chemokine receptors. Invitrogen LIVE/DEAD fixable red dead cell stain kit (L23102) was used to exclude dead cells. Cell surface staining was carried out using the following antibodies; CD3 V450 (clone UCHT1, cat. 560365), CD4 V500 (clone RPA-T4, cat. 560768), CD8 V500 (clone SK1, cat. 561617), CD8 APC-H7 (clone sk1 cat. 560179), CD45RO AF700 (clone UCHL1, cat. 561136), CXCR5 FITC (clone RF8B2, cat. 558112), CXCR3 PE (clone 1C6, cat. 557185), CCR6 PE-Cy7 (clone 11A9, cat. 560620), beta7 BV421 (clone FIB504, cat. 564283), alpha 4 APC-Cy7 (clone 9F10, cat 304328; all antibodies from BD Biosciences). Samples were acquired using the LSR Fortessa flow cytometer (BD Biosciences). Gating strategies are indicated in Supplemental Figs. 3 and 4. Analysis was carried out using FlowJo V3. Wilcoxon rank-sum tests were used to identify differences in chemokine and integrin frequencies between healthy controls and MS patients. Paired Wilcoxon signed-sum test with a Bonferroni correction was used to detect differences in integrin expression within different T cell subsets. Spearman’s correlations were used to detect correlations with T cell subsets and alpha diversity. Statistical tests and box plots were generated using R (V3.5.1).
3. Results
3.1. Relapsing-remitting MS is associated with decreased alpha diversity
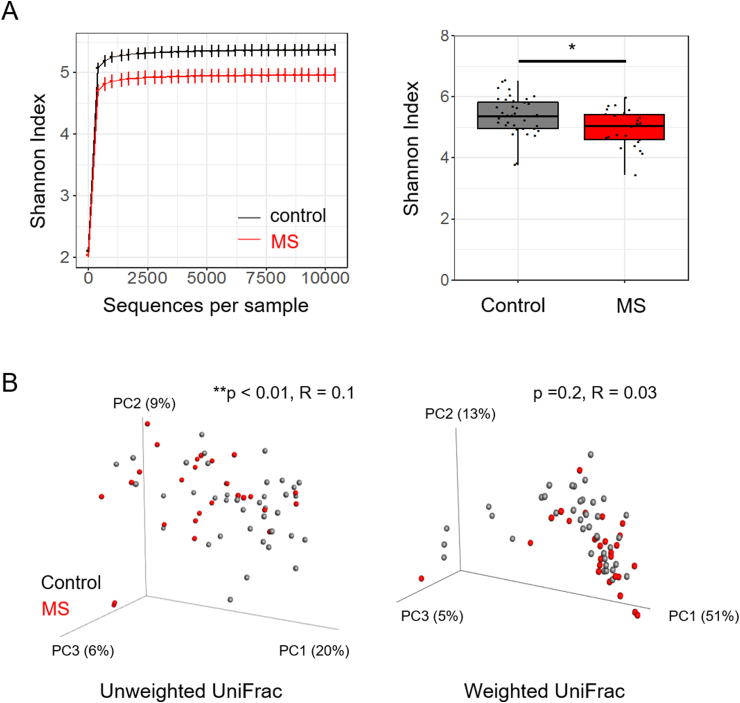
We enrolled 26 RRMS patients and 39 healthy donors matched for age, sex, and BMI (Table 1). The composition of the gut microbiome was identified using high-throughput V4 sequencing of the 16S rRNA gene (S. Fig. 1) [35,36]. To evaluate differences in microbial community structure between patients and controls, we calculated alpha and beta diversity. Alpha diversity refers to the microbial diversity within a sample, while beta diversity refers to microbial diversity across samples. Using Shannon’s index to measure alpha diversity, we observed a moderate but significant reduction in RRMS patients compared to controls (Fig. 1a). Beta diversity was assessed using weighted and unweighted UniFrac coupled with principal coordinates analysis [39]. Unweighted UniFrac identified a significant but weak difference between the two groups (p < 0.01, R = 0.12) (Fig. 1b). Weighted UniFrac analysis considers the relative abundance of taxa and identified no significant differences (p = 0.16, R = 0.03) (Fig. 1b). These results support previous work indicating that MS patients have altered gut microbiomes, suggestive of dysbiosis [27,40].
Fig. 1.
Untreated patients have lower alpha diversity than controls. Rarefied relative abundance of fecal bacteria of controls (n = 39) and untreated MS patients (n = 26) was used to calculate alpha and beta diversity. a) Rarefaction curves were calculated at multiple sequence depths for Shannon index for controls and untreated patients with MS. Graphs depict average ± standard error (left panel). Statistical significance was calculated at the maximum depth (10,385) using a Wilcoxon rank sum test (right panel). b) Beta diversity was calculated using unweighted UniFrac (left panel) and weighted UniFrac (right panel). ANOSIM was used to determine differences in MS and healthy controls. * p < 0.05, ** p < 0.01.
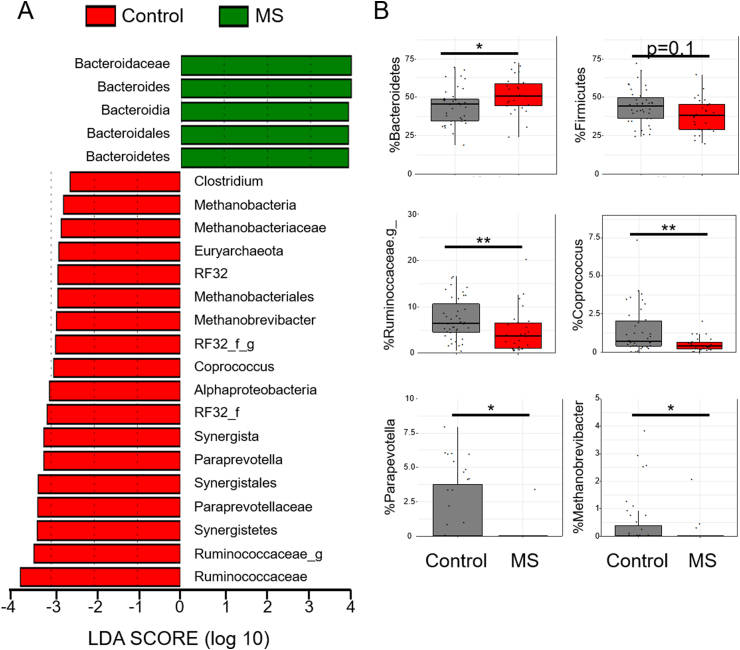
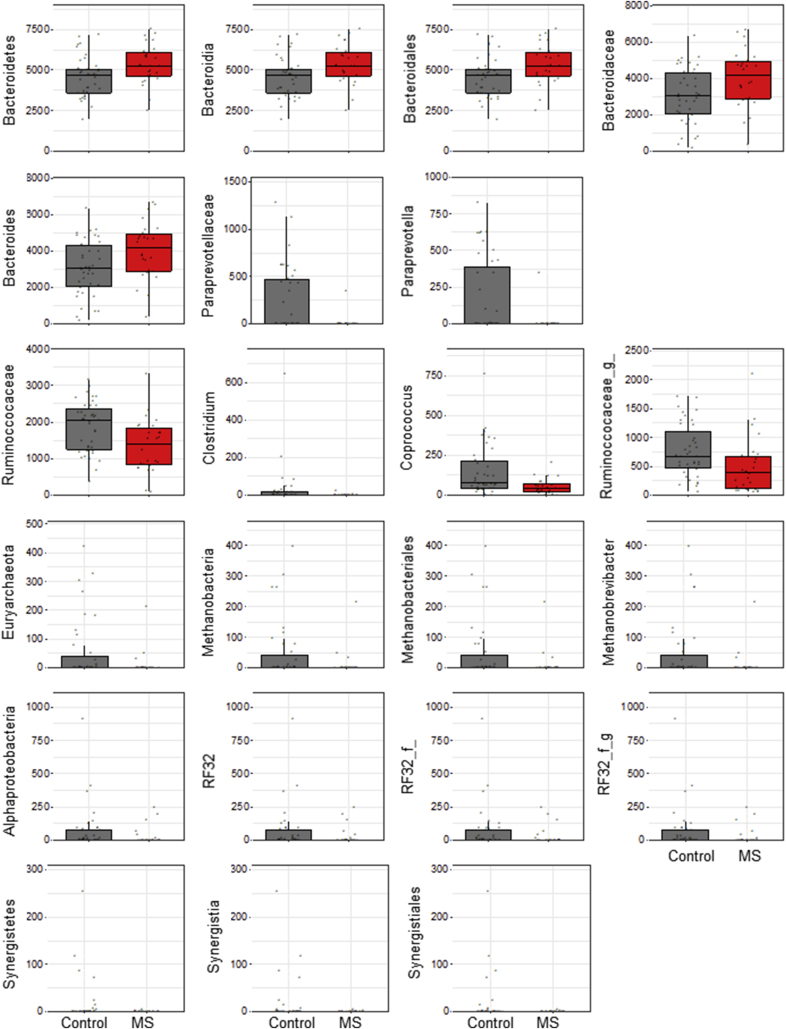
Differential abundance of bacteria between MS and healthy donors was determined using LEfSe [38]. LEfSe identified increased Bacteroidetes phyla in RRMS (Fig. 2; rarefied counts in Supplemental Fig. 2). At the genus level, multiple Firmicutes were reduced in RRMS. Specifically, there were significant decreases of Coprococcus, Clostridium, and an unidentified Ruminococcaceae genus (Fig. 2). Reductions in the genus Paraprevotella (phylum Bacteroidetes), Methanobrevibacter (phylum Euryarchaeota), and an unidentified genus from the phylum Proteobacteria were also noted (Fig. 2), supporting the alpha and beta diversity analyses.
Fig. 2.
Fecal bacterial abundance in controls and patients with MS. Differential abundance was determined between groups using linear discriminant analysis (LDA) effect size (LEfSe). a) green indicates that the bacteria were enriched in the patients with MS (n = 26), red indicates that the bacteria were increased in controls (n = 39). b) Representative dot plots of bacteria abundance. g_ represents an undefined genus of bacteria in the family Ruminoccaceae.
3.2. Circulating T-cells express increased CXCR3 and exhibit increased Th1 and circulating follicular Th1 populations in RRMS
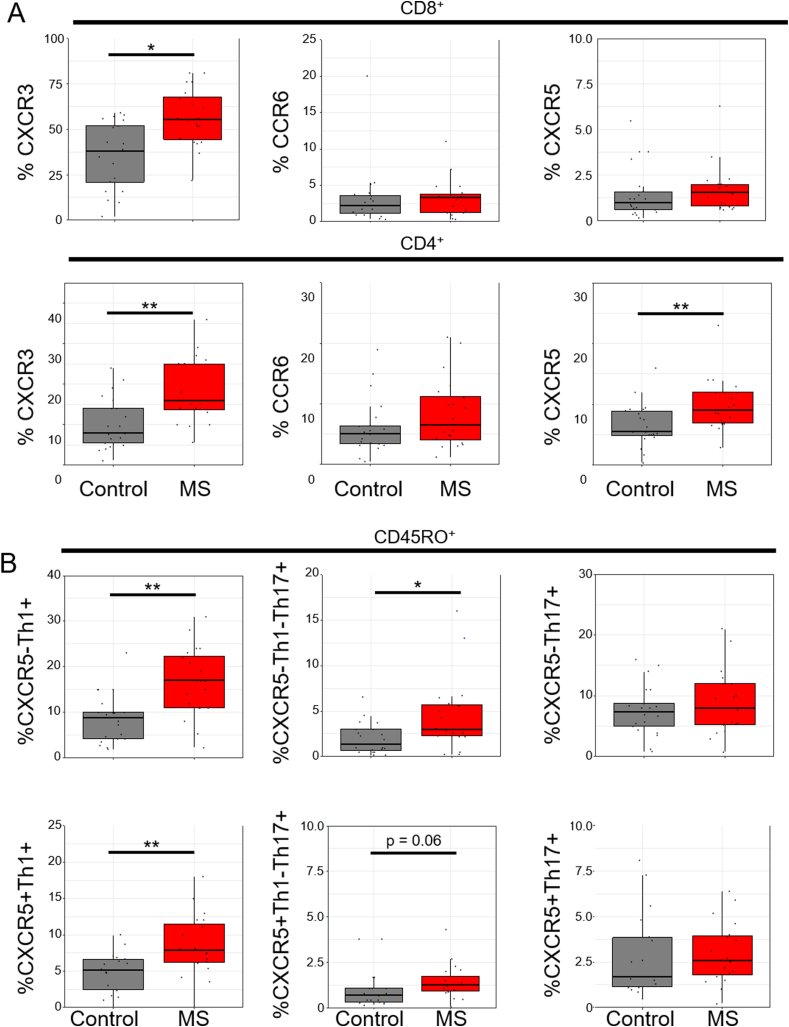
Chemokine expression (CXCR3, CCR6, and CXCR5) was determined on PBMCs using flow cytometry (S. Fig. 3). CXCR3 expression was increased on both CD4 and CD8 T cell subsets from RRMS patients (Fig. 3a). CXCR5 expression on CD4+ T cells was also increased in RRMS, but there were no differences in CCR6 expression (Fig. 3a). Naïve T cells generally express very low levels of these cytokines [41], so subsequent analysis focused on CD45RO+ memory T cells. We subdivided CD4+ CD45RO+ cells into CXCR5+ (cTfh) or CXCR5— (Th) populations [Th1 (CXCR5— CXCR3+ CCR6—), Th17 (CXCR5— CXCR3— CCR6+), Th1–Th17 (CXCR5- CXCR3+ CCR6+), cTfh1 (CXCR5+ CXCR3+ CCR6-), cTfh1-Tfh17 (CXCR5+CXCR3+CCR6+), and cTfh17 (CXCR5+CXCR3—CCR6+) (S. Fig. 3)]. RRMS patients had increased expression of Th1, Th1–Th17, and cTfh1 cells but not Th17 or cTfh17 cells (Fig. 3b), suggesting that circulating memory T cells are polarized towards Th1, Th1–Th17 and cTfh1 phenotypes in this disease.
Fig. 3.
Peripheral T cells from patients with MS are enriched in Th1 cells. Flow cytometry analysis was carried out on PBMC from controls (n = 21) and patients with MS (n = 20). a) CXCR3, CCR6 and CXCR5 expression was determined on total CD8 and CD4 cells. b) Percentage of Th1 (CXCR3+CCR6—), Th1/Th17 (CXCR3+CCR6+) and Th17 (CXCR3—CCR6+) were calculated in CD4+ CD45RO+ T cells that were either CXCR5— or CXCR5+. Data is represented as box plots, whiskers represent 1.5*IQR. Statistical significance was determined using a Wilcoxon’s rank sum test. * p < 0.05, ** p < 0.01.
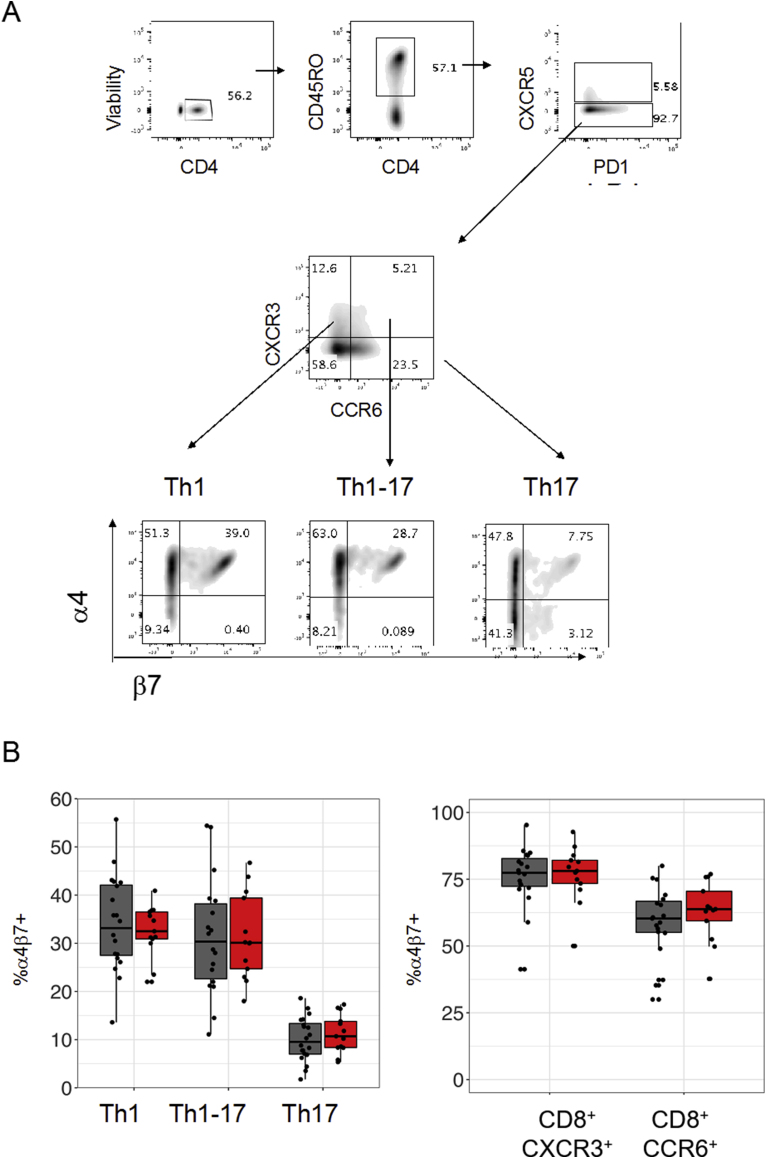
3.3. α4β7 is increased in CXCR3-expressing CD8+, Th1 and Th1-17 T cells in RRMS
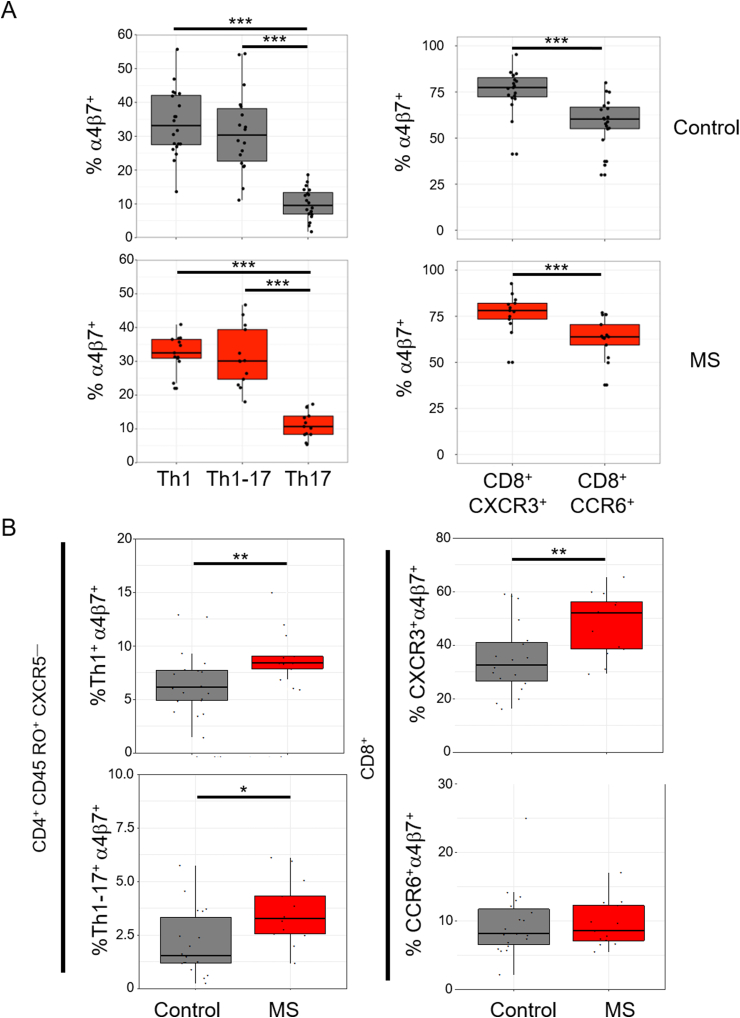
Integrins and chemokine receptors regulate T-cell homing and functional capabilities. The integrin α4β7 is upregulated on T cells that become activated in gut-associated lymphoid tissue and facilitates homing to the intestines, Peyer’s patches and mesenteric lymph nodes [42,43]. We analysed the expression of α4β7 on CD4 and CD8 T-cells (Fig. 4 and S. Fig. 4). In both healthy controls and patients, a subpopulation of Th1, Th1-17 and Th17 T cells express α4β7, with a larger proportion of Th1 and Th1–Th17 cells expressing α4β7 compared to Th17 cells (Fig. 4a). The proportions of α4β7+ subpopulations within each Th subset did not differ between patients and controls (S. Fig. 4b). In both cohorts, CXCR3+ CD8 T cells had a higher proportion of α4β7+ cells than CCR6+ CD8 T cells (Fig. 4a). Similarly, the proportion of α4β7+ subpopulations within the CXCR3+ and CCR6+ CD8 subsets did not differ between patients and controls (S. Fig. 4b). However, the frequency of both CXCR5— CD4+ (e.g. Th1, Th1-17) and CD8 T-cells co-expressing CXCR3 and α4β7 were increased in patients compared to healthy controls. (Fig. 4b). No difference in α4β7 expression was observed among CXCR3+CCR6— cells expressing CXCR5 (e.g. cTfh1).
Fig. 4.
CXCR3+ T cell subsets have enhanced expression of α4β7. (a) Frequency of α4β7+ was calculated as a percentage of the parent populations CD4+CD45RO+CXCR5—CXCR3+CCR6— (Th1), CD4+CD45RO+CXCR5—CXCR3+CCR6+ (Th1-17), CD4+CD45RO+CXCR5—CXCR3—CCR6+ (Th17) (Left panel). Frequency of α4β7+ was calculated as a percentage of the parent populations CD8+CXCR3+ or CD8+CCR6+ (Right panel). (b) Frequency of CXCR3+CCR6—α4β7+ (Th1+α4β7+) and CXCR3+CCR6+α4β7+ (Th1-17+α4β7) was calculated as a percentage of the parent population CD4+CD45RO+CXCR5— T cells (left panels). Frequency of CXCR3+α4β7+ and CCR6+α4β7+ was calculated as a percentage of CD8 T cells expressing (right panels). Boxplots represent expression frequencies of healthy controls (n = 20) and MS patients (n = 13). Wilcoxon’s signed-sum test was used to test for significance. For multiple comparisons, a Bonferroni-correction was applied. * p < 0.05, ** p < 0.01, *** p < 0.001.
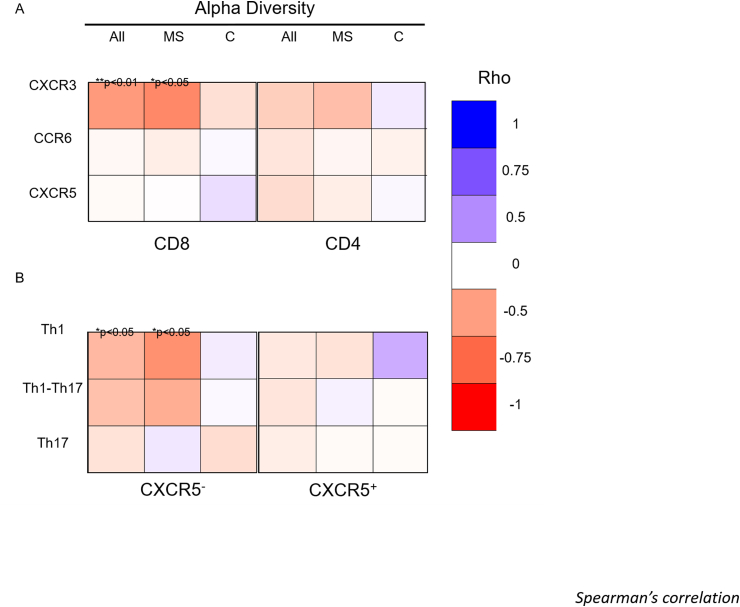
3.4. CXCR3+ CD8 and CXCR3+ Th1 T cells inversely correlate with alpha diversity in MS
Non-parametric Spearman’s rank correlations were used to correlate Shannon’s index with the percentage of circulating CXCR3+, CCR6+ and CXCR5+ CD4 and CD8 T cells. Shannon’s index was also correlated with Th1, Th1-17, Th17, cTfh1, cTfh1-17 and cTfh17 CD4 memory subsets. Correlations were performed using three different groups of donors: all samples, RRMS only and healthy controls only. CXCR3+CD8+ T cells and alpha diversity were inversely correlated among the combined (rho −0.5, **p < 0.01) and RRMS samples (rho −0.6, *p < 0.05) (Fig. 5). Alpha diversity also inversely correlated with circulating Th1 cells from combined (rho −0.4, p < 0.05) and MS samples (rho −0.6, p < 0.05) (Fig. 5). No T cell subsets correlated with alpha diversity among healthy donors.
Fig. 5.
Th1 markers correlate with reduced alpha diversity. Alpha diversity was calculated using the Shannon index. Frequency (%) of chemokine receptors was calculated using flow cytometry. (a) Percentages of CXCR3+, CCR6+ and CXCR5+ were calculated for total CD4 or CD8 T cells. (b) Frequency (%) of Th1 (CXCR3+CCR6—), Th1/Th17 (CXCR3+CCR6+) and Th17 (CXCR3—CCR6+) were calculated in CD4 memory T cells (CD45RO+) that were either CXCR5— and CXCR5+. Spearman’s correlations (rho) between the alpha diversity and chemokine expression were calculated. Colour of squares indicate magnitude of correlation (Rho), significant p values are superimposed on the square. Subject groups were either all MS patients and controls together (All, n = 35), untreated MS patients (MS, n = 15) or healthy controls (C, n = 20).
4. Discussion
We investigated the association between the gut microbiome and circulating T cell subsets in patients with RRMS and healthy controls. Patients exhibited decreased abundance of Coprococcus, Clostridium, and an unidentified Ruminococcaceae genus compared to healthy controls. Correspondingly, CXCR3+ CD4 and CD8 T cells were increased and had increased expression of the gut-homing receptor α4β7. These cell types inversely correlated with alpha diversity, suggesting that the gut-immune axis is aberrant in MS.
We observed reduced alpha diversity in the MS gut microbiomes compared to controls (Fig. 1). Patients had a marked increase in Bacteroidetes with a corresponding reduction in Firmicutes (Fig. 2), in agreement with prior observations [22,44]. Reduced alpha diversity has previously been associated with chronic low-grade inflammation [45] and has been observed in other autoimmune diseases including inflammatory bowel disease [46,47], pre-clinical type 1 diabetes [48,49] and psoriatic arthritis [50] as well as other inflammatory diseases such as obesity [51]. A trend towards reduced alpha diversity, as measured by Shannon index, was also previously reported in RRMS patients with active disease. That dysbiosis normalized during remission [24]. In contrast, others observed shifts in beta diversity without change in alpha diversity in MS patients [[25], [26], [27]]. This variability likely relates to the heterogeneous nature of the disease and underscores the importance of careful patient recruitment and incorporation of clinical metadata into microbiome analyses. Our patient cohort included a subset of patients with active disease (Table 1), and this may contribute to our observed shift in alpha diversity. Further work is necessary to determine whether shifts in alpha diversity are by-products of an activated immune response or whether they drive autoimmunity and disease activity.
Gut dysbiosis commonly manifests as a reduction in community complexity, with a decrease in short chain fatty acid (SCFA) producing bacteria [52]. We observed reductions in several SCFA producers including Lachnospiraceae (genus Coprococcus), Ruminococcaceae, and Clostridiaceae (genus Clostridium) (Fig. 2). A similar reduction of SCFA producers was noted previously [22,44]. SCFAs are important immunoregulators with local and systemic effects [53]. For example, they promote FoxP3 expression, Treg differentiation [[54], [55], [56]] and blood brain barrier integrity [57,58], while attenuating the frequency of Th1 cells [59,60]. They also mitigate the severity of experimental autoimmune encephalomyelitis, an animal model for MS [59,60]. Moreover, Tregs have a reduced suppressive capacity in MS patients [[61], [62], [63]]. Given these observations, SCFA dysregulation resulting from gut dysbiosis may contribute to the reduced Treg suppression and enhanced Th1 responses observed in MS.
SCFA may also be affected indirectly by gut dysbiosis. We observed reduced Methanobrevibacter among MS patients (Fig. 2). These species convert hydrogen to methane, promoting carbohydrate fermentation and SCFA production [64]. Nevertheless, the role of Methanobrevibacter remains to be elucidated, as others observed increased prevalence in MS [27]. The discrepancy may be partially attributed to technical differences. Methanobrevibacter is also associated with constipation and thus its detection may depend on the condition of the stool sample [65]. Future studies are necessary to directly measure SCFAs in MS patients and determine if these metabolites contribute to Treg dysfunction. Other bacterial metabolites may also prove important to disease pathogenesis, and thorough metabolomic characterization is needed.
In our cohort, MS patients had increased expression of CXCR3 on both CD4 and CD8 T cells (Fig. 3a), with a pronounced Th1, Th1–Th17, and cTfh1 bias, as defined by expression of CXCR3 and CXCR5 (Fig. 3b). Increased CXCR3+ Th1 cells were previously reported in MS peripheral blood [66] and cerebral spinal fluid [67] and are known to be spatially associated with demyelination [68]. The increase in cTfh1 cells but not cTfh17 cells had not previously been observed [30,69] (Fig. 3b). Unlike cTfh17 cells, cTfh1 cells produce IFNγ and do not support antibody production [31]. Increased CXCR3 expression was also observed on CD8 T cells (Fig. 3a). As with CD4 T cells, CXCR3 expression in CD8 cells represents prior activation, IFNγ producing capacity and the ability to home to Th1 inflammatory sites [70]. Future studies will need to examine the contributions of cTfh1 and CXCR3+ CD8 T cells to MS pathogenesis.
The α4β7 integrin is a general marker of gut-homing [33,71]. Within the circulating memory CD4 population, α4β7+ CXCR3+ Th1 and Th1-17 cells were increased relative to α4β7+ CCR6+ Th17 cells, indicating that peripheral Th1 cells may be more likely to migrate via the gut than Th17 cells (Fig. 4a). These cells have potential to be pathogenic, as studies have identified α4β7+ memory CD4 T cells in human CSF [72]. Moreover, CXCL10, a CXCR3 ligand, is present in the CSF and lesions of patients and is spatially correlated with demyelination [66,68].
Animal models of MS have directly linked gut microbiota with the functional status of immune cells. When human gut microbes were transplanted to a mouse model of spontaneous brain inflammation, microbiota originating from MS patients were more likely to induce disease than those from their healthy identical twin sibling. Murine lymphocytes were also less able to produce IL-10 after the animals received fecal transplants from MS donors, confirming that the gut microbiome has immunomodulatory effects [25,26]. Th1/Th2 balance is regulated by the gut microbiota [73] and a diet rich in long chain fatty acids (associated with Western diets) also induces a Th1 phenotype and worsens experimental autoimmune encephalomyelitis [59]. Our observations build upon these animal studies and support a relationship between bacterial dysbiosis and immune dysregulation in humans.
The functional significance of dysbiosis for autoimmune disease is still emerging. There are many potential mechanisms through which microbial shifts could have systemic effects. For instance, cross-reactivity (or molecular mimicry) may exist between autoantigen-specific T cells and gut microbiota. Cross-reactivity between Epstein-Barr Virus (EBV) and myelin antigens has long been cited as underlying the development of myelin-specific T-cells in MS. While cross-reactivity is difficult to impossible to directly prove as a causal mechanism in human MS, recent studies (outside of MS) highlight that the billions of unique peptides present in the commensal proteome can be a source for cross-reactivity in auto-reactive settings [[74], [75], [76]] and that candidate antigens with CNS cross-reactivity can be found in the gut [77,78].
Recent work illustrates that gut-derived plasma cells access the CNS in animal models of MS [79]. Future work will determine whether auto-reactive, gut-primed Th1 cells are also recruited to the CNS. Indeed, T-cells are known to migrate into inflamed tissues irrespective of their initial activation site [42]. Additional work will also be needed to establish whether the magnitude of taxonomic shifts observed in MS is likely to convey functional significance. The abundance of bacteria in the gut is often unconnected to their physiologic significance, as phyla comprising a tiny fraction of the microbiome may be disproportionately active metabolically and play major roles in host physiology. Our findings in Fig. 2 agree with other MS human microbiome studies, notably, a decrease in several SCFA producers including Lachnospiraceae (genus Coprococcus), Ruminococcaceae, and Clostridiaceae (genus Clostridium). Follow-up studies will need to evaluate metabolic shifts associated with these taxonomic shifts.
This study correlated changes in the gut microbiome with alterations in circulating inflammatory T cells among MS patients. However, it is important to note that additional studies will be needed to evaluate causation. The extent of gut dysbiosis identified in MS patients using 16S rRNA sequencing is mild to moderate and is inconsistent across studies [22,[24], [25], [26], [27]]. Cross-sectional data, small study sizes, lack of species-level resolution, different experimental protocols and varying geographical locations contribute to this heterogeneity. It is also likely that the metabolic by-products of the microbiome may ultimately prove more important for immune cell activation than individual bacterial taxa. Studies are underway to address these limitations. Understanding the gut/immune axis is likely to be essential for understanding the genetic/environmental interactions in MS disease pathogenesis as well as in explaining the individual level heterogeneity observed in disease severity and response to treatment.
Funding
This work was supported by grants to D.A.H. from the National Institutes of Health (U19 AI089992, R25 NS079193, P01 AI073748, U24 AI11867, R01 AI22220, UM 1HG009390, P01 AI039671, P50 CA121974, R01 CA227473), and the National Multiple Sclerosis Society (NMSS) (CA 1061-A-18, RG-1802-30153), the Nancy Taylor Foundation for Chronic Diseases, and Race to Erase MS.
Study sponsors were not involved in the study design, data collection/interpretation, the decision to submit for publication or the writing of the report.
Author roles
SNC: Conceptualization, investigation, formal analysis, writing original draft, review & editing. MK: Investigation, writing – review & editing. KR: Investigation, formal analysis, writing – review & editing. DAH: Conceptualization, resources, supervision, writing – review & editing. WER: Investigation, writing – original draft, review & editing. EEL: Investigation, writing – original draft, review & editing.
Declaration of competing interest
SNC, MK, KR and WER report no financial disclosures.
DAH has in the past 10 years consulted for the following companies: Bayer Pharmaceuticals, Biohaven Pharmaceuticals, Bristol Myers Squibb, Compass Therapeutics, Eisai Pharmaceuticals, EMD Serono, Genentech, Juno Therapeutics, McKinsey & Co., MedImmune/AstraZeneca, Mylan, Pharmaceuticals, Neurophage Pharmaceuticals, NKT Therapeutics, Novartis Pharmaceuticals, Proclara Biosciences, Questcor Pharmaceuticals, Roche, Sage Therapeutics, Sanofi Genzyme, Toray Industries, Versant Venture.
EEL has served as a consultant for: EMD Serono, Genzyme, Genentech, Biogen, Teva, Celegene and Alexion. She has received research funding from Race to Erase MS and NIH K23NS107624.
Acknowledgements
The authors would like to thank Adam Rosenstein, Tayyab Shah and Benjamin Newman for their work on this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2019.100032.
Contributor Information
William E. Ruff, Email: william.ruff@yale.edu.
Erin E. Longbrake, Email: erin.longbrake@yale.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
S. Fig. 1.
Taxonomy summary of control and MS patient fecal samples. Taxonomy summary of rarefied relative abundance of fecal bacteria of controls (n = 39) and untreated MS patients (n = 26) at the genus level.1
S. Fig. 2.
Rarefied counts of fecal bacteria in controls and patients with MS. Dot plots represent the bacterial counts for MS (n = 26) and controls (n = 39) subjects. f_ and g_ represent undefined family and genus.
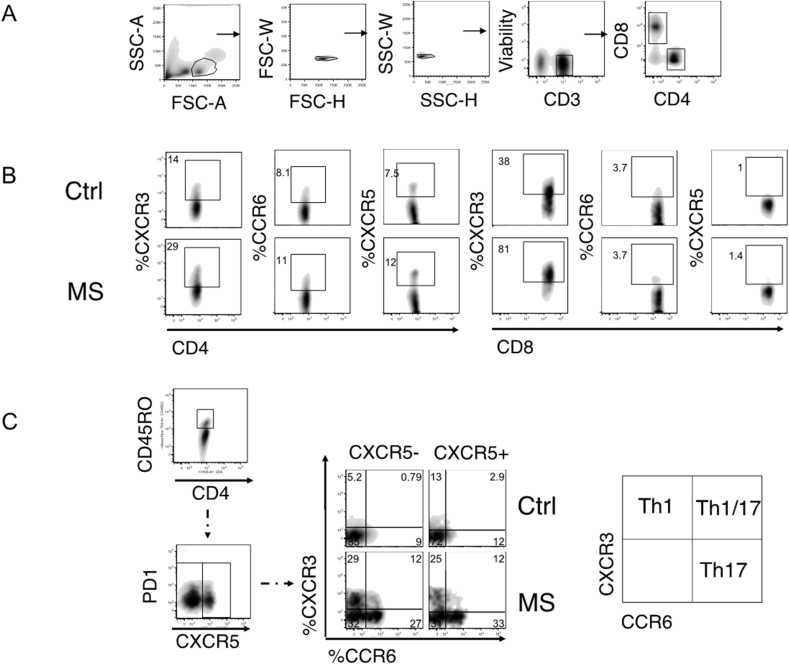
S. Fig. 3.
Flow cytometry gating strategy and representative examples. a) Gating strategy for total CD4 and CD8 T cells. One representative sample is shown. b) Representative plots of the % of CXCR3, CCR6 and CXCR5 chemokine expression from total CD4 and CD8 T cells from healthy controls (Ctrl) and MS patients (MS). c) Representative sample of CXCR5— and CXCR5+ gating on memory T cells (left panel). Representative plots from Ctrl and MS patient showing the % of CD45RO+CXCR5— or CD45RO+CXCR5— Th1, Th1/17 and Th17 populations.
S. Fig. 4:
Flow cytometry gating strategy of homing receptors. a) Gating strategy of α4β7+ is shown for CD4+CXCR5— T cells. CXCR5— T cells are subdivided into Th1 (CXCR3+CCR6—), Th1/Th17 (CXCR3+CCR6+) and Th17 (CXCR3—CCR6+) cells. b) Frequencies of α4β7+ cell in Th subsets from patients (red bars) and controls (gray bars; left panel), frequencies of α4β7+ cells in CXCR3+ and CCR6+ CD8 T cells (right panel).4
References
- 1.Lodygin D., Hermann M., Schweingruber N., Flugel-Koch C., Watanabe T., Schlosser C. beta-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature. 2019;566:503–508. doi: 10.1038/s41586-019-0964-2. [DOI] [PubMed] [Google Scholar]
- 2.Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 3.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple sclerosis. N. Engl. J. Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nylander A., Hafler D.A. Multiple sclerosis. J. Clin. Investig. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.C. International Multiple Sclerosis Genetics. Hafler D.A., Compston A., Sawcer S., Lander E.S., Daly M.J. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 6.Patsopoulos N., Baranzini S.E., Santaniello A., Shoostari P., Cotsapas C., Wong G. 2017. The Multiple Sclerosis Genomic Map: Role of Peripheral Immune Cells and Resident Microglia in Susceptibility. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International c. c. y. e. Multiple sclerosis genetics consortium. Electronic address, C. International multiple sclerosis genetics. Low-frequency and rare-coding variation contributes to multiple sclerosis risk. Cell. 2018;175:1679–16787 e7. doi: 10.1016/j.cell.2018.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson T., Barcellos L.F., Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 9.Marson A., Housley W.J., Hafler D.A. Genetic basis of autoimmunity. J. Clin. Investig. 2015;125:2234–2241. doi: 10.1172/JCI78086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumida T., Lincoln M.R., Ukeje C.M., Rodriguez D.M., Akazawa H., Noda T. Activated beta-catenin in Foxp3(+) regulatory T cells links inflammatory environments to autoimmunity. Nat. Immunol. 2018;19:1391–1402. doi: 10.1038/s41590-018-0236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wekerle H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017;38:483–497. doi: 10.1016/j.it.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y., Goods B.A., Raddassi K., Nepom G.T., Kwok W.W., Love J.C. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7:287ra74. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ota K., Matsui M., Milford E.L., Mackin G.A., Weiner H.L., Hafler D.A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 14.Martin R., Jaraquemada D., Flerlage M., Richert J., Whitaker J., Long E.O. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J. Immunol. 1990;145:540–548. [PubMed] [Google Scholar]
- 15.Pette M., Fujita K., Kitze B., Whitaker J.N., Albert E., Kappos L. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- 16.Sun J.B., Olsson T., Wang W.Z., Xiao B.G., Kostulas V., Fredrikson S. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur. J. Immunol. 1991;21:1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Markovic-Plese S., Lacet B., Raus J., Weiner H.L., Hafler D.A. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J. Exp. Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raddassi K., Kent S.C., Yang J., Bourcier K., Bradshaw E.M., Seyfert-Margolis V. Increased frequencies of myelin oligodendrocyte glycoprotein/MHC class II-binding CD4 cells in patients with multiple sclerosis. J. Immunol. 2011;187:1039–1046. doi: 10.4049/jimmunol.1001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backhed F., Fraser C.M., Ringel Y., Sanders M.E., Sartor R.B., Sherman P.M. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Ruff W.E., Kriegel M.A. Autoimmune host-microbiota interactions at barrier sites and beyond. Trends Mol. Med. 2015;21:233–244. doi: 10.1016/j.molmed.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantarel B.L., Waubant E., Chehoud C., Kuczynski J., DeSantis T.Z., Warrington J. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med. 2015;63:729–734. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Chia N., Kalari K.R., Yao J.Z., Novotna M., Paz Soldan M.M. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cekanaviciute E., Yoo B.B., Runia T.F., Debelius J.W., Singh S., Nelson C.A. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. U. S. A. 2017;114:10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. U. S. A. 2017;114:10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jangi S., Gandhi R., Cox L.M., Li N., von Glehn F., Yan R. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reboldi A., Coisne C., Baumjohann D., Benvenuto F., Bottinelli D., Lira S. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 29.Dominguez-Villar M., Baecher-Allan C.M., Hafler D.A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunill V., Massot M., Clemente A., Calles C., Andreu V., Nunez V. Relapsing-remitting multiple sclerosis is characterized by a T follicular cell pro-inflammatory shift, reverted by dimethyl fumarate treatment. Front. Immunol. 2018;9:1097. doi: 10.3389/fimmu.2018.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babbe H., Roers A., Waisman A., Lassmann H., Goebels N., Hohlfeld R. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bargatze R.F., Jutila M.A., Butcher E.C. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 34.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U. S. A. 2011;108(1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullen T.W., Schofield W.B., Barry N.A., Putnam E.E., Rundell E.A., Trent M.S. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 2015;347:170–175. doi: 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahi S.K., Freedman S.N., Mangalam A.K. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microb. 2017:1–9. doi: 10.1080/19490976.2017.1349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 42.Masopust D., Schenkel J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 43.Habtezion A., Nguyen L.P., Hadeiba H., Butcher E.C. Leukocyte trafficking to the small intestine and colon. Gastroenterology. 2016;150:340–354. doi: 10.1053/j.gastro.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tremlett H., Fadrosh D.W., Faruqi A.A., Zhu F., Hart J., Roalstad S. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol. 2016;23:1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 46.Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozupone C.A., Stombaugh J., Gonzalez A., Ackermann G., Wendel D., Vazquez-Baeza Y. Meta-analyses of studies of the human microbiota. Genome Res. 2013;23:1704–1714. doi: 10.1101/gr.151803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Goffau M.C., Luopajarvi K., Knip M., Ilonen J., Ruohtula T., Harkonen T. Fecal microbiota composition differs between children with -cell autoimmunity and those without. Diabetes. 2012;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kostic A.D., Gevers D., Siljander H., Vatanen T., Hyotylainen T., Hamalainen A.M. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scher J.U., Ubeda C., Artacho A., Attur M., Isaac S., Reddy S.M. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheum. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kriss M., Hazleton K.Z., Nusbacher N.M., Martin C.G., Lozupone C.A. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly Y.M. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 57.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H., Sun J., Wang F., Ding G., Chen W., Fang R. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016;1642:70–78. doi: 10.1016/j.brainres.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Haghikia A., Jörg S., Duscha A., Berg J., Manzel A., Waschbisch A. Dietary fatty acids directly impact central nervous system Autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno M., Noto D., Kaga N., Chiba A., Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Astier A.L., Meiffren G., Freeman S., Hafler D.A. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J. Clin. Investig. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venken K., Hellings N., Thewissen M., Somers V., Hensen K., Rummens J.L. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansen E.E., Lozupone C.A., Rey F.E., Wu M., Guruge J.L., Narra A. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc. Natl. Acad. Sci. U.S.A. 2011;108(1):4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attaluri A., Jackson M., Valestin J., Rao S.S. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol. 2010;105:1407–1411. doi: 10.1038/ajg.2009.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balashov K.E., Rottman J.B., Weiner H.L., Hancock W.W. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorensen T.L., Tani M., Jensen J., Pierce V., Lucchinetti C., Folcik V.A. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorensen T.L., Trebst C., Kivisakk P., Klaege K.L., Majmudar A., Ravid R. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J. Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 69.Romme Christensen J., Bornsen L., Ratzer R., Piehl F., Khademi M., Olsson T. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groom J.R., Luster A.D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Andrian U.H., Mackay C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 72.Kivisakk P., Tucky B., Wei T., Campbell J.J., Ransohoff R.M. Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: relevance for immunotherapy. BMC Immunol. 2006;7:14. doi: 10.1186/1471-2172-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Greiling T.M., Dehner C., Chen X., Hughes K., Iniguez A.J., Boccitto M. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gil-Cruz C., Perez-Shibayama C., De Martin A., Ronchi F., van der Borght K., Niederer R. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881–886. doi: 10.1126/science.aav3487. [DOI] [PubMed] [Google Scholar]
- 76.Ruff W.E., Dehner C., Kim W.J., Pagovich O., Aguiar C.L., Yu A.T. Pathogenic autoreactive T and B cells cross-react with mimotopes expressed by a common human gut commensal to trigger autoimmunity. Cell Host Microbe. 2019;26:100–113 e8. doi: 10.1016/j.chom.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes L.E., Smith P.A., Bonell S., Natt R.S., Wilson C., Rashid T. Cross-reactivity between related sequences found in Acinetobacter sp., Pseudomonas aeruginosa, myelin basic protein and myelin oligodendrocyte glycoprotein in multiple sclerosis. J. Neuroimmunol. 2003;144:105–115. doi: 10.1016/s0165-5728(03)00274-1. [DOI] [PubMed] [Google Scholar]
- 78.Planas R., Santos R., Tomas-Ojer P., Cruciani C., Lutterotti A., Faigle W. GDP-l-fucose synthase is a CD4(+) T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aat4301. [DOI] [PubMed] [Google Scholar]
- 79.Rojas O.L., Probstel A.K., Porfilio E.A., Wang A.A., Charabati M., Sun T. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. 2019;177:492–493. doi: 10.1016/j.cell.2019.03.037. [DOI] [PubMed] [Google Scholar]