Abstract
Introduction
This pilot study evaluated the impact of a diabetes-specific nutritional shake (DSNS) used twice daily by people with type 2 diabetes (T2D) on glycemic response assessed by continuous glucose monitoring (CGM).
Research design and methods
Adults (n=81) with T2D managed by oral medications were studied in a randomized, open-label, three-group parallel study design. The study was conducted in two phases over 14 days: Baseline (days 1–6), during which study participants consumed their habitual self-selected diets (SSD), followed by the Intervention (days 7–14), during which participants were randomized as follows: (1) SSD group received no study product (n=32); (2) DSNS breakfast/afternoon snack (Bkfst/AS) group consumed one DSNS as a breakfast meal replacement and a second to replace their mid-afternoon snack (n=24); (3) DSNS breakfast/prebed snack (Bkfst/PBS) group consumed one DSNS as a breakfast meal replacement and added a second as a prebed snack (n=25). Glucose was assessed by CGM throughout the study. Additionally, participants were asked about snacking behaviors, cravings, and other questions related to the use of DSNS as meal replacements and snacks.
Results
All groups reduced their postprandial glycemic response (positive area under the curve (pAUC, mg/min*dL−1)) and adjusted peak value (mg/dL) when compared with the baseline phase. Participants consuming DSNS in place of their usual breakfast showed greater reductions in pAUC compared with the SSD group (p=0.008) for the DSNS Bkfst/AS group with a trend (p=0.069) for the DSNS Bkfst/PBS group. Adjusted peak value showed greater reductions in both DSNS groups as compared with the SSD group (p=0.002 for DSNS Bkfst/AS and p=0.010 for DSNS Bkfst/PBS). Nocturnal glucose variability was significantly decreased during the intervention phase compared with baseline phase in the DSNS Bkfst/AS group (p=0.020), with no significant differences between groups. After intervention, the DSNS Bkfst/AS group had a significantly lower percentage of participants (17%) reporting cravings for starchy meals/sides compared with before the study (33%) (p=0.046). This group also reported a significant increase in confidence in choosing foods to control their diabetes (from 58.3% to 91.7%, preintervention vs postintervention, respectively, p=0.005).
Conclusions
Use of DSNS to replace breakfast and as an afternoon snack improves both glycemic control and behavioral factors related to dietary management of diabetes.
Trail registration number
Keywords: type 2 diabetes, continuous glucose monitoring, self-management, nutrition intervention
Significance of this study.
What is already known about this subject?
Diabetes-specific nutritional shakes (DSNS) are clinically shown to improve postprandial glucose responses under rigorously controlled experimental conditions. However, it is unknown how replacing meals and snacks with DSNS impacts blood glucose across the day in free-living people with diabetes eating their own diets.
What are the new findings?
The results of this pilot trial using continuous glucose monitoring provide the first evidence in free-living people with diabetes controlled by oral medications only that replacing a daily breakfast and snack with DSNS has relevant benefits on both dietary and glucose management.
Glucose responses at breakfast (positive area under the curve and adjusted peak) improved when subjects replaced their usual breakfast with DSNS compared with the No-Product group.
Subjects who replaced breakfast and a snack with DSNS showed reduced night-time glucose variability compared with baseline period.
Subjects who replaced one meal and one afternoon snack per day with DSNS significantly reduced cravings for starchy foods compared with baseline period.
How might these results change the focus of research or clinical practice?
Optimizing use of DSNS as a dietary approach to manage glycemic control in people with type 2 diabetes may add valuable information to both patients and healthcare providers.
Introduction
Diabetes is a complex disease requiring patients to self-manage their diet, lifestyle, and self-care behaviors in combination with use of antihyperglycemic medications.1–4 Careful attention to diet is an important step toward improving glycemic control. Both the amount and type of carbohydrate affect postprandial glycemia. However, for many patients, determining what to eat and what meal plan to follow is challenging.
Meal replacements are helpful for people with diabetes for being convenient and for providing known calorie amounts with specific macronutrient and micronutrient levels that facilitate meal planning.2 3 Diabetes-specific nutritional shakes (DSNS) are designed with type and amount of carbohydrate and other macronutrient composition to provide quality nutrition while minimizing the impact on postprandial glycemic levels.4–6 Several studies have shown that, in addition to their effects on serum glucose, DSNS may have unique benefits on important gastrointestinal and pancreatic hormones, such as insulin and glucagon-like peptide 1 (GLP-1), and on serum free fatty acids, which mediate glucose metabolism and insulin sensitivity.6–8
Several studies showed that use of meal replacements, including DSNS, to replace one or two meals as part of intensive lifestyle interventions significantly reduced A1C and reduced the need for medications.9–11 In people with type 2 diabetes (T2D), blood glucose is frequently higher in the morning than at other times during the day.12 Additionally, this particular meal may be also skipped or contains high glycemic index carbohydrates and/or saturated fats. For these reasons, one suggested approach has been to use meal replacements for breakfast.13 14 However, there is little evidence on the benefits of using DSNS at any different times during the day.
Optimizing use of DSNS as a dietary approach to manage glycemic control in people with T2D may add valuable information to both patients and healthcare providers. This pilot study was intended to explore the impact of DSNS, used twice daily to replace breakfast and as an afternoon or evening/prebed snack, on glycemic responses assessed over several days by continuous glucose monitoring (CGM). Additional objectives were to examine the potential effects of DSNS on other relevant barriers to successful diabetes self-management.
Research design and methods
Study design
This pilot study is a randomized, multicenter, open-label, parallel, three-arm study conducted at eight clinical centers across North America. Adult participants (at least 30 years of age, male and female), having T2D diabetes, A1C between 7% and 10% and managed by oral antihyperglycemic medications were enrolled. We excluded participants with active disease (cardiovascular, renal, hepatic, cancer), those who were pregnant, night shift workers or following atypical eating pattern other than three main meals and snacks.
The study was conducted in two phases over 14 consecutive days: Baseline (days 1–6) during which participants followed their habitual self-selected diets (SSD) and interstitial glucose data were collected using FreeStyle Libre Pro CGM system (Abbott Diabetes Care, Alameda, CA), which were blinded to participants and staff. During Baseline, participants were asked to maintain their usual diabetes management behaviors (eg, medications, exercise) and typical diet with three main meals and two daily snacks (to be consumed mid-morning and mid-afternoon). This was immediately followed by the Intervention phase (days 7–14) during which participants were randomized, stratified by gender and medication type, to one of three groups: (1) SSD group, in which participants received no study product and were asked to maintain their typical diet and eating patterns as they did during Baseline (n=32); (2) DSNS breakfast/afternoon snack (Bkfst/AS), in which participants were instructed to consume one DSNS as a breakfast meal replacement and one DSNS to replace their mid-afternoon snack (n=24); and (3) DSNS breakfast/prebed snack (Bkfst/PBS), in which participants were instructed to consume one DSNS as a breakfast meal replacement and one DSNS to add as a bedtime snack (n=25). Both groups 2 and 3 replaced the mid-afternoon snack by a DSNS which was consumed either in the afternoon (group 2) or before going to bed (group 3). Study subjects in groups 2 and 3 were not asked to change their mid-morning snacks.
The DSNS used in this study was Glucerna Hunger Smart (Abbott Nutrition, Columbus, Ohio). The nutrition information of this DSNS is shown in the online supplementary table.
bmjdrc-2020-001258supp001.pdf (86KB, pdf)
Participants filled out daily logs to record time of each eating occasion (breakfast, lunch, dinner, and snacks), the wake-up time and the bedtime. Participants’ daily logs were used to identify postprandial glycemic periods and to mark diurnal and nocturnal time frames.
Glucose variables
Unless otherwise specified, glucose variables reflect the average of each participant’s interstitial glucose values obtained by CGM. Postprandial glucose for each meal or snack continued until 120 min after meal/snack. Glucose variability was assessed as the mean amplitude of glycemic excursion, calculated as the arithmetic mean of the differences between consecutive peaks and nadirs (ie, an excursion) using only those excursions for which both segments exceed 1 SD of the blood glucose for the same time period.15
Self-reported outcomes
Questionnaires were administered for participants to comment on several aspects related to diabetes dietary behaviors including snacking, cravings, and confidence in managing their diabetes through diet. At the screening visit, participants were asked to indicate their habitual snacking frequency, time of day and reasons for snacking. To evaluate cravings, participants indicated their frequency of cravings for specific types of foods (salty snacks, chocolate/candy, starchy meals/sides, baked goods, ice cream) during the previous week, before, and after the study, using the Likert scale of 1=Never; 2=Rarely; 3=Sometimes; 4=Frequently; 5=Always. Finally, participants responded to questions asking about their ‘confidence in choosing foods for diabetes control’ during the previous week, before, and after the study using the Likert scale as described above.
Statistical methods
For this pilot study, 30 evaluable participants per group were planned, with 40 participants per group to be randomized, allowing for up to 25% attrition. Evaluability was defined a priori and required participants to have completed both baseline and intervention phases with a minimum of five complete 24 hours ‘days’ (from 24:00 to 23:59 of each successive day) of glucose data and 80% completed daily records across each study phase. Analyses of continuous outcome data included the randomization strata of gender and antihyperglycemia medication as blocking factors, as well as covariates of age, body mass index (BMI), and duration of diabetes. Analysis of the calculated change also included the baseline value as a covariate. Data that were not normally distributed (with/without transformation) were analyzed using non-parametric methods. A p value <0.05 was considered statistically significant and data with a p value of 0.05–0.20 were considered to point out ‘trends’.
Results
Study participants
There were 125 participants randomized with 116 completing the study. A total of 81 participants were evaluated across both baseline and intervention phases. Figure 1 depicts the Consolidated Standards of Reporting Trials flow diagram. Per protocol, evaluable participants were those who were at least 80% adherent to study instructions. During the baseline phase (average 5.0±0.1 days), evaluable participants consumed three main meals with two self-selected snacks (mid-morning between breakfast and lunch and mid-afternoon between lunch and dinner). During the intervention phase (average 6.0±0.1 days), product intake in both DSNS groups averaged at 2.0±0.01 servings per day.
Figure 1.
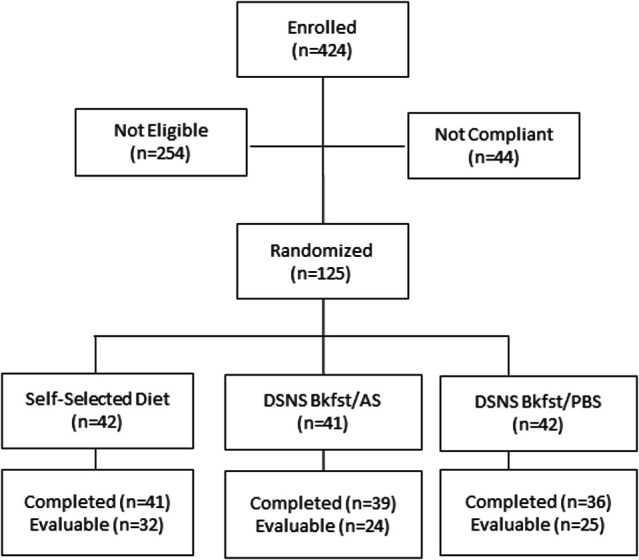
Study Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Bkfst/AS, breakfast/afternoon snack; Bkfst/PBS, breakfast/prebed snack; DSNS, diabetes-specific nutritional shake.
Baseline characteristics are described in table 1; male (59%) and female (41%) participants ranged in age from 40 to 79 years, with BMI ranging from 25 to 40 kg/m2 and A1C from 7% to 10%. Participants were taking oral antihyperglycemic medications as follows: 90% metformin, 53% sulfonylureas, 4% thiazolidinediones. Duration of diabetes ranged from less than 1 to 37 years. Significant differences were observed between the SSD and DSNS Bkfst/PBS groups in BMI (p=0.0071), and duration of diabetes (p=0.0434).
Table 1.
Baseline characteristics among study participants (mean±SD)
| Self-selected diet (n=32) |
DSNS Bkfst/AS (n=24) |
DSNS Bkfst/PBS (n=25) |
Total (n=81) |
|
| Gender, % male | 56 | 58 | 64 | 59 |
| Age (years) | 61±8 | 62±10 | 64±10 | 62±9 |
| BMI | 30±4 | 32±4 | 33±4*§ | 32±4 |
| Waist circumference (cm) | 41±4 | 42±4 | 43±4† | 42±4 |
| Duration of diabetes (years) | 8±6 | 11±8 | 14±10* | 11±8 |
| Measured A1C (%) | 7.9±0.8 | 7.8±0.7 | 7.8±0.7 | 7.8±0.7 |
| Oral antihyperglycemic medications, number of doses/day | 2.2±0.9 | 2.7±1.1 | 2.5±1.0 | 2.4±1.0 |
| Metformin (%) | 87 | 87 | 96 | 90 |
| Thiazolidinones (%) | 6 | 4 | 0 | 4 |
| Sulfonylurea (%) | 47 | 58 | 56 | 53 |
| Ethnicity, % Hispanic | 12 | 21 | 4 | 12 |
| Race (%) | ||||
| White | 72 | 67 | 72 | 70 |
| Black | 25 | 17 | 20 | 21 |
| Asian | 3 | 12 | 0 | 5 |
| American Indian | 0 | 4 | 0 | 1 |
| Other | 0 | 0 | 8 | 2 |
Baseline differences between groups are as follows:
*Significantly different versus self-selected diet group, p<0.05.
†Different versus self-selected diet group, p>0.05 and p<0.2.
‡Different versus DSNS Bkfst/AS group, p>0.05 and p<0.2
Bkfst/AS, breakfast/afternoon snack; Bkfst/PBS, breakfast/prebed snack; BMI, body mass index; DSNS, diabetes-specific nutritional shake.
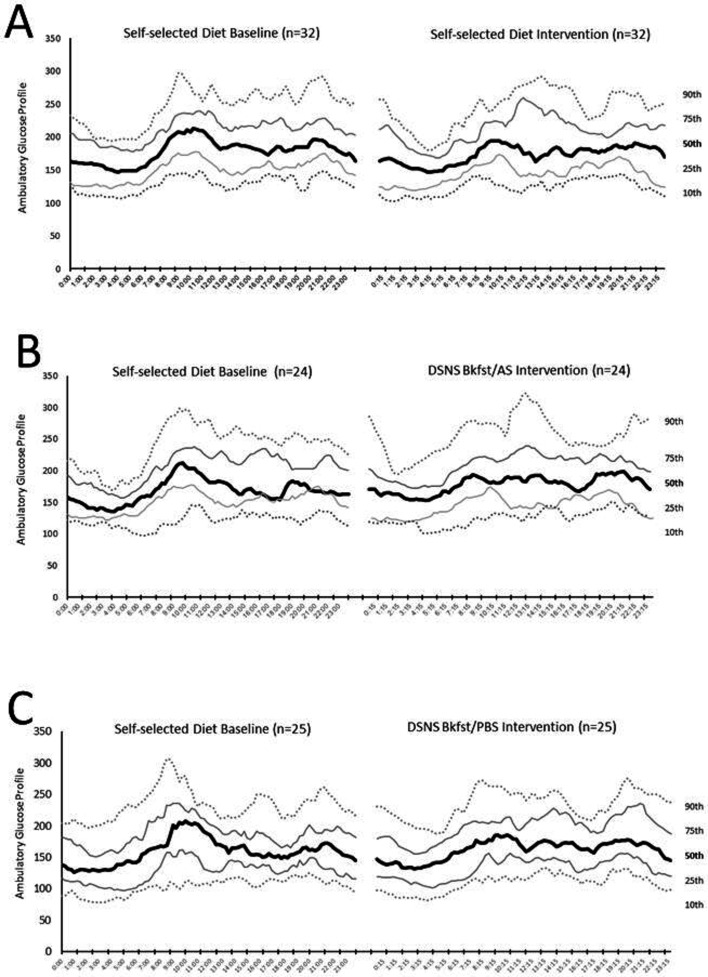
Effect of dietary interventions on glycemic responses
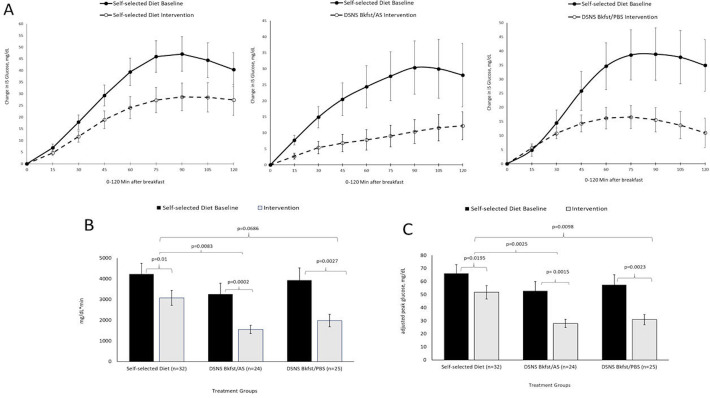
Evaluation of the ambulatory glucose profiles reveals a consistent late-morning/early afternoon elevation in glucose levels, particularly prominent during the baseline phase (figure 2). There was no significant difference in preprandial (fasting) glucose levels among the three study groups (table 2). In the intervention phase, all three groups reduced their 120 min postprandial glycemic response (positive area under the curve (pAUC, mg/min*dL−1)) and adjusted peak value (the greatest change in interstitial glucose within 2 hours after the meal, compared with the interstitial glucose value before the meal, mg/dL) when compared with the baseline phase (figure 3 and table 2). Participants consuming DSNS in place of their usual breakfast showed greater reductions in pAUC compared with the SSD group (p=0.008) for the DSNS Bkfst/AS group with a non-significant trend (p=0.069) for the DSNS Bkfst/AS group. Adjusted peak value showed greater reductions in both DSNS groups as compared with the SSD group (p=0.002 for DSNS Bkfst/AS and p=0.010 for DSNS Bkfst/PBS). While pAUC was analyzed after breakfast, lunch and dinner for all groups, greater reductions in pAUC compared with the SSD group were only shown when participants consumed DSNS to replace their usual breakfast. After lunch and dinner reduction in pAUC was not seen for participants consuming DSNS. It is important to mention that a consistent elevation in glucose levels was only seen for all groups after breakfast and consequently differences among groups could have been detectable. While no significant changes in diurnal glucose variability were observed in either group, nocturnal glucose variability was significantly decreased during the intervention phase compared with baseline phase in the DSNS Bkfst/AS group (p=0.020), with no significant differences between groups.
Figure 2.
Composite ambulatory glucose profiles for all participants in each treatment group during self-selected diet baseline (5+0.1 days) and during intervention phase (6+0.1 days). (A) Self-selected diet. (B) DSNS Bkfst/AS. (C) DSNS Bkfst/PBS. Bkfst/AS, breakfast/afternoon snack; Bkfst/PBS, breakfast/prebed snack; DSNS, diabetes-specific nutritional shake.
Table 2.
Glycemic response results (mean±SEM)
| Variable and treatment groups | Baseline (5±0.1 days) |
Intervention (6±0.1 days) |
Change versus baseline | P value* versus baseline | P value† versus self-selected diet |
| Preprandial (fasting) glucose (mg/dL) | |||||
| Self-selected diet | 184.3±8.0 | 177.4±7.5 | −6.9±4.0 | 0.1193 | – |
| DSNS Bkfst/AS | 186.3±12.8 | 182.0±12.2 | −4.4±5.4 | 0.2368 | 0.5713 |
| DSNS Bkfst/PBS | 173.5±9.8 | 168.3±9.8 | −5.2±5.4 | 0.2714 | 0.7770 |
| Breakfast, positive AUC (mg/dL*min, 0–120 min)‡ | |||||
| Self-selected diet | 4237±514 | 3074±364 | −1162±422 | 0.0100 | – |
| DSNS Bkfst/AS | 3258±529 | 1551±198 | −1708±496 | 0.0002 | 0.0083 |
| DSNS Bkfst/PBS | 3928±596 | 1978±301 | −1950±582 | 0.0027 | 0.0686 |
| Breakfast, adjusted peak value (mg/dL)‡ | |||||
| Self-selected diet | 66.2±6.8 | 51.7±5.1 | −14±6 | 0.0195 | – |
| DSNS Bkfst/AS | 52.7±7.2 | 27.9±3.1 | −25±7 | 0.0015 | 0.0025 |
| DSNS Bkfst/PBS | 57.5±7.7 | 30.8±4.0 | −27±8 | 0.0023 | 0.0098 |
| Daytime§ variability (MAGE, mg/dL) | |||||
| Self-selected diet | 106.5±5.2 | 100.5±4.1 | −6.0±3.6 | 0.1018 | – |
| DSNS Bkfst/AS | 104.5±6.5 | 98.6±6.9 | −5.9±4.6 | 0.2145 | 0.9586 |
| DSNS Bkfst/PBS | 99.5±6.0 | 88.8±5.9 | −10.7±5.9 | 0.0803 | 0.3370 |
| Nocturnal variability (MAGE, mg/dL) | |||||
| Self-selected diet | 63.6±5.4 | 61.4±4.5 | −2.2±4.0 | 0.5936 | – |
| DSNS Bkfst/AS | 70.8±8.8 | 59.5±7.2 | −11.3±4.5 | 0.0204 | 0.4623 |
| DSNS Bkfst/PBS | 60.7±5.1 | 54.9±4.2 | −5.9±4.2 | 0.1793 | 0.7445 |
*Paired t-test or signed-rank test if a variable was declared non-normal by Shapiro-Wilk test (p<0.001).
†Analysis of covariance. The significance level was adjusted for multiple comparisons of treatment group groups using Tukey-Kramer p value adjustments.
‡Begins with the first time point collected after meal and continues until 120 min after meal.
§Daytime (starting from time of waking to time subject went to bed); nocturnal (starting from time subject went to bed to time of waking).5
AUC, area under the curve; Bkfst/AS, breakfast/afternoon snack; Bkfst/PBS, breakfast/prebed snack; DSNS, diabetes-specific nutritional shake; MAGE, mean amplitude of glycemic excursion.
Figure 3.
Mean+SEM glucose responses 0–120 min after breakfast (A), positive AUC (B), and peak value (C). Solid line or dark bar reflects glucose responses across the self-selected diet baseline phase (5+0.1 days); dashed line or light bar reflects glucose responses across the intervention phase (6+0.1 days). AUC, area under the curve; Bkfst/AS, breakfast/afternoon snack; Bkfst/PBS, breakfast/prebed snack; DSNS, diabetes-specific nutritional shake; IS, interstitial.
Effect of dietary interventions on patient-reported outcomes
Table 3 summarizes by treatment group the self-reported cravings for specific food groups/tastes as assessed before the study (preintervention, day 0) and again at the end of the study (postintervention, day 14). Before the study, overall cravings were highest for starchy meals/sides category (34.6% of total sample). Next highest cravings were for salty snacks (25.9%), followed by chocolate/candy (19.7%), ice cream (14.8%), and lowest for baked goods (12.3%). After intervention, only the DSNS Bkfst/AS group reported a significantly lower percentage of participants (17%) reporting cravings for starchy meals/sides compared with before the study (33%), (p=0.0455 for within-group comparison). There were no similar changes for the SSD or DSNS Bkfst/PBS groups or for any other food category.
Table 3.
Patient-reported cravings for specific food categories/tastes*
| Response categories | SSD n=32 |
DSNS Bkfst/AS n=24 |
DSNS Bkfst/PBS n=25 |
Total n=81 |
| Starchy meals/sides, n (%) | ||||
| Preintervention | 8 (25.0) | 8 (33.3) | 12 (48.0) | 28 (34.6) |
| Postintervention | 9 (28.1) | 4 (16.6)† | 9 (32.0) | 22 (27.1) |
| Salty snacks, n (%) | ||||
| Preintervention | 12 (37.5) | 3 (12.5) | 6 (24.0) | 21 (25.9) |
| Postintervention | 8 (25.0)‡ | 5 (20.8) | 7 (28.0) | 20 (24.7) |
| Chocolate/candy, n (%) | ||||
| Preintervention | 9 (28.2) | 4 (16.7) | 3 (12.0) | 16 (19.7) |
| Postintervention | 8 (25.0) | 2 (8.3) | 2 (8.0) | 12 (14.8) |
| Ice cream, n (%) | ||||
| Preintervention | 5 (15.6) | 3 (12.5) | 4 (16.0) | 12 (14.8) |
| Postintervention | 4 (12.5) | 2 (8.3) | 3 (12.0) | 9 (11.1) |
| Baked goods, n (%) | ||||
| Preintervention | 4 (12.5) | 3 (12.5) | 3 (12.0) | 10 (12.3) |
| Postintervention | 4 (9.4) | 5 (20.8) | 2 (8.0) | 11 (13.6) |
*Preintervention versus postintervention was analyzed by paired t-test or signed-rank test if a variable was declared non-normal by Shapiro-Wilk test (p<0.001). Between-group comparisons used analysis of covariance. The significance level was adjusted for multiple comparisons of Treatment Group groups using Tukey-Kramer p value adjustments.
†Significantly different, p<0.05, preintervention versus postintervention.
‡Different p≥0.05 and p<0.2, preintervention versus postintervention.
Bkfst/AS, breakfast/afternoon snack; Bkfst/PBS, breakfast/prebed snack; DSNS, diabetes-specific nutritional shake; SSD, self-selected diet.
Before the study intervention, overall 58% of study participants responded they were ‘frequently/always’ confident in choosing foods for diabetes control. After intervention, responses were significantly increased only in the DSNS Bkfst/AS group (91.7% vs 58.3%, postintervention vs preintervention, respectively, p=0.0047). There were no significant changes for the SSD or DSNS Bkfst/PBS groups.
Discussion
This pilot trial shows that participants with T2D managed with oral antihyperglycemic medication can have further improvements in glycemic control with DSNS use. Using CGM technology, participants who replaced their usual breakfast with a DSNS significantly improved their morning postprandial glycemic excursions and, in those who replaced both breakfast and the afternoon snack, nocturnal glucose variability was improved. Additionally, the use of DSNS to replace breakfast and the afternoon snack was associated with an increase in individuals who reported increased confidence in choosing foods with a positive impact on diabetes control and a reduction in those reporting frequent cravings for starchy meals/sides.
Clinical practice guidelines emphasize the importance of nutrition in diabetes management.1–4 However, patients and healthcare professionals are often limited by the complexity of such recommendations due to the confounding effects of personal, cultural, and other behavioral factors affecting dietary adherence.16 Meal replacements can substitute for a solid food meal or snack with a fixed and known level of calories and nutrients and are clinically demonstrated to be effective in people with diabetes. Therefore, they have been incorporated into guidelines to facilitate adherence to medical nutrition therapy recommendations.17
This pilot study extends the existing scientific evidence and shows that replacing a typical breakfast with DSNS favorably impacts postprandial glycemic responses and replacing a second meal (specifically an afternoon snack) by DSNS reduces overnight glucose variability. The improvement in nocturnal glycemic variability observed in the DSNS Bkfst/AS could be explained by changes in eating and/or lifestyle behaviors associated with the daily intake of two meal replacements. The structured daily intake of two DSNS at a specific time during the day may have had an impact reducing the variability between the highest and the lowest glycemic peak at 24 hours and consequently reduce nocturnal glucose variability. Moreover, previous studies have shown that the type and amounts of foods consumed, including at breakfast, may have effects on glucose metabolism hours after consumption.18 19
As observed in our study, the morning glucose excursion also corresponds to the largest glycemic peak across the day. Controlled feeding studies18–21 have reported that lowering postbreakfast glycemic responses improves glucose variability, suggesting that the early glucose peak is critical to the overall dysglycemia across the day. In free-living people with T2D, glycemic responses are superimposed on numerous internal (ie, chronobiological mechanisms) and external (eg, habitual diet quality, medications, physical activity, social, and emotional) factors which influence glucose levels and variability.22 Interestingly and in the current study, measures of glycemic control in the control group on days 7–14 also decreased, compared with days 1–6. This might be because participants in the control group made changes in their eating and/or lifestyle behavior during the study.
The current study using DSNS adds to the emerging research proposing that both meal composition and timing may be important for glycemic control in patients with T2D.
Beyond glycemic responses, the current study suggests additional benefits of a second DSNS—specifically to replace the afternoon snack—on dietary control and cravings for starchy meals and sides. Behavioral research cites a greater preoccupation with food, lack of control over eating high-carbohydrate foods, and food cravings as significant barriers to successful diabetes self-management.23–26 Replacing a daily breakfast and afternoon snack with a DSNS can lead to increased overall feeling of control over food choices, perhaps by reducing the uncertainty of eating on glycemic responses. The composition of the DSNS (rich in protein, low glycemic index—carbohydrates and fiber) is more likely to induce changes in gastrointestinal hormones (eg, ghrelin, peptide YY (PYY) and GLP-1) which are known to activate selected corticolimbic regions in the brain typically associated with mood, cognitive function, and food cravings.27–29 For instance, DSNS has been shown to induce the secretion of the incretin GLP-1 as compared with standard meal.6 30 GLP-1 is secreted by the enteroendocrine system in response to nutrient ingestion and plays an important role in the regulation of postprandial glucose secretion. Moreover, DSNS was also shown to increase the secretion of PYY and glucagon as compared with oatmeal. The two hormones are major inducers of satiety.30 Overall, the results of the current study are consistent with previous studies suggesting that the specific nutrient composition of diabetes specific nutritional formula(DSNF), (slowly digested carbohydrates and monounsaturated fatty acids) could explain the associated higher levels of incretins as compared with standard nutritional formulas.31 32 Understanding whether the effects observed in the current study are directly attributable to the DSNS or, perhaps, are an indirect consequence of replacing specific foods or changing meal patterns warrants further investigation.
Strengths of this pilot study include its ‘real-world’ setting, where participants were making their own food choices and that CGM glucose data were double blinded and do not influence participants’ behaviors. One limitation of the study is the small sample size. However, the magnitude of objective repeated glucose measurements allowed by CGM helped address this limitation. Additionally, because participants in the study were given no additional diet instruction, there may have been unanticipated impact on eating or other behaviors across the day that could impact these results. Future studies with a larger sample should consider using unblinded (real time) CGM, which would allow participants to evaluate their individual responses to DSNS and track dietary and behavioral modifications that enable them to achieve overall glycemic goals. Further studies are also warranted to understand transcultural responses to the use of DSNS in different settings.
In conclusion, this pilot study extends the existing scientific evidence supporting advantages of DSNS in people with diabetes by suggesting relevant outcomes in both glycemic responses and dietary behaviors in a real-world setting. Collectively, these data suggest that regular use of DSNS as a daily breakfast and subsequent afternoon snack replacement may help reduce barriers and facilitate dietary self-management of diabetes.
Acknowledgments
The authors acknowledge the contributions of the study participants and research staff for their time and efforts. MAGE SAS macro was provided courtesy of the Mayo Foundations for Medical Education and Research, Copyright 2005.
Footnotes
Contributors: VAM, RAH, DSH, ESB, and RR conceptualized the study and played key roles in the data analysis, interpretation, writing and editing. KM, MP, JIM, RMB and OH reviewed and edited the manuscript.
Funding: This study was sponsored by Abbott Nutrition.
Disclaimer: The lead author (VAM) affirms the manuscript is an honest, accurate and transparent account of the study and no important aspect has been omitted.
Competing interests: VAM, RAH, DSH, ESB, and RR are Abbott employees and stockholders. KM and MP received research support for this study from Abbott Nutrition. JIM has consulted for Abbott Nutrition. RMB has received research support, consulted, or has been on the scientific advisory board for Abbott Nutrition, Abbott Diabetes Care, DexCom, Hygieia, Johnson & Johnson, Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, Senseonics and United Healthcare. His technology research is funded in part by NIH/NIDDK. RMB’s employer, non-profit HealthPartners Institute, contracts for his services and no personal income goes to RMB. OH received research support for this study from Abbott Nutrition and has consulted for Abbott Nutrition and Abbott Diabetes Care.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Institutional Review Board of Copernicus Group IRB (IRB Tracking: ABN1-16-657) and conducted in accordance with the principles described in the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.American Diabetes Association 5. Lifestyle Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S46–60. 10.2337/dc19-S005 [DOI] [PubMed] [Google Scholar]
- 2.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract 2015;21 Suppl 1:1–87. 10.4158/EP15672.GLSUPPL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2019 executive summary. Endocr Pract 2019;25:69–100. 10.4158/CS-2018-0535 [DOI] [PubMed] [Google Scholar]
- 4.Hamdy O, Zwiefelhofer D. Weight management using a meal replacement strategy in type 2 diabetes. Curr Diab Rep 2010;10:159–64. 10.1007/s11892-010-0103-9 [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, West DS, Neiberg RH, et al. One-Year weight losses in the look ahead study: factors associated with success. Obesity 2009;17:713–22. 10.1038/oby.2008.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voss AC, Maki KC, Garvey WT, et al. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition 2008;24:990–7. 10.1016/j.nut.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 7.Devitt AA, Oliver JS, Hegazi RA, et al. Glycemia targeted specialized nutrition (GTSN) improves postprandial glycemia and GLP-1 with similar appetitive responses compared to a healthful whole food breakfast in persons with type 2 diabetes: a randomized, controlled trial. Journal of Diabetes Research and Clinical Metabolism 2012;1:20 10.7243/2050-0866-1-20 [DOI] [Google Scholar]
- 8.Mottalib A, Mohd-Yusof B-N, Shehabeldin M, et al. Impact of Diabetes-Specific nutritional formulas versus Oatmeal on postprandial glucose, insulin, GLP-1 and postprandial Lipidemia. Nutrients 2016;8:pii: E443. 10.3390/nu8070443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatti P, di Mauro P, Neri M, et al. Effect of a low-calorie high nutritional value formula on weight loss in T2D diabetes mellitus. Mediterrean J Nutr Metab 2010;1:65–9. [Google Scholar]
- 10.Sun J, Wang Y, Chen X, et al. An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pac J Clin Nutr 2008;17:514–24. [PubMed] [Google Scholar]
- 11.Noronha JC, Nishi SK, Braunstein CR, et al. The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2019;42:767–76. 10.2337/dc18-2270 [DOI] [PubMed] [Google Scholar]
- 12.Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018;84:11–27. 10.1016/j.metabol.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenvers DJ, Schouten LJ, Jurgens J, et al. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr 2014;112:504–12. 10.1017/S0007114514001123 [DOI] [PubMed] [Google Scholar]
- 14.Peng J, Lu J, Ma X, et al. Breakfast replacement with a liquid formula improves glycaemic variability in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr 2019;121:560–6. 10.1017/S0007114518003628 [DOI] [PubMed] [Google Scholar]
- 15.Service FJ, Molnar GD, Rosevear JW, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–55. 10.2337/diab.19.9.644 [DOI] [PubMed] [Google Scholar]
- 16.Mechanick JI, Marchetti AE, Apovian C, et al. Diabetes-specific nutrition algorithm: a transcultural program to optimize diabetes and prediabetes care. Curr Diab Rep 2012;12:180–94. 10.1007/s11892-012-0253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamdy O, Marchetti A, Hegazi RA, et al. The transcultural diabetes nutrition algorithm toolkit: survey and content validation in the United States, Mexico, and Taiwan. Diabetes Technol Ther 2014;16:378–84. 10.1089/dia.2013.0276 [DOI] [PubMed] [Google Scholar]
- 18.Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr 2019;109:1302–9. 10.1093/ajcn/nqy261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson MD, Currie JM, Morgan LM, et al. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia 2003;46:659–65. 10.1007/s00125-003-1081-0 [DOI] [PubMed] [Google Scholar]
- 20.Jakubowicz D, Wainstein J, Ahrén B, et al. High-Energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia 2015;58:912–9. 10.1007/s00125-015-3524-9 [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, Ohta T, Yokoyama J, et al. Effects of low-carbohydrate/high-monounsaturated fatty acid liquid diets on diurnal glucose variability and insulin dose in type 2 diabetes patients on tube feeding who require insulin therapy. Diabetes Technol Ther 2013;15:762–7. 10.1089/dia.2013.0066 [DOI] [PubMed] [Google Scholar]
- 22.Dandona P. Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technol Ther 2017;19:498–506. 10.1089/dia.2016.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng LJ, Wu VX, Dawkes S, et al. Factors influencing diet barriers among outpatients with poorly-controlled type 2 diabetes: a descriptive correlational study. Nurs Health Sci 2019;21:102–11. 10.1111/nhs.12569 [DOI] [PubMed] [Google Scholar]
- 24.Cheng LJ, Wang W, Lim ST, et al. Factors associated with glycaemic control in patients with diabetes mellitus: a systematic literature review. J Clin Nurs 2019;28:1433–50. 10.1111/jocn.14795 [DOI] [PubMed] [Google Scholar]
- 25.Yu JH, Shin MS, Kim DJ, et al. Enhanced carbohydrate craving in patients with poorly controlled type 2 diabetes mellitus. Diabet Med 2013;30:1080–6. 10.1111/dme.12209 [DOI] [PubMed] [Google Scholar]
- 26.Reyes J, Tripp-Reimer T, Parker E, et al. Factors influencing diabetes self-management among medically underserved patients with type II diabetes. Glob Qual Nurs Res 2017;4:1–13. 10.1177/2333393617713097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Klaauw AA, Keogh JM, Henning E, et al. High protein intake stimulates postprandial GLP1 and PYY release. Obesity 2013;21:1602–7. 10.1002/oby.20154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leidy HJ. Increased dietary protein as a dietary strategy to prevent and/or treat obesity. Mo Med 2014;111:54–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Bachlechner S, Denzer-Lippmann MY, Wielopolski J, et al. The effects of different isocaloric oral nutrient solutions on psychophysical, metabolic, cognitive, and olfactory function in young male subjects. Front Psychol 2017;8:1–19. 10.3389/fpsyg.2017.01988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mottalib A, Abrahamson MJ, Pober DM, et al. Effect of diabetes-specific nutrition formulas on satiety and hunger hormones in patients with type 2 diabetes. Nutr Diabetes 2019;9:26. 10.1038/s41387-019-0093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angarita Dávila L, Bermúdez V, Aparicio D, et al. Effect of oral nutritional supplements with Sucromalt and Isomaltulose versus standard formula on glycaemic index, Entero-Insular axis peptides and subjective appetite in patients with type 2 diabetes: a randomised cross-over study. Nutrients 2019;11:1477. 10.3390/nu11071477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Rodríguez CE, Mesa MD, Olza J, et al. Postprandial glucose, insulin and gastrointestinal hormones in healthy and diabetic subjects fed a fructose-free and resistant starch type IV-enriched enteral formula. Eur J Nutr 2013;52:1569–78. 10.1007/s00394-012-0462-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2020-001258supp001.pdf (86KB, pdf)