Abstract
Kaposi sarcoma is one of the acquired immunodeficiency syndrome (AIDS) defining diseases. AIDS-associated Kaposi sarcoma affects primarily the skin and the lungs. Although gastrointestinal involvement is relatively common, biliary tract involvement has rarely been reported. It has been associated mostly with extension from liver disease. We describe an uncommon presentation of disseminated Kaposi sarcoma causing extrahepatic cholestasis due to extrahepatic biliary tract involvement that resolved after sphincterotomy with biliary stenting.
We present a case of a 35-year-old African American male diagnosed with human immunodeficiency virus (HIV) infection in 2005. He presented with AIDS after discontinuation of antiretroviral therapy for one year, subsequently being diagnosed with systemic Kaposi sarcoma. He presented with signs and symptoms of obstructive biliary disease, including jaundice, abdominal pain, fatigue, and fever. We encountered a rare presentation of malignant single extrahepatic biliary stenosis secondary to biliary Kaposi sarcoma. The biochemical pattern markedly improved after endoscopic retrograde cholangiopancreatography with sphincterotomy and stenting. However, and despite the resumption of combined antiretroviral therapy, deep immunosuppression caused worsening clinical condition and death five months after initial presentation.
Certainly, among the multiple etiologies of biliary obstruction in AIDS, Kaposi sarcoma is one to consider.
Keywords: extrahepatic cholestasis, acquired immunodeficiency syndrome, biliary tract, case report, kaposi sarcoma
Introduction
Kaposi sarcoma (KS) is a low-grade vascular tumor associated with human herpesvirus 8 (HHV-8) infection [1,2]. It was first described in 1872 by Moritz Kaposi, a Hungarian dermatologist, initially affecting mostly Mediterranean or Jewish older men. But it was not until 1981 when a demographic change was discovered by Alvin Friedman Kein proving its association with HIV infection [3-5]. There are four variants of its presentation: classic, endemic, posttransplant, and, lastly, the acquired immunodeficiency syndrome (AIDS) associated or epidemic [1,3,5]. The AIDS-associated presentation was for many years the most common AIDS-associated tumor in the United States [6]. Even though KS can develop at any HIV infection stage, generally it occurs in the setting of advanced immune suppression [7]. The incidence has decreased to less than 1% of patients with AIDS after the introduction of combined antiretroviral therapy (cART) [6]. AIDS-associated KS is rapidly progressive and known to initially affect the skin; however, it can extend to mucous membranes and internal organs [1,2,8]. Gastrointestinal involvement is the most common extracutaneous site [2,7]. It is reported that KS can affect any part of the gastrointestinal tract [8]. However, biliary tract involvement has rarely been reported. We present a case with an uncommon presentation of disseminated KS invading the biliary tract and causing extrahepatic cholestasis.
Case presentation
We present the case of a 35-year-old African American male with HIV diagnosed in 2005, initially treated with emtricitabine and tenofovir, although due to lack of insurance he postponed cART for one year. He was admitted to our emergency department with persistent fever, generalized abdominal pain, and jaundice for two weeks and a weight loss of 15 pounds in four weeks. Physical examination on admission showed scleral icterus, small hyperpigmented lesions throughout his trunk, a violaceous nodular enlargement of the hard palate, left axillary lymphadenopathy, a diffusely tender abdomen, and hepatomegaly. Laboratory studies were significant for platelets of 75 k/uL (161-369 k/uL), new-onset conjugated hyperbilirubinemia with a total bilirubin of 23 mg/dL (0.2-1.2 mg/dL), direct bilirubin of 16 mg/dL (0.0-0.2 mg/dL), alkaline phosphatase of 288 IU/L (20-120 IU/L), gamma-glutamyl transferase of 193 IU/L (3-60 IU/L), aspartate aminotransferase of 66 IU/L (0-40 IU/L), alanine aminotransferase of 40 IU/L (5-35 IU/L), and international normalized ratio of 1.3. Computed tomography (CT) of the abdomen revealed hepatomegaly and an ill-defined heterogeneous mass in the peri-portal area with obstruction of the biliary tract with intrahepatic biliary dilation (Figure 1). Endoscopic retrograde cholangiopancreatography demonstrated a non-stenotic erythematous major papilla and a 3-cm stricture in the common hepatic duct (Figure 2). Sphincterotomy and balloon dilation of the stricture were performed, biopsies were taken, and two 7-Fr 12-cm plastic stents were deployed in the right and left hepatic ducts bypassing the stricture. Esophagogastroduodenoscopy with endoscopic ultrasound revealed an erythematous 15-mm nodule in the cardia (Figure 3), with cold biopsies taken and subsequent fine needle biopsy of the peri-portal area performed as well. Inguinal lymph node needle core node biopsy was also performed. Pathology from the porta hepatis mass showed blood clot; however, immunohistochemical staining of the common hepatic duct (Figure 4), stomach nodule (Figure 5), and inguinal lymph node biopsies were positive for HHV-8 KS. cART was commenced, and doxorubicin therapy was started after liver function tests showed improvement. The patient was discharged in view of symptomatic improvement. Liver function tests normalized eight weeks after initial presentation. Nevertheless, his health deteriorated, presenting with hemoptysis, bronchoscopy showed diffuse pulmonary KS, and, subsequently, he had recurrent multiple admission for healthcare-associated pneumonia, cytomegalovirus pneumonitis, pulmonary embolism, and acute tubular necrosis. The patient expired five months after his initial presentation.
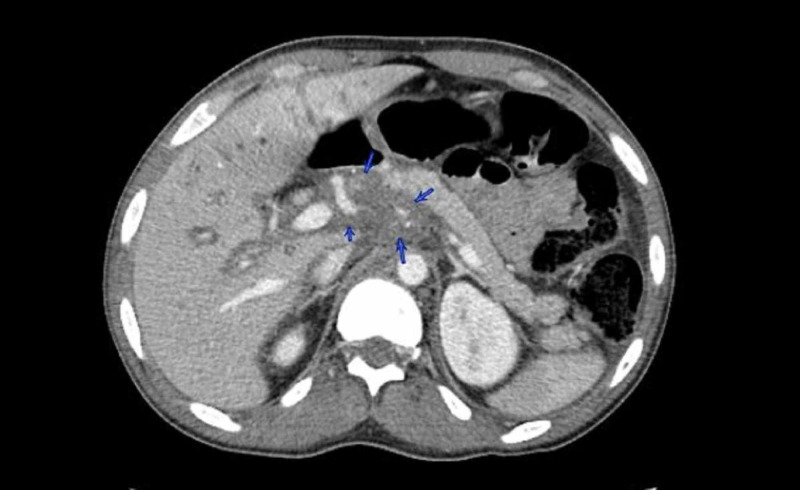
Figure 1. CT of the abdomen.
CT of the abdomen showing an infiltrative process in the portal hepatis to the celiac area (blue arrows) with obstruction of the biliary tract with intrahepatic biliary dilation.
CT, computed tomography
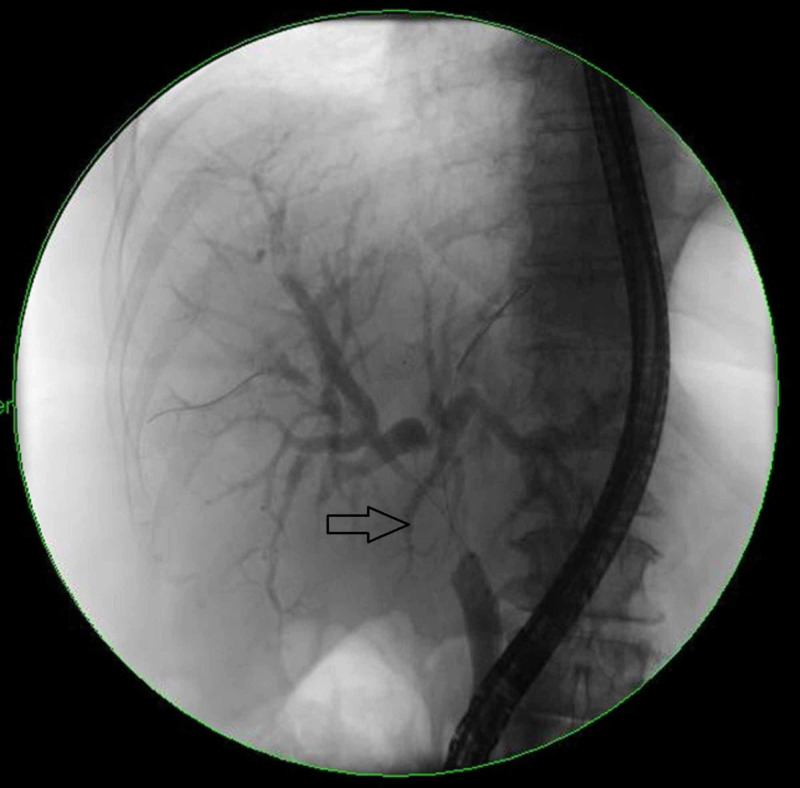
Figure 2. ERCP.
Common hepatic duct with a 3-cm stricture (black arrow).
ERCP, endoscopic retrograde cholangiopancreatography
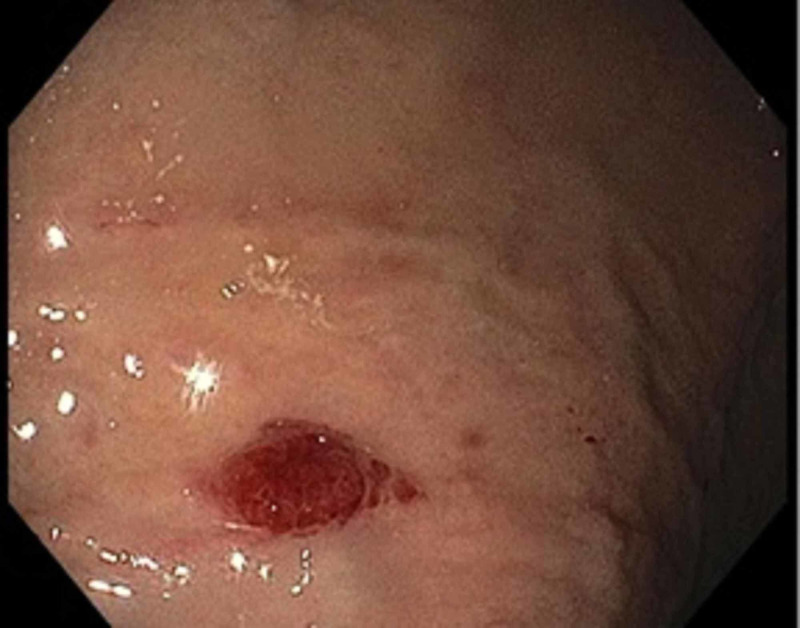
Figure 3. Stomach nodule.
Erythematous 15-mm nodule in the stomach cardia evidenced during EGD.
EGD, esophagogastroduodenoscopy
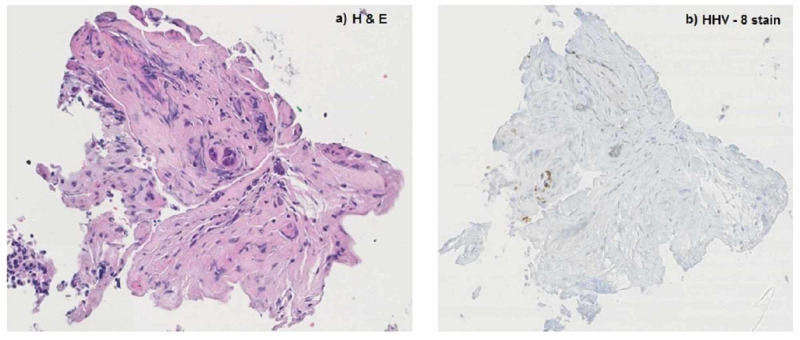
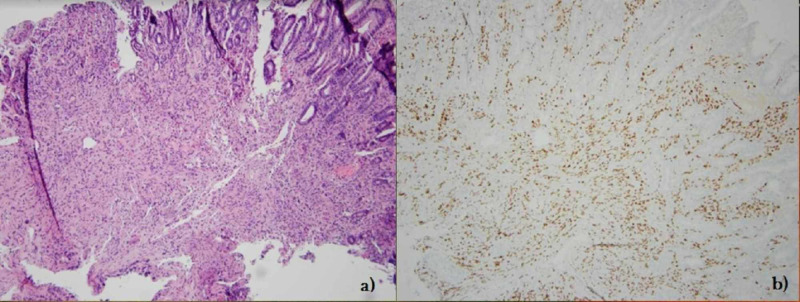
Figure 4. Common hepatic duct biopsy.
H&E stain (a): benign biliary epithelium and scant fibrous fragments, fibrin, and blood. Immunostain for HHV-8 (b): few endothelial cell nuclei are immunoreactive.
H&E, hematoxylin and eosin; HHV-8, human herpesvirus 8
Figure 5. Stomach nodule biopsy.
H&E stain (a): Kaposi sarcoma involving gastric mucosa. (b): Immunostain for HHV-8 is diffusely positive in the endothelial cell proliferation.
H&E, hematoxylin and eosin; HHV-8, human herpesvirus 8
Discussion
Gastrointestinal KS is generally asymptomatic in two-thirds of the cases, and bleeding might be the initial presentation [7,9]. Some patients can present with nonspecific symptoms such as abdominal pain, weight loss, malabsorption, and diarrhea, like the initial clinical presentation of our patient [8,9].
KS involving the biliary tract is infrequent and rarely reported, and an extensive literature review revealed only a few cases. KS has been associated mostly with extension from liver disease presenting with cholangitis and jaundice and may also be caused by extensive disease of the porta hepatis with compression of the extrahepatic biliary tree [10]. A Spanish journal reported a case of biliary tract and gallbladder KS in an AIDS patient without cutaneous involvement that initially presented with pancreatitis; in this case, the involvement of the biliary tract appeared similar to primary sclerosing cholangitis given the beaded pattern [11]. Another study reported a case of a patient with marked changes of sclerosing cholangitis of the intrahepatic bile ducts with postmortem diagnosis of diffuse hepatic KS that caused infiltration of the bile duct [12]. However, our patient had one single 3-cm stricture in the common hepatic duct, which was cannulated with complete resolution of the obstructive pattern. Our patient did not have signs of hepatic involvement nor infiltration extending from the liver. This type of presentation has not been described in the medical literature previously.
Our patient presentation might also be explained by AIDS cholangiopathy, a form of biliary tract inflammation with stricture formation in severely immunosuppressed HIV patients [13,14]. Although the identification of HHV-8 through immunostaining in the tissue biopsy obtained from the biliary tract makes the diagnosis of biliary KS more likely, cutaneous or visceral biopsy is required for the diagnosis of KS. Histopathological features include vascular or spindle cell formations and inflammatory infiltration. Immunohistochemical staining is characteristic of HHV-8 expression, as demonstrated in the common hepatic duct biopsy of our patient [2]. In paraffin-embedded sections, HHV-8 immunostaining has a sensitive (99%) and specific (100%) [8,15].
In patients with advanced symptomatic cutaneous or extracutaneous disease, the role of chemotherapy in addition to standard antiretroviral therapy has been demonstrated to reduce disease progression [2,16]. The current first-line treatment for advanced KS according to JNCCN (Journal of the National Comprehensive Cancer Network) guidelines is liposomal doxorubicin. An alternative option for first-line systemic therapy is paclitaxel [17]. Patients treated with pegylated liposomal doxorubicin or paclitaxel have shown clinical benefit and tumor response, but their side effects are not negligible [16,18]. Newer targeted treatments are being explored; however, the declining incidence of AIDS-related KS makes it difficult to create large-scale trials [19]. HHV-8 infection cannot be eradicated, and complete remission in the setting of advanced disease at presentation is rare; however, reduction or reversion of symptoms and mitigation of end-organ damage can be achieved [17].
As treatment of HIV has improved, a reduction in the incidence of HIV-associated KS has been evidenced; however, in patients without treatment or interrupted therapy, especially those with AIDS, high level of suspicion should be maintained to timely diagnose HHV-8 infection, treat accordingly, and avoid future complications [17,20]. Even though our patient’s symptoms and chemistry improved initially, his marked immunosuppression triggering multiple opportunistic infections caused his death.
Conclusions
KS is an opportunistic malignancy seen commonly in patients with AIDS and can frequently involve the gastrointestinal tract. Any part of the gastrointestinal tract can be affected by KS. Isolated biliary stricture is rarely reported; however, it must be considered among the multiple etiologies of biliary obstruction in AIDS.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Kaposi sarcoma. Radu O, Pantanowitz L. Arch Pathol Lab Med. 2013;137:289–294. doi: 10.5858/arpa.2012-0101-RS. [DOI] [PubMed] [Google Scholar]
- 2.Hepatic Kaposi sarcoma: a case report and review of the literature. Leer-Greenberg BV, Kole A, Chawla S. World J Hepatol. 2017;9:171–179. doi: 10.4254/wjh.v9.i4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moritz Kaposi: idiopathic pigmented sarcoma of the skin. Sternbach G, Varon J. J Emerg Med. 1995;13:671–674. doi: 10.1016/0736-4679(95)00077-n. [DOI] [PubMed] [Google Scholar]
- 4.Moritz Kaposi: a notable name in dermatology. Cohen JM, Burgin S. JAMA Dermatol. 2015;151:867. doi: 10.1001/jamadermatol.2015.1075. [DOI] [PubMed] [Google Scholar]
- 5.An unusual case of invasive Kaposi’s sarcoma with primary effusion lymphoma in an HIV positive patient. Millet A, Singh S, Gittens-Backus G, Dang KA, Shokrani B. Case Rep Oncol Med. 2015;2015:789616. doi: 10.1155/2015/789616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepatic Kaposi’s sarcoma in a patient affected by AIDS: correlation between histology and imaging. Tacconi D, Vergori A, Lapini L, Magnolfi A, Carnevali A, Caremani M. J Ultrasound. 2012;15:215–219. doi: 10.1016/j.jus.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gastrointestinal Kaposi’s sarcoma: case report and review of the literature. Lee AJ, Brenner L, Mourad B, Monteiro C, Vega K, Munoz J. World J Gastrointest Pharmacol Ther. 2015;6:89–95. doi: 10.4292/wjgpt.v6.i3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Primary gastrointestinal Kaposi’s sarcoma in a patient with human immunodeficiency virus. Zapata Laguado MI, Aponte Monsalve JE, Santos JH, Preciado J, Mosquera Zamudio A, Garza Acosta C. Case Rep Oncol. 2018;11:638–647. doi: 10.1159/000492715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaposi sarcoma involving the gastrointestinal tract. Arora M, Goldberg EM. https://pubmed.ncbi.nlm.nih.gov/20827371/ Gastroenterol Hepatol (N Y) 2010;6:459–462. [PMC free article] [PubMed] [Google Scholar]
- 10.Biliary tract pathology in patients with AIDS. Goldin RD, Hunt J. J Clin Pathol. 1993;46:691–693. doi: 10.1136/jcp.46.8.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.[Kaposi's sarcoma of the bile ducts without cutaneous involvement in a patient with AIDS] Segarra P, Abril V, Gil M, Ortega E, Ballester JE, Traves V. Rev Esp Enferm Dig. 1996;88:637–639. [PubMed] [Google Scholar]
- 12.Acquired immunodeficiency syndrome cholangiopathy: spectrum of disease. Cello JP. Am J Med. 1989;86:539–546. doi: 10.1016/0002-9343(89)90381-1. [DOI] [PubMed] [Google Scholar]
- 13.A patient with AIDS and an unusual cause of jaundice. Kohli DR, Lippman HR, Shah TU. Gastroenterology. 2017;152:11–12. doi: 10.1053/j.gastro.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 14.AIDS cholangiopathy in an asymptomatic, previously undiagnosed late-stage HIV-positive patient from Kenya. Gao Y, Chin K, Mishriki YY. Int J Hepatol. 2011;2011:465895. doi: 10.4061/2011/465895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Human herpesvirus 8 immunostaining: a sensitive and specific method for diagnosing kaposi sarcoma in paraffin-embedded sections. Robin Robin, YM YM, Guillou L, Michels JJ, Coindre JM. Am J Clin Pathol. 2004;121:330–334. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 16.A randomized, double-blind study of pegylated liposomal doxorubicin for the treatment of AIDS-related Kaposi’s sarcoma. Cooley T, Henry D, Tonda M, Sun S, O’Connell M, Rackoff W. Oncologist. 2007;12:114–123. doi: 10.1634/theoncologist.12-1-114. [DOI] [PubMed] [Google Scholar]
- 17.AIDS-Related Kaposi Sarcoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. Reid E, Suneja G, Ambinder RF, et al. J Natl Compr Canc Netw. 2019;17:171–189. doi: 10.6004/jnccn.2019.0008. [DOI] [PubMed] [Google Scholar]
- 18.Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cianfrocca M, Lee S, Von Roenn J, et al. Cancer. 2010;116:3969–3977. doi: 10.1002/cncr.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaposi sarcoma. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Nat Rev Dis Primers. 2019;5:9. doi: 10.1038/s41572-019-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HIV-associated Kaposi sarcoma and related diseases. Goncalves P, Uldrick T, Yarchoan R. AIDS. 2017;31:1903–1916. doi: 10.1097/QAD.0000000000001567. [DOI] [PMC free article] [PubMed] [Google Scholar]