Abstract
Autism spectrum disorder (ASD) is associated with the altered functional connectivity of 3 neurocognitive networks that are hypothesized to be central to the symptomatology of ASD: the salience network (SN), default mode network (DMN), and central executive network (CEN). Due to the considerably higher prevalence of ASD in males, however, previous studies examining these networks in ASD have used primarily male samples. It is thus unknown how these networks may be differentially impacted among females with ASD compared to males with ASD, and how such differences may compare to those observed in neurotypical individuals. Here, we investigated the functional connectivity of the SN, DMN, and CEN in a large, well-matched sample of girls and boys with and without ASD (169 youth, ages 8–17). Girls with ASD displayed greater functional connectivity between the DMN and CEN than boys with ASD, whereas typically developing girls and boys differed in SN functional connectivity only. Together, these results demonstrate that youth with ASD exhibit altered sex differences in these networks relative to what is observed in typical development, and highlight the importance of considering sex-related biological factors and participant sex when characterizing the neural mechanisms underlying ASD.
Keywords: autism spectrum disorder, functional connectivity, resting-state functional magnetic resonance imaging, sex differences, sexual differentiation
Introduction
Autism spectrum disorder (ASD) is a prevalent neurodevelopmental condition, which is diagnosed on the basis of challenges with social communication, as well as the presence of repetitive behaviors and circumscribed interests (American Psychiatric Association 2013; Baio et al. 2018). Other behavioral and cognitive domains are also significantly impacted in ASD; these include alterations in the relative salience of social and nonsocial stimuli, as well as differences in social cognition and executive functioning (Lai et al. 2014; Dubey et al. 2015; Chita-Tegmark 2016; Ruta et al. 2017; Lai et al. 2017a). Prior studies in largely male samples have consistently demonstrated that ASD is associated with differences in brain connectivity, including altered intrinsic functional connectivity as assessed using resting-state functional magnetic resonance imaging (fMRI) (Zikopoulos and Barbas 2013; Kana et al. 2014; Hernandez et al. 2015; Hull et al. 2016; Picci et al. 2016). Of particular interest are 3 interrelated neurocognitive networks that, according to the triple network model (Menon 2011; Menon 2019), are hypothesized to offer a parsimonious explanation for the symptoms observed among individuals with ASD, including differences in salience attribution, social cognition, and executive function (Lai et al. 2014; Dubey et al. 2015; Uddin 2015; Chita-Tegmark 2016; Ruta et al. 2017; Lai et al. 2017a). These 3 networks of specific interest are the salience network (SN), default mode network (DMN), and central executive network (CEN). The SN is believed to play a role in detecting and coordinating a response to salient interoceptive and exteroceptive stimuli, including modulating the relative activity of the DMN and CEN, whereas the DMN and CEN have previously been related to social cognition and executive functioning respectively, among other functions (Menon and Uddin 2010; Menon 2011; Uddin 2015; Padmanabhan et al. 2017). Alterations in these networks have also been hypothesized to underlie symptoms observed in other neurodevelopmental and neuropsychiatric disorders, including differences in attention, social functioning, and cognition; however, the specific pattern and directionality of alterations in these networks are thought to differ as a function of the specific disorder (Menon 2011; Menon 2019). In support of the triple network model in ASD, and emphasizing the potential importance of these 3 networks to the neural underpinnings of ASD, previous studies have found that the connectivity within and between these networks is both significantly impacted in ASD and correlated with the magnitude of core ASD traits (Lynch et al. 2013; Nomi and Uddin 2015; Uddin et al. 2015; Yerys et al. 2015; Abbott et al. 2016; Padmanabhan et al. 2017; Lawrence et al. 2019; Nomi et al. 2019). Furthermore, patterns of functional connectivity in these networks have been shown to predict a diagnosis of ASD, as well as changes over time in ASD traits (Anderson et al. 2011; Nielsen et al. 2013; Uddin et al. 2013a; Plitt et al. 2015a; Plitt et al. 2015b; Abraham et al. 2017).
As the prevalence of ASD is approximately 3 to 4 times greater among males than females (Loomes et al. 2017; Baio et al. 2018), almost all studies to date have focused on samples that were primarily or entirely male, with no analyses ever conducted to specifically examine the SN, DMN, and CEN in females with ASD. However, there is growing evidence that girls and boys with ASD differ in their patterns of social attention, social engagement, and executive function (Dean et al. 2014; Head et al. 2014; Lai et al. 2015; Sedgewick et al. 2016; Hull et al. 2017; Harrop et al. 2018a; Harrop et al. 2018b; Sedgewick et al. 2019), domains, which may be related to connectivity of the SN, DMN, and CEN. More broadly, and further suggesting that findings from primarily male samples with ASD may not generalize to female samples, males and females with ASD show significantly different behavioral and cognitive profiles (Baron-Cohen et al. 2014; Frazier et al. 2014; Hiller et al. 2014; Lai et al. 2015; Hull et al. 2017; Evans et al. 2018; Harrop et al. 2018a; Harrop et al. 2018b; Knutsen et al. 2018; Lawson et al. 2018; Moseley et al. 2018; Tillmann et al. 2018; Matheis et al. 2019), as well as distinct patterns of neural activity during cognitive and emotional tasks (Beacher et al. 2012b; Schneider et al. 2013) and brain connectivity (Nordahl et al. 2015; Alaerts et al. 2016; Lai et al. 2017b). Such differences may reflect a pattern of masculinization in both groups and/or the presence of altered sex differences in ASD. For instance, males and females with ASD both show levels of empathizing and systemizing that are shifted from the average neurotypical female profile towards the average neurotypical male profile (Baron-Cohen et al. 2014; Greenberg et al. 2018). Other traits, such as sensorimotor processes, exhibit significant sex differences in adults with ASD but not among neurotypical adults (Moseley et al. 2018). More generally, altered behavioral and neuroimaging sex differences in ASD have been shown to take several forms, including sex differences that are the reverse of those seen in the neurotypical population (i.e., females with ASD resembling neurotypical males, and males with ASD resembling neurotypical females), the presence of greater sex differences in ASD than among neurotypical individuals, and the attenuation of typical sex differences when examining groups with ASD (Beacher et al. 2012a; Beacher et al. 2012b; Bejerot et al. 2012; Supekar and Menon 2015; Hull et al. 2017; Floris et al. 2018; Moseley et al. 2018; O'Neill et al. 2020). The few studies to date that have examined alterations in functional connectivity among females and males with ASD have yielded results that varied depending on the exact measure or network examined. These findings include no significant sex differences in ASD in interhemispheric functional connectivity (whole-brain homotopic connectivity), differences supporting a masculinized neural profile across individuals with ASD (within-network DMN connectivity), or altered sex differences in ASD (cerebellum, posterior superior temporal sulcus, posterior cingulate cortex, and whole-brain connectivity) (Alaerts et al. 2016; Ypma et al. 2016; Kozhemiako et al. 2019; Smith et al. 2019). Taken together, these findings of altered sex differences at both the behavioral and neural level have led to the hypothesis that sex-dependent biological factors may contribute to the presentation of ASD (Lai et al. 2017b). However, despite the proposed centrality of altered SN, DMN, and CEN connectivity to ASD (Menon 2011; Uddin 2015), no studies to date have specifically investigated sex differences in the functional connectivity within and between these key 3 networks among individuals with ASD. It is thus unknown how such connectivity may differ between females and males with ASD, as well as how these patterns may compare with typical sex differences. Importantly, understanding the nature of such differences would allow us to better characterize both the brain-based underpinnings of ASD among females and shed new light on the potential relationship between sex-related neural factors and ASD as a whole.
We, therefore, analyzed the within- and between-network functional connectivity of the SN, DMN, and CEN in a balanced sample of girls and boys with and without ASD to directly examine sex differences in connectivity in ASD and typical development. To the best of our knowledge, this multisite study represents the largest investigation to date of functional connectivity in a pediatric sample of females with ASD. All data were collected using a harmonized data acquisition protocol, and we used both a whole-brain seed-based and a region of interest (ROI)-based network approach to comprehensively examine the functional connectivity of our 3 networks of interest. Our results indicate altered sex differences among youth with ASD in the functional connectivity of the SN, DMN, and CEN, thereby highlighting the importance of considering participant sex and sex-specific biological factors when characterizing the hypothesized neural mechanisms of ASD.
Materials and Methods
Participants
As part of the Gender Exploration of Neurogenetics and Development to Advance Autism Research (GENDAAR; NIMH100028) Consortium, children and adolescents (ages 8–17) were recruited from 4 sites across the United States of America (Harvard Medical School, Seattle Children’s Research Institute, University of California Los Angeles [UCLA], and Yale University). For the ASD group, all participants were required to have a clinical diagnosis of ASD that was confirmed by an experienced, research-reliable clinician using the Autism Diagnostic Observation Schedule Second Edition (ADOS-2) (Lord et al. 2012) and/or the Autism Diagnostic Interview, Revised (ADI-R) (Lord et al. 1994). To maintain reliability on these measures across sites, all lead clinicians double-coded one ADOS-2 and one ADI-R every 6 months, with reliability maintained within each individual site by having the site’s lead clinician double-code 10% of assessments. Virtually all participants with ASD met criteria on both the ADOS-2 and the ADI-R (n = 75/80), with 2 participants meeting criteria on the ADOS-2 but not receiving the ADI-R, and 3 participants meeting criteria on the ADI-R but being subthreshold on the ADOS-2 (−1 point), or not receiving the ADOS-2. In addition to meeting criteria on the ADOS-2 and/or ADI-R, participants in the ASD group were required to have no history of other neurological disorders involving pathology above the brainstem, with the exception of uncomplicated non-focal epilepsy with no active seizures within the last year; nearly all participants with ASD had no history of epilepsy (n = 78/80), with 1 subject having uncomplicated non-focal epilepsy, and 1 subject meeting inclusion criteria but not having their exact epilepsy status retained past study enrollment. Typically developing (TD) control participants were required to have no first- or second-degree relatives with ASD, no evidence of elevated ASD traits (total t-scores < 65 on the parent-report version of the Social Responsiveness Scale, Second Edition [SRS-2]; Constantino and Gruber 2012), and no known developmental, psychiatric or neurological disorders. Exclusion criteria for both groups included any known genetic condition (e.g., Fragile X), premature birth, an inability to comprehend scan instructions, excessive motion, and insufficient high-quality resting-state data. Youth were additionally excluded if they were siblings of another participant in the study; the retained sibling was selected with the goal of matching groups closely by motion during the fMRI scan (as measured by mean relative motion and the number of fMRI-independent components automatically labeled as motion or noise; Pruim et al. 2015b), site/scanner, pubertal development (as measured by the Pubertal Developmental Scale; PDS; Carskadon and Acebo 1993), and separately within each diagnostic group, ASD traits (as measured by the ADOS-2 for the ASD group and the SRS-2 for both the ASD and TD groups). The final sample included 169 participants: 46 girls with ASD, 34 boys with ASD, 48 TD girls, and 41 TD boys. Participants were assigned to the female/girl or male/boy group based on parent-report of biological sex designated at birth; gender identity was not assessed. Informed assent and consent were obtained from all participants and their legal guardians, and the experimental protocol was approved by the Institutional Review Board at each participating site. Anonymized data are publicly available for participants through the National Database for Autism Research (NDAR). As scans from this project are not available on the Autism Brain Imaging Data Exchange (ABIDE; Di Martino et al. 2014; Di Martino et al. 2017), our data are independent from previous analyses that used ABIDE to examine resting-state functional connectivity (Alaerts et al. 2016; Ypma et al. 2016; Yang and Lee 2018; Kozhemiako et al. 2019).
Descriptive statistics are presented in Table 1, with the reported statistical comparisons completed in R using t-tests, chi-squared tests, or their non-parametric equivalent as appropriate (R Core Team 2016). When examining sex differences separately within the ASD and TD groups, girls and boys did not significantly differ on any of the following (all Ps > 0.1): general cognitive ability (i.e., full-scale IQ as measured by the Differential Ability Scales, Second Edition; Elliot 2007), age, handedness, site/scanner, mean relative motion, number of fMRI-independent components labeled as motion or noise, and psychotropic medication status or class (Linke et al. 2017); complete psychotropic medication information is presented in Table S1. There were also no significant differences between girls and boys in overall ASD traits as measured by total SRS-2 scores for all groups (all Ps > 0.2); compared to boys with ASD, girls with ASD had somewhat lower levels of ASD traits as assessed by the ADOS-2, although this difference was not statistically significant (P = 0.08). As expected given the lack of significant differences in age between girls and boys within each diagnostic group, girls exhibited more advanced pubertal development than boys, although this difference only reached statistical significance in the ASD group (ASD: P = 0.01; TD: P = 0.18). When testing for demographic differences separately within the female and male groups, there were no significant differences between ASD and TD youth on any of the following (all Ps > 0.1): age, handedness, site/scanner, mean relative motion, and number of fMRI-independent components labeled as motion or noise. A significant difference was seen in general cognitive ability, such that girls and boys with ASD exhibited significantly lower general cognitive ability scores than their same-sex TD counterparts (both Ps < 0.05). General cognitive ability and pubertal development were therefore included as covariates in all neuroimaging analyses.
Table 1.
Mean and standard deviation of sample descriptives
| ASD | TD | Female versus Male P-values | ASD versus TD P-values | |||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | ASD | TD | Female | Male | |
| Sample size | 46 | 34 | 48 | 41 | — | — | — | — |
| Age (years) | 13.50 ± 2.52 | 13.32 ± 3.04 | 13.15 ± 3.04 | 13.71 ± 2.64 | 0.77 | 0.36 | 0.55 | 0.55 |
| Pubertal Development | 12.84 ± 3.70a | 10.33 ± 3.84b | 12.38 ± 4.63a | 11.12 ± 3.94 | 0.01 | 0.18 | 0.61 | 0.39 |
| General conceptual ability | 99.59 ± 19.95 | 103.74 ± 20.38 | 112.33 ± 14.75 | 114.20 ± 15.62 | 0.37 | 0.57 | <0.001 | 0.01 |
| Handedness (R/L) | 43/3 | 33/1 | 45/3 | 38/3 | 0.63 | 1.00 | 1.00 | 0.62 |
| Scanner (HT/ST/SP/UT/UP/YT) | 4/10/7/8/4/13 | 7/2/9/7/1/8 | 7/3/13/9/7/9 | 5/3/10/11/4/8 | 0.17 | 0.95 | 0.16 | 0.79 |
| Mean relative motion (mm) | 0.18 ± 0.15 | 0.19 ± 0.21 | 0.15 ± 0.13 | 0.13 ± 0.08 | 0.83 | 0.78 | 0.30 | 0.16 |
| # of motion/noise components | 22.33 ± 7.75 | 24.29 ± 7.68 | 20.10 ± 7.95 | 21.56 ± 7.69 | 0.26 | 0.38 | 0.40 | 0.24 |
| SRS-2 total raw | 96.18 ± 31.83b | 98.31 ± 25.82b | 17.13 ± 10.96 | 18.02 ± 14.03 | 0.76 | 0.74 | <0.001 | <0.001 |
| SRS-2 total T-Score | 77.02 ± 11.42b | 75.94 ± 10.35b | 45.02 ± 4.65 | 43.78 ± 5.82 | 0.67 | 0.27 | <0.001 | <0.001 |
| ADOS-2 Comparison Score | 6.58 ± 1.95a | 7.38 ± 2.03 | — | — | 0.08 | — | — | — |
ASD = Autism Spectrum Disorder; TD = Typically Developing; Handedness: R = Right, L = Left. Scanner: HT = Harvard Trio, ST = Seattle Trio, SP = Seattle Prisma, UT = UCLA Trio, UP = UCLA Prisma, YT = Yale Trio. SRS-2 = Social Responsiveness Scale, Second Edition. ADOS-2 = Autism Diagnostic Observation Schedule, Second Edition. Superscripts indicate data missing from 1a or 2b subjects.
MRI Data Acquisition
MRI data were acquired on a Siemens 3 T Trio scanner using a 12-channel headcoil or on a Siemens 3 T Prisma scanner using a 20-channel headcoil after scanner upgrades at 2 sites (Seattle and UCLA). During the resting-state fMRI scan (TR = 2000 ms, TE = 30 ms, field of view [FOV] = 192 mm, 34 slices, slice thickness = 4 mm, in-plane voxel size = 3 × 3mm, acquisition time = 5.5 min; Trio and Prisma parameters were identical), participants viewed a white fixation cross in the center of a black background using MR-compatible goggles (Resonance Technology, Inc., Northridge, CA, USA). For registration purposes, we additionally collected a high-resolution echo planar scan that was co-planar to the fMRI scan to ensure identical distortion characteristics (Trio: TR = 5000 ms, TE = 34 ms, FOV = 192 mm, 34 slices, slice thickness = 4 mm, in-plane voxel size of 1.5 × 1.5 mm, acquisition time = 1.5 min; Prisma parameters were identical except TE = 35 ms). To control for potential scanner differences, 3 phantoms (2 human phantoms and 1 agar phantom) traveled to multiple study sites. Temporal signal-to-noise ratio was calculated for each phantom/scanner combination using Analysis of Functional NeuroImages (AFNI; Table S2) (Cox 1996). A linear mixed model including scanner as a fixed effect and phantom as a random effect revealed a significant main effect of scanner on the temporal signal-to-noise ratio calculated from the phantoms (P = 0.02). We thus included scanner as a covariate in all neuroimaging analyses to statistically control for any effect of scanner. Importantly, data were acquired for an equal proportion of each group (female ASD, male ASD, female TD, male TD) on each scanner, further ensuring that between-group comparisons were not impacted by scanner.
fMRI Data Preprocessing
Standard preprocessing was completed on all resting-state fMRI data using FMRIB’s Software Library (FSL) (Smith et al. 2004) and AFNI (Cox 1996). This included skull stripping in AFNI, motion correction in FSL using the Motion Correction Linear Registration Tool (MCFLIRT) (Jenkinson et al. 2002), and smoothing with a 6 mm full width at half maximum (FWHM) Gaussian kernel in FSL. Resting-state fMRI scans were linearly registered in FSL using FMRIB’s Linear Image Registration Tool (FLIRT) (Jenkinson and Smith 2001; Jenkinson et al. 2002), with each participant’s resting-state fMRI scan first registered to their individual high-resolution matched bandwidth coplanar image (6 degrees of freedom) and then to the MNI152 2 mm standard brain (12 degrees of freedom). To remove potential confounds resulting from head motion, we used Independent Component Analysis (ICA)-based Automatic Removal of Motion Artifacts (ICA-AROMA) (Pruim et al. 2015a; Pruim et al. 2015b) to regress out single-subject components labeled as motion or noise (Table 1). To further reduce potential motion confounds and other noise, resting-state fMRI scans were band-pass filtered (0.1 Hz > t > 0.01 Hz) and the following included as single-subject nuisance regressors: mean cerebrospinal fluid time series, mean white matter time series and mean global time series, as well as their temporal derivatives (Power et al. 2014).
fMRI Data Analysis
To comprehensively examine the SN, DMN, and CEN, we used both a whole-brain and an ROI-based network approach. These methods are complementary in that the former allows one to thoroughly examine the entire extent of each network across the whole brain, whereas the latter focuses only on the hubs of each network but allows for greater statistical power due to the relatively smaller number of multiple comparisons. Group differences that may fall outside, or only partially overlap with, the hub ROIs may not be captured by the ROI-based network approach, but will be observable with the whole-brain approach. Conversely, between-group findings that are not visible when using the whole-brain approach may be easily detectable when using the ROI-based approach because of this approach’s greater statistical power.
For both the whole-brain and the ROI-based network analyses, our planned comparisons focused on the effect of sex within diagnostic group (female ASD vs. male ASD; female TD vs. male TD) and the effect of diagnostic group within sex (female ASD vs. female TD; male ASD vs. male TD). For completeness, we additionally examined the interaction between diagnostic group and sex. All analyses included general cognitive ability, pubertal development, and site/scanner as covariates of non-interest. Effect sizes are reported for all significant between-group contrasts as standardized regression coefficients (βs), reflecting that all group-level comparisons were completed as regressions to allow for the inclusion of nuisance covariates.
To examine whole-brain resting-state functional connectivity of the SN, DMN, and CEN, mean time series were extracted from standard seeds for these networks located in the right orbital frontoinsula for the SN (Seeley et al. 2007), the precuneus for the DMN (Fox et al. 2005), and the right dorsolateral prefrontal cortex for the CEN (Seeley et al. 2007); these seeds have previously been used by our group and others to examine functional connectivity in ASD (Elton et al. 2016; Green et al. 2016; Lawrence et al. 2019). The time series extracted from each 5-mm radius spherical seed was then correlated with that of every other voxel in the brain to obtain correlation maps, which were subsequently transformed into z-score maps using Fisher’s r-to-z transform. Whole-brain group-level analyses were completed in FSL using FMRIB’s Local Analyses of Mixed Effects (FLAME 1 + 2), with variance estimated separately for the ASD and TD groups. As we were primarily interested in functional connectivity within and between the SN, DMN, and CEN, all group analyses were limited to voxels that belonged to one of these networks in any group at a voxel-wise threshold of Z > 3.1 and a corrected cluster threshold of P < 0.05. That is, group analyses were prethreshold masked using a combined mask of the SN, DMN, and CEN across all 4 of our groups (female ASD, male ASD, female TD, male TD). To stringently correct for multiple comparisons, all within- and between-group contrasts were thresholded at Z > 3.1, P< 0.05 (Kessler et al. 2017).
To examine ROI-based network functional connectivity of the SN, DMN, and CEN a 9 × 9 ROI correlation matrix was created for each participant by extracting the mean time series from key nodes of each network and correlating these time series with one another; these ROIs were defined in a prior study with an independent sample (Uddin et al. 2011) and have previously been used to study connectivity in ASD (Uddin et al. 2015; Neufeld et al. 2018). Specifically, these nodes were located in the bilateral frontoinsular cortex (L FIC, R FIC) and anterior cingulate cortex (ACC) for the SN, the posterior cingulate cortex (PCC) and ventromedial prefrontal cortex (vmPFC) for the DMN, and the bilateral dorsolateral prefrontal cortex (L dlPFC, R dlPFC) and bilateral posterior parietal cortex (L PPC, R PPC) for the CEN. After calculating correlations between each pair of ROIs for each subject, these values were converted into z-scores using Fisher’s r-to-z transform and compared between groups using a general linear model in R. Residuals from all general linear models were confirmed to meet the assumptions of independence, normality, and constant variance based on visual inspection of residual histograms and residual plots. A Benjamini and Hochberg false discovery rate (FDR) of 5% was used to correct for multiple comparisons across the 36 ROI pairs, with corrected P-values reported in all cases unless otherwise specified (Benjamini and Hochberg 1995).
To confirm that the results from our whole-brain and ROI-based network analyses were not impacted by medication status (Linke et al. 2017), we used a general linear model to assess the effect of medication on those connectivity measures (i.e., z-scores) which showed significant between-group differences. Exploratory analyses were additionally conducted to examine how the connectivity of those regions that exhibited significant between-group differences may be associated with pubertal development (as measured by the PDS) and ASD traits (as measured by total SRS-2 score and ADOS-2 comparison scores), with FDR-corrected P-values reported.
Results
Whole-Brain Functional Connectivity
The SN, DMN, and CEN exhibited the expected patterns of whole-brain functional connectivity in all 4 groups (Figs S1–S3 and Tables S3–S6). This included SN connectivity with the anterior insula and anterior cingulate cortex, as well as DMN connectivity with the precuneus/posterior cingulate cortex, medial prefrontal cortex/ventromedial prefrontal cortex and angular gyrus, and CEN connectivity with the dorsolateral prefrontal cortex and intraparietal sulcus.
Girls and boys with ASD did not significantly differ in whole-brain SN, DMN, or CEN functional connectivity. TD youth likewise exhibited no significant sex differences in DMN or CEN connectivity. However, the female and male TD groups did demonstrate significant SN differences. Compared to TD girls, TD boys displayed more negative between-network connectivity with the left posterior parietal cortex (Fig. 1A; MNI peak coordinates = −38, −60, 38; β = 0.89; max Z = 4.50). Overall, there were thus no sex differences observed in the ASD group and sex differences in the TD group were limited to the SN.
Figure 1.
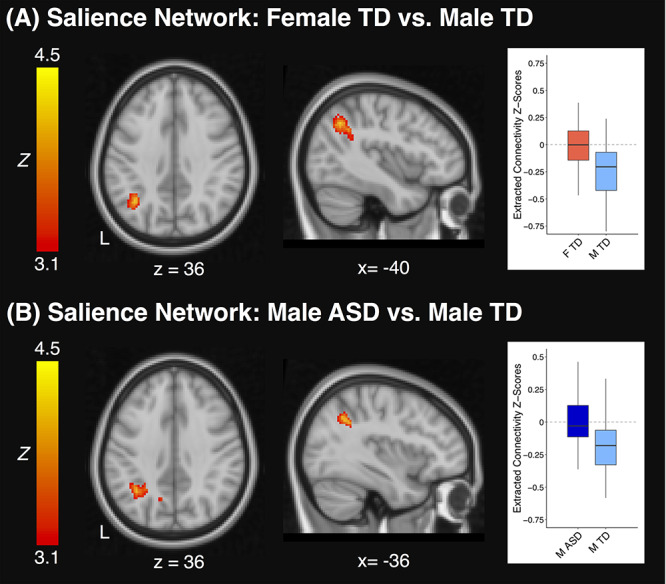
Clusters that displayed a significant difference in salience network functional connectivity between TD girls and boys (A) or between boys with ASD and TD boys (B). Connectivity z-scores extracted from each significant cluster are represented at right, with boxplots displaying the median and interquartile range of connectivity z-scores and whiskers representing the most extreme z-scores within 1.5 times the interquartile range. ASD: Autism Spectrum Disorder; TD: Typically Developing; F: Female; M: Male; L: Left.
When contrasting girls with ASD and TD girls, no significant differences emerged in SN, DMN, or CEN functional connectivity. The male ASD and TD groups significantly differed in SN connectivity only, such that TD boys exhibited significantly more negative connectivity with the left posterior parietal cortex and precuneus than boys with ASD (Fig. 1B; MNI peak coordinates = −28, −58, 26; β = 0.74; max Z = 4.25). To assess whether this difference between boys with ASD and TD boys was impacted by medication among the boys with ASD, connectivity z-scores were extracted from this cluster and compared between medicated and unmedicated boys with ASD; medication did not significantly affect functional connectivity of this cluster (P = 0.7). As a whole, the female ASD group thus displayed no significantly atypical patterns of connectivity when using a whole-brain seed-based approach, whereas boys with ASD exhibited atypical SN connectivity.
When examining the interaction between diagnostic group and sex, there was no significant interaction with regards to connectivity of the DMN or CEN. A diagnostic group by sex interaction was observed in SN connectivity with the left posterior parietal cortex and precuneus (Fig. S4; MNI peak coordinates = −38, −56, 40; β = 1.13; max Z = 3.55) at a commonly used but somewhat less stringent threshold (Z > 2.3, P< 0.05). Connectivity z-scores extracted from this cluster revealed that medicated youth with ASD did not significantly differ from their same-sex unmedicated counterparts (both Ps > 0.6). In sum, the whole-brain seed-based results indicate both that youth with ASD exhibit altered sex differences relative to their TD peers, and that the pattern of atypicalities seen in ASD relative to typical development depends on participant sex.
Exploratory analyses examining the relationship between pubertal development, or ASD traits, and functional connectivity of regions showing between-group differences (Figs 1 and S4) yielded no statistically significant associations (all Ps > 0.1).
ROI-Based Network Functional Connectivity
The female and male ASD groups significantly differed in functional connectivity between the DMN (PCC) and CEN (L PPC), with greater positive connectivity between the DMN and CEN in the female ASD group than the male ASD group (β = 0.87, P = 0.02; Figs 2A and 3A). Girls and boys with ASD exhibited no significant differences in SN functional connectivity. In contrast, TD girls and boys displayed significant sex differences in SN connectivity only, including differences in both within-network connectivity and between-network connectivity (Figs 2B and 3D). TD boys displayed increased positive connectivity within the SN (R FIC with ACC; L FIC with ACC) compared to TD girls (β = 0.63, P = 0.03; β = 0.62, P = 0.03), as well as more negative connectivity between the SN (L FIC) and DMN (PCC; β = 0.67, P = 0.03). Sex differences among TD youth were also observed in functional connectivity between the SN and CEN, with the male TD group exhibiting greater negative connectivity between the right FIC node of the SN and the CEN (L PPC; β = 0.74, P = 0.02), and the female TD group displaying greater positive connectivity between the left FIC node of the SN and the CEN (L PPC; β = 0.64, P = 0.03). As a whole, girls and boys with ASD thus differed only in connectivity between the DMN and the CEN, whereas TD girls and boys differed only in the within- and between-network connectivity of the SN.
Figure 2.
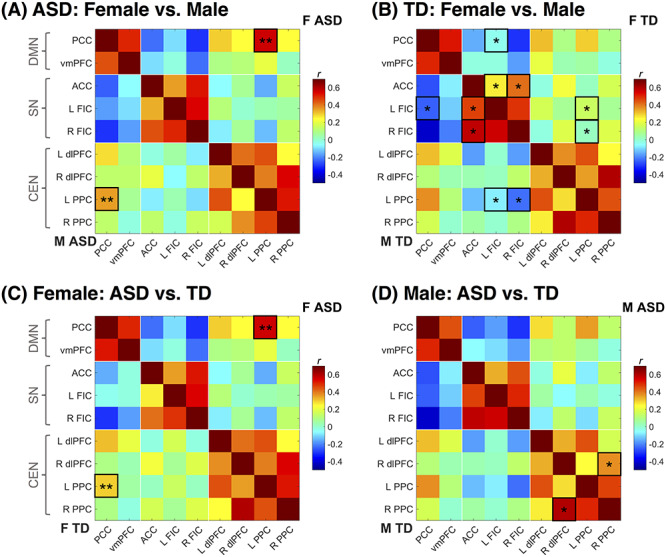
Correlation matrices and significant between-group differences for all ROIs. The upper triangle and lower triangle reflect correlation values extracted from the group identified directly to the upper right or lower left of the correlation matrix, respectively. ASD: Autism Spectrum Disorder; TD: Typically Developing; F: Female; M: Male; DMN: Default Mode Network; SN: Salience Network; CEN: Central Executive Network; ROI: region of interest; *corrected P < 0.05; **corrected P < 0.01.
Figure 3.
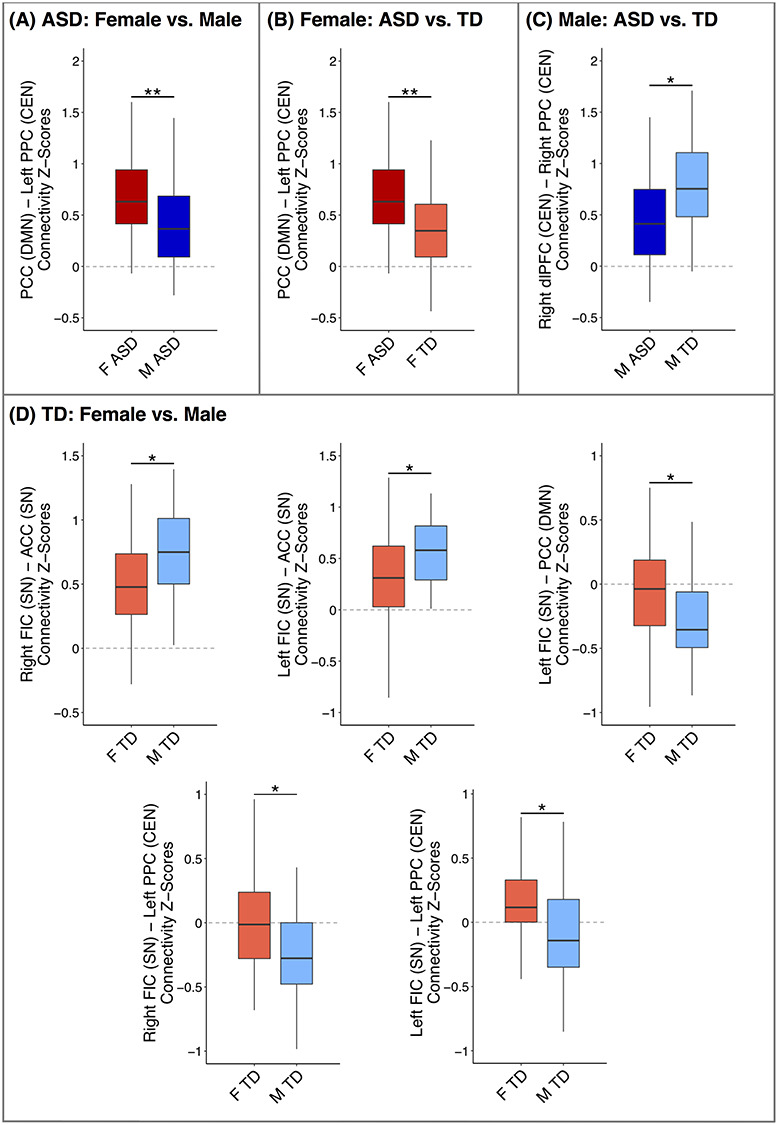
Connectivity z-scores extracted from each ROI pair exhibiting a significant between-group difference, with boxplots displaying the median and interquartile range of connectivity z-scores and whiskers representing the most extreme z-scores within 1.5 times the interquartile range. ASD: Autism Spectrum Disorder; TD: Typically Developing; F: Female; M: Male; DMN: Default Mode Network; SN: Salience Network; CEN: Central Executive Network; ROI: region of interest; *corrected P < 0.05; **corrected P < 0.01.
Significant differences between youth with ASD and their same-sex TD counterparts were detected only in DMN and CEN functional connectivity (Figs 2C, D and 3B, C). Specifically, girls with ASD exhibited increased positive connectivity between the DMN (PCC) and CEN (L PPC) compared to TD girls (β = 0.85, P = 0.002), whereas boys with ASD displayed less positive connectivity within the CEN (R dlPFC with R PPC) than TD boys (β = 0.86, P = 0.02). When analyzing the impact of medication on these connections, medicated girls and boys with ASD exhibited no significant differences from their same-sex unmedicated counterparts (all uncorrected Ps > 0.5). Briefly, the female ASD group was thus characterized by atypical between-network connectivity of the DMN and the CEN, and the male ASD group by atypical within-network connectivity of the CEN.
Interactions between diagnostic group and sex were observed in functional connectivity between the DMN and the CEN (Fig. S5), as well as in the within- and between-network connectivity of the SN (Fig. S5), although they did not quite attain statistical significance after correction for multiple comparisons. More specifically, we found an interaction between diagnostic group and sex in functional connectivity of the DMN (PCC, vmPFC) with the left PPC hub of the CEN (β = 0.97, uncorrected P = 0.002; β = 0.64, uncorrected P = 0.046). An interaction was also noted for functional connectivity within the SN (R FIC with ACC; β = 0.81, uncorrected P = 0.01). Lastly, a diagnostic group by sex interaction was observed for connectivity between the FIC hubs of the SN (L FIC, R FIC) and the left PPC hub of the CEN (β = 0.73, uncorrected P = 0.02; β = 0.89, uncorrected P = 0.004), as well as for connectivity between the ACC hub of the SN and the CEN (L PPC: β = 0.80, uncorrected P = 0.009; R DLPFC: β = 0.76, uncorrected P = 0.02). There was no significant impact of medication status on these connections among either girls with ASD or boys with ASD (all uncorrected Ps > 0.3). In sum, the ROI-based network analyses indicate both that youth with ASD display altered sex differences in the SN, DMN, and CEN compared with TD youth, and that there are sex differences in how these networks are altered in ASD relative to typical development.
When investigating the association between pubertal development, or ASD symptom severity, and functional connectivity of those ROI network hubs that exhibited group differences (Figs 2, 3, and S5), there was no significant relationship between pubertal development, or ASD symptomatology, and connectivity (all Ps > 0.3).
Discussion
Here, we found that youth with ASD exhibited altered sex differences in SN, DMN, and CEN functional connectivity relative to TD youth, and that the nature of neural atypicalities in ASD varied as a function of sex. Connectivity within and between these key higher-order networks are hypothesized to be central to the pattern of symptoms seen in ASD, and prior analyses in largely male samples have found that the functional connectivity of these networks is significantly impacted in ASD (Menon 2011; Nomi and Uddin 2015; Uddin 2015; Abbott et al. 2016; Padmanabhan et al. 2017; Lawrence et al. 2019; Nomi et al. 2019). However, no previous studies have specifically examined the patterns of functional connectivity within and between these 3 networks among females with ASD, even though recent investigations suggest that females and males with ASD display significant behavioral, cognitive, and neural differences (Beacher et al. 2012b; Schneider et al. 2013; Baron-Cohen et al. 2014; Frazier et al. 2014; Hiller et al. 2014; Lai et al. 2015; Nordahl et al. 2015; Alaerts et al. 2016; Hull et al. 2017; Lai et al. 2017b; Evans et al. 2018; Harrop et al. 2018a; Harrop et al. 2018b; Knutsen et al. 2018; Lawson et al. 2018; Moseley et al. 2018; Tillmann et al. 2018; Kozhemiako et al. 2019; Matheis et al. 2019). Notably, such sex differences in ASD have also been shown to differ from those seen in the neurotypical population, suggesting that the biological mechanisms that contribute to sexual differentiation may additionally be implicated in ASD (Beacher et al. 2012b; Supekar and Menon 2015; Alaerts et al. 2016; Moseley et al. 2018; Smith et al. 2019; O'Neill et al. 2020).
When contrasting girls and boys with ASD, we found that girls with ASD exhibited significantly greater functional connectivity between the DMN and the CEN than their male counterparts. These results are broadly consistent with the few published studies to date examining functional connectivity in females with ASD, which, as a whole, have found that females with ASD exhibit increased functional connectivity compared to their male counterparts in a number of connections across the brain (Alaerts et al. 2016; Smith et al. 2019). Our finding of increased functional connectivity between the DMN and the CEN among girls with ASD thus adds to an emerging body of evidence that females with ASD exhibit greater functional connectivity than males with ASD. As females with ASD may be more adept at camouflaging their symptoms than males with ASD, such findings of increased connectivity among affected females in this study and others may reflect protective or compensatory mechanisms (Allely 2019; Hull et al. 2020). With regards to our specific finding of connectivity differences between the DMN and the CEN, functional connectivity between the DMN and the CEN has previously been associated with lab-based measures of executive function as well as IQ, such that greater segregation between these 2 networks is related to better cognitive performance among neurotypical individuals (Kelly et al. 2008; Hampson et al. 2010; Sherman et al. 2014). As girls and boys with ASD exhibit different types of executive function challenges relative to their same-sex peers (Lai et al. 2015; Hull et al. 2017), our finding that girls and boys with ASD differ in their patterns of functional connectivity between the DMN and CEN may relate to the unique executive function difficulties observed in each group. When comparing TD girls and boys, sex differences in SN connectivity were observed. Specifically, we found that TD boys displayed significantly greater connectivity within the SN, as well as a pattern of both less positive and more negative between-network connectivity of the SN with the DMN and CEN. Previous literature examining sex differences among the neurotypical population has focused on adults and indicated both increased and decreased functional connectivity in males relative to females, where the directionality of sex differences may depend on the specific network connections and the age range of the sample (Bluhm et al. 2008; Biswal et al. 2010; Allen et al. 2011; Filippi et al. 2013; Scheinost et al. 2015; Zhang et al. 2016). Functional connectivity analyses focused on whole-brain organizational properties have likewise suggested the presence of sex differences that may depend on participant age (Tian et al. 2011; Wu et al. 2013), with a large-scale developmental study reporting that females exhibited greater network segregation than males (Satterthwaite et al. 2015). Similar to our finding that TD boys displayed greater functional connectivity within the SN than TD girls, one recent study in adolescents demonstrated increased within-network SN connectivity in TD boys relative to TD girls (Teeuw et al. 2019), although another developmental study with a broader age range found no significant differences (Sole-Padulles et al. 2016). Normative sex differences in SN connectivity are also supported by large-scale studies in neurotypical adult samples, which have likewise found that males exhibit greater functional connectivity than females in some SN connections (Biswal et al. 2010; Filippi et al. 2013).
As a whole, our pattern of results demonstrate that youth with ASD exhibit significant sex differences in functional connectivity between the DMN and the CEN that are not present among TD youth, whereas typical sex differences in SN functional connectivity are not observed in ASD youth. A number of previous brain-based studies have similarly demonstrated the existence of altered sex differences in ASD. Recent functional connectivity analyses focused on the cerebellum, posterior superior temporal sulcus, posterior cingulate cortex, and the whole brain, have found that functional connectivity significantly differed between females and males with ASD in the reverse direction of typical sex differences, such that functional connectivity was comparatively masculinized among females with ASD and comparatively feminized among males with ASD (Alaerts et al. 2016; Floris et al. 2018; Smith et al. 2019), although other higher-order networks may instead be comparatively masculinized among males with ASD (Ypma et al. 2016; Floris et al. 2018). Such relative differences in neural feminization and masculinization among the ASD population have also been found when assessing neural activity during a mental rotation task (Beacher et al. 2012b), as well as when analyzing the concentrations of neurometabolites using magnetic resonance spectroscopy (MRS) (O'Neill et al. 2020). Females and males with ASD have additionally been shown to differ from each other even when neurotypical females and males do not, with patterns of cortical and subcortical gray matter volumes differentiating girls and boys with ASD but not TD girls and boys (Supekar and Menon 2015). Neurotypical sex differences have also been reported which are not observed when comparing females and males with ASD, such that typical sex differences in total white matter volume, regional white matter integrity, and regional gray matter volume are attenuated or absent in adults with ASD (Beacher et al. 2012a). Studies analyzing sex hormones or the relative masculinity or femininity of physical features have likewise found reduced sex differences among adults with ASD (Geier and Geier 2007; Bejerot et al. 2012). Taken together, this prior work indicates that individuals with ASD exhibit distinct sex differences relative to neurotypical controls across a wide range of neural and biological measures. Our finding that youth with ASD exhibit unique sex differences in SN, DMN, and CEN functional connectivity is thus both consistent with and expands upon a growing literature demonstrating altered sexual differentiation in ASD. Importantly, such atypicalities suggest in turn that biological factors related to sexual differentiation—such as sex hormones and sex differential gene expression—may contribute to ASD (Arnold 2017; McEwen and Milner 2017). Evidence from previous studies indicate that prenatal sex steroids have organizational effects on the brain (Chura et al. 2010; Lombardo et al. 2012), and that sex differential gene expression likewise contributes to sexual dimorphism in the brain (Rinn and Snyder 2005; Arnold 2017). Such typical sex differences suggest that neurotypical—or arguably “optimal”—brain profiles are sex-specific. Combined with findings of altered sex differences in ASD, this suggests that any deviations from the expected sex-specific neurotypical profile (among females or males) may converge and give rise to core ASD symptomatology in both sexes (Alaerts et al. 2016).
In addition to examining sex differences in ASD and typical development, we also investigated how girls and boys with ASD may differ from their same-sex TD counterparts in SN, DMN, and CEN functional connectivity. When contrasting functional connectivity among girls with ASD and TD girls, girls with ASD displayed significant hyperconnectivity between the DMN and CEN. Several recent analyses have likewise found hyperconnectivity among females with ASD in a range of functional connections across the brain (Alaerts et al. 2016; Yang and Lee 2018; Smith et al. 2019), even though other connections may instead exhibit hypoconnectivity in females with ASD (Alaerts et al. 2016; Ypma et al. 2016; Yang and Lee 2018). A generally mixed pattern of over- and underconnectivity has previously been demonstrated in males with ASD as compared to neurotypical males, with numerous studies suggesting that such variability may depend on the exact connections examined, as well as the specific characteristics of the sample and analysis methods (Muller et al. 2011; Uddin et al. 2013b; Nair et al. 2014; Picci et al. 2016; Hernandez et al. 2017). Our finding that girls with ASD exhibited greater functional connectivity than TD girls between the DMN and CEN, in conjunction with previous studies indicating the existence of both hyper- and hypoconnectivity in males with ASD, suggests that patterns of altered connectivity among females with ASD may similarly depend on the specific network examined as well as sample characteristics. When comparing boys with ASD to TD boys in our analyses, boys with ASD exhibited hyperconnectivity of the CEN and reduced negative connectivity of the SN. Previous studies have likewise found significant alterations in SN and CEN functional connectivity among boys with ASD (Nomi and Uddin 2015; Abbott et al. 2016; Lawrence et al. 2019; Nomi et al. 2019), although the impacted connections and the directionality of such alterations in these networks varies and may also depend on sample attributes (e.g., age) and the selected analysis methods (e.g., task-based vs. resting-state functional connectivity) (Muller et al. 2011; Uddin et al. 2013b; Nair et al. 2014; Hernandez et al. 2017). Taken together, our results demonstrate that girls and boys with ASD exhibit different alterations in functional connectivity when compared with their corresponding same-sex TD peers, such that girls with ASD exhibit significant overconnectivity between the DMN and CEN, whereas boys with ASD display significant alterations in SN and CEN connectivity. This pattern of findings may be specific to ASD, as one recent study in girls and boys with attention-deficit/hyperactivity disorder (ADHD) found that girls with ADHD significantly differed from their same-sex TD counterparts in functional connectivity, but boys with ADHD did not significantly differ from TD boys (Rosch et al. 2018). The currently reported differences between girls and boys with ASD in their pattern of atypicalities relative to TD youth also further highlight the importance of considering participant sex when characterizing functional connectivity in ASD. Additionally, some of the connections that exhibited significant differences between our sample of youth with ASD and their TD counterparts overlap with the connections that displayed altered sex differences in ASD. This suggests that alterations in sexual differentiation among youth with ASD may underlie some of the atypicalities in functional connectivity seen in ASD relative to TD controls, both in the present study and in previous ones that used primarily male ASD samples. The current results are furthermore in line with a brain-based gene expression study, which found that sexually dimorphic processes significantly overlapped with pathways altered in ASD (Werling et al. 2016). As a whole, our findings thus lend additional support to the hypothesis that the biology underlying sexual differentiation may contribute to ASD (Lai et al. 2017b).
Future studies should directly examine how sex-specific biological factors, such as sex hormones and sex differential gene expression, may relate to the atypicalities in functional connectivity seen in ASD. An improved understanding of this relationship may facilitate the creation of biologically based ASD subgroups and more targeted treatments. Additionally, the current investigation focused on functional connectivity differences that were independent of age. However, a recent longitudinal study by our group in a primarily male sample of adolescents with and without ASD found altered developmental trajectories of functional connectivity in ASD (Lawrence et al. 2019). Thus, it will be important to examine trajectories of functional connectivity among both girls and boys with ASD, as well as their TD counterparts. Lastly, atypical sexual differentiation in ASD has also been observed for other measures of brain function and structure (Beacher et al. 2012a; Beacher et al. 2012b; Supekar and Menon 2015; O'Neill et al. 2020). Future studies should directly examine the overlap in such altered sex differences across multiple neuroimaging modalities to foster our understanding of the neural mechanisms that may contribute to such alterations.
In sum, our results demonstrate that youth with ASD exhibited altered sex differences in SN, DMN, and CEN functional connectivity relative to their TD peers, as assessed in a large, well-matched sample using 2 independent analytic approaches. These findings underscore the importance of sex-related biological factors in ASD and the need to consider both females and males when characterizing the neural underpinnings of ASD.
Funding
The National Institute of Mental Health (R01MH100028 to K.A.P., F31MH110140 to K.E.L., and F32MH105167 to S.A.G.), the National Institute of Neurological Disorders and Stroke (T32NS048004 to K.E.L. and F99NS105206 to L.M.H.), the National Science Foundation (Graduate Research Fellowship 1650604 to N.T.P.), the University of California Los Angeles Dissertation Year Fellowship Program, and the ARCS Foundation. We are also grateful for the generous support from the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund. Research reported in this publication was also partially supported by the National Center for Research Resources and by the Office of the Director of the National Institutes of Health under grant numbers C06RR012169, C06RR015431, and S10OD011939. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest: none declared.
Supplementary Material
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, Muller RA. 2016. Patterns of atypical functional connectivity and Behavioral links in autism differ between default, salience, and executive networks. Cereb Cortex. 26(10):4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Milham MP, Di Martino A, Craddock RC, Samaras D, Thirion B, Varoquaux G. 2017. Deriving reproducible biomarkers from multi-site resting-state data: an autism-based example. Neuroimage. 147:736–745. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. 2016. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc Cogn Affect Neurosci. 11(6):1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allely CS. 2019. Understanding and recognising the female phenotype of autism spectrum disorder and the “camouflage” hypothesis: a systematic PRISMA review. Advances in Autism. 5:14–37. [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R et al. 2011. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association 2013. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, Cooperrider JR, Zielinski BA, Ravichandran C, Fletcher PT et al. 2011. Functional connectivity magnetic resonance imaging classification of autism. Brain. 134(Pt 12):3742–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. 2017. A general theory of sexual differentiation. J Neurosci Res. 95(1–2):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen LD, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Rosenberg CR, White T et al. 2018. Prevalence of autism Spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 67(6):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Cassidy S, Auyeung B, Allison C, Achoukhi M, Robertson S, Pohl A, Lai MC. 2014. Attenuation of typical sex differences in 800 adults with autism vs. 3,900 controls. PLoS One. 9(7):e102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Minati L, Baron-Cohen S, Lombardo MV, Lai MC, Gray MA, Harrison NA, Critchley HD. 2012a. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am J Neuroradiol. 33(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Radulescu E, Minati L, Baron-Cohen S, Lombardo MV, Lai MC, Walker A, Howard D, Gray MA, Harrison NA et al. 2012b. Sex differences and autism: brain function during verbal fluency and mental rotation. PLoS One. 7(6):e38355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejerot S, Eriksson JM, Bonde S, Carlstrom K, Humble MB, Eriksson E. 2012. The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. Br J Psychiatry. 201:116–123. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Statistical Methodology. 57(1):289–300. [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S et al. 2010. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 107(10):4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RW, Theberge J, Williamson P. 2008. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 19(8):887–891. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. 1993. A self-administered rating scale for pubertal development. J Adolesc Health. 14(3):190–195. [DOI] [PubMed] [Google Scholar]
- Chita-Tegmark M. 2016. Social attention in ASD: a review and meta-analysis of eye-tracking studies. Res Dev Disabil. 48:79–93. [DOI] [PubMed] [Google Scholar]
- Chura LR, Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Bullmore ET, Baron-Cohen S. 2010. Organizational effects of fetal testosterone on human corpus callosum size and asymmetry. Psychoneuroendocrinology. 35(1):122–132. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. 2012. Social Responsiveness Scale--Second Edition (SRS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29(3):162–173. [DOI] [PubMed] [Google Scholar]
- Dean M, Kasari C, Shih W, Frankel F, Whitney R, Landa R, Lord C, Orlich F, King B, Harwood R. 2014. The peer relationships of girls with ASD at school: comparison to boys and girls with and without ASD. J Child Psychol Psychiatry. 55(11):1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, O'Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, Balsters JH, Baxter L, Beggiato A, Bernaerts S et al. 2017. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data. 4:170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M et al. 2014. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 19(6):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey I, Ropar D, Hamilton AF. 2015. Measuring the value of social engagement in adults with and without autism. Mol Autism. 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot C. 2007. Differential ability scales–second edition: administration and scoring manual. San Antonio, TX: Harcourt Assessment, Inc. [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, Gao W. 2016. Neural connectivity evidence for a categorical-dimensional hybrid model of autism Spectrum disorder. Biol Psychiatry. 80(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SC, Boan AD, Bradley C, Carpenter LA. 2018. Sex/gender differences in screening for autism Spectrum disorder: implications for evidence-based assessment. J Clin Child Adolesc Psychol. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Valsasina P, Misci P, Falini A, Comi G, Rocca MA. 2013. The organization of intrinsic brain activity differs between genders: a resting-state fMRI study in a large cohort of young healthy subjects. Hum Brain Mapp. 34(6):1330–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris DL, Lai MC, Nath T, Milham MP, Di Martino A. 2018. Network-specific sex differentiation of intrinsic brain function in males with autism. Mol Autism. 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, Hardan AY. 2014. Behavioral and cognitive characteristics of females and males with autism in the Simons simplex collection. J Am Acad Child Adolesc Psychiatry. 53(3):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Geier MR. 2007. A prospective assessment of androgen levels in patients with autistic spectrum disorders: biochemical underpinnings and suggested therapies. Neuro Endocrinol Lett. 28(5):565–573. [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, Dapretto M. 2016. Salience network connectivity in autism is related to brain and Behavioral markers of sensory Overresponsivity. J Am Acad Child Adolesc Psychiatry. 55(7):618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DM, Warrier V, Allison C, Baron-Cohen S. 2018. Testing the empathizing-systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proc Natl Acad Sci U S A. 115(48):12152–12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. 2010. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 28(8):1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop C, Jones D, Zheng S, Nowell S, Boyd BA, Sasson N. 2018a. Circumscribed interests and attention in autism: the role of biological sex. J Autism Dev Disord. 48(10):3449–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop C, Jones D, Zheng S, Nowell SW, Boyd BA, Sasson N. 2018b. Sex differences in social attention in autism spectrum disorder. Autism Res. 11(9):1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, Stokes MA. 2014. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol Autism. 5(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Krasileva K, Green SA, Sherman LE, Ponting C, McCarron R, Lowe JK, Geschwind DH, Bookheimer SY, Dapretto M. 2017. Additive effects of oxytocin receptor gene polymorphisms on reward circuitry in youth with autism. Mol Psychiatry. 22(8):1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. 2015. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology. 40(1):171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, Weber N. 2014. Sex differences in autism spectrum disorder based on DSM-5 criteria: evidence from clinician and teacher reporting. J Abnorm Child Psychol. 42(8):1381–1393. [DOI] [PubMed] [Google Scholar]
- Hull JV, Dokovna LB, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. 2016. Resting-state functional connectivity in autism Spectrum disorders: a review. Front Psychiatry. 7:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull L, Lai MC, Baron-Cohen S, Allison C, Smith P, Petrides KV, Mandy W. 2020. Gender differences in self-reported camouflaging in autistic and non-autistic adults. Autism. 24(2):352–363. [DOI] [PubMed] [Google Scholar]
- Hull L, Mandy W, Petrides KV. 2017. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism. 21(6):706–727. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17(2):825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. 2001. A global optimisation method for robust affine registration of brain images. Med Image Anal. 5(2):143–156. [DOI] [PubMed] [Google Scholar]
- Kana RK, Uddin LQ, Kenet T, Chugani D, Muller RA. 2014. Brain connectivity in autism. Front Hum Neurosci. 8:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2008. Competition between functional brain networks mediates behavioral variability. Neuroimage. 39(1):527–537. [DOI] [PubMed] [Google Scholar]
- Kessler D, Angstadt M, Sripada CS. 2017. Reevaluating “cluster failure” in fMRI using nonparametric control of the false discovery rate. Proc Natl Acad Sci U S A. 114(17):E3372–E3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen J, Crossman M, Perrin J, Shui A, Kuhlthau K. 2018. Sex differences in restricted repetitive behaviors and interests in children with autism spectrum disorder: an autism treatment network study. Autism. 23(4):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemiako N, Vakorin V, Nunes AS, Iarocci G, Ribary U, Doesburg SM. 2019. Extreme male developmental trajectories of homotopic brain connectivity in autism. Hum Brain Mapp. 40(3):987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CLE, Lau Z, Lui SSY, Lok E, Tam V, Chan Q, Cheng KM, Lam SM, Cheung EFC. 2017a. Meta-analysis of neuropsychological measures of executive functioning in children and adolescents with high-functioning autism spectrum disorder. Autism Res. 10(5):911–939. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Baron-Cohen S. 2014. Autism. The Lancet. 383(9920):896–910. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lerch JP, Floris DL, Ruigrok AN, Pohl A, Lombardo MV, Baron-Cohen S. 2017b. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res. 95(1–2):380–397. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. 2015. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. 54(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Bookheimer SY, Dapretto M. 2019. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 12(1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LP, Joshi R, Barbaro J, Dissanayake C. 2018. Gender differences during toddlerhood in autism Spectrum disorder: a prospective community-based longitudinal follow-up study. J Autism Dev Disord. 48(8):2619–2628. [DOI] [PubMed] [Google Scholar]
- Linke AC, Olson L, Gao Y, Fishman I, Muller RA. 2017. Psychotropic medication use in autism spectrum disorders may affect functional brain connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2(6):518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S. 2012. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. 32(2):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, Mandy WPL. 2017. What is the male-to-female ratio in autism Spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 56(6):466–474. [DOI] [PubMed] [Google Scholar]
- Lord C, DiLavore PC, Gotham K. 2012. Autism diagnostic observation schedule. 2nd ed. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24(5):659–685. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. 2013. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 74(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheis M, Matson JL, Hong E, Cervantes PE. 2019. Gender differences and similarities: autism symptomatology and developmental functioning in Young children. J Autism Dev Disord. 49(3):1219–1231. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. 2017. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 95(1–2):24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon B. 2019. Towards a new model of understanding - the triple network, psychopathology and the structure of the mind. Med Hypotheses. 133:109385. [DOI] [PubMed] [Google Scholar]
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15(10):483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RL, Hitchiner R, Kirkby JA. 2018. Self-reported sex differences in high-functioning adults with autism: a meta-analysis. Mol Autism. 9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. 2011. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 21(10):2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, Muller RA. 2014. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Hum Brain Mapp. 35(8):4035–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld J, Kuja-Halkola R, Mevel K, Cauvet E, Fransson P, Bolte S. 2018. Alterations in resting state connectivity along the autism trait continuum: a twin study. Mol Psychiatry. 23(7):1659–1665. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, Lainhart JE, Anderson JS. 2013. Multisite functional connectivity MRI classification of autism: ABIDE results. Front Hum Neurosci. 7:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Molnar-Szakacs I, Uddin LQ. 2019. Insular function in autism: update and future directions in neuroimaging and interventions. Prog Neuropsychopharmacol Biol Psychiatry. 89:412–426. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. 2015. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 7:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Iosif AM, Young GS, Perry LM, Dougherty R, Lee A, Li D, Buonocore MH, Simon T, Rogers S et al. 2015. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol Autism. 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Bansal R, Goh S, Rodie M, Sawardekar S, Peterson BS. 2020. Parsing the heterogeneity of brain metabolic disturbances in autism Spectrum disorder. Biol Psychiatry. 87(2):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynch CJ, Schaer M, Menon V. 2017. The default mode network in autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2(6):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci G, Gotts SJ, Scherf KS. 2016. A theoretical rut: revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Dev Sci. 19(4):524–549. [DOI] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Martin A. 2015a. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. Neuroimage Clin. 7:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A. 2015b. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci U S A. 112(48):E6699–E6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. 2015a. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 112:278–287. [DOI] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, Rooij D, Llera A, Buitelaar JK, Beckmann CF. 2015b. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 112:267–277. [DOI] [PubMed] [Google Scholar]
- R Core Team 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rinn JL, Snyder M. 2005. Sexual dimorphism in mammalian gene expression. Trends Genet. 21(5):298–305. [DOI] [PubMed] [Google Scholar]
- Rosch KS, Mostofsky SH, Nebel MB. 2018. ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J Neurodev Disord. 10(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta L, Fama FI, Bernava GM, Leonardi E, Tartarisco G, Falzone A, Pioggia G, Chakrabarti B. 2017. Reduced preference for social rewards in a novel tablet based task in young children with autism Spectrum disorders. Sci Rep. 7(1):3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H et al. 2015. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 25(9):2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT. 2015. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 36(4):1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Regenbogen C, Pauly KD, Gossen A, Schneider DA, Mevissen L, Michel TM, Gur RC, Habel U, Schneider F. 2013. Evidence for gender-specific endophenotypes in high-functioning autism spectrum disorder during empathy. Autism Res. 6(6):506–521. [DOI] [PubMed] [Google Scholar]
- Sedgewick F, Hill V, Pellicano E. 2019. It's different for girls': gender differences in the friendships and conflict of autistic and neurotypical adolescents. Autism. 23(5):1119–1132. [DOI] [PubMed] [Google Scholar]
- Sedgewick F, Hill V, Yates R, Pickering L, Pellicano E. 2016. Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. J Autism Dev Disord. 46(4):1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. 2014. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci. 10:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith REW, Avery JA, Wallace GL, Kenworthy L, Gotts SJ, Martin A. 2019. Sex differences in resting-state functional connectivity of the cerebellum in autism Spectrum disorder. Front Hum Neurosci. 13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Sole-Padulles C, Castro-Fornieles J, Serna E, Calvo R, Baeza I, Moya J, Lazaro L, Rosa M, Bargallo N, Sugranyes G. 2016. Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Dev Cogn Neurosci. 17:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Menon V. 2015. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol Autism. 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuw J, Brouwer RM, Guimaraes J, Brandner P, Koenis MMG, Swagerman SC, Verwoert M, Boomsma DI, Hulshoff Pol HE. 2019. Genetic and environmental influences on functional connectivity within and between canonical cortical resting-state networks throughout adolescent development in boys and girls. Neuroimage. 202:116073. [DOI] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. 2011. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage. 54(1):191–202. [DOI] [PubMed] [Google Scholar]
- Tillmann J, Ashwood K, Absoud M, Bolte S, Bonnet-Brilhault F, Buitelaar JK, Calderoni S, Calvo R, Canal-Bedia R, Canitano R et al. 2018. Evaluating sex and age differences in ADI-R and ADOS scores in a large European multi-site sample of individuals with autism Spectrum disorder. J Autism Dev Disord. 48(7):2490–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. 2015. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 16(1):55–61. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, Phillips J, Feinstein C, Abrams DA, Menon V. 2015. Brain state differentiation and Behavioral inflexibility in autism. Cereb Cortex. 25(12):4740–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V. 2013a. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 70(8):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2013b. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. 2011. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 31(50):18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Parikshak NN, Geschwind DH. 2016. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun. 7:10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X et al. 2013. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 8(2):e55347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee J. 2018. Different aberrant mentalizing networks in males and females with autism spectrum disorders: evidence from resting-state functional magnetic resonance imaging. Autism. 22(2):134–148. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, Strang J, Kenworthy L, Gaillard WD, Vaidya CJ. 2015. Default mode network segregation and social deficits in autism spectrum disorder: evidence from non-medicated children. Neuroimage Clin. 9:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma RJ, Moseley RL, Holt RJ, Rughooputh N, Floris DL, Chura LR, Spencer MD, Baron-Cohen S, Suckling J, Bullmore ET et al. 2016. Default mode Hypoconnectivity underlies a sex-related autism Spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 1(4):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cahill ND, Arbabshirani MR, White T, Baum SA, Michael AM. 2016. Sex and age effects of functional connectivity in early adulthood. Brain Connect. 6(9):700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. 2013. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci. 7:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


