Abstract
Plant–parasitic root-knot nematode Meloidogyne incognita uses an array of effector proteins to establish successful plant infections. Mi-msp-1 and Mi-msp-20 are two known effectors secreted from nematode subventral oesophageal glands; Mi-msp-1 being a putative secretory venom allergen AG5-like protein, whereas Mi-msp-20 is a pioneer gene with a coiled-coil motif. Expression of specific effector is known to cause disturbances in the expression of other effectors. Here, we used RNA-Seq to investigate the pleiotropic effects of silencing Mi-msp-1 and Mi-msp-20. A total of 25.1–51.9 million HQ reads generated from Mi-msp-1 and Mi-msp-20 silenced second-stage juveniles (J2s) along with freshly hatched J2s were mapped to an already annotated M. incognita proteome to understand the impact on various nematode pathways. As compared to control, silencing of Mi-msp-1 caused differential expression of 29 transcripts, while Mi-msp-20 silencing resulted in differential expression of a broader set of 409 transcripts. In the Mi-msp-1 silenced J2s, cytoplasm (GO:0005737) was the most enriched gene ontology (GO) term, whereas in the Mi-msp-20 silenced worms, embryo development (GO:0009792), reproduction (GO:0000003) and nematode larval development (GO:0002119) were the most enriched terms. Limited crosstalk was observed between these two effectors as a sheer 5.9% of the up-regulated transcripts were common between Mi-msp-1 and Mi-msp-20 silenced nematodes. Our results suggest that in addition to the direct knock-down caused by silencing of Mi-msp-1 and Mi-msp-20, the cascading effect on other genes might also be contributing to a reduction in nematode's parasitic abilities.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02353-8) contains supplementary material, which is available to authorized users.
Keywords: Meloidogyne incognita, Mi-msp-1, Mi-msp-20, RNA-Seq, Target, Cascading effect
Introduction
"Sedentary endoparasitic nematodes have evolved very specialised and complex relationships with their hosts. Host–parasite relationships involving Meloidogyne species typify these specialised associations" (Hussey 1985). These specialised associations with the host plants have enabled the Meloidogyne species (root-knot nematodes: RKNs) to economically damage almost all the agricultural and horticultural crops, thus making them the most notorious group of plant–parasitic nematodes (Jones et al. 2013). The soil-dwelling second-stage juveniles (J2s) of RKNs enter the plants through apex or elongation zone of the root, migrate intercellularly to reach the vascular tissue, and develop permanent feeding sites (giant cells) to obtain nourishment throughout their life span (Mitchum et al. 2013). During the process of intercellular migration, a cocktail of cell-wall degrading enzymes are secreted by RKNs that help in softening the cell wall (Danchin et al. 2010; Nguyễn et al. 2014). After reaching the vascular cylinder, the J2s become sedentary, and the giant cells act as "metabolic sink" for the continued supply of food to the nematodes (Davis et al. 2008). For the initiation and maintenance of these giant cells, Meloidogyne spp. secrete several compounds (including effectors) from their dorsal and sub-ventral pharyngeal gland cells through stylet (Kyndt et al. 2013). The nematode derived effector molecules are known to manoeuvre the plant physiology to facilitate their development and reproduction (Gheysen and Mitchum 2011). Previous research based on differential gene expression, cDNA library screening, expressed sequence tag analysis and direct analysis of esophageal gland-secreted proteins resulted in identification and characterisation of several putative parasitism genes of Meloidogyne species (Bakker et al. 2001; Ding et al. 1998; Doyle and Lambert 2002; Jaubert et al. 2002; Lambert et al. 1999; Rosso et al. 1999). However, despite these advances, the current understanding of RKN-host plant interaction is still fractured.
The effector proteins are classified into five broad categories based on their function (Shivakumara et al. 2017). Identification of the triggers for activation and repression of the effectors, and determining their functional sites in the plant–host helps in the elucidation of the process of nematode parasitism. However, with few exceptions, comprehensive knowledge about these secreted proteins is not clear (Huang et al. 2003). Since the identification of β-1,4-endoglucanase (Smant et al. 1998), several effectors have been identified in Meloidogyne species (Dinh et al. 2014; Haegeman et al. 2013; Huang et al. 2003, 2004, 2006; Mantelin et al. 2017; Mitchum et al. 2013; Niu et al. 2016; Rosso et al. 1999; Smant et al. 1998; Xie et al. 2016). Notable among the recently described effectors are—chorismate mutase, venom allergen-like protein and glutathione S-transferase that suppress the host defense during Meloidogyne infection process (Ding et al. 2000; Dubreuil et al. 2007; Long et al. 2006; Rosso et al. 2012; Wang et al. 2007). The effectors, MiMSP40, MeTCTP and MiISE5 were found to suppress the programmed cell death in host plants (Niu et al. 2016; Shi et al. 2018; Zhuo et al. 2017), while Mi8D05 from M. incognita regulated solute and water transport (Xue et al. 2013). Furthermore, MiEFF1, MiCRT and 7H08 in M. incognita were found to target the plant cell nuclei and showed transcriptional activation of host genes (Jaouannet et al. 2013; Lin et al. 2013; Zhang et al. 2015). These studies demonstrate that the successful parasitism by Meloidogyne species is achieved at various interfaces by an interplay and concomitant expression of several effectors (Mitchum et al. 2013).
One pertinent question here is the existence of 'effector crosstalk', i.e., if the nematode effectors interact/influence each other to determine the outcome of nematode parasitism on plants? Scarce experimental evidence is indeed available to support this idea. The in vitro suppression of a sub-ventral gland specific effector gene led to the transcriptional oscillation of other unrelated effector genes specific to the dorsal gland, and vice versa, suggesting the existence of 'effector crosstalk' using reverse-genetics approach (Shivakumara et al. 2016). Unravelling the intricacies of effector–effector interaction was also attempted by Mitchum et al. (2013) using in planta effector screening approach. Recent advances in genomics and transcriptomics have enabled us to investigate the molecular intricacies of effector regulation system of RKNs. Transcriptomic approaches have been used to identify the novel effector molecules in different Meloidogyne species (Haegeman et al. 2013; Li et al. 2016; Petitot et al. 2016; Shi et al. 2018). However, only a few efforts have been made to understand the interaction of different effectors during successful parasitism.
To understand the 'effector crosstalk' phenomenon better, we selected two established M. incognita effectors, Mi-msp-1 and Mi-msp-20, and used a transcriptomic approach. The M. incognita Mi-msp-1 plays a crucial role in host–pathogen interaction (Ding et al. 2000) and is a putative secretory venom allergen AG5-like protein (Hussey et al. 2002). The Mi-msp-1 was found to be expressed in sub-ventral esophageal gland cell (Chaudhary et al. 2019a) and is highly expressed in pre- and post-parasitic M. incognita J2s but not in the adults (Ding et al. 2000). Host-induced gene silencing (HIGS) of Mi-msp-1 was effective in reducing nematode parasitism of eggplants (Chaudhary et al. 2019b). Mi-msp-20 is also a sub-ventral gland specific pioneer gene and is known to contain a coiled-coil motif (Huang et al. 2003). It was found that the host-induced silencing of Mi-msp-20 causes transcriptional alteration of the cell-wall-modifying enzymes and reduced the nematode parasitism on plants (Shivakumara et al. 2017). Here, to investigate the (a) pleiotropic effects of silencing the individual effector genes, (b) effector crosstalk, and (c) molecular mechanism of Mi-msp-1 and Mi-msp-20 genes in the early infection process, we silenced each of these genes separately and studied the changes in transcriptome using RNA-Seq.
Materials and methods
Nematode population
The authenticated population of Meloidogyne incognita was maintained and multiplied on tomato plants (Solanum lycopersicum L. cv. Pusa ruby) in the glasshouse at ICAR-Indian Agricultural Research Institute, New Delhi, India. Egg masses were handpicked and hatched via "modified Baermann's method" (Whitehead and Hemming 1965). The freshly hatched second-stage juveniles (J2s) were used for experimental purpose.
Cloning, sequencing, dsRNA synthesis and in vitro silencing of Mi-msp-1 and Mi-msp-20 genes
Total RNA (~ 500 ng) was extracted from the M. incognita J2s via NucleoSpin RNA Kit (Macherey-Nagel, Düren, Germany) following the manufacturer's instructions, assessed for quality, and reverse transcribed to complementary DNA (cDNA) as described in Shivakumara et al. (2016). The ca. 515 and 598 bp fragments of Mi-msp-1 (AF013289) and Mi-msp-20 (AY134439) were PCR amplified, cloned into the pGEM-T easy vector and insert identity was confirmed by sequencing. Primer details are given in Shivakumara et al. (2016) and Chaudhary et al. (2019a). The purified PCR products of Mi-msp-1 and Mi-msp-20 were used as templates to synthesise the sense and antisense strands, as described in Chaudhary et al. (2019a).
For in vitro silencing of Mi-msp-1 and Mi-msp-20, approximately 10,000 freshly hatched J2s were washed with DEPC treated Milli-Q water and soaked in soaking buffer containing 1 mg ml−1dsRNA (Urwin et al. 2002). Soaking was continued for 24 h in the dark on a slowly moving rotator (as described in Shivakumara et al. 2016). The J2s soaked in water served as control. Two technical replicates were maintained for each treatment and pooled before RNA extraction. All the treatments were repeated once more to generate RNA for the second biological replicate.
RNA extraction, cDNA synthesis, library preparation and RNA-Sequencing
Total RNA from Mi-msp-1 and Mi-msp-20 silenced J2s were extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer's guidelines. The quality assessment, mRNA purification, cDNA synthesis and library preparation were pursued following standard Illumina protocols (Kumar et al. 2014). In total, six libraries were prepared for treatments (Mi-msp-1 and Mi-msp-20 silenced J2s) and control (J2s soaked in water) with two replicates for each sample. For the extraction of total RNA, approximately 20,000 M. incognita J2s were used, and RQ1 RNase-Free DNase treatment (Promega, Madison, WI, USA) was used to remove any DNA contamination. RNA integrity and quality were tested on Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and agarose gel electrophoresis. The RNA concentration was determined by a NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The mRNA was purified from ~ 5 μg of total RNA by using oligodT beads (Illumina® TruSeq® RNA Sample Preparation Kit v2), and the purified mRNA was fragmented in the presence of bivalent cations. Random hexamer primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) were used for first-strand cDNA synthesis. In contrast, DNA polymerase I and RNaseH were used for the second strand cDNA synthesis as per Illumina protocol. The cDNA was cleaned by Agencourt AMPure XP purification kit (Beckman-Coulter, Brea, CA, USA), amplified, quantified using a Nanodrop spectrophotometer and checked again for quality with a Bioanalyzer. Illumina HiSeq platform was used for sequencing the cDNA libraries.
Quantitation and identification of differentially expressed transcripts
To understand the significant biology, we used an approach, wherein we mapped the high quality reads to the already annotated M. incognita transcriptome (Somvanshi et al. 2018). In brief, the NGS QC toolkit was used for quality control of the raw reads (Patel and Jain 2012), and the quantitation was done by mapping the high-quality reads against INRA M. incognita proteome by Kalisto tool (Bray et al. 2016). The DESeq2 package (Love et al. 2014) was used to find the differentially expressed (DE) transcripts between the control (water-soaked sample) and treatments (Mi-msp-1 and Mi-msp-20 dsRNA soaked samples) in R version 3.2.5. The threshold for DEGs was set as |log2 foldchange|≥ 2.0 and ≤ − 2.0 and p value ≤ 0.05. The unique and overlapping genes within the DE datasets were represented using Venny (https://bioinfogp.cnb.csic.es/tools/venny/index2.0.2.html), analysed for significant biology using GO-Elite (Zambon et al. 2012) and also by comparing with the annotated proteome of M. incognita (Somvanshi et al. 2018).
Validation of the expression pattern of five randomly selected transcripts was carried out by quantitative real-time PCR (qRT-PCR). The samples used for qRT-PCR were prepared as described above and used for cDNA preparation. Around 500 ng of RNA was used for reverse transcription by cDNA synthesis kit (Superscript VILO, Invitrogen, Carlsbad, CA, USA), followed by qRT-PCR using SYBR Green Supermix Kit (Eurogentec, Liege, Belgium) on a Realplex2 thermal cycler equipment (Eppendorf, Hamburg, Germany). The 18S rRNA, a constitutively expressed nematode gene, was used as an internal reference. Three biological and three technical replicates were maintained for each sample. The data were analysed by the ΔΔCt method (Livak and Schmittgen 2001), and results were expressed as log2-transformed fold change values. The oligonucleotide primer details for qRT-PCR are provided in supplementary Table 1.
Table 1.
Raw and quality filtered data statistics for Meloidogyne incognita transcriptomes used in this study
| S. no. | Sample name | Sample description | Total no. of reads | Total no. of bases | Total no. of HQ bases (Bases ≥ 20 Phred score) | % HQ bases | Total HQ reads (reads ≥ 70% HQ bases) | Total no of bases in HQ reads | Total no. of HQ bases in HQ reads | % of HQ bases in HQ reads | SRA accession no |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAW | FILTERED | ||||||||||
| 1 | Mi-msp-20_1 | Mi-msp-20 silenced nematode J2s, replication 1 | 26,880,254 | 25,497,268 | SRR7609922 | ||||||
| Forward | 13,440,127 | 1,357,452,827 | 1,303,700,206 | 96.04 | 12,748,634 | 1,287,612,034 | 1,255,138,546 | 97.48 | |||
| Reverse | 13,440,127 | 1,357,452,827 | 1,302,060,938 | 95.92 | 12,748,634 | 1,287,612,034 | 1,260,134,583 | 97.87 | |||
| 2 | Mi-msp-20_2 | Mi-msp-20 silenced nematode J2s, replication 2 | 54,873,818 | 51,951,216 | SRR7609923 | ||||||
| Forward | 27,436,909 | 2,771,127,809 | 2,670,348,497 | 96.36 | 25,975,608 | 2,623,536,408 | 2,563,505,709 | 97.71 | |||
| Reverse | 27,436,909 | 2,771,127,809 | 2,643,617,996 | 95.40 | 25,975,608 | 2,623,536,408 | 2,561,069,061 | 97.62 | |||
| 3 | Mi-msp-1_1 | Mi-msp-1 silenced nematode J2s, replication 1 | 29,658,458 | 28,489,606 | SRR7609920 | ||||||
| Forward | 14,829,229 | 1,497,752,129 | 1,446,797,909 | 96.60 | 14,244,803 | 1,438,725,103 | 1,405,724,346 | 97.71 | |||
| Reverse | 14,829,229 | 1,497,752,129 | 1,446,283,297 | 96.56 | 14,244,803 | 1,438,725,103 | 1,410,970,470 | 98.07 | |||
| 4 | Mi-msp-1_2 | Mi-msp-1 silenced nematode J2s, replication 2 | 28,579,388 | 26,999,684 | SRR7609921 | ||||||
| Forward | 14,289,694 | 1,443,259,094 | 1,390,585,849 | 96.35 | 13,499,842 | 1,363,484,042 | 1,332,597,023 | 97.73 | |||
| Reverse | 14,289,694 | 1,443,259,094 | 1,373,233,871 | 95.15 | 13,499,842 | 1,363,484,042 | 1,329,640,753 | 97.52 | |||
| 5 | Control_1 | Water-soaked (control) nematode J2s replication 1 | 33,110,168 | 31,335,746 | SRR7609918 | ||||||
| Forward | 16,555,084 | 1,672,063,484 | 1,606,748,616 | 96.09 | 15,667,873 | 1,582,455,173 | 1,544,134,819 | 97.58 | |||
| Reverse | 16,555,084 | 1,672,063,484 | 1,601,335,012 | 95.77 | 15,667,873 | 1,582,455,173 | 1,548,532,542 | 97.86 | |||
| 6 | Control_2 | Water-soaked (control) nematode J2s replication 2 | 23,600,570 | 21,985,926 | SRR7609919 | ||||||
| Forward | 11,800,285 | 1,191,828,785 | 1,126,238,936 | 94.50 | 10,992,963 | 1,110,289,263 | 1,071,864,360 | 96.54 | |||
| Reverse | 11,800,285 | 1,191,828,785 | 1,126,239,813 | 94.50 | 10,992,963 | 1,110,289,263 | 1,071,864,360 | 97.03 | |||
Results
RNA-Seq data statistics
As described in methodology part, total RNA was extracted from all the three samples, i.e., (1) water-soaked J2s (control), (2) Mi-msp-1 silenced J2s, and (3) Mi-msp-20 silenced J2s. The sequencing was conducted in the paired-end format using Illumina HiSeq platform with two independent biological replicates for each treatment. The raw reads data for all the samples have been deposited in the Sequence Read Archives (Bio project: PRJNA482761, biosample: SAMN09713929, sample accession number: SRR7609918 to SRR7609923). The statistics for the raw and quality-filtered data is provided in Table 1. A total of 26.8 to 54.8 million raw reads were obtained for the samples, which were quality filtered by NGS QC toolkit v2.3.3 at high stringency. Reads with > 70% high-quality (HQ) bases, each having Phred scores > 20 were selected (HQ reads); resulting in 25.1–51.9 million HQ reads which were used for further downstream analyses (Table 1). For all the RNA-Seq samples, the HQ reads constituted 93.2–97.6% of the total raw reads with an average read length of 101 bp. The replicate correlation was determined by principal component analysis (PCA) using the transcripts per kilobase million (TPM) values. The PCA plot suggested a strong correlation between the biological replicates within each treatment (Fig. 1). The correlation coefficient values between biological replicates of Mi-msp-1 silenced, Mi-msp-20 silenced, and control samples were > 0.99 (Supplementary Table 2). These values suggest the robustness of the RNA-Seq reads data of the replicates.
Fig. 1.
Replicate correlation between the biological replicates used for the RNA-Seq study. a The clustering of the heat maps of gene expression data in each sample shows that the two biological replicates used for various treatments and control samples in the study clustered together. b Principal component analysis (PCA) plot representing the correlation between the two RNA-Seq replicates of each treatment and control samples on X, Y and Z-axis. The Mi-msp-1 sample replicates (represented as msp1-1, msp1-2) are represented between the X/Z-axis, controls on the X/Y axis and the Mi-msp-20 (represented as msp20-1 and msp20-2) on the Z/Y axis. The replicate correlation values for replicate samples of the treatments and control were higher than 0.99
The annotation of the RNA-Seq data was carried out by mapping the HQ reads from each treatment to the pre-annotated M. incognita proteome avoiding the need for creating new transcriptome sequence assembly. A total of 66.0‒73.5% HQ reads from the RNA-Seq samples were found to be mapped to the annotated M. incognita proteome (Table 2), representing 74.9‒78.6% of the total predicted 43,719 transcripts (Table 2).
Table 2.
Read alignment and transcript expression statistics of RNA-Seq samples used in this study against the previously annotated Meloidogyne incognita proteome
| Mi-msp-1_2 | Mi-msp-1_2 | Mi-msp-20_1 | Mi-msp-20_2 | Control_1 | Control_2 | |
|---|---|---|---|---|---|---|
| Read alignment statistics | ||||||
| Total reads | 14,244,803 | 13,499,842 | 12,748,634 | 25,975,608 | 15,667,873 | 10,992,963 |
| Mapped reads | 10,471,662 | 9,887,119 | 8,420,221 | 18,126,388 | 11,511,334 | 7,917,610 |
| Unmapped reads | 3,773,141 | 3,612,723 | 4,328,413 | 7,849,220 | 4,156,539 | 3,075,353 |
| % mapped | 73.51 | 73.23 | 66.04 | 69.78 | 73.47 | 72.02 |
| Transcript expression statistics | ||||||
| Total no. of transcripts | 43,719 | 43,719 | 43,719 | 43,719 | 43,719 | 43,719 |
| Expressed transcripts | 34,262 | 34,241 | 33,049 | 34,369 | 34,344 | 32,761 |
| % expressed | 78.36 | 78.32 | 75.59 | 78.61 | 78.55 | 74.93 |
Differential gene expression in Mi-msp-1 and Mi-msp-20 silenced M. incognita J2s
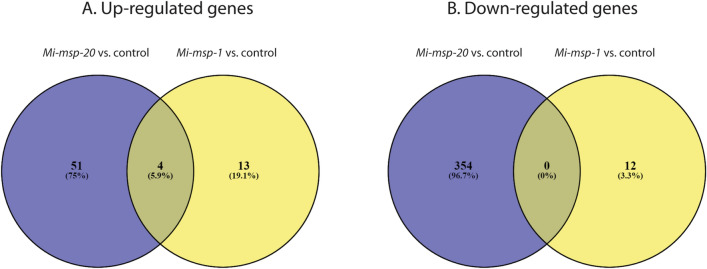
The effect of silencing of Mi-msp-1 and Mi-msp-20 effectors on the transcriptome of M. incognita was determined by comparing the transcripts of Mi-msp-1 and Mi-msp-20 dsRNA soaked J2s to the water-soaked control. A total of 30,503 transcripts were identified as expressed in the RNA-Seq experiment. As compared to the control, 17 transcripts were up-regulated, and 12 were down-regulated in the Mi-msp-1 silenced J2s. In the Mi-msp-20 silenced J2s, 55 transcripts were up-regulated, and 354 were down-regulated (Fig. 2, Supplementary Table 3). The transcripts showing highest up- or down-regulation are presented in Table 3. Amongst the up-regulated transcripts, 13 were unique to Mi-msp-1 silenced worms, and 51 were unique to Mi-msp-20 silenced worms. However, in the down-regulated category, there was no overlap between the transcripts between the treatments and 12 and 354 transcripts were unique to Mi-msp-1 and Mi-msp-20 silenced worms, respectively (Fig. 2b). The complete list of the up-regulated and down-regulated transcripts in both the Mi-msp-1 and Mi-msp-20 silenced worms as compared to controls are given in Supplementary Table 3. Expression of five differentially expressed transcripts (three up-regulated and two down-regulated) was validated by qRT-PCR. The qRT-PCR expression results were in conformity with the expression pattern of those transcripts obtained in RNA-Seq (Supplementary Table 1).
Fig. 2.
Venn diagram showing a up-regulated transcripts and b down-regulated transcripts in the Mi-msp-1 and Mi-msp-20 silenced Meloidogyne incognita J2s with respect to the untreated control J2s. The purple circle represents Mi-msp-20 and the yellow circle represents Mi-msp-1
Table 3.
Top up- and down-regulated annotated genes in Mi-msp-1 and Mi-msp-20 silenced Meloidogyne incognita
| S. no. | Transcript ID | Fold change | Annotation |
|---|---|---|---|
| Down-regulated in Mi-msp-1 silenced worms | |||
| 1 | Minc3s03025g32475 | − 2.18 | Orotidine 5′-phosphate decarboxylase [Caenorhabditis elegans] |
| 2 | Minc3s03057g32601 | − 2.71 | major sperm protein 2 cytoskeletal MSP [Brugia malayi] |
| 3 | Minc3s06758g40280 | − 2.44 | Protein RER1 homolog [C. elegans] |
| Up-regulated in Mi-msp-1 silenced worms | |||
| 4 | Minc3s00357g10916 | 2.08 | Carboxypeptidase [C. elegans] |
| 5 | Minc3s01100g20723 | 2.17 | conserved hypothetical protein |
| 6 | Minc3s04129g35553 | 2.35 | Soluble guanylate cyclase gcy-36 [C. elegans] |
| 7 | Minc3s05491g38327 | 2.01 | Eukaryotic translation initiation factor 4E-3 [C. elegans] |
| 8 | Minc3s05581g38476 | 2.19 | Nuclear Hormone Receptor family [C. elegans] |
| 9 | Minc3s06784g40310 | 2.05 | Integrase core domain-containing protein [B. malayi] |
| 10 | Minc3s07653g41363 | 2.10 | Glycine cleavage system P protein [C. elegans] |
| 11 | Minc3s11057g44585 | 2.63 | Seven WD repeats, AN11 family [C. elegans] |
| Down-regulated in Mi-msp-20 silenced worms | |||
| 12 | Minc3s00279g09287 | − 4.40 | C. briggsae CBR-KLP-12 protein, partial |
| 13 | Minc3s03741g34661 | − 4.39 | UDP-glucuronosyltransferase |
| 14 | Minc3s01347g22989 | − 4.28 | ADP-ribosylation factor 1-like 2 [C. elegans] |
| 15 | Minc3s00168g06605 | − 3.73 | Hepatocyte Growth factor-Regulated TK Substrate (HRS) family [C. elegans] |
| 16 | Minc3s01730g25916 | − 3.66 | Cytochrome Oxidase assembly protein [C. elegans] |
| 17 | Minc3s00049g02640 | − 3.61 | 40S ribosomal protein S14 [B. malayi] |
| 18 | Minc3s06828g40373 | − 3.61 | Prolyl 4-hydroxylase subunit alpha-1 [C. elegans] |
| 19 | Minc3s00535g13913 | − 3.42 | Eukaryotic translation initiation factor 3 subunit K [C. elegans] |
| 20 | Minc3s00698g16238 | − 3.41 | splicing factor |
| 21 | Minc3s02562g30647 | − 3.29 | Aldehyde Dehydrogenase [C. elegans] |
| 22 | Minc3s00001g00036 | − 3.28 | Ras-related protein Rap-1 [C. elegans] |
| 23 | Minc3s05873g38948 | − 3.17 | Quinoid dihydropteridine Reductase [C. elegans] |
| 24 | Minc3s00577g14533 | − 3.17 | CK1 protein kinase |
| 25 | Minc3s00983g19556 | − 3.16 | MAP kinase kinase mkk-4 [C. elegans] |
| 26 | Minc3s06672g40153 | − 3.14 | 2-(3-amino-3-carboxypropyl) histidine synthase subunit 1 [C. elegans] |
| Up-regulated in Mi-msp-20 silenced worms | |||
| 27 | Minc3s05895g38985 | 13.06 | CRE-CLP-2 protein |
| 28 | Minc3s00223g07936 | 3.65 | CRE-UGT-49 protein |
| 29 | Minc3s10828g44395 | 3.05 | CRE-MPZ-1 protein |
| 30 | Minc3s03291g33426 | 2.64 | LD40453p |
| 31 | Minc3s00265g08951 | 2.55 | Putative GPI-anchor transamidase [C. elegans] |
| 32 | Minc3s02068g28088 | 2.54 | CutA1 divalent ion tolerance protein [B. malayi] |
| 33 | Minc3s11545g44967 | 2.50 | Putative U5 small nuclear ribonucleoprotein 200 kDa helicase [C. elegans] |
| 34 | Minc3s02222g28950 | 2.48 | Gut-specific cysteine proteinase [C. elegans] |
| 35 | Minc3s00216g07759 | 2.42 | Zrt (ZRT), Irt-(IRT-) like Protein Transporter [C. elegans] |
| 36 | Minc3s00167g06602 | 2.39 | Invertebrate Lysozyme [C. elegans] |
| 37 | Minc3s02151g28568 | 2.32 | Calponin [C. elegans] |
| 38 | Minc3s05581g38476 | 2.30 | Heat Shock Protein [C. elegans] |
| 39 | Minc3s02166g28658 | 2.30 | Protein kinase C [C. elegans] |
| 40 | Minc3s02068g28084 | 2.30 | AP complex subunit beta [C. elegans] |
| 41 | Minc3s00836g17922 | 2.25 | Cell Division Cycle related [C. elegans] |
In the Mi-msp-1 silenced J2s, GO:0005737 (cytoplasm) was the most enriched gene ontology (GO) term with four hits, whereas RNA-binding was the second most enriched keyword with two hits. Few notable significantly down-regulated transcripts, as compared to control, were orotidine 5′-phosphate decarboxylase, major sperm protein-2 and a C. elegans RER1 protein homolog. Some of the up-regulated transcripts were carboxypeptidase, soluble guanylate cyclase gcy-36, eukaryotic translation initiation factor 4E-3, nuclear hormone receptor family, integrase core domain-containing protein, glycine cleavage system P protein, and a protein with seven WD repeats AN11 family (Supplementary Table 3).
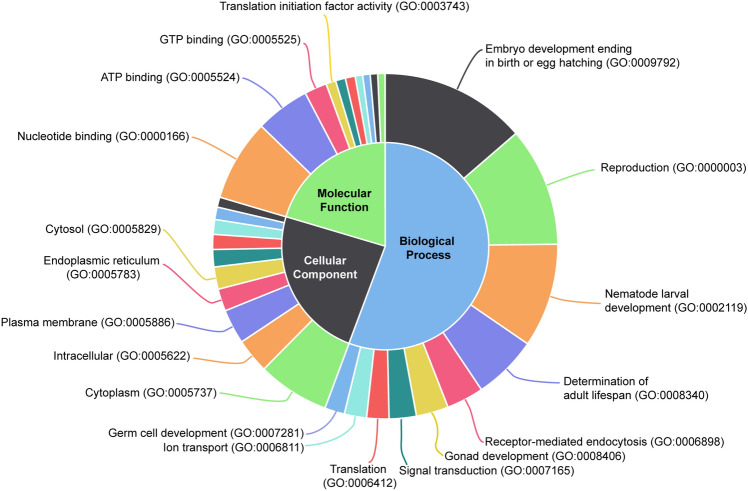
In the Mi-msp-20 silenced J2s, the most enriched KEGG pathways were metabolic pathways (cel01100; 20 transcripts), followed by MAPK signalling pathway (cel04010; 5 transcripts), ErbB signalling pathway (cel04012; 4 transcripts), FoxO signalling pathway (cel04068; 4 transcripts) and arginine and proline metabolism (cel00330; 3 transcripts). The list of enriched ontologies, along with the number of active transcripts and annotation is provided in Supplementary Table 3. The top ten most enriched GO terms were embryo development ending in birth or egg hatching (GO:0009792; 59 transcripts), reproduction (GO:0000003; 48 transcripts), nematode larval development (GO:0002119; 42 transcripts), nucleotide binding (GO:0000166; 33 transcripts) and cytoplasm (GO:0005737; 29 transcripts), determination of adult lifespan (GO:0008340; 26 transcripts), ATP binding (GO:0005524; 22 transcripts), receptor-mediated endocytosis (GO:0006898; 15 transcripts), intracellular (GO:0005622; 14 transcripts), plasma membrane (GO:0005886, 14 transcripts), gonad development (GO:0008406; 13 transcripts), and signal transduction (GO:0007165; 11 transcripts). The top ten enriched ontologies in Mi-msp-20 silenced J2s under each of the three ontology types, i.e., biological processes, cellular component and molecular function are shown in Fig. 3.
Fig. 3.
Donut chart representing the top 10 enriched gene ontologies (GO) in each of the three gene ontology types of biological processes, cellular component and molecular function (the inner circle) in Mi-msp-20 silenced Meloidogyne incognita J2s. The relative area under each GO term/category indicates the number of transcripts mapping to that GO term/category. A complete list can be found in the supplementary Table 3
Discussion
The pre-parasitic second-stage juveniles (J2s) of M. incognita are the host-seeking stage found in soil. Once the J2s locate a suitable host, they enter the roots through the elongation zone. Inside the roots, the juveniles (now early post-parasitic) move intercellularly towards the root tip, take a U-turn and become stationary in the vascular bundle, then initiate the giant cell formation around its head to support feeding. To enter the host roots and move intercellularly, nematode J2s secrete several plant cell-wall-modifying enzymes as well as effector molecules that dissolve the components of root cells. The Meloidogyne sub-ventral gland secretions are necessary for pre-parasitic and early post-parasitic stages, whereas the dorsal gland secretions are vital for post-parasitic stages (Kyndt et al. 2013). In the present investigation, we have selected two genes, Mi-msp-1 and Mi-msp-20, both expressed in sub-ventral glands of the pre-parasitic M. incognita J2s for determining the cascading effects of their RNAi silencing. The M. incognita Mi-msp-1 is a secreted protein of SCP/TAPS protein family (Castillo et al. 2010). It contains a 231 amino acid long open reading frame in which the first 21 amino acids represent a secretion signal (Ding et al. 2000). In contrast, Mi-msp-20 is a pioneer gene with a coiled-coil domain (Huang et al. 2003, 2004). We anticipated that because of the presence of coiled-coil domain, which is a hallmark of regulatory genes, Mi-msp-20 might have a more significant effect on gene expression. Hence in the present study, we undertook an RNA-Seq approach to understand and compare the pleiotropic effects of silencing Mi-msp-1 and Mi-msp-20 effectors and the resultant effector crosstalk at the global level.
Our RNA-Seq experiment showed that silencing of Mi-msp-20 led to differential regulation of 409 transcripts in M. incognita J2s as compared to 29 in Mi-msp-1 silenced J2s. These results demonstrate that the presence of a coiled-coil domain in Mi-msp-20 might be driving the differential expression of a broader set of genes. However, the number of down-regulated transcripts (354) was higher than the number of up-regulated transcripts (55) in Mi-msp-20 silenced J2s. An interesting observation was that 5.9% of the up-regulated transcripts were common between Mi-msp-1 and Mi-msp-20 silenced nematodes suggesting that limited crosstalk exists between the two effectors. However, none of the down-regulated transcripts was common between Mi-msp-1 and Mi-msp-20 silenced worms. It was also shown that both the genes are involved in crosstalk with other cell-wall degrading enzymes (CWDEs) (Shivakumara et al. 2016, 2017; Chaudhary et al. 2019a).
Mi-msp-1 is essential in the initial infection of the host plant (Ding et al. 2000). Host-induced gene silencing of Mi-msp-1 in eggplants decreased the root galling, 36.08–41.20% reduction in the number of eggs per egg mass, and the nematode multiplication factor by 62.37–70.62% as compared to the control plants (Chaudhary et al. 2019b). The reduction in nematode parasitism in Mi-msp-1 silenced transgenic eggplants could be due to the effect of continuous knock-down of Mi-msp-1 in the nematodes parasitising the plants, and also through pleiotropic effects on other genes as shown in the present study important for successful parasitism. Some of these genes could be directly involved in plant parasitism, e.g., C. elegans RER1 protein homolog [involved in protein secretion through secretary pathways (Lee et al. 2004)], carboxypeptidase (implicated in catabolism, protein maturation and regulation), nuclear hormone receptor (gene regulation), a protein with seven WD repeats, AN11 family (multiple functions); while other proteins such as orotidine 5′-phosphate decarboxylase, soluble guanylate cyclase gcy-36 or major sperm protein-2 could have a minor role in plant parasitism.
Similarly, silencing of Mi-msp-20 resulted in a larger perturbance in nematode gene expression and enrichment of diverse pathways and ontologies. It was demonstrated earlier that host-induced gene silencing of Mi-msp-20 in eggplants resulted in 28.22–46.77% reduction in root galling, 22.17–45.86% reduction in the number of egg masses, 25.21–38.48% reduction in the number of eggs per egg mass and a 41.74–66.69% reduction in multiplication as compared to the wild type (Shivakumara et al. 2017). Our results show that genes belonging to the metabolic pathways category were the most affected by silencing Mi-msp-20. Additionally, MAPK, ErbB and FoxO signalling pathways showed enrichment, indicating the involvement of Mi-msp-20 in nematode development, regulation of behavioural quiescence and cellular physiology including cell-cycle control, apoptosis, oxidative stress resistance, glucose metabolism and longevity (Buonanno and Fischbach 2001; Greer et al. 2007; Troemel et al. 2006). The top 10 most enriched GO terms in Mi-msp-20 silenced J2s were embryo development ending in birth or egg hatching, reproduction, nematode larval development, nucleotide binding, determination of adult lifespan, receptor-mediated endocytosis, gonad development and signal transduction. Similar to Mi-msp-1, the reduction in nematode parasitism in Mi-msp-20 silenced eggplants seen earlier (Shivakumara et al. 2017) could be attributed to Mi-msp-20 knock-down as well as its cascading effect on several other genes.
It is to be noted that although our results indicate that as compared to Mi-msp-1, the Mi-msp-20 affects a larger set of genes involved in the broader array of functions; the level of reduction in nematode parasitism through host delivered RNAi was almost equal for both these genes (Chaudhary et al. 2019b; Shivakumara et al. 2017). A model proposing the mode of action of these effector genes is proposed in Fig. 4. However, without further functional evidence, it would be premature to speculate on the details of how both the genes and their cascading effects are involved in reducing nematode parasitism, since the genetics of most of the biological functions in plant–parasitic nematodes is generally poorly understood.
Fig. 4.
Model depicting the probable roles of Meloidogyne incognita effectors Mi-msp-1 and Mi-msp-20 in nematode parasitism on plants. Both these effectors are secreted from subventral oesophageal glands of the nematode M. incognita. Mi-msp-1 codes for an venom-allergen like effector protein and silencing it affects the expression of a fewer number of genes, whereas Mi-msp-20 contain a regulatory coiled-coil domain and silencing it affects expression of a larger number of genes
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file 2 - Supplementary Table 2. Correlation coefficient values of the two biological replicates of Mi-msp-1 and Mi-msp-20 silenced, and control M. incognita J2 samples used for RNA-Seq experiment in this study. (DOCX 14 kb)
Supplementary file 3 - Supplementary Table 3. A list of the up-regulated and down-regulated transcripts in both the Mi-msp-1 and Mi-msp-20 silenced M. incognita J2s as compared to controls along with significant identified pathways. (XLSX 208 kb)
Acknowledgements
Funding from the Department of Biotechnology, Government of India to UR through Grant no. BT/PR5908/AGR/36/727/2012 is acknowledged. The authors thank the Director and the Joint Director (Research), ICAR- Indian Agricultural Research Institute, New Delhi, for extending all the support and facilities to complete the study.
Author contributions
UR conceptualized the study and received the funding for the study; VSS, UR, VP wrote the manuscript; PB collected the biological material for the study; MC performed the validation experiments; VSS, RB, and RNS carried out the bioinformatic analysis of the data and created representations in consultation with UR.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
This article does not contain any studies with human participants, and no animals were harmed for this study by any of the authors.
References
- Bakker J, Gommers F, Smant G, Abad P, Rosso M-N, Dautova M. Single pass cDNA sequencing-a powerful tool to analyse gene expression in preparasitic juveniles of the southern root-knot nematode Meloidogyne incognita. Nematology. 2001;3:129–139. [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotech. 2016;34:525. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Castillo JD, Lawrence KS, Morgan-Jones G, Ramirez CA. Identification of fungi associated with Rotylenchulus reniformis. J Nematol. 2010;42:313. [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S, Dutta TK, Shivakumara TN, Rao U. RNAi of esophageal gland-specific gene Mi-msp-1 alters early stage infection behaviour of root-knot nematode, Meloidogyne incognita. J Gen Plant Pathol. 2019;85:232–242. [Google Scholar]
- Chaudhary S, Dutta TK, Tyagi N, Shivakumara TN, Papolu PK, Chobhe KA, Rao U. Host-induced silencing of Mi-msp-1 confers resistanceto root-knot nematode Meloidogyne incognita in eggplant. Transgenic Res. 2019;28:327–340. doi: 10.1007/s11248-019-00126-5. [DOI] [PubMed] [Google Scholar]
- Danchin EGJ, Rosso M-N, Vieira P, de Almeida-Engler J, Coutinho PM, Henrissat B, Abad P. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc Natl Acad Sci USA. 2010;107:17651–17656. doi: 10.1073/pnas.1008486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EL, Hussey RS, Mitchum MG, Baum TJ. Parasitism proteins in nematode–plant interactions. Curr Opin Plant Biol. 2008;11:360–366. doi: 10.1016/j.pbi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Ding X, Shields J, Allen R, Hussey R. A secretory cellulose-binding protein cDNA cloned from the root-knot nematode (Meloidogyne incognita) Mol Plant Microbe Interact. 1998;11:952–959. doi: 10.1094/MPMI.1998.11.10.952. [DOI] [PubMed] [Google Scholar]
- Ding X, Shields J, Allen R, Hussey RS. Molecular cloning and characterisation of a venom allergen AG5-like cDNA from Meloidogyne incognita. Int J Parasitol. 2000;30:77–81. doi: 10.1016/s0020-7519(99)00165-4. [DOI] [PubMed] [Google Scholar]
- Dinh PT, Brown CR, Elling AA. RNA interference of effector gene Mc16D10L confers resistance against Meloidogyne chitwoodi in Arabidopsis and potato. Phytopathology. 2014;104:1098–1106. doi: 10.1094/PHYTO-03-14-0063-R. [DOI] [PubMed] [Google Scholar]
- Doyle EA, Lambert KN. Cloning and characterisation of an esophageal-gland-specific pectate lyase from the root-knot nematode Meloidogyne javanica. Mol Plant Microbe Interact. 2002;15:549–556. doi: 10.1094/MPMI.2002.15.6.549. [DOI] [PubMed] [Google Scholar]
- Dubreuil G, Magliano M, Deleury E, Abad P, Rosso M. Transcriptome analysis of root-knot nematode functions induced in the early stages of parasitism. New Phytol. 2007;176:426–436. doi: 10.1111/j.1469-8137.2007.02181.x. [DOI] [PubMed] [Google Scholar]
- Gheysen G, Mitchum MG. How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol. 2011;14:415–421. doi: 10.1016/j.pbi.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK–FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, Bauters L, Kyndt T, Rahman MM, Gheysen G. Identification of candidate effector genes in the transcriptome of the rice root knot nematode Meloidogyne graminicola. Mol Plant Pathol. 2013;14:379–390. doi: 10.1111/mpp.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Gao B, Maier T, Allen R, Davis EL, Baum TJ, Hussey RS. A profile of putative parasitism genes expressed in the esophageal gland cells of the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact. 2003;16:376–381. doi: 10.1094/MPMI.2003.16.5.376. [DOI] [PubMed] [Google Scholar]
- Huang G, Dong R, Maier T, Allen R, Davis EL, Baum TJ, Hussey RS. Use of solid-phase subtractive hybridisation for the identification of parasitism gene candidates from the root-knot nematode Meloidogyne incognita. Mol Plant Pathol. 2004;5:217–222. doi: 10.1111/j.1364-3703.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- Huang G, Allen R, Davis EL, Baum TJ, Hussey RS. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci USA. 2006;103:14302–14306. doi: 10.1073/pnas.0604698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey RS. Host–parasite relationships and associated physiological changes. In: Sasser JN, Carter CC, editors. An advanced treatise on Meloidogyne. Vol. I biology and control. Raleigh: NC State University; 1985. pp. 143–153. [Google Scholar]
- Hussey RS, Davis EL, Baum TJ. Secrets in secretions: genes that control nematode parasitism of plants. Braz J Plant Physiol. 2002;14:183–194. [Google Scholar]
- Jaouannet M, Magliano M, Arguel MJ, Gourgues M, Evangelisti E, Abad P, Rosso M-N. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant Microbe Interact. 2013;26:97–105. doi: 10.1094/MPMI-05-12-0130-R. [DOI] [PubMed] [Google Scholar]
- Jaubert S, Laffaire JB, Piotte C, Abad P, Rosso M-N, Ledger TN. Direct identification of stylet secreted proteins from root-knot nematodes by a proteomic approach. Mol Biochem Parasitol. 2002;121:205–211. doi: 10.1016/s0166-6851(02)00034-8. [DOI] [PubMed] [Google Scholar]
- Jones JT, et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 2013;14:946–961. doi: 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, et al. De novo transcriptome sequencing and analysis of the cereal cyst nematode, Heterodera avenae. PLoS ONE. 2014;9:e96311. doi: 10.1371/journal.pone.0096311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J. Nematode feeding sites: unique organs in plant roots. Planta. 2013;238:807–818. doi: 10.1007/s00425-013-1923-z. [DOI] [PubMed] [Google Scholar]
- Lambert KN, Allen KD, Sussex IM. Cloning and characterisation of an esophageal-gland-specific chorismate mutase from the phytoparasitic nematode Meloidogyne javanica. Mol Plant Microbe Interact. 1999;12:328–336. doi: 10.1094/MPMI.1999.12.4.328. [DOI] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Li X, Yang D, Niu J, Zhao J, Jian H. De novo analysis of the transcriptome of Meloidogyne enterolobii to uncover potential target genes for biological control. Int J Mol Sci. 2016;17:1442. doi: 10.3390/ijms17091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Zhuo K, Wu P, Cui R, Zhang L-H, Liao J. A novel effector protein, MJ-NULG1a, targeted to giant cell nuclei plays a role in Meloidogyne javanica parasitism. Mol Plant Microbe Interact. 2013;26:55–66. doi: 10.1094/MPMI-05-12-0114-FI. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long H, Wang X, Xu J. Molecular cloning and life-stage expression pattern of a new chorismate mutase gene from the root-knot nematode Meloidogyne arenaria. Plant Pathol. 2006;55:559–563. [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelin S, Bellafiore S, Kyndt T. Meloidogyne graminicola: a major threat to rice agriculture. Mol Plant Pathol. 2017;18:3–15. doi: 10.1111/mpp.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL. Nematode effector proteins: an emerging paradigm of parasitism. New Phytol. 2013;199:879–894. doi: 10.1111/nph.12323. [DOI] [PubMed] [Google Scholar]
- Nguyễn PV, Bellafiore S, Petitot AS, Haidar R, Bak A, Abed A, Gantet P, Mezzalira I, de Almeida EJ, Fernandez D. Meloidogyne incognita - rice (Oryza sativa) interaction: a new model system to study plant-root-knot nematode interactions in monocotyledons. Rice. 2014;7:23. doi: 10.1186/s12284-014-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Liu P, Liu Q, Chen C, Guo Q, Yin J, Yang G, Jian H. Msp40 effector of root-knot nematode manipulates plant immunity to facilitate parasitism. Sci Rep. 2016;6:19443. doi: 10.1038/srep19443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RK, Jain M. NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitot AS, Dereeper A, Agbessi M, Da Silva C, Guy J, Ardisson M, Fernandez D. Dual RNA-seq reveals Meloidogyne graminicola transcriptome and candidate effectors during the interaction with rice plants. Mol Plant Pathol. 2016;17:860–874. doi: 10.1111/mpp.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso MN, Favery B, Piotte C, Arthaud L, De Boer JM, Hussey RS, Bakker J, Baum TJ, Abad P. Isolation of a cDNA encoding a β-1, 4-endoglucanase in the root-knot nematode Meloidogyne incognita and expression analysis during plant parasitism. Mol Plant Microbe Interact. 1999;12:585–591. doi: 10.1094/MPMI.1999.12.7.585. [DOI] [PubMed] [Google Scholar]
- Rosso MN, Hussey RS, Davis EL, Smant G, Baum TJ, Abad P, Mitchum MG. Nematode effector proteins: targets and functions in plant parasitism. In: Martin F, Kamoun S, editors. Effectors in plant–microbe interactions. New York: Wiley Blackwell Publishing; 2012. pp. 329–356. [Google Scholar]
- Shi Q, Mao Z, Zhang X, Zhang X, Wang Y, Ling J, Lin R, Li D, Kang X, Sun W, Xie B. A Meloidogyne incognita effector MiISE5 suppresses programmed cell death to promote parasitism in host plant. Sci Rep. 2018;8:7256. doi: 10.1038/s41598-018-24999-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumara TN, Papolu PK, Dutta TK, Kamaraju D, Chaudhary S, Rao U. RNAi-induced silencing of an effector confers transcriptional oscillation in another group of effectors in the root-knot nematode, Meloidogyne incognita. Nematology. 2016;18:857. [Google Scholar]
- Shivakumara TN, Chaudhary S, Kamaraju D, Dutta TK, Papolu PK, Banakar P, Sreevathsa R, Singh B, Manjaiah KM, Rao U. Host-induced silencing of two pharyngeal gland genes conferred transcriptional alteration of cell wall-modifying enzymes of Meloidogyne incognita vis-a-vis perturbed nematode infectivity in eggplant. Front Plant Sci. 2017;8:473. doi: 10.3389/fpls.2017.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smant G, Stokkermans JP, Yan Y, de Boer JM, Baum TJ, Wang X, Hussey RS, Gommers FJ, Henrissat B, Davis EL, Helder J, Schots A, Bakker J. Endogenous cellulases in animals: isolation of beta-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc Natl Acad Sci USA. 1998;95:4906–4911. doi: 10.1073/pnas.95.9.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somvanshi VS, Ghosh O, Budhwar R, Dubay B, Shukla RN, Rao U. A comprehensive annotation for the root-knot nematode Meloidogyne incognita proteome data. Data Brief. 2018;19:1073–1079. doi: 10.1016/j.dib.2018.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwin PE, Lilley CJ, Atkinson HJ. Ingestion of double-stranded RNA by preparasitic juvenile cyst nematodes leads to RNA interference. Mol Plant Microbe Interact. 2002;15:747–752. doi: 10.1094/MPMI.2002.15.8.747. [DOI] [PubMed] [Google Scholar]
- Wang X, Li H, Hu Y, Fu P, Xu J. Molecular cloning and analysis of a new venom allergen-like protein gene from the root-knot nematode Meloidogyne incognita. Exp Parasitol. 2007;117:133–140. doi: 10.1016/j.exppara.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Whitehead A, Hemming J. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol. 1965;55:25–38. [Google Scholar]
- Xie J, Li S, Mo C, Wang G, Xiao X, Xiao Y. A novel Meloidogyne incognita effector Misp12 suppresses plant defense response at latter stages of nematode parasitism. Front Plant Sci. 2016;7:964. doi: 10.3389/fpls.2016.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Hamamouch N, Li C, Huang G, Hussey RS, Baum TJ, Davis EL. The 8D05 parasitism gene of Meloidogyne incognita is required for successful infection of host roots. Phytopathology. 2013;103:175–181. doi: 10.1094/PHYTO-07-12-0173-R. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Gaj S, Ho I, Hanspers K, Vranizan K, Evelo CT, Conklin BR, Pico AR, Salomonis N. GO-Elite: a flexible solution for pathway and ontology over-representation. Bioinformatics. 2012;28:2209–2210. doi: 10.1093/bioinformatics/bts366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Davies LJ, Elling AA. A Meloidogyne incognita effector is imported into the nucleus and exhibits transcriptional activation activity in planta. Mol Plant Pathol. 2015;16:48–60. doi: 10.1111/mpp.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo K, Chen J, Lin B, Wang J, Sun F, Hu L, Liao J. A novel Meloidogyne enterolobii effector MeTCTP promotes parasitism by suppressing programmed cell death in host plants. Mol Plant Pathol. 2017;18:45–54. doi: 10.1111/mpp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 2 - Supplementary Table 2. Correlation coefficient values of the two biological replicates of Mi-msp-1 and Mi-msp-20 silenced, and control M. incognita J2 samples used for RNA-Seq experiment in this study. (DOCX 14 kb)
Supplementary file 3 - Supplementary Table 3. A list of the up-regulated and down-regulated transcripts in both the Mi-msp-1 and Mi-msp-20 silenced M. incognita J2s as compared to controls along with significant identified pathways. (XLSX 208 kb)