Abstract
Objective
Calcification serves as a surrogate for atherosclerosis-associated vascular diseases, and coronary artery calcification is mediated by multiple pathogenic factors. Estrogen is a known factor that protects the arterial wall against atherosclerosis, but its role in the coronary artery calcification development remains largely unclear. This study tested the hypothesis that estrogen inhibits coronary artery calcification via the hypoxia-induced factor-1α pathway.
Methods
Eight-week-old healthy female Sprague–Dawley rats were castrated, and vitamin D3 was administered orally to establish. Hypoxia-induced factor-1 inhibitor was administered to test its effect on vascular calcification and expression of bone morphogenetic protein 2 and runt-related transcription factor-2. Vascular smooth muscle cell calcification was induced with CaCl2 in rat aortic smooth muscle cells in the presence or absence of E2(17β-estradiol) and bone morphogenetic protein 2 siRNA intervention.
Results
The estrogen levels in ovariectomized rats were significantly decreased, as determined by ELISA. Expression of hypoxia-induced factor-1α mRNA and protein was significantly increased in vascular cells with calcification as compared to those without calcification (p < 0.01). E2 treatment decreased the calcium concentration in vascular cell calcification and cell calcium nodules in vitro (p < 0.05). E2 also lowered the levels of hypoxia-induced factor-1α mRNA and protein (p < 0.01). Oral administration of the hypoxia-induced factor-1α inhibitor dimethyloxetane in castrated rats alleviated vascular calcification and expression of osteogenesis-related transcription factors, bone morphogenetic protein 2 and RUNX2 (p < 0.01). Finally, bone morphogenetic protein 2 siRNA treatment decreased the levels of p-Smad1/5/8 in A7r5 calcification cells (p < 0.01).
Conclusion
Estrogen deficiency enhances vascular calcification. Treatment with estrogen reduces the expression of hypoxia-induced factor-1α as well as vascular calcification in rats. The estrogen effects occur in a fashion dependent on hypoxia-induced factor-1α regulation of bone morphogenetic protein-2 and downstream Smad1/5/8.
Keywords: Coronary heart disease, estrogen, hypoxia-inducible factor-1α, vascular calcification, bone morphogenetic protein-2
Introduction
Vascular calcification refers to the pathologic process of minerals, such as calcium phosphate (mainly calcium hydroxyphosphate) deposited in the vasculature1 and is mainly characterized by intimal, medial, and valvular calcification.2 In the past 30 years, vascular calcification has been identified as a process of aging and degenerative change, which involves the passive deposition of calcium and phosphorus on the vessel wall.3 Recent studies have shown that vascular calcification is a regulated and active process.4 Coronary artery calcification (CAC) is the most important type of vascular calcification and is closely related to the clinical diagnosis and treatment of coronary heart disease. A new concept has proposed that CAC is not a marker of plaque vulnerability, but severe calcification of the coronary artery remains a big challenge for coronary arterial intervention therapy.5 Thus, there is an urgent need to understand the natural history of vascular calcification.
CAC scores are associated with the atherosclerotic plaque formation, as reported by several large observational studies that have confirmed that coronary calcification assessment predicts future cardiovascular events.6–8 An adverse CAC score change often develops with osteoporosis, particularly in postmenopausal women.9 Researchers have focused on estrogen in a calcification model; however, the findings are controversial. Data have shown that estrogen may retard vascular smooth muscle cell (VSMC) osteoblast-like transformation and downregulate the related osteogenic transcription factors.10 Osako et al.11 and Choi et al.12 found in in vivo and in vitro experiments that E2(17β-estradiol) inhibits RANKL signaling by ERα and reduces calcification. Peng et al.13 reported that estrogen inhibits arterial calcification by promoting autophagy via the ERα signaling pathway. More recent data have shown that in advanced atherosclerotic lesions, E2 can drive, but not inhibit calcification by promoting the differentiation of VSMCs to osteoblast-like cells.14
Hypoxia-inducible factor (HIF)-1α was first discovered in hypoxic hepatoma cells. It is well-established that HIF-1α plays an important role in the progression of atherosclerosis by activating and promoting foam cell formation, inducing endothelial cell dysfunction, apoptosis, and increasing inflammation and angiogenesis.15,16 In contrast, HIF-1α is a marker of the atherosclerotic lesions17 and is considered to be involved in vascular calcification. Osteocalcin, an osteogenic factor, has been shown to induce cartilage ossification of VSMCs and promote vessel calcification via the HIF-1α signaling pathway.18,19 Oral HIF-1α inhibitors inhibit osteoclast activity in ovariectomized mice and prevent bone loss caused by estrogen deficiency.20 It has also been shown that the serum HIF-1α level may be an independent risk factor for the presence of CAC. Nevertheless, the mechanisms underlying CAC have not been established.
Because estrogen is closely related to vascular calcification and osteoporosis, and the HIF-1α signaling pathway plays an important role in vascular calcification. In the present study, we hypothesized that HIF-1α is involved in vascular calcification regulation by estrogen.
Materials and methods
Materials: Eight-week-old SD female rats were purchased from Hunan Jingda Experimental Animal Company (Certificate No. 43004700040271). Calcium chloride, 2-methoxyestradiol (2ME2), E2(17β-estradiol), ICI182780, vitamin D3, and calcium content detection kit were purchased from Sigma (Milwaukee, WI, USA). HIF-1α and bone morphogenetic protein (BMP)-2 antibodies were purchased from Abcam (Cambridge, UK). Rat aortic smooth muscle cell line A7r5 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Wuhan, China). Fetal bovine serum and DMEM/high glucose medium were purchased from Israel Biological (Tel Aviv, Israel). BMP-2 siRNA, siRNA Transfection Medium, and siRNA Transfection Reagent were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). A real-time fluorescence quantitative polymerase chain reaction (RT-PCR) kit and reverse transcription kit were purchased from Promega (Madison, WI, USA). Primers were synthesized by Nanjing Jinsrui Biotechnology (Nanjing, China).
In vivo procedure: The animals used in the study received humane care in compliance with the Guiding Principles for the Care and Use of Animals of Dali University. Forty female Sprague–Dawley rats, eight-weeks old, with one week of adaptive feeding were used. Ovariectomized rats underwent oophorectomies, and sham operation refers to the removal the ovarian peripheral adipose tissue. After three weeks of adaptive feeding, the vascular calcification groups were given vitamin D3 (300,000 U/kg every day at 9 am) by intraperitoneal injection for three consecutive days. The HIF-1α inhibitor, 2ME2, was injected intraperitoneally with 10 mg/kg for another 28 days. Dimethylsulfoxide (DMSO) (2%) with dimethoxy estradiol solution served as the negative control. The doses of 2ME2 and DMSO were adjusted weekly based on the body weight, and the rats were sacrificed by intravenous infusion of air on the 29th day, after which the aorta was harvested.
In vitro experiments: The rat A7r5 VSMC line was cultured in DMEM/high glucose medium containing 10% fetal bovine serum. The fifth to eighth generation cells were used. When the cells were fused at 70–80% confluence, we applied 5 mmol/L CaCl2 treated for nine days to establish the calcification model. The cells were collected for downstream experiments.
Determination of calcification: For VSMC calcification measurement, alizarin red staining was carried out as previously described.21 The calcium concentration, as well as the estrogen concentration, was measured according to the ELISA kit instructions.
RT-PCR was used to detect the expression of HIF-1α and BMP-2 mRNA in the aorta and A7r5 cells. Briefly, total RNA was extracted from rat aortas and A7r5 cells using Trizol. Total RNA concentration and purity were determined by UV spectrophotometry. According to the reverse transcription kit instructions, 2 μg of total RNA was added to a 20-μl system to synthesize cDNA. cDNA (2 μl) was added to a 20-μl reaction system containing 0.2 μl of each of the up- and down-stream primers of the target gene and 2 × GoTaq qPCR Master Mix (10 μl) and amplified on a real-time PCR system. The experiment was repeated three times, and the results were analyzed using the following equation: F = 2 – ΔΔ ct. The primer sequences are shown in Table 1.
Western blotting was used to detect HIF-1α, BMP-2, and p-Smad1/5/8 protein levels in aortas and A7R5 cells. Briefly, total protein in rat aortas and cells was extracted with RIPA lysate supplemented with protease inhibitor. The BCA micro-tube assay was used to determine the protein concentration. An equal amount of protein sample was transferred to a PVDF membrane for 10% SDS–PAGE electrophoresis at 200 mA constant flow for 90 min. The PVDF membrane was placed in a 5% skim milk blocking solution at 4 °C overnight, and the primary antibody diluted with TBST solution was added (HIF-1α, 1:1 000; BMP-2, 1: 1 000, and p-Smad1/5/8, 1:1000) and then incubated at 4°C for 8 h. The next day, the membrane was thrice-washed with TBST (10 min for each wash), and then the secondary antibody was added (1:5000) for 4 h at 4 °C and thrice-washed in TBST again (10 min) before HRP chemiluminescence. The membrane was placed on the imaging panel of the instrument for film acquisition. The data were analyzed by using Image J software. The experiment was repeated three times, and the results were analyzed.
Histology: Paraffin specimens were prepared from rat aortic arch segments; 2–3 μm sections were stained with hematoxylin and eosin and von Kossa.22
Statistical analysis: All values are expressed as the mean ± SD. Raw data were analyzed with SPSS software (version 22.0; Chicago, IL, USA). Statistical analysis for multiple group comparisons was performed using one-way ANOVA, followed by Tukey’s multiple comparison procedure. p values <0.05 were considered to indicate the statistical significance.
Table 1.
Primers sequence.
| Primer sequence | |
|---|---|
| HIF-1α | F: 5′-GCTACAAGAAACCGCCTA-3′ |
| R: 5′-GTTCTTCTGGCTCATAACCC-3′ | |
| BMP-12 | F: 5′-CCATCACGAAGAAGCCATCGAG-3′ |
| R: 5′-CTTCCTGCATTTGTTCCCGAA-3 | |
| β-Actin | F: 5′-TGTGCTGGACTCTGGAGATG-3′ |
| R: 5′-GAAGGAATAGCCACGCTCAG-3 |
Results
During vascular calcification in ovariectomized rats, the serum levels of estrogen decreased, and HIF-1α expression in the aortas increased.
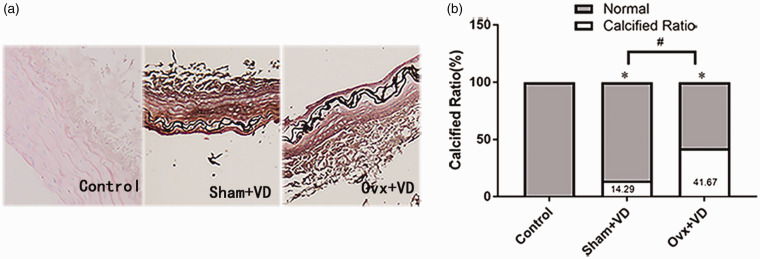
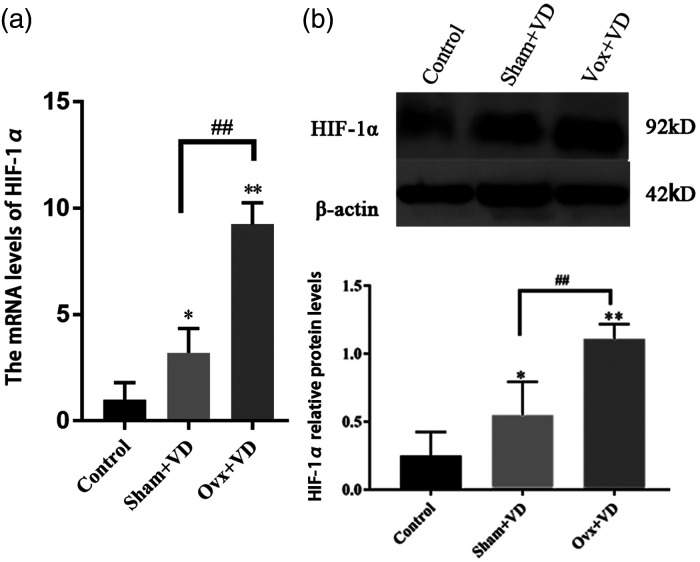
To detect the changes in estrogen and HIF-1α during vascular calcification, rats in sham group (sham operation) and Ovx group (ovariectomy operation) were then treated with 300,000 U/kg of vitamin D3 (VD). The calcification of arteries was stained with Von Kossa, which showed black aggregates deposited at the elastic layers. The calcification area measurement showed that the calcification area of the calcification group increased significantly compared with the normal group (p < 0.05; Figure 1). ELISA results showed a decreased estrogen expression after castration in rats with vascular calcification (Figure S1). The expression of HIF-1α mRNA and protein in calcified aortas was significantly higher than the normal control group (p < 0.01; Figure 2).
Figure 1.
Histopathological examination of thoracic aorta on Vit D3 fed rats treated with or without ovariectomy: (a) histological analyses of thoracic aorta were applied Von Kossa stain (×100); (b) the proportion of calcification area measurement results showed the remarkable increase of calcification in aorta of D3 fed ovariectomized rats (n = 5). #p < 0.05. Compared with the control group, *p < 0.05.
Figure 2.
Effect of ovarian resection on the expression of HIF-1α mRNA (a) and protein (b) in aorta of vitamin D3-treated rats (n = 3). ##p < 0.01. Compared with the control group, *p < 0.05, **p < 0.01.
Abbreviations: sham: sham operation; Ovx: ovariectomized; VD: vit D3 fed.
2. E2 inhibits the protein level of HIF-1α in rat VSMCs and reduces cell calcification.
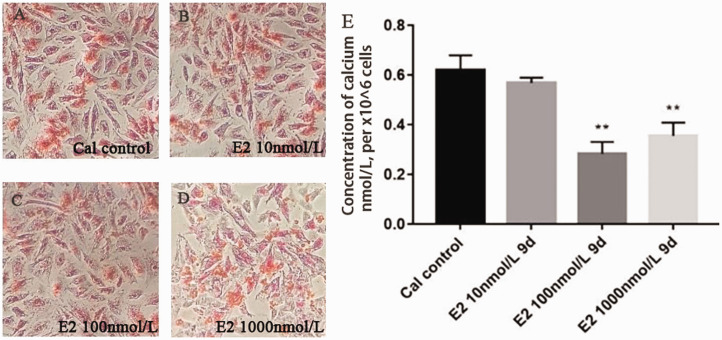
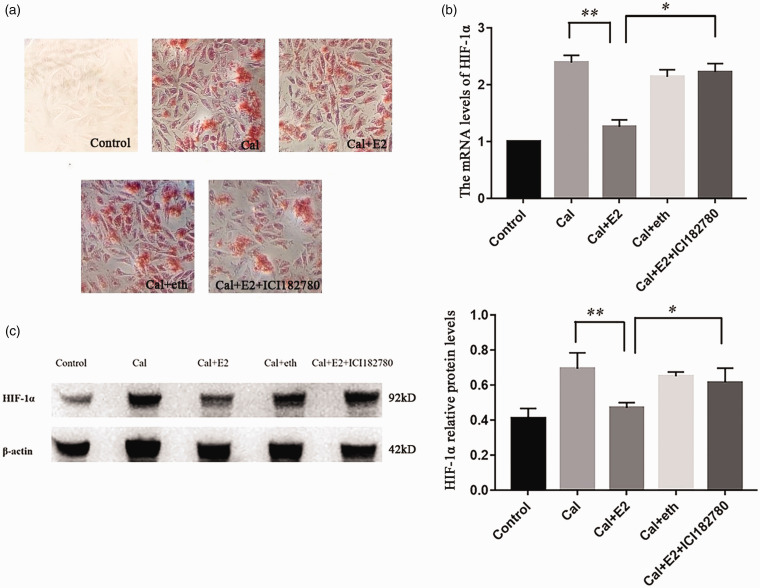
To clarify the relationship between E2 and HIF-1α, the A7r5 cell calcification model was successfully established by treatment with 5 mmol/L CaCl2 for nine days. Different concentrations of E2 (10, 100, and 1000 nmol/L) were used to demonstrate the effect on VSMCs calcium. Compared with the calcified control group (Cal control), the orange–red-calcified nodules under alizarin red staining were remarkably reduced in the E2 intervention group, and we found that E2 reduced the concentration of calcium at the same time (Figure 3). Then, E2 receptor antagonist intervention was applied to verify the effect of E2 on calcification, and on HIF-1α, with ethanol as a vehicle control (eth group), we found that E2 could reduce the mRNA and protein level of HIF-1α, estrogen receptor antagonist ICI182780 abolished the effect of HIF-1α and HIF-1α induced by E2 (Figure 4).
Figure 3.
Calcification of A7r5 cells treated with E2: (a–d) Alizarin Red staining in A7r5 calcification cells showed that E2 could statistically significant decrease in cellular calcium in dose-dependent manner (×100); (e) calcium contents determined by ELISA. Compared with the control group (n = 3). **p < 0.01.
Figure 4.
Calcified A7r5 cells treated with E2 and its antagonist: (a) calcified A7r5 cells treated with E2 and its antagonist, with ethanol as a vehicle control (eth group), alizarin red staining showed that calcification reduction induced by E2 were alleviated by ICI182780 treatment (×100); (b and c) real-time PCR and western blotting demonstrated that HIF-1α were upregulated in the A7r5 calcification treatment, and this augment of HIF-1α in calcification was inhibited by E2. (n = 3) *p < 0.05, **p < 0.01.
Abbreviations: cal: calcification; eth: ethanol vehicle.
3. HIF-1α inhibitor intervention reduces vascular calcification as well as expression of BMP-2 and Runx2 protein levels in ovariectomized rats.
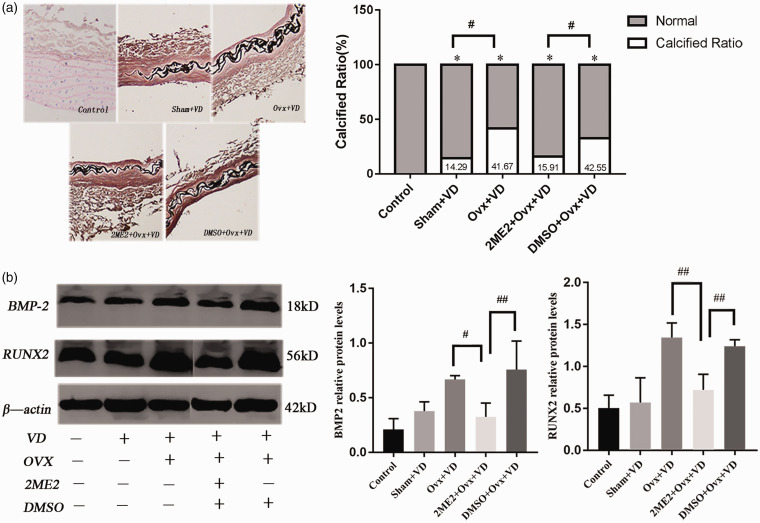
To explore the possible role of HIF-1α regulation by E2, the effect of HIF-1α inhibitor intervention in vivo was evaluated. With 2ME2 treatment of ovariectomized calcification rats for 28 days, von Kossa staining showed that the area of calcification in 2ME2 + Ovx + VD group was significantly smaller than that in the Ovx + VD group (p < 0.05 Figure 5(a)). At the same time, the protein levels of BMP-2 and Runx2 in 2ME2 + Ovx + VD group on aortas were significantly lower than the Ovx + VD group (p < 0.01; Figure 5(b)).
Figure 5.
Calcification and expression of osteogenic factors after 2ME2 intervention in rats with vascular calcification: (a) histological analyses of thoracic aorta stained by von Kossa (×100) and the proportion of stained calcification area were showed that 2ME2 could reduce vascular calcification in vivo (n = 5); (b) the expression of BMP-2 and Runx2 protein level in aorta of ovariectomized vascular calcification rats after 2ME2 intervention (n = 3). #p < 0.05, ##p < 0.01. Compared with the control group, *p < 0.05.
Abbreviations: VD: vit D3 fed; 2ME2: 2-methoxyestradiol; Ovx: ovariectomized; DMSO: DMSO vehicle.
4. BMP-2-p-Smad1/5/8 pathway contributes to the HIF-1α regulation of vascular calcification.
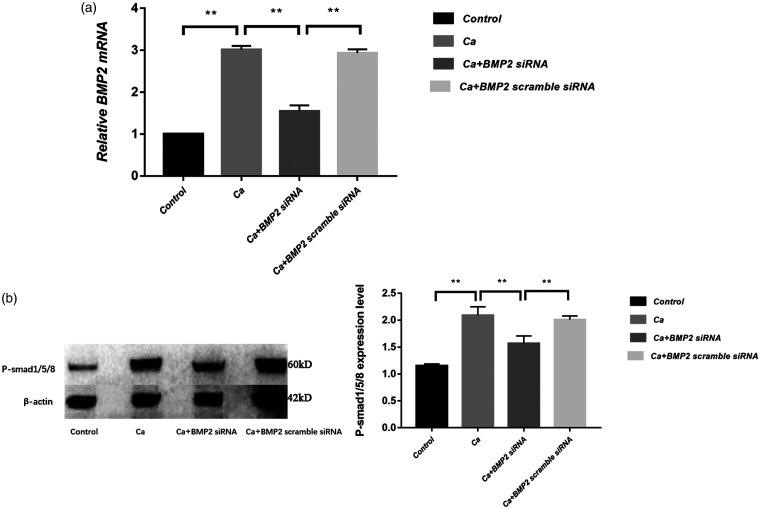
We further used the siRNA interference strategy to silence BMP-2 gene in A7r5 cells and detect the downstream factors. The real-time PCR results confirmed the efficacy of siRNA (Figure 6(a)). Western blotting results showed that the level of p-Smad1/5/8 protein in the calcified group was significantly higher than the control group (p < 0.01). The level of p-Smad1/5/8 protein in the BMP-2 siRNA group was significantly lower than in the calcification group, suggesting that BMP-2 may affect vascular calcification by p-Smad1/5/8 (Figure 6(b)).
Figure 6.
Impact of BMP-2 siRNA interference on expression of p-Smad1/5/8: (a) BMP-2 mRNA measured by real-time PCR showed a successful influence of siRNA on the BMP-2 (n = 3); (b) Western blot showed that BMP-2 siRNA could reduce the protein level of p-Smad1/5/8 (n = 3). **p < 0.01.
Discussion
Using a model of arterial calcification in ovariectomized rats, we found that E2 and HIF-1α changed during vascular calcification. After it was confirmed that E2 regulates HIF-1α in vitro, we showed that a HIF-1α inhibitor effectively alleviated vascular calcification in the rat model and further confirmed that this process was achieved by HIF-1α regulation of BMP-2 and downstream Smad1/5/8.
The prevalence of cardiovascular disease in premenopausal women is only 10–30% of the prevalence in men of the same age. With menopause, the cardiovascular benefits of this gender difference gradually diminish, and cardiovascular disease frequently occurs in older women. The risk is significantly higher, which is approximately fourfold that before menopause.23 Coronary calcification is part of vascular calcification, and the CAC score is an independent predictor of cardiovascular adverse events in postmenopausal women. The results of the Framingham study suggest that the incidence of coronary calcification in men is significantly higher than women, especially for men <60 years of age; the incidence of coronary calcification in women is only 50% that of men.24,25 The lower incidence of coronary calcification in women than men may be related to the protective effect of E2; however, after extensive animal studies and some attempts in treating CAC with estrogen,26,27 the recommendation for hormone therapy is not universal. The reason for this uncertainly lies in the complex biologic effects and multiple pathways of estrogen. Specifically, activation of the RANKL-osteoprotegerin (OPG) axis inhibits calcification, but promotes long-term mortality and HF development in patients with ACS.28 Clearly, we need to identify more direct targets.
HIF-1α is regulated by the hypoxia signaling pathway, and the expression of HIF-1α is significantly increased in hypoxic states. Similar to our animal experiment results, Li et al.29 reported that the serum HIF-1α concentration was also significantly increased with the progression of coronary calcification and positively correlated with the CAC score. Mokas et al.30 found that HIF-1α accelerates osteogenic differentiation and calcification of VSMCs in an inorganic phosphorus-induced cell calcification model. With the exception of the discovery of HIF-1α downregulation by E2, Monsonego-Ornan et al.18 also found that osteocalcin can increase the level of HIF-1α protein in VSMCs, which may involve the glucose metabolic pathway. In our study, we investigated the relationship between HIF-1α and some osteogenic inducers. The similarity between vascular calcification and the osteogenesis process lies in the transformation of VSMCs into osteoblast-like cells. In the process of osteogenic differentiation of VSMCs, BMP-2 and Runx2 can participate in the process of vascular calcification, which are considered to be osteogenic differentiation-inducing factors.31,32 In agreement with our findings, it has been shown that HIF-1α can enhance BMP-2-induced stem-cell osteogenic differentiation,33 mainly via the classical Smads and non-canonical MAPK signaling pathways to regulate the expression of key transcriptional regulators (Sox9 and Runx2). Of note, with the exception of HIF-1α, many estrogen targets involved in bone metabolism and/or postmenopausal osteoporosis may also be involved in the development of arterial calcification. Monocyte chemoattractant protein (MCP)-1, transforming growth factor (TGF-β), M-CSF, matrix metalloproteinases (MMPs), and MMPs tissue inhibitory factor (TIMP) may be the estrogen targets involved in arterial calcification.34
In the current study, after intervention with HIF-1α inhibitor (2ME2), in the ovariectomized calcification group, vascular calcification was alleviated, and the expression of BMP-2 gene and protein was significantly lower than the vehicle group. We silenced the BMP-2 gene in vitro and found that the high calcium-induced VSMC calcification could be effectively alleviated. To further validate the role of BMP-2 in the pathway, Western blotting results showed that p-Smad1/5/8 protein level was downregulated by BMP-2 siRNA. A previous report11 suggested that E2 inhibits the OPG/RANKL signaling pathway by ERa, reduces phosphorylation of Smad-1/5/8, increases the expression of bone matrix Gla protein, and reduces the expression of BMP-2 and the bone transcription factor, Runx2. With the exception of estrogen, gallic acid, a natural compound found in gallnut and green tea, inhibits vascular calcification via the BMP-2-Smad-1/5/8 pathway.35 Together with our results, the data indicated that HIF-1α is closely related to the bone-associated protein, BMP-2, and the p-Smad1/5/8 signaling pathway during vascular calcification. Nevertheless, BMP-2 induced vascular calcification in multiple ways. Hyzy et al.36 reported that BMP-2 induces osteoblast apoptosis. Shu et al.37 showed that BMP-2 induces calcification of VSMCs and upregulates the expression of β-catenin and Runx2, when β-catenin is silenced, the effect of BMP-2 on apoptosis and calcification of VSMCs disappears. There is also a report that BMP-2 downregulates the expression of mir-30b and mir-30c, then increases the expression of Runx2 in cells, and promotes vascular calcification.38
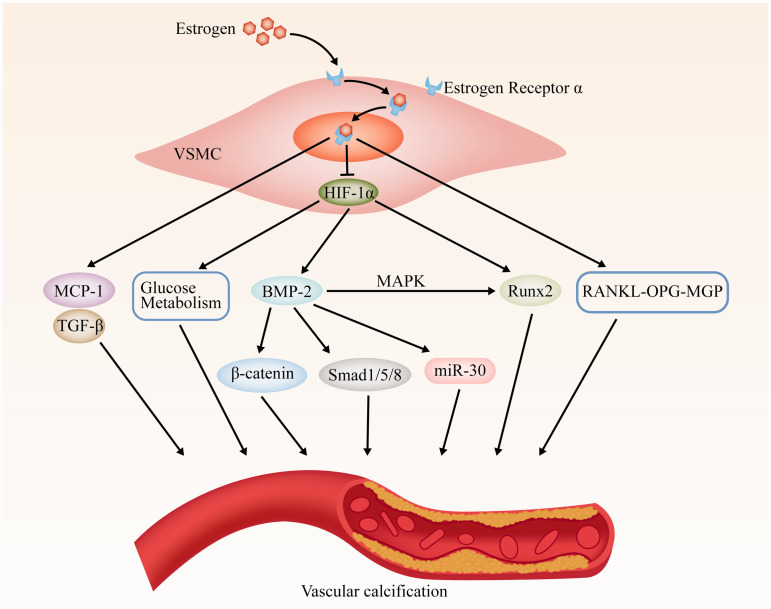
The possible roles of estrogen and HIF-1α in VSMCs osteogenic differentiation and vascular calcification are summarized in Figure 7. During the process of VSMC calcification, E2 may affect the protein levels of BMP-2-p-Smad1/5/8 via HIF-1α and affect VSMC calcification, which may play an important role in vascular calcification and become a new therapeutic target.
Figure 7.
Scheme of estrogen and HIF-1α pathways involved in VSMCs osteogenic differentiation and vascular calcification.
Abbreviations: VSMC: vascular smooth muscle cell; MCP-1: monocyte chemoattractant protein; TGF-β: transforming growth factor β; BMP-2: bone morphogenetic protein 2; MAPK: mitogen-activated protein kinase; Runx2: runt-related transcription factor-2; RANKL: receptor activator of NF-kB ligand; OPG: osteopmtegerin; MGP: matrix Gla protein.
Acknowledgements
We thank International Science Editing (http://www. International scienceediting.com) for editing this manuscript.
Statement of ethics
The animal experiments were approved by the Institutional Animal Care and Use Committee of Dali University, and conformed with the Guide for the Care and Use of Laboratory Animals in China.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the National Nature Science Foundation of China (No. 81560073).
ORCID iD
References
- 1.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol 2014; 34: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 2013; 93: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magne D, Julien M, Vinatier CS, et al. Cartilage formation in growth plate and arteries: from physiology to pathology. Bioessays 2010; 27: 708–716. [DOI] [PubMed] [Google Scholar]
- 4.Giachelli C. The emerging role of phosphate in vascular calcification. Kidney Int 2009; 75: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espitia O, Chatelais M, Steenman M, et al. Implication of molecular vascular smooth muscle cell heterogeneity among arterial beds in arterial calcification. Plos One 2018; 13: e0191976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004; 291: 210–215. [DOI] [PubMed] [Google Scholar]
- 8.Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010; 56: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 9.Christian RC, Liu PY, Sean H, et al. Intimal estrogen receptor (ER)β, but not ERα expression, is correlated with coronary calcification and atherosclerosis in pre- and postmenopausal women. J Clin Endocrinol Metab 2006; 91: 2713–2720. [DOI] [PubMed] [Google Scholar]
- 10.Rzewuska-Lech E, Jayachandran M, Fitzpatrick LA, et al. Differential effects of 17β-estradiol and raloxifene on VSMC phenotype and expression of osteoblast-associated proteins. Am J Physiol Endocrinol Metab 2005; 289: E105–E112. [DOI] [PubMed] [Google Scholar]
- 11.Osako MK, Nakagami HN, Shimizu H, et al. Estrogen inhibits vascular calcification via vascular RANKL system: common mechanism of osteoporosis and vascular calcification. Circ Res 2010; 20: 466–475. [DOI] [PubMed] [Google Scholar]
- 12.Choi BG, Vilahur G, Cardoso L, et al. Ovariectomy increases vascular calcification via the OPG/RANKL cytokine signalling pathway. Eur J Clin Invest 2010; 38: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng YQ, Xiong D, Lin X, et al. Oestrogen inhibits arterial calcification by promoting autophagy. Sci Rep 2017; 7: 3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcrobb LS, Kcy MG, Tsatralis T, et al. Estrogen receptor control of atherosclerotic calcification and smooth muscle cell osteogenic differentiation. Arterioscler Thromb Vasc Biol 2017; 37: 1127. [DOI] [PubMed] [Google Scholar]
- 15.Aarup A, Pedersen TX, Junker N, et al. Hypoxia-inducible factor-1α expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol 2016; 36: 1782–1790. [DOI] [PubMed] [Google Scholar]
- 16.Alessandra B, Giovanni DC, Francesca P, et al. Evidence for markers of hypoxia and apoptosis in explanted human carotid atherosclerotic plaques. J Vasc Surg 2010; 52: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 17.Resar JR, Roguin A, Voner J, et al. Hypoxia-inducible factor 1alpha polymorphism and coronary collaterals in patients with ischemic heart disease. Chest 2005; 128: 787–791. [DOI] [PubMed] [Google Scholar]
- 18.Monsonego-Ornan E, Idelevich A, Rais Y. Bone Gla protein increases HIF-1α-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol 2011; 48: S141-S141. [DOI] [PubMed] [Google Scholar]
- 19.Kapustin AN, Shanahan C, Biology V. Osteocalcin: a novel vascular metabolic and osteoinductive factor? Arterioscler Thromb Vasc Biol 2011; 31: 2169. [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi Y, Sato Y, Kobayashi T, et al. HIF1α is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci USA 2013; 110: 16568–16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John Paul K, Wilkinson FL, Canfield AE, et al. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes: implications for vascular calcification. Circ Res 2006; 98: 1264. [DOI] [PubMed] [Google Scholar]
- 22.Mossbrugger I, Hoelzlwimmer G, Calzada-Wack J, et al. Standardized morphological phenotyping of mouse models of human diseases within the German Mouse Clinic. Verh Dtsch Ges Pathol 2007; 91: 98. [PubMed] [Google Scholar]
- 23.Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol 2009; 29: 279–283. [DOI] [PubMed] [Google Scholar]
- 24.Mcclelland RL, Hyoju C, Robert D, et al. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006; 113: 30–37. [DOI] [PubMed] [Google Scholar]
- 25.Udo H, Massaro JM, Fox CS, et al. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol 2008; 102: 1136–1141.e1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson JC. Does estrogen therapy reduce coronary artery calcification in postmenopausal women? Nat Clin Pract Cardiovasc Med 2007; 4: 654–655. [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007; 356: 2591–2602. [DOI] [PubMed] [Google Scholar]
- 28.Omland T, Ueland T, Jansson AM, et al. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol 2008; 51: 627–633. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Lu WH, Ai R, et al. The relationship between serum hypoxia-inducible factor 1α and coronary artery calcification in asymptomatic type 2 diabetic patients. Cardiovasc Diabetol 2014; 13: 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mokas S, Lariviere R, Lamalice L, et al. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int 2016; 90: 598–609. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Yang HY, Giachelli C. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 2008; 199: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yucheng Y, Bennett BJ, Xuping W, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res 2010; 107: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou N, Wei H, Liao J, et al. HIF-1α potentiates BMP2-induced chondrogenic differentiation but inhibits osteogenic differentiation in stem cells. J Third Military Med Univ 2014; 36: 44. [Google Scholar]
- 34.Saika M, Inoue D, Kido S, et al. 17β-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-alpha. Endocrinology 2001; 142: 2205–2212. [DOI] [PubMed] [Google Scholar]
- 35.Kee HJ, Cho SN, Kim GR, et al. Gallic acid inhibits vascular calcification through the blockade of BMP2-Smad1/5/8 signaling pathway. Vascul Pharmacol 2014; 63: 71–78. [DOI] [PubMed] [Google Scholar]
- 36.Hyzy SL, Olivares-Navarrete R, Schwartz Z, et al. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J Cell Biochem 2012; 113: 3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu R, Xuezhi Z, Xiucai J, et al. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell Physiol Biochem 2014; 34: 2049–2060. [DOI] [PubMed] [Google Scholar]
- 38.Balderman JAF, Hae-Young L, Mahoney CE, et al. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. JAHA 2012; 1: 1225–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]