Abstract
Epidemiological estimates indicate not only an increase in the proportion of older adults, but also an increase in those who continue moderate alcohol consumption. Substantial literatures have attempted to characterize health benefits/risks of moderate drinking lifestyles. Not uncommonly, reports address outcomes in a single outcome, such as cardiovascular function or cognitive decline, rather than providing a broader overview of systems. In this narrative review, retaining focus on neurobiological considerations, we summarize key findings regarding moderate drinking and three health domains, cardiovascular health, Type 2 diabetes (T2D), and cognition. Interestingly, few investigators have studied bouts of low/moderate doses of alcohol consumption, a pattern consistent with moderate drinking lifestyles. Here, we address both moderate drinking as a lifestyle and as an acute event.
Review of health-related correlates illustrates continuing inconsistencies. Although substantive reductions in risk for cardiovascular and T2D events are reported, robust conclusions remain elusive. Similarly, whereas moderate drinking is often associated with enhanced cognition and lower dementia risk, few benefits are noted in rates of decline or alterations in brain structure. The effect of sex/gender varies across health domains and by consumption levels. For example, women appear to differentially benefit from alcohol use in terms of T2D, but experience greater risk when considering aspects of cardiovascular function. Finally, we observe that socially relevant alcohol doses do not consistently impair performance in older adults. Rather, older drinkers demonstrate divergent, but not necessarily detrimental, patterns in neural activation and some behavioral measures relative to younger drinkers. Taken together, the epidemiological and laboratory studies reinforce the need for greater attention to key individual differences and for the conduct of systematic studies sensitive to age-related shifts in neurobiological systems.
1. Introduction
It is widely recognized that the number of older adults is expected to increase dramatically in the next decades. Current projections indicate that individuals 65 years of age and older will constitute approximately 22% of the US population in 2050. In comparison, this age group comprised only about 15% of the population in 2016 (US Census Bureau, 2018). Less appreciated are data illustrating the fact that rates of current drinking in older adults are also on the rise. Recent analyses of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) comparing 2001/2002 and 2012/2013 reports found an 11.8% increase in the prevalence of recent (past year) alcohol consumption among adults between 45 and 64 years of age and an even larger increase (22.4%) among persons 65 and older (Grant et al., 2017) with greater increases among women than men (Dawson, Goldstein, Saha, & Grant, 2015). Most data indicate that while more older adults report current drinking, only a small percentage increase their drinking (Britton & Bell, 2015). Together, these population shifts signal that the number of older adults maintaining non-problem or moderate drinking will increase dramatically in the next decades. Importantly, the health and policy implications of these changes are incompletely understood.
Despite widespread interest in the effects of “moderate drinking lifestyles,” the term remains variably defined (see Kalinowski & Humphreys, 2016; Topiwala & Ebmeier, 2018). Epidemiological and large sample studies often use estimates of typical drinking (i.e., typical standard (std.) drinks/day) (Breslow, Castle, Chen, & Graubard, 2017). However, as discussed in later sections, upper limits for “moderate” drinking are inconsistently applied. Additionally, the alcohol content associated with the definition of a std. drink varies across countries. For example, in the United Kingdom a std. drink is defined as having 8g of absolute alcohol. In the United States, a std. drink is currently defined as 14g of absolute alcohol (NIAAA, 2016). Yet, an alternate definition of 12g is often used (see, Dawson, 2003). These inconsistencies challenge interpretation and international collaborations. Further complicating study of moderate drinking is the fact that drinkers in some studies are classified on the basis of clinical/diagnostic criteria rather than drinking quantity (e.g., Grant et al., 2017). Finally, a subset of published work, composed largely of human laboratory studies, combines these approaches; setting drinking limits and excluding persons who meet clinical criteria (e.g., Gilbertson, Ceballos, Prather, & Nixon, 2009; Marczinski & Fillmore, 2003).
Regardless of nosologic approach, drinking patterns among moderate drinkers are heterogeneous. As evidenced in subsequent sections, some investigators disregard differences in quantity/or frequency among moderate drinkers, treating them as a unitary group. Other researchers subgroup participants by level of drinking (e.g., light vs. heavy), permitting more refined analysis of level of drinking. Yet, uniform definitions for these subgroups are not widely incorporated. Furthermore, drinkers’ outcomes are commonly compared to that of non-drinkers, a reference group that may include individuals who drank previously but are now abstinent (i.e., former drinkers), as well as life-time abstainers. These methodological differences profoundly impact conclusions and recommendations.
Embracing these interpretive challenges, the role of continued drinking with increasing age is a critical area of study. Even healthy aging is accompanied by ubiquitous neuro/biologic adaptations that may impact sensitivity to alcohol’s potential effects. Depending on the biological system, exposure levels, and individual factors, alcohol may impart benefit or enhance risk. A comprehensive review of organ systems and functions is beyond our scope. Therefore, we provide a narrative review. In selecting studies to be included, priority was given to recently published meta-analyses, widely-recognized longitudinal studies, and additional investigations with sufficient detail to permit meaningful comparison/comment. To provide a sense of the evolution of this work, older as well as more recent investigations were considered.
Given the overarching theme of this volume, we constrain our focus to the association between moderate drinking and two common age-related conditions that are, themselves, associated with negative neurobehavioral sequelae: cardiovascular disease (CVD) and Type 2 diabetes (T2D). Following our overview of CVD and T2D, we explore the effects of moderate drinking lifestyles on cognitive status, cognitive decline, and dementia risk.
Although there is a substantial literature regarding moderate drinking lifestyles, there are relatively few data addressing the impact of the consumption of low/moderate alcohol dose among older drinkers, a drinking pattern inherent to the generally accepted definition of moderate drinking lifestyles. With the numbers of current drinkers increasing in older age groups, clarifying the acute effects of alcohol at doses relevant to older adults’ moderate drinking lifestyles is highly pertinent. Thus, we summarize recent work directed to clarifying age-related vulnerability to the acute effects of low/moderate alcohol doses in Section 3.
Finally, sex/gender is a highly relevant biological variable. Yet, as broadly reported, sex differences are often understudied. Thus, to the extent data permit, we address sex differences throughout our review.
2. Moderate drinking lifestyles: Impact on peripheral systems and neurobehavior
2.1. Introduction/rationale
Before proceeding, two caveats bear mentioning. As described above, consistent definitions of standard drinks and “moderate” consumption remain elusive. Furthermore, as evidenced below, the specific domains vary in their reporting practices. To mitigate confusion, to the extent possible, we converted alternate units of measure (i.e., units or std. drinks) to grams. These issues are noted by other investigators (e.g., Peters, Peters, Warner, Beckett, & Bulpitt, 2008) and provide a particularly robust challenge for the cognitive literature. For example, some investigators used definitions consistent with 14g/drink for some types of alcohol beverages (e.g., a 12oz. beer) and 12g/drink for other types of beverages (e.g., assuming a mixed drink has 1.25oz. of liquor). Where sufficient information was lacking, we report measures (e.g., “drinks”) employed by the authors. We retain categorical definitions employed by the cited studies, making no attempt to create a uniform lexicon. Most commonly, drinkers are compared to non-drinking reference groups, which, unless otherwise noted, encompass both lifetime abstainers and currently abstinent previous drinkers (i.e., former drinkers).
2.2. Impact on peripheral systems
There is an extensive literature identifying relationships between peripheral systems, particularly vascular and metabolic functions, and cognitive integrity in older adults, (e.g., Bucur & Madden, 2010; Crichton, Bryan, & Murphy, 2013; Flicker, 2010; Launer, Feskens, Kalmijn, & Kromhout, 1996; Leritz, McGlinchey, Kellison, Rudolph, & Milberg, 2011; Sabia et al., 2019). These functions are impacted by alcohol use, yet relatively few studies examine alcohol as a potential mediator of compromise between peripheral and neural systems (but see, Downer, Jiang, Zanjani, & Fardo, 2015; Harrison et al., 2017; Panza et al., 2008; Ruitenberg et al., 2002). Consequently, while numerous potential mechanisms underlying these relationships have been examined, including alcohol interactions with apolipoprotein E, angiotensin, insulin sensitivity, and inflammatory cytokines (e.g., Herring & Paulson, 2018; Schrieks, Heil, Hendriks, & Beulens, 2015), their relative impact on neurobehavioral functions in aging remains insufficiently characterized. Despite this limitation, the robust, but incompletely consistent, literatures regarding the impact of alcohol consumption on two prevalent age-related conditions, cardiovascular disease (CVD) and Type 2 diabetes (T2D), merit inclusion in any overview of moderate drinking and neurobehavioral outcomes. To aid interpretation, a short glossary of relevant terms is provided in Tables 1 and 2A and B summarize studies addressing cardiovascular health and T2D and are located at the conclusion of Section 2.2.2.
Table 1.
Section 2 glossary.
| Hazard ratio (HR) | Estimates of risk for a given condition (e.g., ischemic stroke) given exposure (e.g., moderate drinking) over time. Commonly reported in survival analyses. |
| Odds ratio (OR) | Comparison of the ratio of persons who were vs. those who were not exposed (e.g., moderate drinkers vs. non-drinkers) among persons with a known outcome (e.g., those who experienced ischemic stroke). OR’s <1.0 reflect reduced risk, OR >1, greater risk. Text identifies significance vs. non-significance. |
| Relative risk/Risk ratio (RR) | Probability of a disease outcome (e.g., presence or absence of ischemic stroke) among those exposed (e.g., moderate drinkers). Parameters same as OR. |
Table 2.
Exemplar analyses of alcohol-associated risk to cardiovascular and type 2 diabetes.
| A: Exemplar cardiovascular meta-analyses | ||||||
|---|---|---|---|---|---|---|
| Reference | Conditions examined | Non-drinker stratificationa | Sex-stratified risk analysisb | # Studies & total sample | Doses & definitions | Comments |
| Roerecke and Rehm (2012) | IHD morbidity & mortality | Yes | Yes | N = 957,684 24 studies | 2.5–12 g/day; 12–24 g/day; 24–36 g/day |
Results support cardioprotective association, but find heterogeneity across sexes and outcomes, with generally weaker effects than those reported earlier |
| Ronksley, Brien, Turner, Mukamal, and Ghali (2011) | CVD mortality, incident CHD, CHD mortality, incident stroke, stroke mortality | Mixed | No | N = 1,184,956 84 studies | <2.5 g/day; 2.5–15 g/day; 15–30 g/day; 30–60 g/day; >60g/day |
2.5–15g/day consistently associated with a 14–25% reduction in risk of all outcomes. Heterogeneous dose-response patterns by outcome measure |
| Larsson, Wallin, Wolk, and Markus (2016) | Ischemic stroke, ICH, SAH | No | Yes | N = 1,102,642 27 studies | <12g/day; 12–24g/day; 24–48 g/day; >48g/day |
Stroke risks vary by drinking pattern and stroke type. ≤24 g/day was associated with reduced risk of ischemic stroke but not intracerebral or subarachnoid hemorrhage |
| Briasoulis, Agarwal, and Messerli (2012) | Hypertension | No | Yes | N = 227,656 16 studies | <10g/day; 10–20 g/day; 20–30 g/day; 30–40 g/day; 40–50 g/day; >50g/day |
Detected sex-dependent associations between stroke and moderate drinking, with protective effects noted only among women. >20 g/day associated with increased risk in both |
| Larsson, Orsini, and Wolk (2015) | Heart failure | No | No | N = 202,378 8 studies | 36g/week; 84g/week.; 168 g/week.; 252g/week |
Observed dose-response function consistent with J-shape: reduced risk at 36 and 84 g/week, but not 168 or 252g/week |
| Wood et al. (2018) | Stroke, MCI, CHD, Heart failure, CVD mortality | N/A | No | N = 599,912 83 studies | 100–200g/week; 200–350g/week; 350 + g/week (0–25g/week and 0–100g/week employed as reference groups) |
Heterogenous relationships across cardiovascular measures; Relative to a reference group including light drinkers, positive linear dose-response associations noted for some outcomes (e.g., stroke; CVD mortality), protective effects for others (e.g., MCI) |
| B: Exemplar type 2 diabetes (T2D) meta-analyses | |||||
|---|---|---|---|---|---|
| Reference | Non-drinker stratificationc | Sex-stratified risk analysisd | # Studies and total sample | Doses and definitions | Comments |
| Knott, Bell, and Britton (2015) | Mixed | Yes | N = 1,902,605 38 studies | No categorical analyses | No T2D risk reduction noted for men at any dose; For women: reduction @ <71g/day; peak reduction @ 31–37g/day |
| Baliunas et al. (2009) | Yes | Yes | N = 477,200 20 studies | No categorical analyses | U-shaped function for both sexes; Protection among women noted <50 g/day, maximal reduction, 40%, @ 24 g/day. For men, protection <60 g/day; maximal reduction, 13% @ 22 g/day |
| Li, Yu, Zhou, and He (2016) | No | Yes | N = 706,716 26 studies | 0–12g/day; 12–24g/day; >24 g/day |
No risk increase noted at any dose. Protection among women maximal @ ~30% reduction and ~25–35g/day. For men: maximal reduction @ ~57% and 20–25 g/day |
| Carlsson, Hammar, and Grill (2005) | No | Mixed | 13 studies | 5–30 g/day; >30 g/day |
At moderate levels (5–30 g/day), approximate reduction of 30% noted across samples; Similar pattern observed when women analyzed separately |
Reflects whether lifetime abstainers were separated from prior drinkers as a reference group. “Mixed” indicates this separation occurred in some, but not all analyses.
Reflects whether dose-response risk functions were individuated for men and women.
Reflects whether lifetime abstainers were separated from prior drinkers as a reference group. “Mixed” indicates this separation occurred in some, but not all analyses.
Reflects whether dose-response risk functions were individuated for men and women.
IHD, Ischemic Heart Disease; CVD, Cardiovascular Disease; CHD, Coronary Heart Disease; ICH, Intracerebral Hemorrhage; SAH, Subarachnoid Hemorrhage; MCI, Myocardial Infarction
2.2.1. Alcohol and cardiovascular health
The so-called “J-shaped curve” describes a dose-response function wherein the impact of an intervening variable shifts from providing benefit to exacting costs in a dose-dependent manner. It has found wide application in studies of alcohol consumption on cardiovascular integrity, wherein relative risks for CVD morbidity, mortality, and related conditions appear reduced at lower (light-to-moderate) levels of consumption, but elevated at heavier levels. Notable examples of large-scale longitudinal examinations consistent with this relationship include the Cardiovascular Health Study (e.g., Bryson et al., 2006), the National Health Interview Survey (e.g., Mukamal, Chen, Rao, & Breslow, 2010), the National Epidemiological Survey on Alcohol and Related Conditions (e.g., Balsa, Homer, Fleming, & French, 2008), the Framingham Heart study (e.g., Walsh et al., 2002), and the Physician’s Health Study (e.g., Malinski, Sesso, Lopez-Jimenez, Buring, & Gaziano, 2004). Similar outcomes have been reported in samples from Great Britain, Germany, France, China and Japan (e.g., Britton, Singh-Manoux, & Marmot, 2004; Inoue et al., 2012; Keil, Chambless, Doring, Filipiak, & Stieber, 1997; Renaud, Gueguen, Schenker, & d’Houtaud, 1998; Yuan, Ross, Gao, Henderson, & Yu, 1997). In contrast, a recent report from the Prospective Urban Rural Epidemiological study included data from 12 countries with heterogeneous incomes/economic status. Relative to never drinkers (lifetime abstainers), current drinking was not associated with risk reduction for incident cardiovascular disease (HR: 0.97), although risk for myocardial infarction was reduced (HR: 0.76; Smyth et al., 2015).
Several meta-analyses (e.g. Larsson et al., 2015; Ronksley et al., 2011) offer some support for cardioprotection at moderate doses. However, they also indicate that protective effects may be weaker, more heterogeneous, and occur at lower levels of consumption than previously reported. For instance, in a meta-analysis of CVD mortality, Ronksley et al. (2011) noted that the lowest risks for coronary heart disease (CHD), CHD mortality, and CVD mortality occurred at 15–30g/day, while stroke mortality risk appeared lowest at <15g/day. A meta-analysis focused on stroke risk, conducted by Larsson et al. (2016), demonstrated that risk for both intracerebral and subarachnoid hemorrhage was reduced among groups consuming either ≤12 or 12–24g/day. Roerecke and Rehm (2012) conducted a meta-analysis of ischemic heart disease mortality and morbidity, in which they stratified by sex and employed a lifetime abstainer reference group. They noted modest evidence of cardioprotection in men and women and demonstrated substantial heterogeneity, challenging interpretations of a unitary “J-shaped” function. A recent meta-analysis conducted by Wood et al. (2018) also observed heterogeneous relationships across cardiovascular health measures. For example, calculating HRs per 100g/week, they found that alcohol consumption was linearly related to stroke (HR 1.14), but inversely related to myocardial infarction (HR.94). Those who drank more than 100g/week had lower life expectancy at age 40, with greater reductions associated with higher levels of weekly drinking.
Earlier work (e.g., Britton et al., 2004; Walsh et al., 2002) suggested stronger cardioprotective effects of moderate consumption among men than in women. More recent meta-analyses of hypertension risk (e.g., Briasoulis et al., 2012; Taylor et al., 2009) observed protective effects only among women. The meta-analysis by Roerecke and Rehm (2012) illustrates the complexity of sex effects; estimations of ischemic heart disease mortality suggested similar risk reductions between men and women drinking 10–20g/day, but indicate that at higher levels (e.g., 50–60g/day) women display markedly increased relative risks whereas men remain at lower risk. Similar susceptibility to these higher doses among women was also apparent for morbidity outcomes. In contrast, greater protective effects were observed for moderately drinking women (~10–30g/day) than were apparent for men at any dose (Roerecke & Rehm, 2012). These observations are consistent with studies focused on women, including the Women’s Health Study (Djousse, Driver, & Gaziano, 2009), which found consumption of 5–15g/day was associated with lower risk of CVD and ~50% lower risk of CVD mortality, relative to abstainers at 12-year follow-up. A recent meta-analysis (Colpani et al., 2018) examining similar levels (8–14g/day) supported these conclusions, noting reductions in CVD, CHD and mortality among women.
2.2.2. Alcohol and type 2 diabetes (T2D)
Review of the T2D literature reveals a generally strong protective effect of moderate drinking, with relative risk reductions of T2D development of ~30% commonly observed among such drinkers (e.g., Carlsson et al., 2005). Several investigations observe maximal risk reductions at lower, relative to higher, doses (e.g., 12g vs. 36g/day; Kao, Puddey, Boland, Watson, & Brancati, 2001), but in contrast to the cardiovascular literature, substantially increased risk at higher levels of consumption is infrequently reported. Inverse linear relationships between consumption levels and T2D are more commonly observed (e.g., Djousse, Biggs, Mukamal, & Siscovick, 2007; Hu, van Dam, & Liu, 2001), with some studies reporting risk reductions of up to 40% at higher levels of consumption (i.e., >50g/day; Conigrave et al., 2001).
Earlier T2D meta-analyses largely supported conclusions of a “U-shaped” function, with moderate consumption producing the greatest risk reduction (relative to abstainers and heavier drinkers), but heavier consumption failing to markedly increased risk relative to abstainers (e.g., Carlsson et al., 2005; Koppes, Dekker, Hendriks, Bouter, & Heine, 2005). More recent analyses have produced mixed results. Whereas Li et al. (2016) observed results consistent with the earlier meta-analyses, a meta-analysis by Baliunas et al. (2009) and a review by Pietraszek, Gregersen, and Hermansen (2010), note protective effects, but observe increased risk at higher doses (e.g., 50–60g/day).
A meta-analysis conducted by Knott et al. (2015) supports a modest “J-shaped” relationship, albeit with several caveats. Their analyses stratified studies on the basis of whether the reference group was composed of only lifetime abstainers or lifetime abstainers and former drinkers. This analysis suggested that when lifetime abstainers are utilized as controls, no protective effect of moderate consumption is evident, i.e., benefit is observed only when moderate drinkers were compared with former drinkers. Moreover, when analyses were stratified by sex, no protective effect was apparent among men, regardless of reference group. Although the lack of T2D protection in men is notable, Knott and colleagues’ observation of lower relative risks among moderate-drinking women is broadly consistent with the larger literature (e.g., Hodge, English, O’Dea, & Giles, 2006; Stampfer et al., 1988). It merits mention that while much of the work includes older adults, age is rarely a specific focus. However, examination of age effects suggests that protection associated with moderate consumption may be greater among older, relative to younger, adults (≥60 vs. <60 years; Li et al., 2016).
2.3. Neurobehavioral outcomes
In this section, we summarize key investigations of the relationship between moderate drinking and specific aspects of cognitive change with age. The first section addresses group differences in cognitive abilities; the second, the association between alcohol use and dementia risk; the third, the relationship between alcohol and cognitive decline; and the fourth, alcohol’s association with structural brain changes. An overview of key studies from this section is provided in Table 3, just prior to in Section 2.4.
Table 3.
Exemplar neurobehavioral studies.
| Reference | Outcome measures | Non-drinker stratificationa | Sex-stratified risk analysisb | Dose ranges and definitions | Comments |
|---|---|---|---|---|---|
| Reas, Laughlin, Kritz-Silverstein, Barrett-Connor, and McEvoy (2016) | Cognitive function | Yes | No | <14g/<28g/day for women/men; 14/28 to <42/<56g/day; >42/>56g/dayc | Observed positive linear relationships between executive function and both amount and frequency of consumption, but U-shaped functions for memory, with the greatest advantages among moderate and infrequent (1–2 days/week) drinkers |
| Herring and Paulson (2018) | Cognitive function; Cognitive decline |
No | No | 14–196g/week | Moderate drinking significantly associated with better cognitive performance across a variety of tasks/domains, but did not have a significant effect on performance decline |
| Hogenkamp et al. (2014) | Cognitive function; Cognitive decline |
No | Male sample | 12 g/day; 24g/day; ≥36g/day |
Moderate consumption associated with performance advantage in set-shifting/executive function task, but not on rates of change in performance over 7-year period |
| Neafsey and Collins (2011) | Dementia; Cognitive decline (Meta-analysis) | Mixed | Mixed | “Light to moderate” (≤1/≤2 drinks/day for women/men); “Heavy” (>3–4 drinks/day)d | Average RR for cognitive risk (dementia/impairment) for moderate drinking was 0.77. Benefit applied to all forms of dementia/impairment, but not rate of cognitive decline |
| Peters et al. (2008) | Dementia; Cognitive decline (Meta-analysis) | No | No | Specific categories not established | Conclude light-to-moderate drinking may be protective against dementia (RR: 0.63) and Alzheimer’s (RR: 0.57) but not vascular dementia or cognitive decline |
| Downer et al. (2015) | Episodic memory; Hippocampal volume |
No | No | “Light” (1–6 drinks/week); “Moderate” (7–14/week); “heavy” (15–34/week)d | Moderate drinking during late life associated with larger hippocampal volumes; Light, but not moderate consumption associated with improvements in episodic memory which were accounted for by hippocampal volume |
| Topiwala et al. (2017) | Cognitive decline; Hippocampal volume |
No | Yes | “Light” (8–56 g/week) “Moderate” (56–112/168 g/week for women/men) “Unsafe” (112/168 +/week) | 112–168g/week associated with over three times the odds for hippocampal atrophy. No associations between drinking and cognitive decline |
| McEvoy et al. (2018) | White matter integrity | Yes | Male Sample | “Very Light” (1–3 drinks/2 weeks); “Light” (4–8/2 weeks); “Moderate” (9–28/2 weeks); “Heavier” (>28/2 weeks)d | U-shaped dose-response function detected for alcohol and fractional anisotropy (FA) measures across multiple tracts, with FA increases peaking at moderate levels |
| Wardzala et al. (2018) | Cognitive decline | No | Yes | “Rare/never” (<14g/week); “Moderate” (<98/196 g/week. for women/men); “Heavy” (>98/196 g/week) | Analyzed rates of decline across a battery of neuropsychological tests and global measures of impairment; Sex-stratified analyses revealed reduced rates of memory decline in moderate drinking women, but measures of global impairment in men |
Reflects whether lifetime abstainers were employed as a reference group. “Mixed” indicates this separation occurred in some, but not all analyses.
Reflects whether dose-response risk functions were individuated for men and women.
1.25oz liquor (~12g alcohol) used to define “1 drink”; conversion to 14g/drink may have overestimated consumption.
Insufficient information to convert “drinks” to grams.
2.3.1. Cognitive status
Early work examining cognitive performance and moderate or social drinking (i.e., 1977–1996, as reviewed in Neafsey & Collins, 2011) tended to report either alcohol-associated decrements in cognitive function (e.g., Parker & Noble, 1977), or no difference relative to non-drinkers (e.g., Parsons, 1986; see Parsons & Nixon, 1998 for review). Similarly, several earlier studies reported no risk reductions for dementia (e.g., Graves et al., 1991; Hebert et al., 1992). As methodological sophistication and attention to moderate/low-risk patterns have matured, conclusions drawn from more recent work have shifted markedly.
A number of subsequent reports focusing on older adults suggested J-shaped relationships, with the greatest risk reductions and/or cognitive advantages noted at light/moderate levels (e.g., Britton et al., 2004). In subgroups of older adults whose average consumption was characterized as either “minimal” or “moderate” drinking, Ganguli, Vander Bilt, Saxton, Shen, and Dodge (2005) observed benefits in multiple neurocognitive tasks, including indices of executive functions, relative to non-drinkers. However, some investigators have reported relative benefit at drinking levels as high as 40 and 80g/day for women and men, respectively (Zuccala et al., 2001). Similarly, based on data from the Framingham Heart Study, Elias, Elias, D’Agostino, Silbershatz, and Wolf (1999) reported better performance on a number of cognitive functions including verbal memory, visual memory, abstract reasoning, and attention at consumption ranges of up to 24–48 and 48–96g/day for women and men, respectively. In more recent work, Herring and Paulson (2018) reported better performance among moderate drinking adults (≤28g/day) over age 65 in several cognitive domains. Advantages persisted across the ~7 year follow-up period for 10 of the 12 measures employed, including measures of executive function, visuospatial skills, verbal fluency, and verbal memory.
In contrast to much of this literature, which utilizes average consumption as a primary predictor, Reas et al. (2016) examined both average quantity and frequency of consumption. They observed positive linear relationships between executive function and both measures of consumption, but U-shaped functions for memory, with the greatest advantages among moderate and infrequent (1–2 days/week) drinkers. Similar advantages among older moderate drinkers were recently noted by Hogenkamp et al. (2014) for executive and psychomotor functions and Downer et al. (2015) for episodic memory function. In contrast to these findings, Topiwala et al. (2017) observed enhanced cognitive functions (lexical/word fluency and recall memory) among women drinking 8–16 g/day and men drinking 8–24 g/day in their early 40 s, but this difference did not persist across the subsequent 30 years of follow-ups.
2.3.2. Dementia risk
Consistent with reports of enhanced cognitive function, a number of investigations have observed protective effects associated with moderate alcohol for dementia risk in aging adults. Ruitenberg et al. (2002) followed ~8000 healthy older adults for approximately 6 years. Daily consumption of 1–3 drinks was associated with substantial risk reductions for development of any dementia (HR: 0.58), with the most robust protection for vascular dementia (HR: 0.30). Subsequent studies examining risks for dementia and/or cognitive impairment have largely supported these risk reductions (e.g., Bachman et al., 2003; Deng et al., 2006; Garcia, Ramon-Bou, & Porta, 2010; Huang, Qiu, Winblad, & Fratiglioni, 2002; Lindsay et al., 2002; Ogunniyi et al., 2006; Richard et al., 2017; Weyerer et al., 2011).
Peters et al. (2008) conducted a meta-analysis that demonstrated risk reductions for any dementia among moderate drinkers (RR: 0.63). The reduction was similar for Alzheimer’s disease (AD) risk (RR: 0.57) but reduction for vascular dementia did not reach significance (RR: 0.89). A meta-analysis conducted by Anstey, Mack, and Cherbuin (2009) supported the observed reductions for any dementia (RR: 0.74), AD (RR: 0.72), and in contrast to Peters et al. (2008), also noted reductions in vascular dementia (RR: 0.75). The meta-analysis reported by Neafsey and Collins (2011) stratified by study date including research conducted from 1998 to 2011. They concluded that the average cognitive RR across studies, including dementia and cognitive impairment, was 0.77. A recent meta-analysis conducted by Xu et al. (2017) found consistent, but weaker relationships (RR: 0.90 for all-cause dementia), with risk reductions confined to low doses (6–12.5g/day).
2.3.3. Cognitive decline trajectories
While observing an alcohol-related reduction in risk of developing dementia and/or cognitive impairment, the previous meta-analyses (Neafsey & Collins, 2011; Peters et al., 2008) did not detect the alcohol-related effect on rate of cognitive decline, noted in other studies (e.g., Ganguli et al., 2005; Wright, Elkind, Luo, Paik, & Sacco, 2006). Similarly, recent work has found little evidence for moderate alcohol-associated alterations in cognitive change across time. Herring and Paulson (2018) conducted a latent growth curve analysis of cognitive performance in older moderate drinkers over approximately 7 years, but found no evidence for alcohol-associated effects on rate of cognitive change. In contrast to the larger literature wherein both sexes are included, Hogenkamp et al. (2014) studied men from the age of 70–77. They noted age-related cognitive decline in men, but despite alcohol-associated advantages at both measurement periods, observed no differential rate of decline between moderate vs. non-drinking men. These conclusions are strengthened by cross-sectional work suggesting that stratification by dose (i.e., light vs. moderate) fails to distinguish trajectories of cognitive decline (Moussas et al., 2015). Though less commonly reported, Topiwala et al. (2017) observed more rapid declines in lexical fluency, but not semantic fluency or word recall, relative to non-drinkers of 14%, 17%, and 16% for light, moderate, and heavy drinkers, respectively, over a 30-year study period. As discussed below, investigations reporting alcohol-associated benefit on rate of decline tend to be either single-sex examinations, or note sex-specific effects.
2.3.3.1. Sex differences
Several large studies investigating women support benefits of moderate consumption on cognitive function, including decelerated rates of decline. Barnes et al. (2007) identified an OR for maintaining optimal cognitive functioning vs. cognitive decline of 1.25 in a large group of ~10,000 older women. Examining data from the similarly large Nurses’ Health Study, Stampfer, Kang, Chen, Cherry, and Grodstein (2005) identified RRs of 0.81 for global cognitive impairment among women drinking less than 15g/day, and 0.85 for “substantial decline” over a 2-year period. Consistent with other null results in decline trajectory, Wardzala et al. (2018) examined alcohol-associated changes in a battery of neuropsychological tests, as well measures of global impairment, with protective effects of moderate alcohol noted only in one memory task. Interestingly, when analyses were stratified by sex, only women displayed the memory improvement, however, moderate drinking men evinced reduced decline in global measures. In another recent study, Sabia et al. (2014) report outcomes dependent on sex and dose. Men consuming ≥36g/day evinced more rapid decline across cognitive domains. At lower levels of consumption, rates were equivalent between moderate and non-drinking men. In contrast, lower levels of drinking (<10g/day) in women was associated with slower rates of decline relative to non-drinking women. These findings are consistent with a number of investigations identifying stronger protective effects of moderate drinking on cognitive function in women than in men (e.g., Dufouil, Ducimetiere, & Alperovitch, 1997; McGuire, Ajani, & Ford, 2007; Stott et al., 2008), and highlight both the import and complexity of considering sex as a critical covariate in neurobehavioral analyses of alcohol effects.
2.3.4. Brain structure
Topiwala et al. (2017) analyzed both structural (MRI) and behavioral measures collected over ~30 years of follow-ups from the Whitehall II cohort in the United Kingdom. They observed dose-dependent acceleration of cognitive decline and hippocampal atrophy. Although deficits in similar measures have been observed in clinical populations with chronic heavy consumption (e.g., Sullivan, Marsh, Mathalon, Lim, & Pfefferbaum, 1995), Topiwala and colleagues observed significantly accelerated decline even among moderate drinkers (~16–24g/day), with odds of right-sided hippocampal atrophy 3.4 times that of non-drinking individuals. This relationship persisted among men when analyses were stratified by sex. Consistent with other sex-dependent cognitive findings, no alcohol-associated insult was observed for women. Similar negative associations between alcohol and total brain, hippocampal, ventricular, and amygdalar volumes have been observed at doses within light-to-moderate ranges (e.g., den Heijer et al., 2004; Ding et al., 2004; Paul et al., 2008). In contrast, Downer et al. (2015) observed alcohol-associated protection in hippocampal volumes among older drinkers from the Framingham Heart Study Offspring Cohort. Moderate drinkers had larger hippocampal volumes relative to non-drinkers, which appeared to account for between-group differences in episodic memory. Similar neuroprotective effects were observed for total brain volume by Gu et al. (2014) and measures of white matter integrity by Mukamal, Longstreth, Mittleman, Crum, and Siscovick (2001) and McEvoy et al. (2018).
Taken together these investigations suggest that among older adults, while moderate drinking may provide at least modest benefit to cognitive function, similar benefits to brain structure are not apparent, with findings often revealing negative volumetric associations. Davis et al. (2014) refer to this contrast between cognitive/behavior outcomes and structural measures as the “alcohol paradox,” given findings that in aging adults volumetric measures correlate positively with cognitive function (e.g., Fletcher et al., 2018; MacLullich et al., 2002). Davis et al. (2014) demonstrate that these positive associations persist among light-to-moderate older drinkers, but observed that despite improved cognitive outcomes, moderate drinkers failed to display the implied volumetric increases. Whether this discontinuity is the product of methodological heterogeneity, may be explained by alterations in peripheral function (i.e., cardiovascular health), or reflects subtle neural alterations requiring more sophisticated physiological measures (e.g., shifts in functional connectivity), remains unknown.
2.4. Summary/limitations
The scope of our current work is insufficient to accommodate granular examination of methods employed by each referenced study. Several observations, however, are broadly applicable. Persistent issues include: (1) inconsistent stratification, classification, and terminology regarding consumption levels (i.e., light vs. moderate vs. heavy); (2) failure to account for patterns of drinking frequency and per occasion quantity, typically overlooked in lieu of average daily consumption; (3) fundamental differences between abstainers and drinkers that are independent of alcohol, but cannot be easily disambiguated or accounted for; and (4) reference group classification, including failures to stratify or otherwise account for potential differences between lifetime abstainers and past drinkers.
The third and fourth issues are particularly challenging. Nearly all of the discussed studies accounted for an extensive collection of covariates. That said, some characteristics of moderate drinkers remain difficult to control statistically. Moderate drinking appears to be a marker of general well-being, expressed as a diverse set of constructs, including prosperity, social functioning, mental and physical health, recreation, and others (e.g., Hansel et al., 2010). Relatedly, moderate drinkers are more likely to exercise regularly and report healthier eating behaviors (e.g., Barefoot et al., 2002), greater utilization of medical services from preventative dental care to mammography (Green, Freeborn, & Polen, 2001). They also report higher rates of subjective well-being (Nekvasil & Liu, 2016). These differences have led to criticism that J- and U-shaped relationships in alcohol-associated health outcome studies may be spurious, resulting from the inability to control for the full constellation of health-related factors that differentiate moderate drinkers from abstainers. Support for such criticism is drawn from observations that J-shaped relationships appear in a host of health measures for which beneficial causal roles of alcohol are difficult to reconcile (reviewed by Fekjaer, 2013), such as liver cirrhosis (Rehm et al., 2010) and postnatal outcomes (Kelly, Leggett, & Cronise, 2009). The challenges in identifying lifestyle factors that differentiate moderate from non-drinkers is compounded by the common practice of including former drinkers with lifetime abstainers in a single reference group. Several studies report that when former drinkers are excluded, alcohol-associated protective effects are far more limited (e.g., Fillmore, Stockwell, Chikritzhs, Bostrom, & Kerr, 2007; Makela, Paljarvi, & Poikolainen, 2005). One leading hypothesis for this discrepancy, proposed by Shaper, Wannamethee, and Walker (1988) often referred to as the “sick quitter” hypothesis, suggests individuals who are not lifetime abstainers often cease use due to negative health consequences. Finally, conclusions regarding the association between moderate drinking and neurobehavioral outcomes remain most tentative. Prospective longitudinal studies with greater attention to reference groups and more sophisticated neurobehavioral measures may aid future interpretation.
3. Acute alcohol effects
3.1. Introduction/rationale
The acute effects of alcohol on brain and behavior have been widely studied, particularly in relatively young drinkers, e.g., between the ages of 21 and 40 using alcohol doses targeting breath alcohol concentrations (BrACs) of ~0.08g/dL (see Weafer & Fillmore, 2012; Wolff, Gussek, Stock, & Beste, 2018; Holloway, 1994, but see Tupler, Hege, & Ellinwood Jr., 1995). There is a paucity of systematic study addressing the effects of lower, socially-relevant, alcohol doses in older adults. Given changing population demographics and drinking patterns, this represents a significant gap in our understanding.
There is, however, a small body of work that has examined postural stability, balance and fine motor coordination using lower alcohol doses. For example, Vogel-Sprott and Barrett (1984) conducted a relatively early study with men between the ages of 19 and 63. Targeting a BrAC of 0.069g/dL, they found that age was negatively associated with balance and fine motor coordination. Jones and Neri (1994) administered an alcohol dose of 0.68g/kg to men, 20–59 years of age. Their data suggested an age-related vulnerability with older drinkers, i.e., those over 40, experiencing greater compromise in postural stability. In contrast to these earlier studies, Wu et al. (2017) applied a more contemporary definition of “older,” studying balance and postural sway in current drinkers over age 65 (mean age ~74) with women comprising 62% of the sample. Before administration of a 0.4g/kg alcohol dose (peak BrACs ~30mg/dL), individuals were assigned to either a good-balance or poor-balance group based on their ability to maintain a stance on one foot (unipedal stance) of > or <30s, respectively. After alcohol, both groups showed greater center of pressure displacement, particularly in the anterior-posterior direction. Only the good-balance group showed a decline in unipedal stance time. The absence of an effect in the poor-balance group was not anticipated. Given their performance at baseline (unipedal mean stance time of only ~13.3s), the outcome may be attributable to a floor effect. Taken together, these studies emphasize the possibility that even low alcohol levels may increase fall risk in older adults. One constraining generalization, is the fact that none of these studies included a placebo control and only the Wu et al. (2017) study included women.
In preparing this review, we conducted a search to identify studies addressing age differences in response to low/moderate alcohol doses. The results of this search, at least to the best of our knowledge, indicate that most of the published work in this domain has been conducted by our laboratory. Given recent attention to this issue (Boissoneault, Frazier, Lewis, & Nixon, 2016; Van Skike, Goodlett, & Matthews, 2019; Wu et al., 2017), we were surprised. Nonetheless, as a consequence, this section relies heavily on research directed by the first author and her colleagues. A short list of key terms is presented in the glossary for this section (Table 4).
Table 4.
Section 3 glossary.
| Cognitive efficiency | References interplay of accuracy and response time in performance. Here, reflected as an efficiency ratio: % accurate/mean RT (for accurate responses) |
| Hit efficiency | Efficiency in correctly identifying target stimuli |
| Correct rejection (CR) efficiency | Efficiency in correctly identifying stimuli as novel, i.e., not presented previously (non-targets) |
| Working memory (WM) | Neurobehavioral processes underlying manipulation of co-occurring stimulus streams/information sources. WM & selective attention rely on top-down cognitive control for differential processing of relevant vs. irrelevant information. |
| Neurophysiological methods | Here, non-invasive EEG methods assessing event related shifts (event related potentials; ERPs) and neural oscillations. The latter are reported in units (db) of spectral power, reflecting the degree to which a specific EEG frequency (e.g., alpha) is observed within a given time window |
As an introduction to this work and to minimize later redundancy, we first summarize our general methods and overarching framework. Across these studies, we applied a double-blind placebo control design and included both men and women. Younger participants were healthy community volunteers between the ages of 25–35 (younger; mean across studies ~28). Older participants ranged from 50 to 70 years of age in earlier studies (mean ~57.2) and 55–70 in more recent work (mean ~62). We recognize that this upper age range excludes a substantive proportion of the aging population. Initial efforts to recruit drinkers beyond 70 were largely unsuccessful. Participants were current social drinkers whose typical drinking patterns fell within National Institute of Alcohol Abuse and Alcoholism (NIAAA, 2009 & 2016) guidelines for low-risk/moderate drinking, i.e., <1 std. drink/day with not more than 3 on any occasion for women, and <2 std. drinks/day with not more than four on any occasion for men. [We should mention that the NIAAA (2016) guidelines recommend that men >65 years of age consume alcohol at levels recommended for women. This recommendation is not included in the USDHHS and USDA (2015) current dietary guidelines and was not applied herein]. Individuals completed a structured clinical research interview and were excluded if meeting criteria for significant mental health disorders including alcohol or drug use disorders. Finally, persons were ineligible if reporting a medical condition that contraindicated alcohol consumption or might confound interpretation. Stratified by age group and sex, individuals were randomly assigned to either a placebo or an active alcohol dose targeting BrACs consistent with an episode of moderate drinking. Age and sex differences in alcohol metabolism were controlled by standardizing alcohol dose on the basis of body water calculations (Watson, Watson, & Batt, 1981). The effectiveness of this procedure is reflected in the absence of main or interaction effects of sex or age on alcohol pharmacokinetics across the alcohol curve, as shown in Fig. 1 Across these studies, average daily alcohol consumption for each group (age by sex) was less than 14g (1 std. drink/day).
Fig. 1.
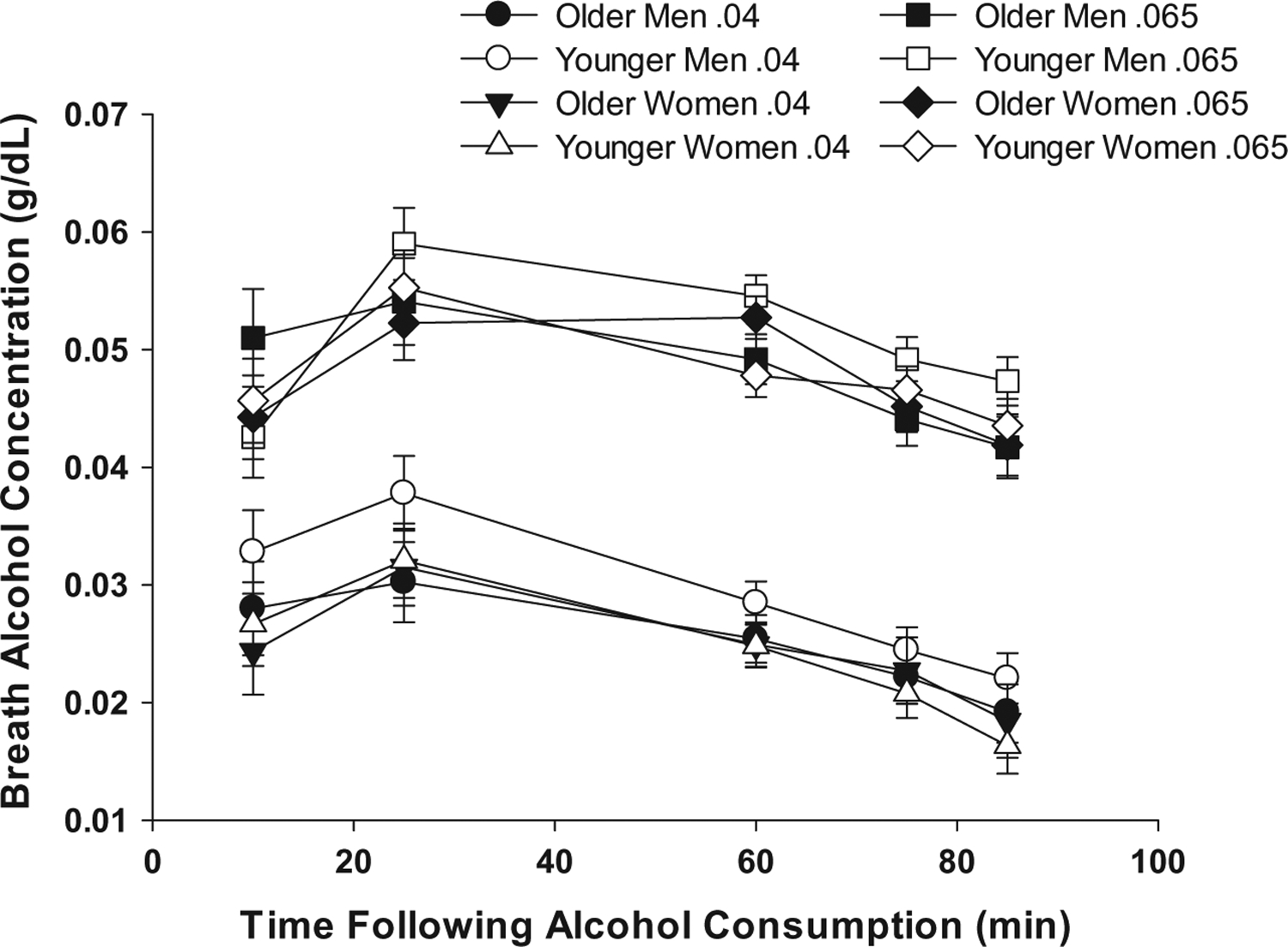
Breath alcohol concentrations (BrACs) across time. BrACs (Means ±SE) for older (55–70) and younger (25–35) healthy, moderate drinking men and women. No age or sex effects are indicated. As expected, the moderate dose (targeted peak BrAC=0.065g/dL) produced higher BrACs.
Within these studies, age and alcohol main effects were expected. These main effects are not of primary interest. Rather, the fundamental question is whether alcohol differentially impacts neurobehavioral processes in older vs. younger healthy social drinkers, i.e., the interaction of alcohol and age. Neurobehavioral functions modulated by the prefrontal cortices and their networks (i.e., executive functions), particularly attention and working memory processes, are impacted by both age and acute alcohol administration (e.g., Andres, Parmentier, & Escera, 2006; Drag, Bieliauskas, Kaszniak, Bohnen, & Glisky, 2009; Weafer & Fillmore, 2008). Leveraging these findings, we interrogate these domains through administration of select laboratory tasks as well as more ecologically valid and complex tasks such as simulated driving.
3.2. Behavioral outcomes
Gilbertson et al. (2009) examined performance on a common test of set-shifting (i.e., Trail Making Test, Form B; Reitan & Wolfson, 1993) on both the ascending and descending limbs of the BrAC curve. As expected, there was an age main effect with the younger cohort completing the task more quickly than did the older group. Of greater relevance was the differential effect of alcohol in the two age groups. On the ascending limb, older adults receiving low dose alcohol (targeted peak BrAC =0.04g/dL) performed more poorly than their age- cohorts receiving placebo. There was no effect of alcohol for older adults at an equivalent BrAC on the descending limb. Interestingly, self-reported ratings of perceived impairment indicated that older adults were relatively unaware of their impairment on the ascending limb. On the descending limb, where alcohol did not impact performance, older adults receiving alcohol reported higher levels of perceived impairment than their cohorts receiving placebo. The younger group was unaffected by alcohol on either limb and reported no differences in perceived impairment.
At peak BrAC, in this same study, participants completed a covert visual attention task (Luck et al., 1994), previously shown to be sensitive to both age (e.g., see Carriere, Cheyne, Solman, & Smilek, 2010) and alcohol (Acons, Chan, Drummond, & Tiplady, 2006). Cognitive efficiency, as reflected in the ability to respond both quickly and accurately, was ascertained (Sklar, Gilbertson, Boissoneault, Prather, & Nixon, 2012). The construct has demonstrated sensitivity to both age (Salthouse, Matlaga, & Wykoff, 1977) and acute alcohol administration (Tiplady et al., 2001) and has been widely applied in studies of alcohol use disorder (Nixon, 1993; Oscar-Berman & Marinkovic, 2007). Efficiency ratios were equivalent for the two age groups in the placebo condition. Alcohol benefitted performance in the younger cohort with those receiving alcohol being more efficient than their age-cohort receiving placebo. Unexpectedly, alcohol did not affect cognitive efficiency in the older group. Further analyses revealed that unlike the other groups, older adults receiving alcohol sacrificed speed for accuracy in maintaining efficient performance.
In an independent sample, similarly selected participants were assigned to one of three groups; targeted BrAC of 0, 0.04, or 0.065g/dL. Participants completed a working memory (WM) task (Boissoneault, Sklar, Prather, & Nixon, 2014; Gazzaley, Clapp, Kelley, McEvoy, Knight, & D’Esposito, 2008) at peak BrAC. The task involved presentation of a short series of individually presented faces and scenes followed by a short delay (WM maintenance period) and then the presentation of a probe stimulus, which was judged as being either present or absent in the previous series. Instructions to remember the faces or the scenes were counterbalanced within participants. Efficiency ratios for “hits” (accurately identifying a stimulus as appearing in the previous series) and “correct rejections” (accurately identifying a stimulus as being novel) were derived. Alcohol had no effect on performance in the younger cohort. For hit efficiency, the older group was inferior to the younger group at both placebo and moderate alcohol doses. Interestingly, the low dose benefitted performance in the older group and resulted in hit efficiency ratios equivalent to that of the younger cohort. Analysis of correct rejections, where detection of novelty is critical, revealed a different pattern. Among older participants, performance was negatively affected at both alcohol doses relative to placebo. For the younger group, performance for the placebo and low dose groups was equivalent, while there was a trend for enhanced efficiency at the moderate dose. The data for Hit and CR efficiency are shown in Fig. 2.
Fig. 2.
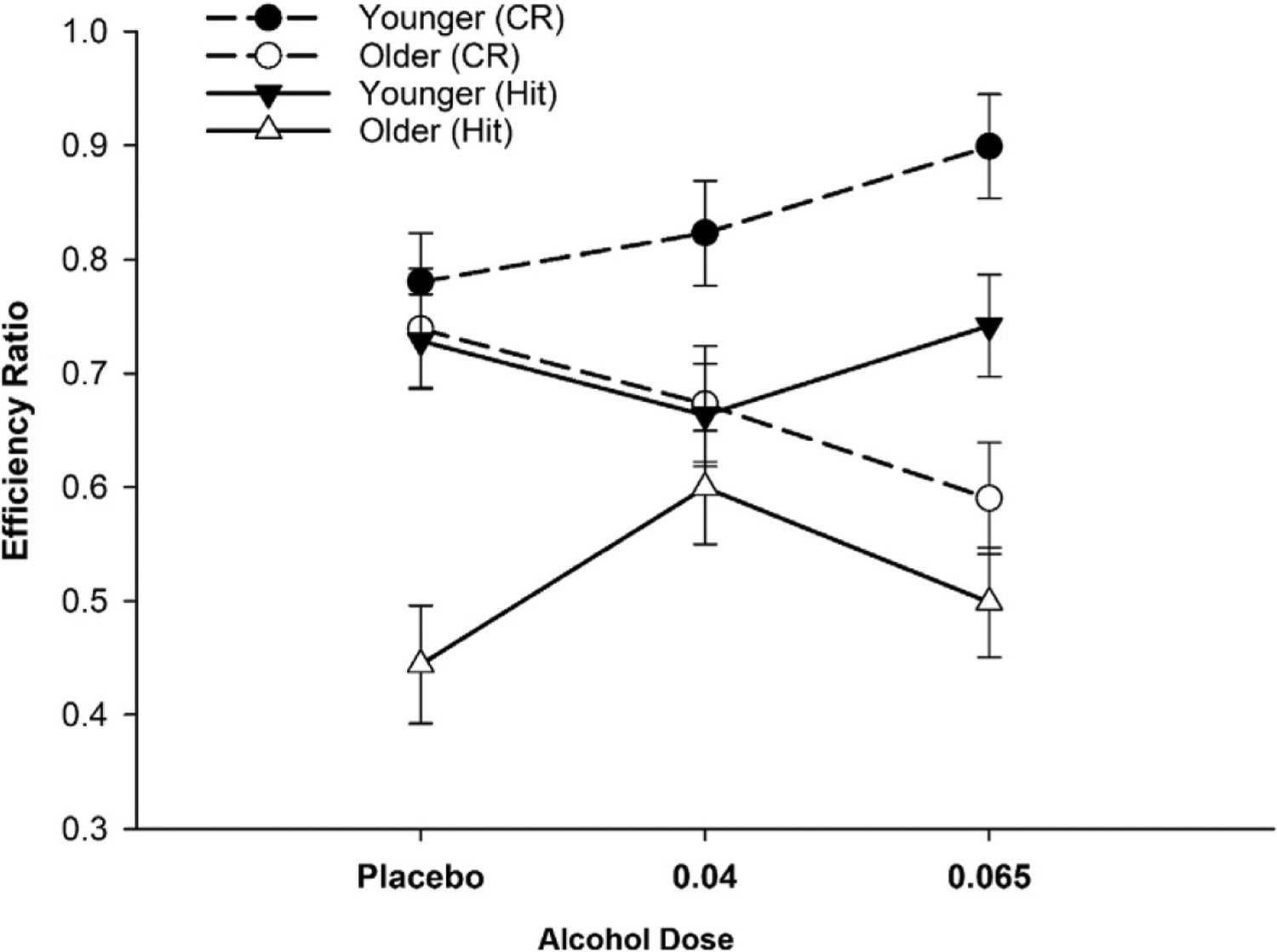
Efficiency ratios for working memory (WM) performance. Age-contingent alcohol effects on WM (Means ±SE). For hit efficiency (triangles), low dose alcohol benefitted older adults (open symbols) relative to placebo (P =0.03). Younger adults were unaffected by alcohol. For CRs (circles), older adults exhibited a dose related decline (P =0.04, at moderate dose). There was an opposite trend for younger adults (P =0.07). Data reported in Boissoneault, J., Sklar, A., Prather, R., & Nixon, S. J. (2014). Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs, 75(5), 870–879.
Lewis, Garcia, Boissoneault, Price, and Nixon (2019) conducted a replication study using identical selection criteria and the same WM task. Both studies showed that older cohorts in the placebo and moderate doses had significantly lower hit efficiency than their younger cohorts receiving these doses, with the older group receiving the low dose achieving performance equivalent to younger cohorts. Similarly, the moderate alcohol dose was associated with reduced correct rejection efficiency in the older cohort as compared to the younger cohorts in both studies. Age-related compromise in correct rejection efficiency at the low dose was observed only in the original study. Taken together, these studies demonstrate differential effects of acute alcohol with age. Furthermore, they suggest that processes related to “attending” and those related to “ignoring” may be impacted differently. Notably, the association between alcohol dose and performance was not linear, and may be facilitatory at some doses. In short, low and moderate alcohol doses are associated with divergent patterns of outcomes in older versus younger drinkers.
Often, there are insufficient women to allow analysis of sex differences (e.g., Boissoneault et al., 2014; Lewis, Boissoneault, Gilbertson, Prather, & Nixon, 2013). The Lewis et al. (2019) paper is an exception. Given identical selection criteria, doses and tasks, participants in the Boissoneault et al., 2014 and Lewis et al., 2019 were combined, permitting sufficient power for analysis of sex differences. Importantly, means for the four groups (2 age by 2 sex) were equivalent on key demographic variables with all groups reporting typical alcohol consumption of between ½ to 1 drink/day. Fig. 1, presented earlier, illustrates the absence of age or sex effects in alcohol metabolism at these doses. It might be noted that the absence of group differences in the BrAC curves is consistent with other reports from this group (Gilbertson et al., 2009; Sklar et al., 2012; Sklar, Boissoneault, Fillmore, & Nixon, 2014). Given earlier discussion, only sex main and interaction effects will be discussed. Hit efficiency was not impacted by the inclusion of sex. To the contrary, complex interactions including sex, age, dose and instruction set were obtained for correct rejection efficiency. The most striking outcome was an age-related divergence with older women in the moderate dose group being disproportionately disadvantaged. In contrast, younger women receiving this dose performed far better than all other groups. Furthermore, the magnitude of this difference was largest when the task demanded that faces be ignored. This pattern eludes ready explanation, but suggests that future work may benefit from greater consideration of (a) existing literature on age differences in face processing (Sullivan, Ruffman, & Hutton, 2007; Wong, Cronin-Golomb, & Neargarder, 2005), and (b) potential age differences in alcohol’s effects on cognitive control processes.
Importantly, ~15% of drivers aged 45–64% and 8% of those 65+ report a current (30day) history of driving after drinking (National Highway Traffic Safety Administration, 2011). Thus, understanding the behavioral outcomes associated with drinking and driving among older adults is of high import. Toward that end, Sklar et al. (2014) examined the age by alcohol interaction in a simulated driving task where driving demands were minimized. They found that core driving skills such as consistency in maintaining speed were compromised in older adults receiving low/moderate doses of alcohol. More recently, Price, Lewis, Boissoneault, Frazier, and Nixon (2018) examined performance in complex scenarios (i.e., country or city settings) and explored drivers’ responses when presented with either relevant (e.g., a pedestrian crossing the street) or irrelevant (e.g. a pedestrian walking on a parallel sidewalk) stimuli. These analyses showed that older adults altered their response strategy contingent on dose and scenario. In less complicated scenarios, the older cohort receiving alcohol became more conservative, decelerating more and braking earlier to relevant stimuli than their cohorts receiving placebo and producing a response pattern largely opposite to that of younger drinkers. In the more complicated metropolitan scenario, those receiving alcohol extended this strategy to irrelevant stimuli, braking and decelerating unnecessarily. There were no sex main or interaction effects with dose. It should be mentioned that to better approximate conditions under which older adults commonly drive after drinking, e.g., after a social event or dinner, the driving task was conducted on the descending BrAC limb in both the Sklar et al. (2014) and Price et al. (2018) studies. Thus, these age-contingent differences in alcohol effects occurred at very low BrACs; ~0.05 and ~0.28g/dL for the moderate and low dosed groups, respectively.
3.3. Neurophysiological outcomes
Complementing behavioral studies of age-differences in vulnerability to acute alcohol are limited investigations using electroencephalographic (EEG) methods. Shifts in the underlying neural activity associated with time-locked events, or event related potentials (ERPs) offer unique opportunities to study individual and group differences in the temporal dynamics of underlying neural processes. One of the most commonly investigated ERP is the P3 (also referenced as the P300). Relative increases in the P3 amplitude presumably reflect heightened neuronal activity, often to target or relevant stimuli. Its latency is typically shorter when tasks are easier and is hypothesized to reflect stimulus evaluation time. Of particular relevance, the P3 is vulnerable to both age and acute alcohol administration (see Polich, 2013 for overview). Lewis et al. (2013) examined the amplitude and latency of the P3 elicited by target stimuli during performance on the covert visual attention task introduced earlier (i.e., Sklar et al., 2012). The P3 was not impacted by alcohol in the younger group. In the older cohort, alcohol blunted P3 amplitudes and extended latencies relative to older adults receiving placebo, a pattern suggesting possible disruption in resource allocation during attentional processing (Polich, 2012). (See Fig. 3, adapted from Lewis et al., 2013).
Fig. 3.
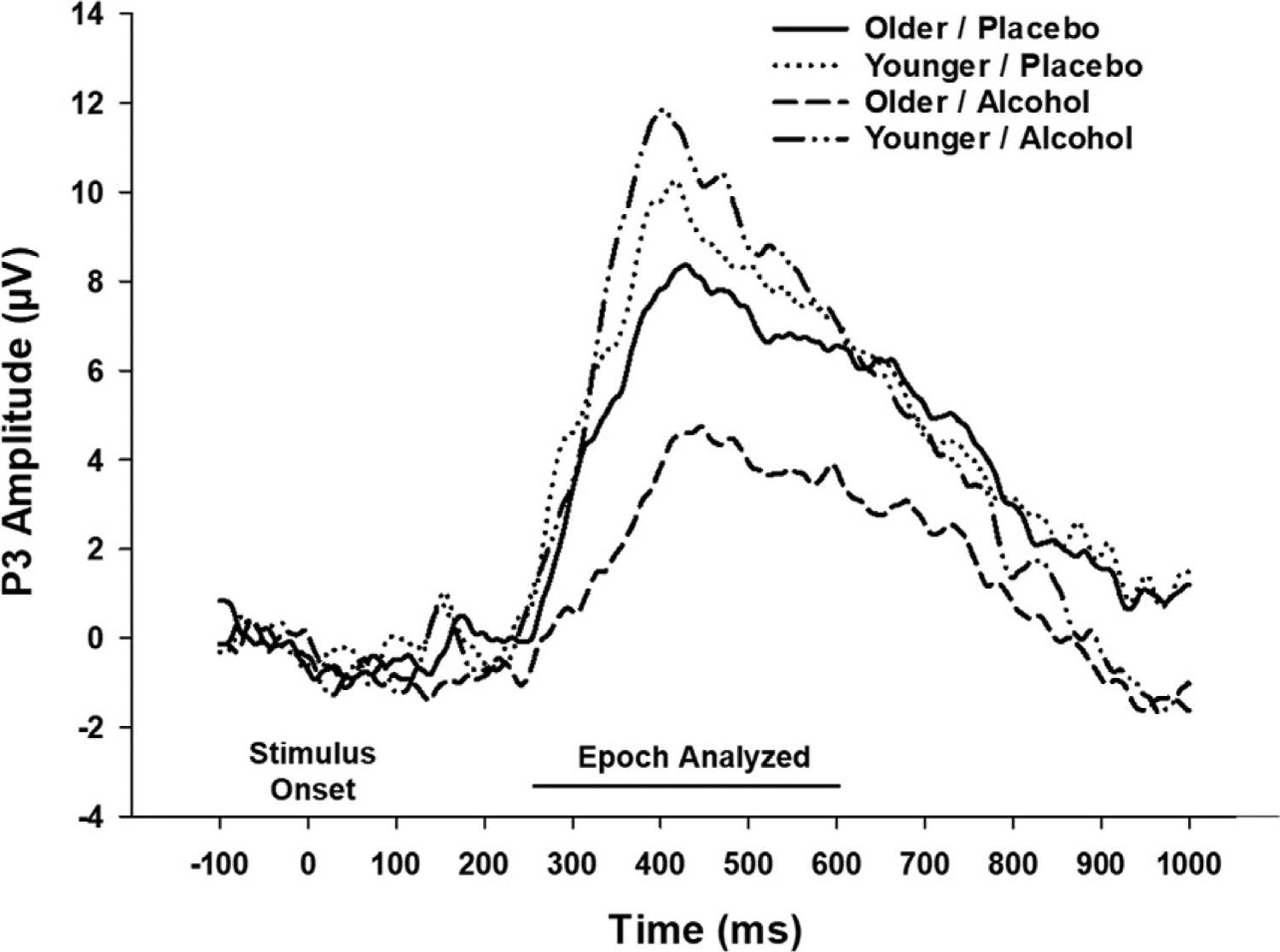
Grand average P3 waveform: Covert visual attention task. Grand average P3 waveform observed in covert visual attention task, differentiated by age and dose (only low and placebo doses used). Data shown were obtained from occipital midline scalp electrode, with similar patterns obtained at the midline parietal site. For older adults, alcohol significantly dampened P3 amplitude (P =0.048) and extended P3 latency (P <0.001), relative to placebo. Younger adults were not impacted by dose. Figure republished with permission from Lewis, B., Boissoneault, J., Gilbertson, R., Prather, R., & Nixon, S.J. (2013). Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alcoholism, Clinical and Experimental Research 37(6), 941–951; Copyright John Wiley and Sons, Inc.
Recently, the group extended its previous work with WM by examining ERP perturbations during the presentation of either “to be attended” or “to be ignored” facial stimuli (Garcia, Lambertus, Lewis, Boissoneault, & Nixon, 2019). Given their sensitivity to respondent age and stimulus content (i.e., faces), they investigated P1 amplitude and N1 latency (Gazzaley et al., 2008; Herrmann, Ehlis, Ellgring, & Fallgatter, 2005; Luck & Kappenman, 2012; Rossion & Jacques, 2012). Although data are not entirely consistent, P1 amplitude is generally shown to be larger to face vs. non-face stimuli, particularly when face stimuli are relevant, i.e., attended to, to task completion (here, WM performance). In contrast, the N170 latency is typically earlier to these stimuli. Studies of age effects (e.g., Gazzaley et al., 2008) suggest that older adults exhibit less robust effects of stimulus relevance for both P1 amplitude and N170 latency. In the current analysis, the investigators examined age by alcohol interactions on these components. For P1 amplitude, when faces were to be attended, only the moderate dose was associated with age-divergent outcomes. In this condition, P1 amplitudes for the older adults were lower than those for the older cohorts at either the low or placebo doses. For the younger cohort, the moderate dose was associated with higher P1 amplitudes than other conditions when face stimuli were to be explicitly ignored, i.e., irrelevant, a different pattern was observed. In that condition, older adults receiving the low dose had higher P1 amplitudes than did the other older groups, whereas this dose was associated with lower P1 amplitudes in the younger group relative to the placebo condition. In short, the degree to which the two age groups demonstrated divergent responses was influenced not only by dose, but also by task demands/stimulus relevance.
Other studies have employed analysis of neural oscillations using indicators of spectral power. Spectral power provides insight regarding the degree to which specific EEG frequency bands are engaged within defined time-locked windows (see Rangaswamy & Porjesz, 2014 for brief review) and are presumed to be temporally associated with sensory or cognitive events. Given their sensitivity to age (Dias et al., 2015; McEvoy, Pellouchoud, Smith, & Gevins, 2001), acute alcohol administration (Boha et al., 2009; Ehlers, Wills, & Havstad, 2012), and task demands (McEvoy et al., 2001; Wang, Rajagovindan, Han, & Ding, 2016), frontal theta power (FTP) and posterior alpha power (PAP) are particularly relevant to the current discussion. FTP has been positively related to performance with higher levels often interpreted as being indicative of mental effort and shifts posited to reflect resource allocation. The association between PAP and performance is contingent on task demands. PAP is presumed to reflect the engagement of inhibitory processes. Thus, PAP is increased during performance on tasks where potentially distracting information is being inhibited, as demanded in the maintenance phase of WM tasks. In contrast, when tasks demand that externally directed visual attention is required, PAP is reduced (see Wang et al., 2016 for discussion).
In the first of these studies, Boissoneault et al. (2016) examined FTP and PAP during the memory maintenance phase of a WM task. Contrary to expectations, there were no age, dose or interaction effects on FTP. PAP, on the other hand, was sensitive to the age by dose interaction. Relative to their age-specific cohort receiving placebo, younger adults receiving the low dose had significantly higher PAP, a pattern expected when inhibitory control must be activated to maintain memory. A similar pattern was observed for the moderate dose group. The reverse pattern was found for older adults. Older adults receiving the low dose had lower PAP relative to the placebo group. A similar outcome, approaching significance (P =0.07), was observed for the moderate vs. placebo doses. As shown in Fig. 4, there were no age differences at the placebo dose. Furthermore, the active doses, relative to placebo, had equivalent effects on PAP within each age group.
Fig. 4.
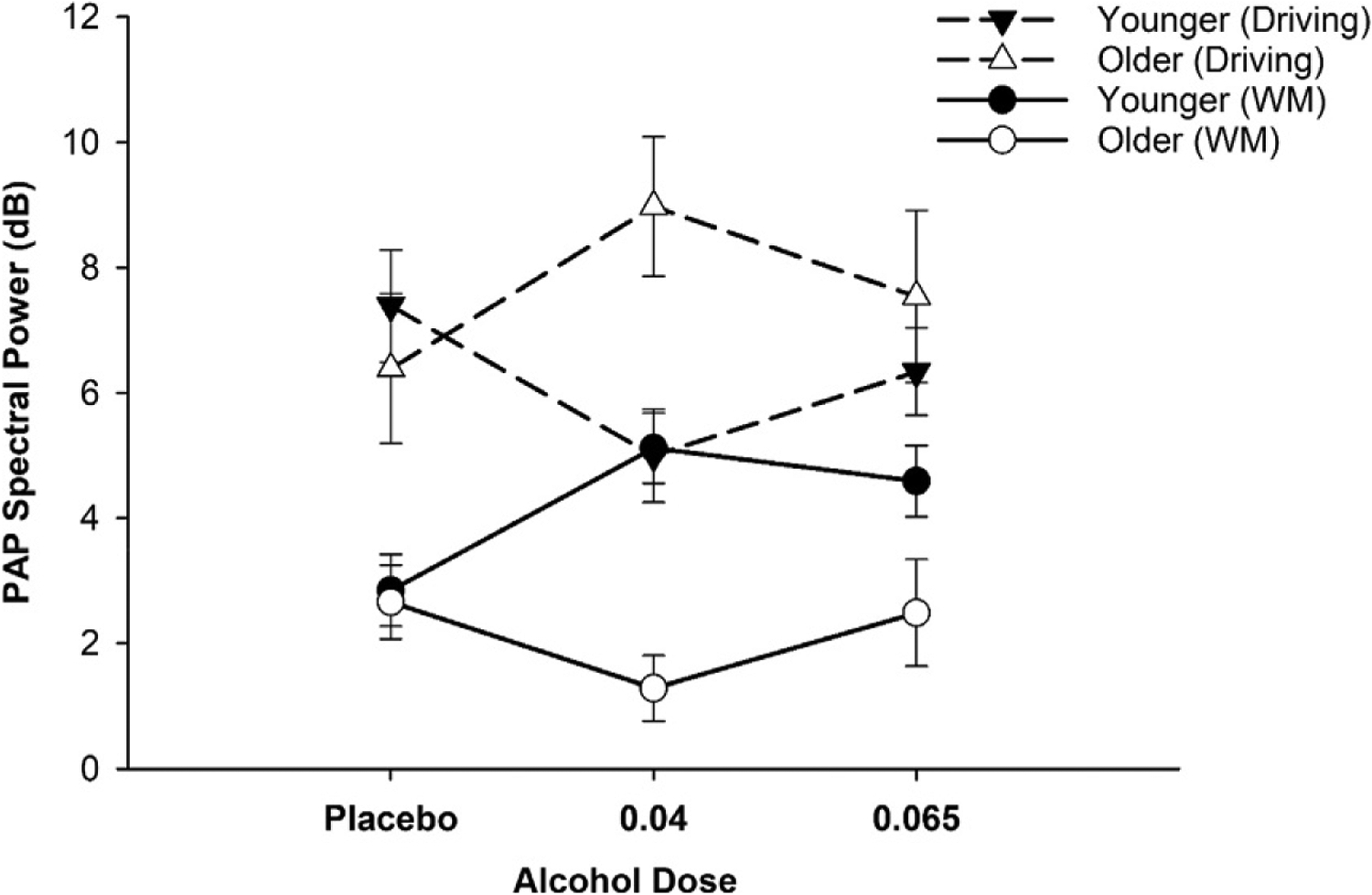
Posterior alpha power (PAP): WM maintenance and simulated driving. Posterior alpha power (PAP) obtained during WM maintenance processing (circles) and simulated driving (triangles). Under alcohol conditions, older (open symbols) and younger (filled symbols) adults demonstrated divergent patterns of neural activity. WM: alcohol-related increase in younger adults was anticipated (placebo vs. low: P =0.002). Older group produced opposite pattern (placebo vs. low: P =0.02). In the drive, PAP was reduced alcohol for younger adults (placebo vs. with low: P =0.026); in the older group, there was an opposite effect (placebo vs. low: P =0.075). Task-related PAP differences at placebo were consistent with performance demands. Data reported in Boissoneault, J., Frazier, I., Lewis, B., & Nixon, S.J. (2016). Effects of age and acute moderate alcohol administration on electrophysiological correlates of working memory maintenance. Alcoholism, Clinical and Experimental Research 40(9), 1874–1883 and Lewis et al., 2016, respectively.
Given the presumed relationship between PAP and inhibitory control, the association between PAP and WM performance was also considered. There was no relationship between PAP and WM performance for the younger cohort. Again, a different outcome was obtained for the older cohort. Among older adults receiving the moderate dose, lower PAP was associated with better performance. This finding is both counterintuitive and provocative. As mentioned above, neurobiological models posit that lower levels of PAP are associated with attention directed to external stimuli (see Wang et al., 2016). Extrapolating from such models, the authors speculated that the older cohort receiving the moderate dose may be directing attention externally, i.e., to the fixation “+,” during the maintenance period rather than directing attention internally, as anticipated. If so, these results are another indicator of age-specific strategic shifts to compensate for alcohol’s effects on inhibitory processes.
In a second study, this group (Lewis, Boissoneault, Frazier, & Nixon, 2016) compared FTP and PAP during simulated driving. FTP was again insensitive to alcohol or age by alcohol interactions. As predicted, there was a significant age by dose interaction for PAP. The age by dose interaction shown in Fig. 4 reflects significant age-related divergence. Under low dose conditions, PAP was reduced in the younger group, presumably reflecting greater attention to external objects/events, as would be anticipated during driving. For older adults, PAP in both alcohol conditions was higher than it was for the placebo group. Although speculative, the pattern suggests that older adults receiving alcohol disproportionately engaged inhibitory processes, perhaps to minimize the influence of distractors in the environment. Taken together, these studies reflect age-specific shifts in neural patterns underlying attention and WM memory that can be observed at both peak BrAC and at much lower alcohol concentrations.
3.4. Summary/limitations
As a whole, these studies illustrate an age-related vulnerability to the acute effects of alcohol. The results are of particular importance for several reasons: (a) The targeted peak BrACs were consistent with drinking levels achieved in typical social settings by moderate drinkers; (b) Age-related vulnerabilities were obtained with a very healthy sample where selection criteria were determined by the intent to assess age and alcohol interactions– without confounds associated with common age-related disorders. Thus, one might anticipate that the effects would be more robust if less healthy adults were selected. (c) Age by alcohol interactions were observed with an older sample that did not exceed age 70; (d) The interaction was obtained at peak and on both limbs of the BrAC curve, and (e) The age-related sensitivity was observed at doses producing no effect in younger adults. Whether processing of relevant (i.e., attending) vs. irrelevant (i.e., ignoring) information is differentially affected is unclear and demands further study. It is also noteworthy that alcohol’s effects in the older cohort were not uniformly negative. For some tasks and some doses, the older group benefitted.
In summary, the primary findings across this developing research are diverging patterns of alcohol effects between young and older healthy non-problem drinkers, obtained at socially relevant alcohol doses and observable in both behavior and neural activation patterns. These effects cannot be attributed to age differences in pharmacokinetics. As shown in Fig. 1, the alcohol metabolism curves are virtually identical for the two age groups. Neither do age differences at baseline account for the differences after alcohol consumption. Thus, the outcomes appear to arise from age-related vulnerability to the acute effects of alcohol consumption, i.e., an age by alcohol interaction.
Most of the published work on age by alcohol interactions explores attention and WM processes; i.e., neurobehavioral processes modulated by the prefrontal cortices and their networks. The driving studies extend the work to more ecologically valid settings, but again, study design has been largely directed to disentangling the impact on directed attention. Research targeting other neurobehavioral domains, functions and measurement methods are needed. Relatedly, with the exception of the spectral power studies, little attention has been directed to identifying mechanisms. For example, given the work indicating age and alcohol related changes in eye-tracking and the impact of these shifts on performance, programmatic study of this (and other) potential mediators and moderators is needed. Finally, greater emphasis must be placed on systematic study of sex differences in response to alcohol across the adult lifespan.
4. Conclusions/future directions
Overall, this review demonstrates the challenges of drawing conclusions regarding the impact of moderate drinking lifestyles and the bouts of drinking that constitute such lifestyles. In this review, we focused on two peripheral systems, cardiovascular health and T2D. They were selected given the prevalence of related health conditions in older adults as well as the fact that several seminal epidemiological and large sample studies have been reported. Furthermore, and of current relevance, cognitive change is often noted as a consequence of compromise in both systems.
Despite the depth and breadth of current research, robust conclusions of risk vs. benefit are often elusive. As noted in text and illustrated in Table 2A and 2B, some outcomes, e.g., a benefit of moderate drinking on risk for ischemic stroke, are commonly observed, but such a benefit does not fully generalize to other cardiovascular conditions. For T2D, a moderate drinking lifestyle is linked to lower risk, but the dose-response benefit applies differentially to women. Critically, the shape of the relationship between alcohol dose and outcomes varies across the two conditions. This finding is highly relevant given the fact that T2D and CVD may be interrelated, at least in some cases.
In reviewing the impact of moderate drinking on neurobehavioral outcomes (see Table 3), conclusions must also be qualified. Moderate drinking appears to, at least modestly, be associated with better cognitive performance, but its impact on rate of decline is unclear with some work showing not only decline in function but increased mortality at ages as young as 40. Finally, the question of whether moderate drinking alters risk for dementia remains incompletely resolved and may await more effective ways of differentiating dementias and their etiology.
There are methodological differences that may contribute to differences across studies including varying definitions of moderate drinking, measurement of/accounting for intervening variables, and the real-world relevance of the dependent/outcome variables (e.g., ecological validity of cognitive tests). Beyond drinking pattern and health condition, other sources of individual variability are accounted for and/or mitigated by study design and sample sizes. That said, commonly ignored individual variables may not only influence the baseline risk for health conditions, but also modulate alcohol’s effects on disease onset and progression. Alternative models explicitly extracting additional neurobiological and socio/environmental factors may facilitate more effective identification of differential risk and enable more effective prevention and intervention efforts.
In contrast to the large literature on moderate drinking lifestyles, only recently, and in limited contexts, has there been programmatic attention directed to the effects of relevant doses of alcohol in older adults. Accepting these caveats, the developing literature suggests that healthy older and younger moderate drinking adults differ little in their neurocognitive performance under placebo conditions, yet show divergent patterns under active alcohol conditions. Importantly, these differences have been observed in both laboratory tasks and simulated driving and using behavioral and neurophysiological measures. Although some data suggest that older women may be particularly sensitive to low/moderate alcohol doses, these results must be replicated and explored in other tasks. This work is provocative and identifies vulnerability in cognitive control systems. It does not, however, address the question of whether those individuals with the greatest age-related vulnerability are at higher risk, with continued moderate drinking for age-related neurobehavioral compromise. This critical question requires the conduct of longitudinal studies across which changes in the effects of acute alcohol and moderate drinking lifestyles can be simultaneously ascertained.
Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse And Alcoholism of the National Institutes of Health under Award Number R01AA19802 (PI: S.J. Nixon) with additional support provided by K01AA026893 (PI: B. Lewis). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Acons K, Chan LS, Drummond G, & Tiplady B (2006). Effects of ethanol and promethazine on awareness of errors and judgements of performance. Journal of Psychopharmacology, 20(5), 661–669. [DOI] [PubMed] [Google Scholar]
- Andres P, Parmentier FB, & Escera C (2006). The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia, 44(12), 2564–2568. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, & Cherbuin N (2009). Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. American Journal of Geriatric Psychiatry, 17(7), 542–555. [DOI] [PubMed] [Google Scholar]
- Bachman DL, Green RC, Benke KS, Cupples LA, Farrer LA, & Group MS (2003). Comparison of Alzheimer’s disease risk factors in white and African American families. Neurology, 60(8), 1372–1374. [DOI] [PubMed] [Google Scholar]
- Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, et al. (2009). Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care, 32(11), 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsa AI, Homer JF, Fleming MF, & French MT (2008). Alcohol consumption and health among elders. The Gerontologist, 48(5), 622–636. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Gronbaek M, Feaganes JR, McPherson RS, Williams RB, & Siegler IC (2002). Alcoholic beverage preference, diet, and health habits in the UNC Alumni Heart Study. American Journal of Clinical Nutrition, 76(2), 466–472. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Cauley JA, Lui LY, Fink HA, McCulloch C, Stone KL, et al. (2007). Women who maintain optimal cognitive function into old age. Journal of the American Geriatrics Society, 55(2), 259–264. [DOI] [PubMed] [Google Scholar]
- Boha R, Molnar M, Gaal ZA, Czigler B, Rona K, Kass K, et al. (2009). The acute effect of low-dose alcohol on working memory during mental arithmetic: I. Behavioral measures and EEG theta band spectral characteristics. International Journal of Psychophysiology, 73(2), 133–137. [DOI] [PubMed] [Google Scholar]
- Boissoneault J, Frazier I, Lewis B, & Nixon SJ (2016). Effects of age and acute moderate alcohol administration on electrophysiological correlates of working memory maintenance. Alcoholism, Clinical and Experimental Research, 40(9), 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J, Sklar A, Prather R, & Nixon SJ (2014). Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs, 75(5), 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Castle IP, Chen CM, & Graubard BI (2017). Trends in alcohol consumption among older Americans: National Health Interview Surveys, 1997 to 2014. Alcoholism, Clinical and Experimental Research, 41(5), 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briasoulis A, Agarwal V, & Messerli FH (2012). Alcohol consumption and the risk of hypertension in men and women: A systematic review and meta-analysis. Journal of Clinical Hypertension, 14(11), 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton A, & Bell S (2015). Reasons why people change their alcohol consumption in later life: Findings from the Whitehall II Cohort Study. PLoS One, 10(3), e0119421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton A, Singh-Manoux A, & Marmot M (2004). Alcohol consumption and cognitive function in the Whitehall II Study. American Journal of Epidemiology, 160(3), 240–247. [DOI] [PubMed] [Google Scholar]
- Bryson CL, Mukamal KJ, Mittleman MA, Fried LP, Hirsch CH, Kitzman DW, et al. (2006). The association of alcohol consumption and incident heart failure: The Cardiovascular Health Study. Journal of the American College of Cardiology, 48(2), 305–311. [DOI] [PubMed] [Google Scholar]
- Bucur B, & Madden DJ (2010). Effects of adult age and blood pressure on executive function and speed of processing. Experimental Aging Research, 36(2), 153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S, Hammar N, & Grill V (2005). Alcohol consumption and type 2 diabetes meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia, 48(6), 1051–1054. [DOI] [PubMed] [Google Scholar]
- Carriere JS, Cheyne JA, Solman GJ, & Smilek D (2010). Age trends for failures of sustained attention. Psychology and Aging, 25(3), 569–574. [DOI] [PubMed] [Google Scholar]
- Colpani V, Baena CP, Jaspers L, van Dijk GM, Farajzadegan Z, Dhana K, et al. (2018). Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: A systematic review and meta-analysis. European Journal of Epidemiology, 33(9), 831–845. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Hu BF, Camargo CA Jr., Stampfer MJ, Willett WC, & Rimm EB (2001). A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes, 50(10), 2390–2395. [DOI] [PubMed] [Google Scholar]
- Crichton GE, Bryan J, & Murphy KJ (2013). Dietary antioxidants, cognitive function and dementia–a systematic review. Plant Foods for Human Nutrition, 68(3), 279–292. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Vidal JS, Garcia M, Aspelund T, van Buchem MA, Jonsdottir MK, et al. (2014). The alcohol paradox: Light-to-moderate alcohol consumption, cognitive function, and brain volume. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences, 69(12), 1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA (2003). Methodological issues in measuring alcohol use. Alcohol Research & Health, 27(1), 18–29. [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Saha TD, & Grant BF (2015). Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug and Alcohol Dependence, 148, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, van Duijn CM, et al. (2004). Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. American Journal of Clinical Nutrition, 80(4), 992–997. [DOI] [PubMed] [Google Scholar]
- Deng J, Zhou DH, Li J, Wang YJ, Gao C, & Chen M (2006). A 2-year follow-up study of alcohol consumption and risk of dementia. Clinical Neurology and Neurosurgery, 108(4), 378–383. [DOI] [PubMed] [Google Scholar]
- Dias NS, Ferreira D, Reis J, Jacinto LR, Fernandes L, Pinho F, et al. (2015). Age effects on EEG correlates of the Wisconsin Card Sorting Test. Physiological Reports, 3(7), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Eigenbrodt ML, Mosley TH Jr., Hutchinson RG, Folsom AR, Harris TB, et al. (2004). Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: The atherosclerosis risk in communities (ARIC) study. Stroke, 35(1), 16–21. [DOI] [PubMed] [Google Scholar]
- Djousse L, Biggs ML, Mukamal KJ, & Siscovick DS (2007). Alcohol consumption and type 2 diabetes among older adults: The Cardiovascular Health Study. Obesity (Silver Spring), 15(7), 1758–1765. [DOI] [PubMed] [Google Scholar]
- Djousse L, Driver JA, & Gaziano JM (2009). Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA, 302(4), 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B, Jiang Y, Zanjani F, & Fardo D (2015). Effects of alcohol consumption on cognition and regional brain volumes among older adults. American Journal of Alzheimer’s Disease and Other Dementias, 30(4), 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA, Kaszniak AW, Bohnen NI, & Glisky EL (2009). Source memory and frontal functioning in Parkinson’s disease. Journal of the International Neuro-psychological Society, 15(3), 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Ducimetiere P, & Alperovitch A (1997). Sex differences in the association between alcohol consumption and cognitive performance. EVA Study Group. Epidemiology of Vascular Aging. American Journal of Epidemiology, 146(5), 405–412. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, & Havstad J (2012). Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Research, 1450, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Silbershatz H, & Wolf PA (1999). Alcohol consumption and cognitive performance in the Framingham Heart Study. American Journal of Epidemiology, 150(6), 580–589. [DOI] [PubMed] [Google Scholar]
- Fekjaer HO (2013). Alcohol-a universal preventive agent? A critical analysis. Addiction, 108(12), 2051–2057. [DOI] [PubMed] [Google Scholar]
- Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, & Kerr W (2007). Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies and new hypotheses. Annals of Epidemiology, 17(Suppl. 5), S16–S23. [DOI] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, et al. (2018). Brain volume change and cognitive trajectories in aging. Neuropsychology, 32(4), 436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker L (2010). Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clinics in Geriatric Medicine, 26(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Vander Bilt J, Saxton JA, Shen C, & Dodge HH (2005). Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology, 65(8), 1210–1217. [DOI] [PubMed] [Google Scholar]
- Garcia CC, Lambertus T, Lewis B, Boissoneault J, & Nixon SJ (2019). Effects of age and acute moderate alcohol consumption on electrophysiological indices of attention. In Presented at the 42nd Annual Research Society on Alcoholism, Minneapolis, MN. [Google Scholar]
- Garcia AM, Ramon-Bou N, & Porta M (2010). Isolated and joint effects of tobacco and alcohol consumption on risk of Alzheimer’s disease. Journal of Alzheimer’s Disease, 20(2), 577–586. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, & D’Esposito M (2008). Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 13122–13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson R, Ceballos NA, Prather R, & Nixon SJ (2009). Effects of acute alcohol consumption in older and younger adults: Perceived impairment versus psychomotor performance. Journal of Studies on Alcohol and Drugs, 70(2), 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AB, van Duijn CM, Chandra V, Fratiglioni L, Heyman A, Jorm AF, et al. (1991). Alcohol and tobacco consumption as risk factors for Alzheimer’s disease: A collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. International Journal of Epidemiology, 20(Suppl. 2), S48–S57. [DOI] [PubMed] [Google Scholar]
- Green CA, Freeborn DK, & Polen MR (2001). Gender and alcohol use: The roles of social support, chronic illness, and psychological well-being. Journal of Behavioral Medicine, 24(4), 383–399. [DOI] [PubMed] [Google Scholar]
- Gu Y, Scarmeas N, Short EE, Luchsinger JA, DeCarli C, Stern Y, et al. (2014). Alcohol intake and brain structure in a multiethnic elderly cohort. Clinical Nutrition, 33(4), 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel B, Thomas F, Pannier B, Bean K, Kontush A, Chapman MJ, et al. (2010). Relationship between alcohol intake, health and social status and cardiovascular risk factors in the Urban Paris-Ile-de-France Cohort: Is the cardioprotective action of alcohol a myth? European Journal of Clinical Nutrition, 64(6), 561–568. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Skelly MJ, Grosserode EK, Lowes DC, Zeric T, Phister S, et al. (2017). Effects of acute alcohol on excitability in the CNS. Neuropharmacology, 122, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Beckett LA, Funkenstein HH, Albert MS, Chown MJ, et al. (1992). Relation of smoking and alcohol consumption to incident Alzheimer’s disease. American Journal of Epidemiology, 135(4), 347–355. [DOI] [PubMed] [Google Scholar]
- Herring D, & Paulson D (2018). Moderate alcohol use and apolipoprotein E-4 (ApoE-4): Independent effects on cognitive outcomes in later life. Journal of Clinical and Experimental Neuropsychology, 40(4), 326–337. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Ehlis AC, Ellgring H, & Fallgatter AJ (2005). Early stages (P100) of face perception in humans as measured with event-related potentials (ERPs). Journal of Neural Transmission (Vienna), 112(8), 1073–1081. [DOI] [PubMed] [Google Scholar]
- Hodge AM, English DR, O’Dea K, & Giles GG (2006). Alcohol intake, consumption pattern and beverage type, and the risk of Type 2 diabetes. Diabetic Medicine, 23(6), 690–697. [DOI] [PubMed] [Google Scholar]
- Hogenkamp PS, Benedict C, Sjogren P, Kilander L, Lind L, & Schioth HB (2014). Late-life alcohol consumption and cognitive function in elderly men. Age (Dordrecht, Netherlands), 36(1), 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway FA (1994). Low-dose alcohol effects on human behavior and performance: A review of post-1984 research. US Department of Transportation, Office of Aviation Medicine DC: Washington: Retrieved from http://www.dtic.mil/get-tr-doc/pdf?AD=ADA286474. [Google Scholar]
- Hu FB, van Dam RM, & Liu S (2001). Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia, 44(7), 805–817. [DOI] [PubMed] [Google Scholar]
- Huang W, Qiu C, Winblad B, & Fratiglioni L (2002). Alcohol consumption and incidence of dementia in a community sample aged 75 years and older. Journal of Clinical Epidemiology, 55(10), 959–964. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nagata C, Tsuji I, Sugawara Y, Wakai K, Tamakoshi A, et al. (2012). Impact of alcohol intake on total mortality and mortality from major causes in Japan: A pooled analysis of six large-scale cohort studies. Journal of Epidemiology and Community Health, 66(5), 448–456. [DOI] [PubMed] [Google Scholar]
- Jones AW, & Neri A (1994). Age-related differences in the effects of ethanol on performance and behaviour in healthy men. Alcohol and Alcoholism, 29(2), 171–179. [PubMed] [Google Scholar]
- Kalinowski A, & Humphreys K (2016). Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction, 111(7), 1293–1298. [DOI] [PubMed] [Google Scholar]
- Kao WH, Puddey IB, Boland LL, Watson RL, & Brancati FL (2001). Alcohol consumption and the risk of type 2 diabetes mellitus: Atherosclerosis risk in communities study. American Journal of Epidemiology, 154(8), 748–757. [DOI] [PubMed] [Google Scholar]
- Keil U, Chambless LE, Doring A, Filipiak B, & Stieber J (1997). The relation of alcohol intake to coronary heart disease and all-cause mortality in a beer-drinking population. Epidemiology, 8(2), 150–156. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Leggett DC, & Cronise K (2009). Sexually dimorphic effects of alcohol exposure during development on the processing of social cues. Alcohol and Alcoholism, 44(6), 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott C, Bell S, & Britton A (2015). Alcohol consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care, 38(9), 1804–1812. [DOI] [PubMed] [Google Scholar]
- Koppes LL, Dekker JM, Hendriks HF, Bouter LM, & Heine RJ (2005). Moderate alcohol consumption lowers the risk of type 2 diabetes: A meta-analysis of prospective observational studies. Diabetes Care, 28(3), 719–725. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Orsini N, & Wolk A (2015). Alcohol consumption and risk of heart failure: A dose-response meta-analysis of prospective studies. European Journal of Heart Failure, 17(4), 367–373. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wallin A, Wolk A, & Markus HS (2016). Differing association of alcohol consumption with different stroke types: A systematic review and meta-analysis. BMC Medicine, 14(1), 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Feskens EJ, Kalmijn S, & Kromhout D (1996). Smoking, drinking, and thinking. The Zutphen Elderly Study. American Journal of Epidemiology, 143(3), 219–227. [DOI] [PubMed] [Google Scholar]
- Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, & Milberg WP (2011). Cardiovascular disease risk factors and cognition in the elderly. Current Cardiovascular Risk Reports, 5(5), 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, Frazier I, & Nixon SJ (2016). Effects of age and acute moderate alcohol administration on neurophysiology during simulated driving. Alcoholism, Clinical and Experimental Research, 40(12), 2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, Gilbertson R, Prather R, & Nixon SJ (2013). Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alcoholism, Clinical and Experimental Research, 37(6), 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Garcia CC, Boissoneault J, Price JL, & Nixon SJ (2019). Working memory performance following acute alcohol: Replication and extension of dose by age interactions. Journal of Studies on Alcohol and Drugs, 80(1), 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Yu FF, Zhou YH, & He J (2016). Association between alcohol consumption and the risk of incident type 2 diabetes: A systematic review and dose-response meta-analysis. American Journal of Clinical Nutrition, 103(3), 818–829. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. (2002). Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. American Journal of Epidemiology, 156(5), 445–453. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, & Hawkins HL (1994). Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance, 20(4), 887–904. [DOI] [PubMed] [Google Scholar]
- Luck SJ, & Kappenman ES (2012). Oxford handbook of event-related potential components. Oxford: Oxford University Press. [Google Scholar]
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, & Wardlaw JM (2002). Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology, 59(2), 169–174. [DOI] [PubMed] [Google Scholar]
- Makela P, Paljarvi T, & Poikolainen K (2005). Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: A follow-up study in a population with a low consumption level. Journal of Studies on Alcohol, 66(6), 722–728. [DOI] [PubMed] [Google Scholar]
- Malinski MK, Sesso HD, Lopez-Jimenez F, Buring JE, & Gaziano JM (2004). Alcohol consumption and cardiovascular disease mortality in hypertensive men. Archives of Internal Medicine, 164(6), 623–628. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, & Fillmore MT (2003). Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology, 11(1), 110–117. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Elman JA, Eyler LT, Franz CE, Hagler DJ, et al. (2018). Alcohol intake and brain white matter in middle aged men: Microscopic and macroscopic differences. NeuroImage: Clinical, 18, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Pellouchoud E, Smith ME, & Gevins A (2001). Neurophysiological signals of working memory in normal aging. Brain Research. Cognitive Brain Research, 11(3), 363–376. [DOI] [PubMed] [Google Scholar]
- McGuire LC, Ajani UA, & Ford ES (2007). Cognitive functioning in late life: The impact of moderate alcohol consumption. Annals of Epidemiology, 17(2), 93–99. [DOI] [PubMed] [Google Scholar]
- Moussas G, Fanouraki I, Pachi A, Asomatou A, Drylli O, Paschalakis G, et al. (2015). Comorbid psychopathology and alcohol use patterns among methadone maintenance treatment patients. Journal of Addiction, 2015, 197652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ, Chen CM, Rao SR, & Breslow RA (2010). Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. Journal of the American College of Cardiology, 55(13), 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ, Longstreth WT Jr., Mittleman MA, Crum RM, & Siscovick DS (2001). Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: The cardiovascular health study. Stroke, 32, 1939–1946. [DOI] [PubMed] [Google Scholar]
- National Highway Traffic Safety Administration. (2011). National survey of drinking and driving attitudes and behaviors: 2008. Annals of Emergency Medicine, 57(4), 405–406. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, & Collins MA (2011). Moderate alcohol consumption and cognitive risk. Neuropsychiatric Disease and Treatment, 7, 465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekvasil N, & Liu D (2016). In U.S., Moderate Drinkers Have Edge in Emotional Health. Gallup, Inc. Retrieved from https://news.gallup.com/poll/188816/moderate-drinkers-edge-emotional-health.aspx. [Google Scholar]
- Nixon SJ (1993). Recent developments in alcoholism: Typologies in women. Recent Developments in Alcoholism, 11, 305–323. [PubMed] [Google Scholar]
- Ogunniyi A, Hall KS, Gureje O, Baiyewu O, Gao S, Unverzagt FW, et al. (2006). Risk factors for incident Alzheimer’s disease in African Americans and Yoruba. Metabolic Brain Disease, 21(2–3), 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinkovic K (2007). Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Santamato A, et al. (2008). Vascular risk factors, alcohol intake, and cognitive decline. The Journal of Nutrition, Health & Aging, 12(6), 376–381. [DOI] [PubMed] [Google Scholar]
- Parker ES, & Noble EP (1977). Alcohol consumption and cognitive functioning in social drinkers. Journal of Studies on Alcohol, 38(7), 1224–1232. [DOI] [PubMed] [Google Scholar]
- Parsons OA (1986). Cognitive functioning in sober social drinkers: A review and critique. Journal of Studies on Alcohol, 47(2), 101–114. [DOI] [PubMed] [Google Scholar]
- Parsons OA, & Nixon SJ (1998). Cognitive functioning in sober social drinkers: A review of the research since 1986. Journal of Studies on Alcohol, 59(2), 180–190. [DOI] [PubMed] [Google Scholar]
- Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, et al. (2008). Association of alcohol consumption with brain volume in the Framingham study. Archives of Neurology, 65(10), 1363–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Peters J, Warner J, Beckett N, & Bulpitt C (2008). Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age and Ageing, 37(5), 505–512. [DOI] [PubMed] [Google Scholar]
- Pietraszek A, Gregersen S, & Hermansen K (2010). Alcohol and type 2 diabetes. A review. Nutrition, Metabolism, and Cardiovascular Diseases, 20(5), 366–375. [DOI] [PubMed] [Google Scholar]
- Polich J (2012). Neuropsychology of P300 In Luck SJ & Kappenman ES (Eds.), The Oxford Handbook of Event-Related Potential Components (pp. 159–188). New York, NY: Oxford University Press. [Google Scholar]
- Price JL, Lewis B, Boissoneault J, Frazier IR, & Nixon SJ (2018). Effects of acute alcohol and driving complexity in older and younger adults. Psychopharmacology, 235(3), 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, & Porjesz B (2014). Understanding alcohol use disorders with neuroelectrophysiology. Handbook of Clinical Neurology, 125, 383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas ET, Laughlin GA, Kritz-Silverstein D, Barrett-Connor E, & McEvoy LK (2016). Moderate, regular alcohol consumption is associated with higher cognitive function in older community-dwelling adults. The Journal of Prevention of Alzheimer’s Disease, 3(2), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. (2010). Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug and Alcohol Review, 29(4), 437–445. [DOI] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D (1993). The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation (2nd ed.). Tucson, Ariz: Neuropsychology Press. [Google Scholar]
- Renaud SC, Gueguen R, Schenker J, & d’Houtaud A (1998). Alcohol and mortality in middle-aged men from eastern France. Epidemiology, 9(2), 184–188. [PubMed] [Google Scholar]
- Richard EL, Kritz-Silverstein D, Laughlin GA, Fung TT, Barrett-Connor E, & McEvoy LK (2017). Alcohol intake and cognitively healthy longevity in community-dwelling adults: The Rancho Bernardo Study. Journal of Alzheimer’s Disease, 59(3), 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, & Rehm J (2012). The cardioprotective association of average alcohol consumption and ischaemic heart disease: A systematic review and meta-analysis. Addiction, 107(7), 1246–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, & Ghali WA (2011). Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. British Medical Journal, 342, d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, & Jacques C (2012). The N170: Understanding the time-course of face perception in the human brain In Luck SJ & Kappenman ES (Eds.), The Oxford handbook of event-related potential components (pp. 115–142). New York (NY): Oxford University Press. [Google Scholar]
- Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, et al. (2002). Alcohol consumption and risk of dementia: The Rotterdam Study. Lancet, 359(9303). [DOI] [PubMed] [Google Scholar]
- Sabia S, Elbaz A, Britton A, Bell S, Dugravot A, Shipley M, et al. (2014). Alcohol consumption and cognitive decline in early old age. Neurology, 82(4), 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Fayosse A, Dumurgier J, Schnitzler A, Empana JP, Ebmeier KP, et al. (2019). Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow-up of Whitehall II cohort study. BMJ, 366, l4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TN, Matlaga BF, & Wykoff MH (1977). Comparative tissue response to six suture materials in rabbit cornea, sclera, and ocular muscle. American Journal of Ophthalmology, 84(2), 224–233. [DOI] [PubMed] [Google Scholar]
- Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, & Beulens JW (2015). The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care, 38(4), 723–732. [DOI] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee G, & Walker M (1988). Alcohol and mortality in British men: Explaining the U-shaped curve. Lancet, 2(8623), 1267–1273. [DOI] [PubMed] [Google Scholar]
- Sklar AL, Boissoneault J, Fillmore MT, & Nixon SJ (2014). Interactions between age and moderate alcohol effects on simulated driving performance. Psychopharmacology, 231(3), 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar AL, Gilbertson R, Boissoneault J, Prather R, & Nixon SJ (2012). Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcoholism, Clinical and Experimental Research, 36(12), 2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth A, Teo KK, Rangarajan S, O’Donnell M, Zhang X, Rana P, et al. (2015). Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: A prospective cohort study. Lancet, 386(10007), 1945–1954. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Arky RA, Hennekens CH, et al. (1988). A prospective study of moderate alcohol drinking and risk of diabetes in women. American Journal of Epidemiology, 128(3), 549–558. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, & Grodstein F (2005). Effects of moderate alcohol consumption on cognitive function in women. New England Journal of Medicine, 352(3), 245–253. [DOI] [PubMed] [Google Scholar]
- Stott DJ, Falconer A, Kerr GD, Murray HM, Trompet S, Westendorp RG, et al. (2008). Does low to moderate alcohol intake protect against cognitive decline in older people? Journal of the American Geriatrics Society, 56(12), 2217–2224. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, & Pfefferbaum A (1995). Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism, Clinical and Experimental Research, 19(1), 110–122. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Ruffman T, & Hutton SB (2007). Age differences in emotion recognition skills and the visual scanning of emotion faces. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 62(1), P53–P60. [DOI] [PubMed] [Google Scholar]
- Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, et al. (2009). Alcohol and hypertension: Gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction, 104(12), 1981–1990. [DOI] [PubMed] [Google Scholar]
- Tiplady B, Drummond GB, Cameron E, Gray E, Hendry J, Sinclair W, et al. (2001). Ethanol, errors, and the speed-accuracy trade-off. Pharmacology, Biochemistry and Behavior, 69(3–4), 635–641. [DOI] [PubMed] [Google Scholar]
- Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, et al. (2017). Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ, 357, j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala A, & Ebmeier KP (2018). Effects of drinking on late-life brain and cognition. Evidence Based Mental Health, 21(1), 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler LA, Hege S, & Ellinwood EH Jr. (1995). Alcohol pharmacodynamics in young-elderly adults contrasted with young and middle-aged subjects. Psychopharmacology, 118(4), 460–470. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Goodlett C, & Matthews DB (2019). Acute alcohol and cognition: Remembering what it causes us to forget. Alcohol, 79, 105–125. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M, & Barrett P (1984). Age, drinking habits and the effects of alcohol. Journal of Studies on Alcohol, 45(6), 517–521. [DOI] [PubMed] [Google Scholar]
- Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, et al. (2002). Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Annals of Internal Medicine, 136(3), 181–191. [DOI] [PubMed] [Google Scholar]
- Wang C, Rajagovindan R, Han SM, & Ding M (2016). Top-down control of visual alpha oscillations: Sources of control signals and their mechanisms of action. Frontiers in Human Neuroscience, 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala C, Murchison C, Loftis JM, Schenning KJ, Mattek N, Woltjer R, et al. (2018). Sex differences in the association of alcohol with cognitive decline and brain pathology in a cohort of octogenarians. Psychopharmacology, 235(3), 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE, Watson ID, & Batt RD (1981). Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. Journal of Studies on Alcohol, 42(7), 547–556. [DOI] [PubMed] [Google Scholar]
- Weafer J, & Fillmore MT (2008). Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology, 201(3), 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, & Fillmore MT (2012). Comparison of alcohol impairment of behavioral and attentional inhibition. Drug and Alcohol Dependence, 126(1–2), 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyerer S, Schaufele M, Wiese B, Maier W, Tebarth F, van den Bussche H, et al. (2011). Current alcohol consumption and its relationship to incident dementia: Results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age and Ageing, 40(4), 456–463. [DOI] [PubMed] [Google Scholar]
- Wolff N, Gussek P, Stock AK, & Beste C (2018). Effects of high-dose ethanol intoxication and hangover on cognitive flexibility. Addiction Biology, 23(1), 503–514. [DOI] [PubMed] [Google Scholar]
- Wong B, Cronin-Golomb A, & Neargarder S (2005). Patterns of visual scanning as predictors of emotion identification in normal aging. Neuropsychology, 19(6), 739–749. [DOI] [PubMed] [Google Scholar]
- Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. (2018). Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet, 391(10129), 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Elkind MS, Luo X, Paik MC, & Sacco RL (2006). Reported alcohol consumption and cognitive decline: The northern Manhattan study. Neuroepidemiology, 27(4), 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HZ, Barry LC, Duan Y, Bohannon RW, Covault JM, & Grady JJ (2017). Acute effects of moderate alcohol consumption on postural stability in older adults. Perceptual and Motor Skills, 124(5), 912–931. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, et al. (2017). Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. European Journal of Epidemiology, 32(1), 31–42. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Ross RK, Gao YT, Henderson BE, & Yu MC (1997). Follow up study of moderate alcohol intake and mortality among middle aged men in Shanghai, China. BMJ, 314(7073), 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccala G, Onder G, Pedone C, Cesari M, Landi F, Bernabei R, et al. (2001). Dose-related impact of alcohol consumption on cognitive function in advanced age: Results of a multicenter survey. Alcoholism, Clinical and Experimental Research, 25(12), 1743–1748. [PubMed] [Google Scholar]
Further reading
- Gureje O, Ogunniyi A, & Kola L (2006). The profile and impact of probable dementia in a sub-Saharan African community: Results from the Ibadan Study of Aging. Journal of Psychosomatic Research, 61(3), 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand BK, Mukamal KJ, & Mittleman MA (2011). Trends in alcohol use among women with and without myocardial infarction in the United States: 1997–2008. Journal of Studies on Alcohol and Drugs, 72(6), 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Gu H, Yan L, Lu H, Wang DJ, Zhou XJ, et al. (2014). Detecting resting-state brain activity by spontaneous cerebral blood volume fluctuations using whole brain vascular space occupancy imaging. NeuroImage, 84, 575–584. [DOI] [PubMed] [Google Scholar]
- Moussa MN, Simpson SL, Mayhugh RE, Grata ME, Burdette JH, Porrino LJ, et al. (2014). Long-term moderate alcohol consumption does not exacerbate age-related cognitive decline in healthy, community-dwelling older adults. Frontiers in Aging Neuroscience, 6, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, & Chikritzhs T (2016). Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. Journal of Studies on Alcohol and Drugs, 77(2), 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JD, Heiser RM, Schooler MP, Brockopp DY, Parshall MB, Cassidy KB, et al. (2002). Characteristics and treatment of patients with heart failure in the emergency department. Journal of Emergency Nursing, 28(2), 126–131. [DOI] [PubMed] [Google Scholar]


