Abstract
Botanical-based natural products are an important resource for medicinal drug discovery and continue to provide diverse pharmacophores with therapeutic potential against cancer and other human diseases. A prototype Traditional Chinese Medicine (TCM) plant extract library has been established at the US National Cancer Institute, which contains both the organic and aqueous extracts of 132 authenticated medicinal plant species that collectively represent the potential therapeutic contents of most commonly used TCM herbal prescriptions. This library is publicly available in 96- and 384- well plates for high throughput screening across a broad array of biological targets, as well as in larger quantities for isolation of active chemical ingredients. Herein, we present the methodology used to generate the library and the preliminary assessment of the anti-proliferative activity of this crude extract library in NCI-60 human cancer cell lines screen. Particularly, we report the chemical profiling and metabolome comparison analysis of four commonly used TCM plants, namely Brucea javanica, Dioscorea nipponica, Cynanchum atratum, and Salvia miltiorrhiza. Bioassay-guided isolation resulted in the identification of the active compounds, and different extraction methods were compared for their ability to extract cytotoxic compounds and to concentrate biologically active natural products.
Keywords: Traditional Chinese Medicine, natural product, NCI-60, anti-proliferative activity
Graphical Abstract

1. Introduction
Traditional Chinese Medicine (TCM) and the accompanied practicing system have evolved over thousands of years in China for the treatment and symptom management of a wide range of medical conditions. The discovery of anti-malarial drug artemisinin (青蒿素, qinghaosu) from the plant Artemisia annua (Asteraceae, 黄花蒿) was originally inspired by a TCM practice recorded more than one thousand years ago, and the award of the 2015 Nobel Prize to Professor Tu Youyou further highlighted the importance of this unique resource for drug discovery [1, 2]. While artemisinin represents an example of single active component from a single plant (A. annua), common TCM practice usually involves the use of a variety of combinations of herbs, animal products, and minerals collectively known as TCM formulae. However, chemically uncharacterized formulae are difficult to standardize and to implement reproducible studies and clinical trials, a factor that has complicated the wide use of TCM and other traditional medicines in monopharmacy-centered modern medical treatment settings. Recent approaches to address these challenges, such as metabolomics and global systems biology, have made a significant impact on understanding the chemical constituents and physiologic pathways involved in TCM efficacy [3]. Through the use of advanced analytical technologies, TCM, as well as other traditional medicines, are currently being explored for their therapeutic potential, societal impact, and general medical practice.
TCM is also an important source of knowledge and source material for study in the field of pharmacognosy. The TCM database@Taiwan [4], a well annotated library of natural products isolated from TCM, currently holds 61,000 distinctive chemical entries. The TCMAnalyzer is a bioinformatics platform incorporating structure, source, and network pharmacology for elucidating the mechanisms of action for TCMs [5]. Biota used as TCM ingredients have shown to be an excellent starting point for the isolation of biologically active and chemically diverse secondary metabolites. Recent examples include (+)- and (−)-linderaspirone A, novel spirocyclopentene dione enantiomers isolated from the TCM plant Lindera aggregata (Lauraceae) [6]; bufospirostenin A and bufogargarizin C, novel steroids with re-arranged A/B rings isolated from the bile and the venom of the TCM toad Bufo bufo gargarizans [7]; and cycloviolacin Y5, an anti-HIV cyclotide isolated from the TCM plant Viola yedoensis (Violaceae) [8]. Moreover, TCM plant-associated endophytes have been investigated as unique and under-explored environments for the isolation of microbes producing unique natural products. For example, the divergolides A-D are novel macrolides isolated from Streptomycetes sp. HKI0576, an endophyte from the TCM mangrove tree Bruguiera gymnorrhiza (Rhizophoraceae) [9].
The primary access to TCM biota for screening and isolation purposes is either from on-site collections or through the purchase of ready-made TCM from specialty vendors. With increasing interest in the identification of the active principles of traditional medicines, well-annotated, publicly-available extract libraries are critical for the advancement of this research field. In early 2000s, NCI joined Harvard Medical School, Beijing University of Chinese Medicine, and Hong Kong Baptist University to co-fund a group of US and Chinese scientists to establish a prototype TCM specimen library, which contained over 200 authenticated medicinal plant and fungal species that collectively represent the therapeutic content of the majority of commonly prescribed TCMs [10]. The library was housed at Harvard University for years with limited public access where its value had not been fully realized. Recently NCI decided to further process a subset of this library and incorporate the resulting extracts into the Natural Products Repository, one of the world’s largest collections of natural products with over 230,000 unique extracts derived from plants, marine organisms, and microbes [11]. The majority of ~80,000 plant samples in this repository were collected from Africa, Central and South America, and Southeast Asia. Addition of the authenticated TCM plant library from China would further increase the diversity of the plant species as well as intrinsic chemical/structural diversity of this national resource of natural products.
Herein, we present the methodology used to generate this extract library and the preliminary assessment of the biological activity in the NCI-60 Human Tumor Cell Line Screen. Chemical identification the active components of four most NCI-60 active TCM species was also conducted namely Brucea javanica (Simaroubaceae), Dioscorea nipponica (Dioscoreaceae), Cynanchum atratum (Apocynaceae), and Salvia miltiorrhiza (Lamiaceae), and differences in extraction efficiency between different processing methods compared. Where applicable and the active principles could be detected in all three different extracts, we conducted a metabolomic analysis or quantified the active principles. This work is presented as a proof-of-concept study and serves to present the potential of this free public resource to the scientific community around the world.
2. Materials and Methods
2.1. TCM plant collection
Collection and authentication of the plant material comprising the NCI library of Traditional Chinese Medicinal Plant Extracts have been reported previously [10].
2.3. Generation of the organic and aqueous extracts of TCM plants
All the TCM plants have been processed using the NCI standard natural products extraction method [12]. In brief, the procedure starts with grinding the plant material to a fine powder with 80% particles in the 0.5–2.0 mm size range. An initial overnight extraction in 1:1 DCM:MeOH combined with a MeOH wash was used to generate the organic extract, followed by an overnight soak in 1:9 MeOH:H2O to generate the aqueous extract. Only 8 of the most active plants in the NCI-60 assay were subjected to the hot water extraction protocol, which was adapted from the traditional TCM preparation methods. The procedure included soaking the grounded particles in cold water for 30 minutes, followed by boiling at 100 °C for 30 minutes, filtering the extract, and a repeat boiling extraction for another 30 minutes. The two hot water extracts were then combined, filtered, and freeze dried.
2.3. General analytical procedures
NMR spectra were recorded at 25 °C on either a 500 MHz Bruker Avance spectrometer, equipped with a triple resonance 5mm CPTCI cryo-probe or a 400 MHz Agilent Inova spectrometer equipped with a 5 mm OneNMR probe. NMR spectral processing and data interpretation was done using MestReNova software, version 8.0. Ultra-Performance Liquid Chromatography (UPLC) high resolution mass spectral (HRMS) data were acquired using on a Waters Acquity UPLC system coupled to a Waters LCT Premier TOF mass spectrometer with an electrospray ionization source. Preparative HPLC was run on a Waters Prep LC 4000 system, equipped with a Delta 600 pump and a 996-diode array detector, using a Phenomenex Kinetex C8 or C18 column [5 μ, 150 × 21.2 mm]. All solvents used for chromatography, UV, and MS were GC/LC-MS or HPLC grade, and the H2O for preparative HPLC was Millipore Milli-Q PF filtered.
2.4. Bioassay-guided natural product isolation
2.4.1. Brucea javanica
A portion of the organic solvent extract N500561 (469 mg) was subjected to preparative HPLC using a Phenomenex Kinetex C8 [5 μm, 150 × 21.2 mm] HPLC column, eluting with a steep gradient from methanol/water (1:9) to methanol. Isocratic conditions at methanol/water (1:9) were employed from time 0 to 5 min, followed by a linear gradient to methanol over 35 min, and finally isocratic methanol for another 8 min; all at a flow rate of 10 mL/min. 48 fractions were collected in one minute increments starting from time zero. The fractions were tested in the NCI-60 one dose assay at 2% of volume of each fraction. Active fractions 14, 15, 20, 21, and 25–28 (combined mass = 5.9 mg) were further purified on a Phenomenex Kinetex C8 [5 μm, 150 × 21.2 mm] HPLC column, eluting with a linear gradient from methanol/water (1:9) to methanol/water (4:6). Isocratic conditions at methanol/water (1:9) were employed from time 0 to 5 min, followed by a linear gradient to methanol/water (4:6) over 35 min, and finally isocratic methanol/water (4:6) for another 8 min; all at a flow rate of 15 mL/min. Ninety six fractions were collected in thirty second increments starting from time = 0 min. Fraction 25 yielded bruceine D (2, 2.0 mg, 0.43% crude extract yield) as a white powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [13]; fraction 64 yielded brusatol (3, 0.8 mg, 0.17% crude extract yield) as a white powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [14]; fraction 73 yielded bruceine A (1, 0.9 mg, 0.19% crude extract yield) as a white powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [15]; and fraction 76 yielded bruceantinol (4, 0.6 mg, 0.13% crude extract yield) as a white powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [16].
2.4.2. Dioscorea nipponica
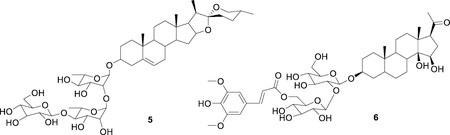
A portion of the organic solvent extract N500157 (200 mg) was subjected to reverse-phase HPLC using a Phenomenex Kinetex C8 [5 μm, 150 × 21.2 mm] HPLC column, eluting with a steep gradient from acetonitrile/water with 0.1% formic acid (1:9) to acetonitrile with 0.1% formic acid. Isocratic conditions at acetonitrile/water with 0.1% formic acid (1:9) were employed from time 0 to 5 min, followed by a linear gradient to acetonitrile with 0.1% formic acid over 50 min, and finally isocratic methanol for another 10 min; all at a flow rate of 10 mL/min. Forty-eight fractions were collected at one-minute intervals, starting from time = 0 min. The fractions were tested in the NCI-60 one dose assay at 2% of volume of each fraction. Active fraction 39 (weight = 34 mg) was purified on a Phenomenex Kinetex C8 [5 μm, 150 × 21.2 mm] HPLC column, eluting with a steep gradient from acetonitrile/water with 0.1% formic acid (25:75) to acetonitrile/water with 0.1% formic acid (35:65). Isocratic conditions at acetonitrile/water with 0.1% formic acid (25:75) were employed from time 0 to 5 min, followed by a linear gradient to acetonitrile/water with 0.1% formic acid (35:65) over 50 min, and finally isocratic acetonitrile/water with 0.1% formic acid (35:65) for another 10 min; all at a flow rate of 10 mL/min. 127 fractions were collected in thirty second increments starting from time = 0 min. Fractions 74–79 yielded dioscin (5, 4.0 mg, 7.0 % crude extract yield) as a white powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [17].
2.4.3. Cynanchum atratum
A portion of the organic solvent extract N500039 (100 mg) was subjected to preparative HPLC using a Phenomenex Kinetex C8 [5 μm, 150 × 21.2 mm] column at a flow rate of 10 mL/min with the following conditions: an initial isocratic hold at methanol/water (10:90) for 5 min, followed by a linear gradient to methanol over 35 min, and a final hold at 100% methanol for 10 min. A total of 48 fractions were collected in one-minute increments starting from time = 0 min. and tested at a single dose (2.5% v/v) in the NCI-60 assay. Additional material was obtained from two successive preparative HPLC collections for a total of 300 mg of processed organic extract material. The major active fraction 26 was pooled (m = 8.5 mg) and further purified on a Phenomenex Kinetex C18 [5 μm, 150 × 21.2 mm] HPLC column at a flow rate of 10 mL/min with the following conditions: an initial isocratic hold at methanol/water (20:80) for 5 min, followed by a linear gradient to methanol/water (60:40) over 25 min, and a final hold at 60% methanol for 10 min. A total of 150 fractions were collected in 20 second increments starting from time = 0 min. Fraction 88 was identified as hancoside A (6; 0.5 mg; 0.17% isolation yield) with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [18].
2.4.4. Salvia miltiorrhiza
A portion of the organic solvent extract N500641 (1 g) was subjected to C18 flash chromatography on a Teledyne Isco CombiFlash Rf system using a 100 g HP C18 RediSep Rf Gold cartridge, eluting with a steep gradient from water to acetonitrile over 20 min, followed by isocratic acetonitrile for another 6 min; all at a flow rate of 40 mL/min. Forty eight fractions were collected in 30 second increments starting from time = 2 min. Fractions 35–45 combined were re-run using a 100 g HP C18 RediSep Rf Gold cartridge, eluting with a steep gradient from acetonitrile/water (65:35) to acetonitrile over 40 minutes where isocratic acetonitrile/water (65:35) conditions were used the initial 16 min, followed by a steep gradient to acetonitrile/water (9:1) over 15 min, followed by a steep gradient to acetonitrile over 4 min, and finally isocratic acetonitrile for 5 min; all at a flow rate of 40 mL/min. Seventy six fractions were collected in 30 second increments starting from time = 2 min. Fractions 17–20 yielded 15,16-dihydrotanshinone (10, 4.6 mg, 0.46% crude extract yield) as a yellow powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [19]; fractions 26–28 yielded cryptotanshinone (9, 28.0 mg, 2.8% crude extract yield) as a yellow powder with chiro-optical, spectroscopic and spectrometric data in agreement to those previously reported [19]; fractions 35–37 yielded semi-pure tanshinone I (7, 15.9 mg, 1.6% crude extract yield) as a yellow powder with spectroscopic and spectrometric data in agreement to those previously reported [19]; and fractions 45–48 yielded tanshinone IIA (8, 25.5 mg, 2.5% crude extract yield) as a yellow powder with spectroscopic and spectrometric data in agreement to those previously reported [19].
2.5. Metabolomic analysis of B. javanica extracts
Samples were prepared at a final concentration of 500 μg/mL in methanol with an internal reference standard of reserpine (2 μg/mL). A 10 μL injection volume of each sample was performed and samples were profiled using a Phenomenex Kinetex C8 UPLC column [2 μm, 50 × 2.1 mm] at a flow rate of 0.6 mL/min and the following conditions: an initial isocratic hold at water (0.1% formic acid) for 2 min, followed by a linear gradient to acetonitrile (0.1% formic acid) over 20 min, and a final isocratic hold at acetonitrile (0.1% formic acid) for 2 min. Peak detection, chromatogram building, isotope removal and final alignment via the Random Sample Consensus (RANSAC) algorithm were performed using MZmine 2 with the default parameters and a minimum height (baseline) setting of 1 × 104.
2.6. Qualitative Analysis of D. nipponica extracts
Qualitative determination and identification of the relative amounts of dioscin (Mw = 868) and analogs Mw = 1030 and 1046 in extracts were done using a Sedere Sedex 85 Evaporative Light Scattering Detector (ELSD). Samples were prepared at a final concentration of 500 μg/mL in methanol injecting 10 μL on the column. A 10 μL injection volume of each sample was performed and samples were profiled using a Phenomenex Kinetex C8 UPLC column [2 μm, 50 × 2.1 mm] at a flow rate of 0.6 mL/min and the following conditions: an initial isocratic hold at water (0.1% formic acid) for 1 min, followed by a linear gradient to acetonitrile (0.1% formic acid) over 13 min, and a final isocratic hold at acetonitrile (0.1% formic acid) for 3 min. Identification of dioscin and analogs were determined using the mass spectrometer and qualitative analysis were done using the ELSD, by comparing the area under the corresponding peaks.
3. Results and discussion
3.1. Access to the NCI library of Traditional Chinese Medicinal Plant Extracts
Each TCM plant has been processed using the standard NCI extraction method for both organic and aqueous extracts [12] (For a list of TCM plants and extraction methodologies used, see Supporting Information Table S1). The final NCI TCM plant extract library contains 664 extracts derived from 332 samples that represent 132 distinct TCM plant species. The extracts are available in 96 and 384 well plates, ready for high throughput screening across a broad array of disease targets. Requests for access to this national resource can be submitted online at https://dtp.cancer.gov/organization/npb/tcm_extracts.htm.
3.2. In vitro anti-proliferative activity of the TCM plant extract library
NCI-60 human tumor cell lines screening [21] of all of the extracts was conducted as the primary assessment of the biological activity of this TCM extract library (Figure 1). Initially, all the organic and aqueous extracts have been assessed for their in vitro cytotoxic activities at a single dose of 100 μg/mL. Of 664 extracts screened, 84 (12%) reached LC50 level against at least 3 different tumor cell lines and were then tested in a full 5-dose response assay. In the 5-dose NCI-60 assay, 21 extracts (3%) were found to exhibit significant cytotoxic activity, representing 17 distinct collection samples with specific part and/or collection location from 8 different TCM plant species.
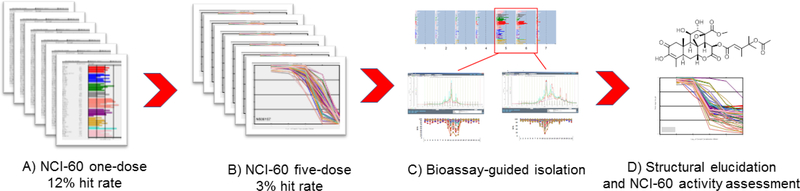
Figure 1.
TCM extract library biological and chemical assessment workflow. A) 664 extracts were tested in the NCI-60 one-dose screen at a concentration of 100 μg/mL. An extract was considered active if more than 3 cell lines achieved cytotoxic activity at the LC50 level. B) 21 extracts passed one-dose activity criteria and were tested in the NCI-60 five-dose screen. An extract was considered active if more than three cell lines achieved growth inhibitory activity at GI50 at a concentration of 10 μg/mL. C) Bioassay-guided isolation was conducted on four NCI-60 active extracts. While the organic, aqueous, and hot water extracts were chemically profiled by LCMS, the bulk of the bioassay-guided fractionation was performed on the organic extract. D) The structures of the isolated active principles were elucidated, and all pure compounds were tested in the NCI-60 five-dose assay.
According to traditional TCM prescriptions, most TCM herbs are extracted with boiled water either along or combined with one or more other herbs based upon the TCM theory of Jun (emperor), Chen (minister), Zuo (assistant) and Shi (courier) and empirical experiences [22]. To compare the historical TCM processing approach with the NCI extraction methods, additional raw materials of the 8 NCI-60 active species were processed again using a hot water extraction protocol adapted from the traditional TCM preparation methods. The extracts were then re-assessed in 5-dose NCI-60 screening, and only those of two species were found to be active (Brucea javanica and Dioscorea nipponica, see details below).
3.3. Chemical and biological characterization of selected TCM species
Four of TCM plant species that exhibited significant in vitro cytotoxicity in 5-dose NCI-60 screening, namely Brucea javanica, Dioscorea nipponica, Cynanchum atratum, and Salvia miltiorrhiza, were subjected to bioassay-guided isolation and the active principles identified upon comparison of spectral data with literature values. In addition, comparative studies of the organic, aqueous, and hot water extraction methods were done to assess their ability to extract and concentrate biologically active natural products.
3.3.1. Brucea javanica
Dried ripe fruit of B. javanica was collected from Huazhou, Guangdong Province in China. The fruit is officially listed in the Chinese Pharmacopoeia and used in the treatment of malaria, dysentery, as well as the topical treatment of warts and corns [23]. In the NCI-60 cell five-dose assay, the organic extract was the most potent identified with an GI50 value of 0.76 μg/mL, while the aqueous and the hot water extracts showed GI50 values of 9.54 μg/mL and 1.45 μg/mL respectively. Notably, the growth inhibition of the hot water extract was only 0.28 log units less potent than that observed for the organic extract. Bioassay-guided isolation identified the active principles of the organic B. javanica extract to be the known quassinoids bruceine A (1), and D (2), brusatol (3), and bruceantinol (4).
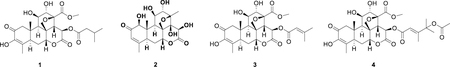
Compounds 1, 3, and 4 showed nanomolar NCI-60 activity, with GI50 values of 87.1, 42.6, and 30.1 nM respectively, while bruceine D (2) lacking a hydroxyl group on C-3 and a small fatty acid ester substituent on C-15, was shown to be significantly less active with a GI50 value of 1.0 μM. These, and other quassinoids have been reported to have potent anti-inflammatory, anti-viral, anti-malarial, as well as anti-proliferative activities [24]. Bruceantin, a C-15 3,4-dimethyl-2-pentenoic acid ester analogue, has been evaluated in human phase I and II clinical trials against various solid tumors but failed due to high toxicity and poor efficacy [24, 25]. This compound has been shown to be an irreversible protein synthesis inhibitor in HeLa cells [26]. Due to inherent toxicity and lack of in vivo anti-tumor activity for this structural class, no further work was conducted on the isolated natural products.
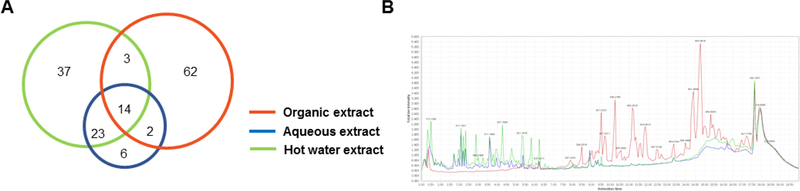
A UPLC-MS metabolomic analysis of the three extraction procedures used to evaluate extracts of B. javanica is presented in Figure 2. A Venn diagram (Figure 2A) of the resulting data shows a significant amount of overlap between all three extracts with the organic extract having the most detected compounds, as well as the greatest number of unique components (77% of total TIC peak count), while the two water extracts had 82% of all peaks in common. Figure 2B shows the organic extract occupying the mid-polarity to non-polar region of the chromatogram, while the secondary metabolite load of the aqueous and the hot water extracts was mainly concentrated in the polar to mid polarity regions of the UPLC chromatogram. All four active principles (1 – 4) isolated from the organic extract were also detected in the aqueous and the hot water extracts, which is likely due to their stability and mid polarity clogP values ranging between −2 and 0.7 (For calculated physicochemical properties of all the natural products isolated in this study, see SI Figure S2).
Figure 2.
Brucea javanica chemical analysis. A) Venn diagram showing the overlap of the secondary metabolites detected by (+)-ESI TIC. Circle area is relative to the number of compounds detected in each extract. B) Reversed-phase C8 UPLC (+)-ESI TIC chromatograms of the three extracts, with the organic extract shown in orange, the aqueous in blue, and the hot water in green.
3.2.2. Dioscorea nipponica
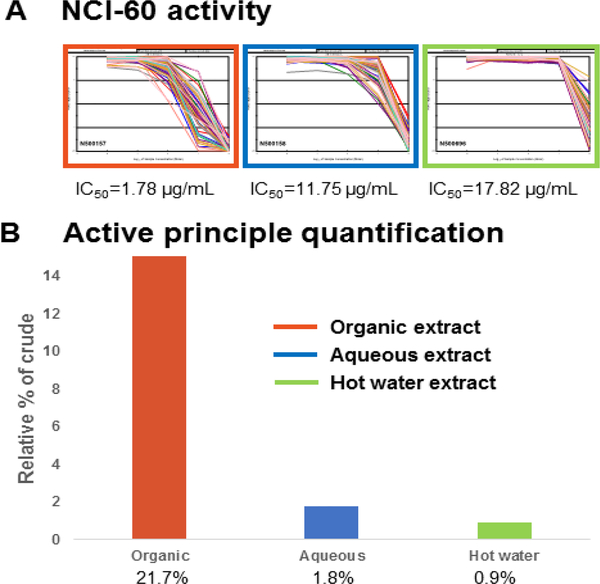
Dried rhizome of D. nipponica was collected from Luanping County, Hebei Province in China. The genus is listed in the Chinese Pharmacopoeia for use in the treatment of diarrhea, asthma, polyuria, diabetes, and rheumatic disease [27]. Furthermore, Di’ao Xinxuekang® capsule, extracted from the roots of Dioscorea opposita, was the first TCM agent approved for use in Europe by the Dutch Medicines Evaluation Board [28]. In the NCI-60 cell five-dose assay, all three D. nipponica extracts showed anti-proliferative activity, with the organic extract most active, followed by the aqueous and the hot water extracts, with GI50 values of 1.78, 11.75, and 17.82 μg/mL, respectively (Figure 3A). Bioassay-guided isolation identified the major NCI-60 active principle in the organic extract to be the steroidal saponin dioscin (5), together with two other related saponins with molecular weights 1030 and 1046 amu. Compound 5 showed modest growth inhibitory activity with an NCI-60 five dose GI50 value of 31.62 μM. Figure 3 shows a comparison of the NCI-60 activity with the amount of saponins found in the three D. nipponica extracts. Compound 5 and related analogs did not exhibit a strong UV chromophore, therefore quantitative analysis was done on a UPLC-based system utilizing the evaporative light scattering detector (ELSD). This methodology has been shown to be applicable for a range of structurally diverse natural products and was able to detect small molecules present in different yields in a crude extract or a fraction of the crude extract [29]. The anti-proliferative activity of D. nipponica was found to be correlated to the amount of saponins detected in the extracts. The organic extract, exhibiting the most potent NCI-60 activity, was determined to contain the highest concentration of dioscin and analogs (21.7% crude weight), while the hot water extract, showing the least potent NCI-60 activity, had the lowest amount of saponins (0.9% crude weight) detected. The clogP value for dioscin at 1.3 (SI, Table S2) is in the mid-polarity range, yet the aqueous and the hot water extracts were found to have significantly less of this compound when compared to the organic extract. The three D. nipponica extraction procedures shown here demonstrate that the organic solvent extraction method gave the best yields for large molecular weight steroid saponins, such as dioscin.
Figure 3.
NCI-60 crude extract activity and saponin quantification of the active principles in the three Dioscorea nipponica extracts. A) NCI-60 five dose all cells plot for the organic (in orange rectangle), aqueous (blue rectangle), and the hot water (green rectangle) extracts. B) Total saponin quantification results.
3.2.3. Cynanchum atratum
Dried root and rhizome of C. atratum were collected from Taibai, Shanxi Province in China. The genus is listed in the Chinese Pharmacopoeia for the treatment of cough and asthma [30]. In the NCI-60 cell five-dose assay, all three C. atratum extracts showed potent activity, with the organic extract most active, followed by the hot water and aqueous extracts, with GI50 values of 0.16, 2.40, and 3.02 μg/mL, respectively. Bioassay-guided isolation identified the major NCI-60 active principle in the organic extract to be the steroidal glycoside hancoside A (6). Compound 6 exhibited low micromolar cytotoxicity against the NCI-60 panel with a GI50 value of 2.45 μM. As with D. nipponica and diosicin, here the extraction of large molecular weight triterpene saponins was shown to be most effective using organic solvents.
3.2.4. Salvia miltiorrhiza
Dried root and rhizome of S. miltiorrhiza was collected from Xi Xia, Henan Province in China. The species is listed in the Chinese Pharmacopoeia for the treatment of blood circulation disorders, menstrual bleeding disorders, inflammation, and angina pectoris [31]. In addition, the plant is a component of many TCM formulae, including Realgar-Indigo naturalis comprised of realgar, Indigo naturalis and S. miltiorrhiza, a TCM used for the treatment of promyelocytic leukemia [32]. In the NCI-60 assay, only the organic solvent extract showed activity at one-dose, while the aqueous and the hot water extracts did not show any growth inhibitory activity at 100 μg/mL in the entire panel. In the five-dose assay, S. miltiorrhiza organic extract showed moderate activity with a GI50 value of 50 μg/mL. Bioassay-guided isolation identified the major NCI-60 active principles in the organic extract to be the phenanthro[1,2-b]furan-10,11-dione analogues tanshinone I (7), tanshinone IIA (8), cryptotanshinone (9), and 15,16-dihydrotanshinone (10).
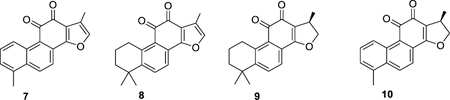
Compounds 8-10 showed low micro molar NCI-60 activity, with GI50 values of 3.72, 2.69, and 2.76 μM respectively, while tanshinone I was found to be unsuitable under reversed-phase chromatography conditions and was not tested due to unsatisfactory purity levels. Commercially available tanshinone I (Sigma-Aldrich) showed an NCI-60 GI50 value of 2.19 μM and exhibited an NCI-60 profile consistent with the crude organic extract of S. miltorrhiza and compounds 8-10. Tanshinone I, tanshinone IIA, and cryptotanshinone are known to have low aqueous solubility and bioavailability [33], as well as high clogP values, the most likely reason why the organic solvent extraction method was the only one shown effective at concentrating these types of natural products.
4. Conclusion
The crude extract library of an authentic collection of TCM herbs described here has been developed to facilitate systematic biological evaluation of the therapeutic potential of traditional Chinese medicines. It is a new addition to the NCI Natural Product Repository, freely available to investigators who are interested in exploring the biological activity and chemical constituents of TCM plants. In a proof-of-concept study of the anti-proliferative activity of this library, 3% of the total extracts, representing 8 out of the 132 total species, have shown significant in vitro cytotoxicity in the NCI-60 human tumor cell lines screening. The standard NCI organic extraction procedure appears to be the most effective approach in terms of extracting NCI-60 active principles from the TCM plants. As NCI-60 activities of most of the plant species in this library have not been reported before, it is likely that additional biological activities might be identified by researchers investigating this extract library using different bioassay endpoints as well. It is hoped that this prototype TCM plant extract library will promote scientific discoveries with therapeutic potentials and enhance international efforts to systematically evaluate commonly used TCM herbal therapies.
Supplementary Material
Acknowledgments
We are grateful to Harvard Medical School, Beijing University of Chinese Medicine, and Hong Kong Baptist University for sharing the TCM plant collections. We thank the Molecular Pharmacology Branch, DTP, DCTD, NCI for performing the NCI 60-cell cytotoxicity assays in support of this study. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tu Y, The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med, 2011. 17(10): p. 1217–20. [DOI] [PubMed] [Google Scholar]
- 2.Tu Y, Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew Chem Int Ed Engl, 2016. 55(35): p. 10210–26. [DOI] [PubMed] [Google Scholar]
- 3.Qiu J, ‘Back to the future’ for Chinese herbal medicines. Nat. Rev. Drug Discovery, 2007. 6(7): p. 506–507. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY-C, TCM Database Taiwan: the world’s largest traditional Chinese medicine database for drug screening in silico. PLoS One, 2011. 6(1): p. e15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, et al. , TCMAnalyzer: A Chemo- and Bioinformatics Web Service for Analyzing Traditional Chinese Medicine. J. Chem. Inf. Model, 2018. 58(3): p. 550–555. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, et al. , A Pair of Windmill-Shaped Enantiomers from Lindera aggregata with Activity toward Improvement of Insulin Sensitivity. Org. Lett, 2010. 12(14): p. 3196–3199. [DOI] [PubMed] [Google Scholar]
- 7.Tian H-Y, et al. , Bufospirostenin A and Bufogargarizin C, Steroids with Rearranged Skeletons from the Toad Bufo bufo gargarizans. J. Nat. Prod, 2017. 80(4): p. 1182–1186. [DOI] [PubMed] [Google Scholar]
- 8.Wang CKL, et al. , Anti-HIV Cyclotides from the Chinese Medicinal Herb Viola yedoensis. J. Nat. Prod, 2008. 71(1): p. 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding L, et al. , Divergolides A-D from a mangrove endophyte reveal an unparalleled plasticity in ansa-macrolide biosynthesis. Angew. Chem., Int. Ed, 2011. 50(7): p. 1630–1634, S1630/1-S1630/23. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg DM, et al. , Developing a library of authenticated Traditional Chinese Medicinal (TCM) plants for systematic biological evaluation--rationale, methods and preliminary results from a Sino-American collaboration. Fitoterapia, 2011. 82(1): p. 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown EC and Newman DJ, The US national cancer institute’s natural products repository; origins and utility. J. Environ. Monit, 2006. 8(8): p. 800–805. [DOI] [PubMed] [Google Scholar]
- 12.McCloud TG, High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules, 2010. 15: p. 4526–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, et al. , Bruceines D, E and H. J. Heterocycl. Chem, 1989. 26(2): p. 493–501. [Google Scholar]
- 14.Harigaya Y, et al. , Spectroscopic studies of brusatol. J. Nat. Prod, 1989. 52(4): p. 740–8. [Google Scholar]
- 15.Phillipson JD and Darwish FA, Bruceolides from Fijian Brucea javanica. Planta Med, 1981. 41(3): p. 209–20. [DOI] [PubMed] [Google Scholar]
- 16.Kupchan SM, et al. , Tumor inhibitors. 100. Isolation and structural elucidation of bruceantin and bruceantinol, new potent antileukemic quassinoids from Brucea antidysenterica. J. Org. Chem, 1975. 40(5): p. 648–54. [DOI] [PubMed] [Google Scholar]
- 17.Huang H-L, Liu R-H, and Shao F, Structural determination of two new steroidal saponins from Smilax china. Magn. Reson. Chem, 2009. 47(9): p. 741–745. [DOI] [PubMed] [Google Scholar]
- 18.Konda Y, et al. , Two New Glycosides, Hancoside and Neohancoside A, from Cynanchum hancockianum. Journal of Natural Products, 1992. 55(10): p. 1447–1453. [Google Scholar]
- 19.Lee S-Y, Choi D-Y, and Woo E-R, Inhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza Bunge. Arch. Pharmacal Res, 2005. 28(8): p. 909–913. [DOI] [PubMed] [Google Scholar]
- 20.Harris ESJ, et al. , Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci. Total Environ, 2011. 409(20): p. 4297–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoemaker RH, The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer, 2006. 6(10): p. 813–823. [DOI] [PubMed] [Google Scholar]
- 22.Qiu J, Traditional medicine: A culture in the balance. Nature (London, U. K.), 2007. 448(7150): p. 126–128. [DOI] [PubMed] [Google Scholar]
- 23.Tang W and Eisenbrand G, Brucea javanica (L.) Merr. Chinese Drugs of Plant Origin. Springer, Berlin, Heidelberg, (1992): p. 207–222. [Google Scholar]
- 24.Fiaschetti G, et al. , Quassinoids: from traditional drugs to new cancer therapeutics. Curr. Med. Chem, 2011. 18(3): p. 316–328. [DOI] [PubMed] [Google Scholar]
- 25.Cuendet M and Pezzuto JM, Antitumor Activity of Bruceantin: An Old Drug with New Promise. J. Nat. Prod, 2004. 67(2): p. 269–272. [DOI] [PubMed] [Google Scholar]
- 26.Liao L-L, Kupchan SM, and Horwitz SB, Mode of action of the antitumor compound bruceantin, an inhibitor of protein synthesis. Mol. Pharmacol, 1976. 12(1): p. 167–76. [PubMed] [Google Scholar]
- 27.Tang W and Eisenbrand G, Dioscorea spp.. Chinese Drugs of Plant Origin. Springer, Berlin, Heidelberg, 1992: p. 459–474. [Google Scholar]
- 28.Chao J, et al. , Major achievements of evidence-based traditional Chinese medicine in treating major diseases. Biochem. Pharmacol. (Amsterdam, Neth.), 2017. 139: p. 94–104. [DOI] [PubMed] [Google Scholar]
- 29.Adnani N, Michel CR, and Bugni TS, Universal Quantification of Structurally Diverse Natural Products Using an Evaporative Light Scattering Detector. J. Nat. Prod, 2012. 75(4): p. 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang W and Eisenbrand G, Cynanchum glaucescens (Decne.) Hand.-Mazz Chinese Drugs of Plant Origin. Springer, Berlin, Heidelberg: p. 417–428. [Google Scholar]
- 31.Tang W and Eisenbrand G, Salvia miltiorrhiza Bge Chinese Drugs of Plant Origin. Springer, Berlin, Heidelberg: p. 891–902. [Google Scholar]
- 32.Wang L, et al. , Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. U. S. A, 2008. 105(12): p. 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, et al. , Enhancement of solubility and dissolution rate of cryptotanshinone, tanshinone I and tanshinone IIA extracted from Salvia miltiorrhiza. Arch Pharm Res, 2012. 35(8): p. 1457–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.