Abstract
Purpose
To enable rigid body motion‐tolerant parallel volumetric magnetic resonance imaging by retrospective head motion correction on a variety of spatiotemporal scales and imaging sequences.
Theory and methods
Tolerance against rigid body motion is based on distributed and incoherent sampling orders for boosting a joint retrospective motion estimation and reconstruction framework. Motion resilience stems from the encoding redundancy in the data, as generally provided by the coil array. Hence, it does not require external sensors, navigators or training data, so the methodology is readily applicable to sequences using 3D encodings.
Results
Simulations are performed showing full inter‐shot corrections for usual levels of in vivo motion, large number of shots, standard levels of noise and moderate acceleration factors. Feasibility of inter‐ and intra‐shot corrections is shown under controlled motion in vivo. Practical efficacy is illustrated by high‐quality results in most corrupted of 208 volumes from a series of 26 clinical pediatric examinations collected using standard protocols.
Conclusions
The proposed framework addresses the rigid motion problem in volumetric anatomical brain scans with sufficient encoding redundancy which has enabled reliable pediatric examinations without sedation.
Keywords: distributed and incoherent sampling, image reconstruction, magnetic resonance, motion correction, parallel imaging
1. INTRODUCTION
Tolerance against motion is desirable in magnetic resonance imaging (MRI). This includes brain MRI, where significant motion‐induced image degradation prevalence has been documented1 and high‐resolution imaging quality may be compromised by head motion.2 Rigid‐body MRI motion correction3, 4 can be tackled via prospective or retrospective techniques. Prospective techniques5 compare advantageously in terms of spin‐history, dephasing confounders, or k‐space density guarantees. Particularly, optical tracking systems have been proposed for head motion estimation, with corrections showing impressive accuracy and latency.6 However, prospective methods require additional hardware and/or scanner modifications, often involving intrusive markers attached to the subject. In addition, satisfactory corrections may not always be possible due to unpredictability or complexity of motion, or maximized sampling efficiency requirements. Retrospective techniques may facilitate scanning or improve prospective results,7 particularly for 3D encodings, where spin‐history is less of a problem, and when using nonlinear reconstruction paradigms8 to deal with nonhomogeneous sampling density after motion.
Motion compensation is strongly dependent on motion estimation from the measured information. Some methods have proposed the use of navigators, where surrogate motion‐sensitive information is interleaved with the main acquisition and correction is applied either prospective or retrospectively.9, 10, 11 Due to variability in time requirements for different MRI sequences, application of a given navigator is usually limited to a specific sequence type. Furthermore, particular care has to be taken to prevent spin‐history or saturation and, sometimes, scanning efficiency may be compromised. Alternatively, sequences can be constructed with relative resilience to motion or, similarly, sampling schemes can be designed to function as implicit navigators. This is the case for spiral and radial trajectories,12, 13, 14 where temporally distributed low‐resolution information is used for motion estimation, with retrospective corrections usually grounded on an intermediate reconstruction of fully formed images for each motion state, often involving nonlinear methods. Finally, other approaches have explored the redundancy of the information sensed by parallel MRI to detect and discard localized inconsistencies in k‐space measurements,15 usually requiring prior image models to limit noise amplification and improve inconsistency detection.16
Building on models of MRI acquisition in the presence of motion,12, 17 some methods have proposed formulations for motion estimation from the k‐space that do not require navigators.18, 19, 20 Our previous work20 introduced a data‐driven reconstruction method for retrospective multi‐shot rigid‐body motion correction or aligned reconstruction taking advantage of the encoding redundancy in the measured data. Performed simulations showed that the ability to solve the aligned reconstruction problem is strongly sensitive to the k‐space encoding order, which suggested that opportunities exist to maximize the sensitivity to motion by appropriate sampling order designs. Consequently, in this paper we introduce the Distributed and Incoherent Sample Orders for Reconstruction Deblurring using Encoding Redundancy (DISORDER) framework as a flexible way to correct for head motion on a variety of spatiotemporal scales and imaging contrasts by optimizing the sample orders for k‐space coverage. In addition, we propose some technical refinements to the aligned reconstruction formulation and extend the simulation domain. The technique is implemented on a 3 T scanner and tested on controlled motion scans and pediatric examinations including magnetization‐prepared rapid acquisition gradient echo (MP‐RAGE), fast spin echo (FSE), fluid attenuated inversion recovery (FLAIR), spoiled gradient echo (SPGR), and balanced steady‐state free precession (bSSFP) sequences. A matlab implementation to reproduce the experiments is made available at https://github.com/mriphysics/DISORDER/releases/tag/1.1.0.
2. THEORY
2.1. Aligned reconstruction
Assuming whitened measurement noise,21 the aligned reconstruction for parallel volumetric imaging can be formulated as:
| (1) |
where x is the image to be reconstructed, θ are the motion parameters, r is the loss function, y is the measured k‐space data, T is a set of rigid motion transformations, S are the coil sensitivities, is the discrete Fourier transform (DFT), and A is a sampling mask. We are interested in reconstructing a 3D image of size with the number of voxels along dimension d from C‐element coil array samples of a discretized k‐space grid of size K. denotes the number of samples within segment m and M is the number of segments in the sequence, with each segment associated to a specific motion state. Detailed information about the terms in Equation 1 can be found in.20 Here we provide a brief description of their structure:
y is a N × 1 vector.
A is a N × KMC block matrix comprising submatrices of size whose entries take the value 1 if the sample e of the segment m corresponds to the k‐space location indexed by k and 0 otherwise.
is a KMC × VMC block diagonal matrix comprising submatrices of size K × V representing 3D DFT's with applied k‐space sampling.
S is a VMC × VM block matrix comprising diagonal submatrices of size V × V whose diagonal elements correspond to the spatial sensitivity of the coil c.
T is a VM × V block matrix comprising unitary22 submatrices of size V × V corresponding to the 3D rigid transformation modeling the motion state m by three translations and three Euler rotation angles codified in the parameter vector .
x is a V × 1 vector.
Equation 1 is a separable nonlinear least squares problem.23, 24 We confront it by iteratively addressing the subproblems:
| (2) |
The first subproblem, reconstructing the image x in the presence of rigid motion,17 can be solved by conjugate gradient (CG).21 As for the second, the solution must null the gradient of the objective function against the motion parameters,20 which is tackled by a Levenberg‐Marquardt (LM) algorithm using a simplified Jacobian.25 A natural initialization is a zero‐motion condition , so the first step corresponds to a standard sensitivity encoding (SENSE) reconstruction. Further in this paper, we describe how to temporally arrange the k‐space samples into segments to improve the aligned reconstruction convergence.
2.2. DISORDER sampling
We focus on Cartesian 3D k‐space grids with uniform sampling as sketched in Figure 1. Figure 1A shows collected samples after the first readout or profile in the direction. Figure 1B shows the first segment, in this example corresponding to the full acquisition of the ‐ plane. Figure 1C shows that segments can be used to define an ordered partition of the ‐‐ grid. Due to short duration, we assume negligible motion during the readout and focus on the phase encode (PE) plane in Figure 1D. We define as the number of profiles per segment, so , and hereinafter we adopt the replacement .
Figure 1.
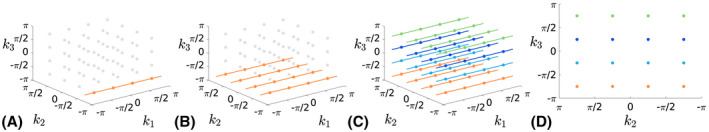
Sketch of volumetric sampling for an exemplary K = 4 × 4 × 4 space (in radians). A, Measured samples after acquiring the first readout. B, Measured samples after acquiring the first segment. C, Measured samples after whole sequence acquisition with color coding used to differentiate each segment. D, View of the ‐ PE plane
By modifying the PE gradients before each readout, it is possible to design different encoding or view orders. These can be defined as a temporally ordered set of profiles with cardinality . Figure 2A shows the first segment of a commonly used Sequential ordering scheme. In this case, due to the partition definition, a segment includes two consecutive planes. Figure 2B, introduces the Checkered traversal. First, a rectangular tiling of the PE plane is built using tiles of size such that . Second, a spectral lexicographic order for the profiles within a tile is defined by , which can be extended to different tiles by translation. Third, interleaved segments are defined such that the same profile is used , with a temporally ordered set of tiles. Finally, the profile sequence to traverse each tile is defined by mapping from the set of profiles within a tile to a temporally ordered set of segments using an electrostatic repulsion criterion with periodic boundary conditions. Hence, a distributed temporal coverage is guaranteed both for the whole spectrum and within each tile. This strategy aids the aligned reconstruction conditioning by reducing the chances for large uncovered spectral areas due to head rotation. Figure 2C presents the Random‐checkered modification. Tiles are built as in the Checkered approach but segments are constructed by a random permutation of the tile elements drawn independently for each tile, so we have . This guarantees a distributed coverage in probability and introduces some incoherence among profiles within and across segments. Figure 2D shows a Random view order where is a random permutation of the profiles conforming the sequence. Note that, considering a free definition of segments, Sequential and Random schemes are particular cases of the Random‐checkered traversal respectively with tiling sizes of 1 × 1 and .
Figure 2.
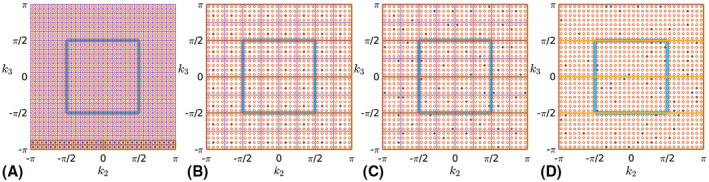
Example of segments for different encoding orders to cover a PE plane (in radians) with using an acquisition partitioned into M = 16 segments with equal number of profiles per segment . Full set of profiles to cover the PE plane as red circles, profiles within a segment filled in blue, underlying tilings () in purple, samples at half the spatial resolution enclosed in cyan, and areas covered by four intra‐segment temporal subdivisions of considered segment enclosed in yellow. A, Sequential; B, Checkered; C, Random‐checkered; and D, Random traversals
View orders should preserve the contrast and the trajectory consistency. We establish the following differentiation:
Non‐steady‐state sequences (MP‐RAGE, FSE, FLAIR). They acquire a fraction of the k‐space or shot after each radiofrequency (RF) preparation. Thus, they induce a natural sampling partition where segments are in correspondence with shots. In addition, magnetic properties are not invariant for the different shot samples. Typically, middle samples within each shot cover the central area of the spectrum,26 which our orders can fulfill by jumping from tile to tile in a Zig‐zag manner. An example is shown in Figure 3 using an elliptical sampling area. The Checkered traversal produces regular segment patterns (first column), with neighboring colors maximally separated within the tile, whereas the Random‐checkered traversal produces non‐regular patterns. Tiling orders generate smooth color transitions across the spectrum for all presented traversals (second column), which translates into smooth magnetic properties of the profiles.
Steady‐state sequences (SPGR, bSSFP). They produce a temporally stable magnetization after reaching the steady‐state, usually facilitated by some preparatory dummy profiles, so the contrast becomes independent of the encoding order. For estimates attempted at the segment level, temporal resolvability of motion will increase with bigger tiling sizes . However, large jumps in the spectrum may induce inconsistencies due to eddy currents, especially for low repetition times, so a trade‐off may be required. If the profiles are covered by the application of M spectral sweeps, analogously to,27 eddy currents can be minimized by an Alternating zig‐zag tiling order where the traversal polarity is reversed for consecutive sweeps. This is illustrated in Figure 4. The segment structure (first column) matches that of Figure 3 but for some minor differences in the Sequential case due to smooth magnetization requirements in shot‐based sequences.26 The Sequential scheme guarantees a smooth passage through k‐space (third and fourth columns). Although quicker k‐space sweeps of our traversals imply larger and steps, these remain substantially lower than for the Random order, which should limit the impact of eddy currents. Finally, the Alternating zig‐zag suppresses the undesirable spikes in the fourth column of Figure 3.
Figure 3.
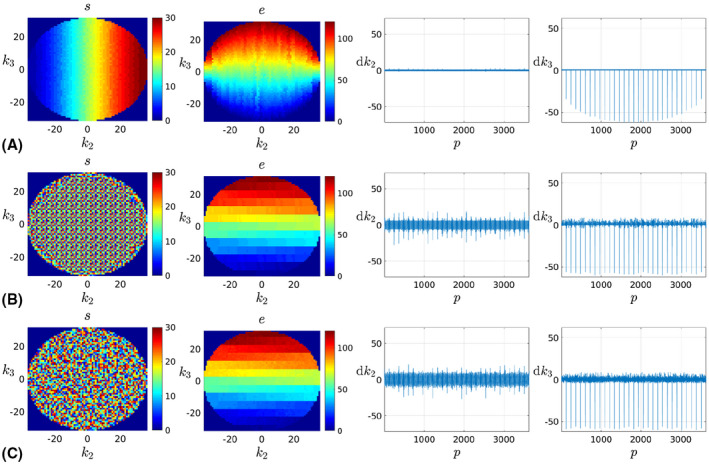
Segment m and tiling e temporal orders together with trajectory derivatives and (left to right) for Zig‐zag tiling orders used in non‐steady‐state sequences. The example corresponds to a case with P = 3630 profiles, M = 30 segments, and tiling pattern . A, Sequential; B, Checkered; and C, Random‐checkered segments
Figure 4.
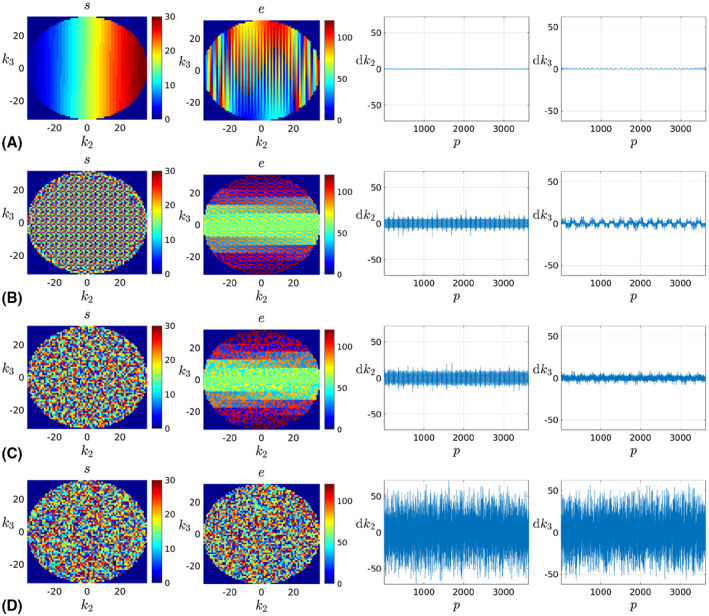
Segment m and tiling e temporal orders together with trajectory derivatives and (left to right) for Alternating zig‐zag tiling orders used in steady‐state sequences. The example corresponds to a case with P = 3630 profiles, M = 30 segments and tiling pattern . A, Sequential; B, Checkered; C, Random‐checkered; and D, Random segments
2.3. Aligned reconstruction refinements
We propose a series of refinements for improved and more efficient aligned reconstructions:
Spatial multiresolution. The spatial and spectral grids for both subproblems in Equation 2 can be refined according to a given multiresolution pyramid as commonly used in image registration.28 In contrast to sequential sampling, the proposed orders allow for motion estimates from samples at coarse scales (area enclosed in cyan in Figure 2) to be completely exploited when reconstructing at fine scales. This is useful for quick aligned reconstructions as adequate motion estimates are often possible at coarse scales.
Temporal multiresolution. Motion estimation can also be attempted at intra‐shot (or intra‐sweep) levels (for instance using the samples enclosed within the yellow areas for the four intra‐segment subdivisions in Figure 2). However, estimates using low‐spatial harmonics localize the structures at coarse scales only and, conversely, motion estimation using high‐spatial harmonics alone is limited by lower SNR and prone to local optima. These limitations can be alleviated using hierarchical estimation refinements by temporally subdividing the samples considered by the motion states within a shot in a coarse‐to‐fine manner.
Coil compression. The two subproblems in Equation 2 can operate on a reduced number of virtual channels.29
Motion compression. The reconstruction subproblem complexity grows with the number of motion states, which can be reduced by motion compression or binning. Estimated motion parameter traces are approximated using piecewise constant functions by Haar wavelet decomposition truncation with threshold τ. Thereby, original motion states are packed into effective states by grouping together those contiguous states with similar motion parameters into an effective motion parameter vector . Thus, the reconstruction complexity is driven by the underlying motion complexity.
Robustness. Accurate intra‐shot corrections may be infeasible, for instance due to temporary inconsistencies in the magnetization. Denoting the real motion parameters by , we can ideally characterize the loss using the sampling noise properties. Sampling noise follows a circularly symmetric complex Gaussian additive stationary distribution and, after whitening, it is independent across channels, so the losses per profile should ideally follow a distribution. To account for the sensitivity of the residuals to the underlying signal, we use trimmed statistics on a logarithmic scale with b ∈ 1, …B indexing the 100c(b) % centile of the loss distribution through k‐space . As we are concerned with anomalously high residuals, robust estimates of the scale and mean of the statistic distribution across segments are obtained respectively by and choosing and . Using these estimates, the statistics are normalized and averaged into , and segments are weighted in the reconstruction by a matrix W with entries , with an acceptance threshold corrected for multiple comparisons.
- Regularization. If outlier segment rejection is activated or the reconstruction is applied to accelerated scans, some form of regularization may be advisable. This is considered by reformulating the reconstruction as:
where λ controls the degree of regularization and corresponds to a shearlet decomposition, which provides nearly optimal approximation rates for piecewise smooth functions with discontinuities on a piecewise smooth surface.30 We resort to an iteratively reweighted least squares (IRWLS) solver, able to produce high‐quality solutions in a few iterations,31 with λ adaptively updated according to32 using a normalized Rayleigh‐quotient trace estimator.33(3)
3. METHODS
3.1. Synthetic experiments
Our contributions are validated using a synthetic dataset built from a neonatal brain axial ground truth (GT) image without perceptible motion artifacts. This corresponds to a multi‐slice TSE sequence acquired on a 3 T philips achieva tx (same scanner as for in‐vivo tests) using a C = 32‐element neonatal head coil array, 0.8 × 0.8 mm in‐plane resolution, 1.6 mm slice thickness, echo time , repetition time , and flip angle . Coil sensitivities were estimated from a separate reference scan.34 We use a 2D dataset and no regularization or outlier rejection for a concise presentation of results. We assume that the simulated 2D k‐space corresponds to the ‐ PE plane of 3D scans and expect the driving conclusions to be extensible to 3D because estimates should be easier along the missing fully sampled readout direction.
Simulations were conducted to compare the conventional sequential order to the various proposed schemes as well as to characterize their performance. The forward model in the presence of rigid motion is applied to the GT to generate synthetically motion corrupted data. Synthesized measures are corrupted with noise levels corresponding to a mean SNR of 30 dB for reconstructions in the absence of motion or acceleration. Different degrees of motion are generated by drawing independent motion states uniformly at random on an interval of rotations [−θ/2, θ/2] around the field of view (FOV) center. Satisfactory convergence in the presence of noise can be ascertained on the assumption of an identifiable global optimum basin by . In this case, the error in the motion parameters is attributed to the uncertainty from the measurement noise and not to partial convergence. Note that we can generally achieve a lower loss for the joint problem () than with the knowledge of the motion parameters () due to the larger complexity of the former. Reconstructions are terminated when and the abscissa scale of the convergence plots was chosen so that iterations have a direct translation into computational costs.
3.2. In vivo experiments
In vivo experiments include the main families of volumetric sequences for brain MRI (see Table 1). We have performed a controlled motion experiment on a consented adult volunteer and applied the method to replace sedation on pediatric subjects scanned after written informed parental consent for an epilepsy study. Imaging is performed using a 32‐channel adult head coil. Data are acquired in the inferior‐superior (IS) , anterior‐posterior (AP) and left‐right (LR) orientation using our scanner implementation of the Random‐checkered traversal. This way, potentially strongest rotations on the sagittal plane are captured by the ‐ coordinates, which may increase the resolvability of intra‐shot motion. In addition, IS readouts allow to easily downweight additional motion sources within the FOV when estimating for motion by restricting the loss function to the superior part of the FOV (2/3 in our implementation). Finally, this orientation facilitates nonselective RF excitation pulses for shorter .
Table 1.
Sequence parameters for the different modalities considered in the experiments
| Subjects | Modality |
|
|
|
α | P |
|
|
R |
|
t | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | MP‐RAGE | 8.3 ms | 3.7 ms | 1.00 s |
|
3630 | 6 × 5 | 1.5 mm | 2 × 2 | 240 × 240 × 220 mm | 1 min 29 s | ||||||
| Pediatric | MP‐RAGE | 7.0 ms | 2.3 ms | 0.90 s |
|
17920 | 8 × 10 | 1.0 mm | 1.4 × 1.4 | 240 × 240 × 188 mm | 3 min 07 s | ||||||
| TSE | 2.50 s | 0.31 s | – |
|
17955 | 9 × 15 | 5 min 42 s | ||||||||||
| FLAIR | 5.00 s | 0.41 s | 1.80 s | 18200 | 10 × 10 | 8 min 30 s | |||||||||||
| SPGR | 8.2 ms | 3.0 ms | – |
|
21654 | 1.3 × 1.3 | 3 min 06 s | ||||||||||
| bSSFP | 6.0 ms | 3.0 ms |
|
2 min 16 s |
Note: is the inversion time, the acquired resolution, R the uniform acceleration factor, the reconstructed FOV, and t the scan duration.
In the controlled motion experiment, the volunteer was first scanned without deliberate motion, and then asked to perform extreme and continuous motion for the entire scan, which was repeated three times. Reconstructed volumes are jointly registered together for error comparisons. The pediatric cohort includes 26 subjects ranging from 3 to 19 years old (mean ± SD of 12 ± 5 years), typically acquiring one MP‐RAGE, TSE, and FLAIR, two SPGRs, and three bSSFPs for an approximate total of 208 tested volumes across all participants. Strongest artifacts in our data are generally arising from motion, so the reported case has been separately chosen for each modality as the most artifacted after reconstruction without motion correction.
3.3. Implementation details
In the in vivo experiments sensitivities are compressed into a number of channels corresponding to a 10% SNR loss. The number of resolution levels is defined as , with ⌊·⌋ denoting the biggest integer lower or equal than the argument and the minimum of the voxel sizes along different directions. As we use 2 × subsampling ratios, we operate at a minimum resolution of 4 mm. In the first iteration at level l, a soft‐masked35 full CG reconstruction is run till loss reduction saturation. Then, the method quickly alternates between reconstruction and motion correction using one CG and one LM iteration with heuristically updated damping and line search. We activate a flag for provisional convergence of the parameters of a given motion state when the maximum update is smaller than a threshold with same values used for motion compression. This saves computations by considering motion updates only on not‐converged parameters. However, this flag is reset to 0 whenever i = n(n−1)/2+1 () to account for the impact of the updated reconstructions in the motion parameter estimates. Joint convergence is achieved when provisional convergence is achieved for all motion states. Then, the method runs a full CG reconstruction with the consolidated motion parameters. If regularized outlier rejection reconstructions are activated, artifacted segments are rejected at levels l such that by densely sampling within and using . If regularization is applied, shearlets are designed based on36 and a final reconstruction is launched with 3 CG iterations within 2 updates of the IRWLS‐induced cost function and λ. Reconstructions are performed on a 8(16) × intel(r) core(tm) i7‐5960x 3.00 GHz CPU, 64 GB RAM, geforce gtx titan x GPU. For further implementation details, readers can refer to the source code.
4. RESULTS
4.1. Validation
In Figure 5, we compare different simulated reconstruction scenarios showing the losses when iterating the method, as solid‐colored lines with joint iterations represented by markers. Losses in the convergence plots are normalized to the minimum of the reference levels , which are shown as dashed lines strongly overlapped for the different alternatives. Figure 6 includes reconstructions with and without motion correction for different reconstruction scenarios and provides absolute value error maps and mean SNR for the compared cases.
Figure 5.
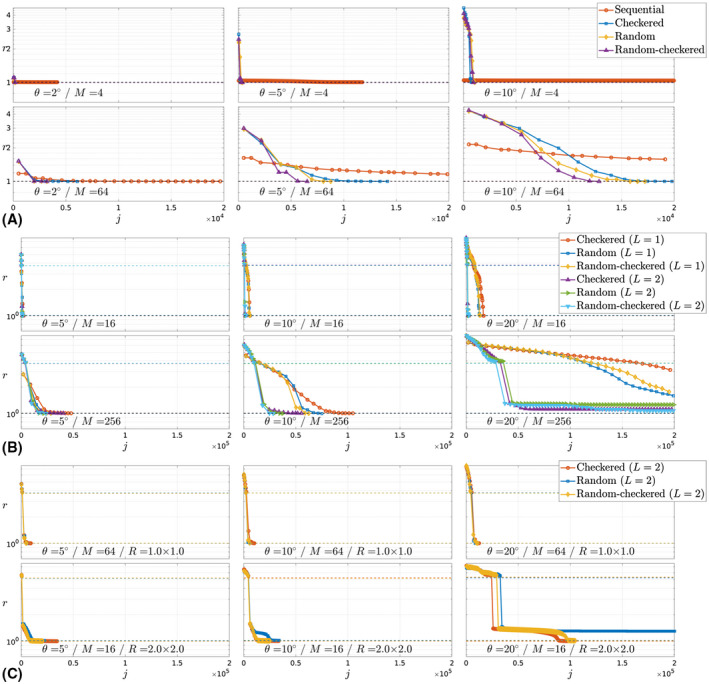
Aligned reconstruction convergence against effective iterations j defined as a single application of the encoding or decoding operator for a single motion state and coil channel at full resolution. A, Different encoding orders, number of segments M ∈ {4, 64} (rows), motion levels (columns), . B, Different encoding orders and number of multiresolution levels L, number of segments M ∈ {16, 256} (rows), motion levels (columns), . C, Different encoding orders, L = 2, acceleration factors R ∈ {1 × 1,2 × 2} with matched number of segments M ∈ {64, 16} (rows), motion levels (columns),
Figure 6.
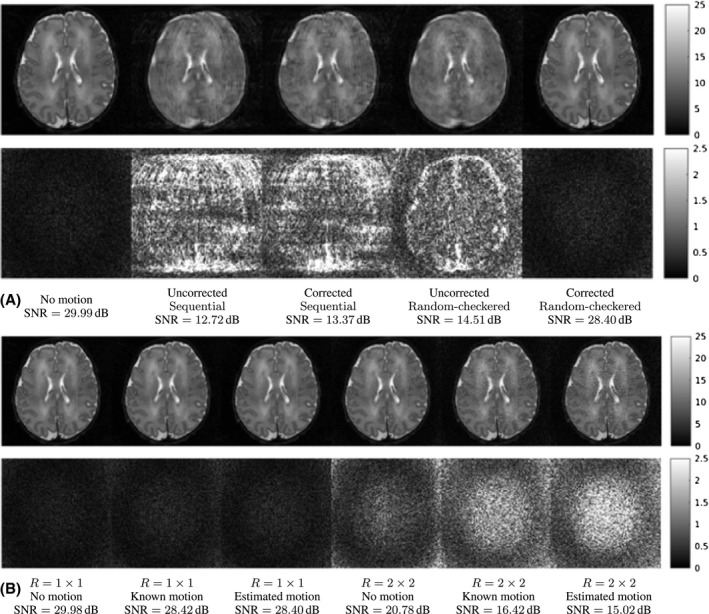
Reconstruction results (A) without and with motion correction for Sequential and Random‐checkered traversals compared to the GT (top row) and corresponding error maps (bottom row) for M = 64/ and (B) for a Random‐checkered traversal without motion, and with known and estimated motion for R = 1 × 1 and R = 2 × 2 (top row) and corresponding error maps (bottom row)
4.1.1. Encoding orders
Figure 5A compares the Sequential, Checkered, Random‐checkered, and Random traversals. Global convergence is achieved for all considered M and θ when using any of the Checkered, Random‐checkered, or Random traversals. In contrast, when using the Sequential order, the method converges to a local optimum or fails to converge in the prescribed iterations except for /M = 4. The loss at the first iteration is always bigger when using nonsequential traversals. This increased inconsistency in the measurement domain relates to the aligned reconstruction sensitivity to motion degradation.
Figure 6A shows reconstructions and errors with and without motion correction for the Sequential and Random‐checkered traversals together with GT motion‐free reconstructions. Motion‐corrected reconstructions using the Random‐checkered data appear similar to the GT despite the strong blurring in uncorrected reconstructions. This is confirmed by the lack of perceptible structure in the residuals and a moderate noise amplification. In contrast, corrections using the Sequential traversal provide only a modest visual benefit.
4.1.2. Multiresolution
Figure 5B compares the Checkered, Random‐checkered, and Random traversals when using a single scale for joint motion estimation and reconstruction (L = 1) and when first approximating the motion solution at half the acquired resolution to initialize the joint problem at full resolution (L = 2). The Sequential traversal was excluded because, as discussed when introducing the multiresolution strategy, it has no opportunity to improve from the poor relative performance showed in Figure 5A by exploiting multiresolution. Plots also include at the coarse scale. Global convergence is achieved for all traversals at all considered configurations except at M = 256/. However, the multiresolution strategy (L = 2) achieves global convergence in less iterations or provides a solution with lower residuals (M = 256/). For moderate levels of motion, convergence is generally quick. For instance, it takes approximately 10 joint iterations i when using the Random‐checkered traversal in a case where random excursions of up to are imposed in every one of the M = 256 segments, probably a more challenging scenario than expected in practice.
4.1.3. Acceleration
Figure 5C tests the ability of the Checkered, Random‐checkered, and Random traversals (using L = 2 scales) to operate in uniformly accelerated regimes as given by different acceleration factors R. We observe convergence to the global solution in all tested scenarios aside from the Random traversal at R = 2 × 2/M = 16/. Considering all conducted simulations, the random checkered traversal is generally providing the quickest solutions.
Figure 6B provides an example of reconstructions and errors in the absence of motion, with known motion and with estimated motion at R = 1 × 1 and R = 2 × 2. The SNR figures for R = 1 × 1 and known motion show a degradation of 1.56 dB with respect to the reference due to noise amplification from nonuniform effective k‐space sampling after motion. No further degradation is introduced from motion estimation errors, as approximately the same SNR figures are obtained for known and estimated motion. R = 2 × 2 acceleration in the absence of motion introduces a degradation of 9.20 dB with respect to the reference, which stems from the reduced number of samples and the g‐factor.37 The presence of motion adds further degradations quantified as 4.36 dB, thus stronger than in the non‐accelerated case. Therefore, without regularization, the limiting reconstruction quality in the presence of motion decreases with larger distances between neighboring k‐space points. Finally, accelerating the scan has also an impact in the uncertainty of motion estimates, as we observe a degradation of 1.40 dB from known to estimated motion, although the errors show no perceptible structure.
4.2. Redundancy for motion tolerance
Figure 7 compares reconstructions without correction, with inter‐shot corrections and when activating intra‐shot corrections in the presence of extreme motion during in vivo data acquisition. Intra‐shot corrections are triggered by subsequent temporal binary subdivisions of the sampled information within each shot until 16 motion states are estimated per shot. We show reconstructions without deliberate motion (GT reconstructions), and reconstructions and absolute differences with respect to the GT using Q = 1, Q = 2, and Q = 3 repeats under extreme motion (Extreme motion reconstructions/errors, Q = {1, 2, 3}). Results for Q = 1 and Q = 2 correspond to the first repeats, with no remarkable differences observed when choosing any other combination. Reconstructions are provided without regularization or outlier rejection. Results without deliberate motion show that inter‐ and intra‐shot corrections do not reduce the reconstruction quality, which demonstrates a safe application of generalized reconstructions in the absence of motion. Degradation is noticeable for uncorrected reconstructions in the presence of motion for all values of Q, although with less coherent ghosts as Q increases due to incoherent blurring by Random‐checkered motion averaging. Inter‐shot corrections increase the reconstruction quality in all cases, with more finely resolved cortical structures as Q increases but with noticeably inferior quality than without deliberate motion. Residual degradation is only partially accounted when using intra‐shot corrections on a single repeat, but can be more satisfactorily addressed with Q = 2 and even more with Q = 3. Namely, the level of deblurring in the fourth and sixth columns of Figure 7C makes corresponding reconstructions visually comparable to those of the first column despite the extreme and continuous motion (estimated excursions up to ). Thus, we can reason that powerful tolerance is achieved for R = 2 × 2 and Q = 2, so that Q = 1 with acceleration (Both alternatives involve the same scanning time but the latter generates a lower g‐factor. The former was used in this experiment because it was more convenient to our scanner implementation of the traversals.) may be adequate for motion tolerance in practice, which has been used to guide the acceleration in the pediatric cohort (see Table 1). However, in contrast to computation times of 2 min (nondeliberate motion), and 11 min (extreme motion, Q = 3) for inter‐shot corrections, corresponding intra‐shot corrections required 21 min and 20 h 36 min. Thus, despite being technically feasible, intra‐shot corrections may have limited applicability due to computational costs. Computational cost increase with the complexity of motion is due to the larger number of iterations for convergence and to the proposed motion compression strategy, with 13/30 binned inter‐shot motion states without deliberate motion and 30/30 with extreme motion (Q = 1), with proportional savings in the reconstruction steps in the former.
Figure 7.
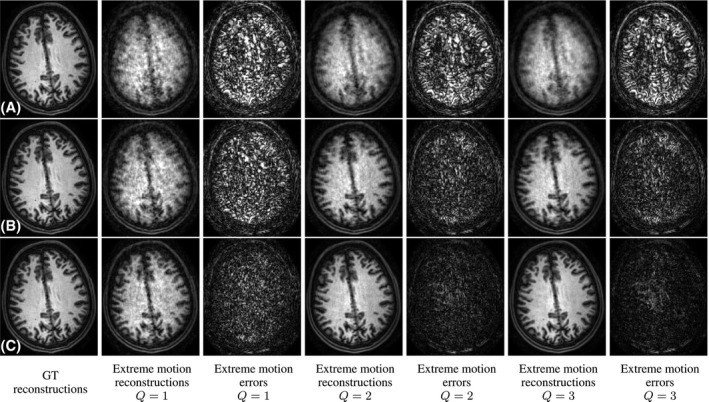
Reconstruction results for extreme motion in vivo. A, Uncorrected; B, inter‐shot corrections; and C, intra‐shot corrections. From left to right, results without deliberate motion and reconstructions and errors in the presence of extreme motion for Q = {1, 2, 3} repeats of a R = 2 × 2 accelerated baseline scan
4.3. Noncompliant subjects
Figure 8 shows worst‐case reconstructions without motion correction, with motion correction alone and with motion correction and the regularized outlier segment rejection. Results are shown for main structural brain MRI modalities, MP‐RAGE, TSE, FLAIR, SPGR, and bSSFP. In all sequences we observe a substantial improvement when activating motion‐corrected reconstructions alone, with better delineated cortical structures. However, subtle artifacts are still present, either in the form of ghosts or of coloured noise. Figure 8C shows that quality can be further improved by rejecting the less consistent segments and performing a regularized reconstruction. In some sequences discarding the artifacted segments seems to reduce residual artifacts from uncorrected fast motion (see for instance fine details in SPGR) while in others it seems to mainly improve the magnetization consistency (see TSE contrast). Across the cohort, we have observed that motion artifact levels always decrease when compensating for motion, with no remarkable differences when activating the corrections in the absence of artifacts. This is along the lines of the quantitative population metrics obtained for the less favourable sequential sampling20 or for multi‐slice scans.38 Worst‐case results of Figure 8 have been judged satisfactory by the practitioners and researchers involved in the project. Therefore, the proposed methodology is delivering reliable examinations for unsedated pediatric subjects challenging to comply to the MRI motion requirements. In this experiment, motion estimates were performed at half the acquired resolution with joint motion estimation and reconstruction always taking less time than final reconstructions at full resolution. Computation times range between 5 min in least artifacted and 40 min in most artifacted volumes in our cohort. Estimated motion traces and outlier segments are reported in Supporting Information Figure S1.
Figure 8.
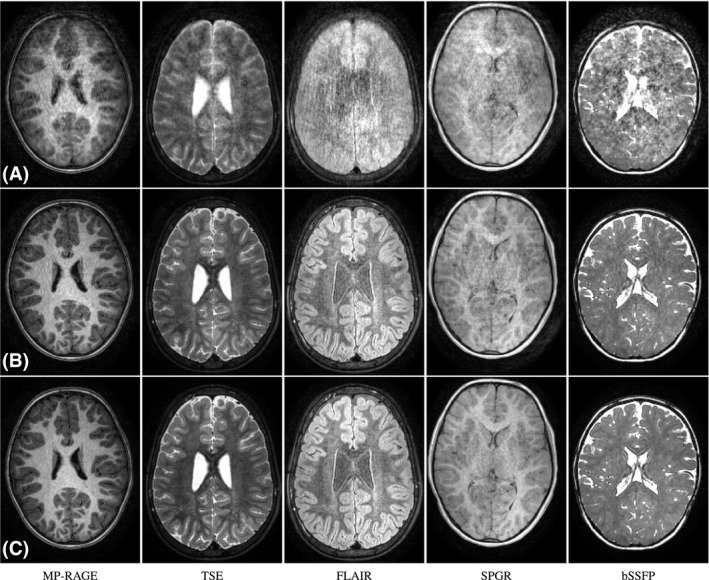
Reconstruction results for pediatric cases with largest intra‐scan degradations. A, Uncorrected; B, motion‐corrected; and C, motion‐corrected and regularized outlier segment rejection reconstructions. From left to right, results for the main families of sequences for structural brain imaging
5. DISCUSSION
We have presented DISORDER, a retrospective framework for motion‐tolerant structural 3D k‐space encoded brain imaging that combines optimized view orders with an improved aligned reconstruction. The proposed distributed and incoherent orders increase the motion sensitivity of the information sampled within a given time window which, provided a certain degree of redundancy, enables the resolvability of motion in the reconstruction. Conducted simulations have shown that reordering the k‐space traversals introduces a significant boost in the ability to estimate the head pose and suppress motion artifacts. Tolerance to motion has been demonstrated in vivo on a controlled experiment involving extreme and continuous motion throughout the examination as well as for the main families of sequences used for structural brain imaging by presenting the reconstruction results on the most challenging datasets from a pediatric cohort of 26 subjects.
Although DISORDER is robust enough in its current form so as to be of practical interest for reliable structural brain MR examinations in noncompliant cohorts, with plans in our center to use it to progressively replace unnecessary sedation or anesthesia in pediatric and neonatal populations,39 it is obviously not free from limitations. First, data consistency may be affected by additional degrading factors. These include inaccuracies in sensitivities but also water‐fat shifts, eddy currents, or flow artifacts. In practice, applying fat suppression when possible, designing the tiles for adequate trade‐offs between eddy currents and motion resolvability in bSSFP sequences, and adequate planning and scanning procedures are usually sufficient to address these issues. Differently, correction of non‐rigid motion components would require an extension of the formulation. Although analogous methodologies40 have shown potential in this context, a robust and efficient extension to non‐rigid motion models will probably require a careful computational design. This may be particularly the case for high‐resolution applications, where both rigid and non‐rigid motion become more important and challenging to correct.2 Moreover, coarse scale motion at ultra high field may require additional corrections of high‐order effects. Finally, in this manuscript we have restricted ourselves to uniform sampling, with further work required to generalize the incoherent and distributed orders and characterize motion correction and resolution retrieval when using variable densities.
In the in vivo experiments of Figure 8, we have shown that inter‐shot corrections can be sufficient in practical brain imaging scenarios requiring motion tolerance. Our underlying assumption is that the subject remains approximately still for a significant portion of the acquisition. In this case, inter‐shot corrections are enough to reconcile the brain pose among the stable periods and data rejection can be applied to the transitions, again, provided that sampling is redundant enough. However, intra‐shot corrections may become more important in challenging situations, as illustrated in Figure 7. Despite its computational limitations, our method is able to provide stable intra‐shot estimates in the absence of motion while offering certain motion correction potential. Although a prior model for the temporal evolution of motion may aid in certain applications, in general, limitations arising from available computational resources and SNR per motion state are likely to complicate intra‐shot tractability.
The situation may perhaps be different if using supervised learning strategies to inform the exploration of the motion parameter space. These may help to improve the spatiotemporal resolvability of motion by aiding the intra‐shot corrections to find better motion solutions. Training may also help to enlarge the motion capture range at a given level of redundancy or decrease the required level of redundancy for a given motion capture range. Although direct learning of motion‐corrected reconstructions could also be attempted, it is likely that, in many circumstances, better results will be obtained when concatenating learned reconstructions with model‐based strategies, as recently suggested in.41 Further integration of both approaches could be tackled, for instance, by incorporating the motion operator into the model‐based learning framework in,42 which may be effective in dealing with the residual penalties from g‐factor amplification due to motion (see Figure 6B). Thereby, future work will explore the opportunities for extending the ranges of motion resilience by supervised learning.
6. CONCLUSION
We have proposed a simple modification of standard 3D Cartesian sequences for structural brain imaging, involving only a distributed and incoherent reordering of the sampled profiles, for high‐quality imaging in the presence of motion. Improved convergence has been demonstrated when using a separable nonlinear least squares formulation for joint motion estimation and reconstruction. Feasibility and conditions for inter‐ and intra‐shot corrections have been characterized by simulations and in vivo reconstructions under extreme motion. The DISORDER method has been successfully applied to replace sedation in a pediatric population scanned using common clinical examination protocols by combining inter‐shot corrections with regularized outlier segment rejection reconstructions. Future work will focus on applying DISORDER to other cohorts and on strengthening its performance by integrating motion learning strategies.
Supporting information
FIGURE S1 First 120 s of estimated motion traces for pediatric cases with largest intra‐scan degradations for each sequence. Left: original motion traces. Right: motion traces with segment opacity given by corresponding reliability w. Solid lines indicate data collection periods for each segment with dotted lines used to connect these
ACKNOWLEDGMENTS
The authors acknowledge the Department of Perinatal Imaging & Health at King's College London.
Cordero‐Grande L, Ferrazzi G, Teixeira RPAG, O'Muircheartaigh J, Price AN, Hajnal JV. Motion‐corrected MRI with DISORDER: Distributed and incoherent sample orders for reconstruction deblurring using encoding redundancy. Magn Reson Med. 2020;84:1–14. 10.1002/mrm.28157
REFERENCES
- 1. Andre JB, Bresnahan BW, Mossa‐Basha M, et al. Towards quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12:689–695. [DOI] [PubMed] [Google Scholar]
- 2. Budde J, Shajan G, Scheffler K, Pohmann R. Ultra‐high resolution imaging of the human brain using acquisition‐weighted imaging at 9.4T. NeuroImage. 2014;86:592–598. [DOI] [PubMed] [Google Scholar]
- 3. Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: a complex problem with many partial solutions. J Magn Reson Imaging. 2015;42:887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godenschweger F, Kägebein U, Stucht D, et al. Motion correction in MRI of the brain. Phys Med Biol. 2016;61:R32–R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: a review. Magn Reson Med. 2013;69:621–636. [DOI] [PubMed] [Google Scholar]
- 6. Schulz J, Siegert T, Reimer E, et al. An embedded optical tracking system for motion‐corrected magnetic resonance imaging at 7T. Magn Reson Mater Phy. 2012;25:443–453. [DOI] [PubMed] [Google Scholar]
- 7. Aksoy M, Forman C, Straka M, Çukur T, Hornegger J, Bammer R. Hybrid prospective and retrospective head motion correction to mitigate cross‐calibration errors. Magn Reson Med. 2012;67:1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adcock B, Hansen A, Roman B, Teschke G. Generalized sampling: stable reconstructions, inverse problems and compressed sensing over the continuum. Adv Imaging Electron Phys. 2014;182:187–279. [Google Scholar]
- 9. Tisdall MD, Reuter M, Qureshi A, Buckner RL, Fischl B, van der Kouwe AJ. Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion. NeuroImage. 2016;127:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson PM, Liu J, Wade T, Tavallaei MA, Drangova M. Retrospective 3D motion correction using spherical navigator echoes. Magn Reson Imaging. 2016;34:1274–1282. [DOI] [PubMed] [Google Scholar]
- 11. Gallichan D, Marques JP. Optimizing the acceleration and resolution of three‐dimensional fat image navigators for high‐resolution motion correction at 7T. Magn Reson Imaging. 2017;77:547–558. [DOI] [PubMed] [Google Scholar]
- 12. Bammer R, Aksoy M, Liu C. Augmented generalized SENSE reconstruction to correct for rigid body motion. Magn Reson Med. 2007;57:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson AG, Velikina J, Block W, Wieben O, Samsonov A. Adaptive retrospective correction of motion artifacts in cranial MRI with multicoil three‐dimensional radial acquisitions. Magn Reson Med. 2013;69:1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pipe JG, Gibbs WN, Li Z, Karis JP, Schar M, Zwart NR. Revised motion estimation algorithm for PROPELLER MRI. Magn Reson Med. 2014;72:430–437. [DOI] [PubMed] [Google Scholar]
- 15. Bydder M, Larkman DJ, Hajnal JV. Detection and elimination of motion artifacts by regeneration of k‐space. Magn Reson Med. 2002;47:677–686. [DOI] [PubMed] [Google Scholar]
- 16. Samsonov AA, Velikina J, Jung Y, Kholmovski EG, Johnson CR, Block WF. POCS‐enhanced correction of motion artifacts in parallel MRI. Magn Reson Med. 2010;63:1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn Reson Med. 2005;54:1273–1280. [DOI] [PubMed] [Google Scholar]
- 18. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free‐breathing MRI. Magn Reson Med. 2008;60:146–157. [DOI] [PubMed] [Google Scholar]
- 19. Loktyushin A, Nickisch H, Pohmann R, Schölkopf B. Blind retrospective motion correction of MR images. Magn Reson Med. 2013;70:1608–1618. [DOI] [PubMed] [Google Scholar]
- 20. Cordero‐Grande L, Teixeira RPAG, Hughes EJ, Hutter J, Price AN, Hajnal JV. Sensitivity encoding for aligned multishot magnetic resonance reconstruction. IEEE Trans Comput Imaging. 2016;2:266–280. [Google Scholar]
- 21. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k‐space trajectories. Magn Reson Med. 2001;46:638–651. [DOI] [PubMed] [Google Scholar]
- 22. Unser M, Thévenaz P, Yaroslavsky L. Convolution‐based interpolation for fast, high‐quality rotation of images. IEEE Trans Image Process. 1995;4:1375–1381. [DOI] [PubMed] [Google Scholar]
- 23. Gan M, Chen CLP, Chen GY, Chen L. On some separated algorithms for separable nonlinear least squares problems. IEEE Trans Cybern. 2018;48:2866–2874. [DOI] [PubMed] [Google Scholar]
- 24. Herring JL, Nagy JG, Ruthotto L. LAP: a linearize and project method for solving inverse problems with coupled variables. Sampling Theory Signal Image Process. 2018;17:127–151. [Google Scholar]
- 25. Ruano AEB, Jones DI, Fleming PJ. A new formulation of the learning problem of a neural network controller. Proceedings of 30th IEEE Conference Decision Control, Brighton, England, 1991; pp. 865–866.
- 26. Busse RF, Brau AC, Vu A, et al. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med. 2008;60:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsao J, Kozerke S, Boesiger P, Pruessmann KP. Optimizing spatiotemporal sampling for k‐t BLAST and k‐t SENSE: Application to high‐resolution real‐time cardiac steady‐state free precession. Magn Reson Med. 2005;53:1372–1382. [DOI] [PubMed] [Google Scholar]
- 28. Unser M, Aldroubi A, Gerfen CR. A multiresolution image registration procedure using spline pyramids. Proceedings SPIE, Wavelet Applications in Signal and Image Processing, vol. 2034, 1993; pp. 160–170. [Google Scholar]
- 29. Buehrer M, Pruessmann KP, Boesiger P, Kozerke S. Array compression for MRI with large coil arrays. Magn Reson Med. 2007;57:1131–1139. [DOI] [PubMed] [Google Scholar]
- 30. Kutyniok G, Lemvig J, Lim WQ. Optimally sparse approximations of 3D functions by compactly supported shearlet frames. SIAM J Math Anal. 2012;44:2962–3017. [Google Scholar]
- 31. Voronin S, Daubechies I. Functional Analysis, Harmonic Analysis, and Image Processing, Contemp Math, vol. 693, chap. An iteratively reweighted least squares algorithm for sparse regularization. Am Math Soc. 2017;391–411. [Google Scholar]
- 32. Li CJ, Zhong YJ. A pseudo‐heuristic parameter selection rule for l 1‐regularized minimization problems. J Comput Appl Math. 2018;333:1–19. [Google Scholar]
- 33. Avron H, Toledo S. Randomized algorithms for estimating the trace of an implicit symmetric positive semi‐definite matrix. J Assoc Comput Mach. 2011;58:1–17. [Google Scholar]
- 34. Allison MJ, Ramani S, Fessler JA. Accelerated regularized estimation of MR coil sensitivities using augmented Lagrangian methods. IEEE Trans Med Imaging. 2013;32:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fuderer M, van den Brink J, Jurrissen M. SENSE reconstruction using feed forward regularization. 12th Proceedings of the International Society for Magnetic Resonance in Medicine, Kyoto, Japan, 2004; p. 2130. [Google Scholar]
- 36. Kutyniok G, Lim WQ, Reisenhofer R. ShearLab 3D: faithful digital shearlet transforms based on compactly supported shearlets. ACM Trans Math Softw. 2016;42:1–5. [Google Scholar]
- 37. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 38. Cordero‐Grande L, Hughes EJ, Hutter J, Price AN, Hajnal JV. Three‐dimensional motion corrected sensitivity encoding reconstruction for multi‐shot multi‐slice MRI: application to neonatal brain imaging. Magn Reson Med. 2018;79:1365–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barton K, Nickerson JP, Higgins T, Williams RK. Pediatric anesthesia and neurotoxicity: what the radiologist needs to know. Pediatr Radiol. 2011;48:31–36. [DOI] [PubMed] [Google Scholar]
- 40. Loktyushin A, Nickisch H, Pohmann R, Schölkopf B. Blind multirigid retrospective motion correction of MR images. Magn Reson Med. 2015;73:1457–1468. [DOI] [PubMed] [Google Scholar]
- 41. Haskell MW, Cauley SF, Bilgic B, et al. Network accelerated motion estimation and reduction (NAMER): Convolutional neural network guided retrospective motion correction using a separable motion model. Magn Reson Med. 2019;82:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aggarwal HK, Mani MP, Jacob M. MoDL: model based deep learning architecture for inverse problems. IEEE Trans Med Imaging. 2019;38:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 First 120 s of estimated motion traces for pediatric cases with largest intra‐scan degradations for each sequence. Left: original motion traces. Right: motion traces with segment opacity given by corresponding reliability w. Solid lines indicate data collection periods for each segment with dotted lines used to connect these


