Abstract
Background & Aims:
Small, 2-dimensional sections routinely used for human pathology provide limited information about bowel innervation. We developed a technique to image human enteric nervous system (ENS) and other intramural cells in 3 dimensions.
Methods:
Using mouse and human colon tissues, we developed a method that combines tissue clearing, immunohistochemistry, confocal microscopy, and quantitative analysis of full thickness bowel without sectioning to quantify ENS and other intramural cells in 3 dimensions.
Results:
We provided 280 adult human colon confocal Z stacks from persons without known bowel motility disorders. Most of our images were of myenteric ganglia, captured using a 20x objective lens. Full thickness colon images, viewed with a 10x objective lens, were as large as 4 x 5 mm 2. Colon from 2 pediatric patients with Hirschsprung disease was used to show distal colon without enteric ganglia, as well as a transition zone and proximal pull-through resection margin where ENS was present. After testing a panel of antibodies with our method, we identified 16 antibodies that bind to molecules in neurons, glia, interstitial cells of Cajal, and muscularis macrophages. Quantitative analyses demonstrated myenteric plexus in 24.5%±2.4% of flattened colon Z-stack area. Myenteric ganglia occupied 34%±4% of myenteric plexus. Single myenteric ganglion volume averaged 3,527,678±573,832 mm 3 with 38,706±5763 neuron/mm 3 and 129,321±25,356 glia/mm 3. Images of large areas provided insight into why published values of ENS density vary up to 150-fold—ENS density varies greatly, across millimeters, so analyses of small numbers of thin sections from same bowel region can produce varying results. Neuron subtype analysis revealed that approximately 56% of myenteric neurons stained with nNOS antibody and approximately 33% of neurons produce and store acetylcholine. Transition zone regions from colon tissues of patients with Hirschsprung disease had ganglia in multiple layers and thick nerve fiber bundles without neurons. Submucosal neuron distribution varied among imaged colon regions.
Conclusions:
We developed a 3-dimensional imaging method for colon that provides more information about ENS structure than tissue sectioning. This approach could improve diagnosis for human bowel motility disorders and may be useful for other bowel diseases.
Keywords: Myenteric plexus, Submucosal ganglia, Nitric oxide, acetylcholine
Introduction:
The enteric nervous system (ENS) controls most aspects of bowel function and is thought to contain as many neurons as spinal cord with ~20 neuron and 4-7 glial cell types1–4. ENS defects may cause debilitating or life-threatening bowel dysfunction, including diverse motility disorders. In many cases, human diseases are well-modeled by mouse mutations that cause striking changes in ENS anatomy5, 6. ENS structural defects are readily visualized in mice via whole mount staining because murine bowel is thin. In contrast, human ENS is buried in opaque tissue making visualization challenging, especially since ENS accounts for <1:1000 cells in bowel wall (our estimate). For this reason, human clinical pathology primarily relies on formalin-fixed paraffin embedded thin sections to visualize ENS. However, even major ENS defects are difficult to appreciate in sectioned bowel (Supplemental Figure 1) and human quantitative analyses provide highly variable results for “normal” colon neuron density7–9. This variability in “normal” makes structural defects causing bowel motility disorders difficult to define. A few approaches demonstrated elaborate ENS structures in human bowel10–17, but methods were not reproducible in our hands, technically challenging and time consuming, or failed to demonstrate ENS in large intact regions. We therefore sought to develop robust, highly reproducible methods to visualize cells controlling human colon motility. Our new approach provides three-dimensional ENS views in large colon regions without sectioning, preserving associations with other bowel cells. This is important because bowel motility requires coordinated muscle contraction and relaxation18 organized by enteric sensory neurons, interneurons, excitatory and inhibitory motor neurons1, 3,19, 20, Interstitial cells of Cajal (ICC)21, enteric glia, muscularis macrophages, platelet derived growth factor receptor alpha-immunoreactive cells (PDGFRa+, or “fibroblast-like cells”), and enteroendocrine cells1, 3. Defining cell type-specific defects that affect bowel motility is most important for life-threatening diseases like Hirschsprung22, 23, achalasia24, gastroparesis25, and chronic intestinal pseudoobstruction (CIPO)26 where improved imaging could provide new ideas about disease pathogenesis or treatment. First, however, we aimed to quantitatively define “normal” ENS anatomy. Here we provide 280 confocal Z-stacks and quantitative data from 14 human adult colons. We also visualized colon ENS from children with Hirschsprung disease, a birth defect where ENS is absent from distal bowel and a hypoganglionic “transition zone” separates aganglionic from proximal ENS-containing colon.
Methods:
All authors had access to data and approved final manuscript.
Mice:
Studies adhere to ARRIVE guidelines27 and Institutional Animal Care and Use Committee at Children’s Hospital of Philadelphia (#16-001041). P0 GFRα1−/− (MGI Cat# 3715269, RRID:MGI:3715269)28 and WT littermate (C57BL/6) were analyzed (9-12 AM). Dam was fed Mouse Diet 5015* (LabDiet), not fasted, housed on corncob (The Andersons, Product 4B) in Lab Products (Seaford, DE) caging.
Human Tissue:
Colon was acquired with Children’s Hospital of Philadelphia Institutional Review Board (IRB#13-010357) and Perelman School of Medicine at University of Pennsylvania approval (IRB#804376) using Abramson Cancer Center Tumor Tissue Bank or pathology de-identified tissue, providing limited clinical data. Jejunum and pancreas were from the Human Pancreas Analysis Program (IRB exempt).
Human colon staining:
Detailed protocol is at protocols.io (Will add link).
Tissue processing:
Adult colons remained at ambient temperature until arrival in pathology following routine hospital procedures. Transfer to sterile ice cold 1X phosphate buffered saline (PBS) occurred 61-112 minutes after resection. Staff pathologists provided regions without recognized abnormalities. Lab received coded specimens in PBS on ice. While in PBS, fat was removed and tissue pinned along edges (serosa up) to Sylgard® 184 Silicone Elastomer (Dow) using insect pins. We stretched while pinning making colon thin, flat, and uniform thickness. Pins were repositioned several times to stretch colon as muscles relaxed increasing area 2.6-fold (N=3, SEM=0.15) compared to un-stretched colon. Pinned tissue was fixed (4% paraformaldehyde, 4°C overnight), transferred to PBS, and edges with pin holes trimmed. Full thickness colon was cut with scissors. Most specimens were 1x1cm2. Larger colonic specimens also stained adequately using this method (e.g., 2x3cm2). Stored tissue (4°C, 50% PBS/50% glycerol/0.05% sodium azide) could be stained months later without obvious tissue degradation. Human jejunum, pancreas, and Hirschsprung colon were processed similar to adult colons. Hirschsprung colon was in ice cold PBS 20-60 minutes after resection. Jejunum and pancreas from transplant donors were in Belzer UW solution29 (Fisher Scientific NC0410019) immediately after resection and stored ~8h before fixation.
Immunohistochemistry:
1x1cm2 fixed colon were processed in 24-well VWR Culture Plates (Non-treated), 2cm diameter, Cat #10861-558). Reagent volumes: 500-1000μL/well (enough to cover tissue). After washing (PBS, 3x5min, room temperature), incubating to permeabilize and remove lipids (100% methanol, 1h, on ice), and treating with Dent’s bleach (5mL 30% hydrogen peroxide, 5mL dimethylsulfoxide, 20mL 100% methanol)30 (2h, room temperature) to permeabilize and quench auto-fluorescence, colon was washed (PBS, 3x5min, room temperature), transferred to 2mL Eppendorf tubes containing blocking solution (4% normal donkey serum, 0.5% Triton X-100, 0.05% sodium azide/PBS) and incubated (3 days, 37°C, New Brunswick Scientific I24 Incubator Shaker, 40-100rpm). Then we incubated with 1-3 primary antibodies (Supplemental Table 1) in blocking solution on shaker (14 days, 37°C, 40-100rpm) using Parafilm® sealed tubes. Unbound primary antibody was removed (PBS/0.05% sodium azide, 3 washes, 2h/wash, plus an overnight wash, on rocker). Secondary antibodies were in PBS/0.05% sodium azide (3 days, 37°C, on rocker). Excess secondary was removed with PBS/0.05% sodium azide (3 washes, 2h/wash, plus additional wash overnight, room temperature, on rocker). Some tested antibodies did not work (Supplemental Table 2).
Dehydration, clearing and mounting after immunohistochemistry:
Colon was dehydrated in 24-well dishes in graded methanol/PBS (~ 500μL/well, extra if needed to cover, 30 minutes/wash: 50% methanol, 70% methanol, 80% methanol, 95% methanol, 100% methanol x 3, room temperature, on rocker). Dehydrated colon incubated in Murray’s Clear (2:1 benzyl benzoate: benzyl alcohol)31 until completely translucent (15-30 min, room temperature) was mounted on glass slides in Murray’s Clear and imaged within 48 hours.
Imaging:
Zeiss LSM 710 confocal microscope (10x and 20x Plan-Apochromat objectives, Zeiss Zen software (version 2.3 14.0.14.201)) Z-axis increments were 4μm (10x objective) or 1μm (20x objective). Each image slice was 900x900 (10x) or 1200x1200 pixels (20x). 10x Z-stacks were stitched to cover large regions. Laser-scanning operated under multi-track to sequentially acquire multi-channel images. Each channel used 100% laser power. Excitation/long-pass emission filters: Alexa Fluor® 647 (excitation: 633 nm, emission: 656-755-nm filter), Alexa Fluor® 594 (excitation: 561 nm, emission: 588-656-nm filter), Alexa Fluor® 488 (excitation: 488 nm, emission: 493-584-nm filter). Tile scan and Zen stitching were used to assemble multi-field images. ImageJ (Java 1.8), Imaris 9.0.2 (Bitplane AG, Zurich Switzerland), Adobe Photoshop CS6, and Inkscape (0.92.3) manipulation was limited to uniform contrast adjustment, cropping, rotating, stitching, assembling Z-stacks, generating three-dimensional projections and videos.
Quantitative analyses employed manual and automated features:
Imaris modules:
Crop 3D, Imaging Processing, Thresholding, Background Subtraction, Surface, Manual Contour, Click Drawing Mode, and Detailed Statistics. ImageJ features: Regions of interest (ROIs), Polygon selection, Straight line tool, Measurements, Scale bar, Z-Project, Split channels, Merge channels, and Duplicate.
Myenteric plexus:
Neurons (HuC/D+PHOX2B+) and glia (S100β+) were manually counted in ganglia using 39 randomly selected colon regions (4 right, 5 fields/subject; 3 left, 5 fields/subject; 1 left, 4 fields). Two-dimensional ganglion areas were manually outlined in flattened Z-stacks (4 right, 4 left colon). Using similar manual outlining, we determined percent colon containing myenteric plexus (defined as ganglia plus thick nerve fiber bundles connecting ganglia), and percent plexus occupied by ganglia (defined as regions with >2 myenteric neurons separated by <1 cell diameter). For three-dimensional analyses, individual ganglion volumes were determined by manually outlining at 5-7μm increments within 20X Z-stacks using Click Drawing mode (Surface Contour module, Imaris). Cell density within ganglia was determined by manually counting within defined volumes.
Neuron subtypes:
Myenteric neurons (HuC/D+) expressing neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT) or vesicular acetylcholine transporter (VAChT) identified by triple-label immunohistochemistry were manually counted within Z-stacks (5 random fields/subject, 4 right, 4 left, 40 total regions). Maximum neuron diameter was determined manually using ImageJ (6 nNOS+/image, 6 ChAT+/image, five 20X images/subject; 4 right, 4 left; forty 20X images total, 240 nNOS neurons, 240 ChAT neurons).
Submucosal plexus:
Ganglia location was defined in Z-stacks using ImageJ.
Statistics:
We used Prism 7 (GraphPad Software, San Diego, CA) D ‘Agostino & Pearson and Shapiro-Wilk normality tests with unpaired t-tests to compare means. Volumes of manually contoured ganglia used Detailed Statistical Analysis (Imaris). Data are presented as mean+/− standard error. Degrees of freedom=6 for analyses.
Results:
Our goal was to establish highly reproducible, inexpensive, simple techniques to visualize human ENS in three dimensions to advance understanding of bowel motility disorders. We tried many published approaches to visualize human ENS10–13, 16, 17,32–35, but human colon was incompletely penetrated by antibody, not transparent after clearing, or methods provided only limited views of ENS anatomy after meticulous challenging microdissection.
Optimal clearing, staining and imaging
To image ENS in full thickness colon without perturbing cellular associations, we modified published methods32, 34 to enhance staining and translucency. Our approach worked routinely with 1x1cm2 colon and can work with larger regions. We reasoned antibodies and light would penetrate better if colon was thin. We therefore pinned and repeatedly stretched colon before fixation to average thickness 800x1000μm (Figure 1A). We kept colon as flat and uniform as possible and avoided drying. We tried staining tissue that had not been stretched prior to fixation, but reagent penetration was poor, and folded tissue made image interpretation difficult.
Figure 1. Imaging cells controlling human colon motility.
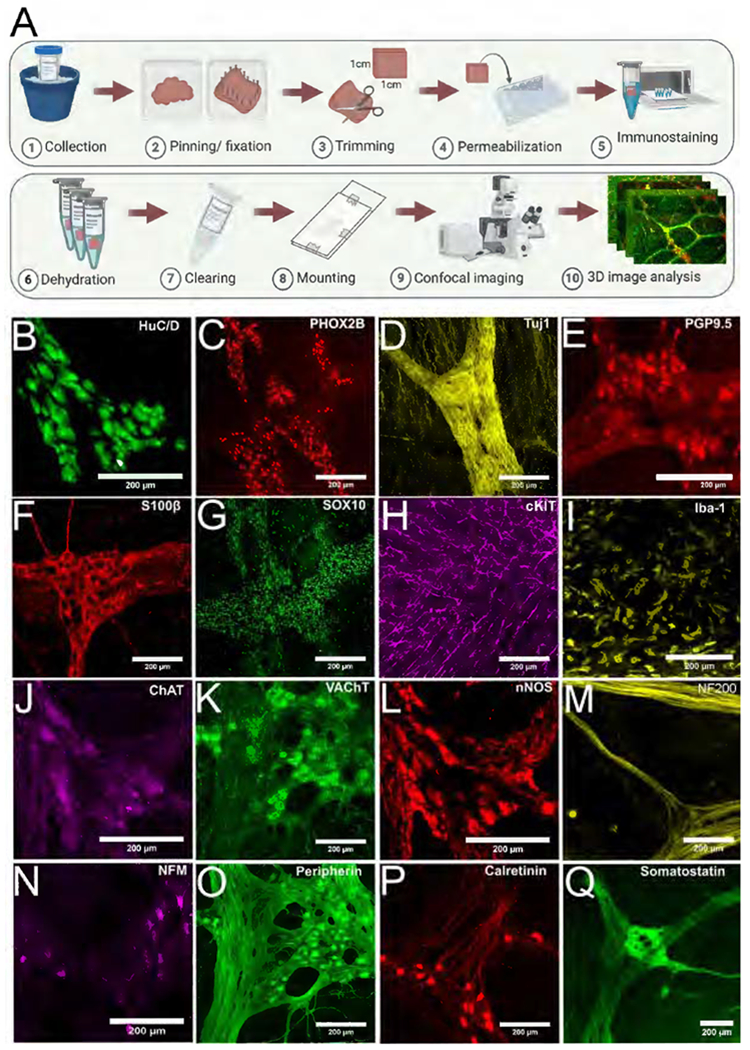
(A) Strategy to image without sectioning. (B-G,J-Q) Human colon myenteric plexus visualized with 14 antibodies. (H,I) Longitudinal muscle ICC and muscularis macrophages visualized with cKIT (H) and Iba-1 (I) antibodies. Scale bars = 200 μm.
Methanol, Dent’s bleach, prolonged 37°C incubation and shaking enhanced staining, but took 23 days (Supplemental Figure 2). Murray’s Clear made tissue translucent, but fluorescence in Murray’s Clear declined over time so we imaged within 48 hours of immersion. Tissue area underwent shrinkage to 66.9 +/−6.6% of original area measured after fixation (Supplemental Figure 3), partially reversing stretching.
Imaging and antibody testing
Colon was easily imaged to a depth of 1000μm allowing full thickness reconstructions of stretched preparations. We identified antibodies binding nerve cell bodies (HuC/D), neuronal and some glial nuclei (PHOX2B), nerve fibers (TuJ1), nerve cell bodies and fibers (PGP9.5), enteric glia (S100β, SOX10), ICC (cKIT), muscularis macrophages (Iba1), and enteric neuron subsets (ChAT, VAchT, nNOS, neurofilament 200, neurofilament M, peripherin, calretinin, somatostatin) (Figure 1B–Q). We generated three-dimensional images of many cell types thought to influence human colon motility and provide 280 adult colon Z-stacks (Will add Blackfynn Link; or at https://drive.google.com/drive/folders/1Tt_A979u7Q5oxFmrOB29SvaERb5nT_Ev?usp=sharing). Our method also worked for human jejunum and pancreas (Supplemental Figure 4, Supplemental Videos 1 and 2). Quantitative analyses focused on adult human colon.
Human colon ENS in three dimensions
To examine normal human colon ENS structure, we analyzed specimens resected for varied clinical indications; all lacked identified motility disorders (Supplemental Table 3) and lacked obvious pathology. Three-dimensional imaging of HuC/D, PHOX2B and S100β stained colon showed enteric neurons clustered into submucosal and myenteric ganglia connected by thick nerve fiber bundles (Supplemental Video 3). Myenteric ganglia appeared larger on average than submucosal ganglia. A few individual neuron cell bodies were within thick circular muscle of all colons (Supplemental Video 3). A rich network of fine nerve fibers and closely associated glia (S100β+) was present within circular and longitudinal muscle, with neurites largely parallel to smooth muscle (Supplemental Video 3). Glia were also closely associated with neuronal soma in ganglia (Supplemental Video 4, Supplemental Figure 5) and nerve fibers were dense near bowel mucosa (Supplemental Video 3).
Human colon myenteric plexus
To establish normal indices, full thickness colon stained for HuC/D, PHOX2B and S100β was imaged parallel to bowel surface (Figure 2A). Confocal Z-stacks were stitched to evaluate large regions (Figure 2A is 4x5mm2). For quantitative analyses, we defined “myenteric plexus” as nerve fiber bundles (containing S100β+ glia) and embedded nerve soma (HuC/D+PHOX2B+). We defined “myenteric plexus ganglia” as regions within fiber bundles containing >2 adjacent neuron cell bodies. Using flattened Z-stacks we determined percent colon containing myenteric plexus or ganglia (Figure 2B). Although myenteric plexus area was similar in left and right colon (left 26.5+/−2.5%, right 22.5+/−4%, P=0.4514), more myenteric plexus (left 43+/−3.2%, right 26+/−3.7%, P=0.0113) and more image area (left 11.6+/−1.7%, right 5.3+/−0.1%, P=0.0116) was occupied by ganglia in left colon. Two-fold differences in density between left and right colon suggest region specific normal ranges.
Figure 2. Human colon myenteric plexus two-dimensional analyses.
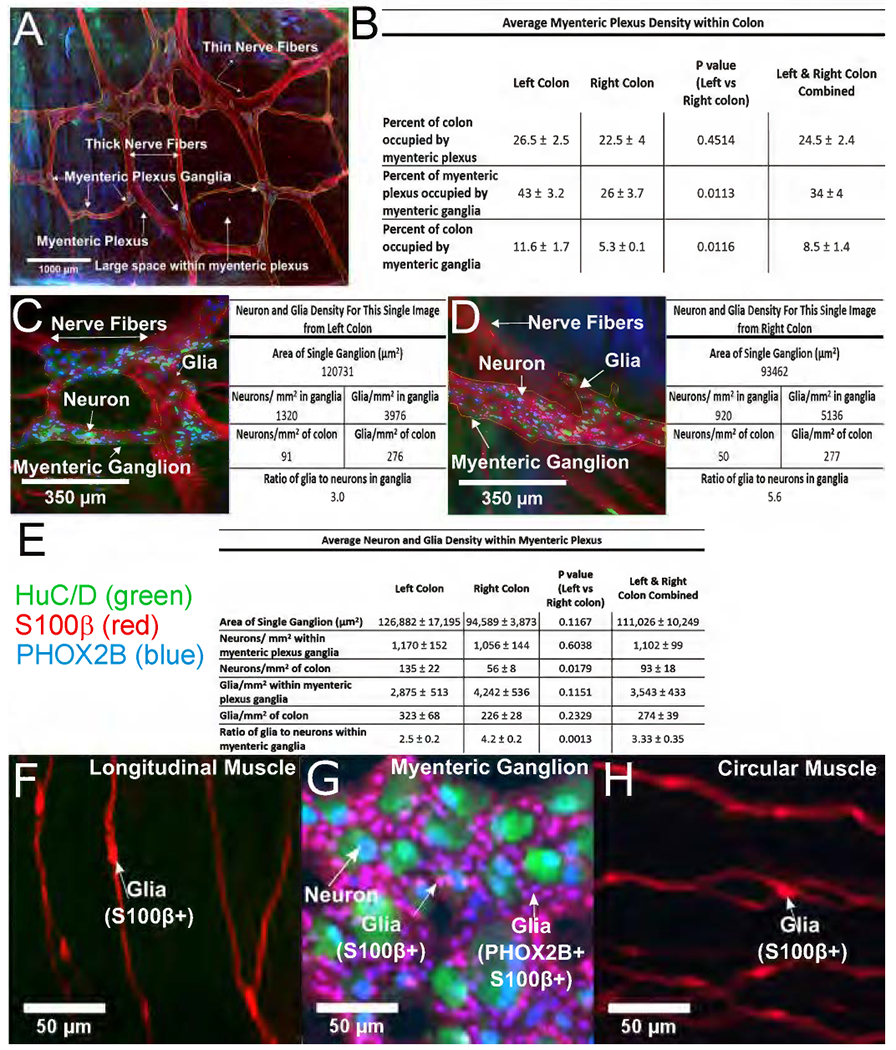
(A) Flattened Z-stack through myenteric plexus (10x objective, stitched fields, 4x5mm2). Small clustered ganglia have HuC/D+ (green) and PHOX2B+ (blue) neurons. Nerve fibers are visualized via glial S100β (red). Myenteric plexus and ganglia within plexus were outlined manually (thin yellow lines). (B) Quantitative data from 4x5mm2 images like (A). (C,D) Flattened Z-stack from colon myenteric ganglia (left (C) or right (D)) stained for HuC/D (green), S100β (red) and PHOX2B (blue) imaged with 20x objective. Table to right of each image shows quantitative data from that specific Z-stack. (E) Quantitative data from 4 right colon, 5 fields/subject; 3 left colon, 5 fields/subject; 1 left colon, 4 fields; Total neurons counted: 1953 right colon, 2603 left colon; Total glia counted: 8021 right colon, 6423 left colon. Single ganglion areas are from 24 right and 19 left colon ganglia. Cells/mm2 within these ganglia are based on 5261 neurons and 16,915 glia. (F,G,H) Colon stained with HuC/D (green), S100β (red), and PHOX2B (blue) antibodies. PHOX2B+ nuclei include all myenteric neurons and glia within myenteric ganglia (G). Glia in longitudinal (F) and circular muscle (H) were not PHOX2B immunoreactive. (A) Scale bar = 1000μm. (C,D) Scale bar=350μm. (F-H) Scale bar=50μm.
We next determined colon myenteric plexus neuronal and glial density by counting HuC/D+, PHOX2B+ and S100β+ cells (Figure 2C–E). We discovered all neurons and glia within myenteric ganglia or thick nerve fiber bundles had nuclear PHOX2B immunoreactivity (Figure 2G). In contrast, glia in thin nerve fibers throughout muscle were not PHOX2B immunoreactive (Figure 2F,H). Using 20X Z-stacks, we counted all stained cells in each image. Data are first presented based on two-dimensional areas, ignoring Z-depth. Quantitative data adjacent to each image were from that specific Z-stack (Figure 2C,D). Areas containing neurons were considered “ganglia” and outlined (see thin yellow lines). Mean density data are in Figure 2E. Although neuron density within myenteric ganglia was similar in left and right colon, neurons/mm2 bowel was greater on left because ganglia occupy a larger percentage of bowel wall (left=135+/−22, right 56+/−8, P=0.0179). In contrast, glial density was not statistically different in right versus left colon (glia/mm2: left=323+/−68, right=226+/−28, P=0.2329) or within myenteric ganglia (left=2,875+/−513, right=4,242+/−536, P=0.1151). Interestingly, within ganglia, ratio of glia to neurons was lower in left than right colon (left=2.5+/−0.2 glia/neuron, right=4.2+/−0.2, P=0.0013). To define cell density within myenteric ganglia in three-dimensions, we manually outlined and counted cells in Z-stacks (Figure 3A,B). Quantitative data for two individual images are provided (Figure 3A,B). Pooled data indicate normal ranges for people without known bowel motility disorders (Figure 3C).
Figure 3. Human colon myenteric plexus two- and three-dimensional analyses.

(A, B) Three-dimensional ganglia volumes (yellow regions) (Supplemental Videos 5 and 6 are from these regions). To right of each image are quantitative data from that specific region. (C) Cell density based on five 20x fields/subject, 8 subjects (4 left, 4 right colon)), 24 right and 19 left colon ganglia volumes, 5261 neurons and 16,915 glial cells. (D-M) Manual neuron and glia counts were obtained after HuC/D (blue), S100β (green) and PHOX2B (red) staining. (D,I) Flattened Z-stacks. (E-H,J-M) Single slices and channels from Z-stack. (N,O) To estimate cell density over large regions we multiplied density within small regions (like Figure 2C) by percentage of bowel with myenteric plexus (using images like 2A). We found little variability in cell density within ganglia, indicated by tight data clusters in individuals. Inter-individual differences primarily reflect percentage of bowel occupied by ganglia in each individual. (A,B,D,I) Scale bars=100 μm. (E,F,G,H,J,K,L,M) Use (E) scale bar=25 μm.
Estimates for large areas were generated by multiplying cell numbers within ganglia (counted at high magnification in three-dimensional Z-stacks) by percent colon containing ganglia (measured at low magnification, Figure 2A). Using five regions/individual, cell density estimates clustered within tight ranges (Figure 3N,O). Variation was greater between subjects with more variation in left versus right colon.
We next asked how biopsy size impacts neuron density estimates, recognizing ENS is not uniformly distributed. To do this we divided a single 4x5mm2 region into 20x1mm2 zones and analyzed ganglion density in each zone (Figure 4A,B). If 1mm2 is evaluated, widely divergent estimates of ganglion density (0-17%) occur depending on zone evaluated. Using 4mm2 areas, ganglion density estimates were more tightly clustered, but still ranged 3.5-11.4%. In contrast, narrow ranges were generated analyzing 9mm2 regions. Thus, limited sampling causes diverse enteric neuron density estimates, with greater precision as area evaluated increases.
Figure 4. Size of region evaluated affects estimated myenteric plexus neuron density.
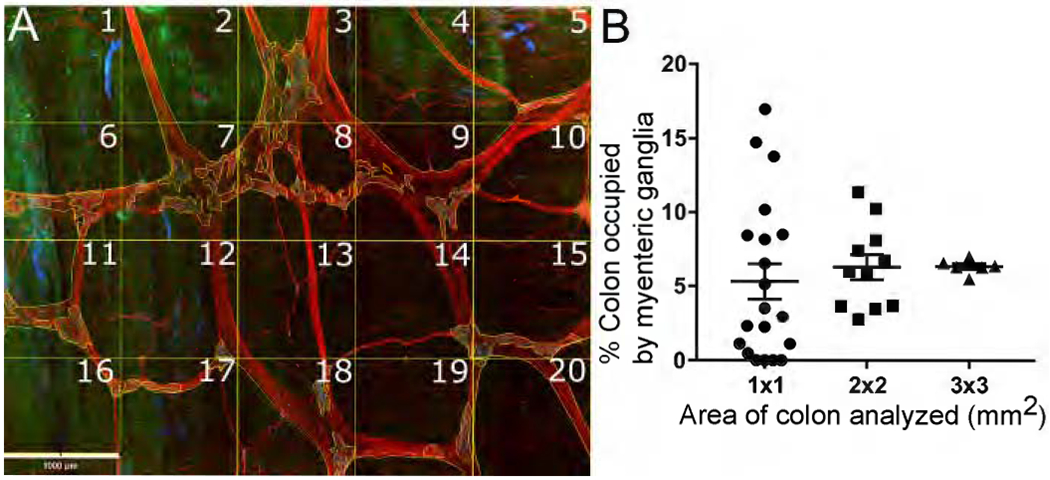
(A) Flattened Z-stack (Figure 2A) was divided into 20 squares (one mm2 each). We determined percentage of each square occupied by ganglia (regions with nerve cell bodies). (B) Estimates of percent colon occupied by ganglia using 1x1mm2, 2x2mm2, or 3x3mm2 regions. Some 1x1mm2 regions have no myenteric plexus; others have up to 17% occupied by ganglia. As size of region evaluated increases, estimates of percent area occupied by ganglia become more uniform. Scale bar=1000μm.
Neuron subtype ratios
Since most myenteric neurons express either nNOS or ChAT and VAChT1, 2, 36–39 we defined ratios of these markers in human colon myenteric plexus using ChAT/nNOS/HuC/D (Figure 5A–H) or ChAT/VAChT/HuC/D staining (Figure 5K–Q). Using Z-stacks to unambiguously distinguish cytoplasmic staining from overlying neurites, we determined percent neurons (HuC/D+) expressing nNOS, ChAT, both, or neither (40 samples, 3359 total neurons evaluated, Supplemental Table 4). In parallel, we compared ChAT to VAChT staining, because murine data suggested ChAT staining is often weak. VAChT was considered “positive” in 1.62-fold more neurons than ChAT, but many VAChT+ cells had faint ChAT staining or high background (Figure 5O–Q, N = 1902 cells). We hypothesize ChAT alone (at least with this antibody) led to systematic cholinergic neuron undercounting. Supplemental Table 4 explains how we adjusted ChAT+ counts based on VAChT/ChAT data to establish “cholinergic” neuron counts (Figure 5I,J). We scored ~50% of myenteric neurons as nNOS+/non-cholinergic (Figure 5I,J), ~28% as cholinergic/nNOS negative, ~5% nNOS+/cholinergic, and ~16% as nNOS−/non-cholinergic. Figure 5I shows inter-individual variability. Supplemental Figure 6 shows ChAT+ neuron diameters were 32% larger than nNOS+ neurons diameters.
Figure 5. Cholinergic/nitrergic neuron ratios.
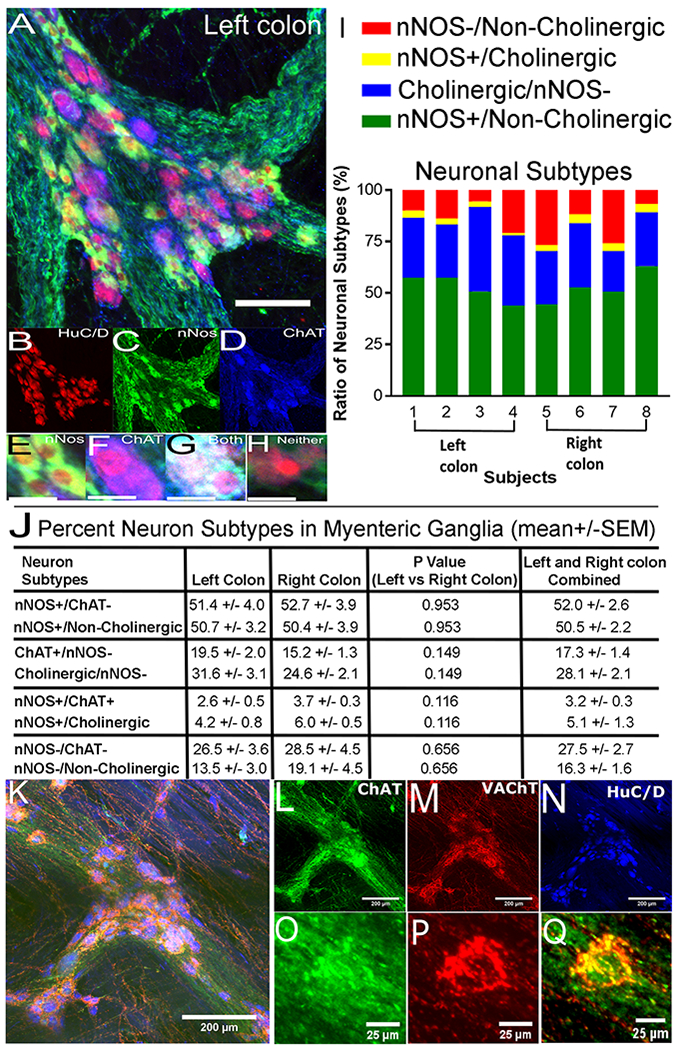
(A) Flattened Z-stack, human colon myenteric ganglion stained for HuC/D (red), nNOS (green) and ChAT (blue). (B-D) Channels for (A). (E-H) Sample neurons from (A): (E) ChAT+/nNOS+, (F) ChAT+/nNOS−, (G) ChAT+/nNOS+, (H) neither ChAT nor nNOS positive. (I) Proportion of neuron classes (8 individuals). (J) Average neuron percentages in each class. (I,J) We initially evaluated 3360 HuC/D+ cells with ChAT and nNOS antibodies. Subsequent analysis of VAChT/ChAT/HuC/D staining showed 1.62-fold more neurons would be identified as cholinergic using combined VAChT/ChAT staining compared to ChAT alone (N = 1902 HuC/D+ cells). “Cholinergic numbers” = “ChAT+ numbers” x 1.62. (K) Flattened Z-stack, human colon myenteric ganglion stained for ChAT (green), VAChT (red) and HuC/D (blue). (L-N) Channels for (K). (O-Q) High magnification single confocal slice of neuron from box in (K). We scored this neuron as VAChT+. ChAT immunoreactivity alone was not well localized and ChAT background too high to score this cell as ChAT+ without VAChT staining. (A) Scale bar=100μm. (B-H,O-Q) Scale bars=25μm. (K-N) Scale bars=200μm. Cell numbers evaluated are in Supplemental Table 4.
Submucosal ganglion location:
Additional parameters could be defined via three-dimensional imaging. For example, human colon submucosal plexus was reported to be in three distinct layers40, but our data suggest ganglia are scattered throughout submucosa, with considerable variability in patterns between evaluated specimens (Supplemental Figure 7). A subset may have ganglia in definable layers.
Hirschsprung disease:
To highlight clinical utility, we stained colon from children with Hirschsprung disease, a problem where enteric neurons are absent from distal bowel. Resected bowel from pull through surgery should contain the entire distal aganglionic region, a hypoganglionic transition zone, and ideally normal ENS at proximal margins. This anatomy is difficult to appreciate in paraffin sections (Figure 6A). In contrast, our three-dimensional method showed proximal resection margin had a rich ENS network (Figure 6C–H). Transition zone was relatively hypoganglionic, with unusual features like rows of neuron along nerve fibers (Figure 6B), myenteric ganglia in more than one layer (Figure 7, slices 23, 29, 42, Supplemental Video 7), thick nerve fiber bundles without neurons (slice 50), and areas without ganglia (slice 144). As expected, distal aganglionic zone was devoid of nerve cell bodies but had extrinsic nerve fibers (Figure 7, Supplemental Video 8).
Figure 6. Whole mount staining in Hirschsprung disease.
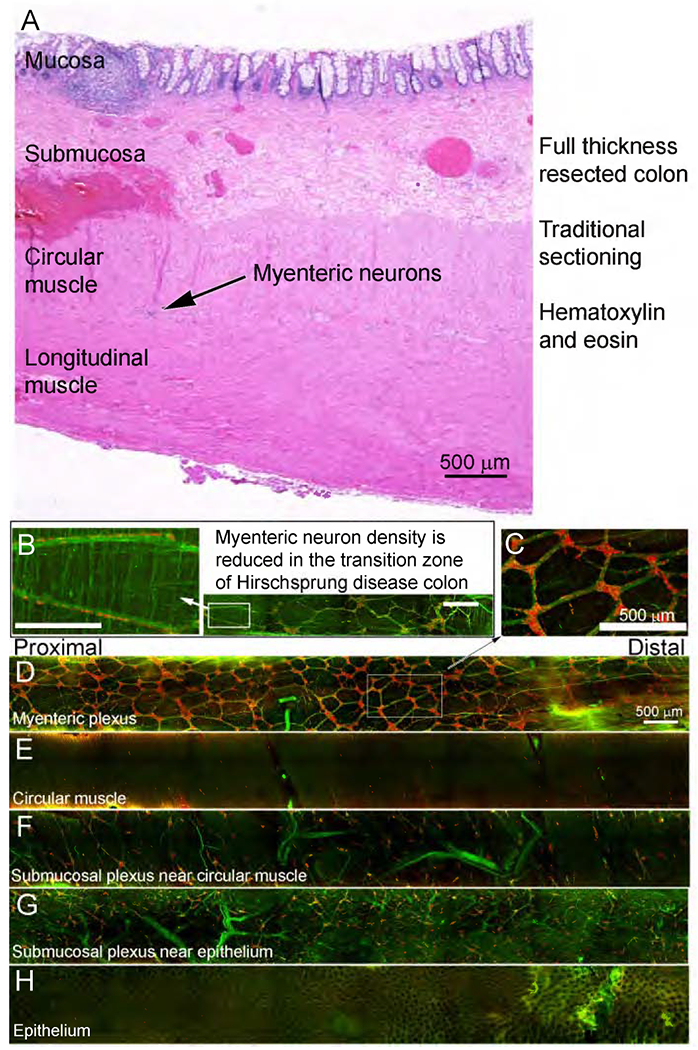
A) Full thickness colon ~ 4.5mm from distal resection margin (H&E stained). (B-H) Two-month old Hirschsprung colon stained for nNOS (green) and HuC/D (red). (B) Magnified region from transition zone with enteric neurons along nerve fibers. (C-H) Most proximal resection margin for Hirschsprung colon. (D-H) Z-stacks slices: (D) 49-97, (E) 95-96, (F) 118-145, (G) 165-176, (H) 208-209. Scale bars=500 μm. Supplemental Video 7 provides additional images from colon region used to generate Figure 6C-H).
Figure 7: Transition zone and distal aganglionic region of Hirschsprung pull through resections.
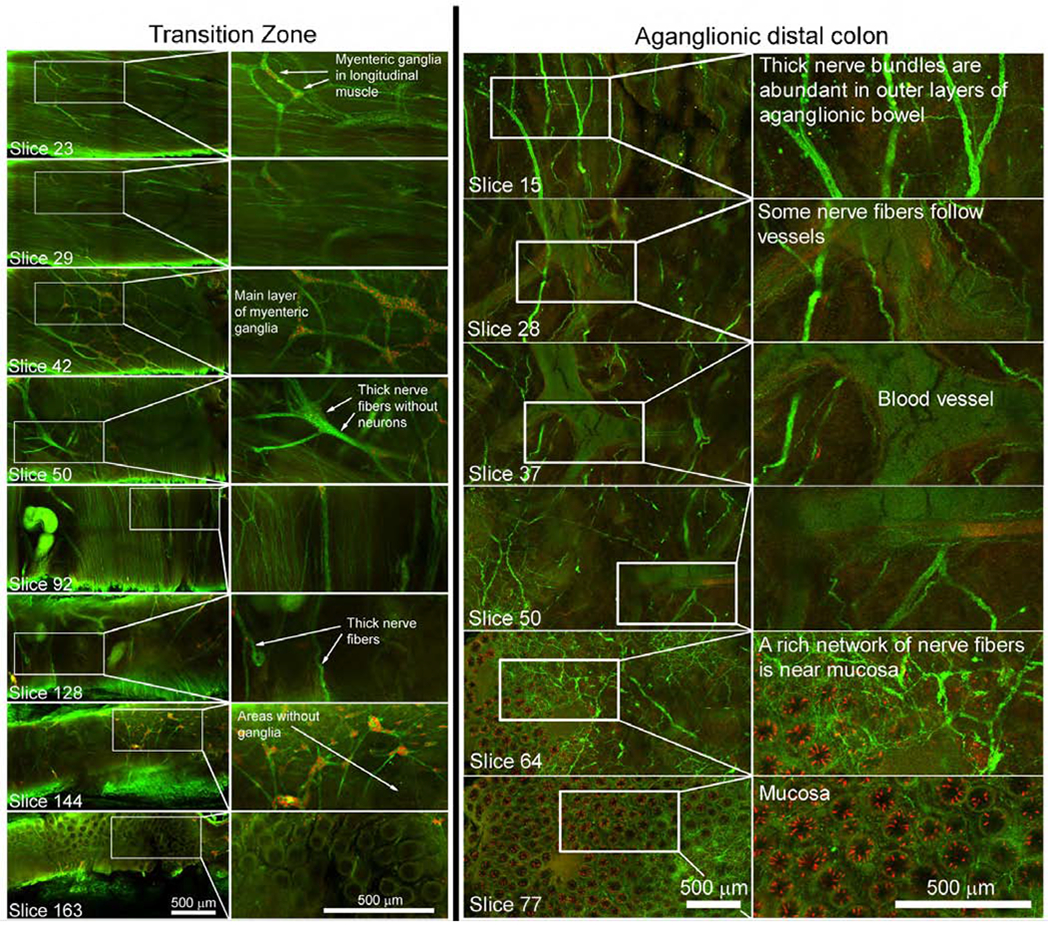
Full thickness Z-stacks stained with HuC/D (red) and nNOS (green) antibodies. Identified single slices from Z-stacks in transition zone (left panel) and aganglionic region (right panel). Higher numbers are closer to mucosa. Scale bars=500μm.
Discussion:
Human bowel was estimated to have ~500 million enteric neurons controlling most aspects of bowel function2, 3. Our data suggest colon alone has ~37 million enteric neurons (~100 neurons/mm2 x 1.4m colon length x 0.15m circumference41 x 1,000,000mm2/m2 x 2.6-fold (stretching) x 0.67-fold (shrinkage with processing)). When ENS is missing or defective, profound bowel dysfunction may occur causing life-threatening problems like Hirschsprung disease22 and neuropathic chronic intestinal pseudo-obstruction26. ENS defects may also underlie achalasia24 and gastroparesis42, where selective nNOS neuron loss was reported. Furthermore, ENS can be damaged by toxins (e.g., chemotherapy)43–45, systemic disease (diabetes, Parkinson’s)46–49, inflammation due to infection50, 51, inflammatory bowel disease52, or necrotizing enterocolitis53, causing long-term dysmotility and visceral hypersensitivity54. Unfortunately, until now, it has been difficult to visualize human ENS in three dimensions. This technical problem limits our understanding of disease mechanisms because full thickness tissue sections often appear “normal” unless changes are dramatic (e.g., complete enteric neuron loss).
Not surprisingly, most of what we know about ENS comes from animal models where bowel is thin and muscle easily dissected from submucosa5, 6, 55. In contrast, human bowel is thick (~1000μm in maximally stretched colon) and muscle layers are difficult to separate from submucosa or each other. For this reason, most human ENS analyses use thin cross-sections (e.g., 5μm), yielding limited data. We hypothesize this problem led to wide variation for “ganglia per 10mm” (13.5-fold range in rectum) and neurons per ganglia (9-fold range in colon) previously reported7. Swaminathan and Kapur further identified a ~150-fold range for “mean number of ganglion cells per cm” in normal colon9 and determined reproducible enteric neuron counts require >5 full circumference sections (estimated at 1.25mm2 = 5 sections x ~0.005mm thick section x ~50mm circumference in 8 week old). Our data suggest 1mm2 still yields quite variable neuron density estimates and that >9 mm2 provides more reliable data. Additional problems arise when evaluating human ENS anatomy using thin sections. ENS organization is difficult to appreciate, so dramatic changes can be missed. Nerve fiber bundle orientation is impossible to discern in sections and small nerve fibers are difficult to see, so changes in neurite density or organization are not appreciated. Decades of mouse work suggests threedimensional imaging provides much greater insight into disease mechanisms and is essential to see many ENS defects (e.g. Supplemental Figure 1). Thus, robust three-dimensional methods to visualize human ENS may provide new insight into bowel motility disorders.
A few prior studies demonstrated human ENS in three-dimensions. Myenteric plexus has been visualized by meticulous “fiber by fiber” removal of longitudinal muscle to expose ENS13, 39, 56. We tried this, but found it difficult to uncover even small regions, and only cells exposed by dissection could be imaged. Three-dimensional human ENS images were also generated by optical clearing and immunohistochemistry using 300μm sections10–12, but we had difficulty replicating this approach, even with significant effort, and little quantitative data were provided from those images.
To overcome these problems, we spent years optimizing clearing and antibody staining for human colon. Our goal was to establish methods that worked well, were easy, and did not require special skill (like microdissection). In addition, we wanted to visualize cells that control bowel motility in three dimensions without sectioning that disrupts cellular connections. For this approach to be useful we need a large library of publicly available images from people without known bowel motility disorders and we need rigorous quantitative data for “normal” ENS anatomy. Our strategy accomplished many of these goals.
Our approach makes colon completely translucent. Large bowel pieces were stained and imaged without sectioning. Confocal imaging permitted visualization of stained cells from serosa to mucosa. Identified antibodies stain neurons, glia, ICC, muscularis macrophages, neuron subtypes and nerve fibers. As expected, some antibodies did not work with our method. We provide 280 three-dimensional Z-stacks of stained adult human colon highlighting how much we miss with traditional tissue sectioning. We performed substantial quantitative analyses to define “normal” adult human colon ENS anatomy. Finally, we show images of Hirschsprung disease colon. This is clinically relevant since “transition zone pull through” is thought to commonly cause Hirschsprung disease post-operative morbidity22, although prolonged processing needed with our approach means it cannot be used for intra-operative decision making. Methods are also applicable to human jejunum and pancreas.
Several observations are worth highlighting. We estimate enteric neurons are <0.1% of total human colon cells. Myenteric and submucosal plexus resembles ENS in other species, but differences in ENS anatomy between species57, 58 means we need human ENS normal values. Irregular ganglia spacing probably explains dramatic variability in reported enteric neuron densities7, 9 since some thin sections include large ganglia and others lack neurons. Within myenteric ganglia, neuron density was fairly uniform (SEM<25% of mean neurons/mm3) and adult human colon had 137+/−20 neurons/myenteric ganglion (Figure 3C: 38,706 neurons/mm3 x 0.0035278 mm3/ganglion = 137 neurons/ganglion). Uniformity of neuron density within myenteric ganglia contrasts with the 1.65-fold difference in ganglion density within myenteric plexus of right versus left colon (P=0.0113). These differences highlight the need to establish region-specific normal ranges for most parameters, although variable ganglion density might simply reflect differences in proximal versus distal colon distensibility.
Enteric glia are much more abundant than neurons even within myenteric ganglia. Our estimates of glial index (glia to neuron ratio) within ganglia (2.5 left; 4.2 right colon) are lower than prior estimates (5.9-7)56, but S100β does not label all glia59, 60. Furthermore, three left colons were from people with diverticulitis where S100β-labeled enteric glia loss was reported61. One novel observation was that PHOX2B-immunoreactive nuclei included S100β+ glia and HuC/D+ neurons, but PHOX2B was not detected in enteric glia outside ganglia. Murine data also show PHOX2B in adult enteric neurons and glia, although mouse images suggest PHOX2B in glia within and outside ganglia62. These data suggest PHOX2B, a gene mutated in some people with Hirschsprung disease22, might influence human enteric glial diversity.
We began to define neuron subtype ratios in human colon myenteric plexus because human intestinal motility disorders may result from quantitative or qualitative ENS defects5, 8,22, 26, 63, 64 and nitric oxide (NO)-producing enteric neurons are particularly susceptible to injury65. Since most myenteric neurons produce either nitric oxide or acetylcholine2, we focused on these subgroups. We found little between-subject variability in non-cholinergic nNOS+ neuron prevalence (~50% of myenteric neurons) similar to prior reports indicating 43-54% of human myenteric neurons were ChAT−/nNOS+ (Supplemental Table 5)36–39, 66–69. Also consistent with past studies reporting 3-10% of human colon myenteric neurons produce nNOS and ChAT, we scored 4-6% of myenteric neurons as nNOS+/cholinergic. We differed substantially from prior studies reporting 36-56% of human colon myenteric neurons were ChAT+/nNOS− and 2-7% ChAT−/nNOS−37–39. Despite using ChAT and VAChT, we scored fewer myenteric neurons as cholinergic/nNOS− (~28%) and more as non-cholinergic/nNOS− (14-19%). We suspect the differences are technical (e.g., less robust staining with this ChAT antibody using our method). We note, however, that neuron subtype ratios vary between mouse strains and are influenced by diet70–75, and human subtype ratios are impacted by age66, diabetes67 and inflammation69, so biological variability between subjects is plausible. Clearly, more remains to be done to define human enteric neuron subtype ratios.
Finally, we include many images from distal Hirschsprung disease colon. Although additional studies are needed to define transition zone and “normal” infant ENS, three-dimensional imaging should facilitate development of intraoperative strategies to avoid transition zone pull through. For example, once transition zone characteristics are well-defined, methods like confocal laser endomicroscopy76 could be used in the operating room to visualize ENS anatomy. Biopsies could also be evaluated three-dimensionally in children treated initially by ostomy, since they undergo pull through surgery months later.
Our study has limitations. Subjects were 28 to 80 years old, with the exception of two infants with Hirschsprung disease. Colons were resected for clinical indications. While we tried to evaluate only pathology-free regions, diverticulitis-associated inflammation could affect ENS. Exact colon regions are not known (i.e., “left” might mean splenic flexure or sigmoid). Specimens were randomly oriented so we cannot distinguish proximal from distal and do not know location along the circumferential colon axis. Finally, technical obstacles should be noted. Staining takes several weeks, so our method cannot be used intraoperatively. Fluorophores fade after immersion in Murray’s Clear, so efficient confocal imaging is required. 3D-imaging takes hours without light sheet microscopy, limiting size of samples that can be imaged. Tissues may dry during prolonged imaging and Murray’s Clear dissolves some plastics. Finally, not all tested antibodies worked well. Nonetheless, our work lays the foundation for future research and suggests new directions for human ENS analyses.
Conclusion:
We can now visualize human ENS in three-dimensions in large colon areas with minimal dissection and no sectioning. Our images make it easy to understand why previously reported enteric neuron density estimates vary up to 150-fold. We hope this method will be widely adopted for defining ENS anatomy in adults and children with life threatening bowel motility disorders where current diagnostic strategies seem most inadequate. We invite others to help us perform quantitative analysis using our images.
Supplementary Material
Acknowledgements:
We thank Pierre Russo for support and encouragement. We appreciate assistance of Deepika Kothakapa, Caitlin Feltcher, Lauren Schmucker, and Andrew Kromer.
Grant support: This work is supported by the Suzi and Scott Lustgarten Endowment (ROH), the Irma and Norman Braman Endowment (ROH), The Children’s Hospital of Philadelphia Research Institute (ROH), NIH grants RO1 DK087715 (ROH), March of Dimes 6-FY15-235 (ROH), The Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (grant no. 1008525) (ROH), Abramson Cancer Center, NIH 5 F30 DK117546-02 (CMW), and NIH SPARC Program OT2OD023859 (to MJH (PI), ROH co-I) and Human Pancreas Analysis Program (HPAP) (URL- https://hpap.pmacs.upenn.edu/), part of Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org) grants UC4 DK112217 (AN).
Study sponsors had no role in study design, data collection, analysis or interpretation of data.
Abbreviations:
- HuC/D
Human HuC / HuD neuronal protein
- nNOS
Neuronal Nitric Oxide Synthase
- CHAT
Choline Acetyltransferase
- VACHT
Vesicular Acetylcholine Transporter
- Tuj1
Beta Tubulin 3
- ROIs
Regions of Interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Authors do not have any relevant conflict of interest.
References:
- 1.Furness JB, Callaghan BP, Rivera LR, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 2014;817:39–71. [DOI] [PubMed] [Google Scholar]
- 2.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012;9:286–94. [DOI] [PubMed] [Google Scholar]
- 3.Schneider S, Wright CM, Heuckeroth RO. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu Rev Physiol 2019;81:235–259. [DOI] [PubMed] [Google Scholar]
- 4.Zeisel A, Hochgerner H, Lonnerberg P, et al. Molecular Architecture of the Mouse Nervous System. Cell 2018;174:999–1014 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol 2013;305:G1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol 2012;366:64–73. [DOI] [PubMed] [Google Scholar]
- 7.Knowles CH, Veress B, Kapur RP, et al. Quantitation of cellular components of the enteric nervous system in the normal human gastrointestinal tract--report on behalf of the Gastro 2009 International Working Group. Neurogastroenterol Motil 2011;23:115–24. [DOI] [PubMed] [Google Scholar]
- 8.Knowles CH, De Giorgio R, Kapur RP, et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut 2010;59:882–7. [DOI] [PubMed] [Google Scholar]
- 9.Swaminathan M, Kapur RP. Counting myenteric ganglion cells in histologic sections: an empirical approach. Hum Pathol 2010;41:1097–108. [DOI] [PubMed] [Google Scholar]
- 10.Liu YA, Chung YC, Pan ST, et al. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol Motil 2013;25:e324–38. [DOI] [PubMed] [Google Scholar]
- 11.Fu YY, Peng SJ, Lin HY, et al. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol 2013;304:G1–11. [DOI] [PubMed] [Google Scholar]
- 12.Liu YA, Chen Y, Chiang AS, et al. Optical clearing improves the imaging depth and signal-to-noise ratio for digital analysis and three-dimensional projection of the human enteric nervous system. Neurogastroenterol Motil 2011;23:e446–57. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth L, Yoneda A, Kader M, et al. Three-dimensional morphology of gut innervation in total intestinal aganglionosis using whole-mount preparation. J Pediatr Surg 2001;36:291–5. [DOI] [PubMed] [Google Scholar]
- 14.Krammer HJ, Karahan ST, Sigge W, et al. Immunohistochemistry of markers of the enteric nervous system in whole-mount preparations of the human colon. Eur J Pediatr Surg 1994;4:274–8. [DOI] [PubMed] [Google Scholar]
- 15.Wattchow DA, Porter AJ, Brookes SJ, et al. The polarity of neurochemically defined myenteric neurons in the human colon. Gastroenterology 1997;113:497–506. [DOI] [PubMed] [Google Scholar]
- 16.Krammer HJ, Karahan ST, Rumpel E, et al. Immunohistochemical visualization of the enteric nervous system using antibodies against protein gene product (PGP) 9.5. Anat Anz 1993;175:321–5. [DOI] [PubMed] [Google Scholar]
- 17.Wedel T, Roblick U, Gleiss J, et al. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat 1999;181:327–37. [DOI] [PubMed] [Google Scholar]
- 18.Keller J, Bassotti G, Clarke J, et al. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol 2018;15:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furness JB. Integrated Neural and Endocrine Control of Gastrointestinal Function. Adv Exp Med Biol 2016;891:159–73. [DOI] [PubMed] [Google Scholar]
- 20.Chambers JD, Bornstein JC, Thomas EA. Insights into mechanisms of intestinal segmentation in guinea pigs: a combined computational modeling and in vitro study. Am J Physiol Gastrointest Liver Physiol 2008;295:G534–41. [DOI] [PubMed] [Google Scholar]
- 21.Blair PJ, Rhee PL, Sanders KM, et al. The Significance of Interstitial Cells in Neurogastroenterology. J Neurogastroenterol Motil 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heuckeroth RO. Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 2018;15:152–167. [DOI] [PubMed] [Google Scholar]
- 23.McKeown SJ, Stamp L, Hao MM, et al. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol 2013;2:113–29. [DOI] [PubMed] [Google Scholar]
- 24.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil 2012;24 Suppl 1:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen LA, Snape WJ Jr. Clinical presentation and pathophysiology of gastroparesis. Gastroenterol Clin North Am 2015;44:21–30. [DOI] [PubMed] [Google Scholar]
- 26.Di Nardo G, Di Lorenzo C, Lauro A, et al. Chronic intestinal pseudo-obstruction in children and adults: diagnosis and therapeutic options. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 27.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 2012;20:256–60. [DOI] [PubMed] [Google Scholar]
- 28.Uesaka T, Jain S, Yonemura S, et al. Conditional ablation of GFRalpha1 in postmigratory enteric neurons triggers unconventional neuronal death in the colon and causes a Hirschsprung’s disease phenotype. Development 2007;134:2171–81. [DOI] [PubMed] [Google Scholar]
- 29.Southard JH, Belzer FO. Organ preservation. Annu Rev Med 1995;46:235–47. [DOI] [PubMed] [Google Scholar]
- 30.Dent JA, Polson AG, Klymkowsky MW. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 1989;105:61–74. [DOI] [PubMed] [Google Scholar]
- 31.Dodt HU, Leischner U, Schierloh A, et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods 2007;4:331–6. [DOI] [PubMed] [Google Scholar]
- 32.Workman MJ , Mahe MM, Trisno S, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hama H, Kurokawa H, Kawano H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci 2011;14:1481–8. [DOI] [PubMed] [Google Scholar]
- 34.Belle M, Godefroy D, Couly G, et al. Tridimensional Visualization and Analysis of Early Human Development. Cell 2017;169:161–173 e12. [DOI] [PubMed] [Google Scholar]
- 35.Erturk A, Mauch CP, Hellal F, et al. Three-dimensional imaging of the unsectioned adult spinal cord to assess axon regeneration and glial responses after injury. Nat Med 2011;18:166–71. [DOI] [PubMed] [Google Scholar]
- 36.Porter AJ, Wattchow DA, Brookes SJ, et al. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology 1997;113:1916–23. [DOI] [PubMed] [Google Scholar]
- 37.Murphy EM, Defontgalland D, Costa M, et al. Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil 2007;19:126–34. [DOI] [PubMed] [Google Scholar]
- 38.Wattchow D, Brookes S, Murphy E, et al. Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterol Motil 2008;20:1298–305. [DOI] [PubMed] [Google Scholar]
- 39.Ng KS, Montes-Adrian NA, Mahns DA, et al. Quantification and neurochemical coding of the myenteric plexus in humans: No regional variation between the distal colon and rectum. Neurogastroenterol Motil 2018;30. [DOI] [PubMed] [Google Scholar]
- 40.Ibba-Manneschi L, Martini M, Zecchi-Orlandini S, et al. Structural organization of enteric nervous system in human colon. Histol Histopathol 1995;10:17–25. [PubMed] [Google Scholar]
- 41.Helander HF, Fandriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol 2014;49:681–9. [DOI] [PubMed] [Google Scholar]
- 42.Farrugia G Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am 2015;44:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa DVS, Bon-Frauches AC, Silva A, et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFkappaB-Dependent Pathway. Sci Rep 2019;9:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macchioni L, Petricciuolo M, Davidescu M, et al. Palmitate lipotoxicity in enteric glial cells: Lipid remodeling and mitochondrial ROS are responsible for cyt c release outside mitochondria. Biochim Biophys Acta Mol Cell Biol Lipids 2018;1863:895–908. [DOI] [PubMed] [Google Scholar]
- 45.Stojanovska V, Sakkal S, Nurgali K. Platinum-based chemotherapy: gastrointestinal immunomodulation and enteric nervous system toxicity. Am J Physiol Gastrointest Liver Physiol 2015;308:G223–32. [DOI] [PubMed] [Google Scholar]
- 46.Chalazonitis A, Rao M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res 2018;1693:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Tredici K, Braak H. Review: Sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathol Appl Neurobiol 2016;42:33–50. [DOI] [PubMed] [Google Scholar]
- 48.Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil 2014;26:611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia 2016;59:404–8. [DOI] [PubMed] [Google Scholar]
- 50.Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest 2015;125:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White JP, Xiong S, Malvin NP, et al. Intestinal Dysmotility Syndromes following Systemic Infection by Flaviviruses. Cell 2018;175:1198–1212 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margolis KG, Gershon MD. Enteric Neuronal Regulation of Intestinal Inflammation. Trends Neurosci 2016;39:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Yang J, Watkins DJ, et al. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther 2013;4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klem F , Wadhwa A, Prokop LJ, et al. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology 2017;152:1042–1054 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young HM, Stamp LA, McKeown SJ. ENS Development Research Since 1983: Great Strides but Many Remaining Challenges. Adv Exp Med Biol 2016;891:53–62. [DOI] [PubMed] [Google Scholar]
- 56.Hoff S, Zeller F, von Weyhern CW, et al. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol 2008;509:356–71. [DOI] [PubMed] [Google Scholar]
- 57.Olsson C, Holmgren S. Autonomic control of gut motility: a comparative view. Auton Neurosci 2011;165:80–101. [DOI] [PubMed] [Google Scholar]
- 58.Christensen J, Stiles MJ, Rick GA, et al. Comparative anatomy of the myenteric plexus of the distal colon in eight mammals. Gastroenterology 1984;86:706–13. [PubMed] [Google Scholar]
- 59.Rao M, Nelms BD, Dong L, et al. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boesmans W, Lasrado R, Vanden Berghe P, et al. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015;63:229–41. [DOI] [PubMed] [Google Scholar]
- 61.Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol 2005;58:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corpening JC, Cantrell VA, Deal KK, et al. A Histone2BCerulean BAC transgene identifies differential expression of Phox2b in migrating enteric neural crest derivatives and enteric glia. Dev Dyn 2008;237:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knowles CH, De Giorgio R, Kapur RP, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol 2009;118:271–301. [DOI] [PubMed] [Google Scholar]
- 64.Uranga-Ocio JA, Bastus-Diez S, Delkader-Palacios D, et al. Enteric neuropathy associated to diabetes mellitus. Rev Esp Enferm Dig 2015;107:366–73. [PubMed] [Google Scholar]
- 65.Rivera LR, Poole DP, Thacker M, et al. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil 2011;23:980–8. [DOI] [PubMed] [Google Scholar]
- 66.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil 2009;21:746–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil 2011;23:131–8, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hetz S , Acikgoez A, Moll C, et al. Age-related gene expression analysis in enteric ganglia of human colon after laser microdissection. Front Aging Neurosci 2014;6:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neunlist M, Aubert P, Toquet C, et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 2003;52:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avetisyan M , Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest 2015;125:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neunlist M, Schemann M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. J Physiol 2014;592:2959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu M, Landreville S, Agapova OA, et al. Retinoblastoma protein prevents enteric nervous system defects and intestinal pseudo-obstruction. J Clin Invest 2013;123:5152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Quelen F, Chevalier J, Rolli-Derkinderen M, et al. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol 2011;589:4341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reichardt F, Chassaing B, Nezami BG, et al. Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. J Physiol 2017;595:1831–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soret R, Chevalier J, De Coppet P, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010;138:1772–82. [DOI] [PubMed] [Google Scholar]
- 76.Sumiyama K, Kiesslich R, Ohya TR, et al. In vivo imaging of enteric neuronal networks in humans using confocal laser endomicroscopy. Gastroenterology 2012;143:1152–1153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


