Abstract
Chronic dysregulated microglial activation may lead to persistent inflammation and progressive neurodegeneration. A previous study reported that ADX88178, a putative metabotropic glutamate receptor 4 (mGluR4) positive allosteric modulator (PAM), exerts anti-inflammatory effects in microglia by activating mGluR4. We employed in vitro models of immortalized microglia cell lines and primary microglia to elucidate the molecular mechanisms responsible for the regulation of inflammatory pathways by ADX88178 and other mGluR4 PAMs. ADX88178 downregulated lipopolysaccharide (LPS)-induced expression of pro-inflammatory mediators, including TNF-α, IL-1β, CCL-2, IL-6, NOS2, miR-155, as well as NO levels, in BV2 cells and primary microglia. Other mGluR4 modulators had divergent activities; VU0361737 (PAM) showed anti-inflammatory effects, whereas the orthosteric group III agonist, L-AP4, and VU0155041 (PAM) displayed no anti-inflammatory actions. In contrast to the earlier report, ADX88178 anti-inflammatory effects appeared to be mGluR4-independent as mGluR4 expression in our in vitro models was very low and its actions were not altered by pharmacological or molecular inhibition of mGluR4. Moreover, we showed that ADX88178 activated Gi-independent, alternative signaling pathways as indicated by the absence of pertussis toxin-mediated inhibition and by increased phosphorylation of cAMP-response element binding protein (CREB), an inhibitor of the NFkB pro-inflammatory pathway. ADX88178 also attenuated NFkB activation by reducing the degradation of IkB and associated translocation of NFkB-p65 to the nucleus. ADX88178 did not exert its anti-inflammatory effects through adenosine receptors, reported as mGluR4 heteromerization partners. Thus, our results indicate that in microglia, putative mGluR4 PAMs activate mGluR4/Gi-independent mechanisms to attenuate pro-inflammatory pathways.
Keywords: mGluR4, microglia, neuroinflammation, metabotropic receptor, lipopolysaccharide
1. Introduction
Brain injury activates pro-inflammatory pathways in glial cells, characterized by increased production of cytokines, chemokines, and microRNAs (miRs) that play important roles in the modulation of damage and repair responses (Perry et al., 2010; Prinz and Priller, 2014). Injury induces diverse microglial phenotypes, some of which serve to promote neurorestoration and repair (Cherry et al., 2014; Colton, 2009; Loane & Kumar, 2016), whereas others contribute to chronic neurotoxic inflammation that can persist from months to years. The pro-inflammatory microglial phenotype is characterized by the release of glutamate and neurotoxic pro-inflammatory molecules such as cytokines including interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, as well as cytotoxic factors such as free radicals and nitric oxide (Barger et al., 2001; Barger et al., 2007; Parker et al., 2002). These sustained inflammatory responses are maladaptive and can contribute to progressive neurodegeneration and long-term neurological dysfunction (Faden et al., 2016; Faden & Loane, 2015; Loane et al., 2014a; Masel et al., 2010).
Glutamate is the principal excitatory neurotransmitter in the brain, which acts on both ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs) (Conn & Pin, 1997; Nicoletti et al., 1996). mGluRs are expressed not only on neurons but also on glial cells, including microglia, and can modulate their functions (Byrnes et al., 2009a; Byrnes et al., 2009b; Loane et al., 2014b; Taylor et al., 2002; Taylor et al., 2003). mGluRs are 7-transmembrane G-protein coupled receptors (GPCR) that are classified into three groups based on their sequence homology, signaling cascade and ligand binding (Conn & Pin, 1997). mGluR4 belongs to group III mGlu receptor that also includes mGlu 6, 7 and 8 receptor subtypes. Similarity in the ligand binding domain of mGluRs has hindered the development of selective agonists that bind directly to the receptor ligand binding site (orthosteric); the lack of subtype selectivity is a major limitation when interpreting pharmacological effects. In contrast, investigation of the role of individual mGluRs has benefited from the more recent development of allosteric modulators. Positive allosteric modulators (PAMs) bind to a separate allosteric site that increases receptor subtype selectivity but may also prime the receptor towards distinct signaling cascades (Foster & Conn, 2017; Kew, 2004; Knoflach et al., 2001; Loane et al., 2009).
We have demonstrated that group I mGluR5 orthosteric agonists and PAMs have strong anti-inflammatory activity at both in vitro models of activated microglia (Byrnes et al., 2009b; Loane et al., 2009; Movsesyan et al., 2004) and in in vivo models of CNS injury (Byrnes et al., 2009a; Loane et al., 2013; Loane et al., 2014b); these observations have been replicated by others (Qiu et al., 2015; Xue et al., 2014; Zhang et al., 2015). Furthermore, we have shown that mGluR5 orthosteric agonist and PAM utilize different signaling mechanisms to exert their anti-inflammatory actions (Bhat et al., 2020).
In a rodent model of Parkinson’s disease, it was reported that the mGluR4 PAM VU0155041 has anti-inflammatory effects associated with decreased levels of Iba1-positive microglia and GFAP-positive astrocytes (Betts et al., 2012). However, it remains unclear if these changes reflect actions mediated through mGluR4. In primary microglia cultures in vitro, expression of mGluR4, mGluR6, and mGluR8 were confirmed at both the mRNA and protein levels and activation of these receptors using the orthosteric group III agonist L-AP4 attenuated microglial activation (Taylor et al., 2003). Although these results suggest that activation of group III mGluR can directly impact microglial responses, L-AP4 lacks subtype specificity and therefore does not allow a precise determination of the group III mGluR subtype responsible for its anti-inflammatory effects.
ADX88178 (5-Methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl) thiazol-2-amine) was initially characterized as a potent mGluR4 PAM that reduced microglia-mediated neuroinflammation in vitro; this anti-inflammatory effect was reported to be attenuated in cultured microglia derived from mGluR4 knockout mice, which suggested that the mGluR4 receptors are necessary for its anti-inflammatory actions (Ponnazhagan et al., 2016). However, the signaling mechanism leading to this effect has not been previously described. Therefore, we examined whether the anti-inflammatory effects of ADX88178 are replicated by other putative mGluR4 PAMs or orthosteric agonists in cultured microglia and if such actions are executed through an mGluR4 canonical Gi-coupled signaling pathway. Our data show that putative mGluR4 agonists have inconsistent anti-inflammatory actions and that anti-inflammatory actions of ADX88178 in microglial cultures appear to involve mGluR4-independent signaling- leading to attenuation of NFkB and CREB phosphorylation.
2. Materials and methods
2.1. Drugs and reagents
mGluR4 PAMs and antagonists: 5-Methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine (ADX88178) was purchased from Sigma. L-(+)-2-Amino-4-phosphonobutyric acid (L-AP4) c/s-2-[[(3,5 Dichlorophenyl)amino]carbonyl]cyclohexanecarboxylic acid sodium salt (VU0155041), N-(4-Chloro-3-methoxyphenyl)-2-pyridinecarboxamide (VU0361737), RS)-α-Methyl-4-phosphonophenylglycine (MPPG), (RS)-α-Cyclopropyl-4-phosphonophenylglycine (CPPG) and (RS)-α-Methylserine-O-phosphate (MSOP) were purchased from Tocris. N-(3-Chloro-4-fluorophenyl)-1H-pyrazolo[4,3-b]pyridin-3-amine (VU0418506) was a generous gift from Dr. Jeffrey Conn (Vanderbilt University). A2aR agonist: 4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride (CGS 21680) and A3 antagonist: 1,4-Dihydro-2-methyl-6-phenyl-4-(phenylethynyl)-3,5-pyridinedicarboxylic acid 3-ethyl-5-[(3-nitrophenyl)methyl] ester (MRS1334) were purchased from Tocris. All drugs were prepared and stored according to manufacturer’s guidelines.
2.2. Drug treatments
mGluR4 PAMs (ADX88178, VU0361737, VU0418506, and VU0155041), and Group III agonist (L-AP4) were applied to microglia 30 minutes prior to LPS stimulation. The group III antagonists (MPPG, CPPG and MSOP), A2AR agonist (CGS 21680), and A3 antagonist (MRS1334) were administered 1 h prior to ADX88178. 10μM PKA inhibitor (H89) or 100μM cell permeable cAMP analogue (dbcAMP) were added 10 minutes prior to addition of ADX88178 (20μM) for 30 minutes, followed by addition of LPS (20ng/mL) for 24h.
2.3. Pertussis toxin
Pertussis toxin was a generous gift from Dr. Nicholas Carbonetti (University of Maryland, Baltimore). BV2 microglia were pre-treated for 6h with 200ng/mL pertussis toxin (PT), a specific inhibitor of Gi/o, or it’s inactive form (PT*) before stimulating the cells with LPS and ADX88178.
2.4. cAMP Assay
BV2 microglia was treated with 1mM of 3-isobutyl-1-methylxanthine (IBMX; Sigma), an inhibitor of cAMP degeneration, for 10 minutes prior to addition of forskolin (100μM) for 10 minutes. BV2 cells were then exposed to ADX88178 for an additional 10 minutes. Cells are washed three times with cold PBS, followed by their lysis in Cell Lysis Buffer 5. Cells were centrifuged at 600xg to remove cellular debris and placed on ice until they were ready to be added to the microplate. cAMP production in BV2 microglia was measured using cAMP Parameter Assay Kit (R&D Systems, Minneapolis, MN) per manufacturer’s protocol. Briefly, primary antibody was added to the microplate provided by the manufacturer for 1h. Wells were aspirated and washed three times with Wash Buffer. 50μL of cAMP Conjugate was added to each well followed by addition of standard, control or samples that were diluted in cell lysis buffer 5. Plate was covered and incubated at room temperature for 2 hours on the shaker. Wash step was repeated followed by the addition of 200μL of Substrate Solution to each well for 30 minutes and then addition of Stop Solution. Absorbance was measured at 450nm using a Synergy HT Multi-Mode Microplate Reader (Biotek). A standard curve was used to calculate the levels of cAMP expressed as pmol/mL.
2.5. BV2 microglia cell culture
BV2 (RRID:CVCL_0182; murine immortalized microglia cells) were grown and maintained in DMEM media (Life Technologies, Carlsbad, CA) that is supplemented with 10% Fetal Bovine Serum (Life Technologies) and 1% penicillin and streptomycin (Millipore Sigma) at 37°C with 5% CO2. BV2 cells were seeded at a density 2×104 cells/well (96-well plate), 0.75×105 cells/well (24-well plate) or 2.5×106 cells (100mm dishes). BV2 cells were incubated with or without various concentrations of mGluR4 PAMs for 30 minutes followed by stimulation with lipopolysaccharide (LPS from Escherichia coli; 20ng/mL; Millipore Sigma) for 6 or 24 hours.
2.6. Primary mouse microglia culture
Primary microglia cultures were prepared from cerebral cortices of P0-P1 C57/BL6 mice as previously described (Loane et al., 2009). In brief, cortices were collected and freed from meninges. Cortices were minced and mechanically dissociated in DMEM/F-12 medium (Life Technologies) and filtered by passing through 40-μm cell strainer. Cells were collected by centrifugation at 1000rpm for 5 minutes and resuspended in DMEM/F-12 medium containing 10% Fetal Bovine Serum (Life Technologies) and 1% penicillin and streptomycin. Cells were cultured on 25 cm2 cell culture flasks for 10–12 days. Floating microglia were harvested from mixed glia by shaking the flasks and reseeding the cells at a density of 50,000 cells/well (96-well plate) for experiments. Forty-eight hours later, cells were treated with mGluR4 PAMs and LPS.
2.7. Gene expression assays
RNA was isolated from cells lysed in Trizol using Direct-zol RNA mini prep kit (ZYMO Research, Irvine, CA). For cDNA synthesis, RNA was reverse-transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) as per manufacturer’s instructions. Thermal cycler reaction parameters for reverse transcription of RNA consisted of 10 minutes at 25°C, 120 minutes at 37°C, 5 minutes at 85°C followed by a hold at 4°C. The cDNA produced by this reaction was the template for the real-time PCR amplification that is carried out by Quant Studio 5 real-time PCR system (ThermoFisher Scientific), using Taqman Universal Master Mix II. Reaction conditions were 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C, 60 seconds at 60°C. The following primers were used in this study: Mm01336189_m1 IL-1β (mouse), Mm00443258_m1 TNF-α (mouse), Mm00446190_m1 IL-6 (mouse), Mm00440502_m1 Nos2/iNOS (mouse), Mm00441242_m1 CCL-2 (mouse), Mm01288386_m1 IL-10 (mouse), Mm00475988_m1 Arg-1 (mouse), Mm99999915_g1 GAPDH (mouse). GAPDH was used as an endogenous control where each gene expression is calculated relative to GAPDH to determine relative expression values (using the 2−ΔΔCt, where Ct is the threshold cycle).
2.8. MicroRNA quantitative PCR
RNA isolation was performed using Direct-zol RNA mini prep kit (ZYMO Research). microRNAs were reverse-transcribed using TaqMan microRNA reverse transcription kit (ThermoFisher Scientific) per manufacturer’s protocol. Thermal cycling conditions used for reverse transcription were 30 minutes at 16°C, 30 minutes at 42°C, 5 minutes at 85°C followed by a hold at 4°C. For real-time PCR amplification of mature miRNA, TaqMan Universal PCR master mix II and TaqMan Small RNA assays were used according to manufacturer’s protocol (mmu480953_mir miR-155, 001973 U6 snRNA). In brief, the reaction setup for real-time PCR of 20μL contain 10μL of TaqMan Universal PCR master mix II, 1μL of TaqMan Small RNA assay, 1.33μL of template cDNA, and 7.67μL nuclease-free water. The cycling condition for real-time PCR involves 40 cycles for enzyme activation step for 10 minutes at 95°C followed by 15 seconds at 95°C and 60 seconds at 60°C. The real-time qPCR results were normalized to endogenous control U6 (Life Technologies) and quantified using comparative Ct method 2−ΔΔCt (Livak and Schmittgen, 2001). PCR reactions were carried out by Quant Studio 5 real-time PCR System (ThermoFisher Scientific).
2.9. Western blotting
Proteins were extracted from whole cell lysates by washing cells with cold PBS three times followed by lysis in RIPA buffer (Teknova, Hollister, CA) containing 1% phosphatase inhibitor cocktail (Phosphatase Inhibitor Cocktail 2 and 3; Millipore Sigma) and protease inhibitor (Millipore Sigma). Proteins were measured using the BCA assay (ThermoFisher Scientific) per manufacturer’s guidelines. 15μg of total protein from each sample was loaded equally to 5–20% SDS-PAGE gradient gels (Bio-rad; Hercules, CA). Proteins were transferred onto nitrocellulose membranes and blocked with 5% BSA in 1X PBS containing 0.01% Tween-20 (PBST) for 1 hour. After blocking, membranes were incubated with primary antibodies, including rabbit anti-metabotropic glutamate receptor 4 (1:500; Abcam), rabbit anti-CREB (1/2000; Cell Signaling), rabbit anti-pCREB (1/2000; Cell Signaling), rabbit anti-NFkB (p65) (1/2000; Cell Signaling), mouse anti-IkB-α (1/2000; Cell Signaling), rabbit anti-H2AX (1/5000; Novus Biologicals), and mouse anti-β-actin (1/20,000; Sigma) overnight at 4°C. After three washes with PBST, membranes were incubated with appropriate HRP-conjugated secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA) for 1h at room temperature. Membranes were washed with PBST and proteins were visualized using Super Signaling West Dura Extended Duration Substrate (ThermoFisher Scientific). Chemiluminescence signal was captured using the ChemiDoc TM XRS+ System and protein bands were quantified using Image J analysis software (NIH, Bethesda, MD). The data reflects the intensity of the target protein normalized to β-actin for total and H2AX for nuclear extracts.
2.10. SiRNA knockdown of GRM4 gene
SMARTpool ON-TARGETplus mouse GRM4 Small Interfering RNA (siRNA) containing a mixture of four targeted siRNAs for mouse mGluR4 and ON-TARGETplus Non-targeting Pool (mock) were purchased from Dharmacon (Lafayette, CO). BV2 microglia cells were seeded in 6-well dishes at density of 0.5×106 cells per well and grown until they reached 60–80% confluency, upon which they were transfected with GRM4 or scrambled siRNAs using Lipofectamine RNAiMAX reagent. Briefly, in separate tubes, Lipofectamine RNAiMAX was diluted in Opti-MEM medium and siRNA was diluted in Opti-MEM. Diluted siRNA was added to diluted Lipofectamine in 1:1 ratio and incubated at room temperature for 5 minutes. siRNA lipid complex was then added to appropriate wells to achieve 25pmol final siRNA concentration. After 6h incubation, the siRNA-lipid complex was removed and cells received normal media until the next day at which point the cells were pre-treated with ADX88178 for 30 minutes, followed by stimulation with LPS and cultured for an additional 24h. GRM4 gene knockdown was confirmed by qPCR.
2.11. Nitrite release
Conditioned media after treatment with ADX88178 or other mGluR4 PAMs and LPS were used to measure nitrite levels using a Griess reagent kit (ThermoFisher Scientific) according to manufacturer’s instruction. Absorbance was measured at 560nm using a Synergy HT Multi-Mode Microplate Reader (Biotek) and normalized with the reference sample. Amount of nitrite (measured in micromoles) released by microglia cells in each sample was calculated by plotting a standard curve.
2.12. TNF-α, IL-1β, and IL-10 ELISAs
Released TNF-α, IL-1β, and IL-10 protein levels were measured from the supernatant of BV2 and primary mouse microglia cells using ELISA kits from R&D Systems (Minneapolis, MN) following manufacturer’s protocol. Briefly, standards and samples were added to a 96-well antibody coated plate and incubated for 2 hours. After several washes with wash buffer, detection antibody was added to the plate for an additional 2 hours at room temperature. Plates were washed and subsequently incubated with horseradish peroxidase-conjugated streptavidin for 20 minutes at room temperature in dark. After several washes, Substrate Solution was added for 30 minutes followed by Stop Solution. Absorbance was measured at 450nm using a Synergy HT Multi-Mode Microplate Reader (Biotek). standard curves were used to calculate levels of released TNF-α, IL-1β, and IL-10 that were expressed in pg/mL. (In Figure 3, they are represented as % of LPS).
2.13. Cell viability assay
Cell viability was measured using a tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) colorimetric end-point assay. After treatment of microglia cultures, 10μL of MTT reagent was added to the cells that contained 100μL of media for a final concentration of 0.5mg/mL of MTT. Development of formazan crystals was monitored continuously while the plate remained in 37°C incubator. After 30 minutes to 1h, formazan crystals were dissolved via addition of 200μL of DMSO or ethanol to each well. Absorbance was measured at 540nm using a Microplate Reader (Biotek). Cytotoxicity was measured using MTT assay and cells images were captured via EVOS cell imaging microscope system (Supplementary Fig. 3). Absorbance measured was directly proportional to cell viability and was compared with non-stimulated control cells.
2.14. Subcellular fractionation
Pre-conditioned media was removed from cells and cells were collected using a cell lifter and washed in ice-cold 1X PBS. Cell suspension was centrifuged at 500xg for 5 minutes at 4°C. Cell pellets were resuspended in digitonin lysis buffer (20mM HEPES, PH 7.2, 80mM KCL, imM EDTA, imM EGTA, imM dithiothreitol, 250mM sucrose, 200μg/mL digitonin, Phosphatase Inhibitor Cocktails 2 and 3 and Protease Inhibitor) for i0 minutes on ice. The lysates were then centrifuged at 1,000xg for 5 minutes at 4°C to pellet the nuclei. The supernatants were transferred to new tubes and centrifuged at 12,000xg for 10 minutes at 4°C to pellet the mitochondria. The supernatants were saved in new tubes and centrifuged at 12,000xg for 10 minutes at 4°C to yield the cytosolic fractions. Total and nuclear lysates were prepared in RIPA buffer for western blotting according to previously described protocol.
2.15. Statistical analysis
Statistical analyses were performed using GraphPad Prism software (San Diego, CA). Values of all experiments are represented as mean ± S.E.M. of at least three independent experiments. Values were compared using one-way ANOVA analysis of variance with Tukey’s post hoc correction. The level of significance was set at *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
3. Results
3.1. ADX88178 attenuates LPS-induced activation of pro-inflammatory pathways, while L-AP4 shows no anti-inflammatory effect in BV2 microglia
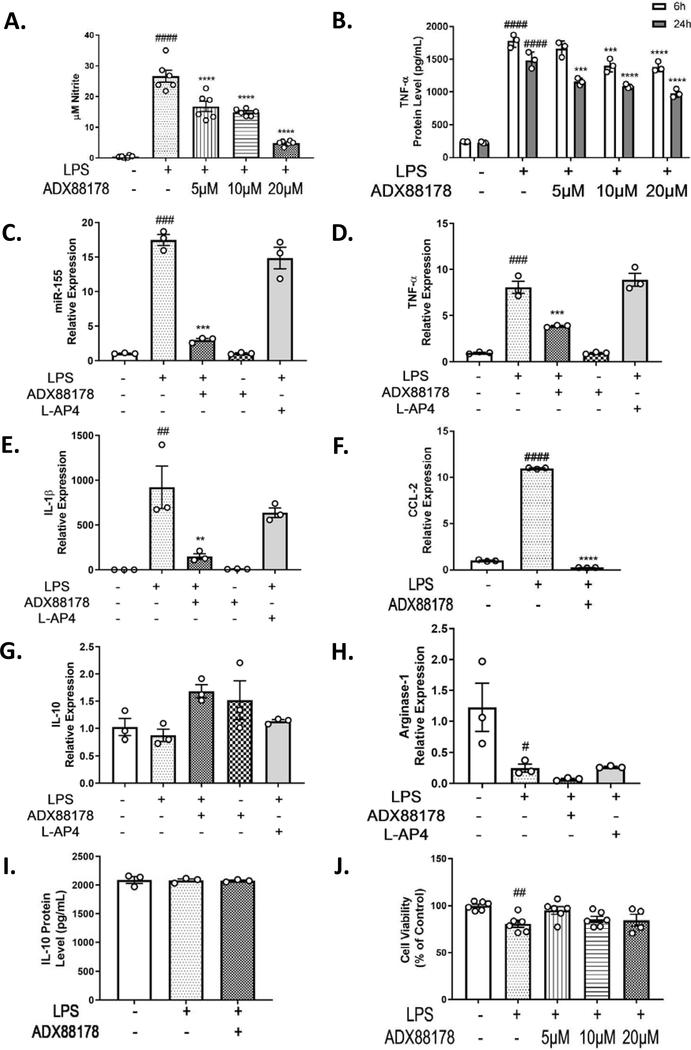
To examine mGluR4 PAM ADX88178 and group III orthosteric agonist L-AP4 anti-inflammatory effects in microglia culture, BV2 microglia were incubated with either ADX88178 or L-AP4 and then stimulated with LPS. LPS concentration response showed LPS 20ng/mL was the lowest LPS concentration that gave a robust nitrite release in BV2 microglia (control vs. LPS 20ng/mL; P<0.0001; Supplementary Fig. 1). Nitrite levels (NO) in the supernatant of BV2 cells stimulated with LPS were significantly increased compared to control cells (control vs. LPS; P<0.0001; Fig. 1A). In our initial concentration-response studies in BV2 microglia, we evaluated the effect of ADX88178 at nanomolar (nM) concentrations in LPS-stimulated BV2 microglia but did not detect significant effects at these low concentrations (Supplementary Fig. 2A). Pretreatment with ADX88178 at 20μM was the lowest concentration that showed optimal inhibition of LPS-induced NO upregulation (Supplementary Fig. 2B). There is evidence that unlike murine models, human macrophages/microglia may have an impaired NO response to inflammatory stimuli due in part to epigenetic inhibition of inducible NO synthase (Gross et al., 2014). In the current study, we used NO as one of several markers of upstream inflammatory cascades and their regulation. We measured TNF-α protein levels at 6h and 24h time points. There was a robust increase in released TNF-α after LPS stimulation followed by a significant concentration-dependent decrease in the presence of ADX88178. As the maximal effect of ADX88178 was observed at 20μM for both time points (24h LPS vs. 24h LPS+ADX88178 20μM, P<0.0001; Fig. 1B), this concentration was used for the remainder of our experiments. We evaluated mRNA expression levels of pro-inflammatory cytokines (TNF-α and IL-1β), inflammatory microRNA (miR-155), chemokine (CCL-2), and anti-inflammatory cytokines (Arginase-1 and IL-10) after treatment with 20μM ADX88178 or 100μM L-AP4. LPS stimulation of BV2 microglia significantly increased expression of miR-155 (LPS vs. control, P<0.001; Fig. 1C), TNF-α (LPS vs. control, P<0.001; Fig. 1D), IL-1β (LPS vs. control, P<0.01; Fig. 1E), and CCL-2 (LPS vs. control, P<0.0001; Fig. 1F). ADX88178 reduced LPS-stimulated levels of the following inflammatory mediators in BV2 microglia: miR-155 (LPS vs. LPS+ADX88178, P<0.001; Fig. 1C), TNF-α (LPS vs. LPS+ADX88178, P<0.001; Fig. 1D), IL-1β (LPS vs. LPS+ADX88178, P<0.01; Fig. 1E), and CCL-2 (LPS vs. LPS+ADX88178, P<0.0001; Fig. 1F); however, L-AP4 did not attenuate any of these LPS-induced inflammatory mediators. Neither ADX88178 nor L-AP4 significant changed mRNA expression of anti-inflammatory cytokines such IL-10 and Arginase-1 (Fig. 1G–H). To confirm, we performed ELISA to measure protein level of anti-inflammatory marker IL-10 in the presence or absence of ADX88178, and we found no significant changes between the treatment groups (Fig. 1I). Treatment with increasing concentrations of ADX88178 did not adversely affect BV2 microglia cell viability when compared with control cells (Fig. 1J). To further show similar cell viability between the treated groups, we took cell images of control, LPS and LPS+ADX88178 treated BV2 cells (Supplementary Fig. 3).
Fig. 1. Effect of ADX88178 and L-AP4 on LPS-induced up-regulation of NO release and pro-inflammatory markers at mRNA and protein levels in BV2 cells.
BV2 microglia cells were pre-treated with ADX88178 (5–20μM) or L-AP4 (100μM) for 30 minutes followed by stimulation with or without LPS (20ng/mL) for the next 24 hours. (A-B) LPS stimulation resulted in a significant increase in nitrite production and TNF-α release (####P<0.0001 vs. control for both). ADX88178 treatment significantly reduced LPS-induced nitrite production starting at 5μM (****P<0.0001 vs. LPS), and TNF-α release at 20μM for both 6h and 24h time points (****P<0.0001 vs. LPS). (C-F) mRNA expression of pro-inflammatory mediators in BV2 microglia were measured at 24h. While L-AP4 exerted no anti-inflammatory effect, ADX88178 pre-treatment resulted in a significant downregulation of LPS-induced mRNA levels of miR-155 and TNF-α (***P<0.001 vs. LPS for both), IL-1β (**P<0.01 vs. LPS) and CCL-2 (****P<0.0001 vs. LPS). (G-H) There was no significant changes in mRNA expression levels of anti-inflammatory cytokines IL-10 and Arginase-1. (I) There was no change in protein levels of IL-10 as measured by IL-10 ELISA. (J) There was no cell cytotoxicity at concentrations of ADX88178 used. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons with post hoc Tukey’s test. ##P<0.01, ###P<0.001, ####P<0.0001 in comparison with control; **P<0.01, ***P<0.001, ****P<0.0001 in comparison with LPS.
3.2. The effects of various mGluR4 agonists vs. ADX88178 on NO release in models of activated microglia cell lines
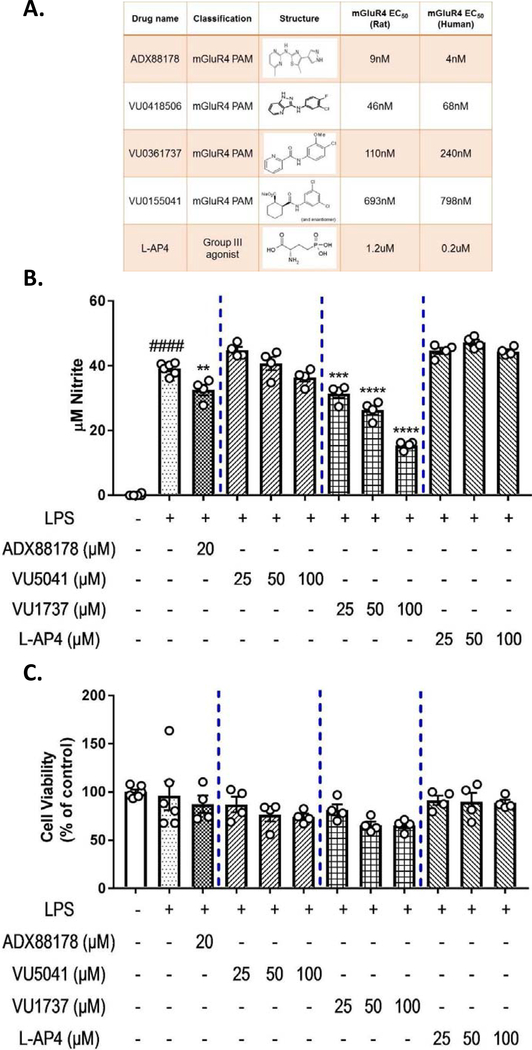
Newly developed mGluR4 PAMs have better Group III subtype selectivity and improved blood brain barrier penetration (Bollinger et al., 2019; Engers et al., 2009; Niswender et al., 2008; Stansley & Conn, 2019; Williams et al., 2009). We examined the effects of multiple mGluR4 PAMs- ADX88178, VU0361737, VU0155041- as well as the orthosteric group III agonist L-AP4 on microglial activation (Fig. 2A). Concentration-response experiments demonstrated that these drugs have divergent anti-inflammatory actions. LPS treated BV2 microglia showed a robust increase in nitrite (control vs. LPS, P<0.0001; Fig. 2B). Pretreatment with ADX88178 caused a significant decrease in levels of NO (LPS vs. LPS+ADX88178 20μM, P<0.01; Fig. 2B). Pre-treatment with VU0361737 also significantly decreased NO levels in a concentration-dependent manner (LPS vs. LPS+VU0361737 100μM, P<0.0001; Fig. 2B). Neither VU0155041 nor L-AP4, at any concentration, reduced LPS-induced NO levels in BV2 microglia (Fig. 2B). To confirm the NO results from BV2 microglia, we examined the effects of these drugs in LPS+IFN-γ stimulated immortalized rat HAPI microglia (Supplementary Fig. 4A). Similarly, ADX88178 (LPS+IFN-γ vs. LPS+IFN-γ+ADX88178 20μM, P<0.0001; Supplementary Fig. 4A) and VU0361737 (LPS+IFN-γ vs. LPS+IFN-γ+VU0361737 100μM, P<0.0001; Supplementary Fig. 3A) exerted the most potent anti-inflammatory effects as measured by released nitrite levels. L-AP4 showed no anti-inflammatory activity, and while VU0155041 had a very modest effect at a very high concentration, VU0418506 showed no anti-inflammatory activity in stimulated HAPI microglia (Supplementary Fig. 4A). To confirm the results of VU0155041 and VU0418506, we evaluated their effects on TNF-α ELISA in LPS-stimulated microglia (Supplementary Fig. 5A–B). While LPS caused a significant increase in TNF-α release in primary mouse microglia (control vs. LPS, P<0.001; Supplementary Fig. 5A) and BV2 microglia (control vs. LPS, P<0.0001; Supplementary Fig. 5B), neither VU0155041 nor VU0418506 attenuated LPS-induced TNF-α protein levels (Supplementary Fig. 5A–B). The cytotoxicity of these drugs was measured using the MTT assay. Treatment of BV2 microglia with the indicated drugs and LPS did not adversely affect cell viability when compared with control cells (Fig. 2C).
Fig. 2. Concentration-response of mGluR4 PAMs vs. ADX88178 for LPS-induced changes in BV2 microglia.
BV2 microglia were pre-treated with ADX88178 and other mGluR4 PAMs and mGluR4 agonist for 30 minutes followed by stimulation with or without LPS (20ng/mL) for the next 24 hours. (A) Representative table shows chemical structure, EC50 and classification of drugs used in this study belonging to the mGluR4 family. (B) LPS stimulation of BV2 microglia resulted in a significant increase in nitrite production (####P<0.0001 vs. control). Pre-treatment with 20μM of ADX88178 significantly reduced LPS-induced nitrite production (**P<0.01 vs. LPS). Pre-treatment with VU0361737 (shown with the last four digits of the drug as VU1737) starting at 25μM also reduced NO levels significantly upon LPS addition (***P<0.001 vs. LPS), while mGluR4 PAM VU0155041 (shown with the last four digits of the drug as VU5041) and orthosteric group III agonist L-AP4 did not attenuate NO production following LPS exposure. (C) There was no cytotoxicity at concentrations of these drugs used. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; **P<0.01, ***P<0.001, ****P<0.0001 in comparison with LPS.
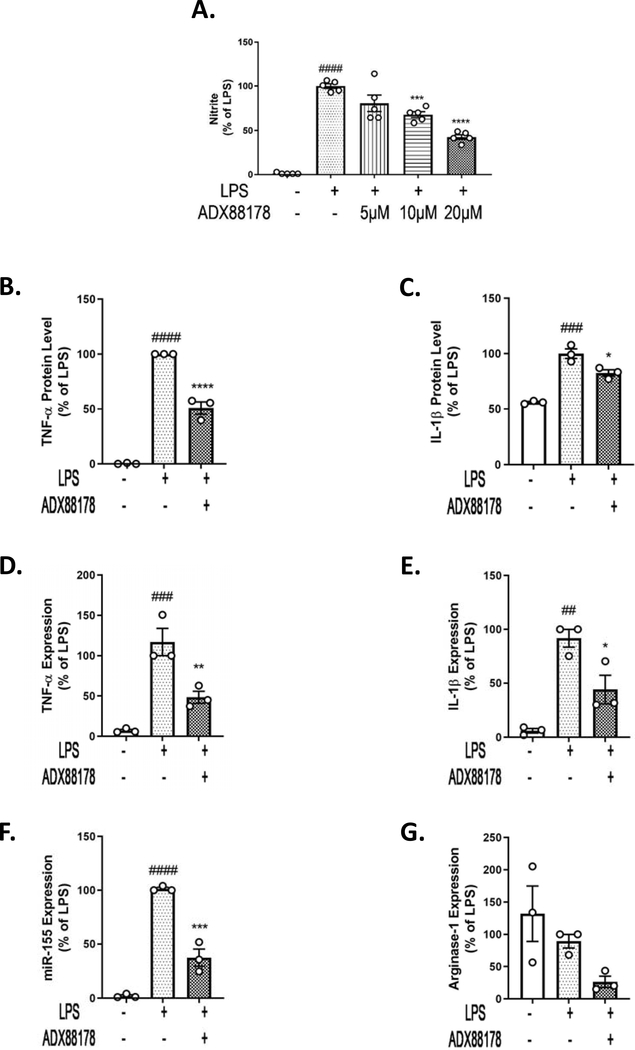
3.3. ADX88178 attenuates LPS-induced activation of inflammation pathways in mouse primary microglia
We validated results obtained in BV2 microglia using primary cultured microglia from mouse brain. There was a significant increase in nitrite levels in the supernatant of primary microglia upon LPS stimulation (control vs. LPS; P<0.0001; Fig. 3A). Pretreatment with ADX88178 caused a concentration-dependent reduction of nitrite levels, with 20μM causing the most significant attenuation in NO levels (LPS vs. LPS+ADX88178 20μM; P<0.0001; Fig. 3A). There was a robust increase in TNF-α (control vs. LPS, P<0.0001; Fig. 3B) and IL-1β (control vs. LPS, P<0.001; Fig. 3C) protein levels after LPS stimulation, which were significantly reduced by ADX88178 (TNF-α: LPS vs. LPS+ADX88178, P<0.0001; Fig. 3B, IL-1β: LPS vs. LPS+ADX88178, P<0.05; Fig. 3C). The mRNA expression of pro-inflammatory cytokines (TNF-α and IL-1β), inflammatory microRNA (miR-155), as well as anti-inflammatory Arginase-1 were also evaluated. LPS stimulation significantly increased expression of TNF-α (control vs. LPS, P<0.001; Fig. 3D), IL-1β (control vs. LPS, P<0.01; Fig. 3E), and miR-155 (control vs. LPS, P<0.0001; Fig. 3F). Similar to data obtained from BV2 microglia, ADX88178 reduced LPS-stimulated levels of the following inflammatory mediators in primary microglia: TNF-α (LPS vs. LPS+ADX88178, P<0.01; Fig. 3D), IL-1β (LPS vs. LPS+ADX88178, P<0.05; Fig. 3E), and miR-155 (LPS vs. LPS+ADX88178, P<0.001; Fig. 3F). No changes in the mRNA level of Arginase-1 was observed for any of the treatments (Fig. 3G).
Fig. 3. ADX88178 attenuates LPS-induced up-regulation of pro-inflammatory markers at the mRNA and protein levels in mouse primary microglia.
Primary microglia cells were pre-treated with ADX88178 (5–20μM) for 30 minutes followed by stimulation with or without LPS (20ng/mL) for 24 hours. (A-C) LPS stimulation resulted in a significant increase in nitrite production, TNF-α release (####P<0.0001 vs. control for both) as well as IL-1β release (###P<0.001 vs. control). Pre-treatment with 20μM of ADX88178 significantly reduced LPS-induced nitrite production (****P<0.0001 vs. LPS), released TNF-α (****P<0.0001 vs. LPS), and IL-1β protein levels (*P<0.05 vs LPS). (D-F) Expression of pro-inflammatory mediators in mouse primary microglia were measured at 24h. ADX88178 pre-treatment resulted in a significant downregulation of LPS-induced mRNA levels of TNF-α (**P<0.01 vs. LPS), IL-1β (*P<0.05 vs. LPS), and miR-155 (***P<0.001 vs. LPS). (G) Level of anti-inflammatory Arginase-1 remained unchanged between treatments. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons with post hoc Tukey’s test. ##P<0.01, ###P<0.001, ####P<0.0001 in comparison with control; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 in comparison with LPS.
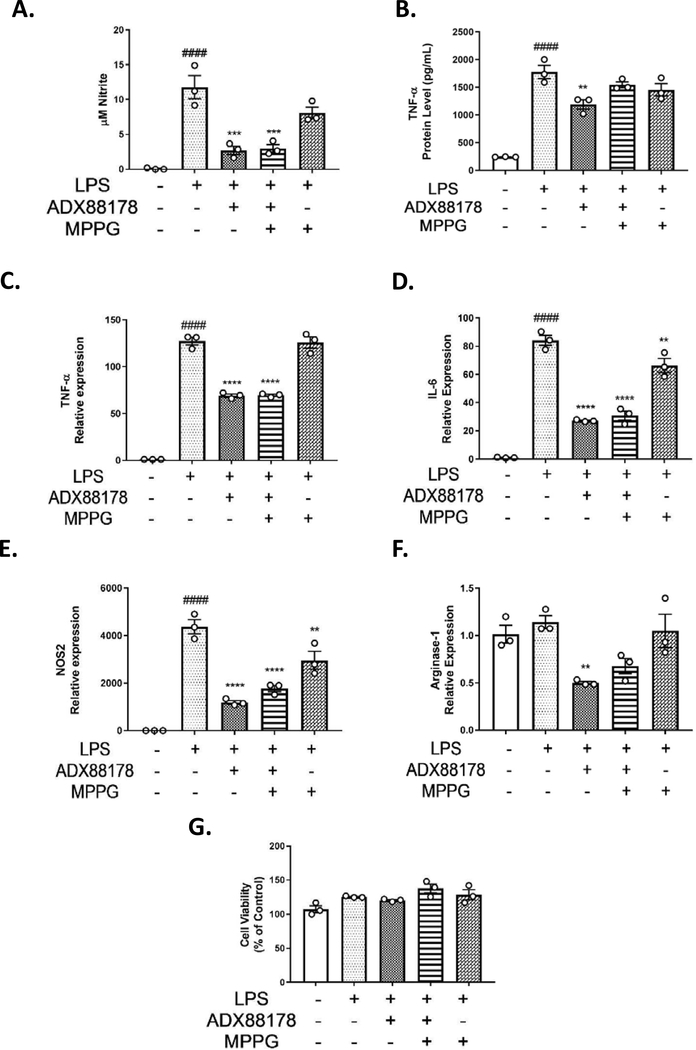
3.4. Effect of the mGluR4 antagonist MPPG on LPS-stimulated mouse primary microglia
Mouse primary microglia were used to examine the effects of partially selective group III inhibitor, MPPG, on anti-inflammatory actions of ADX88178 to address whether the anti-inflammatory effects were mediated by mGlu4 receptor. Addition of LPS to primary microglia caused a robust increase in production of nitrite as well as TNF-α protein levels as measured by ELISA at 24 hours (control vs. LPS, P<0.0001; Fig. 4A–B). Pretreatment with ADX88178 significantly decreased levels of LPS-stimulated NO (LPS vs. LPS+ADX88178, P<0.001; Fig. 4A) and TNF-α (LPS vs. LPS+ADX88178, P<0.01; Fig. 4B). There was no inhibition of ADX88178 anti-inflammatory effects in the presence of MPPG as indicated by NO release and TNF-α ELISA (Fig. 4A–B). LPS stimulation of primary microglia significantly increased mRNA expression of TNF-α, IL-6, and NOS2 (control vs. LPS, P<0.0001 for all three genes; Fig 4C–E). Pre-treatment with ADX88178 reduced LPS-stimulated levels of TNF-α, IL-6 and NOS2 (LPS vs. LPS+ADX88178, P<0.0001 for each gene; Fig. 4C–E), but addition of MPPG did not reverse anti-inflammatory actions of ADX88178 as measured by these markers. No significant change in the mRNA expression of Arginase-1 in MPPG-treated LPS+ADX88178 was found (Fig. 4F). Using the MTT assay in primary microglia, there was no cytotoxicity at any drug concentrations used (Fig. 4G).
Fig. 4. Effect of mGluR4 antagonist, MPPG, in LPS-stimulated mouse primary microglia.
Primary microglia cells were pre-treated with group III antagonist, MPPG, for 1 hour prior to addition of ADX88178 for 30 minutes followed by stimulation with or without LPS (20ng/mL) for 24 hours. (A-B) LPS stimulation resulted in a significant increase in nitrite production and TNF-α release (####P<0.0001 vs. control for both). Pre-treatment with 20mM of ADX88178 significantly reduced LPS-induced nitrite production (***P<0.001 vs. LPS) and TNF-α levels (**P<0.001 vs. LPS). However, MPPG failed to inhibit anti-inflammatory actions of ADX88178 as measured by NO levels and TNF-α protein levels. (C-E) Expression of pro-inflammatory mediators in primary microglia was measured at 6h. LPS stimulation of primary microglia significantly increased expression of TNF-α, IL-6, and NOS2 (####P<0.0001 vs. control for all three genes). ADX88178 pre-treatment resulted in a significant downregulation of LPS-induced levels of TNF-α, IL-1β, and NOS-2 (****P<0.0001 vs. LPS for all three genes). However, MPPG failed to inhibit the anti-inflammatory actions of ADX88178 as measured by these pro-inflammatory gene expressions. (F) There were no significant changes in the gene expression of anti-inflammatory mediator Arginase-1. (G) There was no cytotoxicity at the concentrations of drugs used as measured by MTT assay. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; **P<0.01, ***P<0.001, ****P<0.0001 in comparison with LPS.
3.5. ADX88178 promotes CREB phosphorylation and NFkB inhibition in LPS-activated BV2 microglia
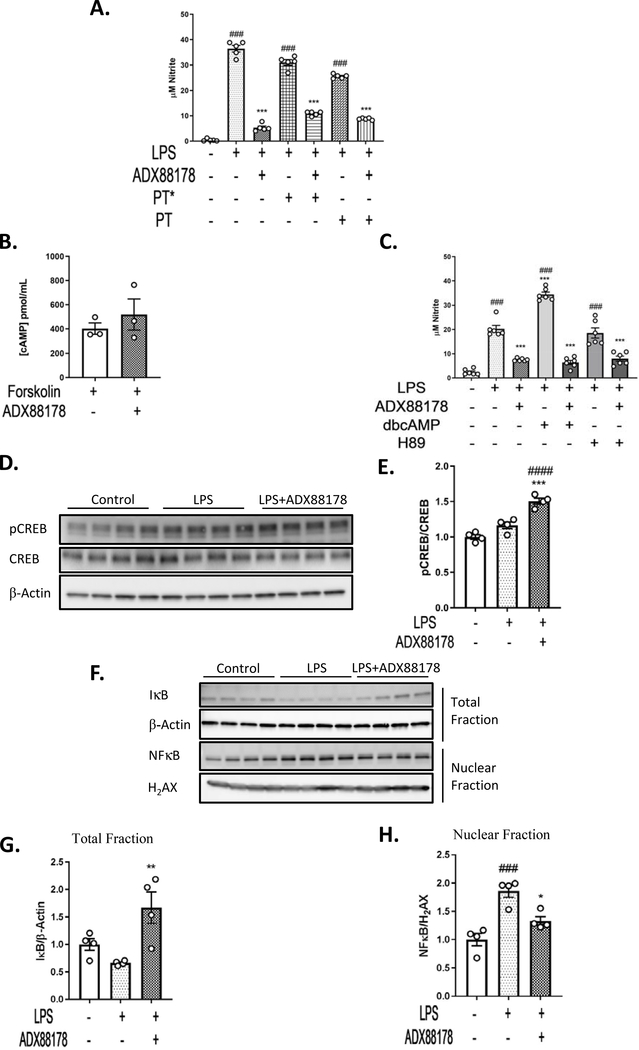
We explored several putative signaling pathways by which ADX88178 may exert its anti-inflammatory actions. Studies have demonstrated that mGluR4 may exert its effects through involvement of Gαi/o (Nicoletti et al., 2011; Niswender & Conn, 2010; Pin & Duvoisin, 1995; Taylor et al., 2003). We investigated important players involved in this signaling pathway in BV2 microglia. Starting at the receptor level, we used pertussis toxin (PT) to inhibit Gαi/o. PT is an active form of pertussis toxin and PT* is an inactive form. There was a significant increase in NO in LPS alone, LPS+PT* and LPS+PT groups (P<0.001 for all three; Fig. 5A) and ADX88178 significantly inhibited NO in LPS alone, LPS+PT* and LPS+PT treatment groups (P<0.001 for all three; Fig. 5A) indicating that ADX88178 does not utilize a PT-sensitive Gαi/o signaling in BV2 microglia. Downstream of Gαi/o receptor, we examined cAMP levels in BV2 microglia in the presence of adenylyl cyclase activator, forskolin. We did not observe any effect of ADX88178 in reducing forskolin-induced cAMP levels (Fig. 5B). Next, we evaluated the effect of upregulation of cAMP through cell permeable cAMP analogue dbcAMP and inhibition of protein kinase A (PKA) through H89. Others have used these compounds in evaluating cAMP-PKA-CREB pathway (Johannessen et al., 2004; Jin et al., 2009; Petrova et al., 1999). LPS+dbcAMP significantly increased NO levels in compare to LPS alone (LPS vs. LPS+dbcAMP, P<0.001; Fig. 5C). However, dbcAMP did not attenuate ADX88178 anti-inflammatory effect. LPS+H89 treatment increased nitrite levels similar to LPS alone (P<0.001 for both; Fig. 5C), and it did not have an additive anti-inflammatory effect to ADX88178. Downstream of PKA is cAMP-response element binding protein (CREB) that is involved in neuroimmune modulation (Moon et al., 2005; Wen et al., 2010; Zou et al., 2017). ADX88178 significantly induced activation of CREB as indicated by increased levels of CREB specific phosphorylation (Ser-133) in LPS-stimulated BV2 microglia (LPS vs. LPS+ADX88178, P<0.001; Fig. 5E). NFkB is an important regulator of pro-inflammatory gene expression-including TNF-α, IL-1β and IL-6 in LPS-activated microglia (Kopitar-Jerala, 2015; Shih et al., 2015). Therefore, we examined the effect of ADX88178 on the NFkB pathway. LPS signals through Toll-Like Receptor 4 (TLR-4) resulting in the degradation of cytoplasmic inhibitory subunit (IkB) and release NFkB (p65) complex into the nucleus. We performed subcellular fractionation to quantify nuclear expression of NFkB and total expression of IkB via western blot. ADX88178 significantly increased IkB levels in the total fraction (LPS vs. LPS+ADX88178, P<0.01; Fig. 5G). Moreover, while LPS caused translocation of NFkB from cytosol into nucleus (control vs. LPS, P<0.001; Fig. 5H), ADX88178 inhibited these changes (LPS vs. LPS+ADX88178, P<0.05; Fig. 5H).
Fig. 5. ADX88178 Promotes CREB Phosphorylation and NFkB Inhibition in LPS-Activated BV2 Microglia.
The mechanism of action of ADX88178 was evaluated by assessing components of classical Gαi/o pathway as well as the non-classical NFkB pathway. (A) Treatment with pertussis toxin (PT) did not inhibit anti-inflammatory action of ADX88178. LPS caused a significant increase in NO levels in LPS alone, LPS+PT* and LPS+PT groups (###P<0.001 for all three). ADX88178 attenuated NO in LPS alone, LPS+PT* and LPS+PT (***P<0.001 for all three). (B) ADX88178 did not attenuate forskolin-induced cAMP level in BV2 microglia as measured by cAMP ELISA. (C) LPS alone and LPS+H89 increased released nitrite levels in BV2 microglia compared to control (###P<0.001 for all three treatments) while LPS+dbcAMP significantly increased NO levels compared to LPS (***P<0.001, LPS vs. LPS+dbcAMP). ADX88178 significantly decreased LPS-induced NO levels in all three treatments (***P<0.001). (D) BV2 cells were pretreated with ADX88178 for 30 minutes followed by stimulation of LPS (20ng/mL) for 30 minutes. Whole cell lysates were used for Western blot analysis. Representative blots for pCREB and CREB. (E) Densitometric analysis shows a significant increase in pCREB/CREB protein level in LPS+ADX88178 treated BV2 microglia compared to LPS alone (***P<0.001, LPS vs. LPS+ADX88178). (F) Representative blots for nuclear and total BV2 microglia fractions. (G) ADX88178 treatment significantly increased IkB protein expression in the total fraction (**P<0.01, LPS total vs. LPS+ADX88178). (H) LPS stimulation of BV2 microglia significantly increased NFkB protein expression in the nuclear fraction (###P<0.001, control vs. LPS) while ADX88178 significantly decreased NFkB nuclear translocation (P<0.05, LPS vs. LPS+ADX88178). All data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ###P<0.001, ####P<0.0001 in comparison with control; *P<0.05, **P<0.01, ***P<0.001 in comparison with LPS for each treatment.
3.6. ADX88178 anti-Inflammatory actions in BV2 microglia appear to be mGluR4-independent
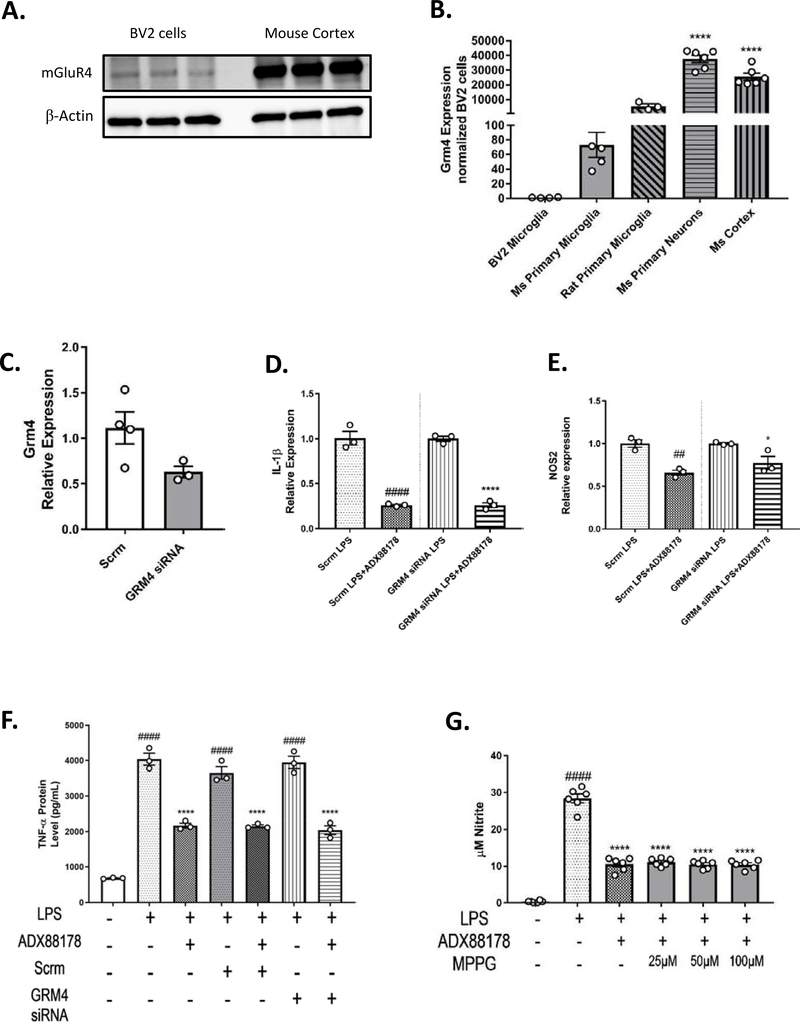
To evaluate the specificity of ADX88178 for mGluR4 receptor and its anti-inflammatory response in microglia cells, we performed mRNA and protein analysis of mGlu4 receptor. Positive controls included mouse cortex and expression levels were compared across many different cell types (Fig. 6A–B). Western blotting of BV2 microglia samples revealed a weak band at the predicted mGluR4 molecular weight of 100–110 kDa when compared to mouse cortex samples (Fig. 6A). Quantitative-PCR analysis revealed that expression of GRM4, gene that codes for mGluR4, in BV2 microglia is 1/20,000 of that in mouse cortex (BV2 microglia vs. mouse (Ms) cortex, P<0.0001; Fig. 6B). To further investigate the anti-inflammatory role of mGluR4, we used siRNA targeted towards GRM4 gene or scrambled (Scrm) siRNA (as control). GRM4 siRNA transfection in BV2 microglia reduced expression of GRM4 by 48% when compared with scrambled siRNA-transfected cells (Fig. 6C). In BV2 microglia, ADX88178 resulted in a significant decrease in LPS-induced release of inflammatory cytokines IL-1β (Scrm LPS vs. Scrm LPS+ADX88178, P<0.0001 and GRM4 siRNA LPS vs. GRM4 siRNA LPS+ADX88178, P<0.0001; Fig. 6D) and NOS2 (NOS2: Scrm LPS vs. Scrm LPS+ADX88178, P<0.01 and GRM4 siRNA LPS vs. GRM4 siRNA LPS+ADX88178, P<0.05; Fig. 6E) in both scrambled siRNA and GRM4-siRNA transfected BV2 microglia. TNF-α protein level was measured from the media of BV2 cells treated with Scrm or GRM4 siRNA. LPS caused a significant increase in TNF-α production in LPS treated cells, Scrm-treated LPS as well as GRM4 siRNA-treated LPS (P<0.0001 for all three groups; Fig. 6F). Pretreatment with ADX88178 significantly decreased levels of LPS-stimulated TNF-α for all three groups (P<0.0001; Fig. 6F) indicating no inhibition of ADX88178 anti-inflammatory effects in the presence of GRM4 siRNA (Fig. 6F). We confirmed our results from primary microglia and used a selective group III antagonist MPPG to evaluate the role of mGluR4 on ADX88178 effects. LPS stimulation of BV2 microglia significantly increased NO levels (P<0.0001) but increasing concentrations of MPPG did not inhibit ADX88178 from attenuating NO release (LPS vs. LPS+ADX88178+MPPG 100uM, P<0.0001; Fig. 6G). Other group III antagonists, MSOP and CPPG, were also tested; they also did not inhibit ADX88178 anti-inflammatory actions (Supplementary Fig. 6).
Fig. 6. ADX88178 does not exert its anti-inflammatory actions through mGlur4 receptor.
Specificity of ADX88178 for mGlu4 receptor was measured. (A) Western immunoblotting using equal amounts of protein from BV2 microglia and mouse cortex revealed that expression of mGluR4 at protein level is very low. (B) At the mRNA level, expression of GRM4 gene in BV2 microglia is significantly lower than in the mouse cortex (****P<0.0001 vs. BV2 microglia). (C-E) qPCR demonstrated a 48% reduction in GRM4 mRNA expression in GRM4 siRNA group compared with scrambled (scrm) control. ADX88178 significantly attenuated LPS-stimulated IL-1β (P<0.0001 LPS vs. LPS+ADX88178 in both scrm control and GRM4 siRNA) and NOS2 (P<0.01, LPS vs. LPS+ADX88178 in scrm control and P<0.05 in GRM4 siRNA) gene expressions. (F) LPS significantly increased TNF-α protein in LPS alone, scrm-treated LPS, and GRM4 siRNA-treated LPS groups (####P<0.0001 vs. control for all three groups) and pretreatment with ADX88178 reduced LPS-induced TNF-α release for all three treatments (****P<0.0001 vs. each respective LPS groups). (G) Group III antagonist, MPPG, did not prevent ADX88178 attenuation of NO levels after LPS stimulation even at the highest concentrations of the antagonist (****P<0.0001, LPS vs. LPS+ADX88178+MPPG 100μM). Statistical analyses: (6C) was analyzed using one-tailed t-test (P=0.073). All remaining data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ##P<0.01, ####P<0.0001 in comparison with control (Scrm LPS in 6D-E); *P<0.05, ****P<0.0001 in comparison with LPS (GRM4 siRNA LPS in 6D-E).
3.7. Adenosine 2A receptor agonist and A3 antagonist do not block actions of ADX88178
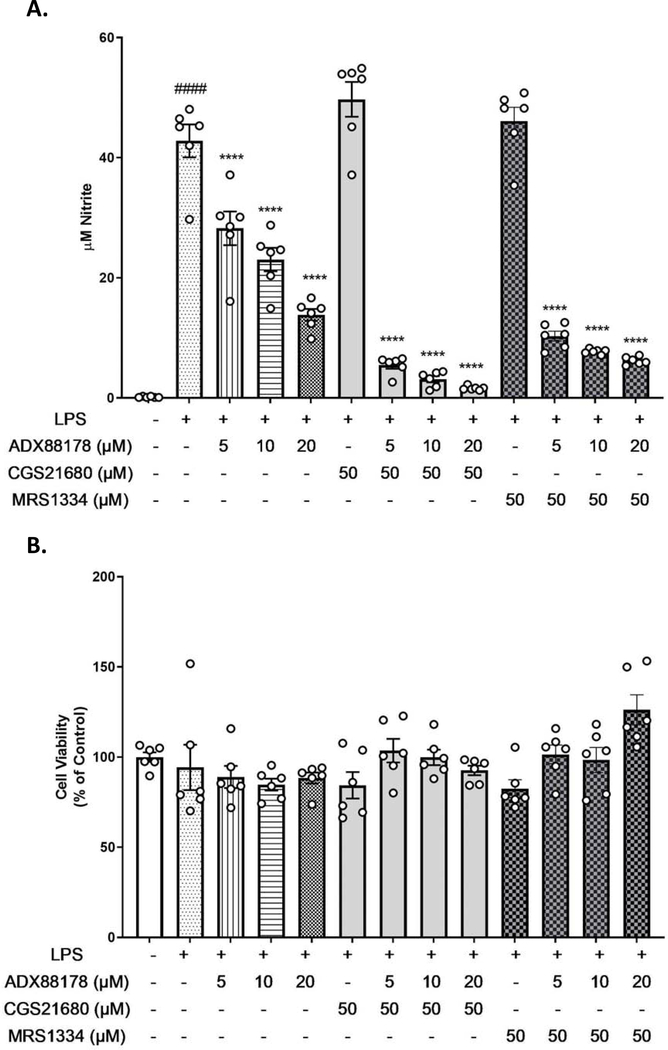
mGluR4 can dimerize with other mGluR subtypes or with adenosine receptors (A2a and A3) (Jones et al., 2012; Klyuch et al., 2012; Lopez et al., 2008). We used an A2a agonist and A3 antagonist to block anti-inflammatory action of ADX88178. CGS21680 is an A2a receptor agonist and MRS1334 is an A3 receptor antagonist. Addition of LPS (20ng/mL) to BV2 microglia significantly increased production of nitrite (control vs. LPS, P<0.0001; Fig. 7A). Pretreatment with increasing concentration of ADX88178 caused a concentration-dependent decrease in LPS-induced NO release (LPS vs. LPS+ADX88178 20μM, P<0.0001; Fig. 7A). Neither CGS21680 nor MRS1334 altered LPS-induced NO release (Fig. 7A). Additionally, pre-treatment with CGS21680 (50μM) or MRS1334 (50μM) did not inhibit ADX88178 anti-inflammatory actions, as shown by NO levels (Fig. 7A). Using MTT assay, no cell cytotoxicity was found at concentrations of the drugs used (Fig. 7B).
Fig. 7. A2aR agonist and A3 antagonist do not block actions of ADX88178.
BV2 microglia were pre-treated with ADX88178 and A2a receptor agonist CGS21680 or A3 receptor antagonist MRS1334 to determine if adenosine receptors are mGluR4 binding partners. (A) LPS stimulation resulted in a significant increase in nitrite release (####P<0.0001 vs. control). Pre-treatment with ADX88178 significantly reduced LPS-induced nitrite production concentration-dependently (****P<0.0001 vs. LPS) while pretreatment with CGS21680 (50μM) or MRS1334 (50μM) prior to LPS stimulation did not cause any changes in the NO levels. Pretreatment with neither CGS21680 nor MRS1334 prior to LPS+ADX88178 caused inhibition of ADX88178 anti-inflammatory action. (B) There was no observed cytotoxicity at any concentration of ADX88178, CGS21680, or MRS1334 alone or in combination with LPS. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA with post hoc Tukey’s test. ####P<0.0001 in comparison with control; ****P<0.0001 comparison with LPS.
4. Discussion
The aim of the present study was to examine the anti-inflammatory effects of mGluR4 PAMs and the mechanisms responsible. We used multiple in vitro models, including immortalized microglial cell lines and primary microglia; evaluated multiple agonists, both orthosteric and PAMs; and examined a number of potential signal transduction pathways.
mGluR4 agonists, both orthosteric and PAMs, have shown anti-inflammatory effects in various experimental neurological disorders-including Parkinson’s disease, experimental autoimmune encephalomyelitis (EAE) and chronic glial activation (Fallarino et al., 2010; Kalinichev et al., 2014; Le Poul et al., 2012; Niswender et al., 2016; Volpi et al., 2016). Astrocyte activation can induce various cytotoxic and pro-inflammatory factors (Mennicken 1999) and mGluR4 is present on activated astrocytes (Guerts 2005, Tang & Lee 2001). Conditioned media from astrocyte cultures incubated with the orthosteric group III agonist, L-AP4, prior to addition of LPS, significantly reduced neuronal cell death (Zhou 2006). In rat models of EAE, treatment with L-AP4 for 28 days limited the induction of RANTES, a chemokine produced by astrocytes that leads to neuroinflammation (Besong 2002). In mGluR4 knockout EAE rats, there was a smaller reduction in RANTES after treatment with L-AP4 (Besong 2002).
A well-known limitation of pharmacological interventions is the potential for off-target activity. PHCCC, one of the earliest drugs to study mGlu4 receptor, is also an mGluR1 antagonist, which complicates interpretation of its actions at mGlu4 receptor (Maj et al., 2003; Marino et al., 2003). VU0364770 is an mGluR4 PAM that has been reported to reverse haloperidol-induced catalepsy in 6-hydroxydopamine (6-OHDA)-lesioned rodent models, but this drug is also a potent inhibitor of monoamine oxidase b (MAO-B) (Jones et al., 2012). Such studies highlight the challenges that are present when using only pharmacological approaches to elucidate specific receptor mediated actions. For these reasons, we used several types of mGluR4 PAMs to study the anti-inflammatory effects of mGluR4 activation in microglia. Based on the previously reported ADX88178 potency and efficacy, we focused on this compound and investigated its regulation of inflammatory signaling cascades.
The putative mGluR4 PAM ADX88178 showed significant anti-inflammatory effects in both BV2 cells and primary microglia cells, as indicated by reductions in IL-1β, TNF-α, CCL-2, IL-6, NOS2, miR-155 as well as attenuated NO release following LPS activation. These results are consistent with those previously reported in the literature (Fallarino et al., 2010; Ponnazhagan et al., 2016). However, other putative mgluR4 agonists showed divergent effects: VU0361737 (PAM) demonstrated anti-inflammatory activity, but neither VU0155041 (PAM) nor the orthosteric agonist L-AP4 showed similar effects. These observations suggest that the anti-inflammatory effects of select mGluR4 PAMs may be mediated through mGluR4-independent mechanisms. This interpretation is in apparent conflict with a recent report claiming that mGluR4 is necessary for ADX88178 attenuation of microglia activation (Ponnazhagan et al., 2016). The latter study used primary microglia generated from a transgenic mouse model with a global knockdown of mGluR4, showing that the LPS-induced elevation of inflammatory markers such as TNF-α, iNOS and MHCII were significantly attenuated through treatment with ADX88178 or L-AP4 in wild-type microglia cultures but not in mGluR4 knockout microglia preparations. To address these differences across studies we examined levels of mGluR4 expression in our models; and evaluated the effects of group III and mGluR4-specific pharmacological antagonists, as well as siRNA knockdown of mGluR4. The low expression levels of mGluR4 in our microglia cell models may argue against a receptor mediated signaling, consistent with the lack of anti-inflammatory effects by the orthosteric agonist L-AP4 or PAMs such as VU0155041. Moreover, neither group III nor mGluR4-specific pharmacological antagonists attenuated the anti-inflammatory actions of ADX88178 in LPS-stimulated BV2 microglia or primary mouse microglia. In addition, molecular knockdown of mGluR4 did not limit ADX88178 anti-inflammatory effects. Together, these results support the conclusion that the observed anti-inflammatory effects of mGluR4 PAMs, such as ADX88178 or VU0361737, in microglial culture models likely reflect mGluR4-independent mechanisms. Although it is possible that ADX88178 may promote anti-inflammatory effects by signaling through mGluR4 in some models, this compound has the ability to signal through additional receptors, and these latter signaling pathways appear to be predominant under conditions of low mGluR4 expression.
We therefore addressed other potentially relevant signaling pathways that could account for ADX88178 anti-inflammatory effects. Group III mGluRs are mainly localized presynaptically and couple Gαi/o to negatively modulate neuronal excitability (Nicoletti et al., 2011; Niswender & Conn, 2010; Pin & Duvoisin, 1995; Taylor et al., 2003). Therefore, we used PT to inhibit Gαi/o in ADX88178 treated preparations; this treatment did not affect ADX88178-dependent anti-inflammatory effects. Downstream of mGlu4 receptor, we examined levels of cAMP in the presence of adenylyl cyclase activator, forskolin (Taylor et al., 2003; Canudas et al., 2004; Kingston et al., 1998). We hypothesized that if ADX88178 worked through mGluR4/Gi-dependent signaling, levels of forskolin-induced cAMP should decrease in the presence of ADX88178. However, ADX88178 did not reduce forskolin-induced cAMP levels in BV2 microglia. Furthermore, we evaluated the effects of PKA inhibitor H89 and cell permeable cAMP analogue dbcAMP. There was a significant increase in LPS+dbcAMP induced NO release, compared to LPS alone, suggesting that cAMP elevation above basal levels increases microglial activation; however, dbcAMP did not attenuate ADX88178 anti-inflammatory effect as measured by NO levels. LPS+H89 increased nitrite release similar to LPS and did not have any additive anti-inflammatory effects to ADX88178, suggesting that inhibition of PKA does not contribute to ADX88178 anti-inflammatory actions. These results suggest that ADX88178 acts independently of the mGluR4/Gi canonical pathway and exerts its actions downstream of cAMP. In other experiments, we examined the levels of pCREB and CREB in BV2 microglia following ADX88178 treatment. CREB is a transcription factor whose activation is mediated via cAMP-dependent protein kinase A (PKA) and plays an important role in inflammatory responses of immune cells (Moon et al., 2005; Wen et al., 2010; Zou et al., 2017). In contrast to the expected effects in a mGluR4/G-coupled system where levels of pCREB should decrease due to inhibition of adenylate cyclase (Ye, 2001), we observed a significant increase in phosphorylation of CREB on Ser-133 in ADX88178-treated BV2 microglia. These results provide additional support for the hypothesis that ADX88178 anti-inflammatory actions involve mGluR4/G,-independent mechanisms. Furthermore, they are consistent with studies showing that in dendritic cells, ADX88178 activated a G-independent pathway that involves phosphatidylinositol-3-kinase (PI3K)-Indoleamine 2,3-dioxygenase 1 (IDO1) to exert an immunoregulatory effect (Volpi et al., 2016). The NFkB pathway plays a key role in regulation of transcriptional activity of pro-inflammatory genes and ADX88178-mediated activation of CREB may inhibit NFkB through blocking the interaction between CREB Binding Protein (CBP) and NFkB complex (Ollivier et al., 1996; Park et al., 2016; Parry & Mackman, 1997; Wen et al., 2010; Zou et al., 2017). Notably, ADX88178 significantly increased the total levels of IkB and reduced LPS-induced translocation of p65 NFkB into the nucleus. Thus, ADX88178 anti-inflammatory actions may involve dual inhibition of NFkB activity-reducing nuclear translocation as well as promoting a CREB-dependent attenuation of pro-inflammatory gene transactivation (Wen et al., 2010; Ye, 2001).
Considering the general property of GPCRs to heterodimerize, we hypothesized that the putative receptors responsible for the observed mGluR4-independent signaling cascades may be among mGluR4 previously established partners, such as adenosine receptors. A2a antagonists and A3 agonists have neuroprotective effects in rodent models of neuroinflammation (Colella et al., 2018; Lee et al., 2006). Moreover, activation of mGluR4 and blockade of A2a receptors confer improved anti-parkinsonian behavioral recovery (Jones et al., 2012; Lopez et al., 2008). Although prior data suggest that ADX88178 is active at human adenosine Ai and human adenosine A3 receptors (Le Poul et al., 2012), given the lack of mRNA expression of A1 receptor in BV2 microglia, we examined other adenosine receptor modulators (A2a agonists and A3 antagonists) in the presence or absence of ADX88178. However, our data are inconsistent with ADX88178 signaling through either adenosine receptors A2a or A3. A model in which ADX88178 signals through mGluR4 heterodimerization partners may help address the apparent conflict between our findings and those of Ponnazhagan and colleagues. Thus, it is possible that the complete absence of mGluR4 in the constitutive knockout model used by Ponnazhagan et al. may have also altered the membrane localization of the putative mGluR4 heterodimerization partners and these changes together are responsible for the lack of activity of ADX88178 or L-AP4 in microglia from their knockout model.
Supplementary Material
Supplementary Fig. 1. LPS concentration-response in BV2 microglia. Different concentrations of LPS were tested on BV2 microglia to establish the lowest concentration that produced a robust nitrite release. (A) LPS 20ng/mL was the lowest concentration that produced a significant NO release in BV2 microglia (####P<0.0001, control vs. LPS 20ng/mL). All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control.
Supplementary Fig. 2. ADX88178 concentration-response in LPS-stimulated BV2 microglia. Nanomolar to micromolar concentrations of ADX88178 were tested on LPS-stimulated BV2 microglia to find the lowest concentration that inhibited NO release. (A) LPS at 20ng/mL produced a significant NO release (####P<0.0001, control vs. LPS), but nanomolar (nM) concentrations of ADX88178 did not inhibit LPS-induced NO release. (B) LPS at 20ng/mL caused significant nitrite release (####P<0.0001, control vs. LPS). The ADX88178 concentration curve was extended to micromolar (μM) concentrations, and ADX88178 at 20pM significantly attenuated LPS-stimulated NO release in BV2 microglia (****P<0.0001, LPS vs. LPS+ADX88178 20pM). All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; ****P<0.0001 in comparison with LPS.
Supplementary Fig. 3. Cell images of BV2 microglia show no cytotoxicity. Cell images of BV2 microglia were taken through EVOS cell imaging system. (A) Control BV2 microglia. (B) LPS 20ng/mL did not show any cell death in comparison to control cells. (C) Treatment with LPS+ADX88178 20μM did not cause cell cytotoxicity.
Supplementary Fig. 4. ADX88178 and VU0361737 attenuate NO release in LPS+IFN-γ stimulated HAPI microglia. (A) HAPI rat microglia were stimulated with LPS (100ng/mL)+IFN-γ (10ng/mL) for maximal nitrite production (####P<0.0001 vs. control). In HAPI microglia cells, ADX88178 (****P<0.0001, LPS+IFN-γ vs. LPS+IFN-γ+ADX88178 20pM) and VU0361737 (****P<0.0001, LPS+IFN-γ vs. LPS+IFN-γ+VU0361737 100pM) produced the most potent anti-inflammatory effects as indicated by released nitrite levels. None of the other tested mGluR4 PAMs or L-AP4 inhibited NO release. (B) HAPI rat microglia were stimulated with LPS 20ng/mL which produced a modest nitrite release (####P<0.0001, control vs. LPS). ADX88178 at 20 μM significantly attenuated NO release (****P<0.0001, LPS vs. LPS+ADX88178 20μM) whereas L-AP4 at 100μM showed only a modest attenuation of NO release. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; *P<0.05, ****P<0.0001 in comparison with LPS+IFN-γ.
Supplementary Fig. 5. Effect of VU0155041 and VU0418506 on LPS-stimulated TNF-α release. mGluR4 PAMs, VU0155041 or VU0418506, were added to LPS-stimulated microglia and TNF-α was measured through ELISA. (A) LPS significantly increased TNF-α release (###P<0.001, control vs. LPS) in primary mouse microglia. VU0155041 did not inhibit LPS-stimulated TNF-α release. (B) LPS significantly increased TNF-α protein levels (####P<0.0001, control vs. LPS) in BV2 microglia; however, VU0418506 did not reverse LPS-mediated TNF-α release. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ###P<0.001, ####P<0.0001 in comparison with control.
Supplementary Fig. 6. Effect of group II/III receptor antagonist CPPG and selective group III mGluR antagonist MSOP in LPS-stimulated BV2 microglia. BV2 microglia were pre-treated with the antagonists for 1 hour prior to addition of ADX88178 for 30 minutes followed by stimulation with or without LPS (20ng/mL) for 24 hours. (A-B) LPS stimulation resulted in a significant increase in nitrite production (####P<0.0001 vs. control for both panels). Pre-treatment with 20μM of ADX88178 significantly reduced LPS-induced nitrite production (****P<0.001 vs. LPS for both panels). However, neither CPPG nor MSOP reversed ADX88178-dependent reduction in NO. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; ****P<0.0001 in comparison with LPS.
Highlights.
ADX88178 attenuates LPS-induced pro-inflammatory pathways in microglia cells.
Other mGluR4 PAMs and orthosteric agonist have divergent immunomodulatory roles.
ADX88178 anti-inflammatory effects are through mGluR4/Gi-independent mechanisms.
ADX88178 anti-inflammatory actions involve NFkB inhibition and CREB activation.
ADX88178 anti-inflammatory effects are not through adenosine receptors.
Acknowledgements
The authors thank Rebecca Henry Ph.D., James Barrett Ph.D., Boris Sabirzhanov Ph.D., Shahnawaz Bhat Ph.D., Oleg Makarevich and Marie Hanscom M.S. for help with the experiments. We thank Dr. Jeffrey Conn and Dr. Colleen Niswender who generously provided us with ADX88178 and VU0418506.
Funding
This work was supported by National Institutes of Health grant R01NS037313 (A.I.F).
Abbreviations
- TBI
Traumatic brain injury
- IL-1β
Interleukin-1β
- TNF-α
Tumor necrosis factor-α
- NOS2/iNOS
Nitric oxide synthase 2
- IL-6
Interleukin-6
- CCL2
C-C motif chemokine ligand 2
- miR-155
MicroRNA-155
- mGluR4
metabotropic glutamate receptor 4
- PAM
positive allosteric modulator
- LPS
lipopolysaccharide
- IL-6
Interleukin 6
- CREB
cAMP-response element binding protein
- NFkB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- IkB
Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor
- miR
microRNA; GPCR: G-protein coupled receptor
- iGluR
ionotropic glutamate receptor
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide
- PT
pertussis toxin
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
Declarations:
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent
Consent for publication is not applicable for this manuscript.
Data availability and authors
All the datasets and materials supporting the conclusions of this article are presented in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76(3):846–54. [DOI] [PubMed] [Google Scholar]
- 2.Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101(5):1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besong G, Battaglia G, D’Onofrio M, Di Marco R, Ngomba RT, Storto M, et al. Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures. J Neurosci. 2002;22(13):5403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts MJ, O’Neill MJ, Duty S. Allosteric modulation of the group III mGlu4 receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson’s disease. Br J Pharmacol. 2012;166(8):2317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat S, Henry RJ, Blanchard AC, Stoica BA, Loane DJ, Faden AI. Enhanced Akt/GSK-3beta/CREB signaling mediates the anti-inflammatory actions of mGluR5 positive allosteric modulators in microglia and following traumatic brain injury in male mice. J Neurochem. 2020:e14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollinger SR, Engers DW, Panarese JD, West M, Engers JL, Loch MT, et al. Discovery, Structure-Activity Relationship, and Biological Characterization of a Novel Series of 6-((1 H-Pyrazolo[4,3- b]pyridin-3-yl)amino)-benzo[ d]isothiazole-3-carboxamides as Positive Allosteric Modulators of the Metabotropic Glutamate Receptor 4 (mGlu4). J Med Chem. 2019;62(1):342–58. [DOI] [PubMed] [Google Scholar]
- 7.Byrnes KR, Loane DJ, Faden AI. Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders. Neurotherapeutics. 2009a;6(1):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia. 2009b;57(5):550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canudas AM, Di Giorgi-Gerevini V, Iacovelli L, Nano G, D’Onofrio M, Arcella A, et al. PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J Neurosci. 2004;24(46):10343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colella M, Zinni M, Pansiot J, Cassanello M, Mairesse J, Ramenghi L, et al. Modulation of Microglial Activation by Adenosine A2a Receptor in Animal Models of Perinatal Brain Injury. Front Neurol. 2018;9:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4(4):399–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. [DOI] [PubMed] [Google Scholar]
- 14.Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation. 2004;1(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engers DW, Niswender CM, Weaver CD, Jadhav S, Menon UN, Zamorano R, et al. Synthesis and evaluation of a series of heterobiarylamides that are centrally penetrant metabotropic glutamate receptor 4 (mGluR4) positive allosteric modulators (PAMs). J Med Chem. 2009;52(14):4115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faden AI, Loane DJ. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics. 2015;12(1):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faden AI, Wu J, Stoica BA, Loane DJ. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 2016;173(4):681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallarino F, Volpi C, Fazio F, Notartomaso S, Vacca C, Busceti C, et al. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat Med. 2010;16(8):897–902. [DOI] [PubMed] [Google Scholar]
- 19.Foster DJ, Conn PJ. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron. 2017;94(3):431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geurts JJ, Wolswijk G, Bo L, Redeker S, Ramkema M, Troost D, et al. Expression patterns of Group III metabotropic glutamate receptors mGluR4 and mGluR8 in multiple sclerosis lesions. J Neuroimmunol. 2005;158(1–2):182–90. [DOI] [PubMed] [Google Scholar]
- 21.Gross TJ, Kremens K, Powers LS, Brink B, Knutson T, Domann FE, et al. Epigenetic silencing of the human NOS2 gene: rethinking the role of nitric oxide in human macrophage inflammatory responses. J Immunol. 2014;192(5):2326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y, Choi IY, Kim C, Hong S, Kim WK. Excretory-secretory products from Paragonimus westermani increase nitric oxide production in microglia in PKC-dependent and -independent manners. Neurosci Res. 2009;65(2):141–7. [DOI] [PubMed] [Google Scholar]
- 23.Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16(11):1211–27. [DOI] [PubMed] [Google Scholar]
- 24.Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, et al. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2012;340(2):404–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinichev M, Le Poul E, Bolea C, Girard F, Campo B, Fonsi M, et al. Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J Pharmacol Exp Ther. 2014;350(3):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kew JN. Positive and negative allosteric modulation of metabotropic glutamate receptors: emerging therapeutic potential. Pharmacol Ther. 2004;104(3):233–44. [DOI] [PubMed] [Google Scholar]
- 27.Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, et al. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37(1):1–12. [DOI] [PubMed] [Google Scholar]
- 28.Klyuch BP, Dale N, Wall MJ. Receptor-mediated modulation of activity-dependent adenosine release in rat cerebellum. Neuropharmacology. 2012;62(2):815–24. [DOI] [PubMed] [Google Scholar]
- 29.Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, et al. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci U S A. 2001;98(23):13402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopitar-Jerala N Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front Mol Neurosci. 2015;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Poul E, Bolea C, Girard F, Poli S, Charvin D, Campo B, et al. A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2012;343(1):167–77. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Jhun BS, Oh YT, Lee JH, Choe W, Baik HH, et al. Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-αlpha production through inhibition of PI 3-kinase/Akt and NF-kappaB activation in murine BV2 microglial cells. Neurosci Lett. 2006;396(1):1–6. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 34.Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol. 2016;275 Pt 3:316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem. 2009;284(23):15629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol. 2014a;73(1):14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loane DJ, Stoica BA, Tchantchou F, Kumar A, Barrett JP, Akintola T, et al. Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury. Neurotherapeutics. 2014b;11(4):857–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, et al. (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45(7):895–906. [DOI] [PubMed] [Google Scholar]
- 39.Marino MJ, Williams DL Jr., O’Brien JA, Valenti O, McDonald TP, Clements MK, et al. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. Proc Natl Acad Sci U S A. 2003;100(23):13668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–40. [DOI] [PubMed] [Google Scholar]
- 41.Mennicken F, Maki R, de Souza EB, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20(2):73–8. [DOI] [PubMed] [Google Scholar]
- 42.Moon EY, Oh SY, Han GH, Lee CS, Park SK. Epac1-mediated Rap1 activation is not required for the production of nitric oxide in BV2, murine microglial cells. J Neurosci Res. 2005;81(1):38–44. [DOI] [PubMed] [Google Scholar]
- 43.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60(7–8):1017–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicoletti F, Bruno V, Copani A, Casabona G, Knopfel T. Metabotropic glutamate receptors: a new target for the therapy of neurodegenerative disorders? Trends Neurosci. 1996;19(7):267–71. [DOI] [PubMed] [Google Scholar]
- 45.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74(5):1345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niswender CM, Jones CK, Lin X, Bubser M, Thompson Gray A, Blobaum AL, et al. Development and Antiparkinsonian Activity of VU0418506, a Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4 Homomers without Activity at mGlu2/4 Heteromers. ACS Chem Neurosci. 2016;7(9):1201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271(34):20828–35. [DOI] [PubMed] [Google Scholar]
- 49.Park T, Chen H, Kevala K, Lee JW, Kim HY. N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J Neuroinflammation. 2016;13(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker LC, Luheshi GN, Rothwell NJ, Pinteaux E. IL-1 beta signalling in glial cells in wildtype and IL-1RI deficient mice. Br J Pharmacol. 2002;136(2):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159(11):5450–6. [PubMed] [Google Scholar]
- 52.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. [DOI] [PubMed] [Google Scholar]
- 53.Petrova TV, Akama KT, Van Eldik LJ. Selective modulation of BV-2 microglial activation by prostaglandin E(2). Differential effects on endotoxin-stimulated cytokine induction. J Biol Chem. 1999;274(40):28823–7. [DOI] [PubMed] [Google Scholar]
- 54.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34(1):1–26. [DOI] [PubMed] [Google Scholar]
- 55.Ponnazhagan R, Harms AS, Thome AD, Jurkuvenaite A, Gogliotti R, Niswender CM, et al. The Metabotropic Glutamate Receptor 4 Positive Allosteric Modulator ADX88178 Inhibits Inflammatory Responses in Primary Microglia. J Neuroimmune Pharmacol. 2016;11(2):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15(5):300–12. [DOI] [PubMed] [Google Scholar]
- 57.Qiu JL, Zhu WL, Lu YJ, Bai ZF, Liu ZG, Zhao P, et al. The selective mGluR5 agonist CHPG attenuates SO2-induced oxidative stress and inflammation through TSG-6/NF-kappaB pathway in BV2 microglial cells. Neurochem Int. 2015;85–86:46–52. [DOI] [PubMed] [Google Scholar]
- 58.Shih RH, Wang CY, Yang CM. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci. 2015;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stansley BJ, Conn PJ. Neuropharmacological Insight from Allosteric Modulation of mGlu Receptors. Trends Pharmacol Sci. 2019;40(4):240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang FR, Lee WL. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J Neurocytol. 2001;30(2):137–43. [DOI] [PubMed] [Google Scholar]
- 61.Taylor DL, Diemel LT, Cuzner ML, Pocock JM. Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer’s disease. J Neurochem. 2002;82(5):1179–91. [DOI] [PubMed] [Google Scholar]
- 62.Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23(6):2150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volpi C, Mondanelli G, Pallotta MT, Vacca C, Iacono A, Gargaro M, et al. Allosteric modulation of metabotropic glutamate receptor 4 activates IDO1-dependent, immunoregulatory signaling in dendritic cells. Neuropharmacology. 2016;102:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185(11):6413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams R, Johnson KA, Gentry PR, Niswender CM, Weaver CD, Conn PJ, et al. Synthesis and SAR of a novel positive allosteric modulator (PAM) of the metabotropic glutamate receptor 4 (mGluR4). Bioorg Med Chem Lett. 2009;19(17):4967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xue F, Stoica BA, Hanscom M, Kabadi SV, Faden AI. Positive allosteric modulators (PAMs) of metabotropic glutamate receptor 5 (mGluR5) attenuate microglial activation. CNS Neurol Disord Drug Targets. 2014;13(4):558–66. [DOI] [PubMed] [Google Scholar]
- 67.Ye RD. Regulation of nuclear factor kappaB activation by G-protein-coupled receptors. J Leukoc Biol. 2001;70(6):839–48. [PubMed] [Google Scholar]
- 68.Zhang ZY, Sun BL, Liu JK, Yang MF, Li DW, Fang J, et al. Activation of mGluR5 Attenuates Microglial Activation and Neuronal Apoptosis in Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats. Neurochem Res. 2015;40(6):1121–32. [DOI] [PubMed] [Google Scholar]
- 69.Zhou F, Yao HH, Wu JY, Yang YJ, Ding JH, Zhang J, et al. Activation of Group M/III metabotropic glutamate receptors attenuates LPS-induced astroglial neurotoxicity via promoting glutamate uptake. J Neurosci Res. 2006;84(2):268–77. [DOI] [PubMed] [Google Scholar]
- 70.Zou ZQ, Chen JJ, Feng HF, Cheng YF, Wang HT, Zhou ZZ, et al. Novel Phosphodiesterase 4 Inhibitor FCPR03 Alleviates Lipopolysaccharide-Induced Neuroinflammation by Regulation of the cAMP/PKA/CREB Signaling Pathway and NF-kappaB Inhibition. J Pharmacol Exp Ther. 2017;362(1):67–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. LPS concentration-response in BV2 microglia. Different concentrations of LPS were tested on BV2 microglia to establish the lowest concentration that produced a robust nitrite release. (A) LPS 20ng/mL was the lowest concentration that produced a significant NO release in BV2 microglia (####P<0.0001, control vs. LPS 20ng/mL). All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control.
Supplementary Fig. 2. ADX88178 concentration-response in LPS-stimulated BV2 microglia. Nanomolar to micromolar concentrations of ADX88178 were tested on LPS-stimulated BV2 microglia to find the lowest concentration that inhibited NO release. (A) LPS at 20ng/mL produced a significant NO release (####P<0.0001, control vs. LPS), but nanomolar (nM) concentrations of ADX88178 did not inhibit LPS-induced NO release. (B) LPS at 20ng/mL caused significant nitrite release (####P<0.0001, control vs. LPS). The ADX88178 concentration curve was extended to micromolar (μM) concentrations, and ADX88178 at 20pM significantly attenuated LPS-stimulated NO release in BV2 microglia (****P<0.0001, LPS vs. LPS+ADX88178 20pM). All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; ****P<0.0001 in comparison with LPS.
Supplementary Fig. 3. Cell images of BV2 microglia show no cytotoxicity. Cell images of BV2 microglia were taken through EVOS cell imaging system. (A) Control BV2 microglia. (B) LPS 20ng/mL did not show any cell death in comparison to control cells. (C) Treatment with LPS+ADX88178 20μM did not cause cell cytotoxicity.
Supplementary Fig. 4. ADX88178 and VU0361737 attenuate NO release in LPS+IFN-γ stimulated HAPI microglia. (A) HAPI rat microglia were stimulated with LPS (100ng/mL)+IFN-γ (10ng/mL) for maximal nitrite production (####P<0.0001 vs. control). In HAPI microglia cells, ADX88178 (****P<0.0001, LPS+IFN-γ vs. LPS+IFN-γ+ADX88178 20pM) and VU0361737 (****P<0.0001, LPS+IFN-γ vs. LPS+IFN-γ+VU0361737 100pM) produced the most potent anti-inflammatory effects as indicated by released nitrite levels. None of the other tested mGluR4 PAMs or L-AP4 inhibited NO release. (B) HAPI rat microglia were stimulated with LPS 20ng/mL which produced a modest nitrite release (####P<0.0001, control vs. LPS). ADX88178 at 20 μM significantly attenuated NO release (****P<0.0001, LPS vs. LPS+ADX88178 20μM) whereas L-AP4 at 100μM showed only a modest attenuation of NO release. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; *P<0.05, ****P<0.0001 in comparison with LPS+IFN-γ.
Supplementary Fig. 5. Effect of VU0155041 and VU0418506 on LPS-stimulated TNF-α release. mGluR4 PAMs, VU0155041 or VU0418506, were added to LPS-stimulated microglia and TNF-α was measured through ELISA. (A) LPS significantly increased TNF-α release (###P<0.001, control vs. LPS) in primary mouse microglia. VU0155041 did not inhibit LPS-stimulated TNF-α release. (B) LPS significantly increased TNF-α protein levels (####P<0.0001, control vs. LPS) in BV2 microglia; however, VU0418506 did not reverse LPS-mediated TNF-α release. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ###P<0.001, ####P<0.0001 in comparison with control.
Supplementary Fig. 6. Effect of group II/III receptor antagonist CPPG and selective group III mGluR antagonist MSOP in LPS-stimulated BV2 microglia. BV2 microglia were pre-treated with the antagonists for 1 hour prior to addition of ADX88178 for 30 minutes followed by stimulation with or without LPS (20ng/mL) for 24 hours. (A-B) LPS stimulation resulted in a significant increase in nitrite production (####P<0.0001 vs. control for both panels). Pre-treatment with 20μM of ADX88178 significantly reduced LPS-induced nitrite production (****P<0.001 vs. LPS for both panels). However, neither CPPG nor MSOP reversed ADX88178-dependent reduction in NO. All values are expressed as mean ± S.E.M. for at least three independent experiments. Data were analyzed using one-way ANOVA for multiple comparisons with post hoc Tukey’s test. ####P<0.0001 in comparison with control; ****P<0.0001 in comparison with LPS.