Abstract
Background:
The urinary tract can be affected by both congenital abnormalities as well as acquired disorders, such as cancer, trauma, infection, inflammation, and iatrogenic injuries, all of which may lead to organ damage requiring eventual reconstruction. As a gold standard, gastrointestinal segment is used for urinary bladder reconstruction. However, one major problem is that while bladder tissue prevents reabsorption of specific solutes, gastrointestinal tissue actually absorbs them. Therefore, tissue engineering approach had been attempted to provide an alternative tissue graft for urinary bladder reconstruction.
Methods:
Human adipose-derived stem cells isolated from fat tissues were differentiated into smooth muscle cells and then seeded onto a triple-layered PLGA sheet to form a bladder construct. Adult athymic rats underwent subtotal urinary bladder resection and were divided into three treatment groups (n = 3): Group 1 (“sham”) underwent anastomosis of the remaining basal region, Group 2 underwent reconstruction with the cell-free scaffold, and Group 3 underwent reconstruction with the tissue-engineered bladder construct. Animals were monitored on a daily basis and euthanisation was performed whenever a decline in animal health was detected.
Results:
All animals in Groups 1, 2 and 3 survived for at least 7 days and were followed up to a maximum of 12 weeks post-operation. It was found that by Day 14, substantial ingrowth of smooth muscle and urothelial cells had occurred in Group 2 and 3. In the long-term follow up of group 3 (tissue-engineered bladder construct group), it was found that the urinary bladder wall was completely regenerated and bladder function was fully restored. Urodynamic and radiological evaluations of the reconstructed bladder showed a return to normal bladder volume and function.Histological analysis revealed the presence of three muscular layers and a urothelium similar to that of a normal bladder. Immunohistochemical staining using human-specific myocyte markers (myosin heavy chain and smoothelin) confirmed the incorporation of the seeded cells in the newly regenerated muscular layers.
Conclusion:
Implantation of PLGA construct seeded with smooth muscle cells derived from human adipose stem cells can lead to regeneration of the muscular layers and urothelial ingrowth, leading to formation of a completely functional urinary bladder.
Keywords: Bladder reconstruction, PLGA, Adipose-dervied stem cells, Tissue engineering, Smooth muscle cells
Introduction
The urinary tract can be affected by both congenital abnormalities as well as acquired disorders, such as cancer, trauma, infection, inflammation, and iatrogenic injuries, all of which may lead to organ damage requiring eventual reconstruction. Enterocystoplasty, which involves the use of a gastrointestinal segment for urinary bladder reconstruction, was first proposed over 100 years ago. Despite its known associated problems, due to the lack of a better alternative, it remains the gold standard today. One major problem is that gastrointestinal tissues absorb specific solutes, whereas bladder tissues prevent their reabsorption [1]. This may lead to complications such as metabolic disorders, mucus production, urolithiasis and malignancy [2, 3]. Due to problems encountered with the use of gastrointestinal segments, numerous investigators have attempted alternative methods, materials, and tissues for bladder replacement or repair [4]. Natural materials such as de-epithelialized bowel segments provide an adequate urothelial covering for bladder reconstruction, but difficulties remain regarding mucosal regrowth, segment fibrosis, or both [5]. In a study on the regeneration of the dog urinary bladder, while the polymer-only bladder construct showed re-epithelialization by urothelial cells, a thickened fibrotic submucosa and a thin layer of muscle fibers were also noted [2].
Attempts to replace the bladder with natural or synthetic materials have shown that bladder tissue, with its specific muscular elastic properties and urothelial permeability functions, cannot be easily replaced. This goal may be attainable with the use of regenerative techniques. Tissue engineering incorporates the principles of cell transplantation, materials science, and engineering toward the development of biological substitutes that can restore and maintain normal organ or tissue function. Donor tissue is dissociated into individual cells, which are implanted directly into the host or expanded in culture, attached to a support matrix, and re-implanted after expansion. Since 1992, urinary bladder tissue specimens have been used as the cell source. These specimens, which are harvested from the bladder wall during surgical interventions, are used as a source of smooth muscle and urothelial cells followed by culture, expansion, seeding on a biocompatible scaffold, and implantation in vivo [5]. Several successful experimental trials have been performed with promising results [6, 7] However, the use of diseased bladder tissue is limited due to its compromised cell quality and the invasive nature of surgical collection, which makes this method unrealistic for clinical applications [8, 9]. Stem or progenitor cells from the oral mucosa, bone marrow, and hair follicles have been used to successfully restore the mucosal and urothelial layers of tissue engineered bladders [10–12]. Adipose tissue provides an additional, abundant source of multipotent cells that have the potential to differentiate into mesenchymal cell lineages, including smooth muscle cells, for urinary bladder reconstruction [13].
Biomaterials possess important physical and biological characteristics, which include a three-dimensional environment that allows cells to develop into new tissues. Synthetic biomaterials are favorable as they are composed of a defined material that can be obtained off-the-shelf with consistent quality from batch to batch. The ideal biomaterial is non-toxic, biocompatible, and supports tissue regeneration without the induction of a severe inflammatory response. It has been reported that the spherical shape of the urinary bladder is important, as it enables the symmetrical distribution of intravesical tension pressure, which is directly proportional to the curvature at any point [14]. Hence, the scaffold material of choice should be flexible and moldable into the shape of the bladder to be reconstructed.
Poly(lactic-co-glycolic acid) (PLGA) has been approved by the American Food and Drug Administration (FDA) and used as a suture material for decades in the clinic and more recently as a drug delivery device and surgical sealant film [15]. PLGA is biodegradable, and its degradation rate is tunable by altering the lactide to glycolide ratio [15]. Commercially available PLGA suture and mesh material is made of 90% glycolide and 10% L-lactide, with a degradation rate ranging from weeks to months in vivo. In this study, a commercial PLGA woven mesh was selected as it fulfills the abovementioned criteria and can easily be applied for clinical use.
We have previously described the method for fabrication of a tissue-engineered bladder construct composing of PLGA mesh which was seeded with smooth muscle cells derived from human adipose stem cells; the construct is leak-proof and mechanically stable [16]. The objective of this study was to evaluate the ability of a prefabricated human tissue-engineered bladder construct using FDA-approved materials for the subtotal reconstruction of the urinary bladder in athymic rats. Important considerations for the successful fabrication of tissue-engineered bladder are highlighted, and recommendations regarding future clinical translation strategies are outlined.
In this study, we hypothesized that the tissue-engineered construct should be fabricated according to the bladder shape, size and wall thickness of a full, native urinary bladder. We also hypothesized that the incorporation of smooth muscle cells is essential to regenerate bladder mucosal layers, while urothelial cells from the native bladder will migrate into the newly regenerated bladder mucosa.
Materials and methods
Bladder construct formation
The fabrication method of bladder construct and HADSCs differentiation to SMCs has been optimized and described previously [16, 17]. Briefly, human adipose-derived stem cells (HADSCs) were isolated from subcutaneous fat obtained with informed consent from patients (aged 20–40 years old) undergoing abdominoplasty. HADSCs were culture expanded in Dulbecco’s modified eagle’s medium supplemented with 10% fetal bovine serum (FBS). At passage 2, HADSCs were seeded in 75 cm2 culture flask with cell seeding density of 5000 cells per cm2 and cultured to 80% confluency. Then, the media was changed to smooth muscle inductive media (MCDB 131 medium, supplemented with 1% FBS and 100 U/ml heparin) to facilitate their differentiation into smooth muscle cells (HADSCs-SM) over a period of 6 weeks. The smooth muscle inductive media was changed every 5 days and the cell passaging was not required within the induction period [17]. Human plasma was obtained from 20 ml of whole blood collected through venipuncture with written consent from the donors (aged 20–30 years old). Plasma was then stored at − 20 °C until the cells were ready for the construct formation. Synthetic absorbable poly(lactic-co-glycolic acid) (PLGA) woven mesh composed of 90% glycolide and 10% L-lactide (VICRYL polyglactin 910; Ethicon, Sommerville, NJ, USA), which has been approved by the FDA for hernia repair, was used as the scaffold material. Three layers of PLGA mesh were sutured together using 5–0 synthetic absorbable sutures (VICRYL) and molded to form the upper two thirds (bladder dome) of the urinary bladder of an athymic rat, i.e. side wall length of 10 mm, dome height of 4–5 mm, and thickness of 1.0–1.2 mm. One million HADSCs-SM were mixed with 500 µl of human plasma and then seeded onto the dome-shaped scaffold. Twenty-five microliters 1 M CaCl2 solution was added to the seeded scaffold to facilitate the polymerization of fibrinogen in the plasma into fibrin. The cell-seeded construct was then incubated in a humidified incubator at 37 °C with 5% CO2 for 2 weeks before implantation.
For the fabrication of cell-free construct, all procedures remained the same as described above except that cells were omitted from the construct.
Treatment groups
Nine female adult athymic rats with body weights ranging 200–250 g underwent subtotal resection of the urinary bladder, where the upper two thirds of the urinary bladder were removed. Group 1 underwent anastomosis of the remaining bladder tissue. Group 2 underwent reconstruction with the cell-free scaffold. Group 3 underwent reconstruction with the tissue-engineered bladder construct (n = 3 per group).
Surgical procedure
Animals were anesthetized using 0.1 ml of a mixture of ketamine, zoletil, and xylazine (KTX) via intravenous injection. A skin incision was made at the midline of the lower abdomen, followed by blunt separation of the underlying abdominal wall muscular layers. Sharp excision of the upper two thirds of the urinary bladder (subtotal resection) was performed. In the resection-anastomosis group, bladder closure was performed by a continuous water-tight stitch using 6–0 suture material (VICRYL) to prevent urinary leakage. In the cell-free scaffold and tissue-engineered construct groups, the excised part was replaced with a construct stitched to the lower part of the native bladder using 6–0 suture material (VICRYL) in a continuous water-tight manner. Synthetic non-absorbable sutures (Prolene, Ethicon, Sommerville, NJ, USA) were used to support the water-tight closure at four specific locations (the 3, 6, 9, and 12 o’clock positions) and also served as a marker of the native bladder-construct junction. The non-absorbable sutures were not removed until the end of study. The bladder was filled with normal saline to test for leakage and additional stitches were added to close any leakage points. The inner surface of the construct was sealed with a biological adhesive substance (Bioglue; CryoLife, Europa Ltd, Surrey, UK) and the outer surface with fibrin sealant (Tisseel; Baxter, Westlake Village, CA, USA). Both substances are FDA-approved for clinical use.
A 10 Fr catheter was placed adjacent to the reconstructed bladder and extended to the exterior space through an abdominal opening secured by a suture. This served as a drainage tube to prevent any intra-abdominal urine collection. Intra-abdominal injection with 0.2 ml of enrofloxacin (Baytril 5%; Bayer, Seoul, Korea) was administered before closure. Closure of the abdominal walls was performed in layers. The analgesic piroxicam (Feldene; Pfizer, Jakarta, Indonesia) was injected intramuscularly after the operation. This was followed by daily antibiotic and analgesic injections for the next 5 days.
Post-surgical monitoring
Post-operative animals were housed individually. Animals were monitored daily for general wellbeing. The drainage tube was monitored for any discharge or displacement.
Radiological, ultrasonic and urodynamic evaluation
Ultrasonic and radiological evaluations studies were performed under general anesthesia on the animal at day 14 and day 90 post-bladder reconstruction. Radiologic assessments were performed using the scout scan function of an in vivo micro-CT machine (SkyScan 1076; Bruker, Antwerp, Belgium) with Omnipaque 300 (GE Healthcare, Malborough, MA, USA) as a contrast agent. The contrast agent was introduced to the bladder using a 20 ml syringe and a 10 Fr catheter via the urethra.
Ultrasonography was performed using a portable ultrasound machine (SonoSite M-Turbo; Fujifilm SonoSite, Bothell, WA, USA) equipped with a linear 10 MHz transducer. Bladder volume was calculated using the formula: [transverse diameter × longitudinal diameter × antero-posterior diameter × 0.52].
Urodynamic evaluations were performed using a modified method [18]. Briefly, the bladder was accessed from the abdomen via a midline incision. A small incision was then made on the bladder dome and a 6 Fr urodynamic catheter was inserted and the Y tubing was connected to an infusion catheter and to a Narco-Biosystem polygraph. A purse-string stitch was performed around the catheter to prevent leakage during filling with contrast agent and to secure the catheter in place midway between the urinary bladder base and roof. Gentle pressure was exerted on the bladder to empty the bladder. Pressure recordings were carried out during continuous manual filling of the bladder with normal saline via the infusion catheter. Bladder filling was done under gravity, with a filling rate of 0.05 ml/second using a 10 ml syringe elevated at around 20–30 cm from the bladder.
Gross examination
Gross, histological and immunohistochemical assessments were performed on the retrieved bladder at the end of the study. Animal euthanization was done by intracardiac pentabarbitol injection upon detection of a decline in animal’s health or at day 90 post-operation. A gross examination was performed before and after bladder removal from the animal and on the interior aspect of the bladder.
Histological evaluation
The bladder specimen was cut in longitudinal sections and fixed with 10% phosphate buffered formalin for 48 h and processed using routine histopathology techniques. Tissue sections with a thickness of 4–5 µm were deparaffinized and stained with hematoxylin and eosin and examined under a light microscope at 40 × and 100 × magnification.
Immunohistochemical evaluation
Deparaffinized sections were pretreated with Tris-buffered saline (Sigma Inc., St Louis, MO, USA) for 5 min. Slides were immersed in pH 9 antigen retrieval buffer (DAKO, Santa Clara, CA, USA) for 20–40 min at 95 °C followed by cooling at room temperature for 20 min and rinsing with running tap water. Non-specific binding was blocked using 10% normal goat serum (Gibco, Life Technologies, Frederick, MD, USA) at 37 °C for 30 min. Mouse monoclonal antibody [1A4] against human α-smooth muscle actin (cat. no. ab7817; Abcam, Cambridge, UK) with 1:100 dilution ratio, mouse monoclonal antibody [3F8] against human smooth muscle myosin heavy chain 1 (cat. no. ab682; Abcam) with 1:100 dilution ratio and mouse monoclonal antibody [4A83] against human smoothelin (cat. no. ab21108; Abcam) with 1:200 dilution ratio were applied as primary antibodies at 4 °C for 14–18 h. Sections were then incubated with goat anti-mouse immunoglobulin tagged with AlexaFluor 488 (Invitrogen, Carlsbad, CA, USA) for 1–2 h followed by counter-staining with DAPI (4′,6-diamidino-2-phenylindole) (Sigma Inc., St. Louis, MO, USA). Stained sections were evaluated using a confocal laser scanning microscope (Nikon A1; Nikon, Tokyo, Japan).
Results
Immediate post-operative follow-up showed good tolerance to surgery with excellent recovery for animals in all groups (resection-anastomosis, cell-free scaffold and tissue-engineered bladder group). Within the 7 days of follow up period, animals of all groups were presented as healthy and active with a non-infected wound site. Evaluations were performed at day 14 before probable complications arose due to technical challenge in maintaining the drainage catheter and post-operative care.
Radiography, ultrasonography and urodynamic measurements in normal bladder
In our previous study, the reference baseline values for various parameters of normal rat bladder function were established [19]. On radiography and ultrasonography, the rat bladder appears oval in shape, with a wall thickness of 1 mm at the bladder dome and 2 mm at the bladder base. The rat bladder volume established using radiography, ultrasonography, and urodynamic study was 1.42 ± 0.03 ml, 1.44 ± 0.05 ml, and 1.40 ± 0.02 ml, respectively. The urodynamic study showed that the rat bladder maintained a low intravesical filling pressure with a mean value of 5.5 ± 0.71 cm H2O and a mean voiding pressure of 18.5 ± 0.71 cm H2O.
Group 1 (resection-anastomosis)
All animals in resection-anastomosis group were presented as healthy and active until the planned euthanization of animals at day 90 post-operation. Radiological and ultrasonography (Fig. 1) examination at 90 days post-operation revealed a normal-appearing urinary bladder shape; the bladder outline was smooth and regular, and the bladder size and volume were within the normal range. No filling defects or calculi were noted within the bladder lumen. The extravesical area was normal with no urinary collection. Ultrasonography showed a return of the bladder dimensions and capacity to normal values with a calculated bladder volume of 1.42 ± 0.03 ml. Urodynamic assessments showed an intravesical filling pressure of 5.4 ± 0.1 cm H2O and voiding pressure of 17 ± 0.5 cm H2O. The gross evaluation showed minimal scar formation at the closure site and a mild inflammatory reaction around the urinary bladder.
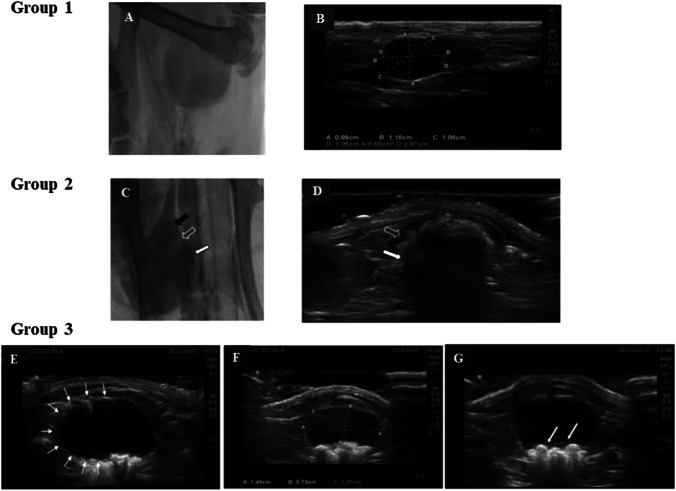
Fig. 1.
A Radiological and B ultrasonography examination of rats with bladder resection-anastomosis (Group 1) at 90 days post-operation. Urinary bladder appears normal in shape; the bladder outline is smooth and regular, and the bladder size and volume are within the normal range. No calculi are noted within the bladder lumen. C Radiography (black arrow points to the native bladder; hollow arrow points to the junction between native and the reconstructed bladder and the white arrow points to the reconstructed bladder) and D ultrasonography examination of rats treated with cell-free scaffold (Group 2) at day 14 post-operation. Urinary bladder appears normal with no evidence of urinary extravasation. An intact scaffold occupying the upper two thirds of the bladder is noted (hollow arrow points to the extravesical space; white arrow points to the newly reconstructed bladder). E–G Ultrasound examination of animal treated with tissue-engineered bladder (Group 3) at day 90 post-operation. Reconstructed urinary bladder appears oval in shape with a smooth and regular outline (white arrow). The bladder size and volume are within the normal range. Presence of urinary bladder calculi white arrow is detected (G)
Group 2 (cell-free scaffold group)
In this group, urinary bladder reconstruction was performed using a cell-free PLGA scaffold. Animals were euthanized once a decline in their health was detected during the daily-based follow-up (7–14 days). Post-reconstructed animals were healthy and active with a non-infected wound site for a variable period of 7–11 days, followed by a decline in animal health afterward. Two weeks post-operation, radiological and ultrasound evaluations (Fig. 1) showed a normal appearing urinary bladder, without any urinary extravasation. Calculated bladder volume was 1.5 ± 0.2 ml. Ultrasonography of the reconstructed bladder at day 14 revealed a full bladder with an intact scaffold occupying the upper two thirds of the bladder. No urinary leakage was detected in the surrounding tissue. The extracted whole bladders maintained their initial configuration. The PLGA scaffold was neither collapsed nor shrunken. No signs of infections were noted. The scaffold-native bladder anastomosis site was intact. No abnormal pathology was detected. Urothelial ingrowth was noted in the retrieved specimens. However, histological preparation of these specimens was nearly impossible as the PLGA mesh, held together by a thin layer of tissue, disintegrated during specimen sectioning. Urodynamic study could not be carried in this group as no animal survived beyond Day 14 till the end of study.
Group 3 (tissue-engineered bladder group)
Animals in this group were presented as healthy and active for a minimum of 7 days, and once the decline in animal health was apparent, euthanisation was performed. One animal remained healthy and active until the targeted euthanisation at day 90 post-operation. Radiological, ultrasound and urodynamic evaluations were performed on day 90 for the animal that survived, followed by euthanasia and bladder retrieval for gross examination as well as histological and immunohistochemical assessments.
Radiological and ultrasound evaluations
Radiography revealed a reconstructed urinary bladder with a normal oval appearance and a smooth and regular outline. The bladder size and volume were within the normal range. An irregular filling defect was present in the bladder lumen, suggesting the presence of urinary bladder calculi. No urinary extravasations were noted. Ultrasonography (Fig. 1) revealed a reconstructed bladder that appeared normal with the bladder dome directed cephalically and the bladder base directed caudally. The bladder lumen corresponded to a hypoechoic region surrounded by the bladder wall. The upper cephalic part of the bladder wall showed a mildly hyperechoic texture, which corresponded to the reconstructed part of the bladder. Multiple urinary bladder calculi appeared as hyperechoic shadows occupying the bladder lumen. The urinary bladder dimensions measured during ultrasound were within the normal range with a calculated bladder volume of 1.48 ml. The bladder wall thickness was 9 mm in the native bladder region and 12 mm in the reconstructed part of the bladder. Neither fluid collections nor calcifications were noted in the surrounding tissues.
Urodynamic evaluation
An intravesicle filling pressure of 5.8 cm H2O was recorded and it remained constantly low during bladder filling assessments. Voiding pressure was triggered by a sharp rise in bladder pressure to 19 cm H2O, followed by a rapid decline after bladder emptying. The maximum filled volume before voiding was 1.5 ml.
Gross examination
Grossly, the newly reconstructed urinary bladder appeared rounded, full, with a size, shape and appearance similar to a normal bladder. There was a mild degree of adhesion with minimal fibrotic changes surrounding the urinary bladder. The PLGA scaffold was no longer visible. The texture and consistency of the inner bladder wall (mucosal) were uniform, smooth, and pinkish in color, with no abnormal growth or stiffness noted. No distinctions could be made between the native and the reconstructed parts of the bladder in terms of consistency and texture. No abnormal pathology or any signs of inflammation were visually apparent. Multiple bladder calculi were found in the bladder lumen (Fig. 2).
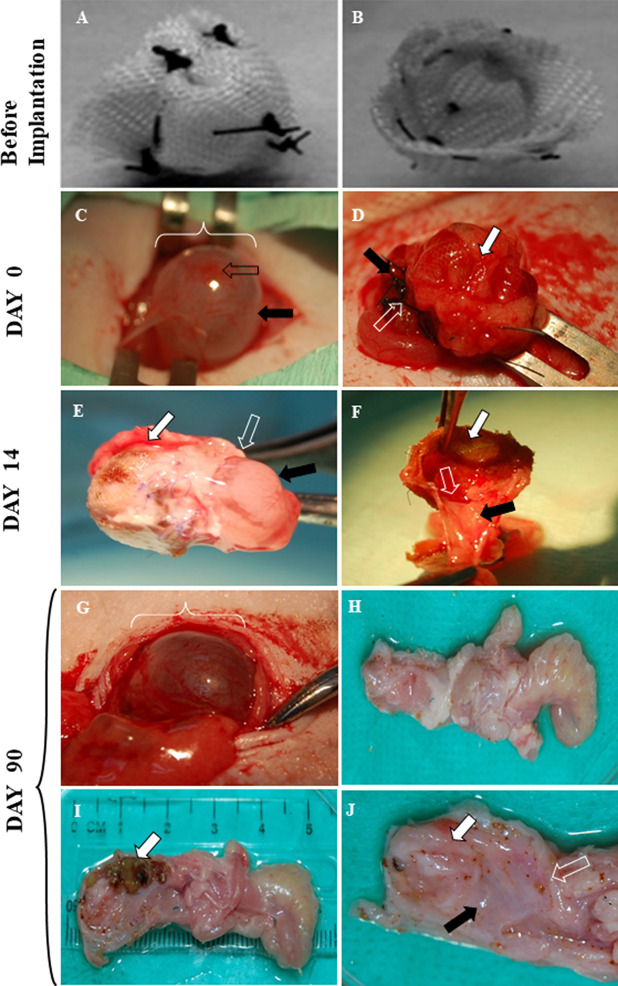
Fig. 2.
Gross appearance of pre-operative, reconstructed and post-implanted urinary bladder at day 0, 14 and day 90 from the tissue-engineered bladder group. A and B show the outer aspect and inner aspect of a urinary bladder construct before implantation, respectively. C Intra-operative pre-reconstruction intact (full normal bladder) with a smooth, rounded bladder dome (black arrow) and bladder body (white arrow). D Intra-operative post-reconstruction bladder with the upper part of the scaffold (white arrow) covered with adhesive and sealant before abdominal closure; the hollow arrow points to sutures at the junction between the scaffold and the lower part (black arrow) of the urinary bladder. E Reconstructed bladder at day 14 post-implantation after removal from the animal. The white arrow indicates the reconstructed (upper) part of the bladder, the black arrow points to the native (lower) part of the bladder and the hollow arrow points to the junction between scaffold and the native urinary bladder. F Reconstructed bladder at day 14 after it was divided into halves showing the inner aspect of the scaffold (white arrow) and the native bladder (black arrow), and the junction between the scaffold and the lower part of the urinary bladder (hollow arrow). Complete tissue ingrowth formed a smooth continuous lining from the inner wall of the native bladder to the scaffold. G Reconstructed bladder at day 90 post-implantation showing a smooth and rounded bladder shape similar to that of a native bladder. H Outer aspect of the extracted bladder and urethra at day 90. I Sagittal view of the extracted bladder at day 90 revealing multiple stones (white arrow) occupying the opened bladder lumen. J Sagittal view of the extracted bladder at day 90 revealing a healthy and pinkish bladder inner lining (white arrow), bladder neck (black arrow) and urethra (hollow arrow). Permission for reproduction of Figures 2A & B have been obtained from Wiley Periodicals, Inc. [16] From: Figure 8 from Salah et al. J Biomed Mater Res Part A, 2013. 101:2237–47. ©2013 WILEY PERIODICALS, INC.
Histological evaluation
Histological examination of the tissue cross-section revealed complete regeneration of the three main layers of the bladder wall; the mucosal layer composed of a stratified urothelium, the submucosal layer with the presence of blood vessels, and the muscular layer with a thickness resembling that of a normal urinary bladder wall with the presence of elongated muscle cells arranged in bundles. No signs of inflammation were observed. No residual PLGA was noted (Fig. 3).
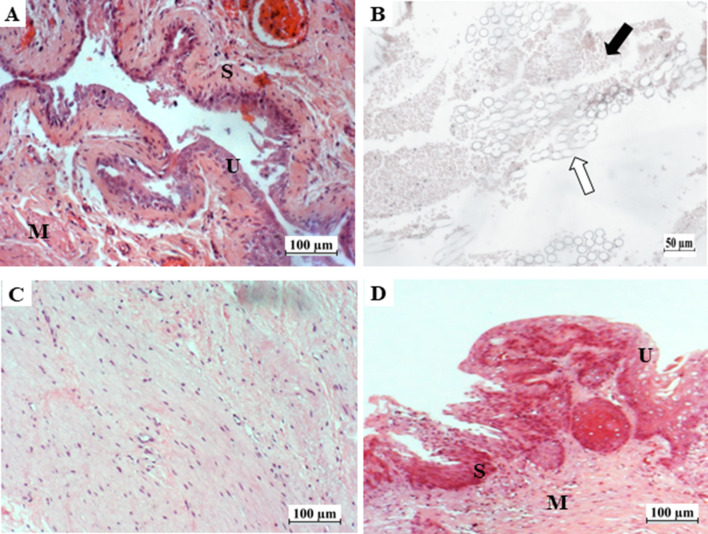
Fig. 3.
Histological photomicrographs of a native urinary bladder and reconstructed bladder at day 14 and day 90 post-implantation (hematoxylin and eosin staining). A Native urinary bladder wall showing the urothelium (U), submucosa (S) and muscular layer (M). B Reconstructed bladder at day 14 post-implantation showing muscle tissue ingrowth (white arrow) within the PLGA scaffold (black arrow). C Muscular layer of the reconstructed urinary bladder at day 90 post-implantation with aligned and elongated cells. D Newly reconstructed bladder wall showing complete regeneration of the urothelium (U), submucosa (S) and muscular layer (M). Scale bar: 100 μm for A, C, D; Scale bar: 50 μm for B
Immunohistochemical evaluation
In tissue specimens from tissue-engineered bladder group, myosin heavy chain and smoothelin were detected in almost 100% of the cells in the muscular layer of the newly regenerated bladder, but not in the native bladder (Fig. 4).
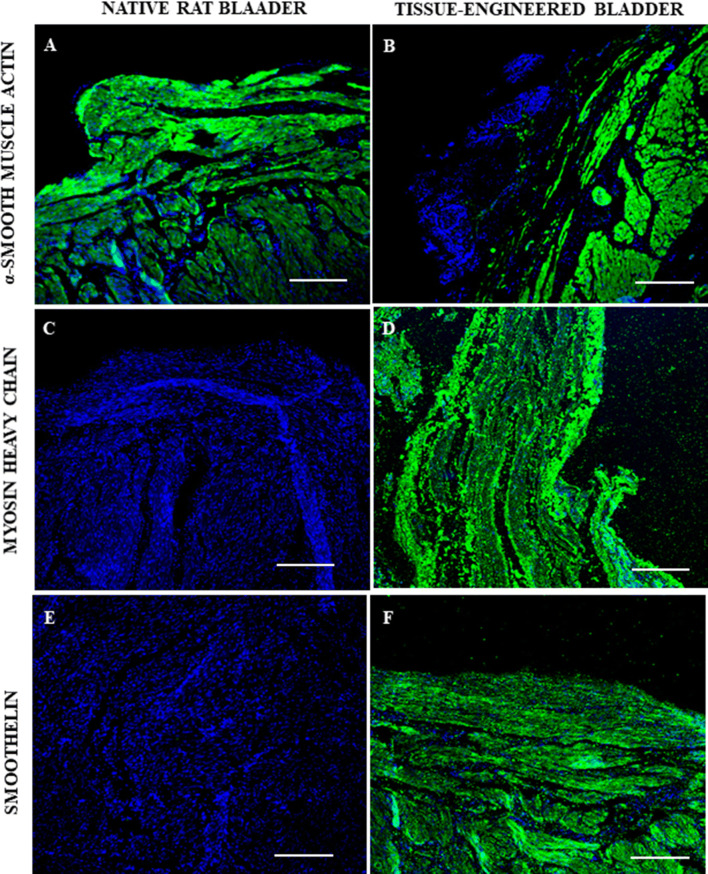
Fig. 4.
Fluorescence photomicrographs of native and newly regenerated bladder walls in the tissue-engineered bladder group at day 90 post-implantation. Immunofluorescent staining (green color) was positive for α-smooth muscle actin in the (A) native rat bladder and in the (B) tissue-engineered bladder. Human myosin heavy chain staining (green color) was negative in the (C) native rat bladder, but positive in the (D) tissue-engineered bladder. Human smoothelin staining (green color) was negative in the (E) native rat bladder but positive in the (F) tissue-engineered bladder. In all photomicrographs, blue color represent nuclei counter-staining with 4′,6-diamidino-2-phenylindole. Scale bar: 100 μm
Discussion
A gross inspection was performed on the bladder constructs that were retrieved after 2 weeks of implantation. The PLGA scaffold remained intact and visible, not collapsed or shrunken, and there were no visible signs of inflammation, infection or necrosis. This is evidence that the PLGA scaffold possessed sufficient mechanical strength against intra-abdominal pressure and forces, and was biocompatible with the host.
In our previous study, a three-layered PLGA mesh seeded with fibrin and cells showed good pressure withholding properties (30.00 cmH2O), a tensile stress at failure of 30.82 ± 3.80 MPa, a maximum tensile strain at failure of 19.42 ± 2.24 mm, a Young’s modulus of 0.035 ± 0.0083 Pa, and a maximum load to yield of 58.55 ± 7.90 N. However, there was some degree of leakage through the mesh. Leakage was completely prevented only when the inner surface of the dome-shaped scaffold was sealed with a biological adhesive substance and the outer aspect was sealed with a layer of fibrin sealant (00.00 ml/min) [16]. The sealing provided by the biological adhesive material, i.e. a plasma-derived fibrin clot and fibrin sealant was only temporary as these materials quickly degrade in vivo, leading to urinary leakage. Degradation would take place between 7 and 14 days, which corresponded to the decline in animal health in this time frame.
Based on this study, we predict that the critical period before urothelial ingrowth may be within the first 2 weeks after implantation. Ideally, native urothelial ingrowth should take place during this period and provide the necessary sealing function of the construct. A two-week period has been reported as being required for complete rat urothelium ingrowth onto an implanted biomatrix [20].
By day 90, the implanted PLGA material was no longer visible grossly or histologically in the retrieved bladder from the animal that survived in the tissue-engineered bladder group. This is evidence that the PLGA material had completely degraded and the seeded smooth muscle cells had formed mature muscular tissue that conferred the newly regenerated bladder with its functional properties.
In our previous findings, we had extensively described and proved the in vitro capability of human adipose derived stem cells differentiation into mature smooth muscle cells and the results were validated through morphological examination, gene expression analysis via PCR, and protein expression evaluation via immunostaining [17]. Use of primary antibodies that react with human antigens and do not cross-react with mouse antigens proved to be a useful approach for cell tracking. Non-cross reactivity with the mouse antigen to myosin heavy chain [3F8] and smoothelin [4A83] has been validated by the supplier (Abcam, Cambridge, UK). Hence, since almost 100% of the cells stained positively for MHC and smoothelin, this is evidence that the cells were of human origin and that they were derived from seeded smooth muscle cells derived from human adipose stem cells. The presence of a stratified urothelial lining of rat origin suggests urothelial migration from the remaining native bladder of the host, thus supporting our hypothesis that the tissue-engineered construct promoted rapid urothelial ingrowth that sealed the newly regenerated bladder from urine leakage. As opposed to most previous strategies using constructs seeded with urothelial cells, we have shown that urothelial ingrowth is possible.
The presence of calculi in the bladder may be the result of crystallization around the PLGA mesh and urinary stagnation during the course of regeneration. Theoretically, there should be no further calculus formation after biomaterial absorption and complete regeneration of the bladder.
In this study, the use of athymic animals allowed the in vivo development of a tissue-engineered bladder containing human cells without immune-mediated rejection. However, the use of these animals poses other challenges. A major challenge was securing the drainage tube and preventing its removal by the animal during the initial post-surgical period. The drainage tube would have prevented the accumulation of urine in the abdominal cavity, even in the case of leakage. Intra-vesical drainage was avoided in order to prevent disruption of the bladder construct. Another challenge was that these rats recovered at a remarkable rate and restored their original bladder function within 3 months post-surgery. Such regenerative capacity would not have been achievable in human patients. Thus, rats may not be the best model organism for the study of bladder regeneration.
Many previous studies have resorted to the use of an acellular tissue matrix derived from xenografts or allografts [1, 6] The advantage is obvious as it mimics the extracellular matrix component of the bladder. However, the need for a consistent source of tissue, standardization to control for donor-to-donor variation, and an effective method for decellularization, sterilization and storage of the biological material are daunting issues. Hence, in our strategy, we employed a synthetic resorbable material, which is already in use in the clinic and FDA approved. The physical properties of the PLGA biomaterial, such as rigidity and flexibility, fulfill the necessary requirements for scaffold shape modulation [21]. The differentiation of human adipose-derived stem cells into smooth muscle cells had been confirmed previously via morphological changes and positive smooth muscle cell marker expression, detected by RT-PCR and immunostaining [17]. Although the use of bladder tissue-derived smooth muscle cells would avoid the need for differentiation, the starting material is limited and would be obtained from diseased tissues. In our approach, plasma polymerization was used to enhance cell delivery and seal the gaps in the PLGA matrix. Plasma has been shown to contain natural growth factors that could accelerate cell proliferation on the surface of the scaffold [22].
In conclusion, implantation of a smooth muscle cells derived from human adipose stem cells seeded-PLGA scaffold (tissue-engineered bladder) could lead to regeneration of the urinary bladder muscular layer and support urothelial ingrowth, leading to a completely functional urinary bladder. Although the findings of this study are preliminary, the presence of seeded cells of human origin in the newly regenerated bladder muscular layer certainly supports this conclusion. However, before the bladder construct can be trialed in the clinic, more work should be done to demonstrate consistent results.
All materials used in the fabrication of the tissue-engineered bladder in this study are available in clinical grade. Adipose-derived stem cells can be obtained from autologous or allogenic sources. Alternatively, other mesenchymal stem cell (MSC) sources such as bone marrow, Wharton’s jelly and even menstrual blood can be explored [23–25]. The processing and expansion of cells should be performed in Good Manufacturing Practice (GMP)-compliant facilities using autologous serum and GMP-grade reagents. Facilities offering clinical-grade allogenic mesenchymal stem cells are now available. Appropriate catheterization and urine drainage for an appropriate period of time must be implemented during surgery to prevent complications during the early phase of bladder tissue regeneration. In view of the slower regenerative capacity in humans compared to rats, the inclusion of urothelial cells in the tissue-engineered construct may be considered. In this case, MSCs can be differentiated into smooth muscle cells, and can also be transdifferentiated into urothelial cells [24, 25]. Biological variations arising from inter-donor tissues may be an issue. Hence, consistent performance of the seeded cells and the quality of human plasma-derived fibrin should be optimized and further defined. A quality control program must be implemented to ensure consistency in these bladder constructs.
Acknowledgements
The study was funded by Universiti Kebangsaan Malaysia Medical Centre Grants: GUP-2017-092, FF-173-2010 and Young Researcher Grant: GGPM-2011-079.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no financial conflicts of interest regarding the publication of this paper.
Ethical statement
Human samples used in this study were obtained and processed with informed consent from patients undergoing abdominoplasty according to procedures approved by the Institutional Research and Ethics Committee (Approval code: FF-173-2010). Experimentation on rats in this study followed guidelines and procedures approved by the Institutional Animal Ethics Committee (Approval code: PP/SURG/2010/ZULKIFLI/17-MARCH/292-MARCH-2010 DECEMBER-2011).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–225. doi: 10.1016/S0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 3.Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004;172:1649–1651. doi: 10.1097/01.ju.0000140194.87974.56. [DOI] [PubMed] [Google Scholar]
- 4.Lam Van Ba O, Aharony S, Loutochin O, Corcos J. Bladder tissue engineering: a literature review. Adv Drug Deliv Rev. 2015;82–83:31–37. doi: 10.1016/j.addr.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81–104. doi: 10.1093/bmb/ldr003. [DOI] [PubMed] [Google Scholar]
- 6.Drewa T, Sir J, Czajkowski R, Wozniak A. Scaffold seeded with cells is essential in urothelium regeneration and tissue remodeling in vivo after bladder augmentation using in vitro engineered graft. Transplant Proc. 2006;38:133–135. doi: 10.1016/j.transproceed.2005.11.086. [DOI] [PubMed] [Google Scholar]
- 7.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 8.Lin HK, Cowan R, Moore P, Zhang Y, Yang Q, Peterson JA, Jr, et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol. 2004;171:1348–1352. doi: 10.1097/01.ju.0000108800.47594.8b. [DOI] [PubMed] [Google Scholar]
- 9.Akbal C, Lee SD, Packer SC, Davis MM, Rink RC, Kaefer M. Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol. 2006;176:1706–1711. doi: 10.1016/j.juro.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 10.Iizuka K, Muraishi O, Maejima T, Kitami Y, Xu YM, Watanabe K, et al. Total replacement of urethral mucosa with oral mucosa in dogs. J Urol. 1996;156:498–501. doi: 10.1016/S0022-5347(01)65913-5. [DOI] [PubMed] [Google Scholar]
- 11.Drewa T, Joachimiak R, Kaznica A, Sarafian V, Pokrywczynska M. Hair stem cells for bladder regeneration in rats: preliminary results. Transplant Proc. 2009;41:4345–4351. doi: 10.1016/j.transproceed.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 12.Wu R, Liu G, Bharadwaj S, Zhang Y. Isolation and myogenic differentiation of mesenchymal stem cells for urologic tissue engineering. Methods Mol Biol. 2013;1001:65–80. doi: 10.1007/978-1-62703-363-3_7. [DOI] [PubMed] [Google Scholar]
- 13.Jack GS, Zhang R, Lee M, Xu Y, Wu BM, Rodríguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 2009;30:3259–3270. doi: 10.1016/j.biomaterials.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damaser MS, Lehman SL. The effect of urinary bladder shape on its mechanics during filling. J Biomech. 1995;28:725–732. doi: 10.1016/0021-9290(94)00169-5. [DOI] [PubMed] [Google Scholar]
- 15.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem SA, Hwei NM, Saim AB, Ho CC, Sagap I, Singh R, et al. Polylactic-co-glycolic acid mesh coated with fibrin or collagen and biological adhesive substance as a prefabricated, degradable, biocompatible, and functional scaffold for regeneration of the urinary bladder wall. J Biomed Mater Res A. 2013;101:2237–2247. doi: 10.1002/jbm.a.34518. [DOI] [PubMed] [Google Scholar]
- 17.Salem SA, Hwie AN, Saim A, Chee Kong CH, Sagap I, Singh R, et al. Human adipose tissue derived stem cells as a source of smooth muscle cells in the regeneration of muscular layer of urinary bladder wall. Malays J Med Sci. 2013;20:80–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Souza AB, Suaid HJ, Suaid CA, Tucci S, Jr, Cologna AJ, Martins AC. Comparison of two experimental models of urodynamic evaluation in female rats. Acta Cir Bras. 2008;23:59–65. doi: 10.1590/S0102-86502008000700011. [DOI] [PubMed] [Google Scholar]
- 19.Salem SA, Hwei NM, Ho CC, Sagap I, Idrus RB, Md Zainuddin Z. Combining radiographic, urodynamic and ultrasonographic technique for the evaluation of the urinary bladder structure and function in an animal model. J Surg Acad. 2011;1:25–32. [Google Scholar]
- 20.Roth CC, Bell CH, Woodson B, Schultz AD, Palmer BW, Frimberger D, et al. Temporal differentiation and maturation of regenerated rat urothelium. BJU Int. 2000;103:836–841. doi: 10.1111/j.1464-410X.2008.08231.x. [DOI] [PubMed] [Google Scholar]
- 21.Ng MH, Chowdhury SR, Morshed M, Tan KK, Tan GH, Phang MY, et al. Effective cell seeding and three-dimensional cell culture for bone tissue engineering. J Biomater Tissue Eng. 2014;4:573–578. doi: 10.1166/jbt.2014.1204. [DOI] [Google Scholar]
- 22.Shoae-Hassani A, Sharif S, Seifalian AM, Mortazavi-Tabatabaei SA, Rezaie S, Verdi J. Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women. BJU Int. 2013;112:854–863. doi: 10.1111/bju.12195. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Zeiai S, Ekblad A, Nordenskjöld A, Hilborn J, Götherström C, et al. Transdifferentiation of autologous bone marrow cells on a collagen-poly(ε-caprolactone) scaffold for tissue engineering in complete lack of native urothelium. J R Soc Interface. 2014;11:20140233. doi: 10.1098/rsif.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Cheng Z, Liu G, Zhao X, Zhong L, Zhu Y, et al. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal Cell Pathol (Amst) 2013;36:63–69. doi: 10.1155/2013/274640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong BF. Transdifferentiation of human adipose-derived stem cells intourothelial cells: potential for urinary tract tissue engineering. Cell Tissue Res. 2012;347:737–746. doi: 10.1007/s00441-011-1317-0. [DOI] [PubMed] [Google Scholar]