Abstract
Background and Purpose
Inhibition of the G‐protein gated ACh‐activated inward rectifier potassium current, I K,ACh may be an effective atrial selective treatment strategy for atrial fibrillation (AF). Therefore, the anti‐arrhythmic and electrophysiological properties of a novel putatively potent and highly specific I K,ACh inhibitor, XAF‐1407 (3‐methyl‐1‐[5‐phenyl‐4‐[4‐(2‐pyrrolidin‐1‐ylethoxymethyl)‐1‐piperidyl]thieno[2,3‐d]pyrimidin‐6‐yl]azetidin‐3‐ol), were characterised for the first time in vitro and investigated in horses with persistent AF.
Experimental Approach
The pharmacological ion channel profile of XAF‐1407 was investigated using cell lines expressing relevant ion channels. In addition, eleven horses were implanted with implantable cardioverter defibrillators enabling atrial tachypacing into self‐sustained AF. The electrophysiological effects of XAF‐1407 were investigated after serial cardioversions over a period of 1 month. Cardioversion success, drug‐induced changes of atrial tissue refractoriness, and ventricular electrophysiology were assessed at baseline (day 0) and days 3, 5, 11, 17, and 29 after AF induction.
Key Results
XAF‐1407 potently and selectively inhibited Kir3.1/3.4 and Kir3.4/3.4, underlying the I K,ACh current. XAF‐1407 treatment in horses prolonged atrial effective refractory period as well as decreased atrial fibrillatory rate significantly (~20%) and successfully cardioverted AF, although with a decreasing efficacy over time. XAF‐1407 shortened atrioventricular‐nodal refractoriness, without effect on QRS duration. QTc prolongation (4%) within 15 min of drug infusion was observed, however, without any evidence of ventricular arrhythmia.
Conclusion and Implications
XAF‐1407 efficiently cardioverted sustained tachypacing‐induced AF of short duration in horses without notable side effects. This supports I K,ACh inhibition as a potentially safe treatment of paroxysmal AF in horses, suggesting potential clinical value for other species including humans.

Abbreviations
- AAD
anti‐arrhythmic drug
- aERP
atrial effective refractory period
- AF
atrial fibrillation
- AFCL
atrial fibrillation cycle length
- AFR
atrial fibrillatory rate
- BCL
basic cycle length
- bpm
beats per minute
- cAF
chronic atrial fibrillation
- cIK,ACh
constitutively active IK,ACh
- EGM
intra‐atrial electrogram
- EP
electrophysiology
- fpm
fibrillations per minute
- HR
heart rate
- ICD
implantable cardioverter defibrillator
- IK,ACh
G‐protein gated ACh‐activated inward rectifier potassium (K+) current
- IKur
ultra‐rapid delayed outward rectifier K+ current
- Kir3.1
α‐subunit of the heterotetramer conducting I K,ACh current
- Kir3.4
α‐subunit of the heterotetramer conducting I K,ACh current
- pAF
paroxysmal atrial fibrillation
- PK
pharmacokinetic
- PPB
plasma protein binding
- SR
sinus rhythm
- SK
small‐conductance Ca2+‐activated K+ channels
- QTc
rate corrected QT interval
What is already known
The G‐protein gated ACh‐activated inward rectifier potassium current (IK,ACh) plays a role in atrial repolarisation.
Discrepancies exist between preclinical and clinical results regarding efficacy of IK,ACh inhibition in AF treatment.
What this study adds
The study provides the selectivity profile of XAF‐1407, inhibiting Kir3.1/3.4 and Kir3.4/3.4 with similar potency.
The study demonstrates efficacy of XAF‐1407 as early AF‐treatment in absence of life‐threatening ventricular side‐effects.
What is the clinical significance
We present first data relating pharmacological IK,ACh inhibition to decreasing cardioversion efficacy over time.
We present preclinical proof‐of‐concept for atrial‐specific ion‐current inhibition as safe treatment of paroxysmal AF
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia with an estimated worldwide prevalence of 3% (Kirchhof et al., 2016). The risk of AF increases substantially with age. Moreover, AF is associated with an impaired quality of life and a twofold increase in all‐cause mortality (Kirchhof et al., 2016). There is an unmet need for safe and effective treatment options that can address the growing number of patients, which is why further research and drug development efforts, focusing on novel pharmacological treatment modalities, are required.
Current anti‐arrhythmic drugs (AADs) are characterised by either poor efficacy or serious non‐cardiac and cardiac adverse effects, including potentially fatal ventricular arrhythmia (Kirchhof et al., 2016).
Class III AADs currently on the market inhibit ion currents conducted by potassium (K+) channels expressed in the both atria and ventricle. Therefore, targeting K+ channels with a predominant expression in atrial myocardial tissue should yield new and effective therapies with a substantially lower risk of ventricular pro‐arrhythmia (Ravens, Poulet, Wettwer, & Knaut, 2013; Skibsbye & Ravens, 2016). Recent studies have identified three possible new targets with high atrial selectivity: the ultra‐rapid delayed outward rectifier K + current (I Kur), small‐conductance Ca 2+‐activated K+ (SK) channels, and the G‐protein gated ACh‐activated inward rectifier K + current (I K,ACh) (Diness et al., 2010; Dobrev et al., 2005; Qi et al., 2014; Ravens et al., 2013; Skibsbye & Ravens, 2016; Voigt & Dobrev, 2016). Tocris Bioscience, The Watkins Building, Atlantic Road, Bristol BS119QD, UK
I K,ACh participates in the late repolarisation of the atrial action potential (AP) and in setting the resting membrane potential. In healthy atrial tissue, I K,ACh is activated by stimulation of muscarinic receptors (M‐receptors) through vagally released ACh (Yamada, Inanobe, & Kurachi, 1998). Increased activation of this current results in shortening of the atrial AP and atrial effective refractory period (aERP) (Shen & Zipes, 2014), which is believed to be an important mechanism in promoting and sustaining AF. However, the initial concept of AF maintenance and aERP shortening by up‐regulation of M‐receptor activated I K,ACh has been refuted (Brundel et al., 2001; Dobrev et al., 2001; Dobrev et al., 2002), and new approaches are now being investigated. In 2005, Dobrev et al. successfully demonstrated an increase of agonist‐independent constitutively active I KACh (cI K,ACh) in patients with chronic AF. Therefore, inhibition of cI K,ACh has been suggested as a new anti‐arrhythmic strategy (Heijman, Ghezelbash, & Dobrev, 2017; Milnes, Madge, & Ford, 2012; Ravens et al., 2013; Skibsbye & Ravens, 2016).
Pharmacological inhibition of this current prolongs aERP and terminates AF with high efficacy in several canine models (El‐Haou, Ford, & Milnes, 2015; Hashimoto, Yamashita, & Tsuruzoe, 2006, 2008; Kovoor et al., 2001; Podd, Freemantle, Furniss, & Sulke, 2016; Walfridsson et al., 2015). However, the positive results from these canine studies could not be confirmed in human phase II clinical trials. In studies investigating the influence of I K,ACh inhibition on AF burden, the compounds BMS 914392, AZD2927, and A7071 had no positive influence on AF burden or aERP length in patients with paroxysmal AF (pAF) and atrial flutter (Podd et al., 2016; Walfridsson et al., 2015).
Hitherto, in vivo pharmacological studies of I K,ACh inhibitors have focused on canine models of AF. These, however, have translated poorly to clinical efficacy in man and have led to the analysis of the role of I K,ACh in other large animal models that better reproduce the human AF phenotypes. Such models should help in developing new and effective AADs.
This study aimed to investigate the efficacy of I K,ACh inhibition by examining the anti‐arrhythmic and electrophysiological properties of the novel putatively potent and highly specific I K,ACh inhibitor, XAF‐1407, in an equine, atrial‐tachypacing‐induced model of persistent AF (Carstensen et al., 2018; Hesselkilde et al., 2019). This animal AF model reproduces important features of human AF and, furthermore, horses spontaneously develop AF both with and without underlying structural heart disease (Reef et al., 2014). Further, the mRNA transcripts encoding the proteins underlying I K,ACh (Kir 3.1/Kir 3.4) are, in horses, predominantly expressed in atrial myocardial tissue, as they are in humans (Haugaard et al., 2015). The present study was designed to characterise the novel I K,ACh inhibitor XAF‐1407 and evaluate the putative atrial selectivity of I K,ACh inhibition and its potential to terminate AF of durations up to 29 days, and as a possible treatment for sustained AF in humans.
2. METHODS
2.1. In vitro electrophysiology and pharmacology
Effects of XAF‐1407 on HEK293 cells (CLS Cat# 300192/p777_HEK293, RRID:CVCL_0045) expressing Kir3.1/3.4, Kir 6.2/SUR2A (provided by Dr Andrew Tinker), Kir 3.4/3.4, Kir 2.1, and CHO cell lines (CLS Cat# 603479/p746_CHO, RRID:CVCL_0213) stably expressing hERG, Kv1.5, Kv4.3, and Nav1.5 were investigated by whole‐cell patch clamp, as well as automated patch clamp. The assessment of cardiac calcium channel activity was performed by counter screening against the pore forming subunit of the cardiac L‐type Ca 2+ channel using a 96‐well plate‐based fluorescence plate.
2.1.1. Maintenance of ion channel expressing cell lines
HEK293 cells expressing Kir3.1/3.4, Kir6.2/SUR2A, Kir3.4/3.4, Kir2.1, and CHO cell lines stably expressing hERG, Kv1.5, Kv4.3, and Nav1.5 were maintained in media containing 10% FCS (HyClone™, FBS; Peribo Science AB/Thermo Fisher Scientific, Bishop's Stortford, UK) and appropriate selection antibiotic (supporting information Table S1). Cells were grown either in suspension or in T‐flasks and routinely passaged. Cells for patch clamping experiments were plated onto glass cover slips prior to use. Cells for automated patch clamping experiments were freshly prepared on each experimental day.
2.1.2. Cloned cardiac ion channel conventional electrophysiology
Standard gigaseal whole‐cell patch clamp techniques were performed at room temperature using glass pipettes (2–5 MΩ). HEKA EPC9/10 amplifiers and Pulse software were used for data acquisition. Series resistance was compensated by >70%. Experimental solutions are given in the supporting information (Tables S2 and S3). Voltage protocols were as follows: Kir3.1/3.4, Kir3.4/3.4, Kir2.1 (VHold −60 mV, +60 mV/100 ms, ramp +140 mV/500 ms, −140 mV/100 ms, 0.1 Hz), hERG (VHold −80 mV, −40 mV/50 ms, +40 mV/1 s, −40 mV/5 s, 0.1 Hz), Kv1.5 (VHold −80 mV, 0 mV/900 ms, −40 mV/100 ms, 0.1 Hz), Kv4.3 (VHold −80 mV, +30 mV/0.5 s, 0.067 Hz), Nav1.5 (VHold −100 or −70 mV, −10 mV/20 ms, 1 or 5 Hz), Kir6.2/SUR2A (VHold −60 mV, −50 mV/200 ms, ramp −70 mV/250 ms, −70 mV/200 ms, 0.1 Hz), and Kv7.1/KCNE1 (VHold −80 mV, −50 mV/30 ms, +60 mV/4 s, −50 mV/4 s, 0.05 Hz). Ionic current recordings were analysed using HEKA software. Kir2, Kir3 as mean current at −120 mV, hERG and Kv7 as peak tail current on repolarisation to −40/−50 mV. Nav1.5 and Kv4.3 as peak current on depolarisation, Kv1.5 as mean current at the end of the depolarising pulse.
2.1.3. Assessment of cardiac calcium channel activity
Counter screening against the pore forming subunit of the cardiac L‐type Ca2+ channel was performed using a 96‐well plate‐based fluorescence plate. Briefly, HEK293 cells expressing Cav 1.2 channel were seeded at 15 x103 cells per well. Cells were loaded with Fluo‐4 AM dye before being equilibrated in the presence of either a range of concentrations of XAF‐1407(1, 3, and 10 μM) or positive control (1‐ and 3‐μM nimodipine; Tocris Bioscience, Bristol, UK) or vehicle. Plates were placed on a temperature‐controlled (37°C) fluorescence plate reader with liquid handling capability. Following recording of baseline fluorescence of each well (1 min per column, 0.2 Hz), a high‐K+ stimulus buffer containing the agonist FPL‐64176 (600 nM; Tocris Bioscience, Bristol, UK) was applied a column at a time during which time fluorescence was measured and recorded (3 min). Compositions of experimental solutions are given in the supporting information (Table S4).
2.1.4. Radioligand binding assessment of pharmacology
The general pharmacology and selectivity of XAF‐1407 was further investigated in panel of radioligand binding assay against 80 receptors, GPCRs, ion channels, and transporters. Details of radioligand binding assay including radioligands, cold‐ligand, conditions, and the reference compound run for each assay are summarised in the supporting information. In assays where radioligand displacement induced by XAF‐1407 was observed to be >50% at a single concentration of 10 μM, assays were rerun using eight concentrations (30 nM to 100 μM) and plotted against concentration and fitted with a sigmoidal function to determine the IC50 value, Hill coefficient (n H), and Ki. For those targets where specific binding was <50% at 10 μM, the IC50 and Ki were assumed to be >10 μM.
2.1.5. Test substance, positive control, bath, and pipette solutions
For electrophysiological studies, a 10‐mM stock solution of XAF‐1407 was prepared in DMSO, frozen, and stored as aliquots at approximately −20°C until use. Serial dilution of the 10‐mM stock in DMSO was performed in glass vials prior to dilution in external bath solution to achieve the desired final perfusion concentration with a vehicle concentration of 0.1%–0.3% DMSO. Compound containing experimental solutions were made fresh throughout the experimental day in glass vials. All drug containing solutions were made up in glassware. Drug delivery systems were constructed from PTFE and glass to minimise drug absorption and adsorption.
2.2. In vivo animal model
2.2.1. Animals
The study was approved by the local ethical committee at the Department of Veterinary Clinical Sciences, University of Copenhagen and The Danish Animal Experiments Inspectorate (license number 2016‐15‐0201‐01128) and was performed in accordance with the European Commission Directive 86/609/ECC. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
Predefined humane endpoints included in the respective animal experiments license were as follows:
No initiation of investigations including pacing from the ICD if a horse displays signs of pain and inflammation or if ventricular tachyarrhythmias are encountered.
If a horse displays dullness when in AF, the ICD should be turned off, and the experiment terminated.
The investigations should be terminated if a horse develops ventricular tachyarrhythmias from one of the investigations, and immediate medical treatment should be initiated. If treatment is unsuccessful, the horse should be killed either by intravenous injection of pentobarbital (Euthasol® 40% ad us. vet.; Virbac, Kolding, Denmark).
Twelve Standardbred mares with a mean age of 7 ± 3 years (range 3–12) were enrolled (461 ± 56 kg, height of 158 ± 4 cm) (Figure 1). Sample size was based on previous experience. Eleven horses completed the whole course of this study while one horse had to be excluded and killed due to complications following implantation of the implantable cardioverter defibrillator (ICD) pacing device (horse #5). Furthermore, one horse was excluded right from the start of the study, as this study age cut‐off was >12 years and a mismatch between the declared age at purchase and the registered date of birth in the horses' official identification papers was registered. Detailed information on the experimental animals is given in the supporting information (Table S5).
FIGURE 1.
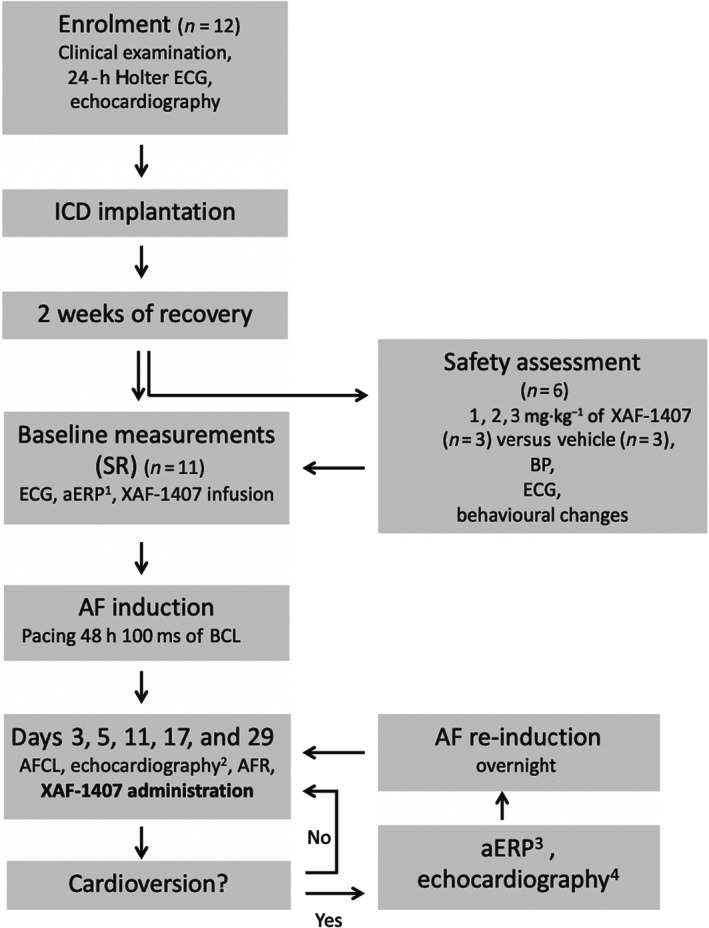
Experimental set‐up (in vivo): Following insertion of the implantable cardioverter defibrillator (ICD) and a recovery period, the safety assessment and baseline measurements were made in sinus rhythm (SR), including measurements of atrial effective refractory period (aERP) which were performed before and after XAF‐1407 infusion (at 1, 2, and 3 h). After initial induction of atrial fibrillation (AF), electrophysiological measurements were made and serial cardioversion attempts made on day 3, 5, 11, 17 and 29. If cardioversion occurred, aERP was measured 1 h after end of the XAF‐1407 infusion, before overnight re‐induction of AF. Echocardiographic examinations were conducted at enrolment (in SR), day 5 and 29 in AF, as well as in SR, if cardioversion occurred
All horses were housed in individual stalls in the same stable, ensuring the possibility of physical and/or visual contact to one or more other horses at all times. Standard bedding material consisted of wood shavings, and all horses had access to a field with one or more other horses on daily basis, except for the 14 days of stall rest following ICD implantation (for further details, see below). The horses were examined daily by a veterinarian licensed in animal experimentation (Certificate No. 32/10/16‐234, Federation of European Laboratory Animal Science Associations [FELASA]) for signs of pain, inflammation, infection, and general well‐being. Daily care, feeding, and stabling management was provided by personnel licensed by The Danish Animal Experiments Inspectorate at the Large Animal Teaching Hospital at University of Copenhagen.
Prior to initiation of the XAF‐1407 AF termination study, six of the horses were treated with three different doses of XAF‐1407 as a safety and tolerance assessment of the drug. The horses included in the study had no history of cardiovascular disease, confirmed by clinical examination, thorough cardiac auscultation, 24‐h Holter ECG monitoring, and echocardiographic examination as previously described (Carstensen et al., 2017).
Following the ICD implantation and a recovery period of 2 weeks, all horses underwent a minimum of six investigations over a period of 1 month (baseline, day 3, day 5, day 11, day 17, and day 29 after AF induction). All investigations were performed on non‐sedated horses standing in their stall, only loosely restrained by a halter, with unrestricted access to water and hay.
As this study was part of a larger study on the mechanisms underlying persistent AF and investigation of novel atrial selective drug treatments, the horses were included in other subsequent studies.
2.2.2. Safety and tolerability assessment
Six of the horses were subjected to a preliminary dose‐ranging safety and tolerability assessment before primary efficacy study (Figure 1). Three doses of XAF‐1407 (Figure 2a) (1, 2, and 3 mg·kg−1) or vehicle (sodium acetate buffer; Sigma Aldrich Denmark A/S, Søborg, Denmark; Th. Geyer Denmark Aps, Roskilde, Denmark) were injected intravenously over a period of 1.5 h as 15‐min infusions with 20‐min time intervals in between subsequent dose escalations (Figure 2b). The six horses were randomly assigned to either the XAF‐1407 (n = 3) or the vehicle (n = 3) group. The operators of the clinical interventions were not blinded, the data analysts for the retrospective BP and ECG monitoring analysis, however, were. Drug or vehicle administration was performed through a 200‐cm Heidelberger extension (Connecta™ PE LINE M/F 200‐cm internal volume 1.4 ml·m−1; Argon Medical Device, The Hague, Netherlands) connected by a three‐way stopcock (BD Connecta™ Three‐way valve 10 cm; Becton Dickinson, Helsingborg, Sweden) to a 12G catheter (Intraflon™ 2 (PTFE), 12G; Vygon, Ecouen, France) in the left jugular vein. Throughout the assessment, continuous non‐invasive monitoring of BP (Figure 2c,d), via a cuff placed around the tail base connected to an electronic pressure transducer (Carescape Monitor B650; GE Healthcare, Brøndby, Denmark), modified base‐apex surface ECG (KRUTECH Televet; Kruuse A/S, Maarslev, Denmark) (Haugaard et al., 2015) as well as behavioural changes (sweating, muscle shivering or weakness, smacking, pawing, neighing, excitement, flehming and drowsiness) was performed for all horses. The XAF‐1407 group underwent periodic blood sampling for pharmacokinetic (PK) analysis drawn into lithium‐heparin tubes (BD Vacutainer® LH 170 I.V. Plus Blood Collection Tubes; BD, Belliver Industrial Estate, Plymouth, UK ) from the right jugular vein at time points T = 0, 5, 10, 15, 25, 35, 40, 50, 60, 70, 75, 85, 105, 125, 145, 165, 185, and 205 min and 24 h. The blood samples were centrifuged at 1,600 x g for 5 min at 4°C, and the supernatant plasma was stored at −80°C until further analysis. The plasma samples were subsequently shipped on dry ice and analysed for XAF‐1407 concentration at Syngene International Ltd., Bangalore, India.
FIGURE 2.
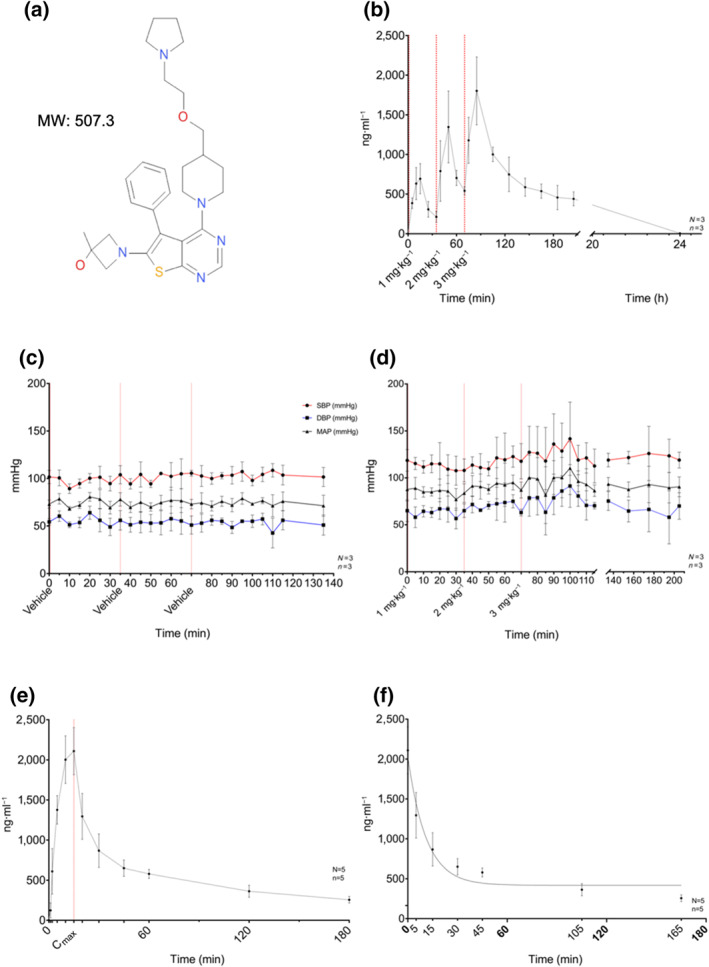
Summary of in vivo pharmacology and pharmacokinetics of XAF‐1407: (a) chemical structure and MW of 508.3 Da (XAF‐1407). (b) Plasma concentration of XAF‐1407 during the safety and tolerability assessment, measured at time points T = 0, 5, 10, 15, 25, 35, 40, 50, 60, 70, 75, 85, 105, 125, 145, 165, 185, and 205 min and 24 h in relation to start of drug administration of the first of three subsequent doses of XAF‐1407 (1, 2, and 3 mg·kg−1). (c) Non‐invasive BP monitoring during the safety and tolerability assessment for XAF‐1407 (n = 3). (d) Non‐invasive BP monitoring during the safety and tolerability assessment for vehicle (NaOAc) (n = 3). (e) Measured average plasma concentration of XAF‐1407 in equine plasma samples repeatedly from start of drug administration to 180 min after the end of the infusion. (f) Measured plasma concentration of XAF‐1407 after bolus injection of 3 mg·kg−1 XAF‐1407 fitted with an exponential 1‐phase decay model
2.2.3. Test compound
The I K,ACh inhibitor XAF‐1407 (Figure 2a, MW 507.3; patent number: WO2013/072694) was provided by Xention Ltd (Newmarket, UK) and dissolved in a 0.1‐M sodium acetate buffer with the pH of ~4 to an intravenous injectable solution with a concentration of 9 mg·ml−1. Osmolarity was corrected by the addition of 0.04054 g·ml−1 dextrose. The solution was prepared within a 2 h of administration and sterile filtered before use (Syringe filter Minisart® NML SFAC; Th. Geyer Denmark ApS, Roskilde, Denmark).
2.2.4. PK profiling after single IV dose
For five horses, venous blood samples were collected in lithium‐heparin tubes (BD Vacutainer® LH 170 I.V. Plus Blood Collection Tubes; BD, Belliver Industrial Estate, Plymouth, UK) on days 17 and 29, respectively, for subsequent PK analysis. XAF‐1407 was administered via an intravenous catheter in the left jugular vein, whereas blood was drawn from the right jugular vein at T = 0, 1, 2, 5, 10, 15, 20, 30, 45, 60, 120, and 180 min.
The blood samples were centrifuged at 1,600 g for 5 min at 4°C, and the supernatant plasma was stored at −80°C until further analysis. The samples were analysed for XAF‐1407 concentration at Syngene International Ltd.
2.2.5. Electrophysiology
Study protocol
An overview of the study protocol is displayed in Figure 1. The overall study period from start of AF induction to end of protocol consisted of 30 days. After recovery from the ICD implantation, all horses underwent electrophysiological (EP) baseline measurements before and after drug treatment (XAF‐1407 3 mg·kg−1 BW, infusion rate 0.2 mg·kg−1·min−1 i.v. over 15 min) in sinus rhythm (SR). After AF induction by atrial tachypacing, pharmacological cardioversion was attempted on days 3, 5, 11, 17, and 29. Throughout all procedures, the horses were monitored by a standard base‐apex surface ECG (KRUTECH Televet; Kruuse,A/S, Maarslev, Denmark) to determine time of cardioversion, to monitor potential arrhythmias or changes in ECG morphology, and to determine atrial fibrillatory rate (AFR). In horses without self‐sustained AF, the ICD was turned off 30 min prior to the scheduled drug treatment. To determine changes in AF cycle length (AFCL) over time, intra‐atrial electrograms (EGMs) were recorded from the ICD leads. If the treatment led to successful conversion to SR, aERP was measured 1 h after the end of drug infusion. Finally, AF was re‐induced by overnight tachypacing.
ICD implantation
As recently described in more detail (Carstensen et al., 2018), all 12 horses were implanted with either dual‐ (n = 8) or single‐chamber (n = 3) ICDs (Maximo® II CRT‐D D284TRK, Maximo® II DR D284DRG, Concerto® C174AWK, Maximo® II VR D284VRC; Medtronic Danmark A/S, Copenhagen, Denmark). Bipolar pacing leads (Tendril™ STS Pacing Leads, 100 cm; St. Judes Medical Denmark A/S, Glostrup, Denmark) were implanted into the right atrium under electrophysiological guidance enabling rapid atrial tachypacing with 600 bpm per 100 ms, using custom‐made pacing software (HRP 2.0; Medtronic Danmark A/S, Copenhagen, Denmark). In brief, the standing and conscious horses were restrained in a stock and sedated using 0.01 mg·kg−1 detomidine (Domosedan® ad us. vet; Orion Pharma Animal Health, Copenhagen, Denmark) and 0.01 mg·kg−1 butorphanol (Torbugesic® ad us. vet; ScanVet Animal Health A/S, Fredensborg, Denmark) followed by a constant‐rate infusion of 180–360 ml·h−1 of 1.0 mg·ml−1 xylazine (Xysol Vet 20 mg·ml−1; ScanVet Animal Health A/S, Fredensborg, Denmark). Furthermore, local anaesthesia was applied subcutaneously, and implantation performed after standard aseptical preparation of the surgical site.
All horses were treated with antibiotics (benzylpenicillin; Panpharma, Luitré, France) and non‐steroidal anti‐inflammatory drugs (flunixin‐meglumin; MSD, Intervet International, Boxmeer, The Netherlands) during and for a minimum of 3 days following implantation.
Before initiation of the experimental protocol, the horses were allowed to recover for 14 days. The first week, all animals were tied up to prevent excessive movement (rolling or lying down) to allow optimal healing of the surgical site and prevention of lead dislodgement. The second week consisted of strict stall rest, but with the possibility of free movement within the stall. After full recovery, all horses had daily access to the field.
Due to complications following ICD implantation (surgical site infection resulting in septicaemia despite of antibiotic treatment), one horse (horse #5) had to be excluded from the study and killed according to the regulations regarding humane end points.
AF induction
Following initial electrophysiological baseline measurements, AF was induced by rapid atrial tachypacing and monitored by base‐apex surface ECG recordings. The AF initiation protocol was designed of constant atrial tachypacing (600 bpm) for the first 48 h, followed by deactivation of the HRP 2.0 software to assess whether AF was self‐sustained. If this was not the case, high‐rate pacing was reinitiated and interrupted on a daily basis until AF was self‐sustained or an investigation was scheduled.
When AF was self‐sustained, the ICD was programmed into a sensing mode where it would start pacing from both leads (each at a rate of 150 bpm), as soon as an atrial rate of <170 bpm was detected. Self‐sustained AF for >24 h was considered stable enough to completely turn off the ICD. The presence of AF was continuously monitored thereafter and confirmed by auscultation or telemetric interrogation of the ICD on daily basis.
Drug treatment
Based on the preliminary safety assessment, a dose of 3 mg·kg−1 XAF‐1407 was considered safe to use in the subsequent studies. Thus, 3 mg·kg−1 XAF‐1407 was administered intravenously to all horses on all investigation days regardless of the horses being in AF or SR. The compound was injected as a 15‐min infusion (rate 0.2 mg·kg−1·min−1).
To study vehicle effect (sodium acetate buffer, NaOAc), an identical volume of vehicle alone was administered to all horses, in the same manner as XAF‐1407, 1 h prior to the active drug treatment on day 11.
For both test compound and vehicle, infusions were administered through a 200‐cm Heidelberger extension (Connecta™ PE LINE M/F 200 cm int. vol. 1.4 ml·m−1; Argon Medical Device, The Hague, The Netherlands); connected by a three‐way stopcock (BD Connecta™ Three‐way valve 10 cm; Becton Dickinson, Helsongborg, Sweden); to a 12G catheter (Intraflon™ 2 [PTFE], 12G; Vygon, Ecouen, France) in the left jugular vein.
Due to paraphlebitis of the left jugular vein, XAF‐1407 was not administered to one horse (horse #9) and due to technical issues into another horse (horse #1), both on day 3.
aERP measurement
The aERP was determined with a decremental S1–S2 protocol, where a premature extra stimulus (S2) was introduced after every eighth paced basic beat (S1) with decrements of 10 ms (pulse width 1 ms, current 3× pacing threshold) until capture was lost. Atrial ERP was defined as the longest S1–S2 interval failing to elicit a new atrial excitation.
This protocol was carried out three consecutive times and averaged at basic pacing cycle lengths (BCL) of 1,000, 800, 500, and 400 ms, respectively. The aERP measurements were performed on baseline day before as well as 1, 2, and 3 h after drug administration. On the following investigation days, aERP measurements were performed 1 h after the end of drug administration if successful cardioversion to SR occurred.
AFCL and AFR
AFCL and AFR were used to assess the degree of cardiac electrical remodelling over time and to evaluate the effect of XAF‐1407 on atrial rate. The parameters were measured 15 min prior to drug administration up to successful cardioversion to SR or 1 h subsequent to drug administration, respectively.
The AFCL was analysed manually by counting consecutive positive atrial deflections (peaks) from EGM recordings, dividing the total recording time (14 s) through the peak count: .
Whereas AFR was calculated from modified base‐apex ECG recordings by using the Cardiolund AFR Tracker software (cardiolund.com) implementing the concept of spatiotemporal QRST cancellation (Stridh & Sörnmo, 2001). For technical reasons, AFR was only calculated for 6 out of 11 horses (horses #7 to #13).
ECG analysis
All horses were monitored by Holter ECG before inclusion, as well as throughout all procedures (KRUTECH Televet; Becton Dickinson). ECG analysis was carried out manually using either Televet 100 6.0.0 software or on‐screen Cardio Calipers 4.0 (Iconico) on lead II. The parameters QRS and rate corrected QT (QTc) interval as well as heart rate (HR), PR interval at predefined time points (T −5, T 15, T 45, and T 60), and P:QRS ratio were analysed.
Additionally, all recordings were analysed for abnormal ventricular complexes (Verheyen et al., 2010). HR, PR, QRS, and QT intervals were measured over 10 consecutive heartbeats at a paper feed of 50 mm·s−1 and 10 mm·mV−1 gain and averaged. The QT measurements were corrected for HR using a piecewise linear regression model specifically tailored for horses, in order to account for the species specific HR range (Pedersen et al., 2016).
To evaluate the influence of XAF‐1407 on atrioventricular node (AV node) conduction, P‐wave to QRS complex ratio was analysed at baseline (n = 8), at resting HR (30–40 bpm), as well as paced at 400‐ms BCL prior to and after drug administration. The atrial rate during SR was determined for 100 subsequent QRS complexes at a paper feed of 50 mm·s−1 and 10 mm·mV−1 gain.
2.2.6. Clinical non‐cardiac side effects
Evaluation of possible non‐cardiac side effects was conducted by close monitoring of behavioural changes during drug administration and up to 2 h after end of infusion. Clinical signs related to the CNS like hyperexcitability, hypersensitivity, and motoric imbalances were registered.
2.3. Data analysis
All data are presented as mean ± SD. Analyses were performed using GraphPad Prism 8 software (GraphPad Prism, RRID:SCR_002798) and R 3.3.1 software (R Project for Statistical Computing, RRID:SCR_001905), with P ≤ 0.05 considered significant. Statistical methods used were paired t‐tests (Figures 6g and 7c), repeated measures one‐way ANOVA (Figure 3d), as well as a linear mixed model with time point as fixed and horse ID as random variable to account for correlations over time (Figures 3c, 5a,b, and 7a,b,d–f). In this repeated measures design, multiple comparisons were performed using either Dunnet's (Figures 3c,d, 5a,b, and 7a,d–f) or Sidak's (Figure 7b) test for multiple comparisons. These post hoc tests were conducted only if the measure of matching effectivity (F in ANOVA and χ 2, df in mixed model) achieved the necessary level of statistical significance (P ≤ 0.05) and significant variance inhomogeneity was not evident. Fisher's exact test has been used to determine significant difference in effect between drug and vehicle (Figure 4b), an exponential 1‐phase decay model to analyse the PK data (Figure 2f) and a piecewise linear regression model to correct QT intervals for HR. Statistical analysis was undertaken only for data sets where each group size was at least n = 5. Group size is defined as the number of independent values, and outliers were included in data analysis and presentation. In Figure 3d, exploratory data points for days 3 (n = 1) and 5 (n = 5) are displayed, even though not included in the statistical analysis, to emphasise the parameters' development over the course of 1 month.
FIGURE 6.
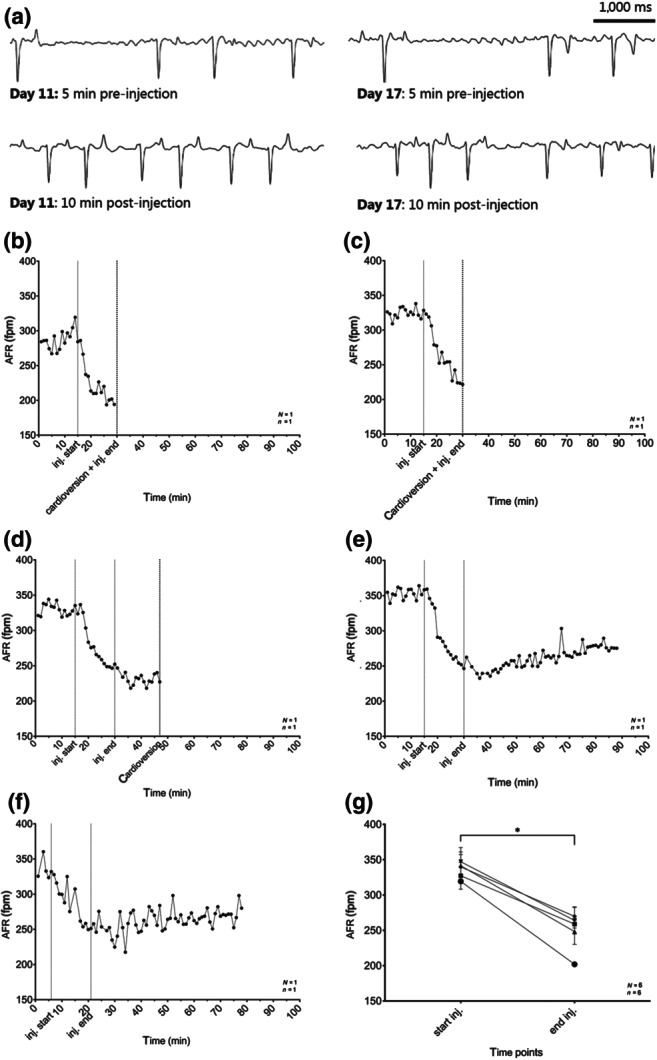
Atrial fibrillatory rates (AFRs) following XAF‐1407 infusion: (a) the depicted ECG traces show the transformation from fine to more coarse fibrillation waves in response to drug administration in 5‐s ECG traces at day 11 (5 min prior to drug administration and 10 min after end of drug administration) versus day 17 (5 min prior to drug administration and 10 min after end of drug administration), however only subsequently leading to cardioversion on day 11. (b–f) Influence of XAF‐1407 administration on AFR for horse #8, representative for the study population: (b) procedure day 3; (c) procedure day 5; (d) procedure day 11; (e) procedure day 17; and (f) procedure day 29. (g) Influence of XAF‐1407 on AFR from start of injection to end of injection at all procedure days (N = 6, n = 6). *P < .05, significantly different as indicated. N, number of horses; n, number of measurements
FIGURE 7.
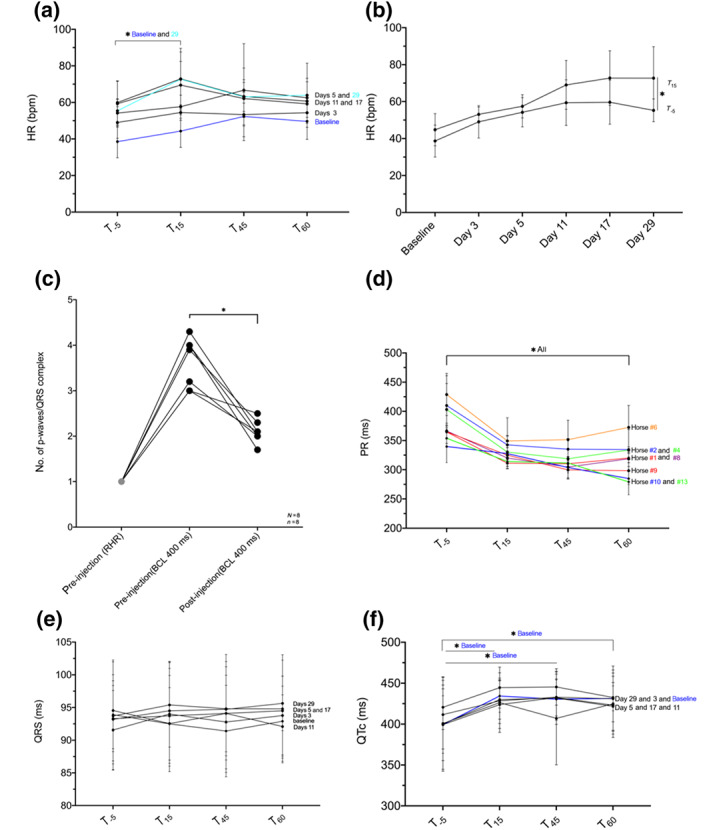
Influence of XAF‐1407 on ECG parameters of AV nodal and ventricular electrophysiology: (a) influence on heart rate (HR) measured on four different time points (minutes) in relation to start of drug administration. (b) Mean difference in HR from before (T −5) to end (T 15) of drug injection at the different procedure days. (c) Change in P:QRS ratio prior to drug injection at resting heart rate (RHR; ~30–40 bpm), prior to drug injection at basic cycle length (BCL) 400 ms and 1 h after drug injection at BCL 400 measured at baseline before AF induction. (d) Influence on PR interval measured on four different time points (minutes) in relation to start of drug administration at baseline before AF induction. (e) Influence on QRS duration measured on four different time points (minutes) in relation to start of drug administration. (f) Influence on QTc measured on four different time points (minutes) in relation to start of drug administration. *P < .05, significantly different as indicated
FIGURE 3.
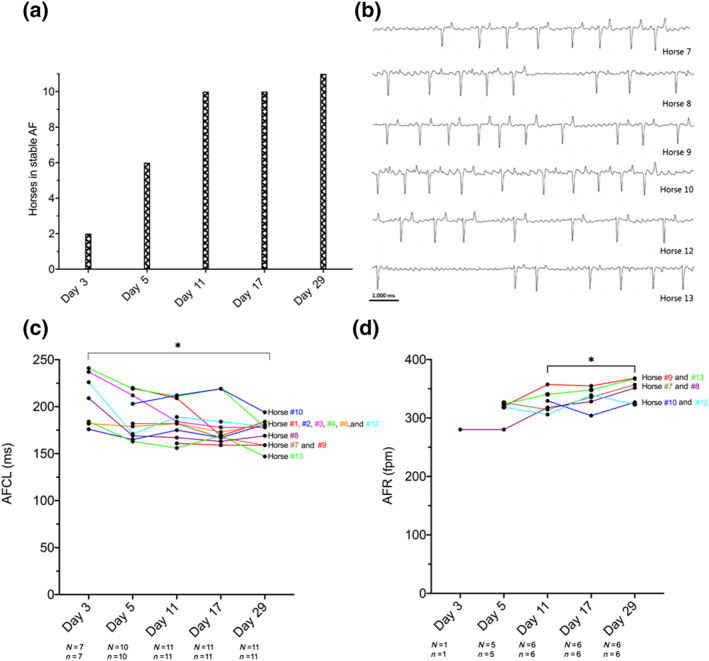
Equine model of persistent AF—AF progression over 1 month: (a) overview of the total number of horses in self‐sustained AF on respective procedure days. (b) Representative ECG (10 s) traces prior to XAF‐1407 administration on day 11. (c) AF cycle length (AFCL) as a measure of electrical remodelling progression over time prior to XAF‐1407 infusion. (d) Atrial fibrillatory rate (AFR) as a measure of electrical remodelling progression over time prior to XAF‐1407 infusion. Data presented as individual time course per horse. *P < .05, significantly different as indicated. AF, atrial fibrillation; N, number of horses; n, number of measurements
FIGURE 5.
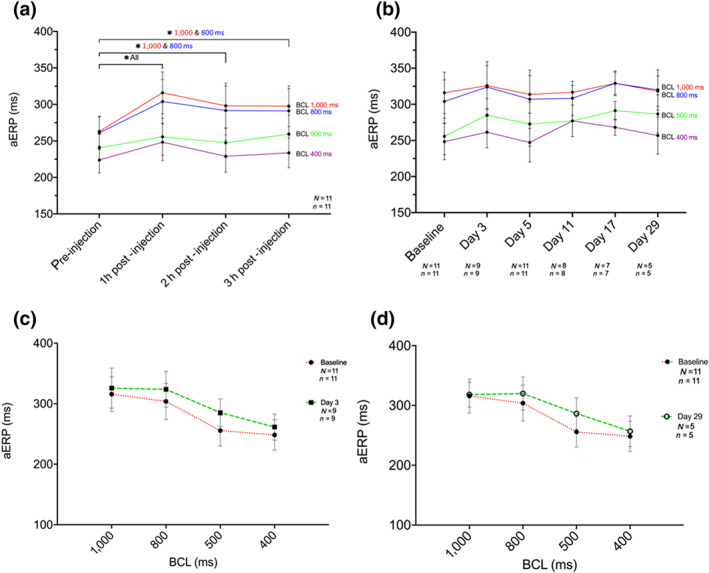
Effect of XAF‐1407 on atrial effective refractory period (aERP): (a) aERP measurements on baseline day before (−5 min) and 60, 120, and 180 min after end of drug administration. (b) aERPs 1 h after end of drug administration and successful cardioversion on procedure days, compared to respective values at baseline. (c) Rate dependency in aERP duration as a function of cycle length at baseline (N = 11, n = 11) and at day 3 (N = 9, n = 9). (d) Rate dependency in aERP duration as a function of cycle length at baseline (N = 11, n = 11) and at day 29 (N = 5, n = 5). *P < .05, significantly different as indicated. N, number of horses; n, number of measurements
FIGURE 4.
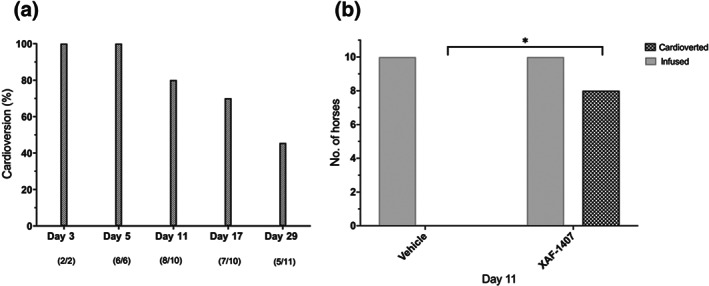
Anti‐arrhythmic properties of XAF‐1407: (a) cardioversion success rate (%) of horses in self‐sustained AF with XAF‐1407 treatment over time. (b) Assessment of anti‐arrhythmic properties of XAF‐1407 versus vehicle (NaOAc) on day 11. * P < .05, significantly different as indicated
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.4. Materials
The compounds used in these studies were supplied as follows: acetic acid by Th. Geyer Denmark ApS, Roskilde Denmark; benzylpenicillin by Panpharma, Luitré, France; butorphanol (Torbugesic®) by ScanVet Animal Health A/S, Fredensborg, Denmark; detomidine (Domosedan®) by Orion Pharma Animal Health, Copenhagen, Denmark; dextrose and sodium acetate buffer by Sigma Aldrich Denmark A/S, Søborg, Denmark; flunixin‐meglumin by MSD, Intervet International, Boxmeer, The Netherlands; FPL‐64176 and nimodipine by Tocris Bioscience (Bristol, UK); pentobarbital (Euthasol) by Virbac, Kolding, Denmark; xylazine (Xysol Vet) by ScanVet Animal Health A/S, Fredensborg, Denmark.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Fabbro, et al., 2019; Alexander, Mathie, et al., 2019).
3. RESULTS
First‐time characterisation of the in vitro and in vivo pharmacology of the putative I KACh inhibitor XAF‐1407 was achieved by extensive recombinant in vitro experiments assessing the effect on cardiac ion current activity and employing an equine model of AF to investigate the compounds electrophysiological effects and anti‐AF properties up to a duration of 1 month. The effect of pharmacological I K,ACh inhibition by XAF‐1407 on atrial and ventricular electrophysiology was assessed by measuring aERP and AFR as well as HR, QRS duration, QTc interval, PR interval, and P:QRS ratio.
3.1. XAF‐1407 is a highly selective I K,ACh inhibitor
In vitro selectivity profiling of a panel of cardiac ion channels revealed that XAF‐1407 is a potent, and highly selective, inhibitor of the ion channels formed by Kir3.1/3.4 heterotetramers and Kir3.4 homotetramers that carry the I K,ACh current (IC50 of 1.1 and 3.2 nM, respectively). In contrast, the IC50 values of other relevant cardiac ion channels were in the micromolar range, with selectivity ratios for Kir3.1/3.4 of >2,000–8,000 up to >27,000 for Nav1.5 and Kir2.1, respectively (Table 1).
TABLE 1.
XAF‐1407 in vitro pharmacology
| Recombinant ion channel | Equivalent cardiac current | IC50 (95% CI) | Hill coefficient (n H) | Selectivity ratio for Kir3.1/3.4 |
|---|---|---|---|---|
| Kir3.1/3.4 | I K,ACh | 0.0011 μM (0.0007–0.0018 μM) | 0.4 ± 0.04 | 1 |
| Kir3.4 | I K,ACh | 0.0032 μM (0.0025–0.0041 μM) | 0.4 ± 0.02 | ~3 |
| Kir2.1 | I K1 | >30 μM (12 ± 5% at 30 μM) | No fit possible | >27,000 |
| Kv11.1 (hERG) | I Kr | 4.2 μM (3.5–5.1 μM) | 0.9 ± 0.06 | ~3,800 |
| Nav1.5 (−100 mV/1 Hz) | I Na, peak | 8.8 μM (8.1 to 9.5 μM) | 2.2 ± 0.2 | 8,000 |
| Nav1.5 (−100 mV/5 Hz) | 5.3 μM (4.4 to 6.5 μM) | 1.8 ± 0.2 | >4,000 | |
| Nav1.5 (−70 mV/1 Hz) | 4.3 μM (3.8 to 4.8 μM) | 1.3 ± 0.1 | >4,000 | |
| Nav1.5 (−70 mV/5 Hz) | 2.6 μM (4.4 to 6.5 μM) | 1.4 ± 0.3 | >2,000 | |
| Kv1.5 | I Kur | 5.8 μM (4.7–7.1 μM) | 1.0 ± 0.08 | >5,000 |
| Kv4.3 | I to,1 | >10 μM (31 ± 7% at 10 μM) | ND | >9,000 |
| Kv7.1/KCNE1 | I Ks | 17 μM (13.8–21 μM) | 1.2 ± 0.15 | >15,000 |
| Kir6.2/SUR2A | I KATP | >10 μM (17 ± 13% at 10 μM) | ND | >9,000 |
| Cav1.2 | I Ca,L | >10 μM (5.8 ± 7.5% at 10 μM) | No fit possible | >9,000 |
Note: Summary of ion channel pharmacology of XAF‐1407 (in vitro): current or fluorescence (Cav1.2 only) inhibition data over a range of drug concentrations were fitted with a sigmoidal function to determine an IC50 and Hill coefficient (n H). Except Kv4.3 and Kir6.2/SUR2A where only a single concentration of 10 μM was tested.
Abbreviations: CI, confidence interval; ND, not determined.
3.2. XAF‐1407: Dose ranging and PK
The presence of the drug in equine plasma was measured and the levels quantitatively compared to in vitro IC50 values to evaluate effect or absence of effect in relation to engagement of drug on proposed target (Figure 2). Drug infusion was tolerated well in all animals without significantly affecting BP (Figure 2c). Following a single intravenous dose of 3 mg·kg−1 of XAF‐1407, a maximal total plasma level (Cmax) of ~2,100 ng·ml−1 was measured (Figure 2f), corresponding to a total concentration of 4.1 μM. in vitro plasma protein binding (PPB) of XAF‐1407 in horse plasma determined the unbound free fraction to be 12%. Peak free unbound XAF‐1407 was around 0.5 μM at Cmax, markedly above the IC50 of Kir3.1/3.4 channels. However, aERP was first measured 60 min after the end of infusion at which time total drug in plasma was 600 ng·ml−1, due to drug distribution into tissue, corresponding to an unbound drug concentration of 0.14 μM.
3.3. Equine model of tachypaced persistent AF
The horses were paced for 48 h to initiate AF. All horses were under the influence of high atrial rates for 29 days, initially due to continuous high‐rate pacing by the ICD and later from self‐sustained AF.
The number of days of pacing required until AF was self‐sustained (>24 h) varied from 2 to 8 days (mean 6 ± 4 days) with one outlier of 20 days (median: 5 days; range: 2–20 days) (Figure 3a). Representative ECG traces of self‐sustained stable AF on day 11 are shown in Figure 3b.
To address the development of AF over time, AFCL and AFR were measured. These two complementary parameters indicate the degree of atrial electrical remodelling, corresponding to the time interval in milliseconds between consecutive atrial depolarisations and number of depolarisations per minute, respectively. AFCL significantly decreased with AF duration (Figure 3c), from day 3 to day 29, with a corresponding significant increase in AFR over the same time (Figure 3d).
3.4. Anti‐arrhythmic properties of XAF‐1407
The cardioversion rate of XAF‐1407 was 100% at day 3 and day 5 following infusion of 3 mg·kg−1 over 15 min (Figure 4a). This finding, however, cannot be supported by statistical significance, due to the small number of animals in stable AF at the respective investigation days. At 29 days of AF, the cardioversion rate had decreased to 45.5%, with AF cardioverted in 5 out of 11 horses following XAF‐1407 infusion. A control infusion of the vehicle solution (NaOAc‐buffer) on day 11 resulted in 0% cardioversion, reflecting a significant difference between vehicle and XAF‐1407 treatment (Figure 4b).
3.5. Electrophysiological properties of XAF‐1407
3.5.1. Atrial effective refractory period
Before initiation of atrial tachypacing, XAF‐1407 significantly prolonged aERP at all basic cycle lengths (BCLs; 1,000, 800, 500, and 400 ms) 1 h after the end of infusion, as well as at 2 and 3 h after the end of infusion at BCL 1,000 and 800 ms, compared to prior infusion. Rate dependency in aERP duration as a function of BCL was evident (Figure 5a). No significant prolongation of aERP was apparent 1 h after the end of drug administration and successful cardioversion on investigation days, compared to respective baseline values before AF induction (Figure 5b). The rate dependency of aERP was retained over the 29 days of pacing (Figure 5c,d).
3.5.2. AFR during drug infusion
A significant drop in AFR during drug infusion was seen in all horses (n = 6) (Figure 6g). AFR recordings of one horse on all investigation days are displayed in Figure 6b–f.
3.6. Influence of XAF‐1407 on nodal function and ventricular electrophysiology
A transient increase in HR after drug administration occurred on all investigation days (Figure 7a), most obvious for time point T 15 (end of drug administration) and statistically significant at baseline and day 29. On the remaining investigation days, only a non‐significant increase was observed. The greatest difference in HR between T −5 and T 15 was 10 ± 2 bpm at day 29 (Figure 7b). The p‐wave to QRS complex ratio at baseline (day 0) prior to and after drug administration was 1:1 at rest (30–40 bpm), between 3:1 and 4:1 under atrial pacing (BCL 400 ms/150 bpm) prior to XAF‐14.7 and 2:1 at 1 h post‐administration of XAF‐1407 (Figure 7c), for all horses. Further, a significant decrease in PR interval following drug administration was observed (Figure 7d).
The administration of XAF‐1407 did not affect QRS duration (Figure 7e). However, it caused a statistically significant increase in QTc lengths at day 0 (baseline), comparing the values prior to drug administration (T −5) to end of administration (T 15) (Figure 7f). On the remaining investigation days, QTc prolongation was observed, although these effects were not statistically significant. Analysis of all ECG recordings revealed no ventricular arrhythmic events.
3.7. Clinical signs
Transient behavioural changes and CNS signs during and up to 1 h following drug administration were evident to varying degrees. It was noticeable that horses that were eating before and at the start of drug infusion stopped eating towards the end of drug infusion (9/11) and the majority (10/11) showed signs of general tension (pawing the ground, change in ear positioning, sceptical facial expression, and flehming). Some displayed signs of hypersensitivity around the muzzle/nostril region (smacking lips, muzzle rubbing, and biting air; 4/11), but only one horse showed pronounced hyper‐excitability/‐sensitivity in terms of uncontrolled head shaking and sensitivity towards noise. All signs were mild and temporary and stopped within 1 h after the end of drug infusion.
4. DISCUSSION
This study investigated the anti‐arrhythmic and electrophysiological properties of an I K,ACh inhibitor, XAF‐1407, for the first time in an equine model of persistent AF.
We found that XAF‐1407 is a potent and highly selective IK,ACh inhibitor that prolongs aERP by approximately 55 ms, decreases AFR shortly after drug infusion, and successfully terminates sustained AF in horses, although with a decreasing efficacy over 4 weeks. At 3 mg·kg−1 XAF‐1407 decreases AV nodal refractoriness and produces a non‐significant QTc prolongation but does not affect QRS duration or promote arrhythmic ventricular activity, consistent with its in vitro ion channel selectivity.
4.1. XAF‐1407 pharmacology
XAF‐1407 is a potent I K,ACh inhibitor with IC50 values in the 1–3 nM range in vitro, with inhibition of other important atrial and ventricular cardiac ion channels in the μM range. The calculated free unbound concentration around 0.5 μM at Cmax implies that the exposure of drug to target in this study is markedly above the IC50 of XAF‐1407 on Kir3.1/3.4 channels. Although PK data show that XAF‐1407 is present in free unbound concentrations sufficient to explain the effect on atrial refractoriness, it cannot be completely ruled out that other ion channels may play a role at these relatively high exposure levels. However, the IC50 values of other relevant cardiac ion channels are in the μM range, of 5.8 μM and 2.6–8.8 μM on Kv1.5 and Nav1.5 channels respectively. Moreover, it should be noted that free drug levels drop dramatically after the infusion has been completed, due to distribution phase into tissue. It therefore seems reasonable to argue that the observed aERP prolongation can be attributed to the inhibition of the I K,ACh current by XAF‐1407.
4.2. XAF‐1407 inhibits Kir3.1/3.4 heterotetramers and Kir3.4 homomers with similar potency
In contrast to previously tested I K,ACh inhibiting compounds like BMS914392/NTC‐801, AZD2927, A7071, and XEN‐R0702 (El‐Haou et al., 2015;Podd et al., 2016 ; Walfridsson et al., 2015), the compound XAF‐1407 inhibited both Kir3.1/3.4 heterotetramers and Kir3.4/3.4 homotetramers with similar potency (IC50 1–3 nM). This feature has been shown to yield greater anti‐AF effects in an ex vivo experimental setting, in which the earlier compound XEN‐R0706 was tested in human atrial tissue preparations from chronic AF (cAF) patients. This resulted in APD90 and aERP prolongations in cAF tissue, whereas no effect was observed in tissue from SR patients (El‐Haou et al., 2015). In direct comparison, a NTC prototype only blocking Kir3.1/Kir3.4 heterotetramers failed to reproduce these results (El‐Haou et al., 2015). These findings contribute to the ongoing debate regarding the molecular and electrophysiological mechanisms underlying agonist‐independent constitutive I K,ACh activity (cI K,ACh) (Brundel et al., 2001; Dobrev et al., 2001; Dobrev et al., 2005; Sharma, Li, Xu, Liu, & Xu, 2011) as a main contributor to aERP shortening and AF maintenance in cAF. These data suggest a putative role of Kir3.4 homotetramers in the development of cI K,ACh activity. Numerous mechanisms have been proposed to underlie this constitutive activity of I K,ACh, including an imbalance between PKC isoforms, an increased activity of the nucleoside diphosphate kinase B, the Kir3.4 homotetramers, and thereby changes in channel stoichiometry or even a functional interaction between these factors (Makary et al., 2011; Voigt, Abu‐Taha, Heijman, & Dobrev, 2014; Voigt et al., 2007). As this study reports a decreasing effect of XAF‐1407 with increasing AF duration and thereby of Kir3.1/3.4 and Kir3.4/3.4 inhibition, this might be an indication towards a dynamic functional coherence of multiple mechanisms.
4.3. XAF‐1407 in an equine model of persistent AF
The equine model used in the present study has been validated in different experimental settings of AF (Carstensen et al., 2018; Haugaard, Hesselkilde, et al., 2015; Haugaard, Pehrson, et al., 2015; Hesselkilde et al., 2019). Even though tachypacing‐induced AF models in general might lack the exact substrate “lone AF” originates from man, choosing an animal species naturally developing lone AF, as the horse (Van Loon et al., 2002), might be of value in the development of AADs. With this study, we were the first to employ a large animal model to relate pharmacological I K,ACh inhibition to a decreasing cardioversion rate over the course of up to 1 month of self‐sustained AF. The results obtained here contrast with those from previous canine models reporting no negative temporal influence on cardioversion rate in dogs with pacing‐induced AF of up to 8 weeks (El‐Haou et al., 2015; Podd et al., 2016; Walfridsson et al., 2015). This model might therefore be closer to providing an explanation for the surprising lack of positive influence on AF burden or aERP length in pAF and atrial flutter patients in clinical trials following I K,ACh inhibitor treatment (Podd et al., 2016; Walfridsson et al., 2015).
Shortening of aERP is still the undisputed hallmark of atrial electrical remodelling (Allessie, Ausma, & Schotten, 2002; De Clercq, Van Loon, Tavernier, Duchateau, & Deprez, 2008; Schotten et al., 2003; Wijffels, Kirchhof, Dorland, & Allessie, 1995), and its prolongation is the commonly used determinant of positive drug effect in pharmacological AF treatment with potassium current inhibiting drugs (Ravens, 2010). In the presence of functional re‐entry, AFCL and the inversely related AFR have become generally accepted surrogate markers for atrial tissue refractoriness (Husser, Stridh, Sornmo, Olsson, & Bollmann, 2004; Platonov, Corino, Seifert, Holmqvist, & Sörnmo, 2014). Both changed over the course of atrial tachypacing with significant decrease and increase, respectively, as an indication of localised and global cardiac remodelling as well as increased electrical complexity, further supporting their use as surrogate parameters for AF remodelling in this model.
Administration of XAF‐1407 resulted in significant prolongation of aERP at day 0, whereas no change in aERP values could be observed later in the study. As aERP measurements were exclusively conducted after successful cardioversion to SR, direct comparison of aERP values before and after drug treatment was not possible on the following investigation days. The respective opposing effects of electrical remodelling and drug treatment could therefore not be separated, and a presumed AF‐mediated aERP shortening driven by an increased constitutive I K,ACh (Dobrev et al., 2002) most likely would be blunted by current inhibition by XAF‐1407.
In contrast to aERP, AFR is measured during AF and thereby addresses atrial refractoriness before, during, and following drug infusion. The significant drop in atrial rate of about 100 fpm, in response to drug administration, indicates that a distinct increase in refractory period in response to pharmacological I K,ACh inhibition occurred in the present study. This evident negative chronotropic response of atrial rate to drug administration throughout the whole study period highlights the ability of XAF‐1407 to prolong refractoriness, whether it led to successful cardioversion or not.
Furthermore, AFR has been proposed as a biomarker for successful cardioversion, as slow atrial rate correlates with high cardioversion probability and vice versa (Hesselkilde et al., 2017; Platonov et al., 2014). This hypothesis further supported by the observed increase in AFR with sustained AF over the 29 days is indicating progression of atrial electrical remodelling and corresponding to a decrease in the cardioversion rate in this study. Similar results have previously been reported for other AADs such as flecainide (Carstensen et al., 2018).
After 1 month of AF, the cardioversion success rate of XAF‐1407 was reduced to 45.5%. Only a few longer duration studies using tachypacing‐induced AF large animal models are available. The cardioversion success rate with vernakalant was reported to be 55% in tachypaced goats with AF for 11 days (Van Hunnik et al., 2016) and 0% in pigs tachypaced for 18 ± 3 days (Diness et al., 2017). Flecainide treatment of tachypaced horses revealed a cardioversion rate of 33% after 55 days and displayed serious life‐threatening cardiac adverse effects (Carstensen et al., 2018).
4.4. XAF‐1407—Cardiac adverse and non‐cardiac side effects
Current available AADs are typified by either poor efficacy or serious non‐cardiac and cardiac adverse effects (Ravens, 2010; Waks & Zimetbaum, 2017). Several AADs such as flecainide, quinidine, and amiodarone, inhibiting multiple ion currents including I K,ACh, are known to enhance AV conduction resulting in unwanted 1:1 conduction. As I K,ACh is an important current involved in regulating impulse generation and AV conduction, infusion of XAF‐1407 was expected to affect nodal function (Drici, Diochot, Terrenoire, Romey, & Lazdunski, 2000). XAF‐1407 enhanced AV node conduction after drug treatment. The observed conduction enhancement is attributed to the pharmacological block of the inhibitory vagal influence of I KACh on I f in AV nodal cells. Vice versa, vagal stimulation through ACh would increase the refractory period of nodal cells, influencing the RMP by inhibition of the pacemaker current I f through its end‐target I KACh.
However, the small HR increase (10 ± 2 bpm) along with the decrease in AFR following XAF‐1407 administration showed no potential for ventricular arrhythmia resulting from sudden 1:1 conduction.
A tendency towards transient QTc prolongation after drug infusion was observed, but we did not observe clinical complications or ventricular arrhythmia. This QTc prolongation could be ascribed to a minor ventricular expression of I K,ACh which previously has been reported in mice, rats, and humans (Wang et al., 2013; Yang et al., 2010) and confirmed in horses (Haugaard, Hesselkilde, et al., 2015). In this context, it is important to emphasise that all measured values are within the physiological range of QTc lengths for Standardbred horses (450–500 ms; Pedersen et al., 2016) and hereby consistent with existing published data, reporting no evidence for negative influence on ventricular electrophysiology by I K,ACh inhibition (El‐Haou et al., 2015; Hashimoto et al., 2008; Podd et al., 2016; Walfridsson et al., 2015).
Ion channels formed by Kir‐family subunits are present not only in cardiac tissue but also in CNS (Humphries & Dart, 2015), linking I K,ACh inhibiting compounds, that penetrate the blood–brain barrier, to CNS and neurological side effects (Skibsbye & Ravens, 2016; Voigt & Dobrev, 2016). XAF‐1407 was specifically designed not to enter the CNS, which was experimentally confirmed in a rat PK and Irwin studies up to 300 mg·kg−1 p.o. (Milnes J, 2016, unpublished data). However, transient CNS signs were evident in a preclinical canine study at the highest explored dose of 10 mg·kg−1 i.v. XAF‐1407 (Milnes J, 2016, unpublished data).
The transient neurological signs observed in the present study may be due to species‐specific differences in the ability of XAF‐1407 to penetrating the blood–brain barrier and thereby being conducive to vagolytic effect of I K,ACh inhibition.
4.5. Limits of the study
The present study should be considered as a contribution to experimental electrophysiology, providing novel insights into the in vitro as well as in vivo pharmacology and electrophysiological properties of XAF‐1407 inhibiting atrial I KACh current, conducted through Kir3.1/3.4 heterotetramers and Kir3.4/3.4 homotetramers with the same potency. The in vitro selectivity profiling of XAF‐1407 was shown using recombinant cell lines expressing cardiac ion channels. These experiments were conducted at room temperature to allow for direct comparison of pharmacology data across the different ion channel assays. However, as for all drugs, it cannot be excluded that the pharmacology of XAF‐1407 may be different at body temperature.
Using the horse as a new large animal species in cardiac electrophysiological research has both advantages and a disadvantage. First, animal size might contribute to this species translational value over smaller species, as horses are one of the few mammalian species that spontaneously develop AF, beside humans. This phenomenon can be connected to atrial size (Kaese & Verheule, 2012). However, due to this species size and thereby the low number of animals in the present study, the stated cardiac safety should be taken with caution, even though no ventricular arrhythmic events were observed. Furthermore, 100% cardioversion rate for day 3 and day 5 might be of low explanatory power due to the small sample size. To minimise factors further reducing power of the present study, only one sex was included. Whether the selection for female individuals implies a potential bias towards efficacy of XAF‐1407 and thereby future investigations is yet unknown.
Referring to the present results concerning electrophysiological properties of XAF‐1407, it is conceivable that drug treatment might have a positive effect in reducing the amount of energy needed for electrical cardioversion, if not successful on its own. However, as direct current cardioversion has not been part of the presented protocol, we cannot provide data supporting this hypothesis. Furthermore, the fact that XAF‐1407 prolongs aERP to the same extent at different HRs suggests that this drug may be suitable as an add‐on therapy in AF patients already receiving rate control treatment. However, this remains to be evaluated in future experiments.
In conclusion, our results have shown that selective inhibition of I K,ACh shortens AFR and terminates AF with decreasing efficacy over time and only minor effects on ventricular electrophysiology not likely to cause any arrhythmic events in horses with tachypacing‐induced AF. This confirms the predominant atrial specificity of I K,ACh and supports the preclinical proof of concept for I K,ACh inhibition as a potential safe treatment strategy for sustained AF in horses and provides further evidence for the important role of I K,ACh in AF in another large animal models beyond the previously used canine models. Employing this large animal model for preclinical screening may help better understand the mechanism of AF and aid in the development of new treatments. However, complimenting such models with human preclinical screening data is expected to be advantageous given the previous failure of other I K,ACh (Kir3.1/Kir3.4) inhibitors in the clinic (El‐Haou et al., 2015; Podd et al., 2016; Walfridsson et al., 2015). Despite the positive outcome of this study in terms of safety and short‐term efficacy, it still remains unclear whether blocking this current alone, is superior to the currently available treatment modalities. Given the complex and incompletely understood mechanisms underlying long‐standing AF, experimental investigation of combinations of atrial‐selective AADs may be considered, as the aim of obtaining sufficient and sustained efficacy needs to go hand‐in‐hand with cardiac safety through maintained atrial selectivity.
AUTHOR CONTRIBUTIONS
The contributions of the authors have been summarised as: 1, experimental conception and/or design; 2, data acquisition and 3, data analysis and/or interpretation and these are indicated for each authors as follows: M.F.F. 1,2,3; H.C. 1; S.D.N. 2,3; E.Z.H. 1,2; C.L. 2,3; M.A.J. 2,3; A.V.L.‐A. 2,3; S.M.S. 1; P.G.P. 3; S.E.‐H. 2; C.J. 2; R.T. 2, R.K. 2; J.W.F. 2; U.S. 1,3; J.T.M. 1,2; U.S.S. 3; T.J. 1,3; R.B, 1,2,3. M.F.F, U.S.S., T.J., and R.B. drafted the manuscript which was revised by all authors who approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST
The authors S.E.‐H., C.J., R.T., R.K., J.F., and J.M. were employees of former Xention Ltd but no longer have financial interest in the experimental compound (XAF‐1407) mentioned within this article. The experimental compound XAF‐1407 was provided free of charge. The other authors (M.F.F., H.C., S.D.N., E.Z.H., C.L., M.A.J., A.V.L.‐A., S.M.S., P.G.P., U.S., U.S.S., T.J., and R.B.) report no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organisations engaged with supporting research.
Supporting information
Table S1. Cell culture conditions
Table S2. Cloned cardiac ion channel conventional electrophysiology. Experimental solutions (external pipette solution composition)
Table S3. Cloned cardiac ion channel conventional electrophysiology. Experimental solutions (internal pipette solution composition)
Table S4. Assessment of cardiac calcium channel activity. Experimental solutions
Table S5. In‐vivo animal model. Animals
Figure S1. Effect of XAF‐1407 on recombinant potassium inward rectifier currents. Original Kir3.1/3.4 (A), Kir3.4/3.4 (B) and Kir2.1 (C) current traces are shown in the absence and presence of XAF‐1407 and following removal of external K+ to assess passive leak. Schematic of the voltage protocol used is shown at the bottom of the figure. A dashed line shows zero current
Figure S2: Effect of XAF‐1407 on recombinant K v 11.1 (hERG) and Na v 1.5 current. Panel A shows the effect of 3 μM XAF‐1407 on Nav1.5 at different holding potentials (‐70mV lower panel or 100 mV upper panel) and frequencies (1 Hz left panel and 5 Hz right panel). Panel B shows original Kv11.1 current records in the absence and presence of 3 and 10 μM XAF‐1407 record from the same cell. A schematic of the voltage protocol is shown inset at the top of each figure. A dashed line shows zero current
Figure S3: Effect of XAF‐1407 on recombinant K v ionic current. Panel A shows original Kv1.5 current records in the absence and presence of 1, 3, 10 and 30 μM XAF‐1407 record from the same cell. Panel B shows original Kv4.3 current records in the absence and presence of 10 μM XAF‐1407. Panel C shows original Kv7.1/KCEN1 current records in the absence and presence of 10 and 30 μM XAF‐1407. A schematic of the voltage protocol is shown above each original trace. A dashed line shows zero current
ACKNOWLEDGEMENTS
The authors acknowledge Medtronic A/S, Denmark, for their kind assistance in terms of technical support and for providing us with ICD pacing units. Furthermore, we would like to thank Jonas Carlson, Department of Cardiology, Lund University for invaluable assistance with the technical AFR analysis. Moreover, we acknowledge all staff members at The Large Animal Teaching Hospital, Department of Veterinary Clinical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen involved in the project. All experiments were performed at the Large Animal Teaching Hospital, Department of Veterinary Clinical Sciences, University of Copenhagen, Taastrup, Denmark. The study was funded by the European Union Horizon 2020 MSCA ITN under Grant Agreement No. 675351, The Independent Research Fund Denmark, DFF‐7017‐00050, The Kustos Foundation of 1881, Kirsten og Freddy Johansens Fond, and Svenningsens Fond. Parts of the data from this manuscript were presented at the 2017 ECEIM Congress, Budapest, Hungary, the 2018 ECAS Congress, Paris, France, and the 2018 Heart Rhythm Society Congress, Boston, MA, USA.
Fenner MF, Carstensen H, Dalgas Nissen S, et al. Effect of selective I K,ACh inhibition by XAF‐1407 in an equine model of tachypacing‐induced persistent atrial fibrillation. Br J Pharmacol. 2020;177:3778–3794. 10.1111/bph.15100
Present address Said El‐Haou, Raymond Tang, Robert Kirby, and John W. Ford, Metrion Biosciences Ltd., Suite 1, Riverside 3, Granta Park, Great Abington, Cambridge, UK.
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Zhu, M. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(S1), S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allessie, M. , Ausma, J. , & Schotten, U. (2002). Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovascular Research, 54(2), 230–246. 10.1016/S0008-6363(02)00258-4 [DOI] [PubMed] [Google Scholar]
- Brundel, B. J. J. M. , Van Gelder, I. C. , Henning, R. H. , Tuinenburg, A. E. , Wietses, M. , Grandjean, J. G. , … Crijns, H. J. (2001). Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: Differential regulation of protein and mRNA levels for K+ channels. Journal of the American College of Cardiology, 37(3), 926–932. 10.1016/S0735-1097(00)01195-5 [DOI] [PubMed] [Google Scholar]
- Carstensen, H. , Hesselkilde, E. Z. , Fenner, M. , Loft‐Andersen, A. V. , Flethøj, M. , Kanters, J. K. , … Buhl, R. (2018). Time‐dependent antiarrhythmic effects of flecainide on induced atrial fibrillation in horses. Journal of Veterinary Internal Medicine, 32(5), 1708–1717. 10.1111/jvim.15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen, H. , Kjær, L. , Haugaard, M. M. , Flethøj, M. , Hesselkilde, E. Z. , Kanters, J. K. , … Jespersen, T. (2017). Antiarrhythmic effects of combining dofetilide and ranolazine in a model of acutely induced atrial fibrillation in horses. Journal of Cardiovascular Pharmacology, 71(1), 1–35. 10.1097/FJC.0000000000000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq, D. , Van Loon, G. , Tavernier, R. , Duchateau, L. , & Deprez, P. (2008). Atrial and ventricular electrical and contractile remodeling and reverse remodeling owing to short‐term pacing‐induced atrial fibrillation in horses. Journal of Veterinary Internal Medicine, 22(6), 1353–1359. 10.1111/j.1939-1676.2008.0202.x [DOI] [PubMed] [Google Scholar]
- Diness, J. G. , Skibsbye, L. , Simó‐Vicens, R. , Santos, J. L. , Lundegaard, P. , Citerni, C. , … Bentzen, B. H. (2017). Termination of vernakalant‐resistant atrial fibrillation by inhibition of small‐conductance Ca2+‐activated K+ channels in pigs. Circulation. Arrhythmia and Electrophysiology, 10(10), 1–13. 10.1161/CIRCEP.117.005125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diness, J. G. , Sørensen, U. S. , Nissen, J. D. , Al‐Shahib, B. , Jespersen, T. , Grunnet, M. , & Hansen, R. S. (2010). Inhibition of small‐conductance Ca2+‐activated K+ channels terminates and protects against atrial fibrillation. Circulation. Arrhythmia and Electrophysiology, 3(4), 380–390. 10.1161/CIRCEP.110.957407 [DOI] [PubMed] [Google Scholar]
- Dobrev, D. , Friedrich, A. , Voigt, N. , Jost, N. , Wettwer, E. , Christ, T. , … Ravens, U. (2005). The G protein‐gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation, 112(24), 3697–3706. 10.1161/CIRCULATIONAHA.105.575332 [DOI] [PubMed] [Google Scholar]
- Dobrev, D. , Graf, E. , Wettwer, E. , Himmel, H. M. , Hála, O. , Doerfel, C. , … Ravens, U. (2001). Molecular Basis of Downregulation of G‐Protein–Coupled Inward Rectifying K+Current (IK,ACh) in Chronic Human Atrial Fibrillation. Circulation, 104(21), 2551–2557. 10.1161/hc4601.099466 [DOI] [PubMed] [Google Scholar]
- Dobrev, D. , Wettwer, E. , Kortner, A. , Knaut, M. , Schuler, S. , & Ravens, U. (2002). Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovascular Research, 54(2), 397–404. 10.1016/s0008-6363(01)00555-7 [DOI] [PubMed] [Google Scholar]
- Drici, M. D. , Diochot, S. , Terrenoire, C. , Romey, G. , & Lazdunski, M. (2000). The bee venom peptide tertiapin underlines the role of I KACh in acetylcholine‐induced atrioventricular blocks. British Journal of Pharmacology, 131(3), 569–577. 10.1038/sj.bjp.0703611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Haou, S. , Ford, J. W. , & Milnes, J. T. (2015). Novel K+ channel targets in atrial fibrillation drug development—Where are we? Journal of Cardiovascular Pharmacology, 66(5), 412–431. 10.1097/FJC.0000000000000277 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, N. , Yamashita, T. , & Tsuruzoe, N. (2006). Tertiapin, a selective IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacological Research, 54(2), 136–141. 10.1016/j.phrs.2006.03.021 [DOI] [PubMed] [Google Scholar]
- Hashimoto, N. , Yamashita, T. , & Tsuruzoe, N. (2008). Characterization of in vivo and in vitro electrophysiological and antiarrhythmic effects of a novel IKACh blocker, NIP‐151: A comparison with an IKr‐blocker dofetilide. Journal of Cardiovascular Pharmacology, 51(2), 162–169. 10.1097/FJC.0b013e31815e854c [DOI] [PubMed] [Google Scholar]
- Haugaard, M. , Hesselkilde, E. , Pehrson, S. , Carstensen, H. , Flethøj, M. , Præstegaard, K. , … Jespersen, T. (2015). Pharmacologic inhibition of small‐conductance calcium‐activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart Rhythm, 12(4), 825–835. 10.1016/j.hrthm.2014.12.028 [DOI] [PubMed] [Google Scholar]
- Haugaard, M. , Pehrson, S. , Carstensen, H. , Flethøj, M. , Hesselkilde, E. , Præstegaard, K. , … Buhl, R. (2015). Antiarrhythmic and electrophysiologic effects of flecainide on acutely induced atrial fibrillation in healthy horses. Journal of Veterinary Internal Medicine, 29(1), 339–347. 10.1111/jvim.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijman, J. , Ghezelbash, S. , & Dobrev, D. (2017). Investigational antiarrhythmic agents: promising drugs in early clinical development. Expert Opinion on Investigational Drugs, 26(8), 897–907. 10.1080/13543784.2017.1353601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselkilde, E. Z. , Carstensen, H. , Flethøj, M. , Fenner, M. , Kruse, D. D. , Sattler, S. M. , … Buhl, R. (2019). Longitudinal study of electrical, functional and structural remodelling in an equine model of atrial fibrillation. BMC Cardiovascular Disorders, 19(1), 1–12. 10.1186/s12872-019-1210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselkilde, E. Z. , Carstensen, H. , Haugaard, M. M. , Carlson, J. , Pehrson, S. , Jespersen, T. , … Platonov, P. G. (2017). Effect of flecainide on atrial fibrillatory rate in a large animal model with induced atrial fibrillation. BMC Cardiovascular Disorders, 17(1), 4–9. 10.1186/s12872-017-0720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, E. S. A. , & Dart, C. (2015). Neuronal and cardiovascular potassium channels as therapeutic drug targets: Promise and pitfalls. Journal of Biomolecular Screening, 20(9), 1055–1073. 10.1177/1087057115601677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husser, D. , Stridh, M. , Sornmo, L. , Olsson, S. B. , & Bollmann, A. (2004). Frequency analysis of atrial fibrillation from the surface electrocardiogram. Indian Pacing and Electrophysiology Journal, 4(3), 122–136. [PMC free article] [PubMed] [Google Scholar]
- Kaese, S. , & Verheule, S. (2012). Cardiac electrophysiology in mice: A matter of size. Frontiers in Physiology, 3 SEP (September), 1–19. 10.3389/fphys.2012.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , … Zeppenfeld, K. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal, 37(38), 2893–2962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- Kovoor, P. , Wickman, K. , Maguire, C. T. , Pu, W. , Gehrmann, J. , Berul, C. I. , & Clapham, D. E. (2001). Evaluation of the role of IKACh in atrial fibrillation using a mouse knockout model. Journal of the American College of Cardiology, 37(8), 2136–2143. 10.1016/S0735-1097(01)01304-3 [DOI] [PubMed] [Google Scholar]
- Makary, S. , Voigt, N. , Maguy, A. , Wakili, R. , Nishida, K. , Harada, M. , … Nattel, S. (2011). Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine‐regulated potassium channels in atrial remodeling. Circulation Research, 109(9), 1031–1043. 10.1161/CIRCRESAHA.111.253120 [DOI] [PubMed] [Google Scholar]
- Milnes, J. T. , Madge, D. J. , & Ford, J. W. (2012). New pharmacological approaches to atrial fibrillation. Drug Discovery Today, 17(13–14), 654–659. 10.1016/j.drudis.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Pedersen, P. J. , Karlsson, M. , Flethøj, M. , Trachsel, D. S. , Kanters, J. K. , Klaerke, D. A. , & Buhl, R. (2016). Differences in the electrocardiographic QT interval of various breeds of athletic horses during rest and exercise. Journal of Veterinary Cardiology, 18(3), 255–264. 10.1016/j.jvc.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Platonov, P. G. , Corino, V. D. A. , Seifert, M. , Holmqvist, F. , & Sörnmo, L. (2014). Atrial fibrillatory rate in the clinical context: Natural course and prediction of intervention outcome. Europace, 16, iv110–iv119. 10.1093/europace/euu249 [DOI] [PubMed] [Google Scholar]
- Podd, S. J. , Freemantle, N. , Furniss, S. S. , & Sulke, N. (2016). First clinical trial of specific IKACh blocker shows no reduction in atrial fibrillation burden in patients with paroxysmal atrial fibrillation: Pacemaker assessment of BMS 914392 in patients with paroxysmal atrial fibrillation. Europace, 18(3), 340–346. 10.1093/europace/euv263 [DOI] [PubMed] [Google Scholar]
- Qi, X. Y. , Diness, J. G. , Brundel, B. J. J. M. , Zhou, X. B. , Naud, P. , Wu, C. T. , … Nattel, S. (2014). Role of small‐conductance calcium‐activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation, 129(4), 430–440. 10.1161/CIRCULATIONAHA.113.003019 [DOI] [PubMed] [Google Scholar]
- Ravens, U. (2010). Antiarrhythmic therapy in atrial fibrillation. Pharmacology & Therapeutics, 128(1), 129–145. 10.1016/j.pharmthera.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Ravens, U. , Poulet, C. , Wettwer, E. , & Knaut, M. (2013). Atrial selectivity of antiarrhythmic drugs. Journal of Physiology, 591(17), 4087–4097. 10.1113/jphysiol.2013.256115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reef, V. B. , Bonagura, J. , Buhl, R. , Mcgurrin, M. K. J. , Schwarzwald, C. C. , van Loon, G. , & Young, L. E. (2014). Recommendations for management of equine athletes with cardiovascular abnormalities. Journal of Veterinary Internal Medicine, 28(3), 749–761. 10.1111/jvim.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotten, U. , Duytschaever, M. , Ausma, J. , Eijsbouts, S. , Neuberger, H. R. , & Allessie, M. (2003). Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation, 107(10), 1433–1439. 10.1161/01.CIR.0000055314.10801.4F [DOI] [PubMed] [Google Scholar]
- Sharma, D. , Li, G. , Xu, G. , Liu, Y. , & Xu, Y. (2011). Atrial remodeling in atrial fibrillation and some related microRNAs. Cardiology, 120(2), 111–121. 10.1159/000334434 [DOI] [PubMed] [Google Scholar]
- Shen, M. J. , & Zipes, D. P. (2014). Role of the autonomic nervous system in modulating cardiac arrhythmias. Circulation Research, 114(6), 1004–1021. 10.1161/CIRCRESAHA.113.302549 [DOI] [PubMed] [Google Scholar]
- Skibsbye, L. , & Ravens, U. (2016). Mechanism of proarrhythmic effects of potassium channel blockers. Cardiac Electrophysiology Clinics, 8(2), 395–410. 10.1016/j.ccep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Stridh, M. , & Sörnmo, L. (2001). Spatiotemporal QRST cancellation techniques for analysis of atrial fibrillation. IEEE Transactions on Biomedical Engineering, 48(1), 105–111. 10.1109/10.900266 [DOI] [PubMed] [Google Scholar]
- Van Hunnik, A. , Lau, D. H. , Zeemering, S. , Kuiper, M. , Verheule, S. , & Schotten, U. (2016). Antiarrhythmic effect of vernakalant in electrically remodeled goat atria is caused by slowing of conduction and prolongation of postrepolarization refractoriness. Heart Rhythm, 13(4), 964–972. 10.1016/j.hrthm.2015.12.009 [DOI] [PubMed] [Google Scholar]
- Van Loon, G. , Duytschaever, M. , Tavernier, R. , Fonteyne, W. , Jordaens, L. , & Deprez, P. (2002). An equine model of chronic atrial fibrillation: Methodology. The Veterinary Journal, 164(2), 142–150. 10.1053/tvjl.2001.0668 [DOI] [PubMed] [Google Scholar]
- Verheyen, T. , Decloedt, A. , De Clercq, D. , Deprez, P. , Sys, S. U. , & Van Loon, G. (2010). Electrocardiography in horses—Part 2: How to read the equine ECG. Vlaams Diergeneeskundig Tijdschrift, 79(5), 337–344. [Google Scholar]
- Voigt, N. , Abu‐Taha, I. , Heijman, J. , & Dobrev, D. (2014). Constitutive activity of the acetylcholine‐activated potassium current IK,ACh in cardiomyocytes In Advances in pharmacology (1st ed., Vol. 70). Elsevier Inc. 10.1016/B978-0-12-417197-8.00013-4 [DOI] [PubMed] [Google Scholar]
- Voigt, N. , & Dobrev, D. (2016). Atrial‐selective potassium channel blockers. Cardiac Electrophysiology Clinics, 8(2), 411–421. 10.1016/j.ccep.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Voigt, N. , Friedrich, A. , Bock, M. , Wettwer, E. , Christ, T. , Knaut, M. , … Dobrev, D. (2007). Differential phosphorylation‐dependent regulation of constitutively active and muscarinic receptor‐activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovascular Research, 74(3), 426–437. 10.1016/j.cardiores.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Waks, J. W. , & Zimetbaum, P. (2017). Antiarrhythmic drug therapy for rhythm control in atrial fibrillation. Journal of Cardiovascular Pharmacology and Therapeutics, 22(1), 3–19. 10.1177/1074248416651722 [DOI] [PubMed] [Google Scholar]
- Walfridsson, H. , Anfinsen, O. G. , Berggren, A. , Frison, L. , Jensen, S. , Linhardt, G. , … Carlsson, L. (2015). Is the acetylcholine‐regulated inwardly rectifying potassium current a viable antiarrhythmic target? Translational discrepancies of AZD2927 and A7071 in dogs and humans. Europace, 17(3), 473–482. 10.1093/europace/euu192 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Liang, B. , Skibsbye, L. , Olesen, S. P. , Grunnet, M. , & Jespersen, T. (2013). GIRK channel activation via adenosine or muscarinic receptors has similar effects on rat atrial electrophysiology. Journal of Cardiovascular Pharmacology, 62(2), 192–198. 10.1097/FJC.0b013e3182965221 [DOI] [PubMed] [Google Scholar]
- Wijffels, M. C. , Kirchhof, C. J. , Dorland, R. , & Allessie, M. A. (1995). Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation, 92(7), 1954–1968. 10.1161/01.CIR.92.7.1954 [DOI] [PubMed] [Google Scholar]
- Yamada, M. , Inanobe, A. , & Kurachi, Y. (1998). G protein regulation of potassium ion channels. Pharmacological Reviews, 50(4), 723–757. [PubMed] [Google Scholar]
- Yang, Y. , Yang, Y. , Liang, B. , Liu, J. , Li, J. , Grunnet, M. , … Chen, Y. H. (2010). Identification of a Kir3.4 mutation in congenital long QT syndrome. American Journal of Human Genetics, 86(6), 872–880. 10.1016/j.ajhg.2010.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cell culture conditions
Table S2. Cloned cardiac ion channel conventional electrophysiology. Experimental solutions (external pipette solution composition)
Table S3. Cloned cardiac ion channel conventional electrophysiology. Experimental solutions (internal pipette solution composition)
Table S4. Assessment of cardiac calcium channel activity. Experimental solutions
Table S5. In‐vivo animal model. Animals
Figure S1. Effect of XAF‐1407 on recombinant potassium inward rectifier currents. Original Kir3.1/3.4 (A), Kir3.4/3.4 (B) and Kir2.1 (C) current traces are shown in the absence and presence of XAF‐1407 and following removal of external K+ to assess passive leak. Schematic of the voltage protocol used is shown at the bottom of the figure. A dashed line shows zero current
Figure S2: Effect of XAF‐1407 on recombinant K v 11.1 (hERG) and Na v 1.5 current. Panel A shows the effect of 3 μM XAF‐1407 on Nav1.5 at different holding potentials (‐70mV lower panel or 100 mV upper panel) and frequencies (1 Hz left panel and 5 Hz right panel). Panel B shows original Kv11.1 current records in the absence and presence of 3 and 10 μM XAF‐1407 record from the same cell. A schematic of the voltage protocol is shown inset at the top of each figure. A dashed line shows zero current
Figure S3: Effect of XAF‐1407 on recombinant K v ionic current. Panel A shows original Kv1.5 current records in the absence and presence of 1, 3, 10 and 30 μM XAF‐1407 record from the same cell. Panel B shows original Kv4.3 current records in the absence and presence of 10 μM XAF‐1407. Panel C shows original Kv7.1/KCEN1 current records in the absence and presence of 10 and 30 μM XAF‐1407. A schematic of the voltage protocol is shown above each original trace. A dashed line shows zero current


