Abstract
Difficulties inherent in the identification of immune correlates of protection or severe disease have challenged the development and evaluation of dengue vaccines. There persist substantial gaps in knowledge about the complex effects of age and sequential dengue virus (DENV) exposures on these correlations. To address these gaps, we were conducting a novel family-based cohort-cluster study for DENV transmission in Kamphaeng Phet, Thailand. The study began in 2015 and is funded until at least 2023. As of May 2019, 2,870 individuals in 485 families were actively enrolled. The families comprise at least 1 child born into the study as a newborn, 1 other child, a parent, and a grandparent. The median age of enrolled participants is 21 years (range 0–93 years). Active surveillance is performed to detect acute dengue illnesses, and annual blood testing identifies subclinical seroconversions. Extended follow-up of this cohort will detect sequential infections and correlate antibody kinetics and sequence of infections with disease outcomes. The central goal of this prospective study is to characterize how different DENV exposure histories within multigenerational family units, from DENV-naive infants to grandparents with multiple prior DENV exposures, affect transmission, disease, and protection at the level of the individual, household, and community.
Keywords: dengue virus, pathogenesis, prospective cohort study, Thailand, transmission
Abbreviations
- DENV
dengue virus
- ELISA
enzyme-linked immunosorbent assay
- FlowNT
flow neutralization assay
- HAI
hemagglutination inhibition
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- JEV
Japanese encephalitis virus
- RT-PCR
reverse-transcriptase polymerase chain reaction
- ZIKV
Zika virus
Dengue virus (DENV) causes a significant burden of disease throughout tropical and subtropical regions of the world, with an estimated 390 million infections annually and 96 million illnesses (1). There is no effective antiviral for DENV infection, and efforts to control the mosquito vector Aedes aegypti have had incomplete success (2). The development of an effective tetravalent DENV vaccine therefore remains an important priority, and several candidate vaccines are in development (3). A single vaccine (Dengvaxia; Sanofi Pasteur, Lyon, France) has been licensed in over 20 countries, including the United States and European Union, but is indicated only for use in people with proof of prior DENV infection. This narrow indication and adverse safety signals in vaccinated dengue-naive individuals has made Dengvaxia uptake poor. Vaccine development and evaluation have been impeded by critical gaps in our understanding of DENV immunology and epidemiology (4).
Infection with any of the 4 DENV serotypes (DENV-1–4) can cause a spectrum of outcomes from asymptomatic infection to dengue hemorrhagic fever or dengue shock syndrome. It has long been recognized that severe dengue illness is more likely to occur in the setting of preexisting immunity to DENV, derived either from placentally transferred maternal antibody in infants or from a prior DENV infection (5, 6). However, preexisting immunity to DENV serotypes has also been shown to be protective against severe disease (7, 8). It is not clear when and how preexisting immunity could predispose toward or protect from illness following a subsequent DENV infection, a knowledge gap that has hindered the development of dengue vaccines and, increasingly, the development of antivirals and immunotherapeutics.
The cross-reactivity and uncertainty inherent in interpreting DENV serotype-specific infection histories based upon serological data render cross-sectional studies, and even short-term prospective studies, suboptimal for characterizing factors associated with dengue pathogenesis and protective immunity. The long-term prospective cohort-cluster study described herein will follow newborn infants and their multigenerational family units through successive DENV infections and over several years, characterizing their dynamic immunological profiles over time and evaluating factors associated with asymptomatic or severe infection outcomes. Nested cluster-based studies of enrolled households experiencing an incident acute dengue illness will allow focused surveillance of individuals at high risk of exposure, some of whom will become infected and thus facilitate studies of immunological correlates of protection and severe disease. We have previously observed that DENV transmission is tightly focused within and immediately surrounding the home of an infectious individual (9, 10). Thus, family units provide a unique opportunity to efficiently study the outcomes of exposure to a given DENV serotype in a multigenerational group of people with shared genetics and overlapping DENV exposure histories.
The objectives and associated hypotheses for the study are as follows:
Objective 1: To correlate pre- and postinfection immunological profiles with disease outcomes across sequential DENV infections in children and adults. Hypothesis 1: Preexisting immunological profiles can be identified that serve as correlates of disease (11) and correlates of protection for subsequent DENV infections (12). Hypothesis 2: The sequence of DENV serotype exposures is associated with risk of severe dengue illness upon subsequent infection (13).
Objective 2: To define potential disease-modifying roles of maternally derived DENV immunity in infants. Hypothesis: Newborns acquire maternal immunity that modulates the clinical outcome of DENV infection within definable windows of time and/or within definable immunological profiles (14).
Objective 3: To define the clinical and immunological outcomes of sequential DENV infections in adults with multiple prior DENV exposures. Hypothesis 1: Adults in DENV-endemic areas experience periodic subclinical infection resulting in immunological boosting and maintenance of cross-protective immunity (8). Hypothesis 2: Immunosenescence in older adults (aged ≥50 years) leads to an increased risk for symptomatic DENV infection due to waning protective immunity.
Objective 4: To identify risk factors for DENV transmission in household units. Hypothesis: The force of infection (i.e., incidence among susceptible individuals) for DENV in households and in communities will differ by virological, entomological, ecological, and household/community factors.
METHODS
General approach
This ongoing, prospective longitudinal cohort-cluster study will follow a cohort of 500 multigenerational family units residing in Kamphaeng Phet province, Thailand. The study began in 2015, will continue through at least 2023, and was actively following 2,870 individuals in 485 families as of May 2019. The study enrolls pregnant women andtheir multigenerational family units, including their newborn infant at the time of delivery (Figure 1). Through active surveillance, cohort subjects with symptomatic DENV infection are identified, and subsequent virus transmission to other family members is evaluated via household-based cluster investigations. Scheduled blood testing is performed annually to detect subclinical infections and to provide baseline and periodic blood samples for immunological analysis. The methods of this observational study are further detailed in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (14).
Figure 1.
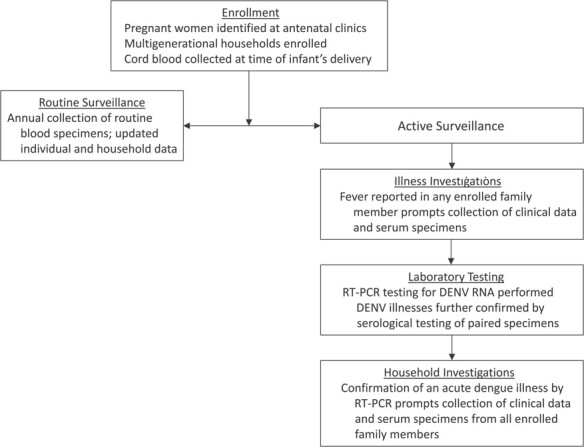
Flowchart of study activities for a prospective family cohort study in Kamphaeng Phet, Thailand, 2015–2019. DENV, dengue virus; RT-PCR, reverse-transcriptase polymerase chain reaction.
Setting
The study is conducted in Kamphaeng Phet province in north-central Thailand (Figure 2). Kamphaeng Phet is located 350 km north of Bangkok and has a population of 700,000 people in a mostly rural to semirural setting. Clinical partners for this study are the 410-bed Kamphaeng Phet Provincial Hospital, situated in the central Muang (capital) district, and the 90-bed Khanu Woralaksaburi district hospital, located approximately 60 km southeast of Muang district. Additionally, pregnant women are recruited from among 52 basic village health centers located in Muang and Khanu Woralaksaburi districts.
Figure 2.
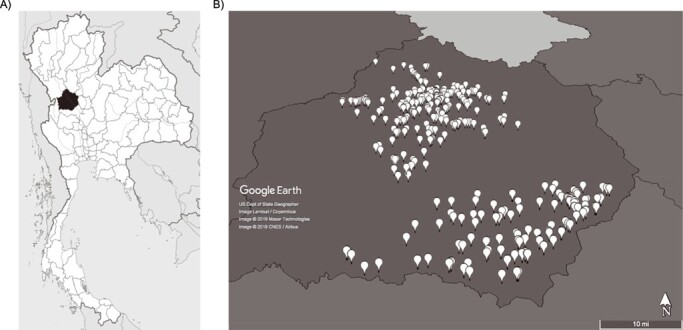
Map of Kamphaeng Phet province, indicating location within Thailand (A) (source: Wikipedia) and the locations of enrolled households (B) (source: Google Earth Pro) for a prospective family cohort study in Kamphaeng Phet, Thailand, 2015–2019. Spatial resolution of the map image is intentionally low, to preclude identification of individual homes.
Enrollment
Enrollment of households is contingent upon the identification of eligible women in the third trimester of pregnancy, who are briefed and screened during antenatal visits. If the pregnant woman consents to participate, her family members are briefed at their home. Inclusion criteria include: 1) residence in Kamphaeng Phet province with no plans to move and 2) at least 4 family members’ consent to participate, including the pregnant woman (aged ≥15 years), her newborn, at least 1 other child <18 years of age, and 1 adult aged ≥50 years. Exclusion criteria include: 1) congenital or acquired immunodeficiency, 2) immunosuppressive therapy within the preceding 6 months, 3) chronic illness that might interfere with study conduct, and 4) receipt of blood products in the past 3 months. Written informed consent is obtained, as is signed assent for children older than 7 years.
All enrolled subjects have a demographic and clinical questionnaire administered (Table 1) and a blood sample drawn for laboratory testing (Table 2). Study staff perform Global Positioning System (GPS) mapping of the home using a hand-held device. When enrolled pregnant women go into labor, study staff collect clinical information about the delivery and coordinate collection of cord blood. Approximately 22 mL of cord blood is collected by the hospital nurse/physician directly from the umbilical cord after delivery.
Table 1.
Data Collection at Each Study Visit for a Prospective Family Cohort Study in Kamphaeng Phet, Thailand, 2015–2019
| Questionnaire | Direct Measurement | Document Review | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Visit | Demographic Data a | Clinical Data b | Household Data c | Weight | Oral Temperature | GPS Mapping | Vaccination Records | Hospital Records, If Applicable | Mosquito Collection d | Human Movement Surveyd |
| Enrollment | X | X | X | X | X | X | ||||
| Annual follow-up | X | X | X | X | X | X | X | |||
| Acute illness investigation | X | X | X | |||||||
| 14- and 28-day convalescent | X | X | ||||||||
| 0-, 14-, and 28-day household contact evaluation | X | X | X | X | X | |||||
Abbreviation: GPS, Global Positioning System.
a Demographic data collected on enrollment include date of birth, sex, education, occupation. These data are updated annually.
b Clinical data collected on enrollment include vaccination history, history of dengue illness, and comorbidities. These data are updated annually and verified against personal vaccination booklets whenever possible. Clinical data collected during acute illness and household investigations include clinical symptoms (onset and duration), care-seeking behavior (hospital and clinic visits, over-the-counter and prescription medications taken), and risk factors (ill contacts, recent travel). If an individual was hospitalized with a febrile illness at a collaborating hospital, hospital records will be reviewed, including physician and nursing notes, laboratory and radiological results, and admission and discharge diagnoses.
c Household data collected on enrollment include housing construction materials, sanitation practices, and water source, location, land use. These data are updated annually.
d Entomological and human movement surveys are performed for all households undergoing household investigations (i.e., in the setting of confirmation of an acute dengue illness by reverse-transcriptase polymerase chain reaction). Trapping of mosquitoes occurs on days 0 and 14; mote deployment on day 0 only. Entomological and movement surveys are also performed for a random subset of households during annual follow-ups.
Table 2.
Specimens and Diagnostic Tests Associated With Each Study Visit for a Prospective Family Cohort Study in Kamphaeng Phet, Thailand, 2015–2019
| Specimen Collection | Diagnostic Testing | |||||||
|---|---|---|---|---|---|---|---|---|
| Study Visits | Serum | PBMC | Cord Blood | HLA | FlowNT 50 | HAI | DENV, ZIKV, JEV IgM, and IgG ELISA | DENV RT-PCR a |
| Enrollment | X | X | X | X | X | X | ||
| Annual follow-up | X | X | X | X | ||||
| Acute illness investigation | X | X | X | X | X | |||
| 14- and 28-day convalescentb | X | X | X | X | ||||
| 0-, 14-, and 28-day household contact evaluationb | X | X | X | X | X | |||
Abbreviations: DENV, dengue virus; ELISA, enzyme-linked immunosorbent assay; FlowNT, flow cytometry-based neutralization assay (50% titers); HAI, hemagglutination inhibition; HLA, human leukocyte antigens; IgG, immunoglobulin G; IgM, immunoglobulin M; JEV, Japanese encephalitis virus; PBMC, peripheral blood mononuclear cells; RT-PCR, reverse-transcriptase polymerase chain reaction; ZIKV, Zika virus.
a DENV RT-PCR is performed during an acute illness investigation (day 0), on the first day of a household investigation (day 0), and on acute specimens collected from household members who develop fever during the 28-day follow-up period.
b Collection of day-28 follow-up specimen is omitted for children younger than 5 years of age for both illness and household investigations, in order to limit the frequency of blood draws in very young children.
Routine (annual) follow-up
All subjects have an annual follow-up questionnaire administered and a routine blood sample drawn by study staff. For 2015–2019, routine follow-up was performed between April and June, prior to the peak DENV transmission (rainy) season in this region. Thus, for some individuals, the first routine follow-up occurred within months of being enrolled. If subjects were unavailable during this period, for example, due to travel outside of the study area, the routine follow-up occurred as early thereafter as possible. Beginning in late 2019, follow-up activities will be shifted to occur at the end of the DENV season (roughly November–December) to maximize the capture of seroconversions following infection (15).
Active surveillance and illness investigations
Active and passive surveillance activities for febrile illnesses are conducted year-round. Participants are instructed to contact study staff if any enrolled subject in the household develops fever. Additionally, study staff contact subjects (via telephone, text, or home visit) weekly to inquire as to whether an acute febrile illness was missed since the previous contact. The threshold for initiating an illness investigation in a study subject is a history of reported fever within the previous 7 days or measured temperature of ≥38°C. Additionally, health-care providers are requested to contact study staff when clinic visits or hospitalizations occur related to acute febrile episodes in enrolled subjects.
Evaluation of a febrile illness involves collection of an acute blood sample and 2 convalescent blood samples: one convalescent sample at 14 days and another at 28 days after acute sample collection. Clinical information—including onset, duration, and severity of symptoms—is recorded by the study team using a prestructured questionnaire at each visit. Study staff inform Kamphaeng Phet Provincial Public Health Office about any acute DENV reverse-transcriptase polymerase chain reaction (RT-PCR)-positive cases within 1–2 days, and they then apply antivector measures per Ministry standards.
Household contact dengue investigation
DENV RT-PCR is performed on acute specimens from illness investigations within 24–48 hours of collection. Confirmation of an acute DENV infection by RT-PCR triggers an expanded evaluation of all enrolled members of the home (a “household investigation”). Household investigations are typically initiated the same day as or day after RT-PCR results are obtained, but they might be delayed up to 3 days over weekends or holidays. Household contacts have blood drawn and clinical data collected on days 0, 14, and 28, regardless of whether or not they are ill.
Laboratory testing
Acute blood samples from illness investigations are tested by DENV RT-PCR within 1–2 days of collection (16). The day-0 blood sample from household investigations (and a household contact’s acute blood sample, if applicable) are also tested by DENV RT-PCR. Serological diagnosis of acute DENV infection is accomplished using enzyme-linked immunosorbent assay (ELISA) and hemagglutination inhibition (HAI) assays (17). Immunoglobulin M (IgM)- and immunoglobulin G (IgG)-capture ELISAs for DENV and HAI assays to detect antibodies to DENV-1–4 are performed on acute and convalescent specimens from illness and household investigations, testing for DENV and Japanese encephalitis virus (JEV) antibodies (18). Dengue IgM of ≥40 U with a DENV IgM-to-IgG ratio of ≥1.8 is defined as “primary” DENV infection; a ratio of <1.8 is defined as “secondary” DENV infection. Further, a 2-fold increase in dengue IgG with an absolute value of ≥100 U indicates secondary infection in the absence of dengue IgM of ≥40 U (18). For HAI, a 4-fold rise in antibody titers for any DENV serotype (greater than or equal to the rise observed for JEV) indicates acute infection. A titer of ≥1:2,560 to any DENV serotype is associated with secondary DENV infection; a maximum titer of 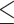 1:1,280 is associated with primary infection. Seroconversion by either HAI or ELISA constitutes serological diagnosis of acute DENV infection in the cohort.
1:1,280 is associated with primary infection. Seroconversion by either HAI or ELISA constitutes serological diagnosis of acute DENV infection in the cohort.
For routinely collected specimens (enrollment and annual follow-up), DENV neutralizing antibody titers are measured using a flow cytometry-based neutralization assay (FlowNT) (19). Fifty percent neutralization titers (FlowNT50) in enrollment specimens are used to determine baseline serostatus, where “DENV-naive” indicates enrollment FlowNT50 titers of <40 for DENV types 1–4; “monotypic” indicates a titer of ≥40 for a single DENV type; and “multitypic” indicates titers of 40 for 2 or more DENV types. Seroconversion is defined as at least a 4-fold rise in FlowNT50 between specimens, for at least 1 DENV type. For the purposes of the present work, seroconversion data are based upon results from HAI assays (FlowNT50 data for the entire cohort are pending at the time of publication). Seroconversion by HAI is defined as a 4-fold rise in titers to at least 1 DENV type, greater than or equal to any rise observed for JEV, for paired specimens.
Entomological procedures
Mosquito collections are performed periodically at the homes of cohort families to coincide with annual (routine) follow-up activities and during all household investigations. Environmental data are collected, such as house infrastructure features, number and types of man-made water storage containers, and other potential mosquito-breeding sites. Adult Aedes mosquitoes are collected from within and around the home using adult mosquito traps (BG-Sentinel; Biogents, Regensburg, Germany) on days 0 and 14 of the contact investigation. Traps are placed early in the morning and retrieved the same evening, thus reflecting 2 single days of mosquito collections to correspond with the timing of acute and convalescent blood specimens. Mosquito samples retrieved from each trap are examined under a microscope to identify their sex and species. Aedes females are subsequently screened for DENV infection by RT-PCR.
Mote deployment
Proximity-detecting motes are deployed during household investigations and sporadically during annual follow-up to identify movement patterns in the cohort. Crossbow’s TelosB motes (TPR2400; Crossbow Inc., San Jose, California) are small electronic devices that detect when they are within range of another mote and log the amount of time that they are in proximity. Information recorded by a mote can be used to determine the amount of time spent at home, the total number of close contacts a subject makes with other subjects, the duration of such contacts, and whether a subject makes contact with certain individuals more often than others (20). These contact patterns are used to inform models of virus transmission within the family unit.
Sample-size calculations
Based on previous studies conducted in Kamphaeng Phet, we assumed an average annual incidence of DENV infection of 7% (21). We therefore anticipate that 7% of children born into the study as infants will experience primary DENV infection each year (220 over 8 years) and that 7% of siblings younger than 18 years of age will experience primary or secondary DENV infection (220 siblings younger than 18 years, 220 adults aged <50 years, and 220 adults aged 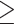 50 years over the 8 year study period). Among these, approximately half of infections will be subclinical infections and half symptomatic infections (22). Assuming that one-half of infections in siblings aged less than 18 years will be secondary DENV infections and all adult infections will be secondary, we expect 550 secondary infections during the 8-year period. We will thus have a power of 83% to detect a 75-unit absolute difference in serotype-specific (preinfection) geometric mean titers between individuals experiencing secondary symptomatic and subclinical DENV infections during the study (objective 1).
50 years over the 8 year study period). Among these, approximately half of infections will be subclinical infections and half symptomatic infections (22). Assuming that one-half of infections in siblings aged less than 18 years will be secondary DENV infections and all adult infections will be secondary, we expect 550 secondary infections during the 8-year period. We will thus have a power of 83% to detect a 75-unit absolute difference in serotype-specific (preinfection) geometric mean titers between individuals experiencing secondary symptomatic and subclinical DENV infections during the study (objective 1).
Statistical analysis
In the present work, seroconversion rates are presented using a denominator of person-months, representing the summed intervals between enrollment and follow-up. Continuous measurements, such as entomological indices, will be compared between groups using analysis of variance (ANOVA) or ANOVA on ranks, as appropriate, based on assessment of normality. Proportional data, such as the proportion of infections that were symptomatic, are compared between groups using χ2 analyses.
In planned future analyses, multivariate analyses will be conducted using generalized estimating equations (GEE) or hierarchical generalized additive models (HGAM), which will include random effects to control for repeated measures within individuals, households, and districts. Age-stratified seroprevalence data, derived from the serotype-specific neutralizing antibody data for all participants on enrollment, will be used to estimate the force of infection for DENV infection using catalytic models as previously described (23). Spatial heterogeneity in the force of infection will be assessed by subdistrict. Serotype-specific force of infection will be estimated, with the infecting serotype for RT-PCR-negative and subclinical infections inferred based upon the dominant DENV serotypes circulating in a given individual’s community in a given year. This approach is supported by prior work indicating highly focal transmission of DENV serotypes and strains in households and communities (9). Sensitivity analyses will explore the impact of these assumptions on the estimates of serotype-specific and overall FOI.
Potential confounders
A major area of interest for this study is the relationship between preexisting dengue immunity and the risk of dengue illness with infection (compared with asymptomatic infection). Age is an important confounder of this association because adults are more likely to have accumulated DENV immunity through multiple prior exposures, and adults have also been shown to have an increased risk of clinical illness with DENV infection (24). Other important confounders might include the presence of medical comorbidities and history of JEV vaccination. All of these variables will be recorded upon enrollment and updated annually. Given that the timing of routine follow-up activities varied during the study period, we will control for the month of follow-up given the seasonality of DENV transmission in the study area. Infecting serotype is an important confounder, related both to prior exposure history and the risk of illness. Analysis will be adjusted for, or stratified by, all of the above confounders in generalized estimating equations or hierarchical generalized additive models as described above.
A second major area of interest for this study is the interplay between individual, household, and community factors and the force of DENV infection within the household. For this analysis, we will stratify on the circulating serotype, calculating the serotype-specific force of infection. An additional possible confounder will be the timing and extent of mosquito-control measures undertaken in and around the home following diagnosis of DENV infection, as routinely performed by the Thai Ministry of Health. For each household investigation, we will seek to gather information on the timing and extent of mosquito control efforts. If this proves to be an important confounder, we will control for it in analysis.
Ethical approvals
This study was approved by Thailand Ministry of Public Health Ethical Research Committee, Siriraj Ethics Committee on Research Involving Human Subjects, Institutional Review Board for the Protection of Human Subjects State University of New York Upstate Medical University, and Walter Reed Army Institute of Research Institutional Review Board (protocol #2119).
RESULTS
Enrollment and retention of study participants
The family cohort study began enrollment in September 2015; 1,216 women in their third trimester of pregnancy were briefed during their routine antenatal care visits, and of these, 811 indicated an interest in participating, and 552 met inclusion criteria for enrollment. As of May 2019, a total of 3,469 individuals in 551 families have been enrolled (Web Figure 1, available at https://academic.oup.com/aje); 599 individuals have been lost to follow-up or withdrawn for reasons of: moved out of study area (245), failure to collect cord blood (183), new failure to meet inclusion criteria (79; e.g., less than 3 family members in home, new diagnosis of immunodeficiency), participant voluntarily withdrew (59), and death unrelated to study procedures (33).
Characteristics of study participants
The median age of participants at enrollment was 21 years (range, 0–93 years) (Figure 3). Among pregnant women, the median age was 24 years (range, 15–42 years), and among family members (i.e., excluding the pregnant woman and her newborn) the median age was 29 years (range, 0–93 years). Of all enrolled participants, 56.9% were female, with 49.3% among enrolled family members.
Figure 3.
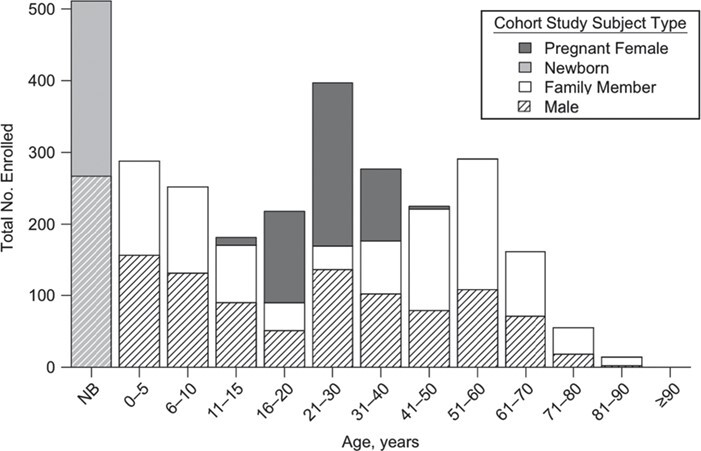
Age and sex composition of the actively enrolled participants in the study, as of May 2019, for a prospective family cohort study in Kamphaeng Phet, Thailand, 2015–2019. NB, newborn.
Enrollment serostatus
Of the 774 individuals enrolled in 2015, 416 completed the first annual follow-up in 2016. The remaining individuals completed follow-up in 2017. Enrollment neutralizing antibody titers DENV-1–4 are available for these 416 individuals and pending for the rest of the cohort. FlowNT50 testing of enrollment specimens demonstrated seroprevalence rates of 89% for newborns (as detected in cord blood), reflecting maternally transferred antibody and, possibly, some in utero infections (Figure 4). Seroprevalence decreased to 46% for children aged 0–5 years and increased steadily thereafter until 100% of individuals aged 30 years or older demonstrated multitypic immunity to DENV.
Figure 4.
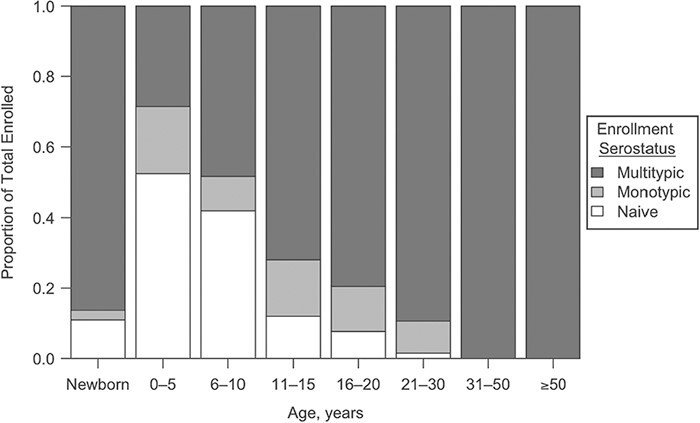
Enrollment serostatus of 416 study participants enrolled in 2015, based upon flow-based cytometry testing for neutralizing antibodies to dengue virus (DENV)-1–4 serotypes, Kamphaeng Phet, Thailand, 2015–2019. “Naive" indicates that an individual lacked detectable antibodies to all 4 serotypes (titer: <1:40); “monotypic” indicates a positive titer to a single DENV serotype; “multitypic” indicates a positive titer for ≥2 DENV serotypes.
Illness and household investigations
A total of 295 illness investigations were conducted between September 2015 and May 2019 among 243 individuals (Figure 5). Illness investigations confirmed 33 acute infections by DENV RT-PCR (11 DENV-1, 8 DENV-2, 4 DENV-3, and 10 DENV-4), prompting 29 household investigations (4 individuals lived in the same home as another person with a confirmed DENV infection); 43 DENV infections were identified during household investigations, including 8 RT-PCR-positive and 35 RT-PCR-negative (serologically confirmed) infections. The associated infection rate in household contacts was therefore 26.7% (43 DENV infections/161 household contacts), consistent with the high household attack rate reported in prior studies in the area (9). Of acute DENV infections in household contacts, 32.5% (13/40) were symptomatic; young children (aged 0–5 years) were the most likely to experience symptomatic DENV infection (50.0%), followed by older children (6–17 years) and young adults (18–30 years) (33.3% (3/9) and 40.0% (4/10), respectively). There were no symptomatic infections detected among household contacts aged 31–50 years (0/4). Seven DENV infections occurred in adults aged 50 years or older, of which 1 infection was symptomatic (14.2%); 80.0% of DENV infections in children aged 0–5 years were primary DENV infections, 55.6% in children aged 5–17 years, and 50.0% in adults aged 16–50 years. Interestingly, in household contacts aged ≥51 years, 71.4% of DENV infections were determined to be “primary” infections by ELISA and/or HAI.
Figure 5.
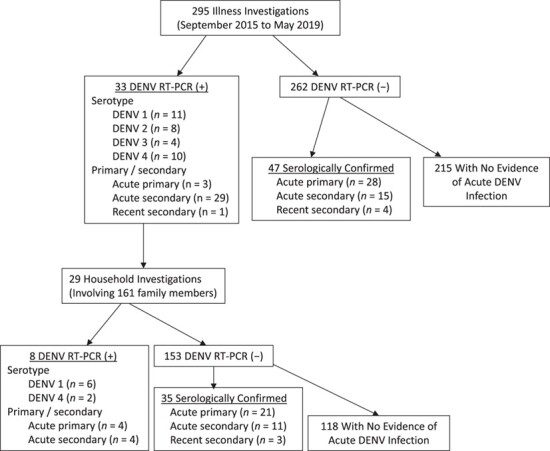
Results from illness and household investigations, including dengue virus (DENV) serotype and primary/secondary infection status of detected infections, Kamphaeng Phet, Thailand, 2015–2019. Note that 4 reverse-transcriptase polymerase chain reaction (RT-PCR)-positive individuals resided in the same house as other tested-positive individuals; thus, 29 household investigations from 33 cases.
Routine follow-up and seroconversions
The median interval of time between enrollment and the first routine follow-up was 13 months (range, 2–20 months), between the first and second routine follow-up was 11 months (range, 5–15 months), and between the second and third routine follow-up was 10 months (range, 6–16 months). In total, 74,343 person-months of observation were accrued between September 2015 and July 2018. We are in the process of completing HAI and FlowNT50 testing to determine seroconversion rates for the second and third years of follow-up for enrolled individuals. Thus, data are presented for seroconversion rates following the first year of enrollment for the cohort participants enrolled in 2015, 2016, and 2017 (Figure 6).
Figure 6.
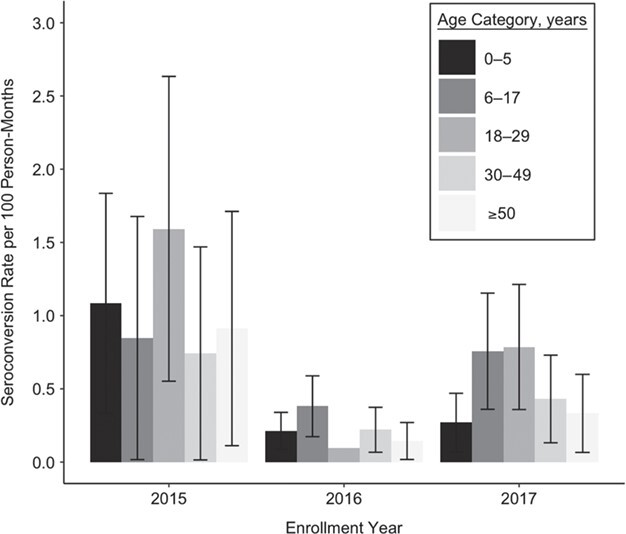
Seroconversion rate (per 100 person-months) according to age group and enrollment cohort, Kamphaeng Phet, Thailand, 2015–2019. Seroconversion was defined as a 4-fold or greater rise in antibody titers to any dengue virus serotype by hemagglutination inhibition (without a higher rise in Japanese encephalitis virus titers) when comparing 2 routinely collected specimens collected, on average, 1 year apart. Error bars represent 95% confidence intervals for estimated rates.
The overall seroconversion rates for the first year of follow-up were: 1.04 DENV infections/100 person-months (95% confidence interval: 0.66, 1.41) for those enrolled in 2015, 0.21/100 person-months (95% confidence interval: 0.15, 0.28) for those enrolled in 2016, and 0.49/100 person-months (95% confidence interval: 0.35, 0.63) for those enrolled in 2017. For cohort participants enrolled in 2015 and 2017, the highest rate of seroconversion was observed in those aged 18–29 years; for those enrolled in 2016, the highest rate was observed in those aged 6–17 years.
DISCUSSION
Recent issues with the safety and immunogenicity of DENV vaccines highlight our limited understanding of how multitypic immune protection from DENV infection and illness is naturally shaped during sequential and accumulated DENV exposures (4). Even less understood are factors associated with the durability of protection into adulthood/advanced age, such as the role of boosting in maintaining protective immunity. The prospective study described herein, being conducted in a hyperendemic and well-characterized locale for DENV transmission in Thailand, aims to address these and other critical gaps in our understanding of DENV transmission and pathogenesis (Figure 7).
Figure 7.
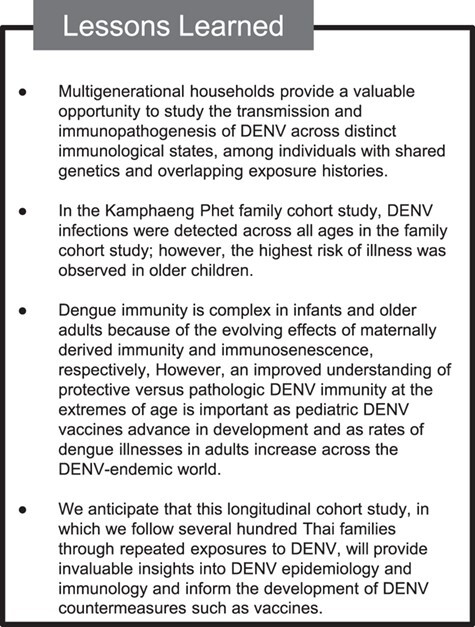
Lessons learned from the Kamphaeng Phet family cohort study for dengue virus (DENV) transmission in households, Thailand, 2015–2019.
We describe a novel family-based cohort study that will follow newborn infants and their multigenerational family units prospectively for the occurrence of sequential DENV infections. The study is an innovative hybrid of cohort and cluster study methodologies, involving the routine characterization of immune profiles prior to and following sequential DENV infections, while employing febrile illness cluster investigations to maximize the capture and characterization of associated infections in the home. These associated infections will manifest across a clinical spectrum from asymptomatic DENV infection to severe disease, allowing the study of correlates of protection and illness (11). The study is currently funded through 2023 (allowing up to 8 years of follow-up); however, it will ideally continue for many years beyond that, permitting the extended characterization of DENV infections and immune profile dynamics in the cohort.
In the first 3 years of the study, seroconversions to DENV occurred across all age groups, from neonates to octogenarians. This is noteworthy given that dengue has typically been studied as a pediatric disease in hyperendemic locales such as Thailand. The mean age of DENV infection is increasing in Thailand (25) and across Asia (24), and so future studies in this cohort will consider the durability of type-specific DENV immunity, the potential for immunosenesence with aging, and differences in the clinical presentation of dengue between children, adults, and the elderly. The capture of instances of immunological boosting is suggested by the relatively high rate of “primary” infection in adults aged >50 years, per standard serological diagnostic criteria. We are developing immunological and statistical criteria to discriminate between boosting events and novel DENV serotype exposures, which will inform our understanding of the natural mechanisms promoting the maintenance of protective immunity in this population.
Future analyses will address the duration of and factors associated with protection from clinical illness afforded by maternal antibodies in the first year of life. This knowledge can inform the identification of optimal ages for DENV vaccination, ideally targeting a window of time associated with minimal interference from maternally derived immunity and with maximal numbers of dengue illnesses averted. The identification of subclinical DENV infections in the setting of waning maternal antibodies in the first year of life poses a challenge, because for these infants “seroconversion” might not manifest as a rise in antibody titers but rather as a “failure to decay.” Therefore, the current reported seroconversion rate in infants is likely an underestimate. We are developing statistical algorithms to facilitate the serological diagnosis of DENV infection in infants, accounting for the rate of decay of maternal antibody.
The early years of this study have encountered some challenges. Unknown at the time of study inception, we now recognize that Zika virus (ZIKV) has been in circulation in Thailand for at least several years (26). The cocirculation of JEV and DENV and high levels of JEV vaccination have complicated the serological diagnosis of DENV infection in Thailand for decades (18, 27). The addition of potential ZIKV exposure to this complex serological milieu makes the diagnosis of DENV infection in the study area more challenging. Serological assays have historically demonstrated poor specificity for distinguishing DENV and ZIKV infections; however, assays such as the NS1 blockade-of-binding ELISA for ZIKV show promise (28) and are being evaluated for possible incorporation into the study. The ability to study newborns longitudinally through sequential incident flavivirus infections (and JEV vaccination) should permit invaluable insights into immunological, epidemiologic, and clinical interactions between DENV, ZIKV, and JEV.
There are multiple sources of potential bias in the study. There is selection bias by nature of the study design, because enrollment is restricted to multigenerational families. We will attempt to control for this with age-adjusted analyses. There might be sampling bias—individuals and families with histories of DENV infection could be more motivated to participate. We have noted that adult men are less likely to enroll and that adults in general have been less likely to volunteer febrile illnesses (these illnesses are more often detected by study staff phone call than by participant-initiated report). We are seeking to address this potential bias by providing educational materials and study results to families, emphasizing the burden of DENV infection in adults in the study area. We have shifted study activities to occur primarily in the evenings in order to increase participation by working adults. Finally, generalizability of study results might be reasonable within Southeast Asia but more limited in countries without a comparably high force of infection or without similar flaviviruses in cocirculation (i.e., the Americas).
In summary, this family cohort study for DENV transmission in Kamphaeng Phet, Thailand, uses a novel multigenerational and longitudinal study design to address fundamental questions in DENV epidemiology and pathogenesis. The study of preexposure immune profiles across all ages will inform correlates of protection and disease severity, with implications for the development of DENV vaccines and therapeutics. The focus on dengue at the household level will help define the full burden of dengue for families and communities. Finally, this study should provide unprecedented epidemiologic data on factors associated with the force of infection for DENV and thereby identify opportunities for population-level interventions to disrupt transmission.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Institute for Global Health and Translational Science, State University of New York Upstate Medical University, Syracuse, New York (Kathryn B. Anderson, Stephen J. Thomas, Timothy P. Endy); Department of Medicine, State University of New York Upstate Medical University, Syracuse, New York (Kathryn B. Anderson, Stephen J. Thomas, Timothy P. Endy); Department of Microbiology and Immunology, State University of New York Upstate Medical University, Syracuse, New York (Kathryn B. Anderson, Stephen J. Thomas, Timothy P. Endy); Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand (Kathryn B. Anderson, Darunee Buddhari, Alden L. Weg, Damon W. Ellison, Louis Macareo, Ananda Nisalak, Alongkot Ponlawat, Stefan Fernandez); Institute for Immunology and Informatics, University of Rhode Island, Providence, Rhode Island (Anon Srikiatkhachorn, Alan L. Rothman); Faculty of Medicine, King Mongkut’s Institute of Technology, Bangkok, Thailand (Anon Srikiatkhachorn); Viral Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, Maryland (Gregory D. Gromowski, Richard G. Jarman); Thai Ministry of Public Health, Nonthaburi, Thailand (Sopon Iamsirithaworn); Emerging Pathogens Institute, University of Florida, Gainesville, Florida (Derek A. T. Cummings); Global Dengue and Aedes-Transmitted Diseases Consortium, Bethesda, Maryland (In-Kyu Yoon).
K.B.A. and D.B. contributed equally as first authors.
This study was funded by the Military Infectious Disease Research Program and the US National Institutes of Health (program project award P01 AI034533).
The authors acknowledge the contributions of Dr. Wiboonwun Prompitayarat, Dr. Anchali Phusong, Dr. Angkana Uppapong, Dr. Chanlika Sukharom from Kamphaeng Phet and Khanuworalaksaburi Hospitals, Chaleaw Saengchan, and clinical, laboratory, and entomology personnel of the Armed Forces Research Institute for Medical Sciences. We thank Dr. Rotjana Khontong and Dr. Taweesak Khanutwong for their support of the field coordination in Kamphaeng Phet, Thailand. We appreciate the involvement of Chandrika Kannadka (Walter Reed Army Institute of Research) in performing the flow neutralization (FlowNT50) testing. We are especially grateful to the children and adults involved in these studies for their participation.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army, the Department of Defense, or the National Institutes of Health. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25.
Conflict of interest: none declared.
REFERENCES
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Screaton G, Mongkolsapaya J. Which dengue vaccine approach is the most promising, and should we be concerned about enhanced disease after vaccination? The challenges of a dengue vaccine. Cold Spring Harb Perspect Biol. 2018;10(6):a029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–340. [DOI] [PubMed] [Google Scholar]
- 5. Halstead SB, Yamarat C. Recent epidemics of hemorrhagic-fever in Thailand—observations related to pathogenesis of a new dengue disease. Am J Public Health Nations Health. 1965;55(9):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halstead SB, Lan NT, Myint TT, et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8(12):1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sabin AB. Research on dengue during World War II. A J Trop Med Hyg. 1952;1(1):30–50. [DOI] [PubMed] [Google Scholar]
- 8. Anderson KB, Gibbons RV, Cummings DA, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis. 2014;209(3):360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas SJ, Aldstadt J, Jarman RG, et al. Improving dengue virus capture rates in humans and vectors in Kamphaeng Phet Province, Thailand, using an enhanced spatiotemporal surveillance strategy. Am J Trop Med Hyg. 2015;93(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoon I-K, Getis A, Aldstadt J, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6(7):e1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buddhari D, Aldstadt J, Endy TP, et al. Dengue virus neutraling antibody levels associated with protection from infection in Thai cluster studies. PLoS Negl Trop Dis. 2014;8(10):e3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189(6):990–1000. [DOI] [PubMed] [Google Scholar]
- 13. Guzmán MG, Kouri G, Valdés L, et al. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11(4):223–227. [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 15. Salje H, Cummings DAT, Rodriguez-Barraquer I, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018;557(7707):719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanciotti RS, Calisher CH, Gubler DJ, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30(3):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7(5):561–573. [DOI] [PubMed] [Google Scholar]
- 18. Innis BL, Nisalak A, Nimmannitya S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40(4):418–427. [DOI] [PubMed] [Google Scholar]
- 19. de Alwis R, de Silva AM. Measuring antibody neutralization of dengue virus (DENV) using a flow cytometry-based technique. Methods Mol Biol. 2014;1138:27–39. [DOI] [PubMed] [Google Scholar]
- 20. Cattuto C, Van den Broeck W, Barrat A, et al. Dynamics of person-to-person interactions from distributed RFID sensor networks. PLoS One. 2010;5(7):e11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Endy TP, Yoon IK, Mammen MP. Prospective cohort studies of dengue viral transmission and severity of disease. Curr Top Microbiol Immunol. 2010;338:1–13. [DOI] [PubMed] [Google Scholar]
- 22. Endy TP, Chunsuttiwat S, Nisalak A, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156(1):40–51. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez-Barraquer I, Buathong R, Iamsirithaworn S, et al. Revisiting Rayong: shifting seroprofiles of dengue in Thailand and their implications for transmission and control. Am J Epidemiol. 2014;179(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger JR, Ooi EE, Kelly DW, et al. Reconstructing historical changes in the force of infection of dengue fever in Singapore: implications for surveillance and control. Bull World Health Organ. 2008;86(3):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummings DAT, Iamsirithaworn S, Lessler JT, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 2009;6(9):e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buathong R, Hermann L, Thaisomboonsuk B, et al. Detection of Zika virus infection in Thailand, 2012–2014. Am J Trop Med Hyg. 2015;93(2):380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruchusatsawat K, Wongjaroen P, Posanacharoen A, et al. Long-term circulation of Zika virus in Thailand: an observational study. Lancet Infect Dis. 2019;19(4):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balmaseda A, Stettler K, Medialdea-Carrera R, et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci U S A. 2017;114(31):8384–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


