Abstract
Negative and positive emotions are known to shape decision-making toward more or less impulsive responses, respectively. Decision-making and emotion processing are underpinned by shared brain regions including the ventromedial prefrontal cortex (vmPFC) and the amygdala. How these processes interact at the behavioral and brain levels is still unclear. We used a lesion model to address this question. Study participants included individuals diagnosed with behavioral-variant frontotemporal dementia (bvFTD, n = 18), who typically present deficits in decision-making/emotion processing and atrophy of the vmPFC, individuals with Alzheimer’s disease (AD, n = 12) who present with atrophy in limbic structures and age-matched healthy controls (CTRL, n = 15). Prior to each choice on the delay discounting task participants were cued with a positive, negative or neutral picture and asked to vividly imagine witnessing the event. As hypothesized, our findings showed that bvFTD patients were more impulsive than AD patients and CTRL and did not show any emotion-related modulation of delay discounting rate. In contrast, AD patients showed increased impulsivity when primed by negative emotion. This increased impulsivity was associated with reduced integrity of bilateral amygdala in AD but not in bvFTD. Altogether, our results indicate that decision-making and emotion interact at the level of the amygdala supporting findings from animal studies.
Keywords: delay discounting, emotion, ventromedial prefrontal cortex, amygdala, voxel-based morphometry
Introduction
Emotions play an important part in many of our decisions (Bechara et al., 2000; Clore and Huntsinger, 2007). Choosing to save for our children’s education rather than buying our dream car not only involves options with different reward magnitude and delays but also options with distinctive affective content. How emotions interact with decision-making processes, however, is still largely unresolved.
The well-established delay discounting task measures the ability to forgo immediate small rewards in favor of larger longer-term rewards (Green and Myerson, 2004). Choosing larger-later rewards over smaller-sooner rewards (e.g. $70 in 40 days over $50 now) is associated with enhanced performance across the lifespan including better academic performance, social relationships and more adaptive social functioning (Hirsch et al., 2008; Shamosh and Gray, 2008). In contrast, the tendency to choose the smaller-sooner rewards over larger-later rewards has been associated with impulsivity-related behaviors and pathological conditions including drug dependence (Bickel and Marsch, 2001), gambling (Reynolds, 2006) or eating disorders (Kekic et al., 2016). Neuroimaging studies investigating delay discounting consistently point to a core network including the ventromedial prefrontal cortex (vmPFC), amygdala, anterior cingulate cortex (ACC) and striatum (McClure et al., 2004; Kable and Glimcher, 2007; McClure et al., 2007; Ballard and Knutson, 2009; Peters and Buchel, 2009). Studies, however, report inconsistent findings, possibly due to focused region-of-interest analyses (Kable and Levy, 2015) or healthy populations where no structural abnormalities are reported (Bjork et al., 2009; Bernhardt et al., 2014; Tschernegg et al., 2015). Greater delay discounting has been associated with reduced grey matter intensity in striatum (Dombrovski et al., 2012; Cho et al., 2013), vmPFC (Bernhardt et al., 2014; Pehlivanova et al., 2018), lateral prefrontal cortex (Bjork et al., 2009), superior frontal gyrus (Schwartz et al., 2010), ACC (Bernhardt et al., 2014), hippocampus (Lebreton et al., 2013), insula (Turel et al., 2018) as well as temporal pole and temporoparietal junction (Pehlivanova et al., 2018). Conversely, greater delay discounting has also been associated with increased grey matter intensity of the striatum (Schwartz et al., 2010; Tschernegg et al., 2015), vmPFC and ACC (Cho et al., 2013) and prefrontal cortex (Wang et al., 2016). Lesion studies have shown that vmPFC lesion increases delay discounting (i.e. impulsivity), compared with healthy controls or with individuals with lesions in other brain regions (Sellitto et al., 2011; Peters and D'Esposito, 2016). In rodents, lesions of the basolateral amygdala (BLA) (Winstanley et al., 2004; Floresco and Ghods-Sharifi, 2007; Ghods-Sharifi et al., 2009) or disconnection between the orbitofrontal cortex (OFC) and the BLA (Churchwell et al., 2009) increases delay discounting similar to lesions to the OFC (Mobini et al., 2002). To our knowledge, no studies have investigated the effect of amygdala damage on delay discounting in humans. Evidence shows that patients with focal amygdala damage have reduced loss aversion (De Martino et al., 2010) and lower scores on tasks of decision-making under risk (Bechara et al., 1999; Bar-On et al., 2003; Hanten et al., 2006; Brand et al., 2007; Weller et al., 2007) or under ambiguity conditions (Brand et al., 2007).
Relevant to this study, some key regions underlying decision-making—vmPFC and amygdala—are known to play a central role in emotion processing (Hommer et al., 2003; Lindquist et al., 2012; Herman et al., 2018; Kelley et al., 2018) and are extensively connected (Haber and Knutson, 2010; Schardt et al., 2010; Patin and Hurlemann, 2011; Plichta and Scheres, 2014). While the vmPFC appears to respond to both negative and positive stimuli (Winecoff et al., 2013; Yang et al., 2020), the amygdala is traditionally known from animal and human lesion studies as the hub for processing negative emotions (LeDoux, 1998; Adolphs et al., 2005). Human neuroimaging studies also support the view for a central role of the amygdala in processing negative emotions (Davis and Whalen, 2001), although amygdala activation during positive emotion processing has been reported as well (Garavan et al., 2001; Hamann and Mao, 2002). Because of their mutual connections, it is not surprising that contextual information such as emotion shifts choices on the delay discounting task toward being more patient or impulsive (Lempert and Phelps, 2016).
The majority of studies show that short (1.5 seconds, Guan et al., 2015) or long (15 seconds, Augustine and Larsen, 2011) exposure to negative emotional pictures increases the propensity to choose smaller-sooner over larger-later rewards, whereas exposure to positive pictures shifts decisions toward choosing larger-later rewards (Guan et al., 2015; Cai et al., 2019). Similar findings were also reported in studies using emotional episodic future thinking as the emotional cue (Liu et al., 2013; Lin and Epstein, 2014; Zhang et al., 2018). Some studies find opposite findings, with effects specific to particular conditions, namely reports of increased delay discounting following positive emotion in extraverted individuals (Hirsh et al., 2010) and decreased delay discounting following fearful faces (Luo et al., 2014). Arousing pictures, regardless of emotion, also tend to increase delay discounting (Wilson and Daly, 2004; Sohn et al., 2015).
This study aimed to identify the relations between decision-making and emotion processing and their biological mechanisms, using a lesion model. Inclusion of patients with behavioral-variant frontotemporal dementia (bvFTD) and Alzheimer’s disease (AD), presenting with atrophy in the key brain regions of the reward and emotion network (vmPFC, limbic lobe) will clarify the role of emotion on delay discounting and the contribution of each brain region in delay discounting. bvFTD is a neurodegenerative condition characterized by marked changes to personality and interpersonal conduct (Piguet et al., 2017) as evidenced by their increase in ‘impulsive, rash or careless actions’ (Rascovsky et al., 2011). Patients with bvFTD also show disruption in emotional processing (Lavenu et al., 1999; Keane et al., 2002; Fernandez-Duque and Black, 2005; Kipps et al., 2009; Kumfor et al., 2013a, 2014a). Atrophy is typically reported in emotion-specific brain regions namely in vmPFC and insula (Seeley et al., 2008), which extends into subcortical regions with disease progression (Landin-Romero et al., 2017). Given their behavioral deficits—decision-making and emotion processing—and atrophy of the vmPFC, we would anticipate a correlation between reduced grey matter intensity in the vmPFC and increased delay discouting, regardless of emotion.
The predominant clinical feature of Alzheimer’s disease in contrast is an impairment in episodic memory (McKhann et al., 2011), mainly attributed to atrophy of structures of the medio-temporal limbic system such as hippocampus and amygdala (Scheltens et al., 1992; McKhann et al., 2011; Poulin et al., 2011) and progressing to parietal, posterior cingulate and frontal cortices with disease (Nestor et al., 2003; Dickerson et al., 2009; Landin-Romero et al., 2017). Early in the disease process, interpersonal behavior and emotion processing are relatively preserved in AD, although some facets of emotion processing and behavior are impaired (Cummings, 1997; Hoefer et al., 2008) and worsen with disease progression (Bidzan et al., 2012; Kumfor et al., 2014b; Bertoux et al., 2015a). AD patients, although overall capable of recognizing emotions, can be severely impaired in retrieving emotions relevant to autobiographical memories for example (Irish et al., 2011; Kumfor et al., 2013a). While emotion processing deficit is considered a core feature of bvFTD (Rascovsky et al., 2011), emotion processing—to some extent—remains comparatively preserved in AD (Lavenu et al., 1999). Despite relatively preserved decision-making and emotion processing compared to bvFTD, we would anticipate emotion to interact with delay discounting performance in AD. We would also expect reduced amygdalar grey matter integrity to increase delay discounting and weaken the interactions between emotions and decision-making.
Few studies have investigated delay discounting in bvFTD and AD. Increased delay discounting has been reported in bvFTD compared to AD (Lebreton et al., 2013; Bertoux et al., 2015b) and in healthy controls (Beagle et al., 2020), while Chiong et al. (2016) reported similar performance between bvFTD, AD and controls. AD patients show a trend for increased delay discounting compared to healthy controls (Lebreton et al., 2013; Bertoux et al., 2015b; Beagle et al., 2020). Brain-behavior associations with delay discounting performance in bvFTD and AD are less clear as most studies only included behavioral data (Bertoux et al., 2015b), only reported patterns of brain atrophy (Lebreton et al., 2013) or investigated brain-behavior correlations across etiologies (Lansdall et al., 2017; Beagle et al., 2020). The only study investigating brain-behavior correlations in bvFTD and AD (Chiong et al., 2016) failed to find significant correlations between brain atrophy and delay discounting probably because of the lack of between-group behavioral differences. Only one study investigated or reported brain-behavior correlations in bvFTD and AD in decision-making tasks other than the delay discounting task (Kloeters et al., 2013). Using the Iowa Gambling Task, this study found that decision-making deficits were attributed to frontal atrophy in bvFTD and to temporal/parietal atrophy in AD.
To identify the influence of emotion on delay discounting, we presented individuals diagnosed with bvFTD or AD, and healthy controls, emotional or neutral pictures before each choice on a delay discounting task. Given their divergent patterns of brain atrophy and clinical features, we predicted that bvFTD would exhibit greater impulsivity overall compared with the other two groups, and that AD would be more impulsive than controls. In addition, we hypothesized that due to their deficits in emotion processing, bvFTD would not show any emotion-induced modulation of delay discounting. In contrast, we expected AD to show a similar emotion-induced modulation of delay discounting than controls, namely increased delay discounting, that is impulsivity, for negative emotions and decreased delay discounting for positive emotions. At the anatomical level, we expected the decision-making deficits to relate to distinct neural structures (Kloeters et al., 2013). Based on lesion studies (Sellitto et al., 2011; Peters and D'Esposito, 2016), we predicted that increased delay discounting in the bvFTD group would correlate with decreased grey matter intensity in the vmPFC, regardless of emotional valence. In the AD group, given the limited vmPFC atrophy, we anticipated that atrophy of the amygdala and other limbic structures would be related to increased delay discounting as demonstrated in animal studies (Winstanley et al., 2004; Floresco and Ghods-Sharifi, 2007; Ghods-Sharifi et al., 2009). In addition, because of its central role in processing negative emotion, we also hypothesized that reduced grey matter intensity in the amygdala in AD would counteract the expected increased delay discounting in the negative condition.
Methods
Participants
Twenty-two patients diagnosed with bvFTD, 15 patients with AD and 15 education- and age-matched healthy controls were recruited from FRONTIER, the frontotemporal dementia research clinic in Sydney, Australia. Calculation of sample size was based on an a priori power analysis using G*Power (Faul et al., 2007). For an alpha level of 0.05, an anticipated effect size of 0.06 (medium) and a power of 0.80, the estimated total sample is 36 participants (12 in each group). All patients underwent a comprehensive neurological examination, a neuropsychological assessment, and a structural brain MRI. Diagnosis was established according to relevant clinical diagnostic criteria at the time of testing for probable or possible bvFTD (Rascovsky et al., 2011) and AD (McKhann et al., 2011). Diagnosis was established by multidisciplinary agreement based on cognitive, clinical and imaging data. Exclusion criteria for patients and controls included: presence of a primary psychiatric disorder, presence of other dementia or neurological disorders, and/or history of alcohol or substance abuse. All healthy controls underwent the comprehensive neuropsychological assessment and the brain MRI and were required to score >88/100 on the ACE-III to ensure they did not have any significant cognitive impairments. All participants or their Person Responsible provided informed consent in accordance with the Declaration of Helsinki. The South Eastern Sydney Local Health District and the University of New South Wales ethics committees approved the study.
Neuropsychological assessment
The ACE-III was used to assess general cognition (Hsieh et al., 2013; So et al., 2018). Disease severity was assessed with the Frontotemporal Lobar Degeneration-Modified Clinical Dementia Rating Scale Sums of Boxes (CDR-FTLD SoB) (Knopman et al., 2008), and disease duration was measured in years from the first onset of symptoms.
Delay discounting task
The ability to delay gratification was assessed with the Monetary Choice Questionnaire (MCQ, Kirby et al., 1999). The MCQ comprises 27 dichotomous choices asking participants to choose between a smaller, immediate monetary reward or a larger, delayed monetary reward (e.g. ‘Would you prefer $15 today or $35 in 13 days?’). Estimates of delay discounting were calculated for all reward magnitudes as well as for each different reward magnitude, categorized as low- ($25–35), medium- ($50–60) and high-magnitude ($75–85) trials. Indifference points were calculated with the classically used hyperbolic discounting equation: V=A/(1+kD) (Mazur, 1987) where V represents the present value of the delayed reward A at delay D, and k is a free parameter that determines the discount rate. Larger values for k indicate a preference for smaller immediate reward. Because of skewness, k values were log-transformed (logk) (Gray et al., 2016). Although the monetary rewards were hypothetical, real and hypothetical rewards lead to similar patterns of discounting (Johnson and Bickel, 2002; Madden et al., 2003). Prior to each choice, an emotional picture (Positive, POS; Negative, NEG; or Neutral, NEU) was presented for 5 seconds and participants were instructed to vividly imagine that they were witnessing the event/content depicted in it (Figure 1).
Fig. 1.
Experimental design. The delay discounting task consisted of three blocks containing either positive (POS), negative (NEG) or neutral (NEU) pictures and presented in randomized order. Participants were first instructed to vividly imagine witnessing the picture and then asked to make a choice on the delay discounting task.
To control that they understood the task correctly, participants completed a training session consisting of three trials, during which they were asked on one random trial to indicate (i) which choice would pay sooner and (ii) which choice would pay greater. Only participants completing the training session and answering correctly the control questions were retained for the analyses.
Participants completed three blocks (POS, NEG or NEU) of the delay discounting task in a randomized order. Each trial began with a fixation cross presented on a 21.5 inch monitor for 500 ms, a picture displayed for 5000 ms and a screen containing both choices displayed until participants responded. An inter-stimulus interval (ISI) of 1000–2000 ms preceded the following trial. Participants indicated their choices by pressing the left or right arrow of a keyboard, according to the choice displayed on the left or the right of the screen. Each block lasted approximately 5 min. The three blocks were separated by a 5-minute break during which participants completed various questionnaires. Stimulus delivery and subjects' responses for both tasks were controlled using E-prime 2.0 software (Psychology Software Tools, Pennsylvania, USA).
The pictures were realistic, high-quality photographs chosen from the Nencki Affective Picture System (NAPS, Marchewka et al., 2014). Pictures were selected on the basis of their original valence rating (1 = very negative, 5 = neutral, 9 = very positive) and ultimately designated as (mean ± standard deviation) positive (7.9 ± 0.2), negative (2.5 ± 0.3) or neutral (5.1 ± 0.2; F(2,80) = 2628.93, P < 0.01). Arousal ratings also differed between positive (4.1 ± 0.1), negative (6.7 ± 0.5) and neutral pictures (4.8 ± 0.4; F(2,80) = 105.97, P < 0.01). Stimuli were matched with respect to their luminance (F(2,80) = 0.63, P = 0.53), contrast (F(2,80) = 2.01, P = 0.14) and entropy (F(2,80) = 2.02, P = 0.14).
Questionnaires
Between each delay discounting block, participants completed the present and future sections of the Zimbardo Time Perspective Inventory, which comprises 37 items ranging from 1 (very untrue) to 5 (very true) and grouped into present-hedonistic, present-fatalistic and future dimensions (Zimbardo and Boyd, 2015).
At the end of the experimental session, participants rated valence and arousal for a subset of pictures (n = 15) of each emotion category using the Self-Assessment Manikin (Lang et al., 1997) and a scale from 1 to 9 (valence: 1 = very negative to 9 = very positive; arousal: 1 = relaxed to 9 = aroused). The picture remained on the screen until the response was recorded.
Statistical analyses
Data were analysed using IBM SPSS Statistics, 24.0 (SPSS Inc., Chicago, Ill., USA). Normally distributed variables, as determined with Shapiro–Wilks tests, were compared across groups using mixed or one-way ANOVAs followed by Sidak post hoc tests. Variables not normally distributed across our sample were analysed by Kruskal–Wallis ANOVA followed by Mann–Whitney U tests. Categorical measures (e.g. sex) were analysed by Chi-square tests. Effect sizes are reported using the partial eta-square (η2).
We investigated delay discounting (logk) with a 3 × 3 mixed ANOVA with within factor of Emotion (POS, NEG or NEU) and between factor of Group (bvFTD, AD and CTRL). Significant interactions were followed by simple effects at each combination of levels of the other factors and followed by Sidak post hoc tests. Additionnally, we investigated effects of Emotion for each reward magnitude separately using the same statistical analysis.
Correlations between the significant delay discounting conditions (Pos, Neg and Neu) in bvFTD and AD and respective valence/arousal ratings (Pos, Neg and Neu) were analysed using Spearman rank coefficient. Only correlations surviving Bonferroni correction for multiple comparisons were kept.
Neuroimaging analyses
MRI acquisition
Participants underwent whole-brain structural MRI on a GE Discovery MR750 3T scanner equipped with an 8-channel head coil. High resolution 3D BRAVO T1-weighted images were acquired using the following parameters: imaging matrix of 256 × 256 × 200, 1 mm isotropic voxel resolution, echo time = 2.5 ms, repetition time = 6.7 ms, inversion time = 900 ms, flip angle = 8°.
Data pre-processing
Voxel-based morphometry (VBM) was conducted using SPM12 (Welcome Department of Cognitive Neurology, London, UK), in Matlab R2018a (Mathworks, Natick, Massachusetts, USA). First, T1-weighted images were segmented into six tissue probability maps in the native space. Both the original T1-weighted and the segmented maps were screened during an image quality control. Two participants (1 bvFTD and 1 AD) were removed for the subsequent pre-processing steps and statistical analyses due to motion during the acquisition or segmentation failure. A DARTEL template was computed using all the grey and white matter probability maps which satisfied our criteria for quality control. Last, grey matter probability maps were spatially normalized to the Montreal National Institute (MNI) space according to the transformation parameters from the corresponding DARTEL template. Images were modulated and smoothed with a Gaussian filter of full width at half maximum of 8 mm.
VBM analyses
Patterns of grey matter intensity decrease were explored using a whole-brain general linear model comprising bvFTD, AD and CTRL groups as well as age and total intracranial volume (to account for individual differences in head size) as regressors of non-interest. The total intracranial volume was assessed in the patient’s space prior to spatial normalization by summing thresholded grey matter, white matter and corticospinal fluid probability maps (threshold = 0.2) and counting non-zero voxels. Differences in grey matter intensities between groups (bvFTD vs control; AD vs control) were assessed using t-tests.
Next, correlations between delay discounting and grey matter intensity were investigated. Scores for each delay discounting condition (POS, NEG or NEU) were entered simultaneously into the design matrix. Age and total intracranial volume were included as regressors of non-interest. Correlations were first investigated between delay discounting and grey matter intensity combining all participants (bvFTD, AD and CTRL). Then, the same analyses described above were conducted to investigate correlations in each patient group combined with controls in order to identify the neural correlates of delay discounting distinct to each patient group. Inclusion of controls has been shown to increase statistical power to detect brain–behavior relationships across the entire brain (e.g. Kumfor et al., 2013b).
Voxel-wise statistical analyses are reported using a cluster size of at least 50 voxels, at statistical threshold of P < 0.001, uncorrected for multiple comparison. This approach minimizes Type I error while balancing the risk of Type II error (Lieberman and Cunningham, 2009). Significant results were overlaid on the Montreal Neurological Institute (MNI) standard brain using MRIcron (https://www.nitrc.org/projects/mricron).
Results
Demographic and neuropsychological profiles
Twenty-two individuals diagnosed with bvFTD, 15 with Alzheimer’s disease and 15 older healthy controls were recruited for this study. Seven participants (4 bvFTD and 3 AD), however, failed the delay discounting task training session, and their data were therefore removed from the analyses. As such, the final samples included 18 bvFTD, 12 AD and 15 CTRL participants. As reported in Table 1, groups were well matched on age (P = 0.636). Although groups are statistically matched on sex (P = 0.080) and education level (P = 0.066), the bvFTD group is marginally composed of more men of lower education than the control or AD groups. Patient groups did not differ on disease duration (P = 0.261) or disease severity (CDR-FTLD Sob, P = 0.989) either. AD patients were however significantly more impaired on general cognition than bvFTD (ACE-III, P < 0.001). Both patient groups had significantly greater disease severity (P < 0.001) and impaired general cognition (P < 0.001) than controls. Excluded participants tended to be more impaired on the ACE-III than their respective samples (bvFTD included: 82.0 ± 9.9, bvFTD excluded: 73.2 ± 14.6, P = 0.15; AD included: 65.9 ± 12.6, AD excluded: 53.6 ± 6.8, P = 0.06).
Table 1.
Demographic and clinical information
| bvFTD (n = 18) | AD (n = 12) | CTRL (n = 15) | P | Post hoc | |
|---|---|---|---|---|---|
| Sex (M:F) | 13:5 | 7:5 | 5:10 | 0.080* | |
| Age | 63.4 ± 9.8 | 65.5 ± 7.3 | 65.7 ± 4.7 | 0.636 | |
| Education (yrs) | 11.7 ± 3.0 | 13.6 ± 3.6 | 13.9 ± 2.7 | 0.066# | |
| Disease duration (yrs) | 8.3 ± 5.9 | 5.1 ± 3.1 | − | 0.261† | |
| ACE-III (/100) | 82.0 ± 9.9 | 65.9 ± 12.6 | 94.8 ± 3.0 | < 0.001 | Patients < Controls; AD < bvFTD |
| CDR-FTLD Sob | 5.3 ± 4.6 | 5.0 ± 3.5 | 0.2 ± 0.2 | < 0.001 | Patients < Controls |
Demographic and clinical information for behavioral-variant frontotemporal dementia (bvFTD), Alzheimer’s disease (AD) and controls (CTRL). Values are mean ± standard deviation. *χ2 test, †Mann–Whitney, #Kruskal–Wallis test. ACE-III = Addenbrooke’s Cognitive Examination—Third edition; CDR-FTLD SoB = Frontotemporal Lobar Degeneration-Modified Clinical Dementia Rating Scale Sums of Boxes (CDR-FTLD SoB). Missing Scores: Education (2 AD); Disease duration (4 AD); ACE-III (1 AD), CDR-FTLD SoB (1 AD, 3 CTRL).
Delay discounting results
Performance on the delay discounting task is illustrated in Figure 2A (and Supplementary Figure 2, which displays individual delay discounting rate for each condition). The ANOVA on delay discounting rate (logk) revealed a significant main effect of Emotion (F(2,84) = 6.316, P = 0.003, ηp2 = 0.131), Group (F(2,42) = 7.504, P = 0.002, ηp2 = 0.263) and an Emotion x Group interaction (F(4,84) = 3.669, P = 0.008, ηp2 = 0.149). Simple effects tests for each level of factor Group showed a main effect of Emotion for AD (F(2,22) = 5.717, P = 0.010, ηp2 = 0.342) but not for bvFTD (F(2,34) = 0.306, P = 0.739, ηp2 = 0.018) or CTRL (F(2,28) = 2.116, P = 0.139, ηp2 = 0.131). AD showed increased delay discounting (i.e. impulsivity) in the NEG compared to POS (P = 0.003) and NEU conditions (P = 0.036). Simple effects tests for each level of factor Emotion showed main effects of Group for all 3 emotions (all P < 0.01). Patients with bvFTD patients were significantly more impulsive than CTRL on all emotion conditions (all P < 0.002) and significantly more impulsive than AD on POS (P = 0.007) and NEU conditions (P = 0.033). AD patients were significantly more impulsive than CTRL on the NEG condition only (P = 0.024).
Figure 2.
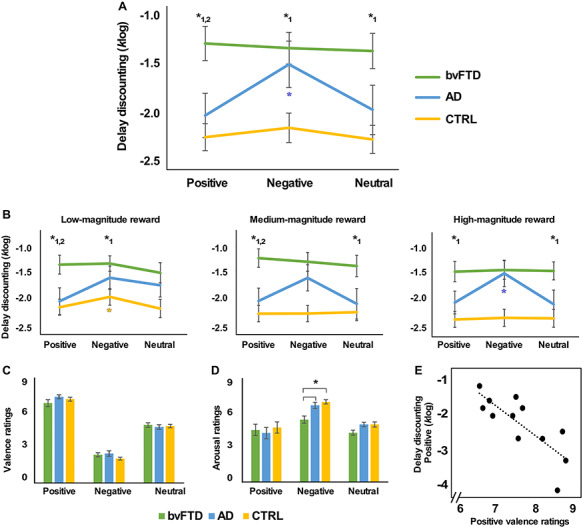
Behavioral results and correlations. A. Average delay discounting rates (k, log transformed) for each Emotion condition (Positive, Negative and Neutral) and Group (behavioral-variant frontotemporal dementia, bvFTD; Alzheimer’s disease, AD; controls, CTRL). B. Delay-discounting rate for low-, medium- and high-magnitude rewards. C, D. Judgement of valence and arousal for each Emotion condition and Group. E. Correlation between delay discounting in the Positive condition and positive valence ratings in the AD group. Graph lines and bars show means and standard error of the mean. * indicates significant post hoc differences (P < 0.05, Sidak corrected for multiple comparisons) for bvFTD < AD, CTRL (*1) and bvFTD = AD < CTRL (*2). Colored * indicates effects of Emotion in each Group.
Figure 2B displays delay discounting rates for each reward magnitude. For low-magnitude rewards, significant main effects of Emotion (F(2,84) = 3.389, P = 0.038, ηp2 = 0.075) and Group (F(2,42) = 5.133, P = 0.010, ηp2 = 0.196) were present but no Emotion x Group interaction (F(4,84) = 1.358, P = 0.256, ηp2 = 0.061). In other words, participants were more impulsive in the NEG than POS (P = 0.011) or NEU (P = 0.030) conditions and the bvFTD group was more impulsive than the CTRL group (P = 0.003). Focused analyses on the control group showed that the CTRL were more impulsive in the NEG compared to POS condition (P = 0.010).
For medium-magnitude rewards, a significant main effect of Group was observed (F(2,42) = 8.359, P = 0.001, ηp2 = 0.285) but not of Emotion (F(2,84) = 2.232, P = 0.114, ηp2 = 0.050) or Emotion x Reward interaction (F(4,84) = 2.118, P = 0.086, ηp2 = 0.092). The bvFTD group was more impulsive than AD (p = 0.022) and CTRL (P < 0.001).
For high-magnitude rewards, significant main effects of Emotion (F(2,84) = 5.142, P = 0.008, ηp2 = 0.109), Group (F(2,42) = 7.061, P = 0.002, ηp2 = 0.252), as well as an Emotion x Group interaction (F(4,84) = 3.694, P = 0.008, ηp2 = 0.150) were present. AD patients were more impulsive in the NEG compared to POS (P < 0.001) and NEU (P = 0.014) conditions. The bvFTD group was more impulsive than CTRL in all conditions (P < 0.05) and than AD in POS (P = 0.036) and NEU (P = 0.031) conditions.
Regarding difference between reward magnitudes, main effects were present in the CTRL (F(2,28) = 6.228, P = 0.006, ηp2 = 0.308) but not in bvFTD (F(2,34) = 1.589, P = 0.219, ηp2 = 0.085) or AD groups (F(2,22) = 0.332, P = 0.721, ηp2 = 0.029). CTRL were more impulsive on low-magnitude trials than medium- (P = 0.011) or high-magnitude trials (P = 0.016).
Questionnaires
Regarding valence ratings, a significant main effect of Emotion (F(2,84) = 419.367, P < 0.001, ηp2 = 0.909) was observed but not of Group (F(2,42) = 0.598, P = 0.555, ηp2 = 0.028) or an Emotion x Group interaction (F(4,84) = 1.324, P = 0.268, ηp2 = 0.059). Valence ratings differed significantly between positive, negative and neutral pictures for all groups (P < 0.001; Figure 2C and D).
Regarding arousal ratings, significant main effects of Emotion (F(2,84) = 26.278, P < 0.001, ηp2 = 0.385) and Group (F(2,42) = 3.892, P = 0.028, ηp2 = 0.156) were observed but no interaction (F(4,84) = 1.753, P = 0.146, ηp2 = 0.077). Negative pictures were judged as more arousing compared to positive and neutral pictures (all P < 0.001) and patients with bvFTD judged pictures as less arousing/more relaxing than CTRL (P = 0.028).
On the Zimbardo Time Perspective Inventory, no significant between-group differences were found (all P values > 0.192) (Present hedonistic: bvFTD = 3.4 ± 0.3, AD = 3.2 ± 0.6, CTRL = 3.5 ± 0.3; Present fatalistic: bvFTD = 2.7 ± 0.7, AD = 2.9 ± 0.5, CTRL = 2.4 ± 0.5; Future: bvFTD = 3.4 ± 0.4, AD = 3.7 ± 0.2, CTRL = 3.5 ± 0.4).
Correlations
Correlations between delay discounting and judgement of valence and arousal were apparent only in AD, where decreased delay discounting in the positive condition correlated with increased judgement of positive valence (r(10) = −0.802, P = 0.002; Figure 2E).
Neuroimaging results
Patterns of atrophy
Patterns of atrophy in the clinical groups were typical of these diseases (Nestor et al., 2003; Seeley et al., 2008; Landin-Romero et al., 2017) (Supplementary Figure 1; Supplementary Table 1). Compared with CTRL, bvFTD showed decreased grey matter intensity in the medial prefrontal cortex, frontal and temporal gyri, ACC, as well as subcortical regions including the hippocampus and striatum. In contrast, AD showed a significant bilateral decrease of grey matter intensity in the medial temporal lobe, including the hippocampus and amygdala, as well as in the precuneus and the insula.
Neural correlates of delay discounting
Correlations between POS, NEG and NEU delay discounting and grey matter intensity revealed that, irrespective of diagnosis, increased delay discounting in the NEG condition was associated with reduced grey matter integrity in the amygdala (P < 0.001, cluster FWE-corrected) and occipital gyrus bilaterally (P < 0.001, uncorrected; Figure 3; Table 2). In contrast, no specific patterns of association emerged for the positive and neutral conditions.
Fig. 3.
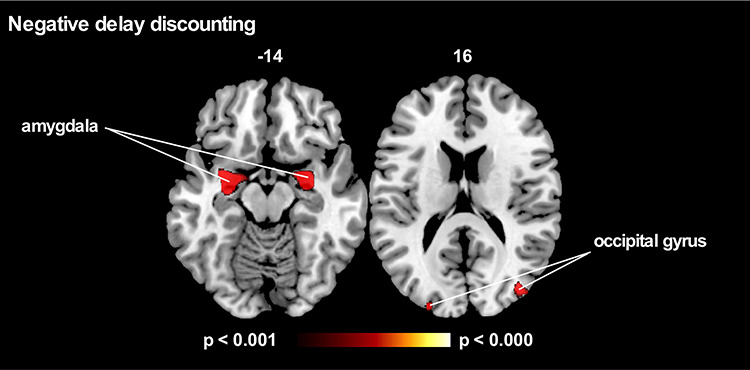
VBM analyses showing regions negatively correlated with delay discounting in the Negative condition irrespective of diagnosis. No clusters survived in the Positive or Neutral conditions (P < 0.001 uncorrected for multiple comparisons). Age and total intracranial volume included as a covariate in all VBM analyses. Clusters are overlaid on the standard MNI brain. The left side of the image is the left side of the brain.
Table 2.
Clusters associated with greater delay discounting in the Positive, Negative and Neutral conditions across all three groups (bvFTD, AD and CTRL).
| Regions | Laterality | MNI | Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Delay discounting: positive condition | |||||
| No significant cluster identified | |||||
| Delayed discounting: negative condition | |||||
| Parahippocampal gyrus, amygdala and hippocampus | L | −31 | −3 | −17 | 3495 |
| Parahippocampal gyrus, amygdala and hippocampus | R | 31 | −2 | −17 | 2639 |
| Middle occipital gyrus | R | 38 | −80 | 16 | 833 |
| Middle occipital gyrus | L | −23 | −93 | 21 | 462 |
| Superior occipital cortex | R | 21 | −94 | 23 | 448 |
| Precuneus | R | 40 | −78 | 38 | 276 |
| Middle frontal gyrus | L | −28 | 40 | 40 | 198 |
| Superior temporal lobe | L | −63 | −9 | 3 | 191 |
| Inferior frontal gyrus | R | 51 | 20 | 20 | 141 |
| Insula | L | −41 | −7 | −6 | 117 |
| Delayed discounting: neutral condition | |||||
| No significant cluster identified | |||||
Further analyses on each patient group combined with controls showed distinct patterns of grey matter intensity in bvFTD and AD correlating with POS, NEG or NEU delay discounting (Figure 4; Table 3). Increased delay discounting in the NEG condition in AD was associated with reduced grey matter intensity in bilateral amygdala, vmPFC, ACC and hippocampus. No such associations were observed in the bvFTD group. Marginal frontal and temporal areas were associated with positive and neutral delay discounting, respectively, in AD and bvFTD.
Fig. 4.
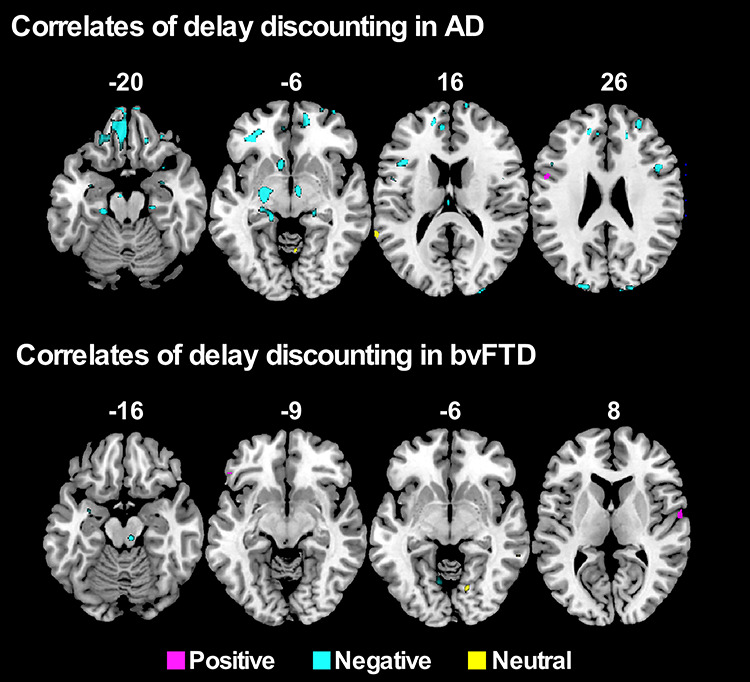
VBM analyses showing regions negatively correlated with delay discounting in AD and bvFTD in the Positive, Negative and Neutral conditions (P < 0.001 uncorrected for multiple comparisons). Age and total intracranial volume included as a covariate in all VBM analyses. Clusters are overlaid on the standard MNI brain. The left side of the image is the left side of the brain.
Table 3.
Clusters associated with greater delay discounting in the Positive, Negative and Neutral conditions for AD and bvFTD groups separately.
| Regions | Laterality | MNI | Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| AD | |||||
| Positive delay discounting | |||||
| Inferior frontal gyrus | L | −53 | 4 | 23 | 382 |
| Negative delay discounting | |||||
| Orbitofrontal frontal cortex | L/R | −35 | 37 | −6 | 7861 |
| ACC | L | −6 | 51 | 6 | 2035 |
| Parahippocampal gyrus and hippocampus | L | −28 | −24 | −32 | 1809 |
| Parahippocampal gyrus and hippocampus | R | 25 | −22 | −29 | 1443 |
| Amygdala | L | −24 | −14 | −7 | 1225 |
| Middle and superior frontal gyrus | R | 26 | 21 | 53 | 1074 |
| Middle frontal gyrus | R | 27 | 55 | 23 | 942 |
| Inferior, middle and superior occipital gyrus | L | −20 | −93 | 21 | 746 |
| Supramarginal gyrus | L | −44 | −39 | 32 | 803 |
| Superior occipital cortex | R | 21 | −95 | 23 | 657 |
| Inferior frontal gyrus | L | −40 | 17 | 16 | 463 |
| Middle and superior frontal gyrus | L | −14 | 3 | 58 | 396 |
| Inferior frontal gyrus | R | 47 | 13 | 25 | 379 |
| Amygdala | R | 31 | −1 | −20 | 326 |
| Inferior temporal gyrus | R | 48 | −18 | −39 | 261 |
| Neutral delay discounting | |||||
| Superior temporal gyrus | L | −66 | −47 | 17 | 75 |
| bvFTD | |||||
| Positive delay discounting | |||||
| Superior temporal gyrus | R | 62 | −6 | 8 | 135 |
| Inferior frontal gyrus | L | −48 | 29 | −13 | 87 |
| Negative delay discounting | |||||
| Lingual gyrus | L | −9 | −64 | −4 | 247 |
| Inferior parietal lobule | L | −61 | −41 | 43 | 148 |
| Supramarginal gyrus | L | −59 | −55 | 36 | 103 |
| Neutral delay discounting | |||||
| Rolandic operculum | R | 15 | −70 | −5 | 186 |
Discussion
This study revealed different patterns of modulation of emotion on decision-making in the two most common younger-onset dementia syndromes, AD and bvFTD, which were associated with specific neural changes. Supporting our hypotheses, bvFTD patients showed greater delay discounting compared to AD and controls, but no modulation according to emotion. In contrast, AD patients showed increased delay discounting in the negative condition, which was associated with greater bilateral amygdala atrophy. No specific pattern of brain atrophy was observed in bvFTD.
The increased impulsivity observed in bvFTD aligns with previous studies reporting impulsive decision-making in this population (Strenziok et al., 2011; Gleichgerrcht et al., 2012; Bertoux et al., 2013, 2015b; Kloeters et al., 2013; Lebreton et al., 2013; Lansdall et al., 2017; Beagle et al., 2020). One recent report, however, failed to show any deficits on the delay discounting task in bvFTD compared with AD and controls (Chiong et al., 2016). The authors argued that this was due to the very early disease stage of their patients. Our findings challenge this interpretation as we find evidence of decision-making deficits on the delay discounting task in patients with a similar disease severity (mean MMSE = 26, converted from ACE-III score, Matias-Guiu et al., 2018).
As anticipated, compared to AD, bvFTD patients failed to show the negative emotion-induced modulation of delay discounting, a finding compatible with a primary deficit in emotion processing in bvFTD. Patients with bvFTD indeed show deficits in recognizing negative emotions (Lough et al., 2006; Goodkind et al., 2015) and emotional expression in faces and voices (Keane et al., 2002; Lavenu and Pasquier, 2005) and emotional blunting (Mendez et al., 2006). Grossmann et al. (2010) showed that bvFTD patients were also less sensitive to negative contextual features when making social decisions: negatively biased scenarios were judged as less negative than controls in bvFTD, whereas positively biased scenarios were rated equally in bvFTD and controls. Alternatively, these findings could be due a failure in decoding the physiological arousal signals in response to negative emotional stimuli. Indeed, previous studies have reported reduced physiological responses (e.g. skin conductance) in response to emotional videos (Kumfor et al., 2019), unpleasant odours (Perry et al., 2017) or pain (Fletcher et al., 2015). bvFTD indeed judged pictures as less arousing than AD and controls, whereas valence ratings were similar across groups. This indicates that the emotional impairment in bvFTD results from a reduced arousal triggered by the pictures rather than a primary deficit in recognizing their emotional content. The specificity of the effect to the negative condition in AD could follow from an effect of arousal on delay discounting rather than an effect of negative emotion per se. Studies have indeed shown that arousing pictures, regardless of emotion, increased delay discounting compared to neutral pictures (Ariely and Loewenstein, 2006; Sohn et al., 2015). Future studies using objectives measures of arousal (e.g. skin conductance) are needed to clarify this point.
Across groups, increased delay discounting for the negative (but not the positive or neutral) condition was associated with reduced grey matter intregrity in bilateral amygdala and occipital gyri. Group-specific analyses indicated that this association was mediated primarily by the AD group which showed reduced grey matter integrity in bilateral amygdala, vmPFC and parahippocampal gyri that correlated with increased delay discounting in the negative condition. These findings indicate that the amygdala is involved in delay discounting, especially within an emotionally negative context. Our findings demonstrate for the first time in humans that amygdala damage increases delay discounting, mirroring animal studies where excitotoxic lesions of the BLA increased delay discounting (Winstanley et al., 2004; Floresco and Ghods-Sharifi, 2007; Ghods-Sharifi et al., 2009). Impact of amygdalar damage on various decision-making tasks has been reported before (Bechara et al., 1999; Bar-On et al., 2003; Hanten et al., 2006; Brand et al., 2007; Weller et al., 2007; De Martino et al., 2010), but never on delay discounting to date.
The direction of the correlation between amygdala integrity and delay discounting was not anticipated given the known role of the amygdala in processing negative emotion. This finding adds to the structural neuroimaging controversy in the field of delay discounting as to whether delay discounting is correlated with increased or decreased grey matter intensity (Cho et al., 2013; Tschernegg et al., 2015; Pehlivanova et al., 2018). Importantly, although central to negative emotion processing, the amygdala is not the only brain region supporting negative emotion processing. Indeed, lesion studies have shown that the amygdala is necessary but not sufficient to process negative emotions as, apart from fear, amygdala damage does not preclude from triggering and feeling other negative emotions (Anderson and Phelps, 2002; Feinstein et al., 2011). One candidate region is the vmPFC, which regulates emotion through top-down inhibition of the amygdala (Andrewes and Jenkins, 2019). Deficient inhibitory control of the vmPFC on the amygdala has been shown to lead to hyper-emotional reactivity and pathologically elevated levels of negative affect (Quirk and Gehlert, 2003; Milad et al., 2006; Rauch et al., 2006; Motzkin et al., 2015). In situations where the affective/emotional signals are absent (i.e. delay discounting with no emotional component or neutral emotion), the amygdala would be less involved, possibly favoring the vmPFC recruitment (Sellitto et al., 2011; Peters and D'Esposito, 2016). This interpretation is consistent with our lack of amygdala involvement in the neutral delay discounting condition. Our study suggests that the amygdala is involved in delay discounting rather than purely in processing emotions, in line with animal studies (Winstanley et al., 2004; Floresco and Ghods-Sharifi, 2007; Ghods-Sharifi et al., 2009).
The association that we found between increased delay discounting in the negative condition and reduced grey matter intensity in the occipital cortex further demonstrates the involvement of broad network during emotion processing and delay discounting task. fMRI and lesion studies have shown that emotional stimuli, particularly arousing, negative, stimuli recruit not only the amygdala but also the visual cortices (Vuilleumier et al., 2004; Sabatinelli et al., 2009; Motzkin et al., 2015). Similarly, involvement of the occipital cortex on delay discounting tasks has been attributed to visual attention (Luo et al., 2009) or vividness of imagined event in episodic delay discounting tasks (Hu et al., 2016) (Luo et al., 2009; Olson et al., 2009).
Some limitations should be acknowledged. Our sample of bvFTD patients was heterogenous in terms of disease severity, disease duration and atrophy pattern compared to AD, which may have precluded other correlations with other brain regions (e.g. vmPFC) to emerge in this group. It should be noted, however, that the absence of correlation in the bvFTD group alone does not indicate that both dementia groups statistically differed. Future studies using larger and more homogeneous groups are needed to resolve these concerns. Importantly, whereas the role of the vmPFC in delay discounting has been clearly demonstrated from lesion studies (Sellitto et al., 2011; Peters and D'Esposito, 2016) and brain stimulation studies (Manuel et al., 2019), evidence from bvFTD is less convincing (Chiong et al., 2016) even in very impaired and homogeneous bvFTD samples. Nevertheless, this limitation does not detract from our main message demonstrating the role of the amygdala in emotional delay discounting.
The absence of the predicted pattern of increased delay discounting in the negative condition in healthy controls when all reward magnitudes were grouped was unexpected. It is likely that this lack of emotion-induced modulation of delay discounting follows from overall reduced variability and impulsivity in our healthy control group, which prevented emotion-related modulations to clearly emerge. Emotion-induced modulation of delay discounting may thus be apparent only under high impulsivity conditions. Supporting this interpretation, our findings show that older controls did exhibit the negative emotion-induced increase in delay discounting but only under the condition of highest impulsivity (i.e. low-magnitude trials). Effects of emotion on delay discounting have been typically reported in young healthy adults (Hirsh et al., 2010; Augustine and Larsen, 2011; Benoit et al., 2011; Liu et al., 2013; Lin and Epstein, 2014; Luo et al., 2014; Guan et al., 2015; Sohn et al., 2015; Zhang et al., 2018). Findings on age-related differences on delay discounting have been mixed. Several studies have reported young individuals to be more impulsive on delay discounting tasks compared to older adults (Green et al., 1999; Whelan and McHugh, 2009; Jimura et al., 2011; Lockenhoff et al., 2011; Eppinger et al., 2012). Other studies, however, have shown no age-related differences (Samanez-Larkin et al., 2011; Roalf et al., 2012; Rieger and Mata, 2015; Seaman et al., 2016) or even increased delay discounting with age (Read and Read, 2004). In sum, although reduced compared to what we could have expected in young individuals, the control group did show emotion-induced modulation of delay discounting. The bvFTD group in contrast showed no emotion-induced modulation of delay discounting for any reward magnitude, further supporting their deficit in emotion processing.
Altogether, this study demonstrates the close connections between emotion processing and decision-making and the conditions under which these vary, in this instance dementia. Our findings have relevance for policymakers when developing health warning messages that aim to dissuade risky behaviors (Nan and Qin, 2019) or for improving negative health behaviors associated with increased delay discounting in clinical populations. A recent study showed promising findings demonstrating that computerized working memory training decreases the rate of delay discounting in older controls (Felton et al., 2019). Improvements in emotion recognition have been reported after computerized emotion recognition training in schizophrenia (Russell et al., 2006) and Huntington’s disease (Kempnich et al., 2017) suggesting an avenue for emotion recognition training as a mean of reducing impulsivity. These clinical interventions based on costs/benefits and emotion detection are however more likely to work in AD than in bvFTD.
Funding
This work was supported by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council (NHMRC) (APP1037746) and the Australian Research Council (ARC) Centre of Excellence in Cognition and its Disorders Memory Program (CE11000102). ALM is supported by the Swiss National Science Foundation, grant no. P300P1_171478 and P4P4PS_183817. OP is supported by an NHMRC Senior Research Fellowship (GNT1103258). RLR is supported by the Appenzeller Neuroscience Fellowship and the ARC Centre of Excellence in Cognition and its Disorders Memory Program (CE110001021). FK is supported by an NHMRC-ARC Dementia Research Development Fellowship (GNT1097026).
Declarations of interest
None.
Supplementary Material
References
- Adolphs R., Gosselin F., Buchanan T.W., Tranel D., Schyns P., Damasio A.R. (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433(7021), 68–72. [DOI] [PubMed] [Google Scholar]
- Anderson A.K., Phelps E.A. (2002). Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. Journal of Cognitive Neuroscience, 14(5), 709–20. [DOI] [PubMed] [Google Scholar]
- Andrewes D.G., Jenkins L.M. (2019). The role of the amygdala and the ventromedial prefrontal cortex in emotional regulation: implications for post-traumatic stress disorder. Neuropsychology Review, 29(2), 220–43. [DOI] [PubMed] [Google Scholar]
- Ariely D. & Loewenstein G. (2006) The heat of the moment: the effect of sexual arousal on sexual decision Making, Journal of Behavioral Decision Making, 19, pp. 87-98 [Google Scholar]
- Augustine A.A., Larsen R.J. (2011). Affect regulation and temporal discounting: interactions between primed, state, and trait affect. Emotion, 11(2), 403–12. [DOI] [PubMed] [Google Scholar]
- Ballard K., Knutson B. (2009). Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuro Image, 45(1), 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On R., Tranel D., Denburg N.L., Bechara A. (2003). Exploring the neurological substrate of emotional and social intelligence. Brain, 126(Pt 8, 1790–800. [DOI] [PubMed] [Google Scholar]
- Beagle A.J., Zahir A., Borzello M., Kayser A.S., Hsu M., Miller B.L., et al. (2020). Amount and delay insensitivity during intertemporal choice in three neurodegenerative diseases reflects dorsomedial prefrontal atrophy. Cortex, 124, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10(3), 295–307. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R., Lee G.P. (1999). Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience, 19(13), 5473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R.G., Gilbert S.J., Burgess P.W. (2011). A neural mechanism mediating the impact of episodic prospection on farsighted decisions. Journal of Neuroscience, 31(18), 6771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B.C., Smallwood J., Tusche A., Ruby F.J., Engen H.G., Steinbeis N., Singer T. (2014). Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self-generated thought and temporal discounting. Neuro Image, 90, 290–7. [DOI] [PubMed] [Google Scholar]
- Bertoux M., de Souza L.C., Sarazin M., Funkiewiez A., Dubois B., Hornberger M. (2015a). How preserved is emotion recognition in Alzheimer disease compared with behavioral variant frontotemporal dementia? Alzheimer Disease and Associated Disorders, 29(2), 154–7. [DOI] [PubMed] [Google Scholar]
- Bertoux M., de Souza L.C., Zamith P., Dubois B., Bourgeois-Gironde S. (2015b). Discounting of future rewards in behavioural variant frontotemporal dementia and Alzheimer's disease. Neuropsychology, 29(6), 933–9. [DOI] [PubMed] [Google Scholar]
- Bertoux M., Funkiewiez A., O'Callaghan C., Dubois B., Hornberger M. (2013). Sensitivity and specificity of ventromedial prefrontal cortex tests in behavioral variant frontotemporal dementia. Alzheimers Dement, 9(5) Suppl, S84–94. [DOI] [PubMed] [Google Scholar]
- Bickel W.K., Marsch L.A. (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96(1), 73–86. [DOI] [PubMed] [Google Scholar]
- Bidzan L., Bidzan M., Pachalska M. (2012). Aggressive and impulsive behavior in Alzheimer's disease and progression of dementia. Medical Science Monitor, 18(3), CR182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Momenan R., Hommer D.W. (2009). Delay discounting correlates with proportional lateral frontal cortex volumes. Biological Psychiatry, 65(8), 710–3. [DOI] [PubMed] [Google Scholar]
- Brand M., Grabenhorst F., Starcke K., Vandekerckhove M.M., Markowitsch H.J. (2007). Role of the amygdala in decisions under ambiguity and decisions under risk: evidence from patients with Urbach-Wiethe disease. Neuropsychologia, 45(6), 1305–17. [DOI] [PubMed] [Google Scholar]
- Cai X.L., Weigl M., Liu B.H., Cheung E.F.C., Ding J.H., Chan R.C.K. (2019). Delay discounting and affective priming in individuals with negative schizotypy. Schizophrenia Research, 210, 180–7. [DOI] [PubMed] [Google Scholar]
- Chiong W., Wood K.A., Beagle A.J., Hsu M., Kayser A.S., Miller B.L., Kramer J.H. (2016). Neuroeconomic dissociation of semantic dementia and behavioural variant frontotemporal dementia. Brain, 139(Pt 2), 578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.S., Pellecchia G., Aminian K., Ray N., Segura B., Obeso I., Strafella A.P. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26(3), 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell, J.C., Morris, A.M., Heurtelou, N.M., Kesner, R.P. (2009). Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behavioral Neuroscience, 123(6), 1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G.L., Huntsinger J.R. (2007). How emotions inform judgment and regulate thought. Trends in Cognitive Sciences, 11(9), 393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.L. (1997). The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology, 48(5) Suppl 6, S10–6. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- De Martino B., Camerer C.F., Adolphs R. (2010). Amygdala damage eliminates monetary loss aversion. Proceedings of the National Academy of Sciences of the United States of America, 107(8), 3788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Bakkour A., Salat D.H., Feczko E., Pacheco J., Greve D.N., et al. (2009). The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex, 19(3), 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski A.Y., Siegle G.J., Szanto K., Clark L., Reynolds C.F., Aizenstein H. (2012). The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological Medicine, 42(6), 1203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B., Nystrom L.E., Cohen J.D. (2012). Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One, 7(5), e36953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007). G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–91. [DOI] [PubMed] [Google Scholar]
- Feinstein J.S., Adolphs R., Damasio A., Tranel D. (2011). The human amygdala and the induction and experience of fear. Current Biology, 21(1), 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton J.W., Collado A., Ingram K.M., Doran K., Yi R. (2019). Improvement of working memory is a mechanism for reductions in delay discounting among mid-age individuals in an urban medically underserved area. Annals of Behavioral Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D., Black S.E. (2005). Impaired recognition of negative facial emotions in patients with frontotemporal dementia. Neuropsychologia, 43(11), 1673–87. [DOI] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Golden H.L., Clark C.N., Slattery C.F., Paterson R.W., et al. (2015). Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain, 138(Pt 11), 3360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S.B., Ghods-Sharifi S. (2007). Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cerebral Cortex, 17(2), 251–60. [DOI] [PubMed] [Google Scholar]
- Garavan H., Pendergrass J.C., Ross T.J., Stein E.A., Risinger R.C. (2001). Amygdala response to both positively and negatively valenced stimuli. Neuroreport, 12(12), 2779–83. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S., St Onge J.R., Floresco S.B. (2009). Fundamental contribution by the basolateral amygdala to different forms of decision making. Journal of Neuroscience, 29(16), 5251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E., Torralva T., Roca M., Szenkman D., Ibanez A., Richly P., et al. (2012). Decision making cognition in primary progressive aphasia. Behavioural Neurology, 25(1), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M.S., Sturm V.E., Ascher E.A., Shdo S.M., Miller B.L., Rankin K.P., et al. (2015). Emotion recognition in frontotemporal dementia and Alzheimer's disease: a new film-based assessment. Emotion, 15(4), 416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.C., Amlung M.T., Palmer A.A., Mac Killop J. (2016). Syntax for calculation of discounting indices from the monetary choice questionnaire and probability discounting questionnaire. Journal of the Experimental Analysis of Behavior, 106(2), 156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Myerson J. (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin, 130(5), 769–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Myerson J., Ostaszewski P. (1999). Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behavioural Processes, 46(1), 89–96. [DOI] [PubMed] [Google Scholar]
- Grossman M., Eslinger P.J., Troiani V., Anderson C., Avants B., Gee J.C., et al. (2010). The role of ventral medial prefrontal cortex in social decisions: converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia, 48(12), 3505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S., Cheng L., Fan Y., Li X. (2015). Myopic decisions under negative emotions correlate with altered time perception. Frontiers in Psychology, 6, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S., Mao H. (2002). Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport, 13(1), 15–9. [DOI] [PubMed] [Google Scholar]
- Hanten G., Scheibel R.S., Li X., Oomer I., Stallings-Roberson G., Hunter J.V., et al. (2006). Decision-making after traumatic brain injury in children: a preliminary study. Neurocase, 12(4), 247–51. [DOI] [PubMed] [Google Scholar]
- Herman A.M., Critchley H.D., Duka T. (2018). The role of emotions and physiological arousal in modulating impulsive behaviour. Biological Psychology, 133, 30–43. [DOI] [PubMed] [Google Scholar]
- Hirsch J.B., Morisano D., Peterson J.B. (2008). Delay discounting: interactions between personality and cognitive ability. Journal of Research in Personality, 42(6), 1646–50. [Google Scholar]
- Hirsh J.B., Guindon A., Morisano D., Peterson J.B. (2010). Positive mood effects on delay discounting. Emotion, 10(5), 717–21. [DOI] [PubMed] [Google Scholar]
- Hoefer M., Allison S. C., Schauer G. F., Neuhaus J. M., Hall J., Dang J. N., et al. (2008) 'Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease', Brain, 131 (Pt 6), pp. 1646-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D.W., Knutson B., Fong G.W., Bennett S., Adams C.M., Varnera J.L. (2003). Amygdalar recruitment during anticipation of monetary rewards: an event-related fMRI study. Annals of the New York Academy of Sciences, 985, 476–8. [DOI] [PubMed] [Google Scholar]
- Hsieh S., Schubert S., Hoon C., Mioshi E., Hodges J.R. (2013). Validation of the Addenbrooke's cognitive examination III in frontotemporal dementia and Alzheimer's disease. Dementia and Geriatric Cognitive Disorders, 36(3-4), 242–50. [DOI] [PubMed] [Google Scholar]
- Hu X., Kleinschmidt H., Martin J.A., Han Y., Thelen M., Meiberth D., et al. (2016). A reduction in delay discounting by using episodic future imagination and the association with episodic memory capacity. Frontiers in Human Neuroscience, 10, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M., Hornberger M., Lah S., Miller L., Pengas G., Nestor P.J., et al. (2011). Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer's disease. Neuropsychologia, 49(9), 2694–702. [DOI] [PubMed] [Google Scholar]
- Jimura K., Myerson J., Hilgard J., Keighley J., Braver T.S., Green L. (2011). Domain independence and stability in young and older adults' discounting of delayed rewards. Behavioural Processes, 87(3), 253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.W., Bickel W.K. (2002). Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior, 77(2), 129–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10(12), 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Levy I. (2015). Neural markers of individual differences in decision-making. Current Opinion in Behavioral Sciences, 5, 100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J., Calder A.J., Hodges J.R., Young A.W. (2002). Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia, 40(6), 655–65. [DOI] [PubMed] [Google Scholar]
- Kekic, M., Bartholdy, S., Cheng, J., McClelland, J., Boysen, E., Musiat, P., O’Daly, O.G., Campbell, I.C., Schmidt, U. (2016). Increased temporal discounting in bulimia nervosa. International Journal of Eating Disorders, 49(12), 1077–81. [DOI] [PubMed] [Google Scholar]
- Kelley N.J., Gallucci A., Riva P., Romero Lauro L.J., Schmeichel B.J. (2018). Stimulating self-regulation: a review of non-invasive brain stimulation studies of goal-directed behavior. Frontiers in Behavioral Neuroscience, 12, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempnich C.L., Wong D., Georgiou-Karistianis N., Stout J.C. (2017). Feasibility and efficacy of brief computerized training to improve emotion recognition in Premanifest and early-symptomatic Huntington's Disease. Journal of the International Neuropsychological Society, 23(4), 314–21. [DOI] [PubMed] [Google Scholar]
- Kipps C. M., Nestor P. J., Acosta-Cabronero J., Arnold R. & Hodges J. R. (2009) 'Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing', Brain, 132 (Pt 3), pp. 592-603. [DOI] [PubMed] [Google Scholar]
- Kirby K.N., Petry N.M., Bickel W.K. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology. General, 128(1), 78–87. [DOI] [PubMed] [Google Scholar]
- Kloeters S., Bertoux M., O'Callaghan C., Hodges J.R., Hornberger M. (2013). Money for nothing - atrophy correlates of gambling decision making in behavioural variant frontotemporal dementia and Alzheimer's disease. Neuroimage Clinical, 2, 263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Kramer J.H., Boeve B.F., Caselli R.J., Graff-Radford N.R., Mendez M.F., et al. (2008). Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain, 131(Pt 11), pp. 2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F., Hazelton J.L., Rushby J.A., Hodges J.R., Piguet O. (2019). Facial expressiveness and physiological arousal in frontotemporal dementia: phenotypic clinical profiles and neural correlates. Cognitive, Affective, & Behavioral Neuroscience, 19(1), 197–210. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J.R., Piguet O. (2013a). Discrete neural correlates for the recognition of negative emotions: insights from Frontotemporal dementia. PLoS One, 8(6), e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J.R., Piguet O. (2013b). The orbitofrontal cortex is involved in emotional enhancement of memory: evidence from the dementias. Brain, 136(Pt 10), pp. 2992–3003. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J.R., Piguet O. (2014a). Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer's disease. Frontiers in Behavioral Neuroscience, 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F., Sapey-Triomphe L. A., Leyton C. E., Burrell J. R., Hodges J. R. & Piguet O. (2014b) 'Degradation of emotion processing ability in corticobasal syndrome and Alzheimer's disease', Brain, 137 (Pt 11), pp. 3061-3072. [DOI] [PubMed] [Google Scholar]
- Landin-Romero R., Kumfor F., Leyton C.E., Irish M., Hodges J.R., Piguet O. (2017). Disease-specific patterns of cortical and subcortical degeneration in a longitudinal study of Alzheimer's disease and behavioural-variant frontotemporal dementia. Neuro Image, 151, 72–80. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1997). International Affective Picture System (IAPS): Technical Manual and Affective Ratings, Gainsville, FL: National Institute of Mental Health Center for the Study of Emotion and Attention. [Google Scholar]
- Lansdall C.J., Coyle-Gilchrist I.T.S., Jones P.S., Vazquez Rodriguez P., Wilcox A., Wehmann E., et al. (2017). Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain, 140(6), 1792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenu I., Pasquier F. (2005). Perception of emotion on faces in frontotemporal dementia and Alzheimer's disease: a longitudinal study. Dementia and Geriatric Cognitive Disorders, 19(1), 37–41. [DOI] [PubMed] [Google Scholar]
- Lavenu I., Pasquier F., Lebert F., Petit H., Van der Linden M. (1999). Perception of emotion in frontotemporal dementia and Alzheimer disease. Alzheimer Disease and Associated Disorders, 13(2), 96–101. [DOI] [PubMed] [Google Scholar]
- Lebreton M., Bertoux M., Boutet C., Lehericy S., Dubois B., Fossati P., et al. (2013). A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biology, 11(10), e1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. (1998). Fear and the brain: where have we been, and where are we going? Biological Psychiatry, 44(12), 1229–38. [DOI] [PubMed] [Google Scholar]
- Lempert K.M., Phelps E.A. (2016). The malleability of intertemporal choice. Trends in Cognitive Sciences, 20(1), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience, 4(4), 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Epstein L.H. (2014). Living in the moment: effects of time perspective and emotional valence of episodic thinking on delay discounting. Behavioral Neuroscience, 128(1), 12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences, 35(3), 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Feng T., Chen J., Li H. (2013). The value of emotion: how does episodic prospection modulate delay discounting? PLoS One, 8(11), e81717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockenhoff C.E., O'Donoghue T., Dunning D. (2011). Age differences in temporal discounting: the role of dispositional affect and anticipated emotions. Psychology and Aging, 26(2), 274–84. [DOI] [PubMed] [Google Scholar]
- Lough S., Kipps C.M., Treise C., Watson P., Blair J.R., Hodges J.R. (2006). Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia, 44(6), 950–8. [DOI] [PubMed] [Google Scholar]
- Luo S., Ainslie G., Giragosian L., Monterosso J.R. (2009). Behavioral and neural evidence of incentive bias for immediate rewards relative to preference-matched delayed rewards. Journal of Neuroscience, 29(47), 14820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Ainslie G., Monterosso J. (2014). The behavioral and neural effect of emotional primes on intertemporal decisions. Social Cognitive and Affective Neuroscience, 9(3), 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden G.J., Begotka A.M., Raiff B.R., Kastern L.L. (2003). Delay discounting of real and hypothetical rewards. Experimental and Clinical Psychopharmacology, 11(2), 139–45. [DOI] [PubMed] [Google Scholar]
- Manuel A.L., Murray N.W.G., Piguet O. (2019). Transcranial direct current stimulation (tDCS) over vm PFC modulates interactions between reward and emotion in delay discounting. Scientific Reports, 9(1), 18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka A., Zurawski L., Jednorog K., Grabowska A. (2014). The Nencki affective picture system (NAPS): introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior Research Methods, 46(2), 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias-Guiu J.A., Pytel V., Cortes-Martinez A., Valles-Salgado M., Rognoni T., Moreno-Ramos T., et al. (2018). Conversion between Addenbrooke's cognitive examination III and mini-mental state examination. International Psychogeriatrics, 30(8), 1227–33. [DOI] [PubMed] [Google Scholar]
- Mazur J.E. (1987). An adjusting procedure for studying delayed reinforcement, Hillsdale, NJ: Erlbaum. [Google Scholar]
- McClure S.M., Ericson K.M., Laibson D.I., Loewenstein G., Cohen J.D. (2007). Time discounting for primary rewards. Journal of Neuroscience, 27(21), 5796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306(5695), 503–7. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R. Jr., Kawas C.H., et al. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement, 7(3), 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M.F., McMurtray A., Licht E., Shapira J.S., Saul R.E., Miller B.L. (2006). The scale for emotional blunting in patients with frontotemporal dementia. Neurocase, 12(4), 242–6. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L., Pitman R.K., Quirk G.J. (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology, 73(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Mobini S., Body S., Ho M.Y., Bradshaw C.M., Szabadi E., Deakin J.F., Anderson I.M. (2002). Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology, 160(3), 290–8. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77(3), 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Qin Y. (2019). How thinking about the future affects our decisions in the present: effects of time orientation and episodic future thinking on responses to health warning messages. Human Communication Research, 45(2), 148–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor P.J., Fryer T.D., Smielewski P., Hodges J.R. (2003). Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Annals of Neurology, 54(3), 343–51. [DOI] [PubMed] [Google Scholar]
- Olson E.A., Collins P.F., Hooper C.J., Muetzel R., Lim K.O., Luciana M. (2009). White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. Journal of Cognitive Neuroscience, 21(7), 1406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patin A., Hurlemann R. (2011). Modulating amygdala responses to emotion: evidence from pharmacological fMRI. Neuropsychologia, 49(4), 706–17. [DOI] [PubMed] [Google Scholar]
- Pehlivanova M., Wolf D.H., Sotiras A., Kaczkurkin A.N., Moore T.M., Ciric R., et al. (2018). Diminished cortical thickness is associated with impulsive choice in adolescence. Journal of Neuroscience, 38(10), 2471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D.C., Datta S., Sturm V.E., Wood K.A., Zakrzewski J., Seeley W.W., et al. (2017). Reward deficits in behavioural variant frontotemporal dementia include insensitivity to negative stimuli. Brain, 140(12), 3346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Buchel C. (2009). Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. Journal of Neuroscience, 29(50), 15727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., D'Esposito M. (2016). Effects of medial orbitofrontal cortex lesions on self-control in intertemporal choice. Current Biology, 26(19), 2625–8. [DOI] [PubMed] [Google Scholar]
- Piguet O., Kumfor F., Hodges J. (2017). Diagnosing, monitoring and managing behavioural variant frontotemporal dementia. Medical Journal of Australia, 207(7), 303–8. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. (2014). Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neuroscience & Biobehavioral Reviews, 38, 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin S.P., Dautoff R., Morris J.C., Barrett L.F., Dickerson B.C., Alzheimer's Disease Neuroimaging I. (2011). Amygdala atrophy is prominent in early Alzheimer's disease and relates to symptom severity. Psychiatry Research, 194(1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Gehlert D.R. (2003). Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Sciences, 985, 263–72. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(Pt 9), 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry, 60(4), 376–82. [DOI] [PubMed] [Google Scholar]
- Read D., Read N.L. (2004). Time discounting over the lifespan. Organizational Behavior and Human Decision Processes, 47, 22–32. [Google Scholar]
- Reynolds B. (2006). A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology, 17(8), 651–67. [DOI] [PubMed] [Google Scholar]
- Rieger M., Mata R. (2015). On the generality of age differences in social and nonsocial decision making. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70(2), 202–14. [DOI] [PubMed] [Google Scholar]
- Roalf D.R., Mitchell S.H., Harbaugh W.T., Janowsky J.S. (2012). Risk, reward, and economic decision making in aging. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 67(3), 289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell T. A., Chu E. & Phillips M. L. (2006) A pilot study to investigate the effectiveness of emotion recognition remediation in schizophrenia using the micro-expression training tool, British Journal of Clinical Psychology, 45 (Pt 4), pp. 579-583. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Lang P.J., Bradley M.M., Costa V.D., Keil A. (2009). The timing of emotional discrimination in human amygdala and ventral visual cortex. Journal of Neuroscience, 29(47), 14864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Mata R., Radu P.T., Ballard I.C., Carstensen L.L., McClure S.M. (2011). Age differences in striatal delay sensitivity during Intertemporal choice in healthy adults. Frontiers in Neuroscience, 5, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt D.M., Erk S., Nusser C., Nothen M.M., Cichon S., Rietschel M., et al. (2010). Volition diminishes genetically mediated amygdala hyperreactivity. Neuro Image, 53(3), 943–51. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P., et al. (1992). Atrophy of medial temporal lobes on MRI in "probable" Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. Journal of Neurology, Neurosurgery and Psychiatry, 55(10), 967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.L., Mitchell A.D., Lahna D.L., Luber H.S., Huckans M.S., Mitchell S.H., et al. (2010). Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuro Image, 50(4), 1392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman K.L., Gorlick M.A., Vekaria K.M., Hsu M., Zald D.H., Samanez-Larkin G.R. (2016). Adult age differences in decision making across domains: increased discounting of social and health-related rewards. Psychology and Aging, 31(7), 737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R., Rascovsky K., Kramer J.H., Weiner M., Miller B.L., et al. (2008). Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology, 65(2), 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M., Ciaramelli E., di Pellegrino G. (2011). The neurobiology of intertemporal choice: insight from imaging and lesion studies. Reviews in the Neurosciences, 22(5), 565–74. [DOI] [PubMed] [Google Scholar]
- Shamosh N.A., Gray J.R. (2008). Delay discounting and intelligence: a meta-analysis. Intelligence, 36(4), 289–305. [Google Scholar]
- So M., Foxe D., Kumfor F., Murray C., Hsieh S., Savage G., et al. (2018). Addenbrooke's cognitive examination III: psychometric characteristics and relations to functional ability in dementia. Journal of the International Neuropsychological Society, 24(8), 854–63. [DOI] [PubMed] [Google Scholar]
- Sohn J.H., Kim H.E., Sohn S., Seok J.W., Choi D., Watanuki S. (2015). Effect of emotional arousal on inter-temporal decision-making: an fMRI study. Journal of Physiological Anthropology, 34, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M., Pulaski S., Krueger F., Zamboni G., Clawson D., Grafman J. (2011). Regional brain atrophy and impaired decision making on the balloon analog risk task in behavioral variant frontotemporal dementia. Cognitive and Behavioral Neurology, 24(2), 59–67. [DOI] [PubMed] [Google Scholar]
- Tschernegg M., Pletzer B., Schwartenbeck P., Ludersdorfer P., Hoffmann U., Kronbichler M. (2015). Impulsivity relates to striatal gray matter volumes in humans: evidence from a delay discounting paradigm. Frontiers in Human Neuroscience, 9, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turel O., He Q., Brevers D., Bechara A. (2018). Delay discounting mediates the association between posterior insular cortex volume and social media addiction symptoms. Cognitive, Affective, & Behavioral Neuroscience, 18(4), 694–704. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M.P., Armony J.L., Driver J., Dolan R.J. (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience, 7(11), 1271–8. [DOI] [PubMed] [Google Scholar]
- Wang Q., Chen C., Cai Y., Li S., Zhao X., et al. (2016). Dissociated neural substrates underlying impulsive choice and impulsive action. Neuro Image, 134, 540–9. [DOI] [PubMed] [Google Scholar]
- Weller J.A., Levin I.P., Shiv B., Bechara A. (2007). Neural correlates of adaptive decision making for risky gains and losses. Psychological Science, 18(11), 958–64. [DOI] [PubMed] [Google Scholar]
- Whelan R., McHugh L.A. (2009). Temporal discounting of hypothetical monetary rewards by adolescents, adults, and older adults. The Psychological Record, 59(2), 247–58. [Google Scholar]
- Wilson M., Daly M. (2004). Do pretty women inspire men to discount the future? Proceedings of the Biological Sciences, 271Suppl 4, S177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A., Clithero J.A., Carter R.M., Bergman S.R., Wang L., Huettel S.A. (2013). Ventromedial prefrontal cortex encodes emotional value. Journal of Neuroscience, 33(27), 11032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A., Theobald D.E., Cardinal R.N., Robbins T.W. (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. Journal of Neuroscience, 24(20), 4718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Tsai S.J., Li C.R. (2020). Concurrent amygdalar and ventromedial prefrontal cortical responses during emotion processing: a meta-analysis of the effects of valence of emotion and passive exposure versus active regulation. Brain Structure & Function, 225(1), 345–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Peng J., Qin L., Suo T., Feng T. (2018). Prospective emotion enables episodic prospection to shift time preference. British Journal of Psychology, 109(3), 487–99. [DOI] [PubMed] [Google Scholar]
- Zimbardo P.G., Boyd J.N. (2015). Putting time in perspective: a valid, reliable individual-differences metric In: Stolarski N.F., Beek W., editors. Time Perspective Theory; Review, Research and Application: Essays in Honor of Philip G. Zimbardo, Cham: Springer International Publishing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


