Abstract
Bleeding is the most common complication of anticoagulant use. The evaluation and management of the bleeding patient is a core competency of emergency medicine. As the prevalence of patients receiving anticoagulant agents and variety of anticoagulants with different mechanisms of action, pharmacokinetics, indications, and corresponding reversal agents increase, physicians and other clinicians working in the emergency department require a current and nuanced understanding of how best to assess, treat, and reverse anticoagulated patients. In this project, we convened an expert panel to create a consensus decision tree and framework for assessment of the bleeding patient receiving an anticoagulant, as well as use of anticoagulant reversal or coagulation factor replacement, and to address controversies and gaps relevant to this topic. To support decision tree interpretation, the panel also reached agreement on key definitions of life-threatening bleeding, bleeding at a critical site, and emergency surgery or urgent invasive procedure. To reach consensus recommendations, we used a structured literature review and a modified Delphi technique by an expert panel of academic and community physicians with training in emergency medicine, cardiology, hematology, internal medicine/thrombology, pharmacology, toxicology, transfusion medicine and hemostasis, neurology, and surgery, and by other key stakeholder groups.
INTRODUCTION
Background
Anticoagulants are used to prevent and treat thrombotic events associated with high morbidity and mortality, such as atrial fibrillation, heart valve replacement, stroke, and venous thromboembolism. They work by altering the normal physiology of the coagulation cascade, resulting in decreased thrombin generation or direct thrombin inhibition. Their main complication is bleeding, from self-limited to life threatening. Most patients with bleeding complications present to the emergency department (ED) for care. Between 2013 and 2015, 17.6% of all patient presentations to the ED for adverse drug events were related to anticoagulant use, the most common class of medications resulting in an adverse event, and approximately half of these cases resulted in hospitalization.1 Recent studies estimate that approximately 228,600 ED visits are due to anticoagulant issues. Bleeding represents approximately 80% of these visits, which exclude fatal bleeding events that never involve ED presentation.1,2
The use of anticoagulants has increased markedly in the past decade. Advances in diagnosis and treatment of venous thromboembolism have led to an increase in the annual incidence of first-time venous thromboembolism, from 73 per 100,000 patients in the mid-1980s to 133 per 100,000 patients in 2009.3 Some patients receive an anticoagulant for a short time, such as those with a provoked deep venous thrombosis. However, recent evidence supports the use of long-term anticoagulation in patients with either unprovoked venous thromboembolism or provoked venous thromboembolism with ongoing risk factors.4–6 Anticoagulants also decrease the risk of ischemic stroke in patients with nonvalvular atrial fibrillation.7 The incidence of atrial fibrillation increases with age, and the increasing geriatric population is leading to larger cohorts of patients receiving long-term anticoagulation. Expanding indications for anticoagulation include venous thromboembolism prophylaxis in the medically complex and oncology patient populations and those with chronic coronary artery disease or peripheral arterial disease.8–12 It is estimated that 4 to 5 million US hospitalized medical patients may qualify for extended venous thromboembolism thromboprophylaxis after discharge and that chronic coronary artery disease and peripheral arterial disease affect 16.8 and 8.5 million Americans, respectively.13,14 As anticoagulation becomes more common, the prevalence of anticoagulated patients and associated bleeding events will increase.
Direct oral anticoagulants have overtaken vitamin K antagonists as preferred anticoagulants for a broad number of indications. As of 2014, the majority of new anticoagulant initiations have been with a direct oral anticoagulant.15–18 The number of patients treated with direct oral anticoagulants has doubled during the past 3 years, from 3 million to 7.6 million.19 Direct oral anticoagulants offer several advantages over vitamin K antagonists, including rapid onset of action, no heparin bridging requirement, short half-life, no routine monitoring, minimal food-drug and drug-drug interactions, and decreased risk of major bleeding events, especially intracranial and fatal ones, as well as noninferiority in preventing thrombotic events.20–23 However, the lack of specific reversal agents for direct oral anticoagulants (until October 2015 for dabigatran and May 2018 for apixaban and rivaroxaban) has been a potential barrier to their use.24 Direct oral anticoagulants encompass several different medications with a variety of targets and drug-specific reversal agents that have only recently been available (Figure 1). Although vitamin K antagonists have been used since the 1940s and clinicians have familiarity treating their bleeding complications, some clinicians may be less familiar with the most current evidence-based approaches.
Figure 1.
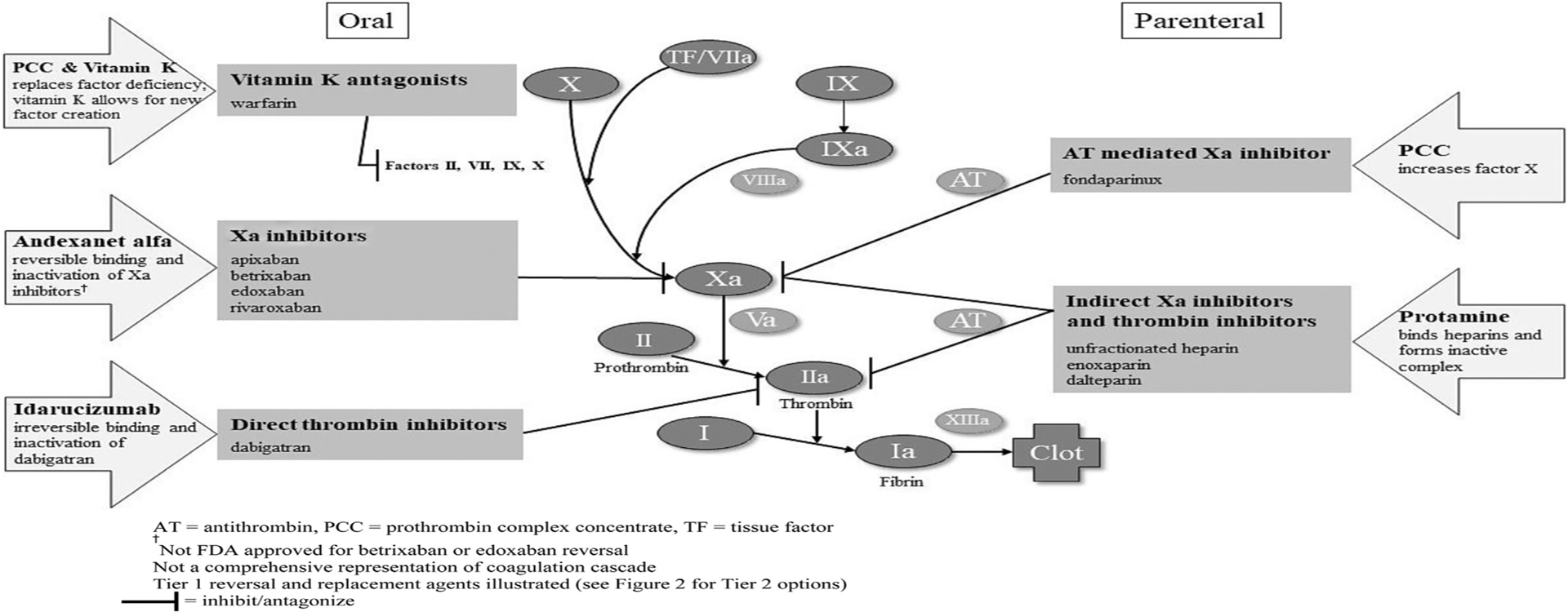
Coagulation cascade, anticoagulants, and reversal or replacement targets.
Importance
The assessment and management of patients with bleeding and without it, but who require emergency procedures, are complex. Clinicians must be familiar with the various anticoagulants and how to rapidly stabilize and risk stratify patients, and, if indicated, administer a reversal agent or factor replacement. Patients may not be capable of accurately communicating the agent, dose, and timing of their anticoagulants. Laboratory tests to detect the presence and effect of anticoagulants are not routinely accessible, and it is unclear whether their results aid treatment (eg, although thrombin and Xa assays were used in trials, reversal use was not predicated on assay results). Unique patient factors such as advanced age, other medications (especially antiplatelet agents), and comorbidities (eg, renal or hepatic dysfunction) must be recognized and considered. Reversal or replacement agents are costly compared with other medications routinely administered in the ED setting; however, their indications involve high-risk patients. Anticoagulated patients can be critically ill with life-threatening bleeding or bleeding at a critical site, or may not be bleeding but require an emergency surgery or procedure with high risk of bleeding that cannot be safely delayed. The assessment and treatment of these patients are fraught with uncertainty and risk, necessitating a decision framework to support timely and sound care.
Definitions of a life-threatening bleeding event or critical site may vary significantly. Several definitions for major bleeding exist in the literature from previous trials, such as International Society and Haemostasis, Thrombolysis in Myocardial Infarction, Global Utilization of Streptokinase and Tissue Plasminogen for Occluded Coronary Arteries, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial, Acute Catheterization and Urgent Intervention Triage Strategy, Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction, Global Registry of Acute Coronary Events, and Platelet Inhibition and Patient Outcomes.25–33 Others have created universal definitions for bleeding severity, such as the Bleeding Academic Research Consortium.34
Confusion persists between reversal (ie, anticoagulant is directly neutralized) and replacement (ie, target clotting factors of the anticoagulant are repleted) strategies. The logistics of administration and pharmacokinetics are significantly different. Current national guidelines have not been updated to reflect the most recently approved reversal agents and thus do not reflect current clinical practice (eg, Food and Drug Administration [FDA] approval of andexanet alfa in May 2018).35 A reversal decision tool that provides the most up-to-date recommendations, highlights the importance of emergency supportive care measures, offers an approach to the risk stratification of patients for whom reversal or replacement agents may benefit, and describes which therapies are likely or not likely to benefit candidate patients would greatly aid clinicians.
Goals of This Investigation
We seek to provide clinicians a consensus decision framework to be used as a bedside tool for assessment and management of patients with bleeding and those without it but who require emergency procedures.
MATERIALS AND METHODS
Study Design and Setting
The American College of Emergency Physicians (ACEP) convened an expert panel on anticoagulation reversal. We performed a structured literature review examining the past decade of publications and guidelines involving vitamin K antagonists, direct oral anticoagulants, heparins, and the corresponding agents used for reversal or factor replacement, including their respective efficacy, safety, and logistics of use. Although the evidence was not graded, we emphasized studies used as the basis for FDA approval of agents. When possible, definitions and recommendations reflected study criteria and treatment processes; otherwise, we relied on the expert panel to standardize definitions and processes. We used a modified Delphi technique to reach consensus.
Literature Review
The panel chairs consulted with medical librarians and performed a structured literature review of PubMed, Scopus, and ClinicalTrials.gov to create a resource library for panel members. The literature search focused on 2 main aspects of anticoagulant reversal or replacement: clinical trials showing the effects of specific reversal or replacement agents, and specialty society guidelines. We searched for key-word terms, shown in Tables E1 to E3 (available online at http://www.annemergmed.com). The panel chairs reviewed abstracts and full articles to select those most relevant. This process yielded 55 clinical trial references and 54 guideline references. We provided panelists with access to the reference library and the opportunity to make additions.
Selection of Participants
Between July and December 2018, ACEP convened a multidisciplinary, geographically and practice-setting diverse expert panel of clinicians (Table 1). ACEP staff selected the cochairs, who in turn selected the panelists. Selection criteria included a publication history, direct clinical experience in evaluation and management of the target patient population, or both. To recruit community emergency physicians and advanced-practice provider-based panelists, ACEP solicited participants through the ACEP Emergency Medicine Practice Committee and Society of Emergency Medicine Physician Assistants, respectively.
Table 1.
Profile of anticoagulant reversal panel experts.
| Discipline or Specialty* | No. of Participants |
|---|---|
| Emergency medicine (academic) | 6 |
| Emergency medicine (community) | 6 |
| Cardiology (general) | 2 |
| Toxicology | 1 |
| Surgery | 1 |
| Neurointensivist | 1 |
| Hematology/transfusion medicine | 1 |
| Intern ist/thrombologist† | 1 |
| Pharmacy | 1 |
| Advanced-practice provider | 1 |
Some panelists were counted in multiple categories.
A thrombologist is a new role for a physician who has developed expertise in anticoagulation and venous thromboembolism management
Methods for Consensus
The Delphi technique is a recognized framework to reach a consensus based on a series of iterative interactions and review of key questions.36 Four rounds of structured voting occurred. The chairs presented results of the initial voting and facilitated extensive discussion at in-person meetings (rounds 2 and 3) on September 17, 2018, and October 1, 2018. The fourth round involved one additional survey of panelist comments and votes. The chairs modified the recommendations according to the previous rounds’ discussion and voting. The final panel recommendations represent consensus and majority opinions.
RESULTS
The panel first focused on reaching agreement on the guidance statement for anticoagulant reversal or factor replacement (Figure 2). We included parenteral agents in the figures and tables because emergency physicians may encounter patients receiving them in addition to oral agents (eg, oncology patient receiving enoxaparin for venous thromboembolism, nursing facility resident treated with unfractionated heparin). We outlined the initial assessment and performance of multiple cognitive and procedural tasks in parallel, particularly determining the amount and last dose of anticoagulant, and the initiation of emergency treatment and supportive care (Figure 3). Immediate supportive care is critical for all patients whether or not a replacement or reversal agent is used. This includes source control by compression, surgery, endoscopy, or interventional radiology. It is important not to anchor on reversal or replacement strategies at the expense of these other critically important actions. The intention of diagnostic testing at this point is to help risk stratify, identify the extent and consequence of bleeding, and uncover contributing comorbid issues (eg, hepatic/renal failure). Concurrent use of aspirin or a P2Y12 inhibitor (eg, clopidogrel, ticagrelor, prasugrel) or parenteral agents (eg, enoxaparin for venous thromboembolism in oncology patients) should be identified. Desmopressin may be useful in the treatment of bleeding associated with antiplatelet agents. The benefit of platelet transfusion is unclear and may in some critical-site bleeding events, such as nontraumatic intracerebral hemorrhage, be harmful in the absence of significant thrombocytopenia.37
Figure 2.
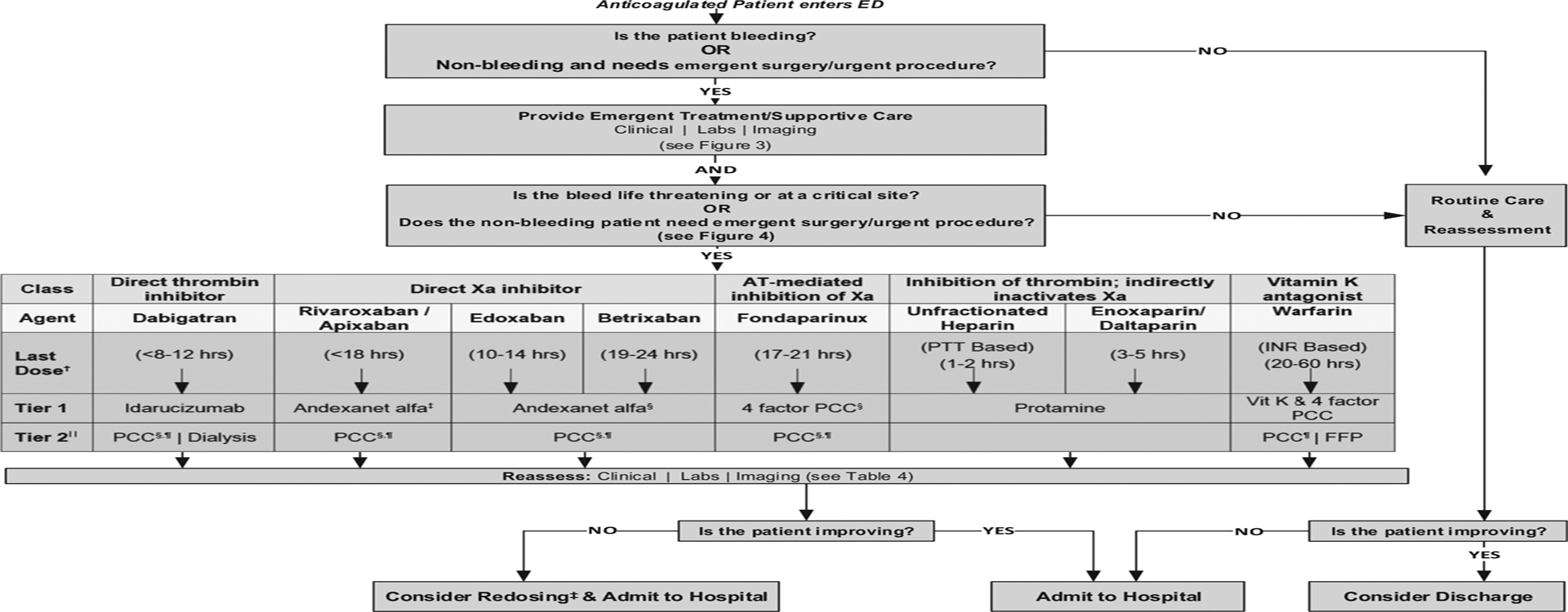
Consensus anticoagulation reversal or replacement decision tree.* *Refer to Table 2 for anticoagulant characteristics and Table 3 for dosing of reversal and replacement agents. AT, Antithrombin; INR, international normalized ratio; PCC, prothrombin complex concentrate; vit K, vitamin K; FFP, fresh frozen plasma. †Based on last dose inclusion criteria used for clinical studies. ‡FDA approved for bleeding only (not emergency surgery/urgent procedure). §Not FDA approved. ‖If tier 1 not available. ¶Includes 4-factor PCC (preferred), 3-factor PCC, and activated PCC.
Figure 3.
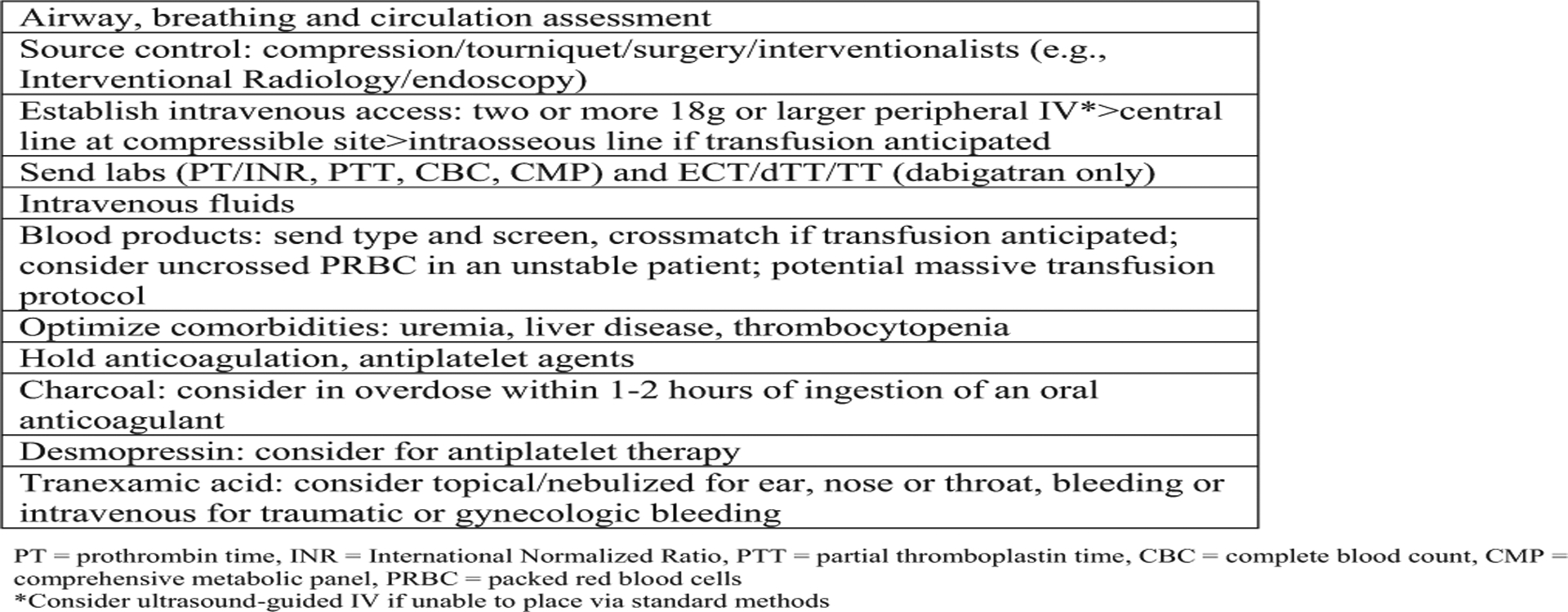
Emergency treatment and supportive care interventions.
In anticoagulant-associated life-threatening or critical-site bleeding or nonbleeding requiring emergency procedures, reversal or replacement agents in addition to supportive measures are essential. Currently, only 2 reversal agents for oral anticoagulants are approved by the FDA: idarucizumab for dabigatran and andexanet alfa for apixaban and rivaroxaban. These specific reversal agents bind the active drug directly and inhibit the anticoagulant effect within minutes. Vitamin K antagonist replacement therapy has included vitamin K and 4-factor prothrombin complex concentrate, 3-factor prothrombin complex concentrate, activated prothrombin complex concentrate, recombinant factor VIIa, or fresh-frozen plasma by replacing factors of the coagulation cascade rather than reversing the anticoagulant. However, the FDA has approved only one agent, the 4-factor prothrombin complex concentrate Kcentra, for this purpose. For the patient receiving a direct oral anticoagulant, replacement with prothrombin complex concentrate or activated prothrombin complex concentrate is presumed to overwhelm the Xa or direct thrombin inhibitor and restore hemostasis. However, supporting evidence is limited: the studies are small case studies or series, have a convenience population without prespecified inclusion or endpoint criteria, and lack FDA approval.38,39 Therefore, we suggest prothrombin complex concentrate for direct oral anticoagulant treatment only if first-line reversal agents (eg, idarucizumab, andexanet alfa) are unavailable. In such cases, 4-factor prothrombin complex concentrate is preferred over 3-factor prothrombin complex concentrate, although clinical data are limited.40,41
Defining which patients benefit from reversal or replacement strategies is pivotal (Figure 4). Risk stratification is ubiquitous in emergency medicine and applies to the bleeding patient receiving anticoagulants or the nonbleeding patient who overdosed or needs an emergency procedure. In the bleeding patient, the most significant risks are life-threatening bleeding events and critical-site bleeding events, both traumatic and nontraumatic. A modified definition of Bleeding Academic Research Consortium type 3 bleeding to define a life-threatening bleeding event is based on laboratory values or vital sign abnormalities attributable to bleeding that also prompts interventions such as transfusions or use of vasoactive medications. Critical sites are based on space-occupying lesions and the predicted morbidity and mortality of hemorrhage (eg, brain, spine). Risk stratification includes clinical context; specifically, the dose and most recent time an anticoagulant was received. For example, a small traumatic subarachnoid hemorrhage with a normal neurologic examination result, associated with a direct Xa inhibitor with a short half-life received more than 2 half-lives ago, may not require a reversal agent. Alternatively, a large intracranial bleeding event with midline shift and National Institutes of Health Stroke Scale score greater than 22 is catastrophic. Use of a replacement or reversal agent would likely be futile and therefore it may be withheld.
Figure 4.

Consensus definitions of the expert panel.
Reversal or replacement carries some risks in addition to cost; patients are returned to their baseline prothrombotic state after administration and could therefore experience a thrombotic event.42,43 Reevaluation of critically ill, high-risk patients has dynamic clinical courses and requires frequent reassessments, especially after treatment with a replacement or reversal agent (Figure 5). Bleeding patients not responding as expected may have additional ongoing processes, such as disseminated intravascular coagulopathy, disseminated intravascular coagulopathy-like syndromes, or traumatic coagulopathies. Reassessment to evaluate for other causes of hemorrhage may include additional or repeated laboratory or imaging studies. Redosing of reversal or replacement agents is controversial because supporting evidence is lacking. However, in the unstable patient with a worsening course, redosing could be indicated, although off label, because the reversal agents’ half-life is shorter than that of the targeted anticoagulant. We detail the key mechanisms of action, metabolism, timing, and dosing for the most common anticoagulants and their corresponding reversal or replacement agents in Tables 2 and 3.
Figure 5.

Reassessment components after reversal or replacement.
Table 2.
Anticoagulant characteristics.
| Drug | FDA Indications | Dosing* | Mechanism | Half-life* | Metabolism and Renal Clearance | Laboratory Effects† |
|---|---|---|---|---|---|---|
| Dabigatran‡ | NVAF, VTE, VTE prophylaxis | NVAF: 150 mg PO bid, (RD) 75 mg PO bid VTE: 150 mg PO bid VTE prophylaxis: 150 mg PO bid (after THR 110 mg PO × 1 followed by 220 mg PO qd) | Direct thrombin inhibitor | 12–17 h | Hydrolyzed to form dabigatran, the active moiety, further metabolized through conjugation 80% renal clearance | ↔/↑PT/INR, ↑↑ PTT, ECT/dTT, TT, ↔ anti-Xa level |
| Rivaroxaban | NVAF, VTE, VTE prophylaxis, chronic CAD or PAD | NVAF: 20 mg PO qd, (RD) 15 mg PO qd VTE: 15 mg PO bid ×21 days followed by 20 mg qd VTE primary (THR/TKR) or secondary prophylaxis: 10 mg qd Chronic CAD or PAD: 2.5 mg PO bid plus aspirin | Direct Xa inhibitor | 5–11.7 h | Hepatic: primary site by CYP3A4/5, CYP2J2, and hydrolysis 33% renal clearance | ↑↑ PT/INR, ↔/↑PTT, ↑anti-Xa level |
| Apixaban | NVAF, VTE, VTE prophylaxis | NVAF: 5 mg PO bid, (RD) 2.5 mg PO bid VTE: 10 mg PO bid ×7 days followed by 5 mg PO bid VTE primary (THR/TKR) or secondary prophylaxis: 2.5 mg PO bid | Direct Xa inhibitor | 6.8–15.2 h | Hepatic: mainly by CYP3A4 25% renal clearance | ↑ PT/INR, ↔/↑ PTT, ↑anti-Xa level |
| Edoxaban‡ | NVAF, VTE | NVAF: 60 mg PO qd, (RD) 30 mg qd VTE: 60 mg PO qd, (RD) 30 mg qd | Direct Xa inhibitor | 11.5 h | Hepatic: minimal, substrate of P-glycoprotein and CYP3A4 50% renal clearance | ↑ PT/INR, ↔/↑ PTT, ↑anti-Xa level |
| Betrixaban | VTE prophylaxis | VTE prophylaxis: 160 mg PO ×l followed by 80 mg PO qd, (RD) 80 mg ×l followed 40 mg qd | Direct Xa inhibitor | 19–27 h | Substrate of P-glycoprotein 6%−13% renal clearance | ↔/↑ PT/INR, ↔/↑ PTT, ↑anti-Xa level |
| Fonda parinux | VTE, VTE prophylaxis | VTE: 5–10 mgSQqd VTE prophylaxis (THR/TKR/hip fracture, abdominal surgery): 2.5 mg SQ qd | AT–dependent (mediated) selective inhibition of Xa | 17–21 h | 77% renal clearance | ↔ PT/INR, ↔/↑ PTT, ↑anti-Xa level |
| Unfractionated heparin | NVAF and valvular atrial fibrillation, VTE, ACS, critical limb ischemia, DIC | NVAF and valvular atrial fibrillation: 70–80 units/kg IV bolus followed by infusion of 18 units/kg per hour or fixed dose of 5,000 units IV bolus followed by infusion of 1,000 units/h VTE: 80 units/kg IV bolus and then 18 units/kg per hour, or fixed dosing of 5,000 units IV bolus followed by 1,000 units/h VTE prophylaxis: 5,000 units SQ every 8–12 h ACS: 60 units/kg (maximum 4,000 units) followed by an initial infusion of 12 units/kg per hour (maximum 1,000 units/h) DIC: Initial, 10,000 units IV bolus and then 5,000 to 10,000 units every 4–6 h |
AT-dependent (mediated) inactivation of thrombin (IIa) and activated factor X (factor Xa) | 1.5 h | Hepatic and reticuloendothelial system Renally cleared only at higher doses; phagocytosis | ↔ PT/INR, PTT, ↑anti-Xa level |
| Enoxaparin | VTE, VTE prophylaxis, STEMI, unstable angina, and non-Q-wave MI, mechanical heart valve prophylaxis | VTE: 1 mg/kg SQ bid or 1.5 mg/kg SQ qd VTE prophylaxis (THR/TKR/abdominal surgery/medical): 30 or 40 mg SQ qd; 30 mg SQ every 12 h STEMI: 30-mg IV bolus followed 15 min later by 1 mg/kg SQ, MAX 100 mg for the first 2 doses; maintenance, 1 mg/kg SQ bid; plus aspirin or (RD for >75 y) 0.75 mg/kg SC every 12 h (maximum 75 mg for the first 2 doses) only) followed by 0.75 mg/kg dosing for the remaining doses Unstable angina and non‒Q‒wave MI: mechanical heart valve: 40 mg SQ every 24 h OR 1 mg/kg SQ bid (say off label?) |
AT-dependent (mediated) selective inhibition of Xa and to a lesser extent thrombin (IIa) | 4.5–7.5 h SQ, 2–4 h IV | Hepatic: primary by desulfation or depolymerization 8%−40% renal clearance |
↔ PT/INR, ↔/↑ PTT, ↑anti-Xa level |
| Dalteparin | VTE prophylaxis, unstable angina, and non-Q-wave MI, mechanical heart valve prophylaxis | VTE in cancer: 200 IU/kg SQ QD ×l mo and then 150 IU/kg SQ qd VTE prophylaxis (THR/abdominal surgery/medical): 2,500–5,000 IU SQ qd UA/NQWMI: 120 IU/kgSQ every 12 h (max 10,000 IU/dose) with concurrent aspirin |
AT-dependent (mediated) selective inhibition of Xa and to a lesser extent thrombin (IIa) | 3–5 h | NA 70% renal clearance |
↔ PT/INR,↔/↑PTT, ↑anti-Xa level |
| Warfarin‡ | NVAF and valvular atrial fibrillation, VTE, VTE prophylaxis (THR/TKR), mechanical heart valve prophylaxis | NVAF and valvular atrial fibrillation: initial 2–5 mg PO qd; adjust dose based on the results of INR VTE and VTE prophylaxis (THR/TKR): initial 2 to 5 mg PO qd; adjust dose based on the results of INR mechanical heart valve: initial 2–5 mg PO qd; adjust dose based on the results of INR |
Blocks the regeneration of vitamin K epoxide, thus inhibiting synthesis of vitamin K-dependent clotting factors, which include factors II, VII, IX, and X, and the anticoagulant proteins C and S proteins C and S | 5–7 days | Hepatic: extensive by CYP2C9 (primary isoenzyme), CYP2C19, CYP2C8, CYP2C18, CYP1A2, and CYP3A4 92% renal clearance |
↑PT/INR, ↔/↑ PTT, anti-Xa level |
NVAF, Nonvalvular atrial fibrillation; VTE, venous thromboembolism; PO, by mouth; bid, twice daily; RD, reduced dose; THR, total hip replacement; qd, once daily; PT, prothrombin time; ECT, Ecarin clotting time; dTT, diluted thrombin time; CAD, coronary artery disease; PAD, peripheral arterial disease; TKR, total knee replacement; SQ, subcutaneous; ACS, acute coronary syndrome; DIC, disseminated intravascular coagulation; IV, intravenous; STEMI, ST-segment elevation myocardial infarction; MI, myocardial infarction; IU, international unit; NQWMI, non-Q-wave myocardial infarction; NA, not applicable.
Assumes normal renal and hepatic function, normal body mass index, and half-lives extended significantly with renal or hepatic failure, depending on the drug.
Laboratory results are not useful unless correlated for a specific drug. Direct oral anticoagulants specifically have varying effects on PT/INR and other laboratory results. Bold up arrows indicate main/significant impact to increase or alter lab result; up arrows indicate likely impact to increase or alter lab result; 2 up arrows indicate significant impact to increase or alter lab result; double sided horizontal arrows indicate may or may not impact lab value; up arrows with double sided horizontal arrow indicates likely impact to increase or alter lab result but also may not alter result.
Requires initial parenteral agent bridge for treatment of acute VTE.
Table 3.
Reversal and factor replacement agent characteristics.
| Drug | Reversal or Replacement Target | Administration Window* | Mechanism | FDA Indications | Half-life | Dosing Regimen | Special Considerations and Cost† |
|---|---|---|---|---|---|---|---|
| Reversal | |||||||
| Idarucizumab | Direct thrombin (factor IIa) inhibitor (dabigatran) | <8–12 h | Humanized monoclonal antibody fragment; neutralizes the anticoagulant effect of dabigatran by irreversibly binding to it and its metabolites | Life‒threatening bleeding Need emergency surgery/urgent invasive procedure Specific for dabigatran | 9.5–10.8 h | 5–g (2 × 2.5 g vials) IV bolus | Redosing was used in ≈2% of patients ≈ $4,452 per administration |
| Andexanet alfa | Direct factor Xa inhibitors (apixaban and rivaroxaban) | <18 h | Recombinant modified human factor Xa (activated factor X) molecule, which acts as a decoy by reversibly binding to factor Xa inhibitors, thereby reducing their availability to act on endogenous factor Xa | Life-threatening or uncontrolled bleeding Specific for direct factor Xa inhibitors, approved only for apixaban and rivaroxaban | 5–7 h | Low dose: ≥8 h since last dose or ≤ 5 mg of apixaban or ≤10 mg of rivaroxaban Initial IV bolus: 400 mg; target infusion rate of 30 mg/min; then IV infusion: 4 mg/min for up to 120 min High dose: <8 h since last dose or unknown and >5 mg of apixaban or >10 mg of rivaroxaban Initial IV bolus: 800 mg; target infusion rate of 30 mg/min; then IV infusion: 8 mg/min for up to 120 min |
Low vs high dose based on timing from last dose and dosage amount of Xa inhibitor May need to redose, although no clinical data currently exist Low dose: ≈ $33,000 per administration High dose: ≈ $59,400 per administration |
| Protamine | UFH and LMWH (enoxaparin, dalteparin) | 1–6 h (unfractionated heparin); SQ doses of UFH take longer to Absorb 3–12 h (low-molecular‒ weight heparin); SQ doses of LMWH take longer to absorb |
A weak anticoagulant that binds heparin and forms inactive complex | Neutralization of heparin or low-molecular-weight heparin | Heparin: 1 mg of protamine will neutralize not less than 100 units of heparin; slow IV injection during 10 min, up to max of 50 mg/dose Dalteparin: 1 mg IV for every 100 anti-Xa IU of dalteparin Enoxaparin: 1 mg IV for every 1 mg of enoxaparin administered in the previous 8 h; if >8 h has elapsed since the last dose, a second infusion of 0.5 mg per 1 mg of enoxaparin may be given | ≈ $10 to $133 per administration (50– to 450–mg range, with higher range representing average dosing in cardiothoracic surgery patients for UFH reversal) | |
| Replacement | |||||||
| 4‒factor PCC‡ (Kcentra in the US) | Approved use: vitamin K antagonists (warfarin) Nonapproved use: DOACs | Varies | Contains nonactivated vitamin K-dependent coagulation factors II, VII, IX, and X, and antithrombotic protein C and protein S, heparin, albumin | Urgent reversal of vitamin K antagonist therapy for treatment of major bleeding, surgical procedures, or both | Factor dependent: 4.2–59.7 h | Warfarin: individualize dose by INR and IBW: INR-based 25–50 IU/kg IV, max 2,500–5,000 IU with concurrent vitamin K or 1,500 IU ×l; may repeat 500 units if bleeding continues DOACs: life ‒ threatening or critical site: 10–25 units/kg and repeated dose in 1–2 h if bleeding continues or if clinically indicated. May consider 50 units/kg up to a suggested maximum of 100 units/kg Nonlife‒threatening or noncritical site: 10 units/kg ×l and repeated dose in 1–2 h if bleeding continues | Warfarin: coadminister with IV vitamin K DOAC: a median (interquartile range) dose of 2,000 IU (1,500–2,000 IU) typically given with less or more needed for patients under or over 65 kg, respectively. Contains heparin so should not be used in patients with confirmed or strongly suspected HIT z$5,075 to $20,300 per administration (25–100 units/kg=1,750 − 7,000 units) |
| 3-factor PCC‡ (Profilnine SD and Bebulin VH in the US) | Factor replacement in patients with hemophilia B Not recommended for DOAC reversal† | Varies | Contains factors II, IX, and X | Hemorrhage in patients with hemophilia B | 24–32 h | No. of factor IX IU required=body weight (kg) × desired factor IX increase (%)×l IU/kg | Not recommended for DOAC reversal ≈$2,818 to $11,270 per administration (25–100 units/kg=1,750–7,000 units) |
| aPCC‡ (FEIBA in the US) | Approved use: factor replacement patients with hereditary factors VIII and IX deficiency and nonhemophiliacs with acquired inhibitors to factors VIII, IX, and XI Nonapproved use: surgical coagulopathy such as trauma or cardiothoracic; DOAC bleeding reversal | Varies | Contains II, VII, IX, and X with primarily activated factor VII and nonactive factors II, IX, and X | Hemorrhage in patients with hereditary factor VII and IX deficiency disease with inhibitor; both for prophylaxis preprocedure and for active bleeding | 72 h (factor II) | Maximum: single dose, 100 units/kg; daily dose, 200 units/kg DOACs: Life‒threatening or critical site: 10–25 units/kg and repeated dose in 1–2 h if bleeding continues or if clinically indicated. May consider 50 units/kg up to a suggested maximum of 100 units/kg Nonlife‒threatening or noncritical site: 10 units/kg ×l and repeated dose in 1–2 h if bleeding continues | ≈ $4,725 to $18,900 per administration (25–100 units/kg=1,750 to 7,000 units) |
| Vitamin K | Vitamin K antagonists (warfarin) | Varies | Vitamin necessary for the hepatic synthesis of factors II, VII, IX, and X Cofactor for a microsomal enzyme that triggers the posttranslational carboxylation of peptide‒bound glutamic acid residues into active coagulation factor | Acquired hypoprothrombinemia and hypoprothrombinemia secondary to anticoagulant | NA | Nonbleeding patients: 2.5–25 mg PO to decrease INR Major bleeding: 5 to 10 mg slow IV injection, in addition to 4-factor PCC |
Initial onset typically 3–6 h if given IV; maximal initial effect on INR usually occurs in ≈16–20 h For bleeding patients receiving warfarin, give vitamin K first and before 4F‒PCC (Kcentra) if 4F‒PCC used Oral: ≈ $67 to $353 per administration (5–25 mg PO) IV: $135 to $270 per administration (5–10 mg IV) |
| Fresh frozen plasma | Vitamin K antagonists (warfarin) | Varies | Comprised plasma proteins such as albumin, immunoglobulins, coagulation factors, complement proteins, and protease inhibitors | Acquired combined coagulation factor deficiency caused by liver disease, or undergoing cardiac surgery or liver transplant TTP | NA | 10–15 mL/kg IV for replacement of coagulation factors in patients with acquired deficiencies | Thawing requires 15–30 min, depending on the quantity Likely ineffective for DOAC reversal ≈$168 per administration (4 units) |
| Other | |||||||
| Tranexamic acid | Not drug specific | NA | Competitive inhibitor of plasminogen activation | IV: patients with hemophilia for short-term use to reduce or prevent hemorrhage and reduce the need for replacement therapy during and after tooth extraction Oral: heavy cyclic menstrual bleeding |
IV: 2 h PO: 11 h | Trauma: 1,000 mg administered IV during 10 min, followed by 1,000 mg infused during the subsequent 8 h | ≈ $18 to $174 per administration (1,000–2,000 mg) |
UFH, Unfractionated heparin; LMWH, low-molecular-weight heparin; US, United States; DOAC, direct oral anticoagulant; aPCC, activated prothrombin complex concentrate; TTP, thrombotic thrombocytopenic purpura.
Assumes normal renal and hepatic function and normal body mass index.
Cost based on typical initial administration for a 70-kg patient, according to the Medi-Span Average Wholesale Price as of June 2019. FFP price is based on a cost of $41.95 per unit (from Shander et al 65).
Dosing of PCC/aPCC products is expressed as units of factor IX.
Complementary treatment strategies include tranexamic acid. The most compelling evidence for tranexamic acid is in traumatic or gynecologic and obstetric hemorrhage.44,45 Case reports suggest that topical or nebulized tranexamic acid can be an effective adjunct in ear, nose, and throat bleeding, such as epistaxis, dental bleeding, or post-tonsillectomy bleeding.46,47 Although tranexamic acid is inexpensive, widely available, and a well-tolerated treatment with minimal thrombotic risks, there are insufficient data to establish a role for it outside of these scenarios.48–50
Nonbleeding anticoagulated patients with either intentional (eg, suicide attempt) or unintentional overdose (eg, acute renal dysfunction, drug-drug interaction, medication error) present unique considerations. Data supporting activated charcoal in overdose of an oral anticoagulant are poor, and it should be considered only in cooperative fully awake patients (eg, obviating the need for a nasogastric tube) who present within 1 to 2 hours of acute ingestion without anticipated need for endoscopy.51 Patients should be monitored for 2 to 5 half-lives of the applicable agent according to renal and hepatic function, drug pharmacokinetics, coingestants, comorbidities, and clinical status. Patients receiving warfarin have a significantly prolonged exposure to bleeding risk (even in the nonbleeding patient), given the long half-life compared with direct oral anticoagulants. Vitamin K is indicated if the international normalized ratio is significantly elevated, even in a nonbleeding patient; factor replacement is generally not indicated in the nonbleeding patient. In addition, recent cases of the drugs K2 or spice contaminated with synthetic vitamin K antagonists with extraordinarily long half-lives should be noted and managed with guidance from a poison control center or a toxicologist.52
Reversal or replacement strategies may be indicated for nonbleeding anticoagulated patients requiring an emergency procedure with significant bleeding risk (eg, ischemic bowel requiring a laparotomy). Treatment decisions involve assessing the risk of procedural-related bleeding without replacement or reversal, the risk of progression of illness requiring the procedure should it be delayed, and the benefit of replacing or reversing the anticoagulant. Considerations include the time of last anticoagulant dose and half-life and comorbidities affecting metabolism and excretion, such as acute renal insufficiency for dabigatran or edoxaban. The specific indication for anticoagulation should also be considered (eg, prophylaxis for a mechanical heart valve versus nonvalvular atrial fibrillation, with CHA2DS2-VASc [congestive heart failure, hypertension, age 75 years or older, disbetes mellitus, stroke, transient ischemic attack or thromboembolism, vascular disease, age 65 to 74 years, sex category (female)] score of 2). If the risks of delay are sufficiently high and the procedure urgently indicated while the anticoagulant is active, reversal or replacement may be indicated and follows the same decision tree as is used for the bleeding patient.38,53–55 Andexanet alfa (as distinct from idarucizumab and 4-factor prothrombin complex concentrate) is not currently FDA approved for nonbleeding patients requiring urgent or emergency procedures or surgeries.
Finally, the timing of restarting anticoagulation after a bleeding episode is an important consideration. It involves balancing the severity, location, and consequence of the bleeding event with the indication of anticoagulation, associated thrombotic risk, and possibility of rebleeding. The timing of resuming anticoagulation should be deferred to the inpatient teams.
LIMITATIONS
Our approach had several limitations. First, we did not perform a systematic literature review consistent with Preferred Reporting Items for Systematic Reviews and Meta-analyses standards.56 Our process permitted discussion of best practices when evidence was lacking; guidelines limited to systematic review were necessarily restricted to interventions with the highest quality of evidence. Second, the topic of anticoagulation reversal or replacement is a dynamic field that will continue to evolve. It is intended to be adaptable to local resource availability (ie, access to specific reversal agents) and will require updating as the evidence evolves. Third, a common criticism of expert panel recommendations is overrepresentation by urban, academic physicians, which risks making the recommendations irrelevant to clinicians practicing in different settings. Although we assembled a panel of clinicians from academic and community settings, as well as urban and rural settings, by definition consensus necessitated compromise. Fourth, these recommendations do not include a cost-benefit analysis. This may be a consideration of treatment; however, the agents have been on the market only a short time and data are lacking. Additionally, multiple confounders of critically ill patients make cost-effectiveness data difficult to interpret. Future considerations include price changes, new and generic agents, and new treatment options. Therefore, the panel chose not to base recommendations on the relative expense of various treatment options.
DISCUSSION
Our multidisciplinary expert panel developed a stepwise guidance framework to aid the emergency physician’s approach to the anticoagulated patient who is bleeding or not bleeding but requiring an emergency procedure. This framework provides interventions that should be considered in all bleeding patients in parallel with a determination of whether the patient is experiencing life-threatening or critical-site bleeding warranting the use of reversal or replacement agents.
There are 3 critical considerations in managing the bleeding patient receiving anticoagulation: Is the bleeding event life threatening, is the site critical, and what are the agent, dose, and most recent time received? The most widely used bleeding definitions come from trial-based literature and are focused on research applications for a specific anticoagulant, patient population, or both.25–31 The Bleeding Academic Research Consortium definition used here is well suited to apply to anticoagulated patients likely to be encountered in the ED.34 Additionally, reversal study definitions of inclusion criteria reflected actual cases and are therefore useful as a bedside tool.42,53,57
This guidance is intended to be a bedside tool for the physicians caring for these critically ill patients. However, nuances were difficult to capture in the simplified format of the decision tree. Definitions of life-threatening bleeding, critical sites, and emergency surgery or urgent procedure in the nonbleeding patient warranting reversal or replacement are sensitive to clinical context. Clinicians should carefully weigh the risks and benefits of the various treatment options, with shared decision making among the patients, families, proxies, and consulting physicians, and appreciate the subtleties of when replacement or reversal is or is not indicated.58,59
Emergency medicine involves a diversity of practice environments and resources, and therefore this framework offers 2 tiers of recommendations. Tier 1 recommendations are most aligned with FDA-approved indications and the highest quality of evidence supporting their use. Tier 2 recommendations are offered as alternatives in circumstances in which tier 1 interventions are not available, have limited supporting data, and may be off label. Poison control centers may serve as an additional resource to guide optimal transfer facility targets based on availability of specific reversal agents.
The available trial data supporting replacement and reversal agents and their costs have generated controversy.60 The lack of a comparator arm in the REVERSE-AD (A Study of the RE-VERSal Effects of Idarucizumab on Active Dabigatran) and ANNEXA-4 (Andexanet Alfa, a Novel Antidote to the anticoagulation Effects of Factor Xa Inhibitors) trials, reports of thrombotic events in treated study subjects, and high cost of andexanet alfa relative to prothrombin complex concentrates generated concerns.61,62 Ongoing investigations aim to address several of these areas, but currently available data are limited.63 Clinical efficacy was demonstrated in the REVERSE-AD and ANNEXA-4 trials but not included in FDA labeling for andexanet alfa. Furthermore, thrombotic events after anticoagulant reversal may be due to the patient’s intrinsic prothrombotic state, the bleeding scenario, or the reversal agent. The thrombotic event rate confidence intervals of all the trials overlap substantially; however, cross comparison of trials is complicated, given their different designs. No thrombotic events occurred in healthy volunteers in phase 3 andexanet alfa trials, and 9.7% experienced an event in the trial of 352 patients with major bleeding.61 Replacement strategies used before andexanet alfa’s availability (eg, prothrombin complex concentrates) are prothrombotic.64 Andexanet alfa’s cost is significant; however, health economic data are currently lacking.
Use of replacement or reversal agents is only one component of care in these critically ill patients. Treatment of the patient with a life-threatening bleeding event may likely require other interventions, such as surgery, interventional radiology, or endoscopy procedures. Specialty consultation to expedite these interventions should be requested in parallel with other treatments. In settings in which patient transfer is required, this should be initiated as early as possible. It may be beneficial to initiate replacement or reversal before transfer as long as it does not prolong transfer for definitive care.
In conclusion, we developed a multidisciplinary anticoagulant reversal and replacement guidance statement supported by literature and consensus definitions to support evaluation and treatment of the bleeding patient and nonbleeding patient requiring emergency invasive procedures. Emergency physicians encounter a majority of anticoagulation-associated hemorrhages and should be supported by hospital resources to rapidly deploy evidence-based treatments. New resources such as reversal and replacement agents offer more treatment options but increase the complexity of treatment decisions. Further research is needed to answer key questions that could further clarify the role of specific reversal or replacement agent treatment regimens to aid targeting of therapies to where they have the most clinical influence.
Supplementary Material
Acknowledgments
The authors acknowledge other panel members and support staff for their contributions to this project: Riane V. Gay, MPA, Travis Schulz, MLIS, and Sandy M. Schneider, MD, from the American College of Emergency Physicians, Dallas, TX; Alan K. Jacobson, MD, from the Department of Medicine, Jerry Pettis VA Medical Center, Loma Linda, CA; and Deborah Kallina, MLIS, and Karen D. Pate, PhD.
Funding and support:By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The expert panel meeting was convened with funds from unrestricted educational grants from Portola Pharmaceuticals and Boehringer Ingelheim to the American College of Emergency Physicians. Dr. Baugh has worked as a consultant for Janssen Pharmaceuticals and previously received research funding from Janssen Pharmaceuticals and Boehringer Ingelheim as a coinvestigator. Dr. Cornutt has received speaker’s fees from Boehringer Ingelheim. Dr. Wilson has worked as a consultant for Janssen Pharmaceuticals, Boehringer Ingelheim, BMS/Pfizer Pharmaceuticals, and Portola Pharmaceuticals, and has also received research funding from them. Dr. Mahan has served on the speaker’s bureau and as a consultant for Boehringer Ingelheim, Janssen Pharma, Portola Pharma, and BMS/Pfizer and as a consultant to Daiichi-Sankyo, WebMD/Medscape, and Pharmacy Times/American Journal of Managed Care. Dr. Pollack is a scientific consultant to Boehringer Ingelheim, Janssen Pharma, Portola Pharma, and BMS/Pfizer; he also received research support from Boehringer Ingelheim, Janssen Pharma, Portola Pharma, Daiichi-Sankyo, CSL Behring, and AstraZeneca. Dr. Milling’s salary is supported by a grant from the National Heart, Lung, and Blood Institute. He serves on the executive committee for the ANNEXA-4 and ANNEXA-I trials, the steering committee for the LEX-209 trial, and the publications committee for the Kcentra trials. He has received consulting fees or research funding from CSL Behring, Portola, Boehringer Ingelheim, Genentech, and Octapharma. He received speaker’s fees from Janssen. Dr. Peacock has received research grants from Abbott, Boehringer Ingelheim, Braincheck, CSL Behring, Daiichi-Sankyo, Immunarray, Janssen, Ortho Clinical Diagnostics, Portola, Relypsa, and Roche. He has served as a consultant to Abbott, AstraZeneca, Bayer, Beckman, Boehringer-Ingelheim, Ischemia Care, Dx, Immunarray, Instrument Labs, Janssen, Nabriva, Ortho Clinical Diagnostics, Relypsa, Roche, Quidel, and Siemens. He has provided expert testimony on behalf of Johnson & Johnson and has stock and ownership interests in AseptiScope Inc, Brainbox Inc, Comprehensive Research Associates LLC, Emergencies in Medicine LLC, and Ischemia DX LLC. Dr. Rosovsky has served as an advisor or consultant to Janssen, BMS, and Portola and has received institutional research support from Janssen and BMS. Dr. Sarode has served as a consultant for CSL Behring and Octapharma and advisor to Portola Pharmaceuticals. Dr. Spyropoulos is a scientific consultant to Janssen, Bayer, Boehringer Ingelheim, Portola, and the ATLAS Group; he also has received research support from Janssen and Boehringer Ingelheim. Dr. Woods is a scientific consultant to Boehringer Ingelheim. Dr. Williams serves as a consultant to Janssen Pharmaceuticals, Boehringer Ingelheim, and Portola Pharmaceuticals.
Footnotes
Although unrestricted educational grants from Portola Pharmaceuticals and Boehringer Ingelheim to ACEP offset the costs of lodging and travel for one in-person meeting, there was no industry involvement in designing, developing, or editing the work product.
REFERENCES
- 1.Shehab N, Lovegrove MC, Geller AI, et al. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316:2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute for Safe Medication Practices. QuarterWatch™ (2016 Annual Report) Part II: Oral Anticoagulants—The Nation’s Top Risk of Acute Injury from Drugs. July 27, 2017. Available at: https://www.ismp.org/resources/quarterwatchtm-2016-annual-report-part-ii-oral-anticoagulants-nations-top-risk-acute. Accessed November 6, 2019.
- 3.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moodley O, Goubran H. Should lifelong anticoagulation for unprovoked venous thromboembolism be revisited? Thromb J. 2015;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–1222. [DOI] [PubMed] [Google Scholar]
- 6.Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 8.Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. [DOI] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. [DOI] [PubMed] [Google Scholar]
- 10.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 11.Lamy A, Eikelboom J, Sheth T, et al. Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: the COMPASS-CABG study. J Am Coll Cardiol. 2019;73:121–130. [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 15.Mahan CE. Practical aspects of treatment with target specific anticoagulants: initiation, payment and current market, transitions, and venous thromboembolism treatment. J Thromb Thrombolysis. 2015;39:295–303. [DOI] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;140:e125–e151; Cir0000000000000665. [DOI] [PubMed] [Google Scholar]
- 17.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Alexander GC, Nazarian S, et al. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy. 2018;38:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziakas PD, Kourbeti IS, Poulou LS, et al. Medicare Part D prescribing for direct oral anticoagulants in the United States: cost, use and the “rubber effect.” PLoS One. 2018;13:e0198674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 21.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 22.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen PB, Skjoth F, Sogaard M, et al. Non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with intracerebral hemorrhage. Stroke. 2019;50:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansell JE. Universal, class-specific and drug-specific reversal agents for the new oral anticoagulants. J Thromb Thrombolysis. 2016;41:248–252. [DOI] [PubMed] [Google Scholar]
- 25.Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. [DOI] [PubMed] [Google Scholar]
- 26.Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. [DOI] [PubMed] [Google Scholar]
- 27.Eikelboom JW, Mehta SR, Anand SS, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 28.Amlani S, Nadarajah T, Afzal R, et al. Mortality and morbidity following a major bleed in a registry population with acute ST elevation myocardial infarction. J Thromb Thrombolysis. 2010;30:434–440. [DOI] [PubMed] [Google Scholar]
- 29.Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–209. [DOI] [PubMed] [Google Scholar]
- 30.Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815–1823. [DOI] [PubMed] [Google Scholar]
- 31.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 32.Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. [DOI] [PubMed] [Google Scholar]
- 33.Khorsand N, Majeed A, Sarode R, et al. Assessment of effectiveness of major bleeding management: proposed definitions for effective hemostasis: communication from the SSC of the ISTH. J Thromb Haemost. 2016;14:211–214. [DOI] [PubMed] [Google Scholar]
- 34.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 35.Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:3042–3067. [DOI] [PubMed] [Google Scholar]
- 36.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–2613. [DOI] [PubMed] [Google Scholar]
- 38.Schulman S, Gross PL, Ritchie B, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118:842–851. [DOI] [PubMed] [Google Scholar]
- 39.Majeed A, Agren A, Holmstrom M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706–1712. [DOI] [PubMed] [Google Scholar]
- 40.Voils SA, Baird B. Systematic review: 3-factor versus 4-factor prothrombin complex concentrate for warfarin reversal: does it matter? Thromb Res. 2012;130:833–840. [DOI] [PubMed] [Google Scholar]
- 41.DeAngelo J, Jarrell D, Cosgrove R, et al. Comparison of 3-factor versus 4-factor prothrombin complex concentrate with regard to warfarin reversal, blood product use, and costs. Am J Ther 2018;25:e326–e332. [DOI] [PubMed] [Google Scholar]
- 42.Connolly SJ, Milling TJ Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Food A Andexanet alfa [package insert]. Silver Spring, MD: Food and Drug Administration; 2018. [Google Scholar]
- 44.Shiraishi A, Kushimoto S, Otomo Y, et al. Effectiveness of early administration of tranexamic acid in patients with severe trauma. Br J Surg. 2017;104:710–717. [DOI] [PubMed] [Google Scholar]
- 45.Brenner A, Shakur-Still H, Chaudhri R, et al. The impact of early outcome events on the effect of tranexamic acid in post-partum haemorrhage: an exploratory subgroup analysis of the WOMAN trial. BMC Pregnancy Childbirth. 2018;18:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz W, Ruttan T, Bundick K. Nebulized tranexamic acid use for pediatric secondary post-tonsillectomy hemorrhage. Ann Emerg Med. 2019;73:269–271. [DOI] [PubMed] [Google Scholar]
- 47.Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771–776. [DOI] [PubMed] [Google Scholar]
- 48.Honda Y, Furugohri T, Morishima Y. Tranexamic acid failed to reverse the anticoagulant effect and bleeding by an oral direct factor Xa inhibitor edoxaban. Pharmacology. 2018;101:92–95. [DOI] [PubMed] [Google Scholar]
- 49.Levy JH, Moore KT, Neal MD, et al. Rivaroxaban reversal with prothrombin complex concentrate or tranexamic acid in healthy volunteers. J Thromb Haemost. 2018;16:54–64. [DOI] [PubMed] [Google Scholar]
- 50.Levine M, Huang M, Henderson SO, et al. Aminocaproic acid and tranexamic acid fail to reverse dabigatran-induced coagulopathy. Am J Ther. 2016;23:e1619–e1622. [DOI] [PubMed] [Google Scholar]
- 51.Ollier E, Hodin S, Lanoiselee J, et al. Effect of activated charcoal on rivaroxaban complex absorption. Clin Pharmacokinet. 2017;56:793–801. [DOI] [PubMed] [Google Scholar]
- 52.Boyack I, Opsha O. Coagulopathic hemorrhage with use of synthetic cannabinoids. Am J Emerg Med. 2019;37:374, e373–374.e374. [DOI] [PubMed] [Google Scholar]
- 53.Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal: full cohort analysis. N Engl J Med. 2017;377: 431–441. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein JN, Refaai MA, Milling TJ Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385: 2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dager WE, Roberts AJ, Nishijima DK. Effect of low and moderate dose FEIBA to reverse major bleeding in patients on direct oral anticoagulants. Thromb Res. 2019;173:71–76. [DOI] [PubMed] [Google Scholar]
- 56.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansen M, Wikkelso A, Lunde J, et al. Prothrombin complex concentrate for reversal of vitamin K antagonist treatment in bleeding and non-bleeding patients. Cochrane Database Syst Rev. 2015;7: CD010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hess EP, Grudzen CR, Thomson R, et al. Shared decision-making in the emergency department: respecting patient autonomy when seconds count. Acad Emerg Med. 2015;22:856–864. [DOI] [PubMed] [Google Scholar]
- 59.Grudzen CR, Anderson JR, Carpenter CR, et al. The 2016 Academic Emergency Medicine consensus conference, shared decision making in the emergency department: development of a policy-relevant patient-centered research agenda May 10, 2016, New Orleans, LA. Acad Emerg Med. 2016;23:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radecki RP, Spiegel RJ. Adventures with andexanet alfa in efficacy, effectiveness, and one-armed studies: May 2019 Annals of Emergency Medicine Journal Club. Ann Emerg Med. 2019;73:545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Food and Drug Administration. Summary Bias for Regulatory Action. May 3, 2018. Available at: https://www.fda.gov/media/113954/download. Accessed November 6, 2019.
- 63.Population Health Research Institute. Trial of andexanet in ICH patients receiving an oral FXa inhibitor. Portola Pharmaceuticals Available at: https://clinicaltrials.gov/ct2/show/NCT03661528?term=Andexanet&rank=7. Accessed November 6, 2019.
- 64.Sorensen B, Spahn DR, Innerhofer P, et al. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care. 2011;15:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shander A, Ozawa S, Hofmann A. Activity-based costs of plasma transfusions in medical and surgical inpatients at a US hospital. Vox Sanguinis. 2016;111:55–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


