Abstract
Laminin-332 is a basement membrane protein composed of three genetically distinct polypeptide chains that actively promote both skin epidermal cell adhesion and migration. Proteolytic fragments of the laminin γ2 chain stimulate migration and scattering of keratinocytes and cancer cells. Sulfur mustard (SM) is a bifunctional alkylating agent that induces separation of basal keratinocytes from the dermal-epidermal junction and invokes a strong inflammatory response leading to delayed wound repair. In the present studies, the role of laminin γ2 in SM-induced skin injury and wound repair was investigated using the mouse ear vesicant model. We found that laminin γ2 chain mRNA was preferentially upregulated in mouse ear skin exposed to SM. In situ hybridization confirmed overexpression of laminin γ2 transcript. Western blot analysis showed increased protein expression of the full-length proform of laminin γ2 and smaller processed fragments of laminin γ2 in skin exposed to SM. Dual immunofluorescence labeling indicated that laminin γ2 fragments are prevalent in suprabasal keratinocytes behind the leading edge in areas of hyperplasia in injured skin. In addition, co-expression of laminin γ2 and the senescent marker, p16INK4a was found to overlap with the hyperplastic migratory epithelial sheet. This observation is similar to hypermotile keratinocytes reported in invasive carcinoma cells. Overall, our studies indicate that laminin γ2 is preferentially expressed in skin post SM exposure and that protein expression appears to become progressively more fragmented. The laminin γ2 fragments may play a role in regulating SM-induced skin wound repair.
Keywords: laminin-332, vesicant sulfur mustard, skin wound healing, cellular migration, basement membrane
Skin exposure to the alkylating agent, sulfur mustard (SM), results in a wound that has some unique characteristics when compared to other wound types (e.g., incisional, chemical, or thermal wounds). The main difference between SM-induced wounds and other wound types is the increased time for repair of the alkylated wound (Shakarjian et al., 2010; Graham and Schoneboom, 2013). Incisional cutaneous wound repair follows several distinct phases in an ordered progression including coagulation, inflammation, migration/proliferation, and remodeling (Martin, 1997; Singer and Clark, 1999; Su and Richmond, 2015). However, skin exposed to SM induces fluid-filled blisters accompanied by a strong inflammatory response that causes extensive tissue damage (Casillas et al., 1997; Sabourin et al., 2002; Shakarjian et al., 2006; Gerecke et al., 2009; Joseph et al., 2011; Mouret et al., 2015; Achanta et al., 2018; Chang et al., 2018b). In addition, a chronic recurrent state of inflammation often occurs. Delayed wound repair may contribute to scarring commonly seen in SM injured skin.
Human skin exposed to SM induces focal separation of the epidermis at the dermal-epidermal junction (DEJ) of skin within 24 hr (Monteiro-Riviere et al., 1999; Chang et al., 2018a). The blister plane induced by alkylating agents such as SM coincides precisely with the histological location of laminin-332 (LM-332). In normal healthy skin, basal keratinocytes adhere to the basement membrane and the underlying dermis by stable anchoring complexes (made of type VII collagen) attached to anchoring filaments composed of LM-332 (Marinkovich et al., 1993; Rousselle et al., 1997, 2019; Brittingham et al., 2006; Marinkovich, 2008; Rousselle and Beck, 2013). LM-332 is a glycoprotein made up of three polypeptide chains (α3, β3, and γ2) that are distinct gene products (see Fig. 1 for structure of LM-332). It is expressed and secreted as a full-length heterotrimer in the extracellular matrix (Marinkovich et al., 1992; Matsui et al., 1995b; Uitto and Pulkkinen, 1996; Aumailley and Rousselle, 1999; Aumailley et al., 2003). Once secreted, all three chains of LM-332 are proteolytically processed and initiate keratinocyte adhesion for basement membrane attachment, a critical step in wound repair (Gagnoux-Palacios et al., 1996, 2001; Amano et al., 2000; Sasaki et al., 2001; Koshikawa et al., 2005; Aragona et al., 2017). In vitro studies show multiple proteolytic processing sites in the amino-terminal region of γ2 chains (Gilles et al., 2001; Ogawa et al., 2004; Hamasaki et al., 2011). After cutaneous injury, the proteolytic processing of the γ2 chain in particular is found in abundance in the leading edge of migrating keratinocytes (Kainulainen et al., 1998; Gagnoux-Palacios et al., 2001; Katayama et al., 2003; Rousselle et al., 2019). The proteolytic fragments of laminin γ2 chain are known to functionally change the laminin from cell adhesion to cell migration during wound healing and in invasive carcinoma cells (Pyke et al., 1994; Matsui et al., 1995a; Kainulainen et al., 1998; Katayama et al., 2003; Katayama and Sekiguchi, 2004; Nguyen et al., 2006; Ogawa et al., 2007; Hamasaki et al., 2011; Rousselle and Beck, 2013; Yasuda et al., 2019).
Figure 1.

Schematic diagram of laminin-332 (LM-332). LM-332 is a heterotrimer made up of three separate polypeptide chains (α3 shown in green, β3 shown in yellow, and γ2 shown in purple). Each chain contains a long arm in the C terminus and epidermal growth factor-like globular domains (EGF) domains in the N-terminus. The three chains are folded into a cross-shaped trimer, which are held together by an α-helical coiled-coil structure in the long arm region. LM-332 can be further processed at several proteolytic cleavage sites (indicated by arrows) (A). The proform of the γ2 chain of LM-332 (150 kDa) can be proteolytic processed to the mature form (105 kDa) when incorporated into three polypeptide chains. During injury and wound repair, the γ2 chain of LM-332 can be further cleaved into fragments (e.g., 80 and 45 kDa) of laminin γ2, which is also known as cell-scattering factor. (B) shows the specific laminin γ2 antibody (LCC) target epitopes in the C-terminus of the long form coiled-coil region; the antibody recognizes the proform (γ2) and processed forms (γ2′, and γ2x) of the laminin γ2 chain. (C) shows the specific laminin γ2 antibody (L4) target epitopes in the region of the globular domain; the antibody recognizes the proform (γ2) and the globular processed forms including the short arm of laminin γ2 chain (γ2-sa), and the proteolytic fragment of laminin γ2 chain (γ2-pf).
During acute normal wound injury, keratinocytes proliferate and migrate as a sheet for wound closure. Motility of the migratory keratinocytes is critical for proper re-epithelization and wound repair (Raja et al., 2007; Rousselle et al., 2019). In chronic wounds and in early neoplastic invasion, studies show that co-expression of laminin γ2 and p16INK4a is associated with hypermotility of migratory keratinocytes (Natarajan et al., 2005, 2006). p16INK4a is a cell senescent marker expressed by epidermal keratinocytes in the migrating front and in invasive lesions (Natarajan et al., 2003; D’Arcangelo et al., 2017). The co-expression of laminin γ2 and p16INK4a suggests promotion of epithelial migration in keratinocytes that have left the cell cycle. Skin exposed to SM results in severe hyperplasia which is consistently observed in the epithelium (Chang et al., 2009, 2013, 2014). The present studies examined the expression of laminin γ2 and the senescence marker, p16INK4a in an attempt to pinpoint their spatial and temporal expression in vivo. A better understanding of the role of laminin γ2 in wound repair may lead to the development of therapies to promote healing following SM-induced skin injury.
MATERIALS AND METHODS
Animals and Exposures
A mouse ear vesicant model was used in all studies (Chang et al., 2018b). All SM exposures were performed at Battelle Memorial Institute (Columbus, OH). Male CD1 mice (25–35 g, N = 10 per group; Charles River Laboratories, Portage, MI) were anesthetized with ketamine and xylazine. Next, 5 μl of 97.5 mM SM (0.08 mg) was diluted in CH2Cl2 and placed on the ventral side (inner ear) of the right ear to induce SM skin wounds. The left ear was used as a vehicle control. Skin punch biopsies were collected and processed immediately for histopathology, immunohistochemistry, in situ hybridization and mRNA and protein expression at 24, 72, and 168 hr post exposure.
RNA Extraction and TaqMan Real-Time Polymerase Chain Reaction
Total RNA was isolated from frozen punch biopsies using TRIzol reagent (Invitrogen Corporation, Carlsbad, CA). Total RNA (1 μg) for each sample was reverse-transcribed into cDNA using a first-strand synthesis system (Invitrogen Corporation), including a minus reverse transcriptase reaction as a control. The mRNA expression levels of the three LM332 polypeptide chains, the laminin subunit α3 gene (LAMA3), the laminin subunit β3 gene (LAMB3), and the laminin subunit γ2 gene (LAMC2), were measured using primers and probe sets designed by TaqMan gene expression assays (Applied Biosystems, Foster City, CA); hypoxanthine-guanine-phosphoribosyl-transferase was used as an endogenous control. Real-time polymerase chain reaction (RT-PCR) was performed on an ABI Prism 7900 real time PCR system (Applied Bio-systems). All template controls were negative after amplification.
In Situ Hybridization for Laminin γ2 Chain mRNA
In situ hybridization was performed using a DIG-RNA labeling kit (Roche, Indianapolis, IN) following the manufacture’s protocol to detect mouse laminin γ2 mRNA expression. Briefly, 163 bp (2346 f – 2509 rc) mouse laminin γ2 (NCBI reference sequence XM_011247927.1) cDNA was made from total RNA from mouse skin (Invitrogen Corporation). This cDNA was used as a template for RT-PCR, and the product was inserted into a pCRII-TOPO vector (Invitrogen Corporation) for cloning as per the manufacturer’s instructions. Once the insert orientation was confirmed, the insert (plus some vector) was cut using Kpn-I and Xba-I. Sense and antisense primers were designed containing the T7-promoter plus mouse laminin γ2, or the Sp6 promoter plus mouse laminin γ2. These primers were used in PCR reactions with the cut insert to produce the template for making the RNA sense and antisense probes (DIG RNA labeling Kit (SP6/T7) kit, Roche). These DIG-labeled probes were used to hybridize to 10 μm mouse skin sections.
Tissue sections that were embedded in paraffin were deparaffinized, rehydrated through a series of graded ethanol baths, and finally into water. Sections were fixed in 4% paraformaldehyde, digested with proteinase K (37°C for 15 min), and washed with DEPC-treated PBS. In situ hybridization was performed using a modified protocol from Roche. Slides were treated with ISH pre-hybridization solution (BioChain Institute, Hayward, CA) for 3 hr at 50°C. RNA probes were diluted (100 ng/mL) into ISH hybridization solution (BioChain Institute), denatured at 80°C for 5 min, applied to tissue sections, and incubated at 50°C for 12–16 hr. Slides were sequentially washed in 2× SSC, 50°C, 20 min; 1.5× SSC, 50°C, 20 min; and twice in 0.2× SSC, 37°C, 20 min. Tissue sections were then incubated against 1× NaCl-Tris-EDTA buffer (NTE) for 10 min, RNase for 30 min, and 1× NTE again for 10 min. Slides were incubated with 1× blocking buffer (Roche) for 1 hr at room temperature, followed by anti-digoxigenin-AP antibody (1:500 with blocking buffer) (Roche) for 1 hr. Color was developed by NTB/BCIP according to the manufacturer’s recommendations (Roche). Slides were mounted with Permount (Fisher Scientific, Fair Lawn, NJ). The sense RNA probes were applied to adjacent sections for nonspecific probe binding as negative controls, and these probes were consistently negative.
Western Blotting
Skin samples were pulverized and homogenized with stainless steel grinding beads (OPS Diagnostics, LLC, Lebanon, NJ) using a GenoGrinder in the presence of protease inhibitors (Complete Mini, EDTA-free Protease Inhibitor Cocktail Tablets, Roche) in RIPA buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% Nadeoxycholate, and 0.1% SDS) and assayed for protein concentration using BCA Protein Assay Reagent (Pierce Chemical, Rockford, IL). Pooled protein lysates (12 μg, N = 10 per group) were analyzed using 5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Life Sciences Group, Hercules, CA). The membranes were blocked with 5% nonfat milk solution and incubated with a mouse anti-laminin γ2 monoclonal antibody (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C, followed by goat anti-mouse IgG horseradish peroxidase conjugated secondary antibody (1: 6,000; BioRad). Proteins were detected by chemiluminescence (SuperSignal West Pico Chemiluminescence Substrate System, Pierce Chemical). GAPDH was used to monitor equal protein loading.
Histology
Tissues were fixed in 10% (vol/vol) neutral buffered formalin and embedded in paraffin. Seven-micrometer skin sections were prepared and stained with hematoxylin and eosin (H&E, Goode Histolabs, New Brunswick, NJ). Slides were scanned on an Olympus VS120 Virtual Microscopy system (Waltham, MA).
Immunofluorescence and Confocal Microscope
Paraffin-embedded tissue sections (7–10 μm) were deparaffinized and rehydrated. Antigens were unmasked using heat-mediated citrate buffer. Double immunofluorescent studies were carried out by blocking the sections with CAS-BLOCK (Invitrogen Corporation) or 10% donkey serum for 1 hr at room temperature, followed by sequential incubation with a goat anti-laminin γ2 (LCC, 1;100, Santa Cruz Biotechnology, Inc.), and either rabbit anti-p16INK4a (1:30, Santa Cruz Biotechnology, Inc.), or rabbit polyclonal anti-laminin γ2 (L4, a gift from Dr. Takako Sasaki) (Sasaki et al., 2001), diluted in PBS/0.05% Tween-20/1.5% Normal Donkey Serum (NDS) or appropriate IgG controls. After overnight incubation at 4°C, slides were treated with appropriate secondary antibodies (donkey anti-goat Alexa Fluor 568 or donkey anti-rabbit Alexa Fluor 488 1:1,000, Invitrogen) for 1 hr at room temperature in the dark. Sections were counterstained with DAPI and mounted in ProLong Gold anti-fade reagent (Invitrogen Corporation). 3D Z-stacks of confocal images were collected using a Zeiss LSM 510 Meta Confocal Microscope equipped with multitrack laser lines to select from UV 405 nm, tunable argon, and two HeNe lasers (594 and 633 nm). Confocal projection images were created to be viewed down into the image stack along the Z-axis.
Statistics
The mRNA expression levels between SM treated and control samples at each time point (N = 10 per group) are expressed as fold change and presented as mean ± standard error (SE). The mean was calculated and analyzed for significance at P ≤ 0.05 using ANOVA followed by the unpaired Student’s t test. Naïve unexposed controls were compared to SM treated skin (SigmaStat, Systat, San Jose, CA).
RESULTS
Structural Changes of Mouse Ear Skin Exposed to SM
Control mouse ear skin shows tight DEJ between the epidermis and the dermis below (Fig. 2A). Following SM exposure, damage to the epithelium increases with time; necrosis of basal cells becomes apparent as does the appearance of subepidermal blisters (Fig. 2B–D). Over time, SM induces blister formation as shown by the separation of the epidermis and dermis in the basement membrane zone (Fig. 2B). Exposure to SM also causes an influx of inflammatory cells to the wound area. Severe epidermal hyperplasia was also observed in skin post SM exposure (Fig. 2C–D).
Figure 2.

Structural changes in mouse ear skin following exposure to sulfur mustard (SM). Tissue sections from mouse ears were prepared for histology post SM exposure and stained with hematoxylin and eosin. The ventral (inner surface) ear skin of unexposed control tissue (A) and 24, 72, and 168 hr post SM (B–D) are presented. SM-induced microblisters as shown by the separation of epidermis from the dermis below (indicated by black arrows) at 24 hr post exposure (B). Mouse ear epidermis increased from 1 to 2 cells thick to more than 5–6 cells thick post SM exposure. Severe epidermal hyperplasia (H) was observed in skin at 72–168 hr post SM exposure (indicated by arrows with a dashed line, C–D). All images are shown in the same magnification, scale bar = 50 μm.
Differential Expression of LM-332 (α3β3γ2) in SM-Induced Skin Wounds
To quantify mRNA expression of LM-332, we used TaqMan gene expression assays and observed differential gene expression for the three chains (LAMA3, LAMB3, and LAMC2) of LM-332 after SM exposure (Fig. 3). Significant downregulation of mRNA expression of the laminin α3 chain (LAMA3, 0.4-fold) and β3 chain (LAMB3, 0.3-fold) was observed in skin 24 hr post SM exposure. In contrast, mRNA for the laminin γ2 (LAMC2) chain increased with time and was preferentially upregulated in SM-induced skin wounds at 72 hr (23.8-fold), and 168 hr (9.4-fold) post exposure.
Figure 3.

Laminin-332 mRNA expression in mouse ear skin following exposure to sulfur mustard (SM). Three separate LM-332 gene products were evident in mouse ear skin exposed to SM; LM α3 shown in green, LM β3 shown in yellow, and LM γ2 shown in purple. mRNA expression levels are normalized to unexposed control samples and expressed as relative fold change. The relative fold change of the unexposed control sample is considered as one-fold (marked by the dotted line). Data are presented as mean ± SE (N = 10) and analyzed for statistical significance using ANOVA and the unpaired Student’s t test. Statistic data with P ≤ 0.05 (marked by *) are considered as significantly different from the unexposed control sample.
In Situ Hybridization of the Laminin-γ2 Chain in SM-Induced Skin Injury
To detect the γ2 chain mRNA transcripts in skin post SM exposure, in situ hybridization using both sense and antisense probes was performed. In control tissues, laminin γ2 chain mRNA was distributed throughout the interfollicular and follicular epithelium on the ventral side of mouse ear skin (Fig. 4A). At 24 hr post SM exposure, the laminin γ2 chain transcripts continue to be expressed in the interfollicular epithelium; reduced amounts were expressed in hair follicles (Fig. 4B). By 72–168 hr post SM, expression of laminin γ2 chain transcripts were mainly distributed in basal keratinocytes with some positive cells near the adjacent suprabasal keratinocytes (Fig. 4C–D, marked by arrows). In this study, SM was directly applied to the ventral ear skin, but not the dorsal side of the ear skin. However, contralateral ear damage was also apparent in the dorsal ear skin. The numbers of laminin γ2 chain positive cells in the dorsal side of the ear skin also increased with time (Fig. 4E–G).
Figure 4.
In situ hybridization of laminin γ2 chain transcripts in mouse ear skin treated with sulfur mustard (SM). The antisense laminin γ2 probe hybridized to mouse ear skin is shown: (A) unexposed control skin; (B–D), 24–168 hr post SM ventral ear skin; (E–G), 24–168 hr post SM dorsal ear skin, scale bar = 50 μm. The images of the epidermis of the ventral (inner surface) ear skin is oriented to the top; whereas the epidermis of the dorsal (outer surface) ear skin is oriented to the bottom. Arrows delineate laminin γ2 chain positive cells.
Detection of the Laminin γ2 and its Fragments in SM Treated Mouse Ear Skin
To confirm expression of laminin γ2 protein in skin following SM exposure, Western blot analysis was performed using a specific monoclonal antibody against laminin γ2. This antibody recognizes epitopes of laminin γ2 with protein sizes of 150, 105, and 80 kDa (Fig. 1B). Laminin γ2 protein expression at band sizes of 150 and 105 kDa was evident in control tissue (Fig. 5). Following SM exposure, the skin expressed the 150 and 105 kDa bands of laminin γ2 as well as an 80 kDa band of laminin γ2. The 105 and 80 kDa bands are the two major processed forms of laminin γ2 and these increased with time 24–168 hr post SM exposure. Mouse ear tissue also overexpressed the 80 kDa form of laminin γ2 72–168 hr post SM (Fig. 5).
Figure 5.
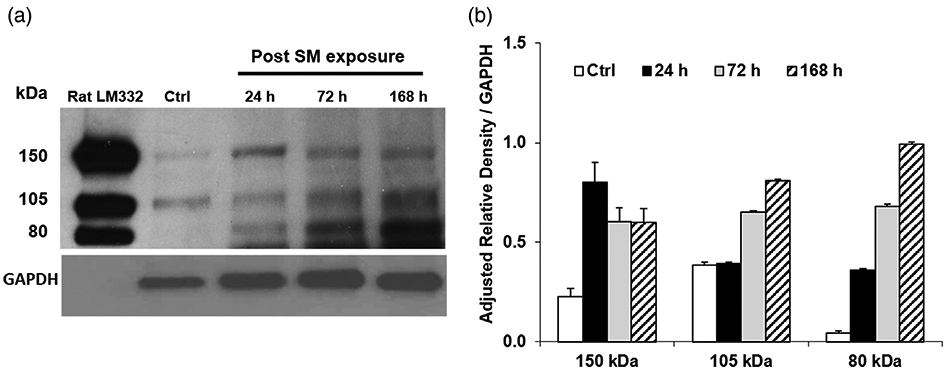
Effects of sulfur mustard (SM) on laminin γ2 protein expression in mouse ear skin. (A) Representative Western blot of laminin γ2. Lysates were prepared from mouse ear skin 24, 72, and 168 hr following SM treatment and analyzed for expression of laminin γ2 using LCC antibodies which recognize epitopes in the long arm coiled-coil region near C-terminus of the protein. Note significant upregulation of the proform (~150 kDa) and two major processed (~105 and ~80 kDa) forms of laminin γ2 expression that were observed after 72 and 168 hr post SM exposure. Only two bands (~150 kDa, γ2, and ~105 kDa, γ2′) of laminin γ2 were expressed in control skin. Purified rat laminin-332 was used as positive control; laminin γ2 (LCC) antibodies cross-reacts with both rat and mouse laminin γ2. (B) Laminin γ2 protein expression in mouse ear skin following SM exposure. Densitometry of the western blot data was performed using Image J. The adjusted relative density (ARD) normalized to GAPDH expressed as the integrals (area mean density) for the 150 kDa bands were untreated control, 0.23; 24 hr, 0.80; 72 hr, 0.61; 168 hr, 0.60. The ARD normalized to GAPDH expressed as the integrals for the 105 kDa bands were untreated control, 0.38; 24 hr, 0.39; 72 hr, 0.65; 168 hr, 0.81. The ARD normalized to GAPDH expressed as the integrals for 80 kDa bands were untreated control, 0.04; 24 hr, 0.36; 72 hr, 0.68; 168 hr, 0.99. Values are expressed as means ± standard error of the mean.
Localization of Laminin-γ2 Fragments in Mouse Ear Skin
To localize laminin γ2 in mouse ear skin, we performed a dual-label confocal immunofluorescence study using two specific laminin γ2 antibodies. These antibodies recognize several epitopes of laminin γ2: LCC (laminin-γ2) antibody binds to the long coiled-coil region near the C-terminus of the protein and recognizes laminin γ2, γ2′, and γ2x (Fig. 1B), and L4 (laminin γ2) antibody which targets the processed short arm of laminin γ2 (including γ2, γ2-sa, and γ2-pf) near the EGF-like globular domain IV (Fig. 1C). Laminin γ2 was expressed in the basal keratinocytes and hair follicles of control skin samples as detected by both laminin γ2 (LCC and L4) antibodies (data not shown). In skin exposed to SM, laminin γ2 was predominately localized in the hyperplastic epidermis behind the leading edge in SM injured skin at 72 hr post exposure. Using the LCC antibody, the laminin γ2 was expressed in the cytoplasm of basal cells. In addition, intense fluorescence for LCC was observed mostly in the outer layers of the suprabasal epithelium (in red, Fig. 6A). Using the L4 antibody, intense laminin γ2 fluorescence was evident throughout the suprabasal epithelium (in green, Fig. 6B). Co-expression of the two laminin γ2 antibodies (in yellow, Fig. 6C) were observed in some basal keratinocytes and in the outermost epithelial cell layer of SM-induced skin wounds 72 hr post exposure.
Figure 6.

Effects of sulfur mustard (SM) on laminin γ2 expression in mouse ear skin. Tissue sections from mouse ear skin were analyzed 72 hr post SM exposure with laminin γ2 specific primary antibodies targeting epitopes in different regions of laminin γ2 polypeptide chain. Laminin γ2 (LCC) recognizes epitopes in the long arm coiled-coil region near C-terminus (shown in red, A). Laminin γ2 (L4) recognizes epitopes in the short arm globular domain (shown in green, B). Merged image shows co-expression of laminin γ2 (LCC) and laminin γ2 (L4) in yellow (C). Projected confocal microscopic images are presented with the epidermis on top; the basement membrane in the DEJ indicated by the white dotted line; leading edge indicated by arrows. Nuclei counterstained with DAPI (shown in blue), scale bar = 10 μm.
Co-Expression of p16INK4a and Laminin γ2 at the SM-Induced Wound Margin
We next analyzed expression of the senescent marker, p16INK4a, and laminin γ2 (LCC) using confocal microscopy. At 72 hr post SM exposure, strong expression of laminin γ2 was observed in the suprabasal keratinocytes of the hyperplastic epithelium (shown in red, Fig. 7A). Numerous p16INK4a positive cells were also visible throughout the epidermis. In particular, p16INK4a expression was observed in the outermost layers of the hyperplastic epidermis (shown in green, Fig. 7B). Co-expression of the senescent marker (p16INK4a) and laminin γ2 was found in areas of epidermal hyperplasia of SM-induced skin wounds (shown in yellow, Fig. 7C).
Figure 7.

Co-expression of p16INK4a and laminin γ2 in sulfur mustard (SM)-induced skin wounds. Mouse ear tissue sections 72 hr post SM exposure were analyzed by dual labeling immunofluorescence confocal microscopy for laminin γ2 (shown in red, A) and the senescence marker, p16INK4a (shown in green, B). Merged image shows co-localization of p16INK4a and laminin γ2 in yellow (C). Projected confocal microscopic images of the 72 hr SM damaged skin sample are presented with epidermis on the top; the basement membrane in the DEJ is indicated by the white dotted line. Nuclei were counterstained with DAPI (shown in blue), scale bar = 10 μm.
DISCUSSION
LM-332 is a vital adhesive glycoprotein located in the basement membrane zone and acts to connect the epidermis to the dermis, a function important in maintaining skin integrity. Gene mutations in LM-332 as well as structural damage to the protein may cause it to lose its adhesive properties; this can result in separation of the epidermis from the dermis and skin blistering (Uitto et al., 1994, 1997; Hirsch et al., 2017; Rousselle et al., 2019). LM-332 protein is a heterotrimer that requires the transcription and translation of three chains (α3, β3, and γ2) before becoming assembled and secreted into the extracellular matrix for keratinocyte attachment (Matsui et al., 1995b; Cheng et al., 1997; Champliaud et al., 2000; Rousselle and Beck, 2013). In the present studies, we found that all three chains of LM-332 are transcribed in approximately equal amounts in control mouse ear skin. However, in skin treated with SM, there is substantial overexpression of mRNA for the laminin γ2 chain. This was also evident in in situ hybridization experiments where increased levels of laminin γ2 transcript were found mainly in basal keratinocytes, hair follicles, and in the leading edge of SM-induced skin wounds. Laminin γ2 is known to have dual functions. It acts as an adhesion factor for cell attachment in the basement membrane, as well as promoting cell migration (Miyazaki et al., 1993; Rousselle et al., 2019). The overexpression of γ2 chain in SM-induced skin wounds may play a role in stimulating migration during wound repair.
The laminin γ2 protein appears as several bands at distinct molecular weights in SDS polyacrylamide gels. These laminin γ2 fragments may have slightly different functions (Sasaki et al., 2001; Ogawa et al., 2004, 2007; Hamasaki et al., 2011). The laminin γ2 is secreted as a proform (γ2, 150 kDa) into the extracellular matrix and is partially processed to a 105 kDa (γ2′) form that is important for cell attachment (Gagnoux-Palacios et al., 1996, 2001). The 80 kDa fully processed form (γ2x) represents the coiled-coil rod region alone without globular domains. In vitro studies have shown that monomeric globular domains of laminin γ2 can be further proteolytically processed into additional fragments including γ2-sa (80 kDa) and γ2-pf (45 kDa) which when purified, have been found to stimulate cellular migration (Sasaki et al., 2001; Ogawa et al., 2004, 2007; Sato et al., 2014). In our model, western blot data showed significant increases of laminin γ2 at the protein level, suggesting de novo laminin γ2 synthesis in skin post SM exposure. We found only two bands (150 and 105 kDa) in unexposed control tissues. The 80 kDa band (γ2x), which was not observed in control skin, first appeared in SM treated tissues after 24 hr, becoming more abundant after 72–168 hr post SM. The LM-γ2x (80 kDa) fragments may be generated from LM-γ2 (150 kDa, γ2, and 105 kDa, γ2′ forms) following cleavage by matrix metalloproteinases (MMPs) in SM treated skin. Our previous studies showed upregulation of MMPs in SM-induced skin wounds (Shakarjian et al., 2006; Gerecke et al., 2009; Chang et al., 2014, 2018b).
The short arm globular domain fragments of laminin γ2 are reported to promote cellular migration in invasive carcinoma cells (Gagnoux-Palacios et al., 2001; Katayama et al., 2003; Tsubota et al., 2010). In our model, the laminin γ2 fragmentation observed post SM exposure likely plays a role in promoting cellular migration for wound repair. However, SM-induced skin wound progression is often prolonged or recurring. Hyperplasia, stimulated by inflammation and injury, corresponds to proliferation and migration of keratinocytes for wound healing. Since hyperplasia becomes prominent in vesicant skin wounds by 72 hr post SM exposure (Chang et al., 2009, 2013, 2014), we examined laminin γ2 expression in the hyperplastic epidermis using two specific laminin γ2 antibodies. One laminin γ2 antibody (LCC, Fig. 1B) targets regions of the coiled-coil long arm and recognized the full-length proform (γ2, 150 kDa), the partly processed form (γ2′, 105 kDa), and the coiled-coil long arm minus the globular domain (γ2x, 80 kDa) of the laminin-γ 2 chain. The second laminin γ2 antibody (L4, Fig. 1C) targets regions of the short arm globular domain and recognized the full-length proform (γ2, 150 kDa) and the short arm globular fragments (γ2-sa, ~80 kDa; γ2-pf, ~45 kDa) of the laminin γ2 chain. It is of interest that the two antibodies showed different expression patterns. The long arm of laminin γ2 expression identified by the LCC antibody was mostly restricted to basal keratinocytes and suprabasal keratinocytes in the hyperplastic epithelium (Fig. 6A). We speculate that the expression observed in the basal keratinocytes may represent the full-length proform laminin γ2 (γ2, Fig. 1B) required for cell attachment in the basement membrane whereas those expressed in the suprabasal keratinocytes may represent the processed forms (γ2′ and γ2x, Fig. 1B) important in cell migration.
If instead we examine the distribution of the L4 antibody that recognizes the short arm fragments of laminin γ2 (γ2-sa, and γ2-pf, Fig. 1C); we observe that the processed short arm fragments of laminin γ2 co-express with LCC in the suprabasal keratinocytes. The co-expression recognized by L4 and LCC antibodies are likely fragments of laminin γ2. The short arm fragments of laminin γ2 (γ2-sa, and γ2-pf) identified by the L4 antibody have a wider distribution with expression throughout the epidermis (Fig. 6B). Co-expression of LCC and L4 in the suprabasal layers suggest keratinocytes migration behind the leading edge of SM-induced skin wounds (Fig. 6C). This compliments our western blot data which showed increased expression of the unprocessed-full size, laminin γ2 proform (150 kDa), and the preferentially expressed processed forms of γ2 (105 and 80 kDa) in tissues 72–168 hr post SM exposure. Furthermore, the laminin γ2 fragments appeared to express in keratinocytes that left the cell-cycle for migration. This is confirmed by our dual-label immunofluorescent studies using specific antibodies targeting laminin γ2 and the senescent marker p16INK4a. Laminin γ2 and p16INK4a mainly co-localize in suprabasal keratinocytes. Co-expression of these proteins has been reported in invasive carcinoma cells (Natarajan et al., 2005, 2006). In our studies, the co-expression of laminin γ2 and p16INK4a in the suprabasal keratinocytes indicate that they are more similar to the molecules expressed by invasive epithelial carcinoma cells and chronic ulcers than those expressed by superficial skin wounds. This suggests that the suprabasal keratinocytes in SM-induced skin wounds become hypermotile for wound repair. However, studies show SM-induced wound healing is prolonged, lasting beyond 7 days post exposure, and is frequently associated with hypertrophic scar formation (Graham and Schoneboom, 2013). The motility of the migrating keratinocytes is a critical factor to promote wound healing in skin post SM exposure. Longer term studies are required to elucidate the role of laminin γ2 fragments in SM-induced skin wounds. In addition, SM exposure results in cell dysfunction and triggers the endoplasmic reticulum stress (ERS) response. In a previous report, we observed that the ERS-induced apoptotic gene, GADD153/CHOP, had a similar expression pattern to that of the senescent marker, p16INK4a (Chang et al., 2013). This suggests that cell survival and apoptosis are occurring simultaneously. Wound repair is complex; skin exposed to SM may cause extreme cellular damage which can lead to many perturbations in cell growth and differentiation leading to a delay in wound healing.
Overall, our results indicate that laminin γ2 is preferentially expressed in skin post SM exposure. The laminin γ2 protein expression appeared fragmented and expressed throughout the suprabasal keratinocytes in SM-induced skin wounds. Co-expression of laminin γ2 with p16INK4a was also observed in suprabasal keratinocytes suggesting motility of the migrating keratinocytes behind the leading edge in skin wounds post SM exposure. Laminin γ2 and its fragments may play a role in regulating SM-induced wound repair. These observations may provide information regarding novel targets for the development of countermeasures against SM damage. Alternatively, our data may help to identify targets that might enhance the migration of epithelial cells for wound repair while minimizing the potential for scarring.
ACKNOWLEDGEMENTS
This research was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), grant number U54AR055073. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. This work was also supported in part by National Institutes of Health grants P30ES005022, and T32ES007148.
Grant sponsor: National Institute of Arthritis and Musculoskeletal and Skin Diseases; Grant numbers: ES005022, T32ES007148, U54AR055073.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Literature Cited
- Achanta S, Chintagari NR, Brackmann M, Balakrishna S, Jordt SE. 2018. TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation. Toxicol Lett 293:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, Keene DR, Hudson DL, Nishiyama T, Lee S, et al. 2000. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem 275:22728–22735. [DOI] [PubMed] [Google Scholar]
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD, Blanpain C. 2017. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun 8:14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, El Khal A, Knoss N, Tunggal L. 2003. Laminin 5 processing and its integration into the ECM. Matrix Biol 22:49–54. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Rousselle P. 1999. Laminins of the dermo-epidermal junction. Matrix Biol 18:19–28. [DOI] [PubMed] [Google Scholar]
- Brittingham R, Uitto J, Fertala A. 2006. High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun 343:692–699. [DOI] [PubMed] [Google Scholar]
- Casillas RP, Mitcheltree LW, Stemler FW. 1997. The mouse ear model of cutaneous sulfur mustard injury. Toxicol Method 7:381–397. [Google Scholar]
- Champliaud MF, Virtanen I, Tiger CF, Korhonen M, Burgeson R, Gullberg D. 2000. Posttranslational modifications and beta/gamma chain associations of human laminin alpha1 and laminin alpha5 chains: Purification of laminin-3 from placenta. Exp Cell Res 259:326–335. [DOI] [PubMed] [Google Scholar]
- Chang YC, Gordon MK, Gerecke DR. 2018a. Expression of laminin 332 in vesicant skin injury and wound repair. Clin Dermatol (Wilmington) 2(1), 115. [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Sabourin CL, Lu SE, Sasaki T, Svoboda KK, Gordon MK, Riley DJ, Casillas RP, Gerecke DR. 2009. Upregulation of gamma-2 laminin-332 in the mouse ear vesicant wound model. J Biochem Mol Toxicol 23:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Soriano M, Hahn RA, Casillas RP, Gordon MK, Laskin JD, Gerecke DR. 2018b. Expression of cytokines and chemokines in mouse skin treated with sulfur mustard. Toxicol Appl Pharmacol 355:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Wang JD, Hahn RA, Gordon MK, Joseph LB, Heck DE, Heindel ND, Young SC, Sinko PJ, Casillas RP, et al. 2014. Therapeutic potential of a non-steroidal bifunctional anti-inflammatory and anti-cholinergic agent against skin injury induced by sulfur mustard. Toxicol Appl Pharmacol 280:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Wang JD, Svoboda KK, Casillas RP, Laskin JD, Gordon MK, Gerecke DR. 2013. Sulfur mustard induces an endoplasmic reticulum stress response in the mouse ear vesicant model. Toxicol Appl Pharmacol 268:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. 1997. Self-assembly of laminin isoforms. J Biol Chem 272:31525–31532. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo D, Tinaburri L, Dellambra E. 2017. The role of p16 (INK4a) pathway in human epidermal stem cell self-renewal aging and cancer. Int J Mol Sci 18(7), 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Allegra M, Spirito F, Pommeret O, Romero C, Ortonne JP, Meneguzzi G. 2001. The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J Cell Biol 153:835–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Vailly J, Durand-Clement M, Wagner E, Ortonne JP, Meneguzzi G. 1996. Functional re-expression of laminin-5 in laminin-gamma2-deficient human keratinocytes modifies cell morphology, motility, and adhesion. J Biol Chem 271:18437–18444. [DOI] [PubMed] [Google Scholar]
- Gerecke DR, Chen M, Isukapalli SS, Gordon MK, Chang YC, Tong W, Androulakis IP, Georgopoulos PG. 2009. Differential gene expression profiling of mouse skin after sulfur mustard exposure: Extended time response and inhibitor effect. Toxicol Appl Pharmacol 234:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles C, Polette M, Coraux C, Tournier JM, Meneguzzi G, Munaut C, Volders L, Rousselle P, Birembaut P, Foidart JM. 2001. Contribution of MT1-MMP and of human laminin-5 gamma2 chain degradation to mammary epithelial cell migration. J Cell Sci 114:2967–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JS, Schoneboom BA. 2013. Historical perspective on effects and treatment of sulfur mustard injuries. Chem Biol Interact 206:512–522. [DOI] [PubMed] [Google Scholar]
- Hamasaki H, Koga K, Aoki M, Hamasaki M, Koshikawa N, Seiki M, Iwasaki H, Nakayama J, Nabeshima K. 2011. Expression of laminin 5-γ2 chain in cutaneous squamous cell carcinoma and its role in tumour invasion. Br J Cancer 105:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, et al. 2017. Regeneration of the entire human epidermis using transgenic stem cells. Nature 551:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph LB, Gerecke DR, Heck DE, Black AT, Sinko PJ, Cervelli JA, Casillas RP, Babin MC, Laskin DL, Laskin JD. 2011. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Exp Mol Pathol 91:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen T, Hakkinen L, Hamidi S, Larjava K, Kallioinen M, Peltonen J, Salo T, Larjava H, Oikarinen A. 1998. Laminin-5 expression is independent of the injury and the microenvironment during reepithelialization of wounds. J Histochem Cytochem 46:353–360. [DOI] [PubMed] [Google Scholar]
- Katayama M, Sanzen N, Funakoshi A, Sekiguchi K. 2003. Laminin gamma2-chain fragment in the circulation: A prognostic indicator of epithelial tumor invasion. Cancer Res 63:222–229. [PubMed] [Google Scholar]
- Katayama M, Sekiguchi K. 2004. Laminin-5 in epithelial tumour invasion. J Mol Histol 35:277–286. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. 2005. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem 280:88–93. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP. 2008. Discovery of basement membrane components. J Invest Dermatol 128:E3–E4. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Keene DR, Rimberg CS, Burgeson RE. 1993. Cellular origin of the dermal-epidermal basement membrane. Dev Dyn 197:255–267. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. 1992. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem 267:17900–17906. [PubMed] [Google Scholar]
- Martin P 1997. Wound healing—Aiming for perfect skin regeneration. Science 276:75–81. [DOI] [PubMed] [Google Scholar]
- Matsui C, Nelson CF, Hernandez GT, Herron GS, Bauer EA, Hoeffler WK. 1995a. Gamma 2 chain of laminin-5 is recognized by monoclonal antibody GB3. J Invest Dermatol 105:648–652. [DOI] [PubMed] [Google Scholar]
- Matsui C, Wang CK, Nelson CF, Bauer EA, Hoeffler WK. 1995b. The assembly of laminin-5 subunits. J Biol Chem 270:23496–23503. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Kikkawa Y, Nakamura A, Yasumitsu H, Umeda M. 1993. A large cell-adhesive scatter factor secreted by human gastric carcinoma cells. Proc Natl Acad Sci USA 90:11767–11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Inman AO, Babin MC, Casillas RP. 1999. Immunohistochemical characterization of the basement membrane epitopes in bis(2-chloroethyl) sulfide-induced toxicity in mouse ear skin. J Appl Toxicol 19:313–328. [DOI] [PubMed] [Google Scholar]
- Mouret S, Wartelle J, Batal M, Emorine S, Bertoni M, Poyot T, Clery-Barraud C, Bakdouri NE, Peinnequin A, Douki T, et al. 2015. Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice. Toxicol Lett 232:68–78. [DOI] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD 2nd, Guo Z, Hopkinson S, Lazar AJ, Brenn T, Jones JC, Rheinwald JG. 2006. A keratinocyte hypermotility/growth-arrest response involving laminin 5 and p16INK4a activated in wound healing and senescence. Am J Pathol 168:1821–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD 2nd, Jones JC, Rheinwald JG. 2005. Co-expression of p16INK4a and laminin 5 by keratinocytes: A wound-healing response coupling hypermotility with growth arrest that goes awry during epithelial neoplastic progression. J Investig Dermatol Symp Proc 10:72–85. [DOI] [PubMed] [Google Scholar]
- Natarajan E, Saeb M, Crum CP, Woo SB, McKee PH, Rheinwald JG. 2003. Co-expression of p16(INK4A) and laminin 5 gamma2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture. Am J Pathol 163:477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NM, Pulkkinen L, Schlueter JA, Meneguzzi G, Uitto J, Senior RM. 2006. Lung development in laminin gamma2 deficiency: Abnormal tracheal hemidesmosomes with normal branching morphogenesis and epithelial differentiation. Respir Res 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Tsubota Y, Hashimoto J, Kariya Y, Miyazaki K. 2007. The short arm of laminin gamma2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin beta4 chain. Mol Biol Cell 18:1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Tsubota Y, Maeda M, Kariya Y, Miyazaki K. 2004. Regulation of biological activity of laminin-5 by proteolytic processing of gamma2 chain. J Cell Biochem 92:701–714. [DOI] [PubMed] [Google Scholar]
- Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, Tryggvason K. 1994. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol 145:782–791. [PMC free article] [PubMed] [Google Scholar]
- Raja SK, Garcia MS, Isseroff RR. 2007. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front Biosci 12:2849–2868. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Beck K. 2013. Laminin 332 processing impacts cellular behavior. Cell Adh Migr 7:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Keene DR, Ruggiero F, Champliaud MF, Rest M, Burgeson RE. 1997. Laminin 5 binds the NC-1 domain of type VII collagen. J Cell Biol 138:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Montmasson M, Garnier C. 2019. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol 75–76:12–26. [DOI] [PubMed] [Google Scholar]
- Sabourin CL, Danne MM, Buxton KL, Casillas RP, Schlager JJ. 2002. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. J Biochem Mol Toxicol 16:263–272. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Mann K, Brakebusch C, Yamada Y, Fassler R, Timpl R. 2001. Short arm region of laminin-5 gamma2 chain: Structure, mechanism of processing and binding to heparin and proteins. J Mol Biol 314:751–763. [DOI] [PubMed] [Google Scholar]
- Sato H, Oyanagi J, Komiya E, Ogawa T, Higashi S, Miyazaki K. 2014. Amino-terminal fragments of laminin gamma2 chain retract vascular endothelial cells and increase vascular permeability. Cancer Sci 105:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakarjian MP, Bhatt P, Gordon MK, Chang YC, Casbohm SL, Rudge TL, Kiser RC, Sabourin CL, Casillas RP, Ohman-Strickland P, et al. 2006. Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure. J Appl Toxicol 26:239–246. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. 2010. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci 114:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. 1999. Cutaneous wound healing. N Engl J Med 341:738–746. [DOI] [PubMed] [Google Scholar]
- Su Y, Richmond A. 2015. Chemokine regulation of neutrophil infiltration of skin wounds. Adv Wound Care (New Rochelle) 4:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota Y, Ogawa T, Oyanagi J, Nagashima Y, Miyazaki K. 2010. Expression of laminin gamma2 chain monomer enhances invasive growth of human carcinoma cells in vivo. Int J Cancer 127:2031–2041. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L. 1996. Molecular complexity of the cutaneous basement membrane zone. Mol Biol Rep 23:35–46. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, Christiano AM. 1994. Molecular basis of the dystrophic and junctional forms of epidermolysis bullosa: Mutations in the type VII collagen and kalinin (laminin 5) genes. J Invest Dermatol 103:39S–46S. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, McLean WH. 1997. Epidermolysis bullosa: A spectrum of clinical phenotypes explained by molecular heterogeneity. Mol Med Today 3:457–465. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Nakagawa M, Kiyokawa H, Yoshida E, Yoshimura T, Koshikawa N, Itoh F, Seiki M. 2019. Unique biological activity and potential role of monomeric laminin-γ2 as a novel biomarker for hepatocellular carcinoma: A review. Int J Mol Sci 20 (1), 226. [DOI] [PMC free article] [PubMed] [Google Scholar]



